-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEstimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
Leigh Johnson and colleagues model the HIV epidemic in South Africa, using national level health statistics as inputs, to estimate the number of deaths avoided and extra years of life gained due to antiretroviral programs.
Published in the journal: . PLoS Med 14(12): e32767. doi:10.1371/journal.pmed.1002468
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002468Summary
Leigh Johnson and colleagues model the HIV epidemic in South Africa, using national level health statistics as inputs, to estimate the number of deaths avoided and extra years of life gained due to antiretroviral programs.
Introduction
Substantial declines in adult all-cause mortality have been observed in South Africa since the mid-2000s [1–4], consistent with trends in other African countries [5–8]. Although these reductions are commonly attributed to the impact of antiretroviral treatment (ART) on HIV-related mortality, there has been no formal assessment of the extent to which the observed reduction in mortality is explained by the introduction of ART. A number of other factors could partly explain the reductions in mortality. First, adult HIV incidence in South Africa and other African countries peaked in the 1990s and has since declined substantially [9–11]. Some of the reduction in mortality may thus be due to the stage of the HIV epidemic. Second, HIV may be evolving towards a less virulent form [12,13]. Third, reductions in all-cause mortality could be a reflection of reductions in non-HIV mortality. By fitting mathematical models to mortality and HIV prevalence data, it is possible to evaluate which factors best explain the observed reductions in adult mortality.
Most modelling studies have relied on data from ART cohorts established for research purposes, such as those participating in the International Epidemiology Databases to Evaluate AIDS (IeDEA) collaboration [14]. However, estimation of mortality from these cohorts is challenging, and few modelling studies have considered the biases that may arise. Mortality rates are highly heterogeneous between cohorts [15,16], and there is concern that research cohorts may not be representative of routine ART services in developing countries. There is also concern that average follow-up times in ART cohorts are typically short, such that mortality estimates are biased towards the high mortality observed at short ART durations [17]. Even when mathematical models allow for differences in mortality by ART duration, they typically do so using a piecewise-constant assumption [18,19], which leaves potential for exaggeration of mortality at longer ART durations. Finally, many patients who die while on ART are not recorded as having died and are instead classified as lost to follow-up [20], leading to underestimation of mortality.
South Africa is an ideal setting in which to evaluate the impact of ART on mortality at a population level, as it has one of the highest rates of vital registration in sub-Saharan Africa [21], with around 94% of all adult deaths recorded in recent years [22–25]. It also has well-established ART research cohorts, many of which participate in the IeDEA–Southern Africa (IeDEA-SA) collaboration [26]. By linking patient records to the South African vital registration system, researchers have been able to identify an estimated 96.2% of all adult deaths in patients with ID numbers [22]. South Africa’s public sector ART programme began in 2004, and although the early rollout of ART was hindered by the Mbeki government [27], ART access subsequently improved dramatically [28], with the South African ART programme becoming the biggest in the world.
This study aims to evaluate how much of the reduction in adult mortality in South Africa is attributable to the impact of ART, using a mathematical modelling approach. As most previous mathematical models of HIV in Africa have been calibrated only to HIV prevalence data, this study also aims to assess how sensitive model estimates of HIV mortality are to the inclusion of mortality data in the calibration process.
Methods
The Thembisa model was used to simulate HIV transmission, disease progression, and mortality in South Africa. The model has previously been described [19], and S1 Text provides a more detailed explanation of the model parameters most relevant to the present analysis; the model is also freely available online (https://www.thembisa.org, Version 3.2 downloads). Briefly, the model is a deterministic model that divides the population into age - and sex-specific cohorts and ‘risk groups’ that are defined in terms of marital status and propensity for concurrent partnerships. Non-HIV mortality assumptions are based on an analysis of South African cause-of-death statistics [2]. The simulation of the HIV epidemic starts in 1985, based on an assumed initial prevalence of HIV in women in the high risk group. HIV incidence is simulated based on assumptions about rates of partnership formation, commercial sex activity, marriage, coital frequency, and condom use (all of which vary in relation to age and sex), as well as assumed probabilities of transmission per sex act. Probabilities of HIV transmission per act of unprotected sex depend on sex, relationship type, and the HIV stage of the HIV-positive partner. Transmission probabilities are assumed to be reduced after ART initiation, depending on assumed rates of viral suppression, and the female-to-male transmission probability is also assumed to be reduced if the male partner is circumcised.
Progression of HIV disease
After acquiring HIV, adults (ages 15 years and older) are assumed to progress through 5 stages of HIV infection in the absence of ART (acute infection, CD4 ≥ 500 cells/μl, CD4 350–499 cells/μl, CD4 200–349 cells/μl, and CD4 < 200 cells/μl). Rates of progression between CD4 stages and untreated HIV mortality rates depend on the individual’s age and sex. Due to uncertainty regarding the average untreated HIV survival time and the effects of age and sex on HIV survival, prior distributions are assigned to represent the uncertainty around these parameters (Table 1). The model allows for a possible change in HIV virulence over time; a prior distribution represents the uncertainty around parameter E, the relative rate of progression between CD4 stages for each additional calendar year (Table 1). In year t, the rate of progression from one CD4 stage to the next is proportional to Et − 1999. Any change in virulence is likely to affect set point viral loads (SPVLs) [29–31], which in turn affect transmission probabilities [32–34]. The model therefore allows for a change over time in HIV transmission probabilities, with the transmission probability per sex act in year t being proportional to Eαϕ(t − 1999), where α is the ratio of the increase in infectivity (on a log scale) to the increase in disease progression (on a log scale) for each unit change in SPVL (Table 1), and ϕ is a constant (2.5).
Tab. 1. Comparison of prior and posterior distributions. 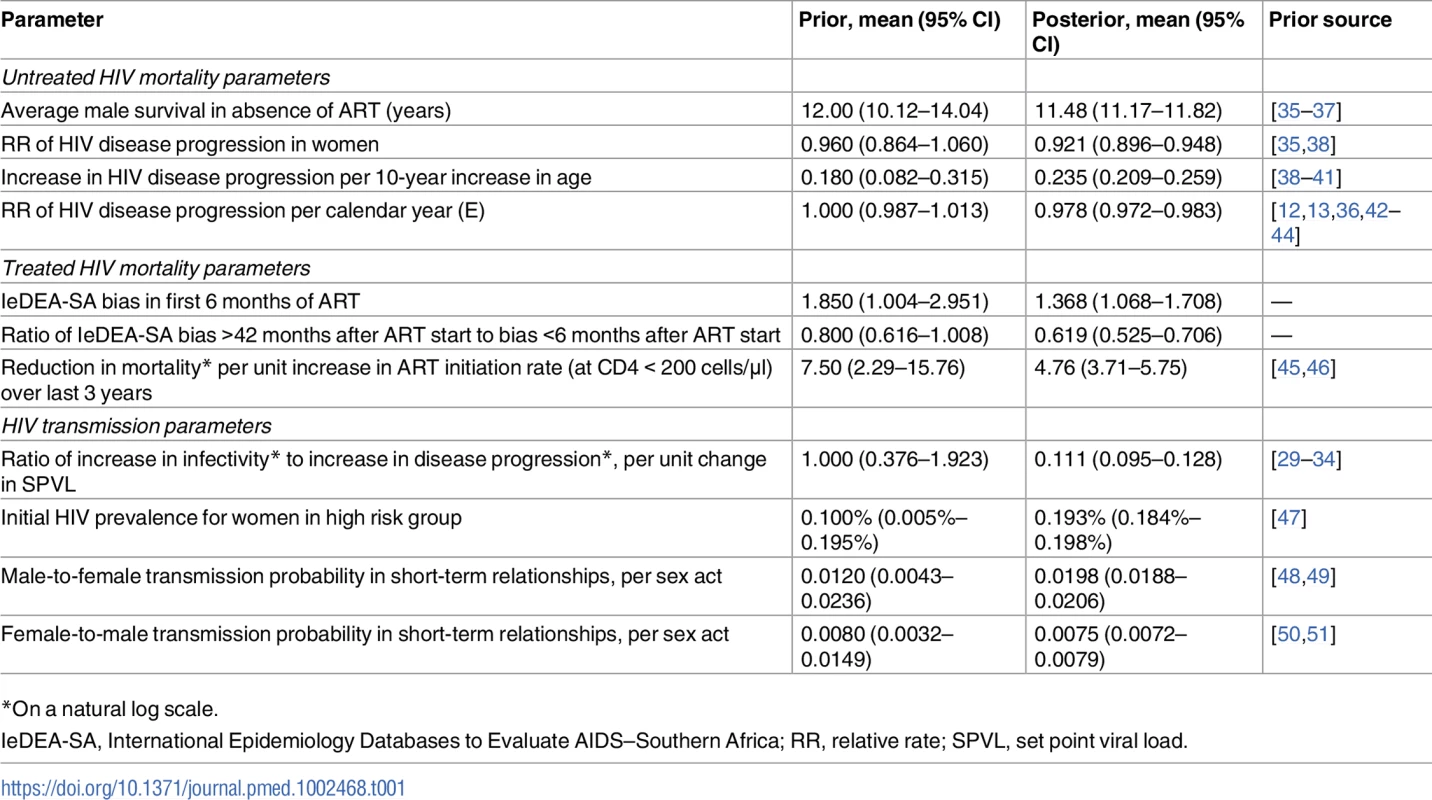
*On a natural log scale. The model simulates HIV testing and diagnosis and assumes that once individuals have been diagnosed, they can start ART immediately (if eligible) or defer ART. The model allows for changes over time in South African ART eligibility criteria, and allows for differences in ART initiation rates between ART-eligible diagnosed adults depending on sex and CD4 stage. The model has been calibrated to match the reported numbers of adults on ART in the public and private sectors [28].
Once individuals have started ART, their HIV-related mortality rates depend on their age, sex, CD4 stage at ART initiation, and time since ART initiation. These rates are based on relative survival models fitted to IeDEA-SA data; the models estimate HIV-specific mortality in excess of background (non-HIV) mortality after linking patient records to the national death registry [52]. To allow for the possibility that the IeDEA-SA estimates may be biased, 2 multiplicative adjustment factors are specified: one for the first 6 months after ART initiation and one for ART durations of over 42 months (adjustments at other durations are interpolated from these). Prior distributions are specified to represent the uncertainty around the first adjustment and the ratio of the second to the first (Table 1). The model allows the mortality rates in untreated individuals with CD4 < 200 cells/μl and treated individuals with baseline CD4 < 200 cells/μl to depend on the average rate of ART initiation over the last 3 years (since high rates of ART initiation mean that most patients starting ART with CD4 < 200 cells/μl will start ART soon after crossing the 200-cells/μl threshold, leaving relatively few untreated individuals at very low CD4 counts).
Calibration and validation
A Bayesian approach was adopted in fitting the model to South African HIV prevalence and mortality data. Although most of the HIV transmission and sexual behaviour parameters were fixed at the posterior means estimated when the model was previously fitted to HIV prevalence data [19], prior distributions were specified to represent the uncertainty in the 4 transmission parameters given in Table 1. Prior distributions were also specified for the 7 disease progression and mortality parameters given in Table 1. A likelihood function was defined to represent the model fit to the all-cause recorded death statistics over the 1997–2014 period [1], disaggregated by sex and 5-year age group, and adjusted for incomplete registration [22] and lower completeness in the period prior to 2004 [53]. Only the deaths in the 20–59-year age range were included for calibration purposes, as HIV contributes relatively little to mortality at older ages. The likelihood function also represents the model fit to age-specific HIV prevalence data from national antenatal clinic surveys (1997–2013) and household surveys (in 2005, 2008, and 2012) [54]. Posterior estimates of the model parameters that provide the best fit to the HIV prevalence and recorded death data were simulated using incremental mixture importance sampling [55]. Further technical details are provided in S1 Text. Posterior model estimates were validated by comparing the model estimates of the proportions of HIV-positive adults in different CD4 categories to survey estimates of the corresponding proportions. Model results were also compared to those obtained when only the HIV prevalence data were used in the likelihood function (i.e., the approach that has traditionally been used in the calibration of HIV models).
Counterfactual scenarios
The model simulations were compared with simulations in 2 counterfactual scenarios: a worst-case scenario that considers what would have happened in the absence of any ART and a best-case scenario that considers what would have happened if South Africa had followed WHO guidelines on ART eligibility immediately upon publication [56–60], with diagnosed patients starting ART an average of 6 months after reaching ART eligibility [61]. Adult life years saved by ART were estimated by subtracting the adult population size (individuals aged 15 years and older) in the worst-case scenario from that in the main scenario, and summing the differences over the period from 1 July 2000 to 30 June 2014. Similarly, potential life years gained in the best-case scenario were estimated by summing the differences in adult population size between the best - and worst-case scenarios.
Results
Model calibration
The model was in close agreement with recorded levels of adult mortality in South Africa, which rose rapidly to peak in 2006 before declining (Fig 1). Similar consistency was noted when the model was compared with recorded death statistics over each 5-year age range (Fig D in S1 Text). The model was also consistent with age - and sex-specific HIV prevalence levels in South African national surveys (Figs E and F in S1 Text). Model validations were performed against surveys of CD4 distributions in HIV-positive South African populations, and model estimates were found to be in reasonable agreement with survey data (Fig J in S1 Text). However, when the model was calibrated only to HIV prevalence data, model estimates of mortality substantially exceeded observed mortality after 2003 (Fig L in S1 Text).
Fig. 1. Trends in modelled and recorded deaths in adults (ages 20–59 years). 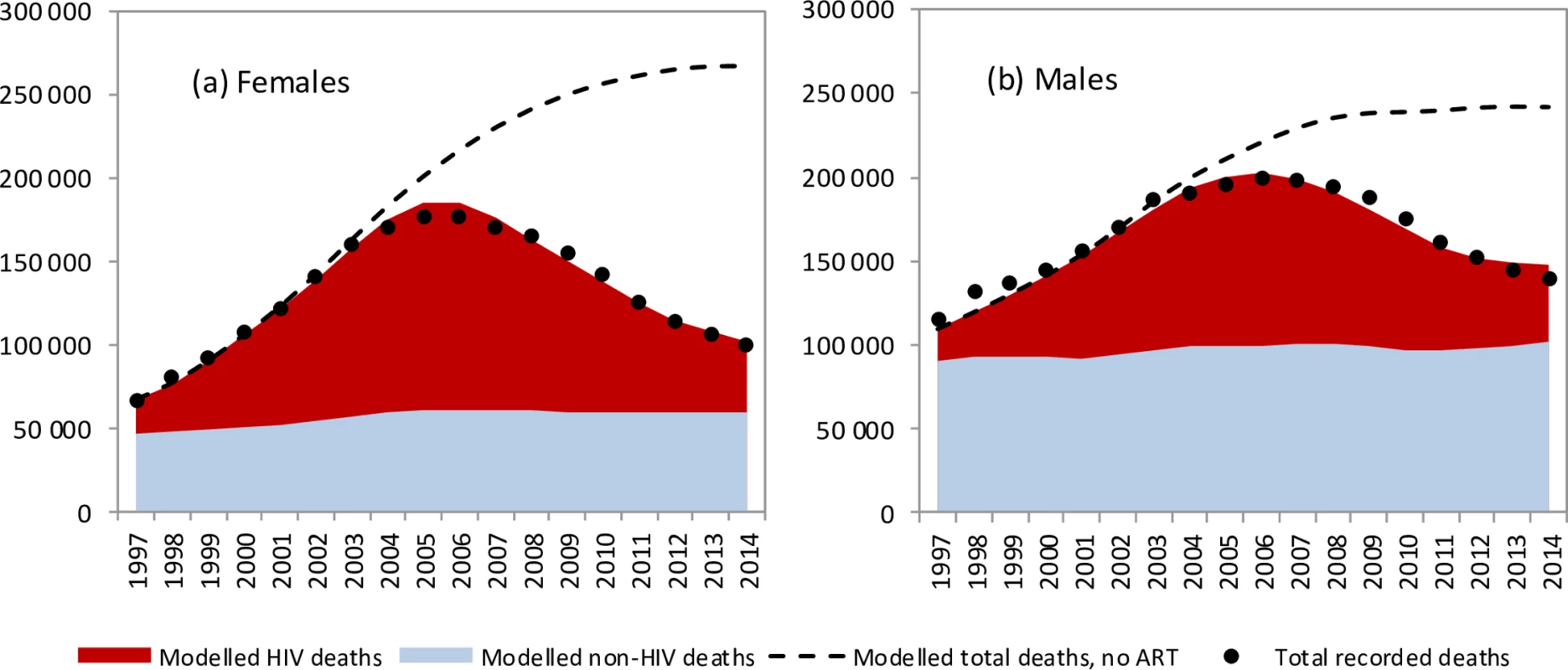
Modelled and recorded HIV deaths in (a) females and (b) males for the years 1990–2014. Shaded areas represent posterior means from the main analysis, and dashed lines represent model estimates from the ‘no ART’ counterfactual. Dots represent recorded death estimates, after adjustment for incomplete vital registration. Posterior estimates of model parameters
Posteriors estimates of model parameters were in most cases consistent with prior distributions (Table 1). However, there was evidence of a change towards lower HIV virulence over time (relative rate of disease progression of 0.978 [95% CI: 0.972–0.983] per calendar year). The bias in the IeDEA-SA estimates of mortality differed by time since ART initiation: during the first 6 months after ART start, mortality rates in the model were estimated to be 1.37 (95% CI: 1.07–1.71) times those estimated from IeDEA-SA cohorts, but at >42 months after ART initiation, mortality rates in the model were estimated to be lower than those estimated in IeDEA-SA cohorts.
Mortality attributable to HIV/AIDS
The model estimates that in the 1985–2014 period, 2.70 million adult HIV-related deaths occurred in South Africa (95% CI: 2.66 million–2.75 million). Adult HIV deaths are estimated to have peaked at 231,000 per annum in 2006 (95% CI: 227,000–235,000) and declined to 95,000 in 2014 (95% CI: 91,000–99,000). Although HIV/AIDS initially accounted for more deaths in women than in men, numbers of HIV/AIDS deaths declined to similar levels in men and women in 2014 (Fig 1). The age pattern of HIV/AIDS mortality over the 2005–2006 period (1 July 2005–30 June 2006) differed for men and women, with HIV-related deaths in women being highest in the 25–34-year age range, and those in men being highest in the 30–39-year age range (Fig 2). Non-HIV mortality rates were substantially lower in women than in men, with the result that HIV accounted for a greater fraction of deaths in women (up to 81% of deaths in women aged 25–29 years over the 2005–2006 period). The model estimated a 17% greater number of adult HIV-related deaths up to 2014 if it was calibrated only to HIV prevalence data (3.16 million, 95% CI: 2.83 million–3.55 million). The greatest divergence in HIV mortality estimates, compared to that in the main analysis, occurred in the more recent years: using only HIV prevalence data to calibrate the model yielded an estimate of 161,000 HIV deaths (95% CI: 138,000–186,000) in 2014, 69% (95% CI: 46%–97%) higher than when the model was calibrated to both HIV prevalence and mortality data.
Fig. 2. Age and sex differences in mortality levels (1 July 2005–30 June 2006). 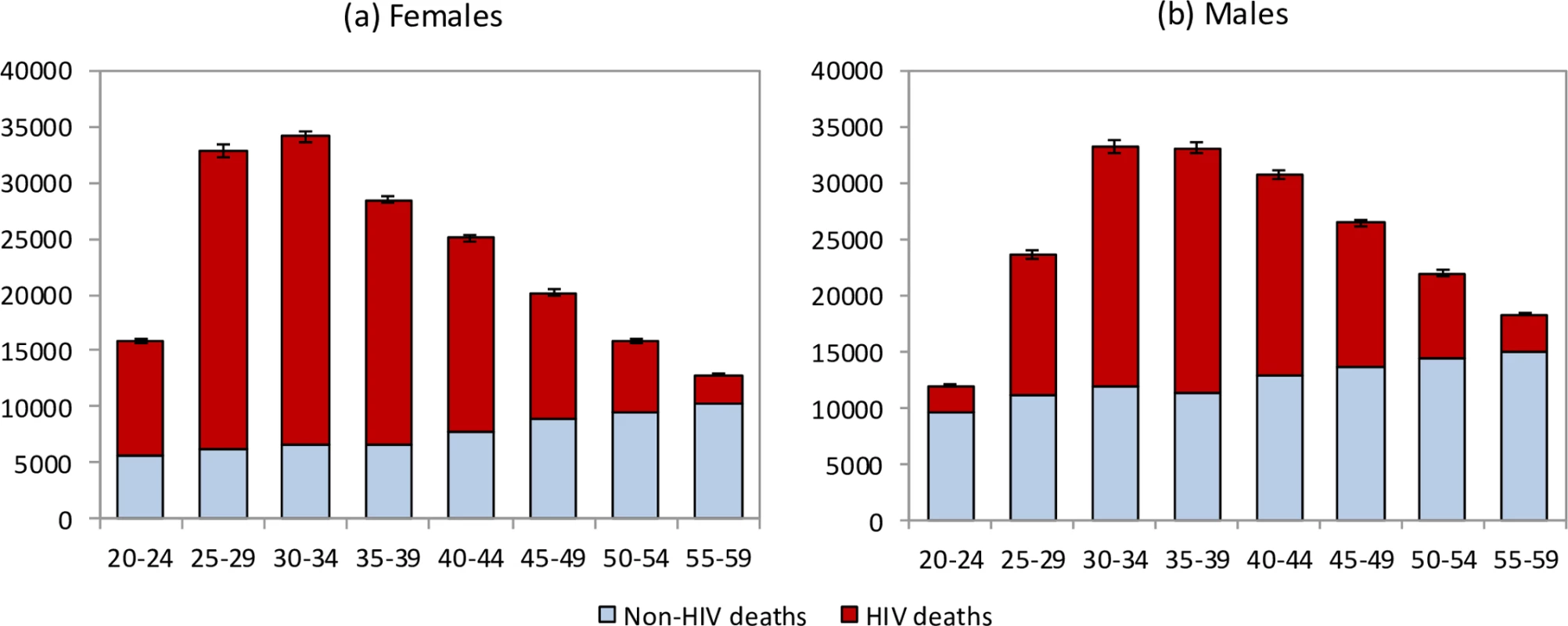
HIV and non-HIV deaths by 5-year age group in (a) females and (b) males. Bars represent posterior means from the main analysis. Error bars represent 95% confidence intervals around model estimates of total deaths (HIV and non-HIV combined). In the period up to 2005, most HIV-related deaths in South African adults occurred in individuals who were undiagnosed, but in recent years an increasingly high proportion of HIV-related deaths occurred in individuals who had been diagnosed but were ART-naïve (Fig 3). In 2014, the model estimates that 18.3% (95% CI: 17.2%–19.5%) of adult HIV-related deaths occurred in individuals who were undiagnosed, 41.7% (95% CI: 34.6%–48.0%) occurred in individuals who were diagnosed but ART-naïve, 10.2% (95% CI: 8.6%–11.9%) occurred in individuals within 6 months of starting ART, and 29.8% (95% CI: 24.6%–36.2%) occurred in individuals who had started ART more than 6 months prior to death.
Fig. 3. HIV-related deaths in adults for the years 1990–2014 by level of engagement in HIV care. 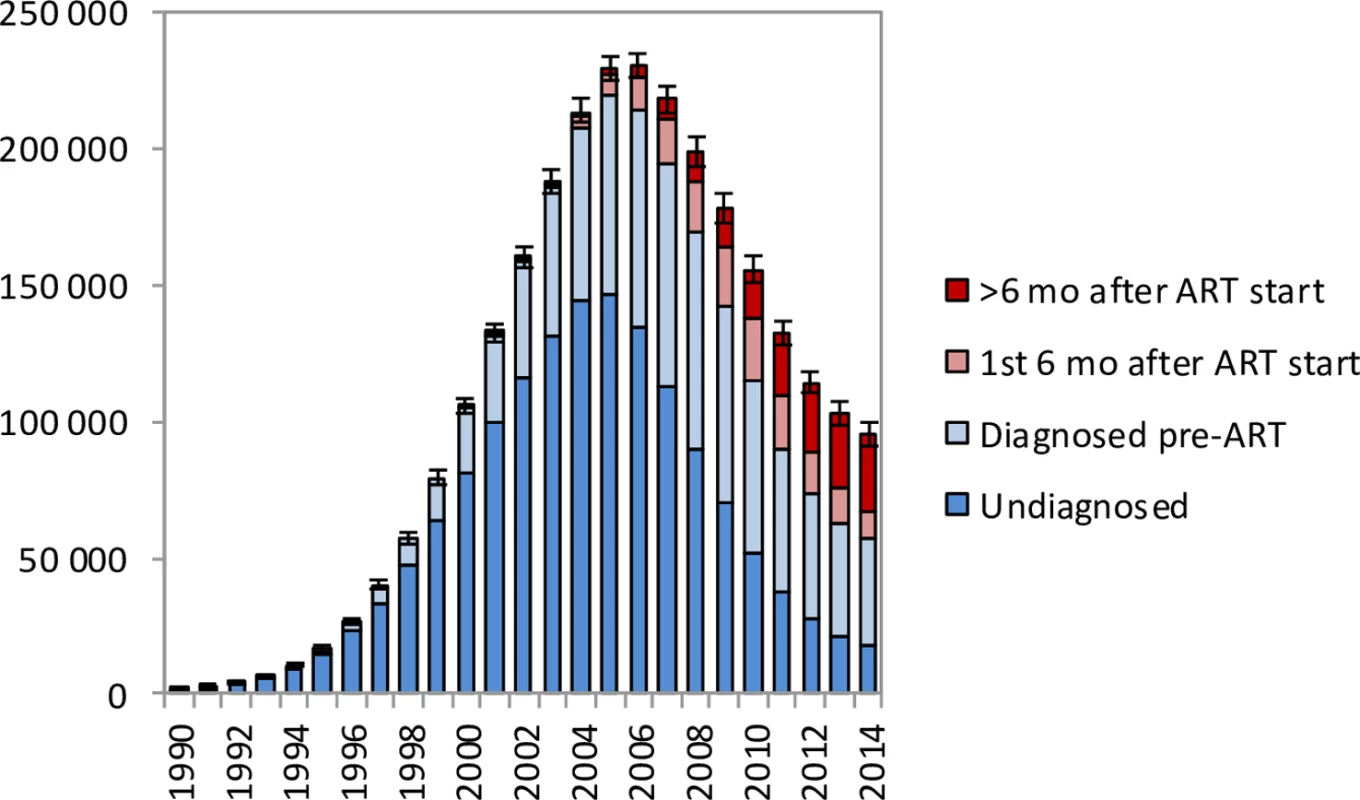
Bars represent posterior means from the main analysis. Error bars represent 95% confidence intervals around model estimates of total HIV-related deaths in adults. Life years saved by ART
The model estimates that in the absence of ART, adult HIV-related deaths would have continued to increase over time, reaching 376,000 per annum in 2014 (Fig 1). In the 2000–2014 period, the South African ART programme is estimated to have saved 6.15 million life years in adults (95% CI: 5.52 million–6.69 million), and the cumulative number of adult HIV-related deaths was reduced by 1.72 million (95% CI: 1.58 million–1.84 million) relative to what would have been expected in the absence of ART. This compares with a potential saving of 8.80 million (95% CI: 7.90 million–9.59 million) life years and 2.20 million (95% CI: 2.00 million–2.37 million) fewer HIV-related deaths if South Africa had moved swiftly to implement WHO guidelines and had achieved high levels of ART uptake in HIV-diagnosed individuals (Fig 4A). A higher number of life years saved (6.71 million, 95% CI: 5.56 million–8.02 million) and HIV deaths averted (1.85 million, 95% CI: 1.64 million–2.07 million), relative to the ‘no ART’ counterfactual, was also estimated when the model was fitted only to HIV prevalence data. The annual percentage reduction in HIV-related mortality due to ART increased steadily after the start of the public sector ART rollout in 2004, reaching 74.7% (95% CI: 73.3%–76.1%) in 2014 (Fig 4B). However, a much more dramatic reduction in HIV-related mortality could have been achieved in the best-case scenario, if ART rollout had been more timely.
Fig. 4. Impact of ART on mortality. 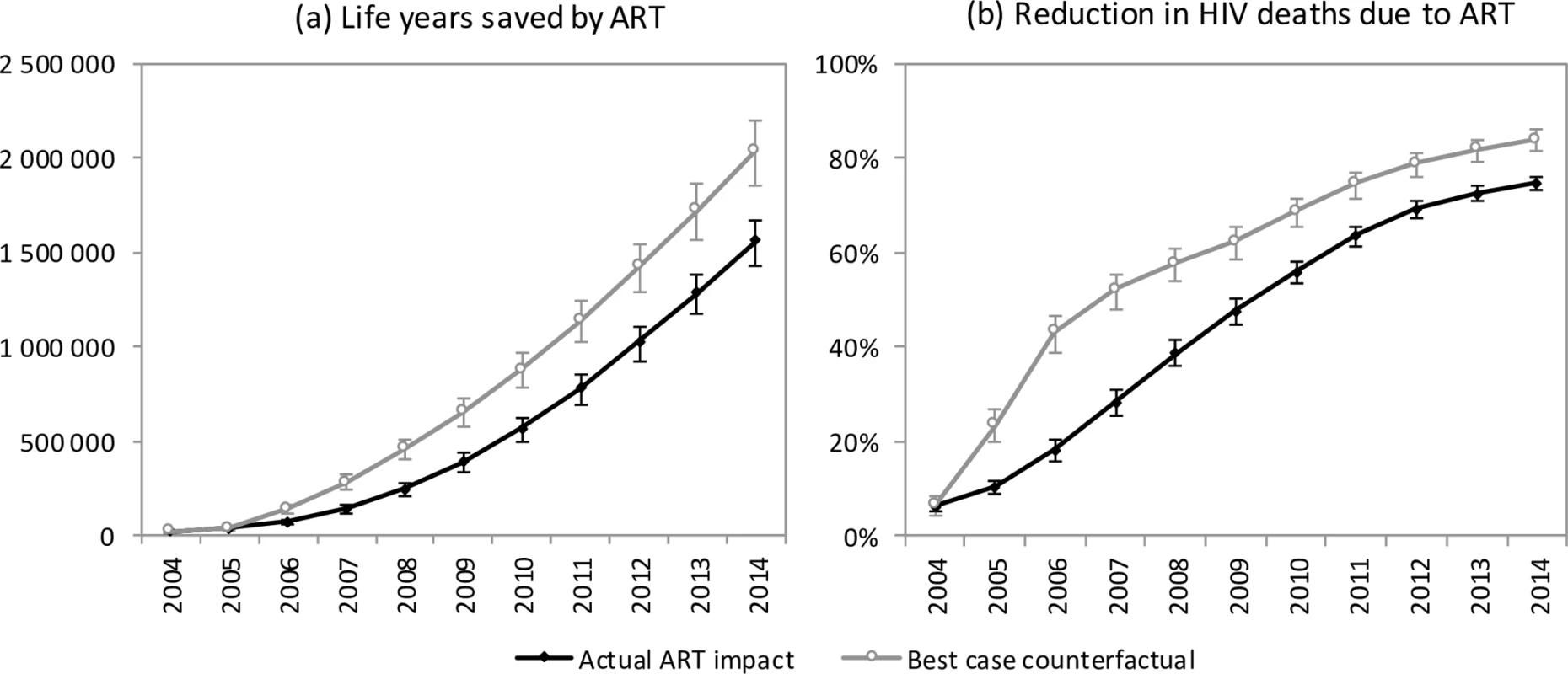
Life years saved by ART (a) and reduction in HIV deaths due to ART (b) for the years 2004–2014. Error bars represent 95% confidence intervals around model outputs. Savings are calculated relative to a ‘no ART’ counterfactual. In the ‘actual ART impact’ scenario, actual rates of ART enrolment in South Africa are entered into the model, and the model results are compared to the ‘no ART’ counterfactual. In the ‘best-case counterfactual’, the rates of ART enrolment entered into the model are those that would have been expected if South Africa had adopted WHO guidelines immediately upon publication and promoted ART rollout more aggressively. Discussion
HIV/AIDS has had a profound impact on adult mortality in South Africa, causing 2.7 million deaths in the 1985–2014 period. It is encouraging to see the dramatic impact that ART has had, saving 6.2 million adult life years in the period up to 2014, and reducing AIDS mortality in adults to only a quarter of what it would have been in the absence of ART. However, the slow pace of ART rollout, against a backdrop of political resistance to ART in the early 2000s [27], has had tragic consequences: our model estimates that the saving in life years could have been 2.7 million greater if the South African government had promoted ART more aggressively and adopted WHO guidelines in a more timely manner. These results are based on a model that has been calibrated to detailed and extensive HIV prevalence data, mortality data, HIV testing data, ART programme data, and estimates of mortality rates in ART research cohorts. Our model estimates that over 40% of adult HIV-related deaths in 2014 occurred in individuals who had been diagnosed but had not started ART. Our model also suggests that the observed mortality decline in South Africa over the last decade cannot be attributed only to the impact of ART, and changes in HIV virulence could also be partly responsible for the reduction.
Our estimates of AIDS mortality in South Africa are similar to those obtained in the Second National Burden of Disease Study (Fig N in S1 Text), which analysed cause-of-death data in South Africa, recoding deaths that did not appear to have been correctly recorded in the South African vital registration system [2]. However, our estimates of AIDS mortality levels are substantially lower than those published by the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the Institute for Health Metrics and Evaluation [62] (Fig N in S1 Text). Similar lack of agreement between model estimates of mortality in South Africa and recorded levels of mortality have been noted in a previous comparison of 10 different HIV models [63], and similar inconsistencies between WHO estimates and estimates from Demographic Surveillance System sites have been observed in Uganda [8]. The vast majority of these models have not been calibrated to mortality data, and the lack of agreement with recorded death statistics is thus not surprising. Indeed, our model also overestimated recorded levels of adult mortality when it was calibrated only to HIV prevalence data, with the estimated levels of AIDS mortality in 2014 in this analysis being about 69% greater than in the main analysis. There have been few prior attempts to fit HIV models to recorded death statistics in the countries most heavily affected by HIV, and those models that have done so limited their focus to mortality in the pre-ART era [9,64,65]. It is important that efforts are made to integrate mortality data into HIV model calibration procedures if these models are to reflect accurately the impact of ART.
A number of factors could explain why other models have tended to overestimate HIV mortality in South Africa. Most importantly, few previous models have considered the possibility of changing HIV virulence. Our results suggest that HIV may be evolving towards a less virulent form, consistent with evidence from other African studies [12,13]. These changes may be the result of a trade-off between virulence and transmission potential [13,66], or they may be the result of the fitness costs associated with HIV mutating to escape cytotoxic T lymphocyte responses [12,67].
Most mathematical models of ART impact are parameterized using mortality data from ART research cohorts, but this analysis suggests that these data are biased in complex ways. South African cohorts participating in the IeDEA-SA collaboration are predominately urban, and many of the associated programmes operate with strong support from non-governmental organisations and academic institutions, making them untypical of the general public health sector. The higher socioeconomic status of patients in urban cohorts and the higher staff-to-patient ratios in IeDEA-SA cohorts might be expected to contribute to lower mortality rates in the IeDEA-SA cohorts compared to the general public health sector [68–70]. However, this bias may become less significant at longer durations after ART initiation because patients frequently move between services or interrupt ART [71,72], while the vital registration system continues to record deaths after patients have left their original IeDEA-SA treatment cohorts. In addition, the bias may be offset by a counteracting bias due to the assumption of piecewise-constant mortality over different treatment durations.
A limitation of this analysis is that we have calibrated the Thembisa model to all-cause mortality data rather than HIV-related mortality data, without making any allowance for uncertainty in non-HIV mortality rates. Cause of death is poorly recorded in the South African vital registration system [73], with HIV-related causes being particularly susceptible to misclassification [74]. It is thus not possible to derive reliable estimates of HIV-related deaths directly from the vital registration data unless assumptions are made about patterns of misreporting, as in the Second National Burden of Disease Study [2]. The non-HIV mortality assumptions in the Thembisa model are derived from the Second National Burden of Disease Study, and the cause-of-death data are therefore to some extent already implicit in the model. Non-HIV mortality rates over the 20–59-year age range are assumed to have declined by 15%–34% in men and by 7%–20% in women over the 1995–2014 period; assuming a more modest decline in non-HIV mortality would lead to a smaller estimated impact of HIV on South African mortality.
Another limitation of this analysis is that we have not allowed for changes in ART effectiveness over time, such as might be expected following the introduction of tenofovir and fixed dose combinations. However, a recent analysis of IeDEA-SA data suggests that there has been little change in ART mortality rates over time, after controlling for changes in baseline CD4 count and changes in ART duration [75]. Some bias could also be introduced by the assumption of a constant annual change in HIV virulence: other analyses suggest that the change in virulence might be more rapid in the early stages of the epidemic, and the trend might not be consistently in the same direction [44,76,77]. More sophisticated modelling is required to identify the likely mechanisms behind the putative virulence changes. It is also worth noting that we have not considered uncertainty in several drivers of HIV incidence, and it is thus not possible to draw conclusions about the effect of changes in virulence on trends in HIV incidence.
This study does not consider the macroeconomic consequences of the ART programme in South Africa. It is likely that increased labour productivity and reduced costs of orphan care have contributed positively to South Africa’s gross domestic product [78], and one study estimated that output per capita could increase by 12% in South Africa if all HIV-positive individuals received ART [79]. However, a recent review found little consistency between studies that quantified the macroeconomic impact of HIV [80]. Further macroeconomic modelling is required to estimate the economic benefits of the ART-related savings in life years. This study also does not consider the impact of ART on South Africa’s tuberculosis (TB) epidemic, which has been shown to be substantial in other modelling studies [81]. Independently of the ART programme, there have been several other TB interventions that may have reduced TB mortality in HIV-positive individuals, such as isoniazid preventive therapy for HIV-positive individuals, improved TB diagnosis, TB case finding in HIV-positive individuals, and improvements in TB treatment [82]. Although we have attributed much of the improvement in untreated HIV-positive mortality in South Africa to changes in HIV virulence, it is possible that some of the improvement was in fact due to these advances in TB care and prevention.
This analysis demonstrates that the reductions in adult mortality that have been observed in South Africa are not attributable to the stage of the HIV epidemic. In fact, AIDS mortality rates would have continued to rise in the absence of ART (Fig 1), and a simple comparison of mortality rates in recent years to those in 2006 therefore does not do justice to the extent of the mortality reduction achieved by ART. The reductions in adult mortality are also not attributable to improvements in non-HIV mortality, as declines in non-HIV mortality rates since 1997 have been relatively modest [4,83].
Although South Africa has recently adopted a policy of universal ART eligibility, many challenges remain in reducing AIDS mortality. The high proportion of HIV-related deaths occurring in individuals who have been diagnosed but have not started ART suggests that many individuals who know they are HIV-positive and are ART-eligible are either reluctant or unable to start ART. This is consistent with the experience of a recent trial of treatment-as-prevention in rural South Africa, in which only 47% of newly diagnosed adults linked to care within 6 months of diagnosis [84], and estimates from the same community show that most HIV deaths occur pre-ART [85]. In order for ART to be more effective, both in reducing AIDS mortality and in preventing HIV transmission, it will be critical to ensure more timely ART initiation after HIV diagnosis. Simplified models of ART initiation are needed in order to achieve greater uptake of ART following diagnosis, for example, same-day ART initiation or expedited ART initiation [86,87]. In addition, education programmes may be required in order to raise awareness of the benefits of early ART initiation, and to address misconceptions about ART [88]. Our model also suggests that an increasingly high proportion of HIV-related deaths (30% in 2014) occur more than 6 months after ART initiation. Interventions to improve ART retention and increase viral suppression will therefore also be important in reducing future levels of AIDS mortality in South Africa.
Supporting Information
Zdroje
1. Statistics South Africa. Mortality and causes of death in South Africa, 2014: findings from death notification. Pretoria: Statistics South Africa; 2015 [cited 2016 Mar 13]. Available from: http://www.statssa.gov.za/publications/P03093/P030932014.pdf.
2. Bradshaw D, Msemburi W, Dorrington R, Pillay-van Wyk V, Laubscher R, Groenewald P. HIV/AIDS in South Africa: how many people died from the disease between 1997 and 2010? AIDS. 2016;30 : 771–8. doi: 10.1097/QAD.0000000000000947 26509830
3. Dorrington R, Bradshaw D, Laubscher R. Rapid mortality surveillance report 2012. Cape Town: South African Medical Research Council; 2014 [cited 2014 Apr 21]. Available from: http://www.mrc.ac.za/bod/RapidMortalitySurveillanceReport2012.pdf.
4. Pillay-van Wyk V, Msemburi W, Laubscher R, Dorrington RE, Groenewald P, Glass T, et al. Mortality trends and differentials in South Africa from 1997 to 2012: second National Burden of Disease Study. Lancet Glob Health. 2016;4:e642–53. doi: 10.1016/S2214-109X(16)30113-9 27539806
5. Reniers G, Slaymaker E, Nakiyingi-Miiro J, Nyamukapa C, Crampin AC, Herbst K, et al. Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA). AIDS. 2014;28(Suppl 4):S533–42. doi: 10.1097/QAD.0000000000000496 25406756
6. Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, Munthali F, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371 : 1603–11. doi: 10.1016/S0140-6736(08)60693-5 18468544
7. Stoneburner R, Korenromp E, Lazenby M, Tassie JM, Letebele J, Motlapele D, et al. Using health surveillance systems data to assess the impact of AIDS and antiretroviral treatment on adult morbidity and mortality in Botswana. PLoS ONE. 2014;9(7):e100431. doi: 10.1371/journal.pone.0100431 25003870
8. Asiki G, Reniers G, Newton R, Baisley K, Nakiyingi-Miiro J, Slaymaker E, et al. Adult life expectancy trends in the era of antiretroviral treatment in rural Uganda (1991–2012). AIDS. 2016;30 : 487–93. doi: 10.1097/QAD.0000000000000930 26765939
9. Johnson LF, Hallett TB, Rehle TM, Dorrington RE. The effect of changes in condom usage and antiretroviral treatment coverage on HIV incidence in South Africa: a model-based analysis. J Roy Soc Interface. 2012;9 : 1544–54. doi: 10.1098/rsif.2011.0826 22258551
10. Nagelkerke NJ, Arora P, Jha P, Williams B, McKinnon L, de Vlas SJ. The rise and fall of HIV in high-prevalence countries: a challenge for mathematical modeling. PLoS Comput Biol. 2014;10(3):e1003459. doi: 10.1371/journal.pcbi.1003459 24626088
11. Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: Joint United Nations Programme on HIV/AIDS; 2013 [cited 2014 Apr 13]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
12. Payne R, Muenchhoff M, Mann J, Roberts HE, Matthews P, Adland E, et al. Impact of HLA-driven HIV adaptation on virulence in populations of high HIV seroprevalence. Proc Natl Acad Sci U S A. 2014;111:E5393–400. doi: 10.1073/pnas.1413339111 25453107
13. Blanquart F, Grabowski MK, Herbeck J, Nalugoda F, Serwadda D, Eller MA, et al. A transmission-virulence evolutionary trade-off explains attenuation of HIV-1 in Uganda. Elife. 2016;5:e20492. doi: 10.7554/eLife.20492 27815945
14. Yiannoutsos CT, Johnson LF, Boulle A, Musick BS, Gsponer T, Balestre E, et al. Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect. 2012;88(Suppl 2):i33–43. doi: 10.1136/sextrans-2012-050658 23172344
15. May MT, Hogg RS, Justice AC, Shepherd BE, Costagliola D, Ledergerber B, et al. Heterogeneity in outcomes of treated HIV-positive patients in Europe and North America: relation with patient and cohort characteristics. Int J Epidemiol. 2012;41 : 1807–20. doi: 10.1093/ije/dys164 23148105
16. Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9):e1001718. doi: 10.1371/journal.pmed.1001718 25203931
17. Braitstein P, Brinkof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367 : 817–24. doi: 10.1016/S0140-6736(06)68337-2 16530575
18. Stover J, Brown T, Marston M. Updates to the Spectrum/Estimation and Projection Package (EPP) model to estimate HIV trends for adults and children. Sex Transm Infect. 2012;88(Suppl 2):i11–6. doi: 10.1136/sextrans-2012-050640 23172341
19. Johnson LF, Chiu C, Myer L, Davies MA, Dorrington RE, Bekker LG, et al. Prospects for HIV control in South Africa: a model-based analysis. Glob Health Action. 2016;9 : 30314. doi: 10.3402/gha.v9.30314 27282146
20. Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS ONE. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790 19495419
21. Setel PW, Macfarlane SB, Szreter S, Mikkelsen L, Jha P, Stout S, et al. A scandal of invisibility: making everyone count by counting everyone. Lancet. 2007;370 : 1569–77. doi: 10.1016/S0140-6736(07)61307-5 17992727
22. Johnson LF, Dorrington RE, Laubscher R, Hoffmann CJ, Wood R, Fox MP, et al. A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. J Int AIDS Soc. 2015;18 : 20628. doi: 10.7448/IAS.18.1.20628 26685125
23. Dorrington RE, Bradshaw D. Maternal mortality in South Africa: lessons from a case study in the use of deaths reported by households in censuses and surveys. J Pop Res. 2011;28 : 49–73. doi: 10.1007/s12546-011-9050-9
24. Dorrington RE, Bradshaw D, Laubscher R, Nannan N. Rapid mortality surveillance report 2015. Cape Town: South African Medical Research Council; 2016 [cited 2017 Jan 23]. Available from: http://www.mrc.ac.za/bod/RapidMortalitySurveillanceReport2015.pdf.
25. Statistics South Africa. Mortality and causes of death in South Africa, 2011: findings from death notification. Pretoria: Statistics South Africa; 2014 [cited 2014 Apr 15]. Available from: http://beta2.statssa.gov.za/publications/P03093/P030932011.pdf.
26. Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41 : 1256–64. doi: 10.1093/ije/dyr080 21593078
27. Nattrass N. AIDS and the scientific governance of medicine in post-apartheid South Africa. Afr Aff (Lond). 2008;107 : 157–76.
28. Johnson LF, Dorrington RE, Moolla H. Progress towards the 2020 targets for HIV diagnosis and antiretroviral treatment in South Africa. South Afr J HIV Med. 2017;18:a694. doi: 10.4102/sajhivmed.v18i1.694
29. Hansmann A, Schim van der Loeff MF, Kaye S, Awasana AA, Sarge-Njie R, O’Donovan D, et al. Baseline plasma viral load and CD4 cell percentage predict survival in HIV-1 - and HIV-2-infected women in a community-based cohort in the Gambia. J Acquir Immun Defic Syndr. 2005;38 : 335–41.
30. Lavreys L, Baeten JM, Chohan V, McClelland RS, Hassan WM, Richardson BA, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42 : 1333–9. doi: 10.1086/503258 16586394
31. O’Brien TR, Rosenberg PS, Yellin F, Goedert JJ. Longitudinal HIV-1 RNA levels in a cohort of homosexual men. J Acquir Immun Defic Syndr. 1998;18 : 155–61.
32. Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205 : 358–65. doi: 10.1093/infdis/jir747 22241800
33. Quinn T, Wawer M, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342 : 921–9. doi: 10.1056/NEJM200003303421303 10738050
34. Fideli US, Allen SA, Musonda R, Trask S, Hahn BH, Weiss H, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17 : 901–10. doi: 10.1089/088922201750290023 11461676
35. Todd J, Glynn JR, Marston M, Lutalo T, Biraro S, Mwita W, et al. Time from HIV seroconversion to death: a collaborative analysis of eight studies in six low and middle-income countries before highly active antiretroviral therapy. AIDS. 2007;21(Suppl 6):S55–63.
36. Glynn JR, Sonnenberg P, Nelson G, Bester A, Shearer S, Murray J. Survival from HIV-1 seroconversion in Southern Africa: a retrospective cohort study in nearly 2000 gold-miners over 10 years of follow-up. AIDS. 2007;21 : 625–32. doi: 10.1097/QAD.0b013e328017f857 17314525
37. Eligibility for ART in Lower Income Countries (eART-linc) Collaboration, Wandel S, Egger M, Rangsin R, Nelson KE, Costello C, et al. Duration from seroconversion to eligibility for antiretroviral therapy and from ART eligibility to death in adult HIV-infected patients from low and middle-income countries: collaborative analysis of prospective studies. Sex Transm Infect. 2008;84(Suppl i):i31–6.
38. Time from HIV-1 seroconversion to AIDS and death before widespread use of highly-active antiretroviral therapy: a collaborative re-analysis. Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Concerted Action on SeroConversion to AIDS and Death in Europe. Lancet. 2000;355 : 1131–7. 10791375
39. Isingo R, Zaba B, Marston M, Ndege M, Mngara J, Mwita M, et al. Survival after HIV infection in the pre-antiretroviral therapy era in a rural Tanzanian cohort. AIDS. 2007;21(Suppl 6):S5–13. doi: 10.1097/01.aids.0000299405.06658.a8 18032939
40. Hogg RS, Strathdee SA, Craib KJ, O’Shaughnessy MV, Montaner JS, Schechter MT. Lower socioeconomic status and shorter survival following HIV infection. Lancet. 1994;344 : 1120–4. 7934494
41. Pezzotti P, Phillips AN, Dorrucci M, Lepri AC, Galai N, Vlahov D, et al. Category of exposure to HIV and age in the progression to AIDS: longitudinal study of 1199 people with known dates of seroconversion. BMJ. 1996;313 : 583–6. 8806246
42. Herbeck JT, Müller V, Maust BS, Ledergerber B, Torti C, Giambenedetto S, et al. Is the virulence of HIV changing? A meta-analysis of trends in prognostic markers of HIV disease progression and transmission. AIDS. 2012;26 : 193–205. doi: 10.1097/QAD.0b013e32834db418 22089381
43. Touloumi G, Pantazis N, Pillay D, Paraskevis D, Chaix ML, Bucher HC, et al. Impact of HIV-1 subtype on CD4 count at HIV seroconversion, rate of decline, and viral load set point in European seroconverter cohorts. Clin Infect Dis. 2013;56 : 888–97. doi: 10.1093/cid/cis1000 23223594
44. Pantazis N, Porter K, Costagliola D, De Luca A, Ghosn J, Guiguet M, et al. Temporal trends in prognostic markers of HIV-1 virulence and transmissibility: an observational cohort study. Lancet HIV. 2014;1:e119–26. doi: 10.1016/S2352-3018(14)00002-2 26424120
45. Boulle A, Bock P, Osler M, Cohen K, Channing L, Hilderbrand K, et al. Antiretroviral therapy and early mortality in South Africa. Bull World Health Organ. 2008;86 : 678–87. doi: 10.2471/BLT.07.045294 18797643
46. Adam MA, Johnson LF. Estimation of adult antiretroviral treatment coverage in South Africa. S Afr Med J. 2009;99 : 661–7. 20073293
47. Küstner H, Swanevelder J, van Middelkoop A. National HIV surveillance—South Africa, 1990–1992. S Afr Med J. 1994;84 : 195–9. 7974040
48. Pettifor AE, Hudgens MG, Levandowski BA, Rees HV, Cohen MS. Highly efficient HIV transmission to young women in South Africa. AIDS. 2007;21 : 861–5. doi: 10.1097/QAD.0b013e3280f00fb3 17415041
49. Auvert B, Ballard R, Campbell C, Caraël M, Carton M, Fehler G, et al. HIV infection in a South African mining town is associated with herpes simplex virus-2 seropositivity and sexual behaviour. AIDS. 2001;15 : 885–98. 11399961
50. Mahiane SG, Legeai C, Taljaard D, Latouche A, Puren A, Peillon A, et al. Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. AIDS. 2009;23 : 377–83. 19198042
51. Baeten JM, Richardson BA, Lavreys L, Rakwar JP, Mandaliya K, Bwayo JJ, et al. Female-to-male infectivity of HIV-1 among circumcised and uncircumcised Kenyan men. J Infect Dis. 2005;191 : 546–53. doi: 10.1086/427656 15655778
52. Johnson LF, Mossong J, Dorrington RE, Schomaker M, Hoffmann CJ, Keiser O, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10(4):e1001418. doi: 10.1371/journal.pmed.1001418 23585736
53. Dorrington RE, Moultrie TA, Timæus IM. Estimation of mortality using the South African Census 2001 data. CARe Monograph 11. Cape Town: Centre for Actuarial Research; 2004 [cited 2015 Dec 3]. Available from: http://www.commerce.uct.ac.za/Research_Units/CARE/Monographs/Monographs/Mono11.pdf.
54. Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, et al. South African national HIV prevalence, incidence, and behaviour survey, 2012. Cape Town: Human Sciences Research Council; 2014 [cited 2014 Apr 16]. Available from: http://www.hsrc.ac.za/en/research-outputs/view/6871.
55. Raftery AE, Bao L. Estimating and projecting trends in HIV/AIDS generalized epidemics using incremental mixture importance sampling. Biometrics. 2010;66 : 1162–73. doi: 10.1111/j.1541-0420.2010.01399.x 20222935
56. World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach—2003 revision. Geneva: World Health Organization; 2004 [cited 2004 Sep 13]. Available from: http://www.who.int/hiv/pub/prev_care/en/arvrevision2003en.pdf.
57. World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. Geneva: World Health Organization; 2009 [cited 2010 Jul 30]. Available from: http://www.who.int/hiv/pub/arv/advice/en/index.html.
58. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013 [cited 2013 Sep 3]. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/en/.
59. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: World Health Organization; 2015 [cited 2016 Apr 24]. Available from: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/.
60. Gupta S, Granich R. When will sub-Saharan Africa adopt HIV treatment for all? South Afr J HIV Med. 2016;17:a459.
61. Mujugira A, Celum C, Thomas KK, Farquhar C, Mugo N, Katabira E, et al. Delay of antiretroviral therapy initiation is common in East African HIV-infected individuals in serodiscordant partnerships. J Acquir Immun Defic Syndr. 2014;66 : 436–42. doi: 10.1097/QAI.0000000000000192 24798765
62. Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, et al. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3:e361–87. doi: 10.1016/S2352-3018(16)30087-X 27470028
63. Eaton JW, Bacaër N, Bershteyn A, Cambiano V, Cori A, Dorrington RE, et al. Assessment of epidemic projections using recent HIV survey data in South Africa: a validation analysis of ten mathematical models of HIV epidemiology in the antiretroviral therapy era. Lancet Glob Health. 2015;3:e598–608. doi: 10.1016/S2214-109X(15)00080-7 26385301
64. Oster E. Estimating HIV prevalence and incidence in Africa from mortality data. B E J Econom Anal Policy. 2010;10 : 80.
65. Lopman B, Gregson S. When did HIV incidence peak in Harare, Zimbabwe? Back-calculation from mortality statistics. PLoS ONE. 2008;3(3):e1711. doi: 10.1371/journal.pone.0001711 18320032
66. Shirreff G, Pellis L, Laeyendecker O, Fraser C. Transmission selects for HIV-1 strains of intermediate virulence: a modelling approach. PLoS Comput Biol. 2011;7(10):e1002185. doi: 10.1371/journal.pcbi.1002185 22022243
67. Fryer HR, Frater J, Duda A, Roberts MG, Phillips RE, McLean AR. Modelling the evolution and spread of HIV immune escape mutants. PLoS Pathog. 2010;6(11):e1001196. doi: 10.1371/journal.ppat.1001196 21124991
68. Burch LS, Smith CJ, Phillips AN, Johnson MA, Lampe FC. Socioeconomic status and response to antiretroviral therapy in high-income countries: a literature review. AIDS. 2016;30 : 1147–62. doi: 10.1097/QAD.0000000000001068 26919732
69. Charalambous S, Grant AD, Churchyard GJ, Mukora R, Schneider H, Fielding KL. Clinic-level factors influencing patient outcomes on antiretroviral therapy in primary health clinics in South Africa. AIDS. 2016;30 : 1099–109. doi: 10.1097/QAD.0000000000001014 26752280
70. Vella V, Govender T, Dlamini S, Taylor M, Moodley I, David V, et al. Retrospective study on the critical factors for retaining patients on antiretroviral therapy in KwaZulu-Natal, South Africa. J Acquir Immun Defic Syndr. 2010;55 : 109–16.
71. Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low - and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. 2015;20 : 365–79. doi: 10.1111/tmi.12434 25418366
72. Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health. 2011;16 : 1297–313. doi: 10.1111/j.1365-3156.2011.02828.x 21718394
73. Joubert J, Bradshaw D, Kabudula C, Rao C, Kahn K, Mee P, et al. Record-linkage comparison of verbal autopsy and routine civil registration death certification in rural north-east South Africa: 2006–09. Int J Epidemiol. 2014;43 : 1945–58. doi: 10.1093/ije/dyu156 25146564
74. Birnbaum JK, Murray CJ, Lozano R. Exposing misclassified HIV/AIDS deaths in South Africa. Bull World Health Organ. 2011;89 : 278–85. doi: 10.2471/BLT.11.086280 21479092
75. Johnson LF, Keiser O, Fox MP, Tanser F, Cornell M, Hoffmann CJ, et al. Life expectancy trends in adults on antiretroviral treatment in South Africa. AIDS. 2016;30 : 2545–50. doi: 10.1097/QAD.0000000000001197 27428744
76. Roberts HE, Goulder PJ, McLean AR. The impact of antiretroviral therapy on population-level virulence evolution of HIV-1. J Roy Soc Interface. 2015;12 : 20150888. doi: 10.1098/rsif.2015.0888 26609066
77. Herbeck JT, Mittler JE, Gottlieb GS, Mullins JI. An HIV epidemic model based on viral load dynamics: value in assessing empirical trends in HIV virulence and community viral load. PLoS Comput Biol. 2014;10(6):e1003673. doi: 10.1371/journal.pcbi.1003673 24945322
78. Resch S, Korenromp E, Stover J, Blakley M, Krubiner C, Thorien K, et al. Economic returns to investment in AIDS treatment in low and middle income countries. PLoS ONE. 2011;6(10):e25310. doi: 10.1371/journal.pone.0025310 21998648
79. Ferreira PC, Pessôa S, Dos Santos MR. The economic impact of AIDS on income and human capital. Econ Inquiry. 2011;49 : 1104–16.
80. Haacker M. The economics of the global response to HIV/AIDS. Oxford: Oxford University Press; 2016.
81. Bacaër N, Ouifki R, Pretorius C, Wood R, Williams B. Modeling the joint epidemics of TB and HIV in a South African township. J Math Biol. 2008;57 : 557–93. doi: 10.1007/s00285-008-0177-z 18414866
82. Churchyard GJ, Mametja LD, Mvusi L, Ndjeka N, Hesseling AC, Reid A, et al. Tuberculosis control in South Africa: successes, challenges and recommendations. S Afr Med J. 2014;104 : 244–8. 24893501
83. Reniers G, Blom S, Calvert C, Martin-Onraet A, Herbst AJ, Eaton JW, et al. Trends in the burden of HIV mortality after roll-out of antiretroviral therapy in KwaZulu-Natal, South Africa: an observational community cohort study. Lancet HIV. 2017;4:e113–21. doi: 10.1016/S2352-3018(16)30225-9 27956187
84. Iwuji CC, Orne-Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. Uptake of home-based HIV testing, linkage to care, and community attitudes about ART in rural KwaZulu-Natal, South Africa: descriptive results from the first phase of the ANRS 12249 TasP cluster-randomised trial. PLoS Med. 2016;13(8):e1002107. doi: 10.1371/journal.pmed.1002107 27504637
85. Bor J, Rosen S, Chimbindi N, Haber N, Herbst K, Mutevedzi T, et al. Mass HIV treatment and sex disparities in life expectancy: demographic surveillance in rural South Africa. PLoS Med. 2015;12(11):e1001905. doi: 10.1371/journal.pmed.1001905 26599699
86. Rosen S, Maskew M, Fox MP, Nyoni C, Mongwenyana C, Malete G, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015. doi: 10.1371/journal.pmed.1002015 27163694
87. Wilkinson L, Duvivier H, Patten G, Solomon S, Mdani L,Patel S, et al. Outcomes from the implementation of a counselling model supporting rapid antiretroviral treatment initiation in a primary healthcare clinic in Khayelitsha, South Africa. South Afr J HIV Med. 2015;16 : 367.
88. Katz IT, Dietrich J, Tshabalala G, Essien T, Rough K, Wright AA, et al. Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS Behav. 2015;19 : 704–14. doi: 10.1007/s10461-014-0920-y 25304330
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 12- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Cell salvage and donor blood transfusion during cesarean section: A pragmatic, multicentre randomised controlled trial (SALVO)
- Re-emerging and newly recognized sexually transmitted infections: Can prior experiences shed light on future identification and control?
- Antiretroviral therapy and population mortality: Leveraging routine national data to advance policy
- Psychosocial and socioeconomic determinants of cardiovascular mortality in Eastern Europe: A multicentre prospective cohort study
- Research on HIV cure: Mapping the ethics landscape
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- Effects of women’s groups practising participatory learning and action on preventive and care-seeking behaviours to reduce neonatal mortality: A meta-analysis of cluster-randomised trials
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
- Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: A modeling study
- Sexually transmitted infections—Research priorities for new challenges
- Healthcare provider perspectives on managing sexually transmitted infections in HIV care settings in Kenya: A qualitative thematic analysis
- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Dual-strain genital herpes simplex virus type 2 (HSV-2) infection in the US, Peru, and 8 countries in sub-Saharan Africa: A nested cross-sectional viral genotyping study
- Association between infrastructure and observed quality of care in 4 healthcare services: A cross-sectional study of 4,300 facilities in 8 countries
- Bridging the quality chasm in maternal, newborn, and child healthcare in low- and middle-income countries
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



