-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation between infrastructure and observed quality of care in 4 healthcare services: A cross-sectional study of 4,300 facilities in 8 countries
In a cross-sectional study of 4,300 facilities in 8 countries, Hannah Leslie and colleagues examine the association between infrastructure and observed quality of care in 4 healthcare services.
Published in the journal: . PLoS Med 14(12): e32767. doi:10.1371/journal.pmed.1002464
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002464Summary
In a cross-sectional study of 4,300 facilities in 8 countries, Hannah Leslie and colleagues examine the association between infrastructure and observed quality of care in 4 healthcare services.
Introduction
The first decade of the 2000s saw a dramatic increase in global health activity, with double-digit increases in international development assistance for health [1], reflecting the global focus on the HIV epidemic and intensified efforts to meet the Millennium Development Goals (MDGs) [2]. Two lessons learned in the pursuit of the health MDGs have particular salience for the current effort to achieve Sustainable Development Goal (SDG) 3: ensuring healthy lives and promoting well-being for all at all ages [3]. First, measurement can drive progress. With the assistance of several global initiatives, including the Countdown to 2015 and the Global Burden of Disease Study, countries closely tracked and compared population coverage of essential health services. As a result, remarkable global and national increases in coverage of services such as facility-based delivery and measles vaccination were achieved [2]. Improvements in health-related indicators that were MDG targets outstripped those in non-MDG targets by nearly 2-fold [4]. Second, for many conditions, increased access to care is insufficient to improve population health when care is of poor quality. In areas such as maternal and newborn health, studies from India, Malawi, and Rwanda have demonstrated that expanded access to formal healthcare has failed to yield survival benefits [5–7]. It is increasingly apparent that the path to achievement of SDG 3 will require similar attention to the measurement and improvement of healthcare quality as the MDG era brought to healthcare access [8,9].
Quality of care has been defined as the “degree to which health services for individuals and populations increase the likelihood of desired health outcomes and are consistent with current professional knowledge” [10]. Efforts to operationalize this broad definition have included the identification of key characteristics of quality, namely care that is safe, timely, effective, equitable, efficient, and people centered [11,12]. Health system theorists further agree that the delivery of high-quality care is contingent on adequate readiness of the health system or program and, once delivered, should yield impacts from improved health to client satisfaction [13,14]. In the same vein, measures of healthcare quality have traditionally been divided into 3 domains: structure or inputs to care, process or content of care, and outcomes of care [15]. Each domain has advantages and disadvantages: inputs are the necessary foundations for care but are not sufficient to describe its content or effects, process measures pertain directly to care delivery but are challenging to collect, and outcome measures assess the ultimate goal of the health system but reflect many factors beyond the health system itself.
In low - and middle-income countries (LMICs), information on healthcare quality is sparse [16]. A major source of data on health system performance has been standardized facility surveys, with over 100 unique surveys completed in the last 5 years alone [17–22]. Implementation of facility surveys is costly and typically supported by multilateral donor organizations such as the World Bank; World Health Organization (WHO); Global Fund for AIDS, Tuberculosis and Malaria; and the US Agency for International Development [20,21]. Among the most commonly used facility surveys is the Service Availability and Readiness Assessment (SARA), developed by WHO [22]. The SARA aims to measure facility readiness to provide essential care and hence focuses on inputs such as infrastructure, equipment, supplies, and health workers. Completion of a SARA survey costs a minimum of US$100,000 to generate national estimates for a small to medium country; more complex sampling to generate regional estimates can require several times that amount [23]. Other facility surveys also focus on input measures. For example, of 20 survey tools assessing health facility quality and readiness for family planning, 7 are limited to structural quality alone; across all 20 tools, indicators of structure are collected 5 times more frequently than indicators of process [18]. A review of 8,500 quality indicators used to assess performance-based financing programs found that over 90% measured structural aspects of quality [24]. The emphasis on input-based measures shapes health system research and monitoring: in the growing area of effective (quality-adjusted) coverage assessment, multiple studies look to input-based measures to estimate capacity to provide high-quality care [25–27].
The reliance on inputs to measure quality in LMICs reflects the notion that these are necessary for good care. However, while some inputs are clearly essential for care provision (e.g., health workers must be present; drugs must be in stock), it is not clear that overall availability of inputs is related to health processes or outcomes [28–31]. With growing attention to quality of care as a driver of future health gains and scarce resources available for measurement, selecting the right measures is important. Is infrastructure a reasonable proxy for quality of clinical care?
In this paper, we compare structural and process quality of 4 essential health services—family planning, antenatal care (ANC), delivery care, and sick-child care—using data from nationally representative samples of health facilities in 8 LMICs. The aims of this work are to describe facility inputs and observed adherence to guidelines for good clinical care for these services and to assess the strength of the relationship between these measures.
Methods
Ethical approval
The original survey implementers obtained ethical approval for data collection; primary data do not include identifiable patient information. The Harvard University Human Research Protection Program approved this secondary analysis as exempt from human subjects review.
Study design and sample
The Service Provision Assessment (SPA) is a standardized survey designed to measure the capacity of health systems in LMICs. It is conducted by the Demographic and Health Survey Program of the US Agency for International Development in coordination with a national statistics agency in the countries surveyed. All health facilities in each country are listed, and a nationally representative sample is selected. The facility assessment includes a standard set of tools: an audit of facility services and resources, interviews with healthcare providers, and direct observation of the provision of clinical services.
In this analysis, we pooled data from all SPA surveys conducted between 2007 and 2015 that included observations of family planning, ANC, delivery care, and/or sick-child care. The surveys were from Haiti (2013), Kenya (2010), Malawi (2014), Namibia (2009), Rwanda (2007), Senegal (2013–2014), Tanzania (2015), and Uganda (2007). Surveys in Kenya, Senegal, Tanzania, and Uganda are nationally representative samples of the health system; those in Haiti, Malawi, Namibia, and Rwanda are censuses or near censuses. Observations were conducted in all services in all countries with the exception of delivery care, which was observed only in Kenya and Malawi. Patients are selected for observation using systematic random sampling from a list of those presenting for services on the day of the visit; assessment included up to 5 observations per provider and up to 15 observations per service. Children under 5 presenting with illness (as opposed to injury or skin or eye infection exclusively) were eligible for inclusion; when possible, new ANC clients and new family-planning clients were oversampled 2 to 1 relative to returning clients. For this analysis, we limited ANC observations to women’s first visit to the facility to standardize expected clinical actions. We excluded facilities with a single observation to limit variation.
Facility infrastructure: Service readiness
We calculated infrastructure indices for each clinical service based on WHO definitions of service readiness [22]. We extracted the cross-cutting domains of basic amenities (e.g., safe water) and precautions for infection prevention (gloves, sanitizer) from the general service readiness index as an essential foundation for all services. We combined these with the 4 domains defined for each service-specific readiness score: staff and training, equipment, diagnostics (as applicable), and medicines and commodities. Each domain consists of specific tracer items such as functional blood pressure cuff, hemoglobin test, and valid iron pills for ANC (see S1 Table for items by service). Items that were not included on the survey for a given country were excluded from the calculation for that country. Some items were skipped if a facility did not have the service or capacity underlying the item—for example, stool microscopy in facilities without laboratory testing. We set these items to 0, reflecting the lack of capacity to use the item in that facility. In rare cases, facility managers provided invalid responses or no responses, leading to missing values; we imputed 0 for these items in the absence of evidence for their availability (and functional status) at the facility. Frequency of unasked, skipped, and missing items is reported in S1 Table. We computed domain scores as the mean availability of items and averaged across cross-cutting and service-specific domains to create an index from 0 to 1 for each service; each domain contributes equally to the final infrastructure index, regardless of the number of items it comprises.
Observed clinical quality
Clinical observations consisted of an observer filling out a checklist of actions that providers are expected to complete during each patient visit; observers are members of the assessment team, typically nurses, who have completed training and evaluation on assessment procedures. We created indices of observed clinical quality for each service using international guidelines for evidence-based care or previously validated indices of quality [32–35]. Indices each contain between 16 and 22 items across domains such as patient history, physical exam, and counseling/management. S2 Table lists the items in each index and average performance by country. Each observation was scored based on percentage of items performed; observations were averaged within service in order to generate an average of quality of care per service delivered at each facility, weighted by the inverse probability of sampling clients within each facility.
Analysis plan
We predefined infrastructure and observed clinical quality using international guidelines for both and identifying matching variables in the SPA. We identified unadjusted correlation as the appropriate analysis for a linear relationship and, in the absence of a predetermined threshold of inputs necessary for good clinical quality, used cross validation to rigorously test threshold options without overfitting to the observed data. We considered assessment of the full sample and of the sample limited to facilities with more than 1 observation; we selected the latter as the main analysis due to a priori concerns about measurement error in data from a single observation, i.e., that single observations may be less reliable than multiple observations in conveying underlying quality.
Statistical analysis
We provided descriptive statistics of service-specific facility characteristics, including whether the facility is a hospital versus a health center, clinic, or dispensary; whether it was publicly or privately managed; whether it is located in an urban or rural area; and the number of observations per facility. We also calculated mean and standard deviation of the service-specific infrastructure index and observed clinical quality in each country and assessed correlation of infrastructure across services and clinical quality across services. We calculated the intraclass correlation (ICC) by country to quantify variation.
We generated scatterplots of infrastructure and observed clinical quality with a linear curve to visualize the association and calculated Pearson’s correlation coefficient for each association. The smooth curve in each scatterplot was fitted using a generalized additive model to capture potential nonlinear effect; the shaded area represents the 95% confidence interval around the smoothed curve. Histograms of infrastructure and observed clinical quality were plotted along the 2 axes. We divided facilities into quintiles of infrastructure and plotted median clinical quality and the interquartile range (IQR) across quintiles to visualize variability of process quality within levels of structural inputs.
We attempted to identify a minimum threshold of inputs required for good quality clinical care. We tested for nonlinearity in the relationship between infrastructure and observed quality by fitting linear spline models with a single knot. Because we do not have prior knowledge about the location of the knot, we started from a range of cutoff values between the minimum and maximum of infrastructure. For each cutoff value, we fit a linear spline model of observed clinical quality on infrastructure with a new variable taking the values of the marginal increase of service infrastructure above the cutoff value. We obtained prediction error using 10-fold cross validation for each candidate value [36]. We picked the cutoff value with the smallest prediction error as the location of the knot for the final model for each country and service. We assessed the statistical significance (p ≤ 0.05) of the marginal spline in this model to determine whether the spline meaningfully changed the association from the basic linear model.
Cross-country analyses are weighted so that each country contributes equally to the sample; within-country analyses are unweighted due to the restrictive selection criteria applied to the final analytic sample. Analyses were conducted in Stata version 14.1 (StataCorp, College Station, Texas) and R version 3.3.1 (the R Foundation for Statistical Computing).
Results
Of 8,501 facilities selected, 8,254 (97.1%) were assessed; 4,354 facilities had at least one valid observation in the selected services (32,531 total observations). The analytic sample comprised 1,407 facilities for ANC, 1,842 for family planning, 227 for delivery, and 4,038 for sick-child care. Because observations were sampled based on availability of patients on the day of visit, facilities excluded from the analysis were disproportionately smaller clinics and health centers. Hospitals made up approximately 25% of the sample for ANC, family planning, and sick-child care and 71% of the facilities for delivery care (Table 1). Approximately 27% of facilities were privately managed, ranging from 22% in family planning to 30% in sick-child care. The number of observations per facility varied from 3.42 in ANC to 4.71 in sick-child care.
Tab. 1. Characteristics of facilities providing family planning, antenatal, sick-child, and delivery care in 8 countries, 2007–2015. 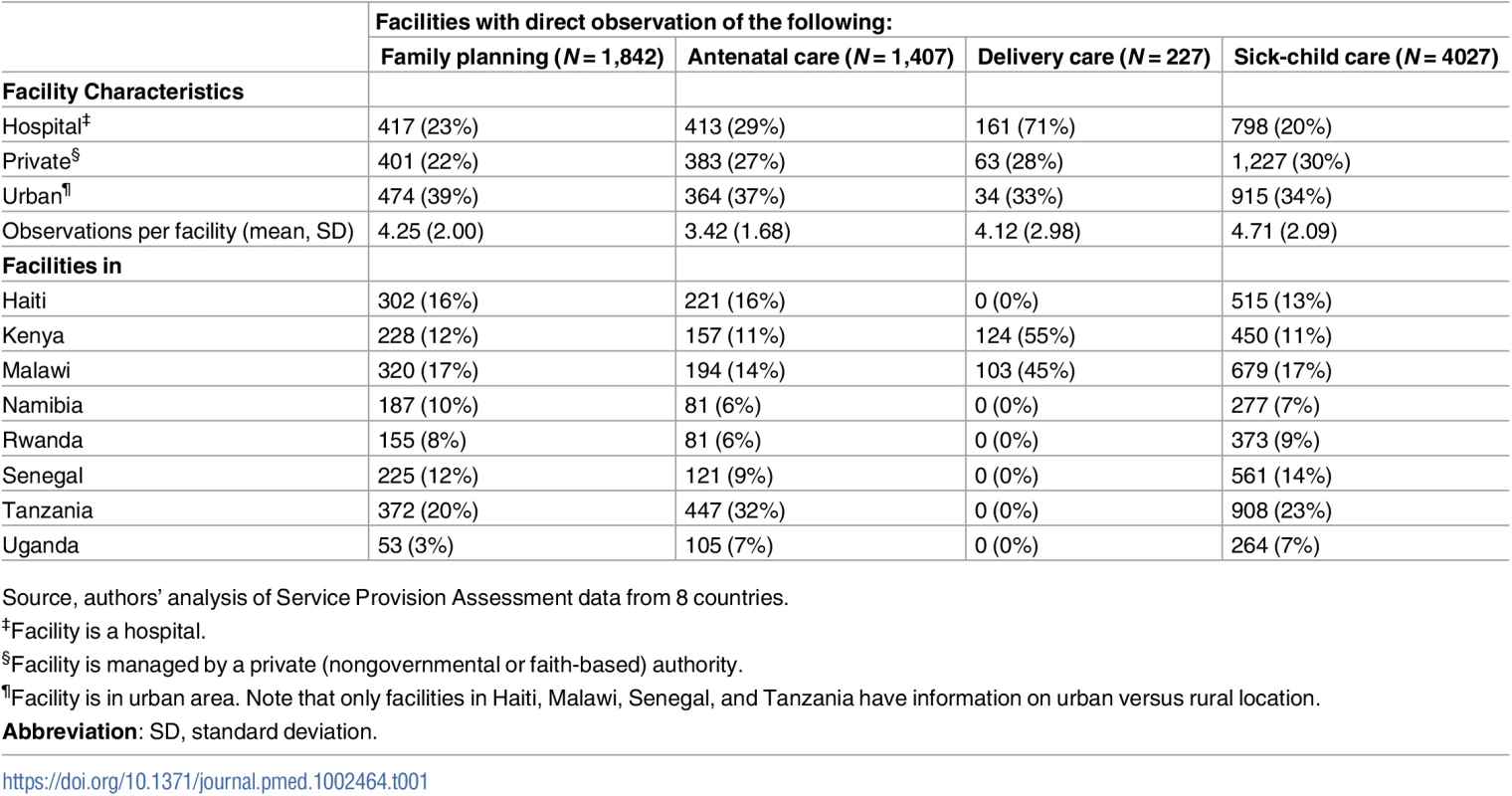
Source, authors’ analysis of Service Provision Assessment data from 8 countries. Facilities in the sample demonstrated moderate levels of infrastructure across all services (Table 2). Infrastructure was highest in family planning (averaging 0.70 in Rwanda to 0.80 in Kenya) and lowest in sick-child care (averaging 0.59 in Haiti and Rwanda to 0.70 in Namibia). Observed clinical quality was low in all services, with an average of 60% of clinical actions completed in ANC and delivery care compared to 48% in family planning and only 37% in sick-child care. Although infrastructure in different clinical areas was correlated by definition due to shared basic amenities and infection control domains, the magnitude of the correlation ranged from a minimum of 0.41 for delivery care and family planning to a maximum of 0.69 for ANC and sick-child care (Panel A in S3 Table). Correlation was lower for clinical quality across services, with negative correlation for delivery care with ANC and sick-child care and the largest correlation at 0.32 for ANC and family planning (Panel B in S3 Table). In all services, the ICC for within - versus between-country variance was higher for observed clinical quality (Panel C in S3 Table), indicating that clinical quality varied relatively more between countries than did infrastructure.
Tab. 2. Summary statistics of infrastructure and observed clinical quality in facilities providing family planning, antenatal, sick-child, and delivery care. 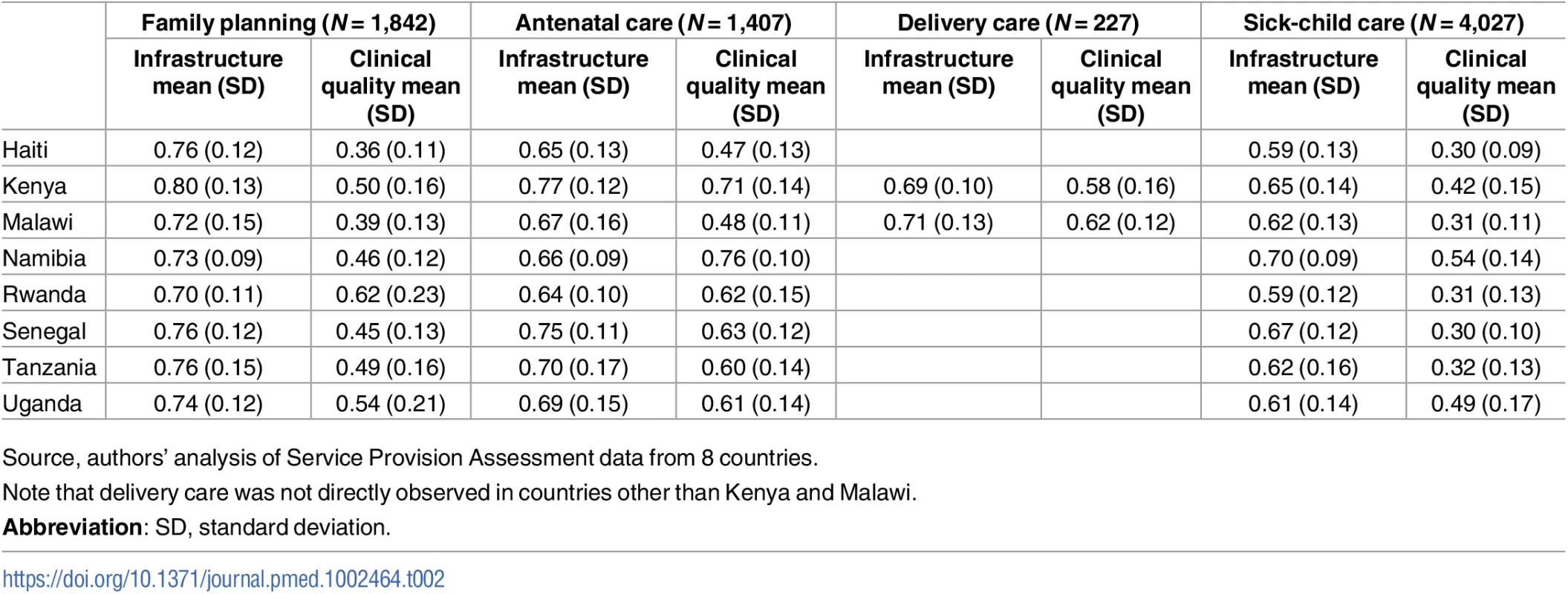
Source, authors’ analysis of Service Provision Assessment data from 8 countries. The association between infrastructure and observed clinical quality for each service is shown by country in Fig 1 (family planning and ANC) and Fig 2 (labor and delivery care and sick-child care). The variation in observed clinical quality in particular is evident in the range of the scatter along the y-axis and the flatter histograms in most, though not all, plots. Across different countries and services, the association was consistently positive but weak, with highly variable magnitude across countries by service, ranging across all analyses from −0.03 (family planning in Senegal) to 0.40 (ANC in Tanzania). Median correlation across all services and countries was 0.20.
Fig. 1. Association between infrastructure and observed clinical quality for family planning and antenatal care. 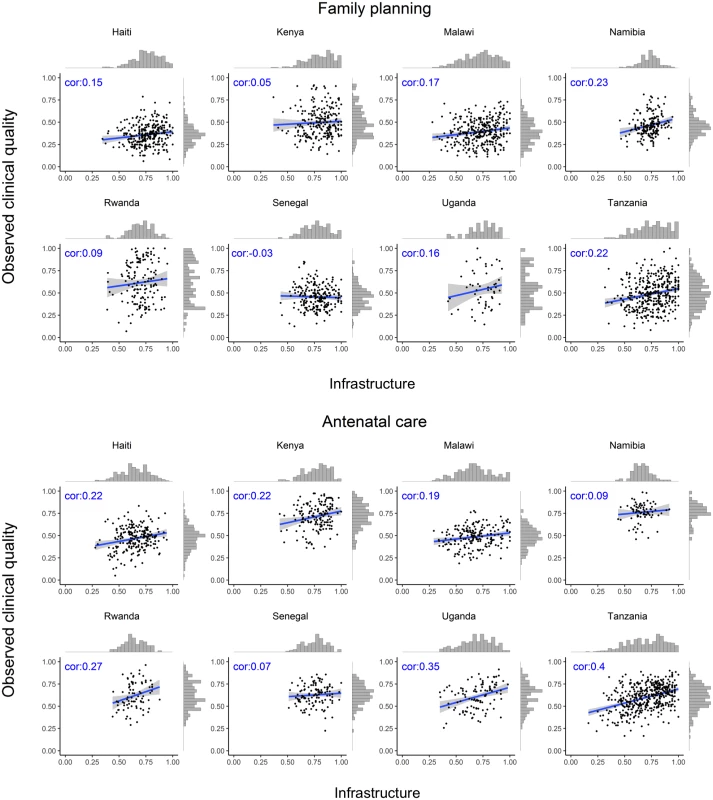
cor, correlation coefficient. Fig. 2. Association between infrastructure and observed clinical quality for labor and delivery care and sick-child care. 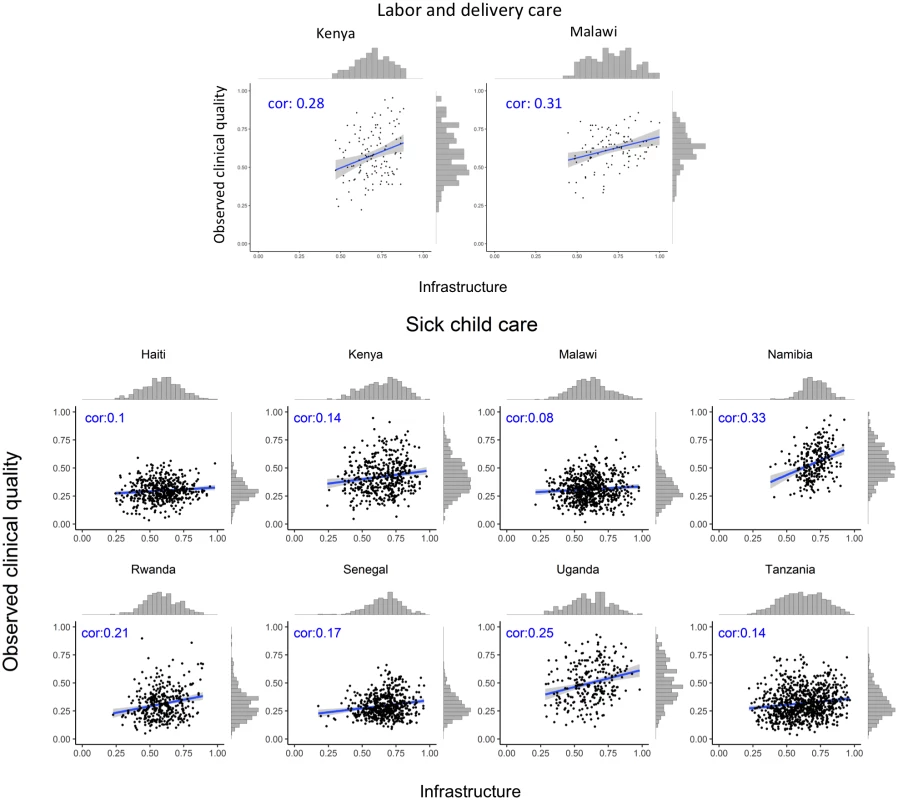
cor, correlation coefficient. Fitting spline models failed to identify a significant inflection point in any country for any service (S1 Fig). The association between infrastructure and observed clinical quality was generally linear across countries and services.
The boxes in Fig 3 display median and IQR of observed quality by quintile of infrastructure pooled across all countries. The modest association between inputs and observed clinical quality are evident in ANC and delivery care in particular. Even as infrastructure increases, however, variability in observed clinical quality remains high: IQR in the highest quintile of infrastructure barely differs from that in the lowest quintile, with the exception of sick-child care, for which the IQR increases from 0.16 to 0.26 as infrastructure increases (Table 3).
Fig. 3. Range of observed clinical quality across quintiles of infrastructure. 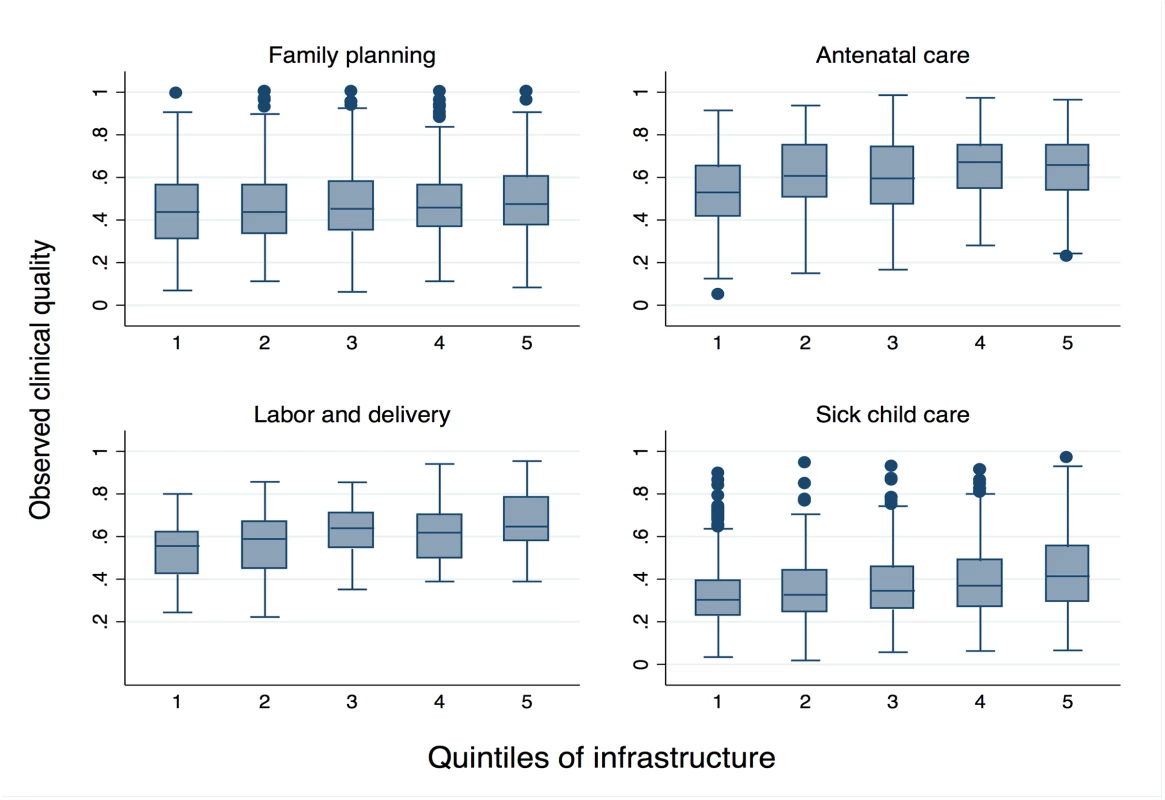
Tab. 3. Median and interquartile range of observed clinical quality by quintile of infrastructure. 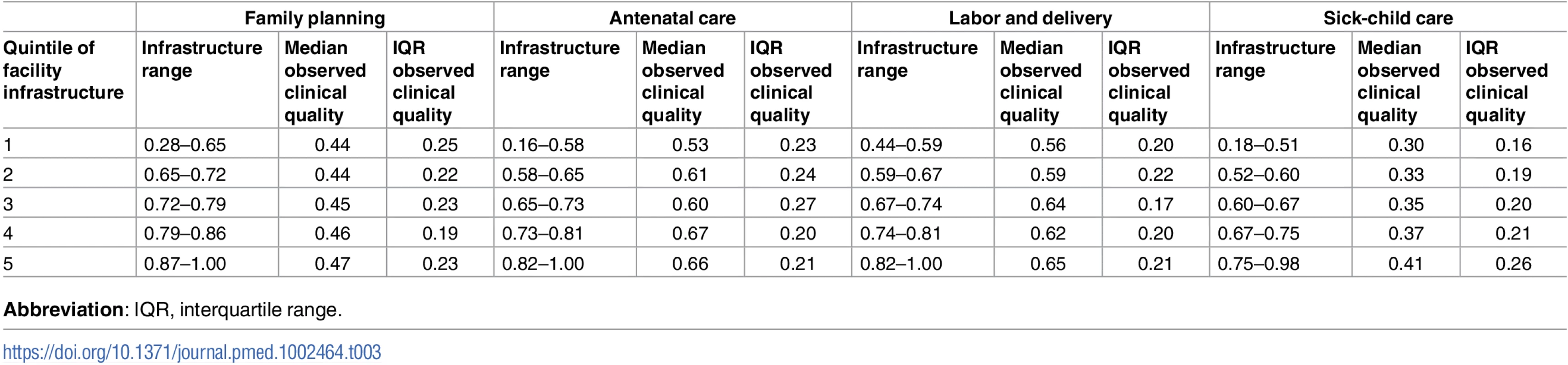
Abbreviation: IQR, interquartile range. Discussion
Across multiple clinical services in 8 countries, correlation between inputs and adherence to evidence-based care guidelines was weak: within each service, facilities with similar levels of infrastructure provided widely varying care. Observed clinical quality tended to be more variable and lower than infrastructure in nearly all countries and services, suggesting that using inputs as a proxy for quality of care as delivered would be both unreliable and systematically biased to overstate quality. These results were based on a sample stripped of likely outliers (facilities with a single observation of clinical care per service) in order to minimize noise in the association of inputs and process quality. Even in these generally larger facilities, gaps in readiness to provide essential care and particularly in observed clinical quality were evident in all services and countries. Although inputs to care should serve as an essential foundation for high-quality care, these data did not suggest the existence of a minimum threshold of inputs necessary for providing better care within the range of infrastructure observed here. It is possible that such a threshold exists at extremely low levels of facility infrastructure.
The deficiencies in facility infrastructure found in this study are similar to prior assessments of structural inputs [16,37,38] and suggest that even the hospitals and larger facilities overrepresented in this analysis lack key elements of basic amenities, equipment, and medications required to provide basic services. Cross-national estimates of process quality measures are scarcer, but a growing body of evidence from national and subnational studies attests to high variability and low attainment in measures of clinical process quality in low-resource settings [39–41]. Our finding of lower process quality than inputs affirm findings in diverse settings such as India [42], Bangladesh [43], and South Sudan [44].
Measuring the necessary inputs to care provided limited insight on the process quality of care delivered in primary care services as well as in more resource-intensive delivery care. These findings amplify a study of pay-for-performance interventions in Rwanda, demonstrating that increased availability of inputs for delivery care explained an insignificant fraction of increased delivery volume [45]. Although we would not expect perfect correlation due to the breadth of the infrastructure measures relative to the specific items of evidence-based care, the limited associations and high variability in observed clinical quality at similar levels of facility infrastructure was striking, even for well-equipped facilities. More surprisingly, some facilities were able to provide above-average care quality at quite low levels of infrastructure. While several of the elements of observed clinical quality in these services—particularly the primary care services of family planning, ANC, and sick-child care—could be completed with no supplies, rudimentary equipment such as thermometers and blood pressure cuffs are required. Low to modest correlation in the assessed measures suggests that performance on global standards for readiness bears little relevance for performance on global standards for provision of care. Our findings underscore the importance of direct measurement of the process of care as delivered to provide meaningful insight on the current state of quality and the key areas for improvement. The importance of measuring care processes is bolstered by the growing evidence of a know–do gap, in which providers often underperform their knowledge tests [40,41,46].
This work calls into question the utility of health facility assessments such as the SARA and other surveys focusing on inputs in their current configuration: if facility infrastructure is only weakly correlated with the delivery of care, it is likely to be even less correlated to outcomes. Subnational and cross-national comparison of inputs to care will thus serve little purpose in understanding how the health system is performing in improving population health. Assessment of infrastructure, including the functioning of basic amenities and equipment and the availability of medicines and supplies, is important for proper health system management, but such information is required on a local level and with high frequency if it is to be actionable. Procurement and other supply chain information systems offer a better source for this information than expensive and infrequent facility surveys. Bolstering their capacity—and in particular the analysis and use of such data for monitoring and improvement purposes—is a global health priority [47].
Given the limited resources for health system measurement—including health worker time—information collected must be justified based on its value in understanding and improving health system performance; methods of data collection should be optimized for the intended purpose. Health facility assessments can provide valuable standardized information on the health system just as Demographic and Health Surveys do on the population. Improvements to current health facility assessments should be pursued, including attempts to identify a minimum set of input indicators that reflect overall structural capacity and in standardizing indicators of healthcare processes or impacts that best capture the quality of care for subnational, national, and cross-national monitoring and performance assessment. Consideration of a range of quality indicators and methods to collect them is warranted, such as vital registration, focused direct observations, patient exit and community surveys, and stronger measures of healthcare-sensitive health outcomes, including patient-reported outcomes, in facilities and after discharge.
To our knowledge, this is the first multi-country, multiservice comparison of inputs and process quality measures in low-resource settings. The analysis was based on 32,566 direct observations of clinical care from the most comprehensive health facility assessment in widespread use [17]. We limited our sample to facilities with multiple observations to minimize the impact of single, potentially nonrepresentative observations and defined infrastructure using the essential equipment and supplies pertinent to the type of care being observed, as defined by WHO. However, there are several limitations to this study. Direct observation of care can increase provider efforts via the Hawthorne effect [48], although limiting to facilities with multiple observations should mitigate its impact on the results. Observer error or inability to observe procedures taking place prior to the clinical encounter could introduce variability in measurement unrelated to the quality of clinical care provided. Variation in assessing each visit could attenuate the relationship between infrastructure and average quality [49]. The small number of countries precludes assessment of changes in the observed associations over time; the selected surveys spanned 2007–2015; while it is likely that efforts to achieve the MDGs affected facility infrastructure and observed clinical quality in these countries, it is not possible to assess such effects in these data or to determine whether such changes might have strengthened or weakened the association between them. The cross-sectional nature of the data makes it impossible to identify associations between long-term availability of equipment and supplies and clinical care quality as well as to disentangle reverse causality such as shortages due to high patient demand. These factors could contribute to the variability in the observed data; any data source addressing these concerns would require longitudinal data collection.
As the quality of care assumes a more prominent role in national and global efforts to improve population health outcomes, accurate measurement is vital. Healthcare providers and physical inputs, such as buildings, medicines, and equipment, are an essential foundation for delivering healthcare. However, we found that these structural measures provide little insight on the quality of services delivered to patients. Expanding measurement of processes and outcomes of care is imperative to achieve better health outcomes and improve performance of health systems.
Supporting Information
Zdroje
1. Dieleman J, Campbell M, Chapin A, Eldrenkamp E, Fan VY, Haakenstad A, et al. Evolution and patterns of global health financing 1995–2014: development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. The Lancet. 2017. Epub April 19 2017. doi: 10.1016/S0140-6736(17)30874-7 28433256
2. United Nations. The Millennium Development Goals Report 2015. New York, New York: United Nations, 2015.
3. United Nations Development Program. Sustainable Development Goals. Geneva, Switzerland: United Nations, 2015.
4. Lim SS, Allen K, Bhutta ZA, Dandona L, Forouzanfar MH, Fullman N, et al. Measuring the health-related Sustainable Development Goals in 188 countries: a baseline analysis from the Global Burden of Disease Study 2015. The Lancet. 2016;388(10053):1813–50. https://doi.org/10.1016/S0140-6736(16)31467-2.
5. Randive B, Diwan V, De Costa A. India's Conditional Cash Transfer Programme (the JSY) to Promote Institutional Birth: Is There an Association between Institutional Birth Proportion and Maternal Mortality? PLoS One. 2013;8(6):e67452. Epub 2013/07/05. doi: 10.1371/journal.pone.0067452 23826302.
6. Godlonton S, Okeke EN. Does a ban on informal health providers save lives? Evidence from Malawi. Journal of Development Economics. 2016;118 : 112–32. doi: 10.1016/j.jdeveco.2015.09.001 26681821
7. Can Institutional Deliveries Reduce Newborn Mortality? Evidence from Rwanda [Internet]. IDEAS. 2014 [cited 10 October 2017]. https://ideas.repec.org/p/ran/wpaper/1072.html.
8. Sobel HL, Huntington D, Temmerman M. Quality at the centre of universal health coverage. Health Policy Plan. 2016;31(4):547–9. Epub 2015/10/01. doi: 10.1093/heapol/czv095 26420642.
9. Kruk ME, Pate M, Mullan Z. Introducing The Lancet Global Health Commission on High-Quality Health Systems in the SDG Era. The Lancet Global health. 2017;5(5):e480–e1. Epub 2017/03/18. doi: 10.1016/S2214-109X(17)30101-8 28302563.
10. Institute of Medicine. Medicare: A Strategy for Quality Assurance. Washington DC: National Academy Press; 1990.
11. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press; 2001. 360 p.
12. Tunçalp Ӧ, Were W, MacLennan C, Oladapo O, Gülmezoglu A, Bahl R, et al. Quality of care for pregnant women and newborns—the WHO vision. BJOG: An International Journal of Obstetrics & Gynaecology. 2015;122(8):1045–9.
13. World Health Organization. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies: World Health Organization Geneva; 2010.
14. Bruce J. Fundamental Elements of the Quality of Care: A Simple Framework. Studies in Family Planning. 1990;21(2):61–91. doi: 10.2307/1966669 2191476
15. Donabedian A. The quality of care: How can it be assessed? JAMA. 1988;260(12):1743–8. 3045356
16. O'Neill K, Takane M, Sheffel A, Abou-Zahr C, Boerma T. Monitoring service delivery for universal health coverage: the Service Availability and Readiness Assessment. Bull World Health Organ. 2013;91(12):923–31. Epub 2013/12/19. doi: 10.2471/BLT.12.116798 24347731.
17. Nickerson JW, Adams O, Attaran A, Hatcher-Roberts J, Tugwell P. Monitoring the ability to deliver care in low - and middle-income countries: a systematic review of health facility assessment tools. Health Policy Plan. 2015;30(5):675–86. doi: 10.1093/heapol/czu043 24895350
18. Sprockett A. Review of quality assessment tools for family planning programmes in low - and middle-income countries. Health Policy Plan. 2016. Epub 2016/10/16. doi: 10.1093/heapol/czw123 27738020.
19. Johns Hopkins University. PMA2020 Data: Johns Hopkins University; 2017 [22 May 2017]. https://pma2020.org/pma2020-data.
20. Demographic and Health Surveys Program. The DHS Program—Service Provision Assessments (SPA): USAID; 2017 [22 May 2017]. http://dhsprogram.com/What-We-Do/survey-search.cfm?pgType=main&SrvyTp=type.
21. World Bank. Central Microdata Catalog 2017 [22 May 2017]. http://microdata.worldbank.org/index.php/catalog/central?sk=Service Delivery Indicators Survey.
22. World Health Organization. Service Availability and Readiness Assessment (SARA): An annual monitoring system for service delivery. Geneva, Switzerland: 2013.
23. World Health Organization. Service Availability and Readiness Assessment (SARA) Reference Manual. Geneva, Switzerland: World Health Organization, 2013.
24. Gergen J, Josephson E, Coe M, Ski S, Madhavan S, Bauhoff S. Quality of Care in Performance-Based Financing: How It Is Incorporated in 32 Programs Across 28 Countries. Global Health: Science and Practice. 2017;5(1):90–107. doi: 10.9745/ghsp-d-16-00239 28298338
25. Nguhiu PK, Barasa EW, Chuma J. Determining the effective coverage of maternal and child health services in Kenya, using demographic and health survey data sets: tracking progress towards universal health coverage. Tropical Medicine & International Health. 2017;22(4):442–53. doi: 10.1111/tmi.12841 28094465
26. Baker U, Okuga M, Waiswa P, Manzi F, Peterson S, Hanson C. Bottlenecks in the implementation of essential screening tests in antenatal care: Syphilis, HIV, and anemia testing in rural Tanzania and Uganda. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2015;130(Suppl 1):S43–50. Epub 2015/06/10. doi: 10.1016/j.ijgo.2015.04.017 26054252.
27. Marchant T, Tilley-Gyado RD, Tessema T, Singh K, Gautham M, Umar N, et al. Adding Content to Contacts: Measurement of High Quality Contacts for Maternal and Newborn Health in Ethiopia, North East Nigeria, and Uttar Pradesh, India. PLOS ONE. 2015;10(5):e0126840. doi: 10.1371/journal.pone.0126840 26000829
28. Gage AJ, Ilombu O, Akinyemi AI. Service readiness, health facility management practices, and delivery care utilization in five states of Nigeria: a cross-sectional analysis. BMC pregnancy and childbirth. 2016;16(1):297. Epub 2016/10/08. doi: 10.1186/s12884-016-1097-3 27716208.
29. Armstrong CE, Martinez-Alvarez M, Singh NS, John T, Afnan-Holmes H, Grundy C, et al. Subnational variation for care at birth in Tanzania: is this explained by place, people, money or drugs? BMC Public Health. 2016;16 Suppl 2 : 795. Epub 2016/09/17. doi: 10.1186/s12889-016-3404-3 27634353.
30. Leslie HH, Fink G, Nsona H, Kruk ME. Obstetric Facility Quality and Newborn Mortality in Malawi: A Cross-Sectional Study. PLoS Med. 2016;13(10):e1002151. Epub 2016/10/19. doi: 10.1371/journal.pmed.1002151 27755547.
31. Acharya LB, Cleland J. Maternal and child health services in rural Nepal: does access or quality matter more? Health Policy Plan. 2000;15(2):223–9. 10837046
32. World Health Organization. Integrated Management of Childhood Illness: Chart Booklet. Geneva, Switzerland: World Health Organization, 2014 March. Report No.
33. Department of Reproductive Health and Research, Family and Community Health. WHO Antenatal Care Randomized Trial: Manual for the Implementation of the New Model. Geneva, Switzerland: World Health Organization, 2002.
34. World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, Switzerland: World Health Organization, 2016.
35. Tripathi V, Stanton C, Strobino D, Bartlett L. Development and Validation of an Index to Measure the Quality of Facility-Based Labor and Delivery Care Processes in Sub-Saharan Africa. PLoS One. 2015;10(6):e0129491. Epub 2015/06/25. doi: 10.1371/journal.pone.0129491 26107655.
36. Dudoit S, van der Laan MJ. Asymptotics of cross-validated risk estimation in estimator selection and performance assessment. Statistical Methodology. 2005;2(2):131–54. https://doi.org/10.1016/j.stamet.2005.02.003.
37. Kruk ME, Leslie HH, Verguet S, Mbaruku GM, Adanu RMK, Langer A. Quality of basic maternal care functions in health facilities of five African countries: an analysis of national health system surveys. The Lancet Global Health. 2016. doi: 10.1016/S2214-109X(16)30180-2
38. Leslie HH, Spiegelman D, Zhou X, Kruk ME. Service readiness of health facilities in Bangladesh, Haiti, Kenya, Malawi, Namibia, Nepal, Rwanda, Senegal, Uganda and the United Republic of Tanzania. Bull World Health Organ. 2017;95(11).
39. Gathara D, English M, van Hensbroek MB, Todd J, Allen E. Exploring sources of variability in adherence to guidelines across hospitals in low-income settings: a multi-level analysis of a cross-sectional survey of 22 hospitals. Implementation Science. 2015;10(1):60. Epub 2015/05/01. doi: 10.1186/s13012-015-0245-x 25928803
40. Mohanan M, Vera-Hernandez M, Das V, Giardili S, Goldhaber-Fiebert JD, Rabin TL, et al. The know-do gap in quality of health care for childhood diarrhea and pneumonia in rural India. JAMA pediatrics. 2015;169(4):349–57. Epub 2015/02/17. doi: 10.1001/jamapediatrics.2014.3445 25686357.
41. Leonard KL, Masatu MC. The use of direct clinician observation and vignettes for health services quality evaluation in developing countries. Soc Sci Med. 2005;61(9):1944–51. Epub 2005/06/07. doi: 10.1016/j.socscimed.2005.03.043 15936863.
42. Chavda P, Misra S. Evaluation of input and process components of quality of child health services provided at 24 × 7 primary health centers of a district in Central Gujarat. Journal of Family Medicine and Primary Care. 2015;4(3):352–8. doi: 10.4103/2249-4863.161315 26288773
43. Hoque DME, Rahman M, Billah SM, Savic M, Karim AQMR, Chowdhury EK, et al. An assessment of the quality of care for children in eighteen randomly selected district and sub-district hospitals in Bangladesh. BMC Pediatrics. 2012;12(1):197. Epub 2012/12/28. doi: 10.1186/1471-2431-12-197 23268650
44. Berendes S, Lako RL, Whitson D, Gould S, Valadez JJ. Assessing the quality of care in a new nation: South Sudan's first national health facility assessment. Tropical medicine & international health: TM & IH. 2014;19(10):1237–48. Epub 2014/08/20. doi: 10.1111/tmi.12363 25134414.
45. Ngo DK, Sherry TB, Bauhoff S. Health system changes under pay-for-performance: the effects of Rwanda’s national programme on facility inputs. Health Policy Plan. 2016;32(1):11–20. doi: 10.1093/heapol/czw091 27436339
46. Leonard KL, Masatu MC. Professionalism and the know—do gap: Exploring intrinsic motivation among health workers in Tanzania. Health economics. 2010;19(12):1461–77. doi: 10.1002/hec.1564 19960481
47. Health Data Collaborative. Health Data Collaborative Progress Report 2016–2017. Health Data Collaborative, 2017 May 2017. Report No.: 1.
48. Leonard K, Masatu MC. Outpatient process quality evaluation and the Hawthorne Effect. Soc Sci Med. 2006;63(9):2330–40. Epub 2006/08/05. doi: 10.1016/j.socscimed.2006.06.003 16887245.
49. Lüdtke O, Marsh HW, Robitzsch A, Trautwein U. A 2 × 2 taxonomy of multilevel latent contextual models: Accuracy–bias trade-offs in full and partial error correction models. Psychological Methods. 2011;16 : 444–67. 21787083
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 12- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Cell salvage and donor blood transfusion during cesarean section: A pragmatic, multicentre randomised controlled trial (SALVO)
- Re-emerging and newly recognized sexually transmitted infections: Can prior experiences shed light on future identification and control?
- Antiretroviral therapy and population mortality: Leveraging routine national data to advance policy
- Psychosocial and socioeconomic determinants of cardiovascular mortality in Eastern Europe: A multicentre prospective cohort study
- Research on HIV cure: Mapping the ethics landscape
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- Effects of women’s groups practising participatory learning and action on preventive and care-seeking behaviours to reduce neonatal mortality: A meta-analysis of cluster-randomised trials
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
- Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: A modeling study
- Sexually transmitted infections—Research priorities for new challenges
- Healthcare provider perspectives on managing sexually transmitted infections in HIV care settings in Kenya: A qualitative thematic analysis
- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Dual-strain genital herpes simplex virus type 2 (HSV-2) infection in the US, Peru, and 8 countries in sub-Saharan Africa: A nested cross-sectional viral genotyping study
- Association between infrastructure and observed quality of care in 4 healthcare services: A cross-sectional study of 4,300 facilities in 8 countries
- Bridging the quality chasm in maternal, newborn, and child healthcare in low- and middle-income countries
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: An evaluation from multi-country surveys and stakeholder interviews
- Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial
- The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention
- Estimating the impact of antiretroviral treatment on adult mortality trends in South Africa: A mathematical modelling study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



