-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaZika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review
In a systematic review, Nicola Low and colleagues use a causality framework to examine the evidence for zika virus infection as a cause of congenital brain abnormalities and Guillain–Barré syndrome.
Published in the journal: . PLoS Med 14(1): e32767. doi:10.1371/journal.pmed.1002203
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002203Summary
In a systematic review, Nicola Low and colleagues use a causality framework to examine the evidence for zika virus infection as a cause of congenital brain abnormalities and Guillain–Barré syndrome.
Introduction
An “explosive pandemic of Zika virus infection” [1] in 2015 caught the world by surprise. The Pan American Health Organization (PAHO) and World Health Organization (WHO) published an alert about a possible association with increases in reports of congenital abnormalities and Guillain–Barré syndrome (GBS) on December 1, 2015 [2]. On February 1, 2016, WHO declared a Public Health Emergency of International Concern [3]. Microcephaly at birth is a clinical finding that can include a range of brain malformations resulting from a failure of neurogenesis [4]. Infections acquired in pregnancy, including cytomegalovirus and rubella, are established causes [4]. GBS is an immune-mediated ascending flaccid paralysis, which typically occurs within a month of an infection, such as Campylobacter jejuni or cytomegalovirus [5]. As of October 20, 2016, 67 countries have reported autochthonous transmission of the mosquito-borne flavivirus Zika since 2015, and 27 of these countries have reported cases of congenital brain abnormalities, GBS, or both [6]. The emergency committee recommended increased research [3] to provide more rigorous scientific evidence of a causal relationship as a basis for the global health response.
Unexplained clusters of rare but serious conditions require urgent assessment of causality, balancing speed with systematic appraisal. Bradford Hill is widely credited for his proposed framework for thinking about causality in epidemiology, which listed nine “viewpoints” from which to study associations between exposure and disease (S1 Text, p2) [7]. Since then, the list has been modified (S1 Text, p2; S1 Table) [8]. Bradford Hill emphasised that his viewpoints were not rules but, taken together, the body of evidence should be used to decide whether there is any other more likely explanation than cause and effect.
The level of certainty required before judging that Zika virus is a cause of microcephaly and GBS is contentious [9]. Most assessments have been based on rapid but nonsystematic appraisals [10–12]. Based on rapid reviews, WHO has stated that there is “scientific consensus that Zika virus is a cause of microcephaly and GBS” since March 31, 2016 [13]. On April 13, a narrative review stated that there was “a causal relationship between prenatal Zika virus infection and microcephaly” [11]. Evidence about the causal relationship between Zika virus and GBS has not yet been assessed in detail. We previously described a causality framework for Zika virus and plans for a systematic review (S1 Text, p3; S2 Table), with a preliminary overview of 21 studies, published up to March 4, 2016 [14]. The objectives of this study are to reassess the evidence for causality and update the WHO position about links between Zika virus and (a) congenital brain abnormalities, including microcephaly, in the foetuses and offspring of pregnant women and (b) GBS in any population, and to describe the process and outcomes of an expert assessment of the evidence.
Methods
We describe three linked components: the causality framework, the systematic reviews, and the expert panel assessment of the review findings. The WHO Zika Causality Working Group convened the expert panel of 18 members with specialist knowledge in the fields of epidemiology and public health, virology, infectious diseases, obstetrics, neonatology, and neurology (membership of the expert panel is provided in the Acknowledgments).
Zika Causality Framework
In February 2016, we developed a causality framework for Zika virus by defining specific questions for each of ten dimensions, modified from Bradford Hill’s list (S2 Table): temporality; biological plausibility; strength of association; exclusion of alternative explanations; cessation; dose–response relationship; animal experimental evidence; analogy; specificity; and consistency of findings. This review covered 35 questions about congenital brain abnormalities, including microcephaly, and 26 questions about GBS. We also listed seven groups of cofactors that might increase the risk of an outcome in the presence of Zika virus [15].
Systematic Review
Our protocol was registered on March 21, 2016 in the database PROSPERO (CRD42016036693) [16]. We report the methods in full according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17] in S1 Text (p3-4).
To report our findings, we use the term item for an individual record, e.g., a case report. Occasionally, the same individuals or data were reported in more than one publication (item). To avoid double counting, we organised these items into groups. We chose a primary publication (the item with the most complete information) to represent the group, to which other items were linked (S4 Table, S6 Table).
Eligibility
We included studies of any design and in any language that directly addressed any research question in the causality framework (S1 Text, p3).
Information sources and search strategy
We searched multiple electronic databases and websites (protocol [16] and S1 Text, p3) and included published peer-reviewed studies, ongoing studies, and non-peer reviewed sources. For the dimension addressing analogous causes of the outcomes and for cofactors, we did not conduct systematic searches.
We conducted our first search from the earliest date to April 11, 2016 and updated the search on May 30 and July 29. We selected items and extracted data systematically on included items up to May 30 and reported on nonsystematically identified studies up to July 29, 2016.
Study selection and data extraction
We screened titles, abstracts, and full texts by liberal accelerated screening (S1 Text, p3) [18]. For data extraction, one reviewer extracted data and a second reviewer checked the extracted data. We used case definitions and laboratory diagnostic test interpretations as reported by study authors.
Synthesis of findings and assessment of methodological quality
We tabulated study-level data and clinical information from case reports, case series, cross-sectional studies, case-control studies, and cohort studies. We assessed methodological quality for these designs using checklists [19]. Each reviewer recorded an overall judgement to indicate whether study findings did or did not provide support for each causality dimension. We assigned a judgement of sufficient evidence about a causality dimension if the consensus assessments were supportive for at least half of the specific questions. We appraised the body of evidence according to the Grading of Research Assessment Development and Evaluation (GRADE) tool, as suggested for urgent health questions [20], but did not apply upgrading or downgrading because these concepts could not be applied consistently across the range of study designs.
Expert Panel
In a series of web and telephone conferences between April 18 and May 23, 2016, we presented our approach to the assessment of causality, the causality framework, and our synthesis of evidence to the expert panel. We discussed these topics with the experts during the conferences and through email discussions. We then drafted summary conclusions about the most likely explanation for the reported clusters of cases of microcephaly and GBS. The expert panel members reached consensus statements to update the WHO position (Fig 1).
Fig. 1. Timeline of Zika causality review, February 1 to August 2016. 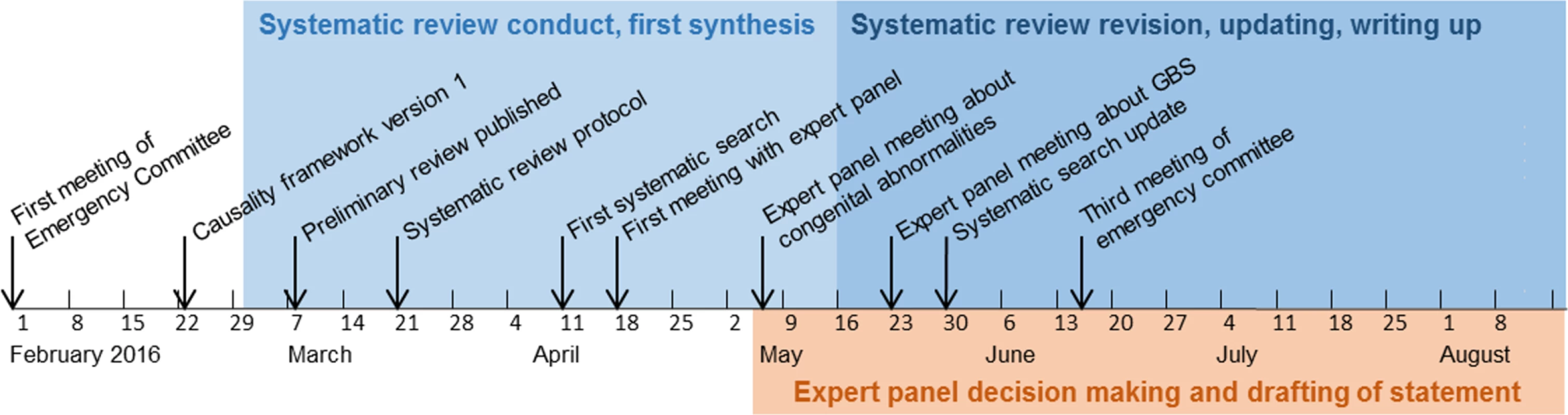
A Public Health Emergency of International Concern was announced on February 1, 2016 in response to clusters of microcephaly, GBS, and other neurological disorders. Results
We found 1,091 unique items published from 1952 to May 30, 2016 (S1 Fig, S3 Table). Most excluded items were reviews or editorials and commentaries (44%, n = 479). We included 106 items from 87 groups (Table 1), of which 83% were published in 2016. For both outcomes, the majority of items were clinical, individual-level case reports, case series, or population-level surveillance data.
Tab. 1. Summary of included items according to outcome, study design, and causality dimension. 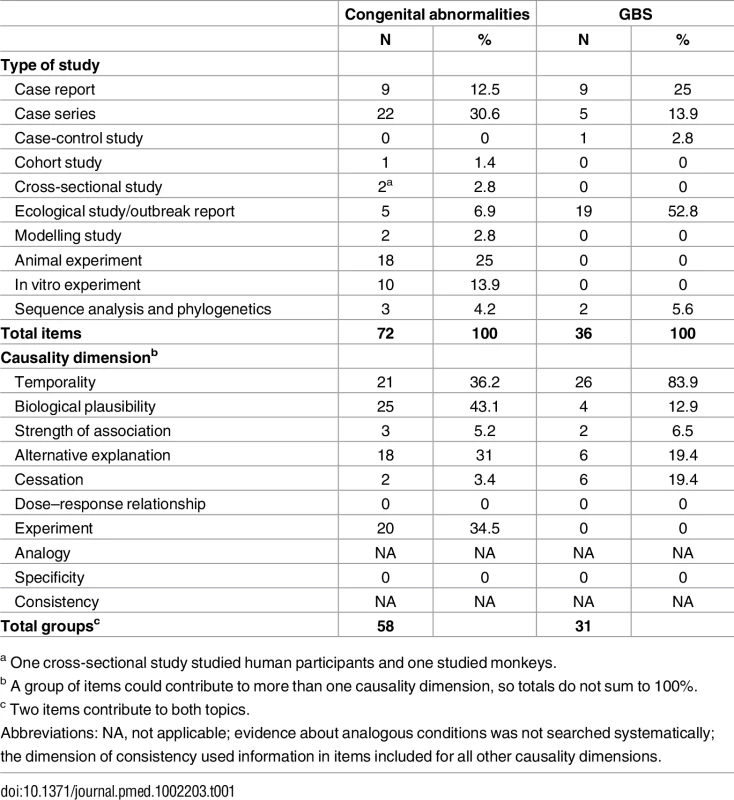
a One cross-sectional study studied human participants and one studied monkeys. Congenital Brain Abnormalities
A total of 72 items belonging to 58 groups addressed questions related to congenital brain abnormalities up to May 30, 2016 [13, 21–93]. Table 2 summarises the characteristics of 278 mother–infant pairs described in included studies.
Tab. 2. Geographic, clinical, and microbiological characteristics of mother–infant pairs. 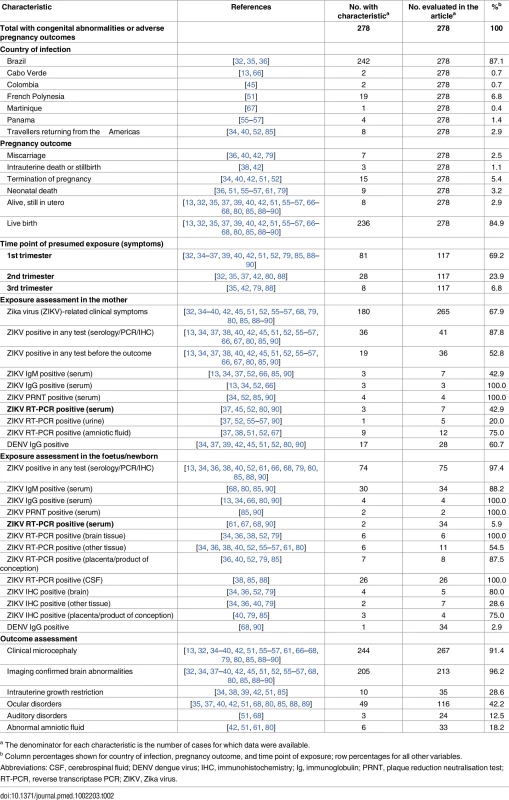
a The denominator for each characteristic is the number of cases for which data were available. Table 3 summarises the assessment for each causality dimension, S4 Table provides an extended description of study findings, and S5 Table summarises the quality of the body of evidence.
Tab. 3. Summary of reviewers’ assessments of evidence about Zika virus infection and congenital abnormalities, by causality dimension. 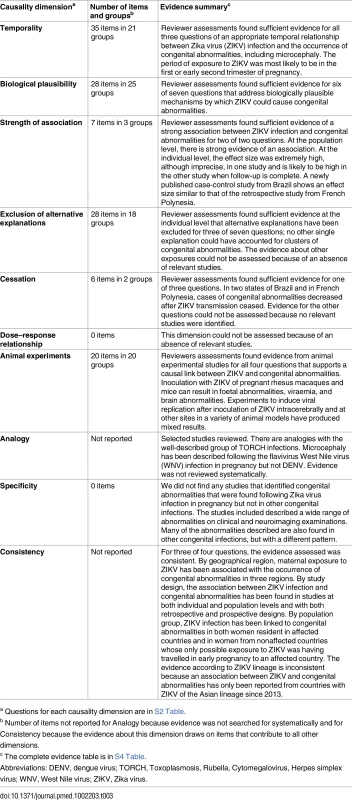
a Questions for each causality dimension are in S2 Table. Temporality
Thirty-five items [30–37, 39–43, 45–47, 49, 51, 52, 57, 60, 65, 67, 68, 73–76, 78, 80, 85, 86, 88–90] in 21 groups addressed temporality (S4 Table). Overall, 67.9% (180/265) of women reported symptoms of Zika virus infection during pregnancy (Table 2). Confirmed infection preceded a diagnosis of microcephaly in a small proportion of cases because many reports were published before laboratory confirmation testing was available. Of the 36 mothers with laboratory-confirmed Zika virus infection, 19 (52.8%) were diagnosed before the detection of foetal malformations or miscarriage [40, 42, 45, 52, 67]. Two detailed case reports show timelines of recent infection followed by neuroimaging evidence of brain abnormalities and subsequent birth with microcephaly [42] or foetal infection [52]. The most likely time point of exposure was the first or the early second trimester, based on individual case reports and three modelling studies [47, 49, 60]. At the population level, epidemic curves of reported Zika virus illness increased in parallel with reported cases of microcephaly, with a lapse of 30 to 34 wk in two states of Brazil (Pernambuco and Bahia) (S2 Text) [57, 60].
Biological plausibility
Twenty-eight items [29, 30, 34, 36–38, 40, 41, 44, 51, 52, 54–58, 61, 63, 64, 67, 69, 72, 75, 77, 79, 81, 85, 91–93] in 25 groups addressed biological plausibility (S4 Table). These studies suggest a teratogenic effect of Zika virus on the developing brain. Detailed investigations about a woman with Zika virus infection whose pregnancy was terminated found that isolated viral particles from the foetal brain, but not other tissues, were capable of replication in cell culture [52]. Zika virus RNA was also found in foetal brain tissue in three other studies [34, 36, 38]. Zika virus from both the African and the Brazilian (Asian) lineages replicates in different types of neural progenitor cells (NPCs) [44, 69, 91]. The phosphatidylserine-sensing receptor tyrosine kinase AXL is a potential entry point into human cells and is expressed in developing human cerebral cortex tissue [29, 64]. In vitro studies using NPCs and cerebral organoids show that Zika virus replicates in neural tissue and can disturb the cell cycle and lead to apoptosis [44, 69, 77, 81, 91].
Strength of association
We reviewed seven items [42, 46, 47, 49, 60, 78, 86] in three groups up to May 30, 2016 (S4 Table). Two studies suggest that the association between Zika virus infection in pregnancy and congenital brain abnormalities is likely to be very strong [42, 47]. In Rio de Janeiro, investigators compared 72 women with positive reverse transcriptase PCR (RT-PCR) results for Zika virus with 16 women with other causes of rash [42]. Follow-up was more intensive in women with Zika virus infection than those without. Of 42 Zika-infected women with one or more ultrasound scans, 12 (29%) had abnormal scans. All 16 women without Zika virus infection were reported to have had one normal routine scan, but no follow-up data were reported. The preliminary description of the data suggests a marked increase in the risk of congenital abnormalities. In French Polynesia, investigators reconstructed a hypothetical cohort of pregnant women from different sources of data, including eight retrospectively identified cases of microcephaly [47]. They estimated that the risk of microcephaly would be 53.4 times (95% confidence interval 6.5–1,061.2) higher in women with Zika virus infection than in uninfected women if exposure had occurred in the first trimester. Methods and assumptions were clearly described, but the estimate was imprecise and was obtained from indirect data sources. One case-control study in Pernambuco, Brazil, was ongoing at the time of the first searches. The Microcephaly Epidemiology Research Group enrolled 32 cases and 62 controls and found a crude odds ratio of 55.0, 95% CI 8.66–∞) between neonatal microcephaly and laboratory-confirmed Zika virus infection in pregnancy [94].
At population level, state-level data in Brazil showed a positive correlation between case reports of Zika-like illness and cases of microcephaly [49]. These data also show a higher prevalence of microcephaly in 15 states that had reported cases (2.8 per 10,000 live births) than in four states with no reported cases (0.6 per 10,000 live births) [46] (prevalence ratio 4.7, 95% CI 1.9–13.3).
Exclusion of alternative explanations
Twenty-eight items [30–39, 41–43, 45, 51, 52, 65, 68, 73–76, 79, 80, 85, 88–90] in 18 groups addressed alternative explanations (S4 Table). No alternative single infectious cause could have resulted in large clusters of cases of microcephaly in different places. Acute dengue virus infection was excluded in 12 studies. Four studies excluded maternal exposure to alcohol or medication, or genetic causes of congenital abnormalities [34, 35, 37, 51]. No study excluded exposure to environmental toxins or heavy metals.
Cessation
Six items [46, 49, 57, 60, 78, 86] in two groups addressed this dimension (S4 Table). Surveillance reports of Zika-like illness in northeastern Brazil in 2015 declined [57, 60] either due to seasonality of the vector or population immunity. Reports of microcephaly declined with a similar pattern in Bahia state [60]. In Pernambuco state, a similar pattern was observed but a dengue epidemic occurred simultaneously, so the decline in microcephaly cases might not be attributable to the Zika virus outbreak alone (S2 Text) [57]. We did not find any data on trends in microcephaly cases in countries other than Brazil.
Dose–response relationship
We did not find any relevant studies.
Experiments in animals
We reviewed 20 items [21–28, 48, 50, 53, 59, 62, 69–71, 82–84, 87] (S4 Table). Studies in the 1950s–1970s show that experimental inoculation of Zika virus resulted in illness, cerebral lesions, and viral replication in the brain in some but not all species tested [21–25, 27, 28]. Some effects might have been enhanced by the numerous serial passaging and subsequent viral adaptation of the original Ugandan Zika strain MR766 and the choice of genetically susceptible animal models. More recent animal studies have shown evidence of neurotropism in immunocompromised mice and in foetal or infant (suckling) immunocompetent mice [48, 71, 82] but not in adult immunocompetent mice [50, 53]. Real-time reports are documenting studies of Macaque monkeys experimentally infected with a Brazilian strain and a French Polynesian strain of Zika virus (both Asian lineage) during pregnancy [70]. High and persisting viraemia was observed in one animal. Foetal autopsy revealed viral RNA in some tissues, but the brain tissue was negative for Zika virus and showed no histopathological lesions or clinical microcephaly.
Analogy
Clinical observations linking clusters of babies born with microcephaly and an earlier outbreak of Zika virus infection in Brazil are analogous to the discovery in 1941 of congenital rubella syndrome [95]. Cytomegalovirus and toxoplasmosis in pregnancy can both cause microcephaly, intracranial calcification, and ocular and auditory defects [96] (cited in [43]). Two cases of microcephaly were reported amongst 72 women infected with the neurotropic flavivirus West Nile virus infection in pregnancy [97]. A review of 30 studies of dengue virus infection in pregnancy found evidence of vertical transmission but did not mention microcephaly or other congenital brain abnormalities as possible complications [98].
Specificity of association
We did not find any studies that described neuroimaging or clinical features found only in association with Zika virus infection.
Consistency
Findings that support Zika virus infection as a cause of congenital brain abnormalities have come from different kinds of epidemiological studies and laboratory studies in both humans and animals (S4 Table). Case reports of pregnancies affected by Zika virus have come from the Americas, the Pacific region (Table 2), and West Africa [13, 66]. The prevalence of microcephaly has not been higher than expected in all countries with Zika virus transmission, however. Congenital brain abnormalities or the presence of Zika virus in products of conception has also been found in pregnant travellers returning from Zika-affected countries [34, 40, 52], showing consistency across populations. There have been no reports of congenital brain abnormalities from countries affected by the African lineage [99]. One in vitro study found that Brazilian (Asian lineage) and African Zika strains both replicated in murine and human cell cultures and organoids [69, 77].
Summary of quality of evidence
The body of evidence includes a wide range of study designs and populations in both humans and animals (S5 Table). Much of the evidence in humans comes from uncontrolled or ecological study designs that have inherent biases for ascertaining causal associations. Of two studies that quantified the strength of association, effect sizes were very large but also imprecise [47, 94]. One of three comparative studies was at low risk of bias [94]. Evidence from animal studies is, by its nature, indirect. We could not formally assess publication bias; our search strategy was wide, but we found very few studies with findings that were not consistent with causality.
Guillain–Barré Syndrome
We found 36 items belonging to 31 groups that addressed questions related to GBS [54–57, 67, 78, 100–122]. We summarise the findings according to clinical characteristics of 118 individuals diagnosed with GBS in Table 4.
Tab. 4. Geographic, clinical, and microbiological characteristics of people with GBS. 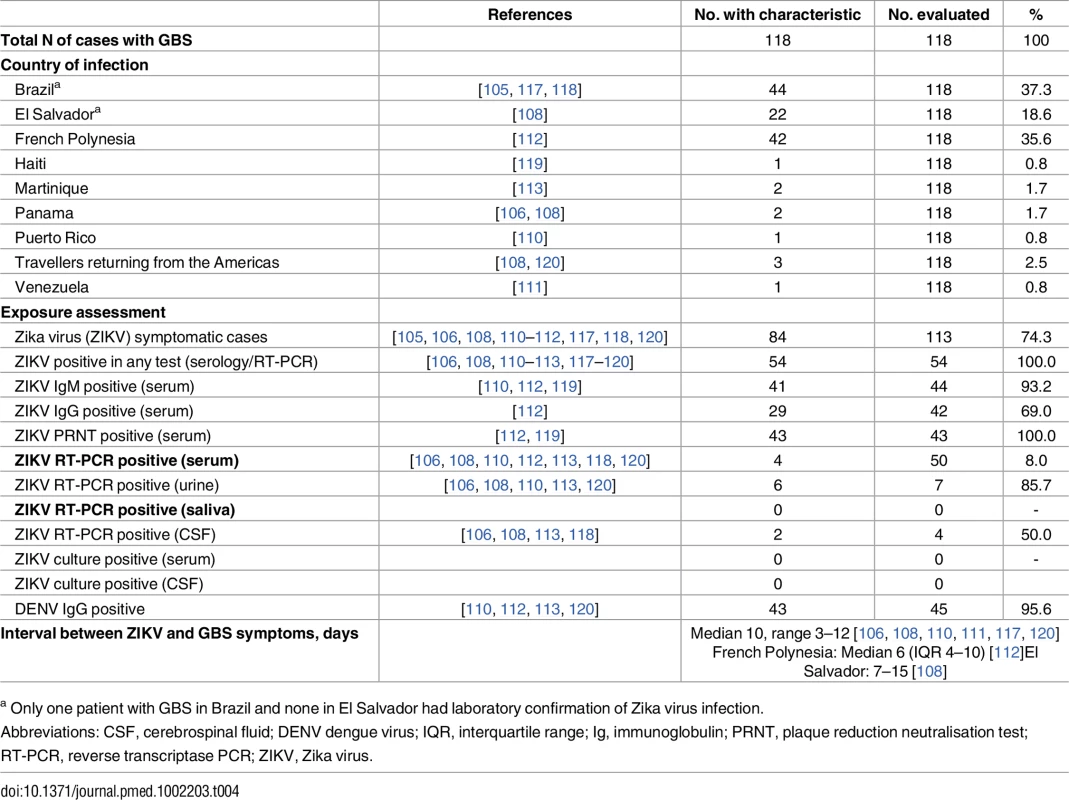
a Only one patient with GBS in Brazil and none in El Salvador had laboratory confirmation of Zika virus infection. Table 5 summarises the reviewers’ assessments by causality dimension, S6 Table provides an extended description of study findings, and S7 Table summarises the quality of the body of evidence.
Tab. 5. Summary of reviewers’ assessments of evidence about Zika virus infection and GBS, by causality dimension. 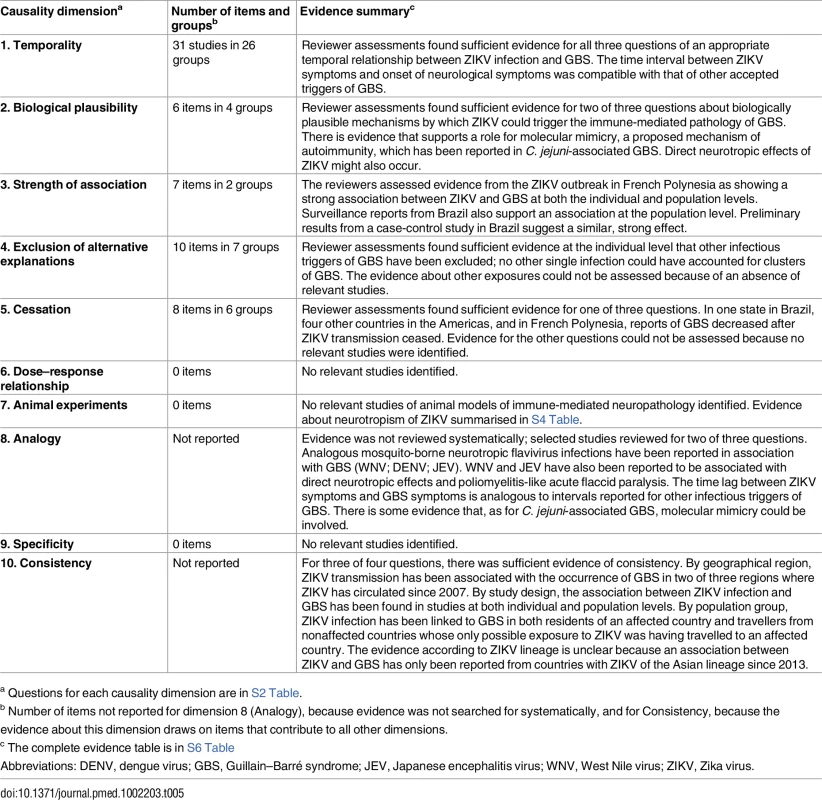
a Questions for each causality dimension are in S2 Table. Temporality
We included 31 items [55–57, 67, 78, 100–112, 115–117, 120–122] in 26 groups (S6 Table). At the individual level, symptoms of Zika virus infection were reported before the onset of GBS symptoms in cases in French Polynesia, Brazil, El Salvador, Panama, Puerto Rico, and Venezuela, and in returning travellers from Haiti, Suriname, and Central America. All patients with GBS had laboratory-confirmed Zika virus infection except for 42 of 44 in Brazil and all those in El Salvador. The intervals between Zika virus infection and neurological symptoms delays of 3 to 12 d [108, 111, 112] are consistent with a postinfectious autoimmune mechanism [5]. In one ecological study in Bahia, Brazil, a lag of 5 to 9 wk between the epidemic peaks of cases with acute exanthematous illness and GBS was attributed to data collection issues [78].
At the population level, 11 countries in Latin America (Brazil, Colombia, El Salvador, French Guiana, Honduras, Venezuela, Suriname) and the Caribbean (Dominican Republic, Jamaica, Martinique) and French Polynesia have reported an increase in GBS cases during outbreaks of Zika virus infection [56, 57, 67, 104, 107, 109]. Surveillance reports show sporadic GBS cases in association with Zika-like illness in four countries but without an increase above background level (Guadeloupe, Haiti, Panama, Puerto Rico). One study reported on surveillance data about acute flaccid paralysis in children in 20 South Pacific islands. The number of expected cases of acute flaccid paralysis was <1 per year in these small countries, and an increase during periods of Zika virus transmission was only observed in the Solomon Islands [115].
Biological plausibility
We reviewed six items [54, 102, 111, 112, 114, 116] in four groups (S6 Table). Anti-ganglioside antibodies, whose presence supports the clinical diagnosis of GBS, were found in the serum of a third of patients in a case-control study in French Polynesia [112] and in one patient from Venezuela [111]. The case-control study and two in silico studies also provide some evidence for molecular mimicry of Zika virus epitopes and host antigens [112]. Studies of predicted epitopes and human antigens suggested peptide sharing between Zika virus and human proteins [54, 114]. Several experimental studies with human neural stem cells and mouse models have shown some evidence for neurotropism of Zika virus (S4 Table, biological plausibility).
Strength of association
We reviewed seven items [101–104, 112, 116, 122] in two groups identified up to May 30, 2016. One published case-control study enrolled 42 cases of GBS during the Zika outbreak in French Polynesia and compared them with 98 patients hospitalised with nonfebrile illness (S6 Table) [112]. Several alternative causes of GBS were excluded. Evidence of Zika virus infection was much more common in GBS cases than controls (odds ratios 59.7, 95% CI 10.4–+∞ defined as IgM or IgG positivity and 34.1, 95% CI 5.8–+∞ defined as presence of neutralising antibodies). Cases and controls were matched, but there was no additional adjustment for confounding. In Brazil, surveillance data showed a 19% increase in reports of GBS cases in 2015 compared with 2014 [101]. Information received after May 30 found a second case-control investigation conducted in Brazil that enrolled controls from the community and is ongoing; preliminary results suggest a similar, strong effect (Sejvar J., personal communication).
Alternative explanations
We included ten items [102, 110, 112, 113, 116–121] in seven groups (S6 Table). In several studies, other infections that can trigger GBS were excluded, such as C. jejuni, Mycoplasma pneumoniae, HIV, Epstein–Barr virus, and herpes simplex virus. No single infectious trigger would have resulted in GBS outbreaks in multiple locations.
Cessation
Eight items [56, 57, 78, 103, 104, 109, 122] in six groups addressed cessation (S6 Table). In surveillance reports from six countries (Brazil, Colombia, El Salvador, French Polynesia, Honduras, and Suriname), the incidence of GBS declined as reports of Zika virus infection fell.
Dose–response relationship, experiments in animals, and specificity
We did not find any relevant studies.
Analogy
Clusters of cases of GBS have been reported in association with outbreaks of C. jejuni gastroenteritis [123]. The incidence of GBS estimated from studies in French Polynesia of 0.24 per 1,000 Zika virus infections [112] is at the lower end of estimates for C. jejuni (0.3 [124] and 1.17 [125] per 1,000). The reported latency between gastrointestinal symptoms and onset of paralysis of approximately 9 d (range 1–23 d) [124, 126, 127] is similar to Zika virus-associated cases. Other mosquito-borne neurotropic flaviviruses have been reported as possible triggers of GBS in case reports and case series: dengue virus [128], West Nile virus [129], Japanese B encephalitis virus [130, 131], or yellow fever 17D vaccination [132]. An acute poliomyelitis-like flaccid paralysis, resulting from direct neural infection, presumably of anterior horn cells, has also been reported as a clinical consequence of these viruses [129, 133, 134]. Putative biological mechanisms include up-regulation of major histocompatibility class I and II molecules of peripheral nerve cells and subsequent immune-mediated cell destruction [135], auto-antibodies directed against heat shock proteins [136], galactocerebrosides [137], or myelin basic protein (MBP), and proliferation of MBP-specific T-cells [138].
Consistency
The link between Zika virus and GBS has been made in studies of different designs at individual and population levels (S6 Table). Clusters of GBS have been seen in multiple countries during epidemics of Zika virus but have not been reported in all those in which Zika virus outbreaks have occurred. Outbreaks of GBS in which gene sequencing has been done were associated with Zika virus of the Asian lineage.
Summary of quality of evidence
The body of evidence includes a wide range of study designs and populations in humans (S7 Table). A majority of the evidence reviewed was from uncontrolled or ecological study designs that have inherent biases for ascertaining causal associations. The only study that examined the strength of association found a very large but imprecise estimate of the effect size but did not have serious risks of bias [112]. There was no evidence of indirectness. We could not formally assess publication bias, but we had a broad search strategy, and we did find evidence that outbreaks of GBS have not been seen in all countries with Zika virus transmission.
Cofactors that might act in the presence of Zika virus
We prespecified seven categories of cofactors (S2 Table). The most widely discussed was past dengue virus infection [112]. A mechanism known as antibody-dependent enhancement might be involved, when IgG antibodies against viral envelope proteins resulting from a prior infection bind to virus particles of a subsequent infection, leading to enhanced replication and potentially more severe illness [139]. Evidence from in vitro experiments suggests cross-reactivity between dengue and Zika virus antibody responses and antibody-dependent enhancement of Zika virus by dengue antibodies [139, 140]. In several of the studies that we reviewed, evidence of past dengue virus infection was reported (S1 Text, p4-5). We did not systematically review evidence for other cofactors but report additional narrative findings in S1 Text (p4-5).
WHO Expert Panel Conclusions
Based on the evidence identified up to July 29, 2016, the expert panel concluded that
the most likely explanation of available evidence from outbreaks of Zika virus infection and clusters of microcephaly is that Zika virus infection during pregnancy is a cause of congenital brain abnormalities including microcephaly and
the most likely explanation of available evidence from outbreaks of Zika virus infection and GBS is that Zika virus infection is a trigger of GBS [141].
The expert panel recognises that Zika virus alone may not be sufficient to cause either congenital brain abnormalities or GBS. The panel does not know whether these effects depend on as yet uncharacterised cofactors being present, nor does it know whether dengue virus plays a part, as this is carried by the same species of mosquito and has circulated in many countries during the same period.
Discussion
Up to May 30, 2016, we found evidence that supported a causal association between Zika virus infection and congenital brain abnormalities, including microcephaly, with at least one study addressing one or more specific questions for eight of ten causality dimensions and between Zika virus infection and GBS, with at least one study about one or more specific questions in seven of ten dimensions. There are methodological weaknesses, inconsistencies, and gaps in the body of evidence for both sets of conditions. Studies found after the cut-off for our first searches did not change our conclusions but strengthened the evidence about biological plausibility, strength of association, and exclusion of alternative explanations.
Interpretation of the Review Findings
The expert panel’s conclusions support causal links between Zika virus and congenital brain abnormalities and GBS and address Bradford Hill’s question, “…is there any other answer equally, or more, likely than cause and effect?” [7]. The conclusions consider both the epidemiological context of unexpected clusters of different types of neurological conditions in countries that have experienced their first outbreaks of Zika virus infection and the strengths and weaknesses of a systematically reviewed body of evidence about ten dimensions of causality (S4 Table, S5 Table, S6 Table and S7 Table). Empirical observations cannot “prove” causality, however [7, 142], and discussions have been intense [9]. A cause can be identified without understanding all the necessary component causes or the complete causal mechanisms involved [142, 143]. In the case of GBS, the infections that precede it are often referred to as “triggers” of the immune-mediated causal pathways involved in pathogenesis.
The body of evidence about Zika virus and congenital abnormalities (72 items included) has grown more quickly than that for GBS (36 items). Research efforts might have concentrated on congenital abnormalities because clusters of affected infants were so unusual, especially in Brazil, where rubella has been eradicated. In contrast, GBS is an established postinfectious neurological disorder, and some commentators have already assumed Zika virus as another cause [12]. Whilst only one case-control study from French Polynesia has been published so far [112], clusters of GBS during outbreaks of Zika virus infection have been reported from several other countries [144], and case-control studies are ongoing in Brazil, Colombia, Mexico, and Argentina.
Comparative studies from French Polynesia suggest that the risk of both microcephaly or of GBS is at least 30 times higher in people who had Zika virus infection compared to those who did not [47, 94, 112], although confidence intervals are wide. The true effect size might be weaker because the earliest studies investigating causality are often overestimates [145]. Even if the methods of forthcoming studies in Brazil [42] and elsewhere reduce confounding and bias, the increase in the risk of disease amongst those with Zika virus infection is likely to remain substantially raised. Inconsistencies in the evidence still need investigation, however. Disease clusters were not seen in Africa [146], but congenital abnormalities and GBS are rare complications that might not be detected in countries with small populations or poor surveillance systems. In the case of microcephaly, terminations of potentially affected pregnancies might have resulted in underascertainment [147].
Current evidence does not show which specific environmental and host factors interact with Zika virus. A cofactor that increases the risk of neurological damage could help to explain why surveillance reports show clusters of microcephaly or GBS in some geographical areas but not others. Dengue virus has been suggested as a possible cofactor or another component cause [143]. One major limitation to interpretation of data about causality and cofactors is the lack of accurate and accessible diagnostic tools, owing to the short duration of viraemia, cross-reactivity with other flaviviruses, and lack of standardisation [148].
Strengths and Limitations
The strengths of our study are that we appraised evidence of causality systematically but rapidly and transparently within a structured framework. We searched both published and unpublished sources. The systematic review process could not eliminate publication bias but reduced the risk that only positive reports in favour of causation would be evaluated. There were limitations to the process, too. Our search strategy did not cover the literature about analogous conditions or cofactors systematically. We did not select studies or extract data in duplicate, but additional reviewers checked the extracted data independently. The included studies used a variety of case definitions for microcephaly and GBS, and we could not standardise these, so misclassification is possible, but this limitation did not change the overall conclusions. Our rapid assessment of quality was not quantitative; we did not find a tool that covered all review questions and study designs appropriately and were not able to standardise the GRADE tool across study designs in the time available [20].
Implications for Policy and Research
The conclusions of the expert panel facilitate the promotion of stronger public health measures and research to tackle Zika virus and its effects. The evidence gaps that we identified provide researchers with research questions, and WHO has published a Zika virus research agenda [149]. Better diagnostic tests will allow more accurate assessment of Zika virus in tissues and of population-level immunity. Research about Zika virus and acute flaccid paralysis is needed to define the clinical and electrophysiological pattern, mechanisms of causality, and to distinguish between the roles of autoimmunity and direct effects on anterior horn cells or neurons. Basic research will also further the development of vaccines, treatments, and vector control methods. For the populations currently at risk, cohort studies are needed to determine both absolute and relative risks of pregnancies affected by asymptomatic and symptomatic Zika virus infection and the role of cofactors and effect modifiers, and to define the full range of physical and developmental abnormalities that comprise the congenital Zika virus syndrome.
From Rapid Systematic Review to Living Systematic Review
Our systematic review deals with multiple neurological disorders and more detailed questions about causality than other reviews. We reached the same conclusion as Rasmussen et al. [11], but the larger number of studies allowed a more comprehensive and balanced summary of evidence and of evidence gaps. In addition, our review addresses the association between Zika virus and GBS. We also plan to examine other acute neurological disorders (S1 Text).
Our review will quickly become outdated because the pace of new publications is outstripping the time taken for the review process. The concept of a “living systematic review” has been proposed as a way to combine rigour with timeliness for intervention research [150] through the development of methods to incorporate new evidence as soon as it is available and make evidence summaries available immediately. We are working on methods to produce a living systematic review of the Zika causality framework that will incorporate new studies, provide frequent open access updates, and allow cumulative meta-analyses of both aggregate and individual patient data from rigorous prospective studies as these become available. The declaration by journal editors to improve access to data during public health emergencies [151, 152] could be combined with the living systematic review approach to improve timeliness and open access to research about causality [153].
In summary, rapid and systematic reviews with frequent updating and open dissemination are now needed, both for appraisal of the evidence about Zika virus infection and for the next public health threats that will emerge. This rapid systematic review found sufficient evidence to conclude that Zika virus is a cause of congenital abnormalities and is a trigger of GBS.
Supporting Information
Zdroje
1. Fauci AS, Morens DM. Zika Virus in the Americas—Yet Another Arbovirus Threat. N Engl J Med. 2016;374(7):601–4. Epub 2016/01/14. doi: 10.1056/NEJMp1600297 26761185
2. Pan American Health Organization, World Health Organization. Epidemiological Alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas—1 December 2015. 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Last accessed 17.08.2016.
3. Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387(10020):719–21. Epub 2016/02/16. doi: 10.1016/S0140-6736(16)00320-2 26876373
4. Woods CG, Parker A. Investigating microcephaly. Arch Dis Child. 2013;98(9):707–13. doi: 10.1136/archdischild-2012-302882 23814088
5. Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388(10045):717–27. doi: 10.1016/S0140-6736(16)00339-1 26948435
6. World Health Organization. Situation report. Zika virus, microcephaly, Guillain-Barré syndrome—20 October 2016. 2016. http://apps.who.int/iris/bitstream/10665/250590/1/zikasitrep20Oct16-eng.pdf. Last accessed 24.10.2016.
7. Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58 : 295–300. PubMed Central PMCID: PMC1898525. 14283879
8. Gordis L. Chapter 14. From Association to Causation: Deriving Inferences from Epidemiologic Studies. Epidemiology. Philadelphia: Saunders Elsevier; 2009. p. 227–46.
9. Doshi P. Convicting Zika. BMJ. 2016;353:i1847. Epub 2016/04/09. doi: 10.1136/bmj.i1847 27056643
10. Frank C, Faber M, Stark K. Causal or not: applying the Bradford Hill aspects of evidence to the association between Zika virus and microcephaly. EMBO Mol Med. 2016;8(4):305–7. Epub 2016/03/16. PubMed Central PMCID: PMC4818755. doi: 10.15252/emmm.201506058 26976611
11. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects—Reviewing the Evidence for Causality. N Engl J Med. 2016;374(20):1981–7. Epub 2016/04/14. doi: 10.1056/NEJMsr1604338 27074377
12. Smith DW, Mackenzie J. Zika virus and Guillain-Barre syndrome: another viral cause to add to the list. Lancet. 2016;387(10027):1486–8. Epub 2016/03/08. doi: 10.1016/S0140-6736(16)00564-X 26948432
13. World Health Organization. Zika situation report. Zika virus, Microcephaly and Guillain-Barré syndrome—31 March 2016. 2016. http://www.who.int/emergencies/zika-virus/situation-report/31-march-2016/en/. Last accessed 17.08.2016.
14. Broutet N, Krauer F, Riesen M, Khalakdina A, Almiron M, Aldighieri S, et al. Zika Virus as a Cause of Neurologic Disorders. N Engl J Med. 2016;374(16):1506–9. Epub 2016/03/10. doi: 10.1056/NEJMp1602708 26959308
15. Solomon T. Flavivirus encephalitis and other neurological syndromes (Japanese encephalitis, WNV, Tick borne encephalits, Dengue, Zika virus). Int J Infect Dis. 2016;45 : 24. doi: 10.1016/j.ijid.2016.02.086 72245053
16. Low N, Krauer F, Riesen M. Causality framework for Zika virus and neurological disorders: systematic review protocol 2016. CRD42016036693. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016036693.
17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed Central PMCID: PMC2707599. doi: 10.1371/journal.pmed.1000097 19621072
18. Khangura S, Konnyu K, Cushman R, Grimshaw J, Moher D. Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1 : 10. PubMed Central PMCID: PMC3351736. doi: 10.1186/2046-4053-1-10 22587960
19. National Institute for Health and Clinical Excellence. Developing NICE guidelines: the manual appendix H. London: National Institute for Health and Clinical Excellence, 2015. https://www.nice.org.uk/process/pmg20/resources/developing-nice-guidelines-the-manual-appendix-h-2549711485. Last accessed 26.10.2016.
20. Thayer KA, Schunemann HJ. Using GRADE to respond to health questions with different levels of urgency. Environ Int. 2016;92–93 : 585–9. doi: 10.1016/j.envint.2016.03.027 27126781
21. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509–20. Epub 1952/09/01. 12995440
22. Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46(5):521–34. Epub 1952/09/01. 12995441
23. Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50(5):442–8. Epub 1956/09/01. 13380987
24. Reagan RL, Stewart MT, Delaha EC, Brueckner AL. Response of the Syrian hamster to eleven tropical viruses by various routes of exposure. Tex Rep Biol Med. 1954;12(3):524–7. 13216647
25. Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg. 1958;52(3):263–8. Epub 1958/05/01. 13556872
26. Andral L, Bres P, Serie C. Yellow fever in Ethiopia. III. Serological and virological study of the forest fauna. [French]. Bull World Health Org. 1968;38(6):855–61. 0008222620.
27. Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35(2):183–93. Epub 1971/01/01. 5002906
28. Way JH, Bowen ET, Platt GS. Comparative studies of some African arboviruses in cell culture and in mice. J Gen Virol. 1976;30(1):123–30. Epub 1976/01/01. doi: 10.1099/0022-1317-30-1-123 1245842
29. Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of Zika Virus Infection in Human Skin Cells. J Virol. 2015;89(17):8880–96. Epub 2015/06/19. PubMed Central PMCID: PMC4524089. doi: 10.1128/JVI.00354-15 26085147
30. Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47(1):6–7. Epub 2016/01/06. doi: 10.1002/uog.15831 26731034
31. Ventura CV, Maia M, Bravo-Filho V, Gois AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387(10015):228. Epub 2016/01/18. doi: 10.1016/S0140-6736(16)00006-4 26775125.
32. Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. Epub 2016/01/29. doi: 10.15585/mmwr.mm6503e2 26820244
33. Ventura CV, Maia M, Ventura BV, Linden VV, Araujo EB, Ramos RC, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016;79(1):1–3. Epub 2016/02/04. doi: 10.5935/0004-2749.20160002 26840156
34. Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374(10):951–8. Epub 2016/02/11. doi: 10.1056/NEJMoa1600651 26862926
35. de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, et al. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016. Epub 2016/02/13. doi: 10.1001/jamaophthalmol.2016.0267 26865554.
36. Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, et al. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(6):159–60. Epub 2016/02/20. doi: 10.15585/mmwr.mm6506e1 26890059
37. Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16(6):653–60. Epub 2016/02/22. doi: 10.1016/S1473-3099(16)00095-5 26897108
38. Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, et al. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis. 2016;10(2):e0004517. Epub 2016/02/26. PubMed Central PMCID: PMC4767410. doi: 10.1371/journal.pntd.0004517 26914330
39. Werner H, Fazecas T, Guedes B, Lopes Dos Santos J, Daltro P, Tonni G, et al. Intrauterine Zika virus infection and microcephaly: correlation of perinatal imaging and three-dimensional virtual physical models. Ultrasound Obstet Gynecol. 2016;47(5):657–60. Epub 2016/03/01. doi: 10.1002/uog.15901 26923098
40. Meaney-Delman D, Hills SL, Williams C, Galang RR, Iyengar P, Hennenfent AK, et al. Zika Virus Infection Among U.S. Pregnant Travelers—August 2015-February 2016. MMWR Morb Mortal Wkly Rep. 2016;65(8):211–4. Epub 2016/03/05. doi: 10.15585/mmwr.mm6508e1 26938703
41. Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet. 2016;387(10023):1051–2. Epub 2016/03/06. doi: 10.1016/S0140-6736(16)00625-5 26944027.
42. Brasil P, Pereira JP Jr., Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro—Preliminary Report. N Engl J Med. 2016. Epub 2016/03/05.
43. Miranda-Filho Dde B, Martelli CM, Ximenes RA, Araujo TV, Rocha MA, Ramos RC, et al. Initial Description of the Presumed Congenital Zika Syndrome. Am J Public Health. 2016;106(4):598–600. Epub 2016/03/10. doi: 10.2105/AJPH.2016.303115 26959258
44. Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18(5):587–90. Epub 2016/03/10.
45. Villamil-Gomez WE, Mendoza-Guete A, Villalobos E, Gonzalez-Arismendy E, Uribe-Garcia AM, Castellanos JE, et al. Diagnosis, management and follow-up of pregnant women with Zika virus infection: A preliminary report of the ZIKERNCOL cohort study on Sincelejo, Colombia. Travel Med Infect Dis. 2016;14(2):155–8. Epub 2016/03/11. doi: 10.1016/j.tmaid.2016.02.004 26960750
46. Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, et al. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(9):242–7. Epub 2016/03/11. doi: 10.15585/mmwr.mm6509e2 26963593
47. Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–32. Epub 2016/03/20. PubMed Central PMCID: PMC4909533. doi: 10.1016/S0140-6736(16)00651-6 26993883
48. Deng YQ, Zhao H, Li XF, Zhang NN, Liu ZY, Jiang T, et al. Isolation, identification and genomic characterization of the Asian lineage Zika virus imported to China. Sci China Life Sci. 2016;59(4):428–30. Epub 2016/03/20. doi: 10.1007/s11427-016-5043-4 26993654
49. Faria NR, Azevedo Rdo S, Kraemer MU, Souza R, Cunha MS, Hill SC, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352(6283):345–9. Epub 2016/03/26. PubMed Central PMCID: PMC4918795. doi: 10.1126/science.aaf5036 27013429
50. Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, et al. Characterization of a Novel Murine Model to Study Zika Virus. Am J Trop Med Hyg. 2016;94(6):1362–9. Epub 2016/03/30. PubMed Central PMCID: PMC4889758. doi: 10.4269/ajtmh.16-0111 27022155
51. Besnard M, Eyrolle-Guignot D, Guillemette-Artur P, Lastere S, Bost-Bezeaud F, Marcelis L, et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill. 2016;21(13). Epub 2016/03/31.
52. Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 2016;374(22):2142–51. Epub 2016/03/31. doi: 10.1056/NEJMoa1601824 27028667
53. Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl Trop Dis. 2016;10(5):e0004658. PubMed Central PMCID: PMC4858159. doi: 10.1371/journal.pntd.0004658 27149521
54. Homan J, Malone RW, Darnell SJ, Bremel RD. Antibody mediated epitope mimicry in the pathogenesis of Zika virus related disease. 2016. http://biorxiv.org/content/biorxiv/early/2016/03/19/044834.full.pdf. Cited 9 November 2016.
55. Pan American Health Organization. Epidemiological Alert. Zika virus infection 24 March 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=33937&lang=en=com_docman&task=doc_view&Itemid=270&gid=33937&lang=en. Last accessed 17.08.2016.
56. Pan American Health Organization. Epidemiological Alert. Zika virus infection 08 April 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=34144&lang=en. Last accessed 17.08.2016.
57. Pan American Health Organization. Epidemiological Update. Zika virus infection—28 April 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=34327&lang=en. Last accessed 17.08.2016.
58. Pylro V, Oliveira F, Morais D, Orellana S, Pais F, Medeiros J, et al. Exploring miRNAs as the key to understand symptoms induced by ZIKA virus infection through a collaborative database. 2016. http://biorxiv.org/content/biorxiv/early/2016/03/06/042382.full.pdf. Cited 9 November 2016.
59. Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, et al. Natural history of Asian lineage Zika virus infection in macaques. bioRxiv. 2016.
60. Reefhuis J, Gilboa SM, Johansson MA, Valencia D, Simeone RM, Hills SL, et al. Projecting Month of Birth for At-Risk Infants after Zika Virus Disease Outbreaks. Emerg Infect Dis. 2016;22(5):828–32. PubMed Central PMCID: PMC4861542. doi: 10.3201/eid2205.160290 27088494
61. European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome. First update 21 January 2016. 2016. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Last accessed 17.08.2016.
62. Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJF, Neyts J. The viral polymerase inhibitor 7-deaza-2'-C-methyladenosine is a potent inhibitor of in 1 vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl Trop Dis. 2016;10(5) e0004695. doi: 10.1371/journal.pntd.0004695 27163257.
63. Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352(6287):816–8. Epub 2016/04/12. doi: 10.1126/science.aaf6116 27064148
64. Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18(5):591–6. Epub 2016/04/04. PubMed Central PMCID: PMC4860115. doi: 10.1016/j.stem.2016.03.012 27038591
65. Hazin AN, Poretti A, Turchi Martelli CM, Huisman TA, Microcephaly Epidemic Research G, Di Cavalcanti Souza Cruz D, et al. Computed Tomographic Findings in Microcephaly Associated with Zika Virus. N Engl J Med. 2016;374(22):2193–5. Epub 2016/04/07.
66. World Health Organization. Zika situation report. Zika virus, Microcephaly and Guillain-Barré syndrome—07 April 2016. 2016. http://www.who.int/emergencies/zika-virus/situation-report/7-april-2016/en/. Last accessed 17.08.2016.
67. Pan American Health Organization. Epidemiological Alert. Zika virus infection 31 March 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=34041&lang=en. Last accessed 17.08.2016.
68. Microcephaly Epidemic Research Group. Microcephaly in Infants, Pernambuco State, Brazil, 2015. Emerg Infect Dis. 2016;22(6):1090–93. PubMed Central PMCID: PMC4880105. doi: 10.3201/eid2206.160062 27071041
69. Campanati L, Higa LM, Delvecchio R, Pezzuto P, Valadão AL, Monteiro FL, et al. The Impact of African and Brazilian ZIKV isolates on neuroprogenitors. 2016. http://biorxiv.org/content/biorxiv/early/2016/03/31/046599.full.pdf. Cited 9 November 2016.
70. Zika Experimental Science Team. ZIKV-003 and ZIKV-005: Infection with French Polynesian and Asian lineage Zika virus during the first pregnancy trimester. 2016. https://zika.labkey.com/project/OConnor/ZIKV-003/begin.view? Last accessed 10.05.2016.
71. Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19(5):720–30. Epub 2016/04/14. PubMed Central PMCID: PMC4866885. doi: 10.1016/j.chom.2016.03.010 27066744
72. Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr., et al. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe. 2016;19(5):705–12. Epub 2016/04/14. PubMed Central PMCID: PMC4866896. doi: 10.1016/j.chom.2016.03.008 27066743
73. de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, Coeli RR, Rocha MA, Sobral da Silva P, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901. Epub 2016/04/15. PubMed Central PMCID: PMC4830901. doi: 10.1136/bmj.i1901 27075009
74. Cavalheiro S, Lopez A, Serra S, Da Cunha A, da Costa MD, Moron A, et al. Microcephaly and Zika virus: neonatal neuroradiological aspects. Childs Nerv Syst. 2016;32(6):1057–60. Epub 2016/04/16. PubMed Central PMCID: PMC4882355. doi: 10.1007/s00381-016-3074-6 27080092
75. Guillemette-Artur P, Besnard M, Eyrolle-Guignot D, Jouannic JM, Garel C. Prenatal brain MRI of fetuses with Zika virus infection. Pediatr Radiol. 2016;46(7):1032–9. Epub 2016/04/20. doi: 10.1007/s00247-016-3619-6 27090801
76. Cordeiro MT, Pena LJ, Brito CA, Gil LH, Marques ET. Positive IgM for Zika virus in the cerebrospinal fluid of 30 neonates with microcephaly in Brazil. Lancet. 2016;387(10030):1811–2. Epub 2016/04/23. doi: 10.1016/S0140-6736(16)30253-7 27103126.
77. Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165(5):1238–54. Epub 2016/04/28. PubMed Central PMCID: PMC4900885. doi: 10.1016/j.cell.2016.04.032 27118425
78. Paploski IA, Prates AP, Cardoso CW, Kikuti M, Silva MM, Waller LA, et al. Time Lags between Exanthematous Illness Attributed to Zika Virus, Guillain-Barre Syndrome, and Microcephaly, Salvador, Brazil. Emerg Infect Dis. 2016;22(8):1438–44. Epub 2016/05/05. doi: 10.3201/eid2208.160496 27144515
79. Noronha L, Zanluca C, Azevedo ML, Luz KG, Santos CN. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz. 2016;111(5):287–93. Epub 2016/05/05. PubMed Central PMCID: PMC4878297. doi: 10.1590/0074-02760160085 27143490
80. Moron AF, Cavalheiro S, Milani H, Sarmento S, Tanuri C, de Souza FF, et al. Microcephaly associated with maternal Zika virus infection. BJOG. 2016;123(8):1265–9. Epub 2016/05/07. doi: 10.1111/1471-0528.14072 27150580
81. Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, et al. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016;19(2):258–65. Epub 2016/05/11. doi: 10.1016/j.stem.2016.04.014 27162029
82. Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, et al. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell Res. 2016;26(6):645–54. Epub 2016/05/14. PubMed Central PMCID: PMC4897185. doi: 10.1038/cr.2016.58 27174054
83. Li C, Xu D, Ye Q, Hong S, Jiang Y, Liu X, et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 2016;19(1):120–6. Epub 2016/05/18. doi: 10.1016/j.stem.2016.04.017 27179424
84. Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165(5):1081–91. Epub 2016/05/18. PubMed Central PMCID: PMC4874881. doi: 10.1016/j.cell.2016.05.008 27180225
85. Culjat M, Darling SE, Nerurkar VR, Ching N, Kumar M, Min SK, et al. Clinical and Imaging Findings in an Infant With Zika Embryopathy. Clin Infect Dis. 2016. Epub 2016/05/20. doi: 10.1093/cid/ciw324 27193747.
86. Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med. 2016;375(1):1–4. Epub 2016/05/26. PubMed Central PMCID: PMC4945401. doi: 10.1056/NEJMp1605367 27222919
87. Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10(4):e0004682. Epub 2016/05/24. PubMed Central PMCID: PMC4836712. doi: 10.1371/journal.pntd.0004682 27093158
88. Ventura CV, Maia M, Travassos SB, Martins TT, Patriota F, Nunes ME, et al. Risk Factors Associated With the Ophthalmoscopic Findings Identified in Infants With Presumed Zika Virus Congenital Infection. JAMA Ophthalmol. 2016;134(8):912–8. Epub 2016/05/27. doi: 10.1001/jamaophthalmol.2016.1784 27228275
89. Miranda HA 2nd, Costa MC, Frazao MA, Simao N, Franchischini S, Moshfeghi DM. Expanded Spectrum of Congenital Ocular Findings in Microcephaly with Presumed Zika Infection. Ophthalmology. 2016;123(8):1788–94. Epub 2016/05/30. doi: 10.1016/j.ophtha.2016.05.001 27236271
90. Hanaoka MM, Kanas AF, Braconi CT, Mendes EA, Santos RA, Ferreira LCdS, et al. A Zika virus-associated microcephaly case with background exposure to STORCH agents. 2016. http://biorxiv.org/content/early/2016/05/10/052340. Cited 9 November 2016.
91. Garcez PP, Nascimento JM, Mota de Vasconcelos J, Madeiro da Costa R, Delvecchio R, Trindade P, et al. Combined proteome and transcriptome analyses reveal that Zika virus circulating in Brazil alters cell cycle and neurogenic programmes in human neurospheres. 2016. https://peerj.com/preprints/2033/. Cited 9 November 2016.
92. Ganguly B, Ganguly E. Disruption of human astn2 function by ZIKV ns4b gene as a molecular basis for Zika viral microcephaly. 2016. http://biorxiv.org/content/early/2016/05/20/054486. Cited 9 November 2016.
93. Frumence E, Roche M, Krejbich-Trotot P, El-Kalamouni C, Nativel B, Rondeau P, et al. The South Pacific epidemic strain of Zika virus replicates efficiently in human epithelial A549 cells leading to IFN-beta production and apoptosis induction. Virology. 2016;493 : 217–26. doi: 10.1016/j.virol.2016.03.006 27060565
94. de Araujo TV, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo AP, et al. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016.
95. Gregg NM. Congenital Cataract Following German Measles in the Mother, 1941. Epidemiol Infect. 1941;107(1):35–46.
96. Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22(1):99–126, Table of Contents. PubMed Central PMCID: PMC2620634. doi: 10.1128/CMR.00023-08 19136436
97. O'Leary DR, Kuhn S, Kniss KL, Hinckley AF, Rasmussen SA, Pape WJ, et al. Birth outcomes following West Nile Virus infection of pregnant women in the United States: 2003–2004. Pediatrics. 2006;117(3):e537–45. doi: 10.1542/peds.2005-2024 16510632
98. Pouliot SH, Xiong X, Harville E, Paz-Soldan V, Tomashek KM, Breart G, et al. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv. 2010;65(2):107–18.
99. Musso DG, D. J. Zika Virus. Clin Microbiol Rev. 2016;29(3):487–524. Epub 2016/04/01. doi: 10.1128/CMR.00072-15 27029595
100. Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536–43. Epub 2009/06/12. doi: 10.1056/NEJMoa0805715 19516034
101. World Health Organization WHO. Zika situation report. Neurological syndrome and congenital anomalies—5 February 2016. 2016. Available from: http://apps.who.int/iris/bitstream/10665/204348/1/zikasitrep_5Feb2016_eng.pdf?ua=1. Last accessed 26.10.2016.
102. Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika virus infection complicated by Guillain-Barre syndrome—case report, French Polynesia, December 2013. Euro Surveill. 2014;19(9). Epub 2014/03/15.
103. Institut de veille sanitaire. Virus Zika Polynésie 2013–2014, Ile de Yap, Micronésie 2007—Janvier 2014. 2014. http://www.invs.sante.fr/Publications-et-outils/Points-epidemiologiques/Tous-les-numeros/International/Virus-Zika-en-Polynesie-2013-2014-et-ile-de-Yap-Micronesie-2007-Janvier-2014. Last accessed 17.8.16.
104. Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44(7):302–7. Epub 2014/07/09. doi: 10.1016/j.medmal.2014.04.008 25001879
105. Pan American Health Organization. Epidemiological Update. Neurological syndrome, congenital anomalies, and Zika virus infection—17 January 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32879&lang=en. Last accessed 17.08.2016.
106. Pan American Health Organization. Epidemiological Alert. Zika virus infection -3 March 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=33486&lang=en. Last accessed 17.08.2016.
107. Pan American Health Organization. Epidemiological Alert. Zika virus infection -10 March 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=33659&lang=en. Last accessed 17.08.2016.
108. Pan American Health Organization. Epidemiological Alert. Zika virus infection -17 March 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=33768&lang=en. Last accessed 16.08.2016.
109. Pan American Health Organization. Epidemiological Update. Zika virus infection—21 April 2016. 2016. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=34243&lang=en. Last accessed 17.08.2016.
110. Thomas DL, Sharp TM, Torres J, Armstrong PA, Munoz-Jordan J, Ryff KR, et al. Local Transmission of Zika Virus—Puerto Rico, November 23, 2015-January 28, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(6):154–8. Epub 2016/02/20. doi: 10.15585/mmwr.mm6506e2 26890470
111. Reyna-Villasmil E, Lopez-Sanchez G, Santos-Bolivar J. [Guillain-Barre syndrome due to Zika virus during pregnancy]. Med Clin (Barc). 2016;146(7):331–2. Epub 2016/03/08.
112. Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387(10027):1531–9. Epub 2016/03/08. doi: 10.1016/S0140-6736(16)00562-6 26948433
113. Roze B, Najioullah F, Ferge JL, Apetse K, Brouste Y, Cesaire R, et al. Zika virus detection in urine from patients with Guillain-Barre syndrome on Martinique, January 2016. Euro Surveill. 2016;21(9). Epub 2016/03/12.
114. Lucchese G, Kanduc D. Zika virus and autoimmunity: From microcephaly to Guillain-Barre syndrome, and beyond. Autoimmun Rev. 2016;15(8):801–8. Epub 2016/03/29. doi: 10.1016/j.autrev.2016.03.020 27019049
115. Craig AT, Butler MT, Pastore R, Paterson BJ, Durrheim DN. Update on Zika virus transmission in the Pacific islands, 2007 to February 2016 and failure of acute flaccid paralysis surveillance to signal Zika emergence in this setting. Bull World Health Organ. 2016. doi: 10.2471/blt.16.171892
116. Watrin L, Ghawche F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barre Syndrome (42 Cases) Occurring During a Zika Virus Outbreak in French Polynesia. Medicine (Baltimore). 2016;95(14):e3257. Epub 2016/04/09.
117. Fontes CA, Dos Santos AA, Marchiori E. Magnetic resonance imaging findings in Guillain-Barre syndrome caused by Zika virus infection. Neuroradiology. 2016;58(8):837–8. Epub 2016/04/14. doi: 10.1007/s00234-016-1687-9 27067205
118. Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, et al. Guillain-Barre syndrome associated with Zika virus infection. Lancet. 2016;387(10026):1482. Epub 2016/04/27. doi: 10.1016/S0140-6736(16)30058-7 27115821
119. Kassavetis P, Joseph JM, Francois R, Perloff MD, Berkowitz AL. Zika virus-associated Guillain-Barre syndrome variant in Haiti. Neurology. 2016;87(3):336–7. Epub 2016/05/11. doi: 10.1212/WNL.0000000000002759 27164708
120. Duijster JW, Goorhuis A, van Genderen PJ, Visser LG, Koopmans MP, Reimerink JH, et al. Zika virus infection in 18 travellers returning from Surinam and the Dominican Republic, The Netherlands, November 2015-March 2016. Infection. 2016. Epub 2016/05/23.
121. van den Berg B, van den Beukel JC, Alsma J, van der Eijk AA, Ruts L, van Doorn PA, et al. [Guillain-Barre syndrome following infection with the Zika virus]. Ned Tijdschr Geneeskd. 2016;160(0):D155. Epub 2016/05/28. 27229696
122. Yung CFT, K. C. Guillain-Barre Syndrome and Zika Virus: Estimating Attributable Risk to Inform Intensive Care Capacity Preparedness. Clin Infect Dis. 2016. Epub 2016/05/27. doi: 10.1093/cid/ciw355 27225243.
123. Jackson BR, Zegarra JA, Lopez-Gatell H, Sejvar J, Arzate F, Waterman S, et al. Binational outbreak of Guillain-Barre syndrome associated with Campylobacter jejuni infection, Mexico and USA, 2011. Epidemiol Infect. 2014;142(5):1089–99. doi: 10.1017/S0950268813001908 23924442
124. McCarthy N, Giesecke J. Incidence of Guillain-Barre syndrome following infection with Campylobacter jejuni. Am J Epidemiol. 2001;153(6):610–4. 11257070
125. Tam CC, Rodrigues LC, Petersen I, Islam A, Hayward A, O'Brien SJ. Incidence of Guillain-Barre syndrome among patients with Campylobacter infection: a general practice research database study. J Infect Dis. 2006;194(1):95–7. doi: 10.1086/504294 16741887
126. Mishu B, Blaser MJ. Role of infection due to Campylobacter jejuni in the initiation of Guillain-Barre syndrome. Clin Infect Dis. 1993;17(1):104–8. 8353228
127. Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barre syndrome. N Engl J Med. 1995;333(21):1374–9. doi: 10.1056/NEJM199511233332102 7477117
128. Carod-Artal FJ, Wichmann O, Farrar J, Gascon J. Neurological complications of dengue virus infection. Lancet Neurol. 2013;12(9):906–19. Epub 2013/08/21. doi: 10.1016/S1474-4422(13)70150-9 23948177
129. Sejvar JJ, Bode AV, Marfin AA, Campbell GL, Ewing D, Mazowiecki M, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11(7):1021–7. PubMed Central PMCID: PMC3371783. doi: 10.3201/eid1107.040991 16022775
130. Xiang JY, Zhang YH, Tan ZR, Huang J, Zhao YW. Guillain-Barre syndrome associated with Japanese encephalitis virus infection in China. Viral Immunol. 2014;27(8):418–20. doi: 10.1089/vim.2014.0049 25140441
131. Ravi V, Taly AB, Shankar SK, Shenoy PK, Desai A, Nagaraja D, et al. Association of Japanese encephalitis virus infection with Guillain-Barre syndrome in endemic areas of south India. Acta Neurol Scand. 1994;90(1):67–72. 7941960
132. McMahon AW, Eidex RB, Marfin AA, Russell M, Sejvar JJ, Markoff L, et al. Neurologic disease associated with 17D-204 yellow fever vaccination: a report of 15 cases. Vaccine. 2007;25(10):1727–34. doi: 10.1016/j.vaccine.2006.11.027 17240001
133. Solomon T, Kneen R, Dung NM, Khanh VC, Thuy TT, Ha DQ, et al. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351(9109):1094–7. doi: 10.1016/S0140-6736(97)07509-0 9660579
134. Chung CC, Lee SS, Chen YS, Tsai HC, Wann SR, Kao CH, et al. Acute flaccid paralysis as an unusual presenting symptom of Japanese encephalitis: a case report and review of the literature. Infection. 2007;35(1):30–2. doi: 10.1007/s15010-007-6038-7 17297587
135. Argall KG, Armati PJ, King NJ, Douglas MW. The effects of West Nile virus on major histocompatibility complex class I and II molecule expression by Lewis rat Schwann cells in vitro. J Neuroimmunol. 1991;35(1–3):273–84. 1955569
136. Loshaj-Shala A, Regazzoni L, Daci A, Orioli M, Brezovska K, Panovska AP, et al. Guillain Barre syndrome (GBS): new insights in the molecular mimicry between C. jejuni and human peripheral nerve (HPN) proteins. J Neuroimmunol. 2015;289 : 168–76. doi: 10.1016/j.jneuroim.2015.11.005 26616887
137. Meyer Sauteur PM, Huizinga R, Tio-Gillen AP, Roodbol J, Hoogenboezem T, Jacobs E, et al. Mycoplasma pneumoniae triggering the Guillain-Barre syndrome: A case-control study. Ann Neurol. 2016;80 : 566–580. doi: 10.1002/ana.24755 27490360.
138. Tseng YF, Wang CC, Liao SK, Chuang CK, Chen WJ. Autoimmunity-related demyelination in infection by Japanese encephalitis virus. J Biomed Sci. 2011;18 : 20. PubMed Central PMCID: PMC3056755. doi: 10.1186/1423-0127-18-20 21356046
139. Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17(9):1102–8. Epub 2016/06/25. doi: 10.1038/ni.3515 27339099
140. Paul LM, Carlin ER, Jenkins MM, Tan AL, Barcellona CM, Nicholson CO, et al. Dengue Virus Antibodies Enhance Zika Virus Infection. 2016. http://biorxiv.org/content/early/2016/04/25/050112. Cited 9 November 2016.
141. World Health Organization. Zika causality statement. Zika virus infection: update on the evidence for a causal link to congenital brain abnormalities and Guillain-Barré syndrome. Update of WHO Statement published on 31 March 2016. Last updated 7 September 2016. Last accessed 24.10.2016. http://www.who.int/emergencies/zika-virus/causality/en/.
142. Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95 Suppl 1:S144–50.
143. Rothman KJ. Causes. Am J Epidemiol. 1976;104(6):587–92. 998606
144. Dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, et al. Zika Virus and the Guillain-Barre Syndrome—Case Series from Seven Countries. N Engl J Med. 2016. Epub 2016/09/01.
145. Vandenbroucke JP. Case reports in an evidence-based world. J R Soc Med. 1999;92(4):159–63. PubMed Central PMCID: 1297135. 10450190
146. Solomon T, Baylis M, Brown D. Zika virus and neurological disease—approaches to the unknown. Lancet Infect Dis. 2016;16(4):402–4. Epub 2016/03/01. doi: 10.1016/S1473-3099(16)00125-0 26923117
147. Aiken AR, Scott JG, Gomperts R, Trussell J, Worrell M, Aiken CE. Requests for Abortion in Latin America Related to Concern about Zika Virus Exposure. N Engl J Med. 2016;375(4):396–8. doi: 10.1056/NEJMc1605389 27331661
148. Haug CJ, Kieny MP, Murgue B. The Zika Challenge. N Engl J Med. 2016;374(19):1801–3. Epub 2016/03/31. doi: 10.1056/NEJMp1603734 27028782
149. World Health Organization. Zika virus research agenda. October 2016. http://www.who.int/reproductivehealth/zika/zika-virus-research-agenda/en/. Last accessed 24.11.2016.
150. Elliott JH, Turner T, Clavisi O, Thomas J, Higgins JPT, Mavergames C, et al. Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap. PLoS Med. 2014;11(2):1–6.
151. Dye C, Bartolomeos K, Moorthy V, Kieny MP. Data sharing in public health emergencies: a call to researchers. Bull World Health Organ. 2016;94(3):158. PubMed Central PMCID: PMC4773943. doi: 10.2471/BLT.16.170860 26966322
152. Modjarrad K, Moorthy VS, Millett P, Gsell PS, Roth C, Kieny MP. Developing Global Norms for Sharing Data and Results during Public Health Emergencies. PLoS Med. 2016;13(1):e1001935. PubMed Central PMCID: PMC4701443. doi: 10.1371/journal.pmed.1001935 26731342
153. Tracz V, Lawrence R. Towards an open science publishing platform. F1000Res. 2016;5 : 130. PubMed Central PMCID: PMC4768651. doi: 10.12688/f1000research.7968.1 26962436
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Evaluating Hospital-Based Surveillance for Outbreak Detection in Bangladesh: Analysis of Healthcare Utilization Data
- Effect of a Primary Care Walking Intervention with and without Nurse Support on Physical Activity Levels in 45- to 75-Year-Olds: The edometer nd onsultation valuation (PACE-UP) Cluster Randomised Clinical Trial
- Patient Safety Incidents Involving Sick Children in Primary Care in England and Wales: A Mixed Methods Analysis
- Biomarker-Defined Subsets of Common Diseases: Policy and Economic Implications of Orphan Drug Act Coverage
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Priority-Setting for Novel Drug Regimens to Treat Tuberculosis: An Epidemiologic Model
- Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review
- Association of Body Mass Index with DNA Methylation and Gene Expression in Blood Cells and Relations to Cardiometabolic Disease: A Mendelian Randomization Approach
- Socioeconomic Inequalities in Body Mass Index across Adulthood: Coordinated Analyses of Individual Participant Data from Three British Birth Cohort Studies Initiated in 1946, 1958 and 1970
- Mosquito-Disseminated Insecticide for Citywide Vector Control and Its Potential to Block Arbovirus Epidemics: Entomological Observations and Modeling Results from Amazonian Brazil
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland
- Master Regulators of Oncogenic Response in Pancreatic Cancer: An Integrative Network Biology Analysis
- Sick Children Crying for Help: Fostering Adverse Event Reports
- What Is the Purpose of the Orphan Drug Act?
- Novel Vector Control Approaches: The Future for Prevention of Zika Virus Transmission?
- Artificially Sweetened Beverages and the Response to the Global Obesity Crisis
- Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp)
- Bolstering Community Cooperation in Ebola Resurgence Protocols: Combining Field Blood Draw and Point-of-Care Diagnosis
- Defining Abnormal Fetal Growth and Perinatal Risk: Population or Customized Standards?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
- What Is the Purpose of the Orphan Drug Act?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



