-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBiomarker-Defined Subsets of Common Diseases: Policy and Economic Implications of Orphan Drug Act Coverage
Aaron Kesselheim and colleagues examine orphan-designated drugs approved between 2009 and 2015 in the United States.
Published in the journal: . PLoS Med 14(1): e32767. doi:10.1371/journal.pmed.1002190
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1002190Summary
Aaron Kesselheim and colleagues examine orphan-designated drugs approved between 2009 and 2015 in the United States.
Summary Points
The Orphan Drug Act of 1983 was intended to incentivize the development of pharmaceutical products for rare diseases by providing manufacturers with the opportunity to earn grants, tax credits, fee waivers, and seven years of post-approval market exclusivity for the approved indication.
Over the past decade, the number of orphan drug designations has roughly doubled, with a simultaneous increase in those that target biomarker-defined subsets of common diseases.
Among all orphan-designated drugs approved in 2009–2015 indicated for biomarker-defined disease subsets, we examined the circumstances surrounding the drug’s discovery and development, secondary approvals, off-label uses, subsequent revenues, and the reported monthly cost.
Orphan-designated drugs to treat biomarker-defined subsets of common conditions have a number of characteristics that make them ill-suited to the orphan drug designation, including short development times and rapid expansion of off-label indications after approval. Application of the Orphan Drug Act in these cases risks wasting resources that might be better focused on truly rare conditions.
Congress passed the Orphan Drug Act in 1983 to incentivize the development of pharmaceutical products for rare diseases that might not otherwise be financially viable because of small potential patient populations [1]. Companies can apply for an orphan drug designation from the Food and Drug Administration (FDA) based on the rarity of the targeted disease—defined by a prevalence of fewer than 200,000 patients annually in the United States—and providing a medically plausible basis for believing that their drug or biologic product would aid in its treatment, prevention, or diagnosis. The product is then subject to safety and efficacy testing and formal FDA review and approval.
Orphan drug designation provides manufacturers with the opportunity to earn special research grants from a pool of over US $20 million per year, and subsequent FDA approval of the product carries additional incentives: companies receive tax credits for incurred clinical trial costs (50% tax credit for expenses incurred during clinical testing, maximum of US $30 million), waiver of the FDA approval user fee (currently approximately US $2.4 million), and seven years of post-approval market exclusivity for the approved indication [2]. This legislation has been largely considered a success, with proponents arguing that it has contributed to the commercialization of many drugs in the past 30 years [3]. The number of orphan drug designations has increased from an average of 63 per year in the first two decades of the legislation (1984–2003) to over 200 per year in the past decade (Fig 1). In 2015 alone, 353 products received Orphan Drug Act designations at various stages in their pre-FDA-approval testing process.
Fig. 1. Orphan drug designations per year. 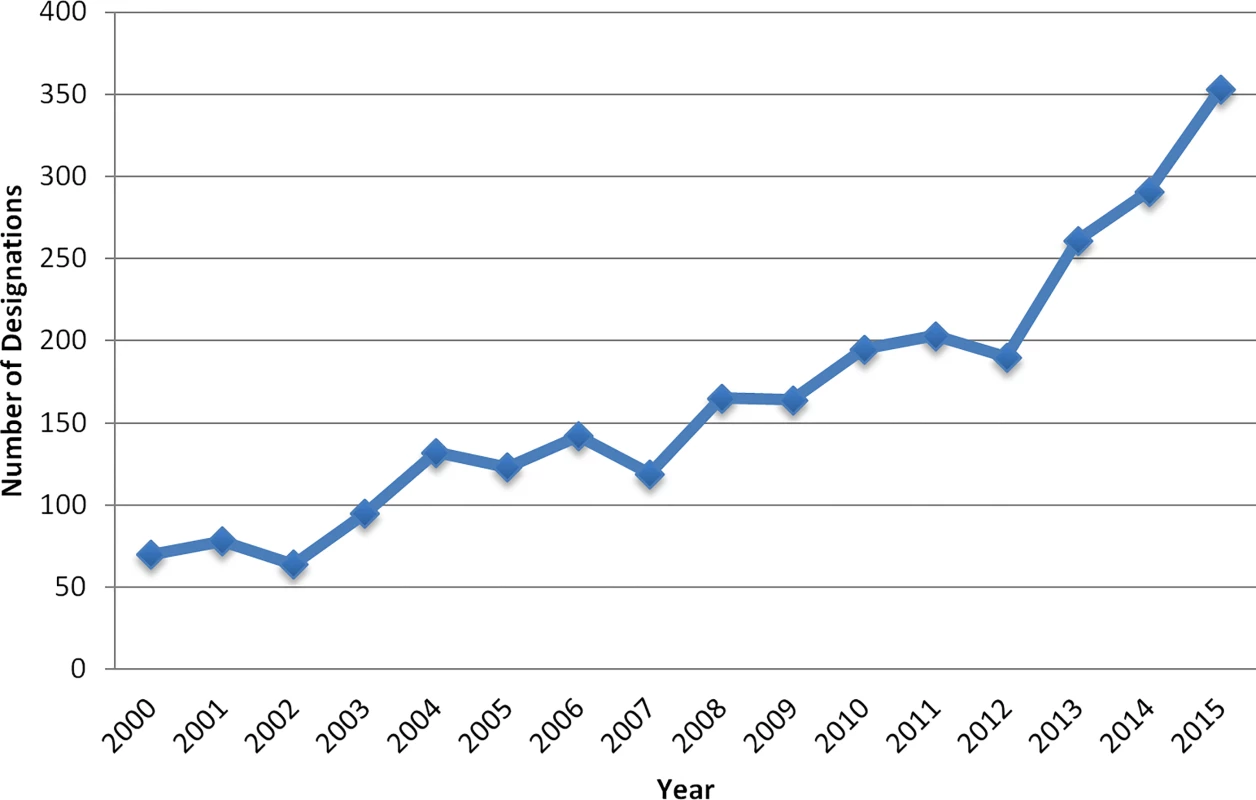
Yearly Numbers of Drug Products as Qualifying for Orphan Drug Status by the FDA (2000–2015)
Recently, the landscape surrounding use of this act has begun to change. Over the past decade, as orphan drug approvals have comprised an increasing share of all FDA-approved drugs, one contributor to this rise has been an increase in orphan-designated drugs that target biomarker-defined disease subsets [4]. For example, while non-small cell lung cancer was once divided into squamous cell carcinoma and adenocarcinoma, scientists now consider it a heterogeneous disease made up of numerous different genetic aberrancies. About 5% of non-small cell lung cancers have been found to have a rearrangement in the ALK gene, and three targeted chemotherapy agents—crizotinib (Xalkori), ceritinib (Zykadia), and alectinib (Alecensa)—have been approved for patients with lung cancer demonstrating ALK mutations. All were designated as orphan drugs [5].
With increasing investment in precision medicine, biomarker-defined disease subsets will play an increasingly central role in drug development. We sought to determine to what extent drugs targeting biomarker-defined subsets of more common diseases have been classified as orphan drugs over the past decade. Because the intent of the Orphan Drug Act was to help incentivize for-profit pharmaceutical manufacturers to invest in drugs important for patients with rare diseases, such a shift may signal the need for changes to the legislation.
Analysis of Orphan-Designated Drugs (2009–2015)
Data Sources and Collection
Using the FDA’s database of approved drugs, we compiled a list of all therapeutic drugs approved with formal orphan designation from 2009 to 2015 (excluding products used in diagnosis, like contrast agents). We then determined the drug’s primary therapeutic area and whether the orphan-designated drug targeted a biomarker-defined rare subset of a disease. A biomarker-defined subset was specified for this purpose as any drug approved based on its efficacy in a subset of a more prevalent disease characterized by a particular genetic variant or other specified diagnostic test.
Data Extraction
For each drug, we examined the circumstances surrounding the drug’s discovery and development. We collected certain key characteristics of the pivotal trials leading to the approval of the drugs. A pivotal trial is a clinical trial labeled by the FDA as most important in providing support for the indication(s) for which a drug is approved. From the FDA medical reviews and the published pivotal trials, we identified the total number of participants exposed to the drug and whether the trial tested a surrogate endpoint (e.g., disease response, disease progression) or clinical endpoint (e.g., overall survival). We also identified the date of the Investigational New Drug application, signaling the initiation of human clinical trials, and the date of New Drug Application to determine the length of time each drug spent in active development.
Next, we identified other special FDA designations—priority review status, accelerated approval, and fast track—attached to the drugs in the cohort via the Drugs@FDA database [6]. A fourth designation, breakthrough therapy, was instituted in 2012, midway through the study time period, so we excluded it from the analysis. The FDA confers priority review status on therapeutics that “offer major advances in treatment, or provide a treatment where no adequate therapy exists” [7]. Accelerated approval allows for earlier marketing of agents that fill an unmet need for a serious medical condition by relying on surrogate endpoint data for initial approval [8]. The fast-track designation can be provided to similarly promising agents, allowing for closer coordination between agency and manufacturer throughout the development phase, in addition to a more streamlined review process.
We identified whether the drug had been subsequently approved for other uses, such as other subsets of the same disease or other indications. For the oncologic drugs, we searched the National Comprehensive Cancer Network (NCCN) Database for approved off-label uses. An off-label use is a use for an indication that was not part of either the original labeled indication or an additional FDA approved indication. The NCCN database is a valid proxy to ascertain the extent of off-label use, as insurance companies use it to determine coverage [9].
Finally, from the company’s U.S. Securities and Exchange Commission filings, we identified the 2014 revenues attributed to sale of the drug in the United States (and worldwide sales, if available), the most recent year of data we could consistently find. Additionally, we assessed the reported monthly cost for each drug in 2014 using the Memorial Sloan Kettering DrugAbacus drug pricing database for the oncology drugs [10] and consumer-reported databases for non-oncologic drugs [11]. We also used the DrugAbacus database to assess the prices of non-biomarker-derived oncologic orphan drugs and non-orphan oncologic drugs that were approved during the same time period (2009–2015). The dollar values reported are not specific to each clinical indication.
Findings
From 2009 to 2015, 229 new drugs were approved, of which 84 (37%) had an orphan designation. The annual rate of orphan-designated drug approvals peaked in 2015, when 21/45 of the drugs in our cohort (47%) were approved with an orphan designation [1]. See S1 Appendix for full list of approved orphan drugs.
Among the 84 orphan-designated drugs, 13 (16%) were for biomarker-derived subsets of more prevalent diseases. Eleven of these addressed oncology indications. For example, afatinib (Gilotrif) was approved as an orphan drug in 2013 to treat patients with non-small cell lung cancer with an EGFR positive mutation, a rare variant affecting about 10% of patients [3]. Two non-oncologic drugs met inclusion criteria: ivacaftor (Kalydeco), approved in 2012 to treat the class III Gly551Asp mutation of cystic fibrosis, and lumacaftor/ivacaftor (Orkambi), approved in 2015 to treat the F508del mutation of cystic fibrosis (Table 1).
Tab. 1. Approvals of New Orphan-Designated Drugs Indicated for Biomarker-Defined Subsets of More Common Diseases, 2009–2015 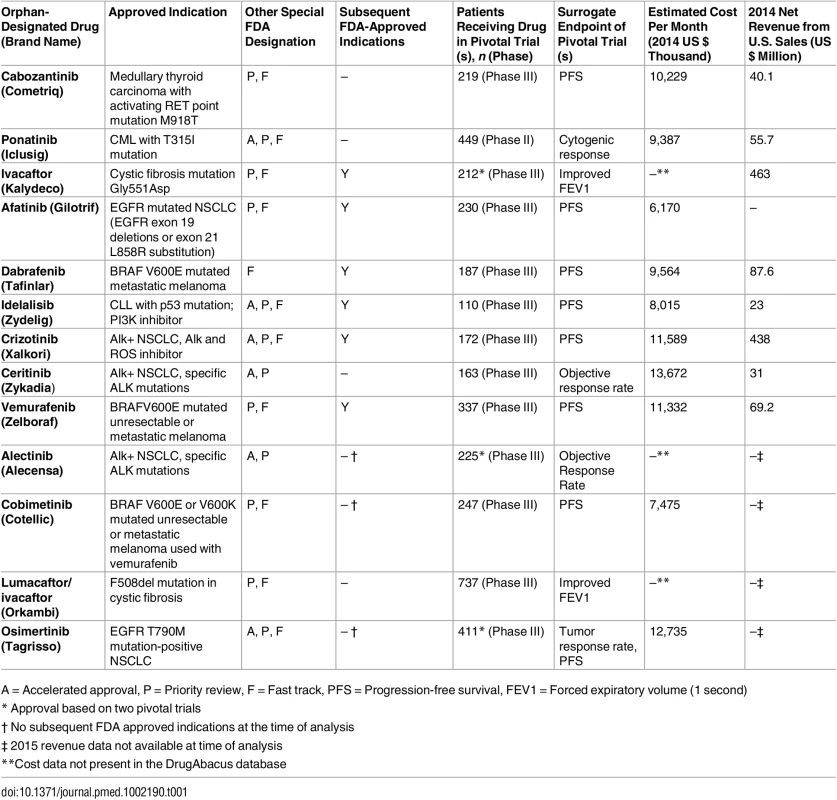
A = Accelerated approval, P = Priority review, F = Fast track, PFS = Progression-free survival, FEV1 = Forced expiratory volume (1 second) Orphan drugs for biomarker-defined disease subsets represented a substantial proportion of drugs approved for oncology indications during the study time period. A total of 89 drugs were approved for oncology indications, of which 39 (44%) received orphan designation. Of the 39 orphan-designated oncology drugs, 11 (28%, or 12% of all newly approved oncology drugs) were for biomarker-derived disease subsets.
Features of Drug Development
Three of the drugs in our sample were developed by smaller biotechnology firms that were subsequently acquired by larger pharmaceutical companies. Idelalisib (Zydelig) emerged from Calistoga Pharmaceuticals, a company focused on developing PI3K inhibitors that was later bought by Gilead Sciences [2]. Crizotinib (Xalkori) was initially attributed to Sugen, a small biotechnology firm based in California, which was later acquired by Pharmacia and subsequently Pfizer. Vemurafenib (Zelboraf) was developed in part at Plexxikon, which was later acquired by Daiichi Sankyo in 2011.
Clinical development times for this subset of orphan-designated drugs were short. All 13 drugs received at least one other expedited FDA designation besides the Orphan Drug Act designation (Accelerated Approval, Priority Review, Fast Track), with 12 receiving at least two other designations. Four of the drugs received all four designations. The median time between the initiation of human clinical trials and the submission of the full trial results dossier to the FDA was 4.6 years (interquartile range [IQR]: 3.4–5.6 years). Approvals were granted after a median FDA review period of 5.3 months.
The pivotal clinical trials for the 13 drugs in our sample had a median of 225 participants receiving active therapy, ranging from 110 (idelalisib) to 737 (lumacaftor/ivacaftor). One pivotal trial was phase II, while the others were phase III (nine of which were randomized). All trials tested surrogate endpoints, such as progression-free survival or objective response rate.
Orphan Drug Pricing and Revenue
The median monthly cost for the ten biomarker-derived orphan drugs indicated for oncologic conditions included in the DrugAbacus database in 2014 dollars was US $9897, ranging from US $6,170 per month for afatinib (Gilotrif) to US $13,672 per month for ceritinib (Zykadia). By contrast, the median for non-biomarker defined oncologic orphan drugs approved during the same time period (n = 8) was US $12,764 per month (range: US $9,240–US $64,260). Finally, the median for non-orphan oncology drugs approved during the same time period (n = 19) was US $8,701 per month (range: US $5,535–US $77,554) (Fig 2).
Fig. 2. Monthly cost of three subgroups of oncologic drugs. 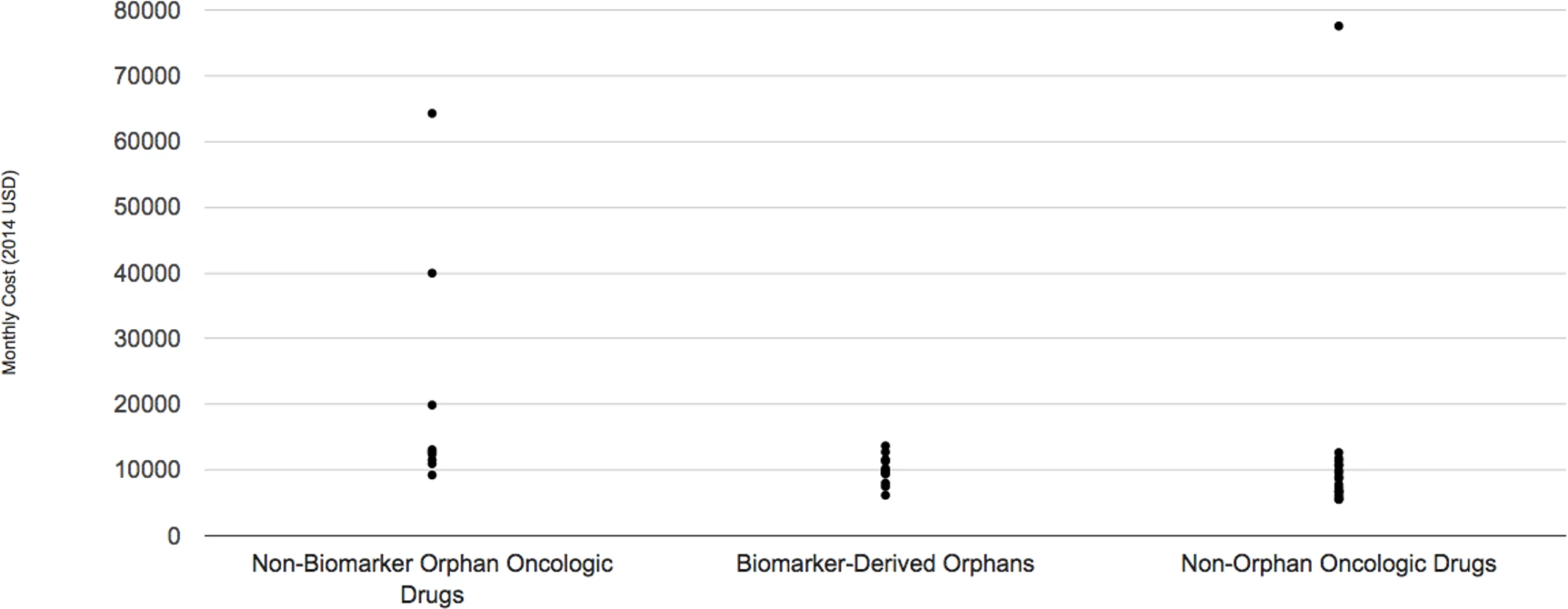
See S1 Appendix for raw data used in these figures. We found 2014 U.S. sales revenues for 8 of the 9 drugs that were approved during or before 2014 (afatinib is manufactured by a European company that did not report this information). We found a wide range of revenues attributed to sales of the drug in the United States in 2014, from US $31 million (ceritinib) to US $463 million (ivacaftor). Most manufacturers did not report revenue arising from international sales, although we did observe that vemurafenib’s manufacturer reported US $302 million worldwide sales and dabrafenib’s manufacturer reported US $204 million worldwide sales in that year.
Subsequent Use of Orphan Drugs
The majority of the drugs in our sample were used to treat additional indications after their original approvals. As of January 2016, 7 of the 14 drugs had been formally FDA-approved for at least one other use. Ivacaftor (Kalydeco) was approved in 2012 to treat a specific mutation in cystic fibrosis, but its approval has since been expanded to include eight additional cystic fibrosis mutations. Additionally, in 2015, the FDA approved the use of ivacaftor in combination with lumacaftor (Orkambi) to treat a more common cystic fibrosis mutation (F508 deletion) occurring in 70% of cystic fibrosis patients.
The eight drugs indicated for oncologic conditions and approved before 2014 had at least one off-label use listed in the NCCN database (none of the products approved in 2015 were yet listed in the database). For example, NCCN supports use of ceritinib (Zykadia), approved in 2014 for ALK+ non-small cell lung cancer, for soft tissue sarcoma and inflammatory myofibroblastic tumors with the ALK translocation. Dabrafenib (Tafinlar), approved to treat BRAFV600E metastatic melanoma, can be used off label for selected subsets of central nervous system malignancies and non-small cell lung cancer.
Discussion
We found that biomarker-defined orphan drugs, most of which relate to oncologic indications, now make up a substantial minority of orphan-designated drug approvals. This subset is characterized by short development times and high prices that are consistent with the costs of other non-orphan drugs. However, these orphan-designated drugs are also available for use outside the biomarker-defined disease subset for which they were originally approved, as nearly all of the drugs in our sample were subsequently associated with other supplemental indications.
Among the justifications for the Orphan Drug Act’s incentives are that drugs for rare diseases are costly to develop and test, and the small numbers of patients receiving them would not provide sufficient return on manufacturers’ investment. But these justifications may not apply equally to all drugs for rare diseases. In our analysis, we found that biomarker-defined orphan-designated drugs can be developed based on trials in small numbers of patients and relatively short development times. By comparison, the median clinical trial time period for non-orphan-designated cancer drugs during a similar time period was 6.9 years (IQR 6.5–8.0 years) [12]. They also sustain high prices after approval as well as broad coverage by insurers in the United States [13].
The biomarker-defined orphan-designated drugs in our sample were frequently associated with secondary approvals or guideline-supported off-label uses, although we did not assess actual off-label prescribing rates [14,15]. Is the Orphan Drug Act relevant when drugs find such additional uses after approval [16,17]? Applying the orphan designation in such circumstances wastes resources that might otherwise be applied to more deserving drugs. For example, the FDA’s US $2.17 million user fee is waived for orphan-designated drugs, and studies show that the FDA takes a highly flexible posture in its review, accepting less rigorous, smaller-scale premarket efficacy testing than for non-orphan drugs [18,19]. The fact that many drugs approved as orphan-designated products on the basis of biomarker-defined populations quickly and readily lead to additional recommended indications suggests that the FDA should reconsider whether biomarker-defined subsets of more common diseases are truly “rare diseases” in the same way as rare cancers or enzyme deficiencies, and whether they are similarly deserving of these regulatory incentives [20,21]. Scientific advances lead to the uncovering of more biomarkers, and the increasing number of biomarker-defined subsets of more common diseases that will inevitably result should lead to a re-examination of how a “rare disease” is defined in the United States to determine the applicability of the Orphan Drug Act. For example, instead of being defined based on the number of patients with a certain disease, the drug class or genomic drug target could be the basis for the designation. Thus, a drug designed to treat ALK mutations would qualify for orphan designation status if the number of patients with that mutation across all cancers, rather than just lung cancer, was less than the 200,000-patient threshold.
The prices of oncology drugs did not differ substantially whether the drug targeted a rare disease (either biomarker-defined or not) or a more common form of cancer, suggesting that pricing does not reflect either a premium for the small patient populations or a discount due to the smaller trials and generally shorter development times characterized by these products. While we do not have insight into confidential rebates that private payors may have negotiated with manufacturers, such a result suggests that drug pricing in the U.S. market is largely insensitive to development costs—which would vary based on whether the product was studied in a small or large population—and is instead more closely tied to manufacturers’ ability to set prices [22]. Recent efforts to tie oncology drug pricing closer to value may lead to changes in these trends in future years [23].
In summary, we found a substantial number of new orphan-designated drugs intended to treat biomarker-defined subsets of more common diseases. These diseases have a number of characteristics that make them ill-suited to the orphan drug designation, including rapid expansion of recommended indications after approval. Application of the Orphan Drug Act to these diseases risks wasting regulatory resources that might be better focused on truly rare conditions.
Supporting Information
Zdroje
1. Orphan Drug Act of 1983. 21 U.S.C. 360bb(a)(2) (2008). https://www.gpo.gov/fdsys/pkg/STATUTE-96/pdf/STATUTE-96-Pg2049.pdf Accessed 2 Sept 2016.
2. Reardon S. Regulators adopt more orphan drugs. Nature. 2014;508 : 16–17. doi: 10.1038/508016a 24695293
3. Field MJ, Boat TF. Committee on Accelerating Rare Diseases Research and Orphan Product Development Board on Health Sciences Policy. Washington, DC: National Academies Press. 2010.
4. Herder M. When everyone is an orphan: against adopting a U.S.-styled orphan drug policy in Canada. Account Res. 2013;20(4):227–69. doi: 10.1080/08989621.2013.793120 23805831
5. Daniel MG, Pawlik TM, Fader AN, Esnaola NF, Makary MA. The Orphan Drug Act: Restoring the Mission to Rare Diseases. Am J Clin Oncol 2015;00(00):1. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000421-900000000-99097
6. Food and Drug Administration. Drugs@FDA: FDA approved drug products. Drugs@FDA. 2016. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails
7. Food and Drug Administration. Fast track, breakthrough therapy, accelerated approval and priority review. Sept 14, 2015. http://www.fda.gov/forpatients/approvals/fast/ucm20041766.htm
8. Darrow JJ, Kesselheim AS. Drug Development and FDA Approval, 1938–2013. N Engl J Med 2014;370(26).
9. Conti R, Bernstein A, Villaflor V, Schilsky R, Rosenthal M, Bach P. Prevalence of off-label use and spending in 2010 among patent-protected chemotherapies in a population-based cohort of medical oncologists. J Clin Oncol. 2013;31(9):1134–9. doi: 10.1200/JCO.2012.42.7252 23423747
10. Memorial Sloan Kettering Cancer Center Abacus Database. 2015. http://app.drugabacus.org/abacus-mskcc
11. Nocera J. The $300,000 Drug. The New York Times. July 19, 2014. http://www.nytimes.com/2014/07/19/opinion/joe-nocera-cystic-fibrosis-drug-price.html?_r=0
12. Kesselheim AS, Myers JA, Solomon DH, Winkelmayer WC, Levin R, Avorn J. The prevalence and cost of unapproved uses of top-selling orphan drugs. PLoS ONE. 2012;7(2):1–7.
13. Faden L, Huskamp H. Medicare Part D Coverage and Reimbursement of Orphan Drugs. National Academies Press; 2010.
14. Gupta SK, Nayak RP. Off-label use of medicine: Perspective of physicians, patients, pharmaceutical companies and regulatory authorities. Journal of Pharmacology & Pharmacotherapeutics. 2014;5(2):88–92.
15. Casali PG. The off-label use of drugs in oncology: a position paper by the European Society for Medical Oncology (ESMO). Ann Oncol. 2007;18(12):1923–1925. doi: 10.1093/annonc/mdm517 18083693
16. Fugh-Berman A, Melnick D. Off-label promotion, on-target sales. PLoS Med 2008;5(10):e210. doi: 10.1371/journal.pmed.0050210 18959472
17. Wellman-Labadie O, Zhou Y. The US Orphan Drug Act: rare disease research stimulator or commercial opportunity? Health Policy 2010;95(2–3):216–28. doi: 10.1016/j.healthpol.2009.12.001 20036435
18. Kesselheim AS, Myers JA, Avorn J. Characteristics of clinical trials to support approval of orphan vs nonorphan drugs for cancer. JAMA. 2011;305(22):2320–6. doi: 10.1001/jama.2011.769 21642684
19. Mitsumoto J, Dorsey ER, Beck CA, Kieburtz K, Griggs RC. Pivotal studies of orphan drugs approved for neurological diseases. Ann Neurol. 2009;66(2):184–90. doi: 10.1002/ana.21676 19743448
20. Herder M. Orphan drug incentives in the pharmacogenomic context: policy responses in the USA and Canada. J Law Biosci. 2016;lsv060.
21. Gibson S, Tigerstrom B von. Orphan drug incentives in the pharmacogenomic context: policy responses in the US and Canada. J Law Biosci. 2015;2(2):263–91. doi: 10.1093/jlb/lsv013 27774196
22. Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States: origins and prospects for reform. JAMA 2016;316(8):858–871. doi: 10.1001/jama.2016.11237 27552619
23. Bach PB. Indication-specific pricing for cancer drugs. JAMA 2014;312(16):1629–1630. doi: 10.1001/jama.2014.13235 25279433
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Evaluating Hospital-Based Surveillance for Outbreak Detection in Bangladesh: Analysis of Healthcare Utilization Data
- Effect of a Primary Care Walking Intervention with and without Nurse Support on Physical Activity Levels in 45- to 75-Year-Olds: The edometer nd onsultation valuation (PACE-UP) Cluster Randomised Clinical Trial
- Patient Safety Incidents Involving Sick Children in Primary Care in England and Wales: A Mixed Methods Analysis
- Biomarker-Defined Subsets of Common Diseases: Policy and Economic Implications of Orphan Drug Act Coverage
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Priority-Setting for Novel Drug Regimens to Treat Tuberculosis: An Epidemiologic Model
- Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review
- Association of Body Mass Index with DNA Methylation and Gene Expression in Blood Cells and Relations to Cardiometabolic Disease: A Mendelian Randomization Approach
- Socioeconomic Inequalities in Body Mass Index across Adulthood: Coordinated Analyses of Individual Participant Data from Three British Birth Cohort Studies Initiated in 1946, 1958 and 1970
- Mosquito-Disseminated Insecticide for Citywide Vector Control and Its Potential to Block Arbovirus Epidemics: Entomological Observations and Modeling Results from Amazonian Brazil
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland
- Master Regulators of Oncogenic Response in Pancreatic Cancer: An Integrative Network Biology Analysis
- Sick Children Crying for Help: Fostering Adverse Event Reports
- What Is the Purpose of the Orphan Drug Act?
- Novel Vector Control Approaches: The Future for Prevention of Zika Virus Transmission?
- Artificially Sweetened Beverages and the Response to the Global Obesity Crisis
- Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp)
- Bolstering Community Cooperation in Ebola Resurgence Protocols: Combining Field Blood Draw and Point-of-Care Diagnosis
- Defining Abnormal Fetal Growth and Perinatal Risk: Population or Customized Standards?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
- What Is the Purpose of the Orphan Drug Act?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



