-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMaster Regulators of Oncogenic Response in Pancreatic Cancer: An Integrative Network Biology Analysis
Florian Markowetz and colleagues study transcriptional mechanisms influenced by mutated KRAS, which is common in pancreatic ductal adenocarcinomas, and possible implications for disease characteristics and prognosis.
Published in the journal: . PLoS Med 14(1): e32767. doi:10.1371/journal.pmed.1002223
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002223Summary
Florian Markowetz and colleagues study transcriptional mechanisms influenced by mutated KRAS, which is common in pancreatic ductal adenocarcinomas, and possible implications for disease characteristics and prognosis.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most lethal human malignancy, with a 5-y survival of 4% [1]. There are very few treatment options, with the only chance of a cure being surgical resection if the tumour is detected at an early, confined stage. Chemotherapeutic options are used in the palliative setting but have toxic side effects and do not extend survival for more than a few months. Pancreatic cancers display vast intratumoural heterogeneity with respect to their mutational profiles [2], but more than 90% of cases have a mutation in the KRAS oncogene, which almost exclusively is located in codon 12 [3].
Other highly recurring mutations in PDAC include INK4/ARF, P53, and SMAD4 [4]. Recent studies [4–6] on the genomic landscape of PDAC have identified alterations in genes related to chromatin remodeling, DNA damage repair, and axon guidance, as well as focal amplifications in druggable genes—including ERBB2, MET, and FGFR1—in a small subset of patients. Several recent studies [7–9] have described the transcriptomic landscape of PDAC and identified different subtypes with a link to survival.
Here, we took advantage of seven existing transcriptomic studies containing in total 560 samples to explore KRAS biology (see S1 Computational Analysis). The role of KRAS in PDAC initiation is well known [10], and cancer growth and survival often directly depend on it [11]. However, the mechanisms by which KRAS contributes to PDAC progression, in particular its interactions with downstream pathways, have not been equally well characterised [12]. The aims of our study were to identify transcription factors determining the transcriptional response to oncogenic KRAS and to explore what impact their activity has on the development of the disease and patient outcome.
Methods
This study did not have a protocol or prospective analysis plan. To achieve the first aim, we used master regulator analysis, a well-established network biology strategy [13–16], which combines a transcriptional signature with a coexpression network to identify key transcription factors. For the second aim, we used clustering techniques to identify patient subtypes based on transcription factor activities and characterised these subtypes by survival analysis, integration of mutation data, and methods that infer immune activity from gene expression profiles. For clarity, the Methods description is split into three main sections: defining a transcriptional signature for oncogenic KRAS, identification of master regulators of KRAS response, and characterisation of PDAC subtypes. All code and scripts to reproduce our analysis are available as annotated documents as part of the supplementary information (S1 Computational Analysis, S2 Computational Analysis).
1. Defining a Transcriptional Signature for Oncogenic KRAS
Here, we describe how we derived the gene expression signature of oncogenic KRAS, which provides the focus point of our study.
Murine ductal cell line
KF508 is a murine ductal pancreatic cell line we received from the Tuveson lab. The cells were isolated from a genotype KrasLSL-G12D mouse according to a published protocol [17]. Briefly, the pancreas of a KRASLSL-G12D mouse was dissected, minced, and digested at 37°C in Hank’s balanced salt solution (HBSS) (Hank’s balanced salt solution + 5 mmol/L glucose + 0.05 mmol/L CaCl2) containing 2 mg/mL type V collagenase (Sigma, St. Louis, Missouri). After 15 min, the digested material was filtered through a 105 μm nylon mesh (Small Parts, Inc.). Fragments trapped on the mesh were digested further in 0.05% trypsin–0.53 mmol/L ethylenediaminetetraacetic acid (Life Technologies) for 2 min. Proteases were inactivated by addition of DMEM/F12 (Invitrogen) supplemented with 10% fetal bovine serum. The tissue was washed three times in HBSS to remove collagenase completely. The ductal fragments were plated on 2.31 mg/mL rat tail collagen type I (BD Biosciences) precoated plastic dishes for growth in monolayer. It is noteworthy that the cells were spontaneously immortalized—i.e., they continued to proliferate after a few passages without any additional changes to promote immortalisation. The cells were transferred to plastic after five passages. The mouse is very well characterised in the literature [10, 18, 19]. The cell line was generated from a mouse with C57Bl6/129 background of unknown age and sex, and a positive E-cadherin expression was used to validate epithelial origin (2016 email from Dr. Kris Frese to Shivan Sivakumar, unreferenced). KF508 cells contain the mutant version of the Kras allele (G12D) preceded by a Lox-Stop-Lox cassette inhibiting transcription. Infection with Cre-expressing adenovirus (adeno-cre) deletes the floxed stop sequence, permitting Cre-mediated recombination and expression of oncogenic KrasG12D, whilst adenovirus-mock was used as a negative control. For adenoviral infections, cells were transduced with 1x107 PFU adenoviral-mock or adenoviral-cre per mL of media (virus was obtained from the University of Iowa).
Media to propagate ductal cells
The ductal cell medium was adapted from the Schreiber protocol. A bottle of DMEM/F12 media was heated to 37°C for 30 min. The media was supplemented with 1.22mg/ml nicotinamide (Sigma), 5 mg/ml glucose (Sigma), 5% ITS + cell culture supplement (VWR), 5% Nu-Serum IV (VWR), and 20 ng/ml Epidermal Growth Factor (Peprotech). This mixture was subsequently filter sterilized through a 0.22 μM filter. To check expression of oncogenic Kras, the Ras GTPase activation kit from Millipore was used.
Gene expression profiling
The microarray experiment was performed by the genomics core facility in the CRUK Cambridge Institute. Briefly, total RNA was extracted from six sets of mock - and cre-treated ductal cells using the Qiagen RNeasy kit. The RNA was labelled and hybridized. This experiment was carried out on two Illumina Mousev2 BeadChips using 24 arrays (1 sample per BeadChip). These arrays interrogate 46,235 randomly distributed bead types, and in this experiment there was a mean of 21 beads per bead type (standard deviation of 6). In total, there are 20,562 genes being interrogated in this experiment, with 8,472 genes being interrogated by more than one bead type and 12,014 bead types not being assigned to a gene symbol. Differential expression analysis between the two groups (“mock” versus “cre”) was carried out using the limma Bioconductor package [20]. Mouse Ensembl IDs were mapped to human Ensembl IDs in Ensembl 50, using biomart (http://www.ensembl.org/biomart).
2. Identification of Master Regulators
Here, we describe the data and methods to build a coexpression network for PDAC and how it is used to identify the transcription factors determining the KRAS signature, the so-called master regulators.
Collection of 560 gene expression profiles from 7 independent studies
Raw gene expression data files were obtained from Gene Expression Omnibus (GEO) for a total of five independent, pancreatic-related studies [8,18–22]. GEO accession numbers for these five studies are GSE17891 (n = 26), GSE15471 (n = 36), GSE16515 (n = 36), GSE32676 (n = 25), and GSE2109 (n = 17). All five downloaded datasets were generated on the Affymetrix GeneChip® Human Genome U133 Plus 2.0 array. The ICGC microarray gene expression data (n = 242) plus clinical metadata and mutational profiles were directly obtained from the authors of Bailey et al. 2016 [9]. TCGA RNA-seq data [22] (n = 178) was obtained with permission from the Cancer Genomics Hub (https://cghub.ucsc.edu). Clinical metadata for the TCGA cohort was downloaded via cBioPortal [21].
Processing of gene expression datasets
QC analysis was performed using affy and affyPLM [22]. R packages and normalization was performed with RMA (robust multichip averaging) method [23]. Batch effects in the ICGC cohort were removed by applying the ComBat algorithm [24]. TCGA RNA-seq data was transformed with the variance-stabilizing transformation method [25].
Identification of core disease processes
Network inference was done using the RTN [13, 14] package and coexpression analysis using partial correlations. We applied the pcor.shrink function from the corpcor [26] with default parameters. The significance of each partial correlation was computed using the fdrtool R package [13,14,27,28]. Master regulators were identified with the msviper functionality of the viper package [29], and community detection was performed by using the fastgreedy.community function in the igraph R package [30].
3. Characterisation of PDAC Subtypes
The core disease processes define three subtypes, which we comprehensively characterized.
Pathway analysis
Pathway and gene ontologies enrichment analyses were performed in R by hypergeometric test or gene set enrichment analysis as indicated. Kyoto Encyclopedia of Genes and Genomes (KEGG) and two GO gene set collections obtained from KEGG.db and GO.db databases in R were used.
Characterization of PDAC subtypes
In order to detect the similarity between tumour samples based on the activity of the 27 master regulators, we used viper [29] to perform a single-sample analysis and compute a matrix with activity scores for each one of the 27 regulators. The distance between the tumour samples was computed with the signature distance method with the default parameters in viper [29]. PDAC subgroups were identified by applying hierarchical clustering with agglomerative average linkage.
Survival analysis
Survival analysis was performed using the Kaplan–Meier estimator, comparison among groups was analysed using log-rank tests, and multivariate analysis was performed using the Cox proportional hazard model. For the ICGC cohort, we adjusted the survival differences for age, gender, and tumour stage covariates. For the TCGA cohort, we modelled the interaction between tumour subtype (group label) and a binary (yes/no) targeted therapy indicator, adjusting for age, gender, tumour stage, and radiation treatment indicator. The two binary treatment indicators considered (targeted therapy and radiation treatment) were retrieved from “targeted_molecular_therapy” and “radiation_treatment_adjuvant” labels, respectively, of the associated TCGA clinical metadata. These indicators refer to whether the patient had adjuvant and/or postoperative pharmaceutical or radiation therapy. More specific information about the treatment regime was not possible to obtain.
Analysis of mutational data
We used the previously identified landscape [9] of somatic mutations and copy number alterations in the ICGC cohort to investigate the frequency of samples with mutations in 39 key genes in pancreatic cancer [5]. Somatic mutations were filtered out to include only alterations with high and moderate impact on the basis of their predicted effect on the protein [31]. Copy number alterations were restricted to losses of at least one copy and to amplifications of more than five copies.
Analysis of leukocyte infiltration and stromal content
ESTIMATE [32] was used to gauge the degree of leukocyte infiltration and stromal content within the tumour microenvironment for each of the three derived pancreatic cancer subtypes. The Wilcoxon rank sum test was used to elucidate whether the computed immune and stromal infiltrates were significantly different across the subtypes.
We compared the samples by their enrichment for the Hedgehog, Notch, and cell cycle pathways using single-sample gene set enrichment analysis (ssGSEA).We used the R implementation of ssGSEA: GSVA (gene set variation analysis) with default parameters. Three gene set collections were downloaded from the Broad Institute Molecular Signature Database (MSigDB): the curated KEGG, BIOCARTA v5.0, and C5 Gene Ontology collections [33]. These were aggregated and filtered in order to build a single collection containing only immunological pathways. For each sample, Hedgehog, Notch and cell cycle enrichment was determined by running ssGSEA, using a curated gene set for each pathway. We furthermore calculated the enrichment for each immunological pathway in the collection by running ssGSEA across all the samples. First-order partial correlation coefficients between Hedgehog/Notch and the immunological pathways were computed using the R package “ppcor” to correct for the strong positive correlation between Hedgehog and Notch (see S2 Computational Analysis). p-values were multiplied by the number of correlations to correct for multiple testing-associated type I errors. Associations between Hedgehog and Notch and an immunological pathway were deemed “significantly associated” if one or the other had p < 0.05.
Gene expression deconvolution
We used the deconvolution tool CIBERSORT to estimate the fraction of immune cells in the bulk tumour using gene expression data [34]. CIBERSORT uses the expression profiles of purified samples to derive a matrix of 22 immune cell type signatures (LM22), which can be used to elucidate the immune microenvironment composition of bulk tumour samples [34]. We preprocessed the discovery and validation datasets according to CIBERSORT requirements. For the microarray data, probes mapping to more than one gene were removed from the analysis. For genes mapping to more than one probe, we selected the probe with the highest variance value across the sample space.
Data were quantile normalised and CIBERSORT was run with 1,000 permutations. A threshold of p < 0.05 defined a successful CIBERSORT deconvolution. First-order partial correlations were used to evaluate whether any significant associations existed between any of the leukocyte cell types and the Hedgehog/Notch pathways. The ICGC and TCGA cohorts were treated as discovery and validation cohorts, respectively. ICGC cohort p-values were adjusted for multiple testing by multiplying by the number of partial correlation tests. A threshold of p < 0.05 defined significance in both cohorts. The t-statistics of the partial correlation between Hedgehog/Notch and the leukocyte fractions was used as a comparison metric (see S2 Computational Analysis).
Immune checkpoint gene expression, such as those for PD-1 and PD-L1, were tested for association with both the Hedgehog and Notch pathways through the use of first-order partial correlation analysis. 44 pancreatic cancer cell lines were downloaded from the Broad Institute Cancer Cell Line Encyclopedia. These were classified into “Hedgehog,” “cell cycle,” or “Notch” by means of the nearest shrunken centroids method implemented via the R package “pamr.” This was then verified by comparing the subtype classification with each cell line’s ssGSEA-derived enrichment score for the Hedgehog, cell cycle, and Notch pathways.
Results
Network Analysis Identifies KRAS-Specific Master Regulators
A KRAS-specific transcriptional signature enables us to understand the regulatory patterns induced in the presence of this mutation through downstream network analysis. To facilitate this analysis, we first derived a transcriptional signature of oncogenic KRAS by using a previously reported murine pancreatic ductal cell line with an inducible Lox-Stop-Lox (LSL) cassette in front of the KRASG12D oncogene to regulate transcription [35]. Comparing the transcriptomes of induced KRAS-on cells with KRAS-off control population in six replicates, we identified 667 differentially expressed probe sets (Fig 1A). Using gene set enrichment analysis, we found that the KRAS signature was enriched for many transcript changes in the pathways associated with the MAPK pathway, regulation of cell growth, and regulation of the actin cytoskeleton, which is in line with established functions of KRAS (S1 Table). Given that these cell lines are derived from murine samples, genes in the signature were mapped to their equivalent human orthologue where possible and removed from the signature if no orthologue exists.
Fig. 1. Oncogenic KRAS is regulated by three groups of master regulators. 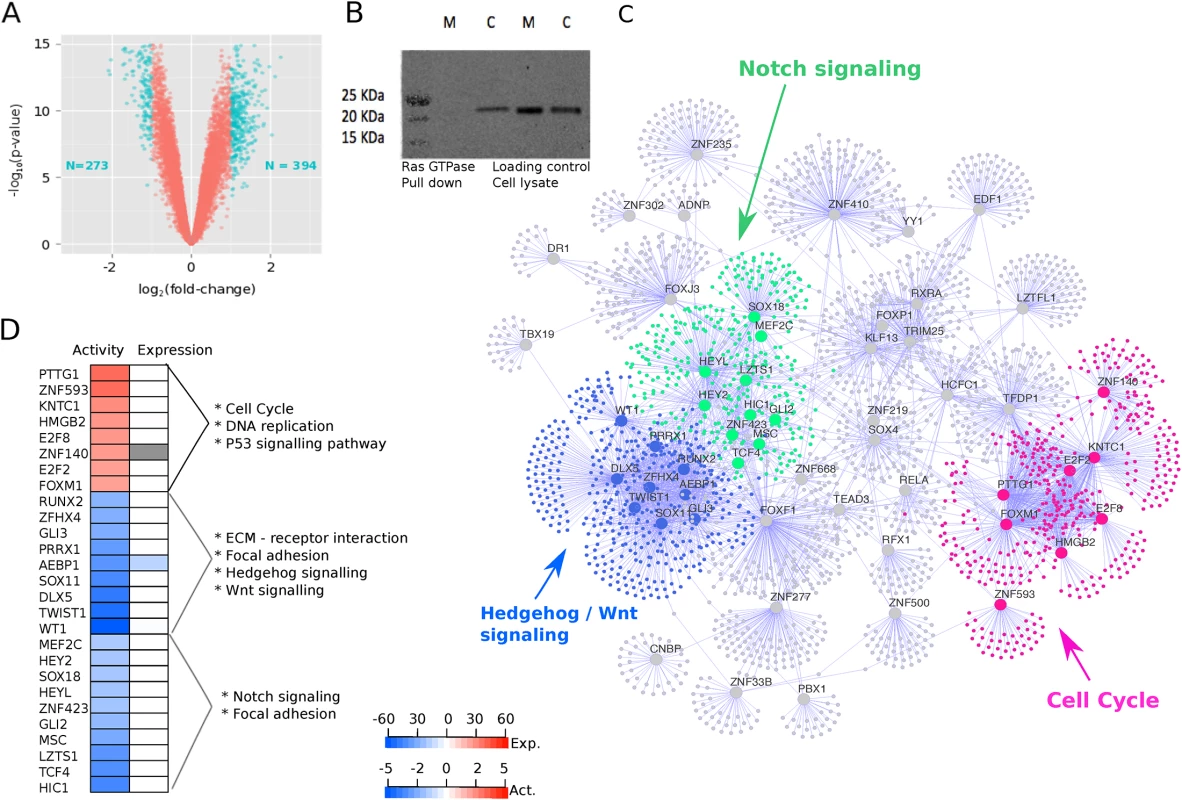
A. Volcano plot showing the magnitude of the differential gene expression between murine mock ductal cells and murine cre ductal cells (with activated oncogenic Kras). Each dot represents one probe with detectable expression in both conditions. The coloured dots mark the threshold (p < 0.05 and log2 fold-change > 1) for defining a gene as differentially expressed. B. Ras GTPase assay shows increased GTPase activity in cre cells blotted with pan-Ras antibody (M, mock; c, cre). C. Visual representation of master regulators (MRs) identified with msVIPER analysis (p < 0.01). The nodes in the networks represent the 55 master regulators (large dots) and the corresponding inferred targets (smaller dots). The edges in the network represent the regulatory relationship between regulators and the inferred targets. The colours highlight the community structure of the network identified via greedy optimization of modularity. The three groups of nodes correspond to a total of 27 master regulators and represent three distinct disease processes enriched for cell cycle (pink), Hedgehog/Wnt signalling (blue), and Notch signalling (green) pathways. D. For the 27 MRs in the three core processes, the heat map shows their activity (first column) and differential expression in the KRAS signature (second column) as obtained by Virtual Inference of Protein-activity by Enriched Regulon (VIPER) analysis. “Expression” refers to the differential expression value after KRAS induction (cell line experiment). The colour code of in the heat map corresponds to the t-statistic value obtained after limma differential expression analysis, with blue representing down-regulated genes and red representing up-regulated genes after KRAS activation. “Activity” refers to the differential protein activity value after KRAS induction with red or blue representing activation or inactivation, respectively. The protein activity score is quantitatively inferred by the aREA algorithm in VIPER by systematically analysing expression of genes coexpressed with the transcription factor (TF). To study which transcription factors (TFs) act as master regulators for the observed transcriptional changes, we used 560 gene expression profiles collected from seven independent studies [8, 9, 36–40] to infer a coexpression network between transcription factors and their potential targets. Details of datasets are described in the Methods section. We used different safeguards against biases potentially incurred during data integration. Every dataset was normalized independently and corrected for batch-effects if it contained sample subgroups (S2 Fig). We then used a shrinkage estimate of partial correlations [26] to infer a network between regulators and their targets in each dataset. The shrinkage estimate automatically adapts to differences in sample size and ensures reliable results across studies. A significant partial correlation corresponds to an edge in the network, and the set of all inferred targets of a regulator is called its “regulon.” We confirmed that the regulons were well conserved between the seven networks (S3 Fig). We then converted the p-values of all edges into Z-scores and combined them into a single, integrated network using Stouffer’s meta-analysis score [28], which weights the significance of an edge by the size of each study to ensure that larger cohorts contribute more to the integrated network. Thus, the final integrated network consists of edges showing consistent results across all seven studies.
In the next step, we superimposed the transcriptional KRAS signature onto the regulatory network using the VIPER (Virtual Inference of Protein-activity by Enriched Regulon analysis) algorithm [5, 9, 13, 14, 29, 40]. VIPER tests for significant overlap between genes in the transcriptional signature and genes in each regulon. 55 regulons were significantly enriched for signature genes, and we called the corresponding TFs “master regulators” (MRs) of activated oncogenic KRAS (S4 Fig). Several MRs have been previously implicated in PDAC, such as GLI3, AEBP1, and CASP5, while some of the MRs have not been associated with PDAC before, such as TCF21, TWIST1, and FOXF2.
To identify the core biological processes represented by these master regulators, we used a community detection algorithm on the transcriptional network and identified Notch signaling, Hedgehog/Wnt signalling, and cell cycle as close clusters of 27 of the 55 MRs (S5 Fig). Eight of the 27 MRs had an overall activation in protein gene up-regulation activity, and the other 19 had repressed protein activity (Fig 1D). The Notch and Hedgehog/Wnt clusters cluster closer together in the network, whereas the cell cycle MRs form a distinct group (Fig 1C).
KRAS Master Regulators Define Three Core Regulatory Processes
Based on the expression of genes within each regulon, we inferred the activity of the MRs in each patient sample (S1 Computational Analysis). These activity scores clearly defined three disease processes (Fig 2). This result indicates that even though PDAC has KRAS as a dominant oncogene driver, it develops along one of three distinct paths, which can be identified by their underlying biological pathways.
Fig. 2. Clustering of master regulators into different functional groups. 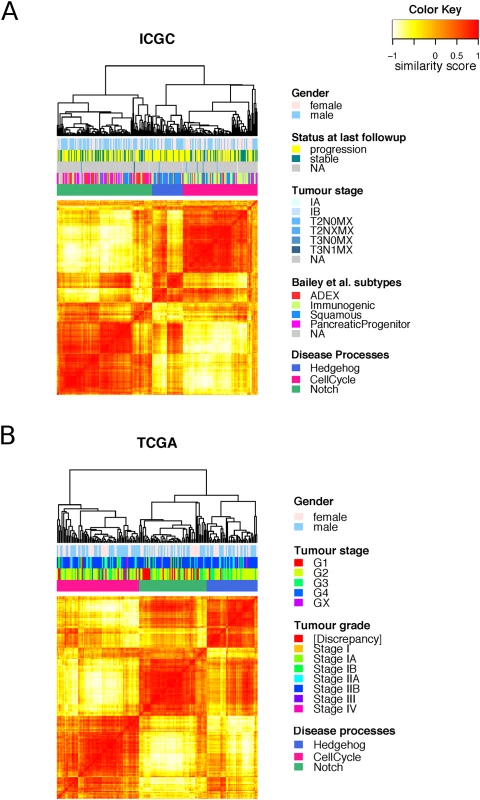
Heat maps showing the similarity between the samples in the A. ICGC and B. TCGA cohorts as measured by “signature distance” between the MRs activity profiles [27]. Unsupervised analysis identified three classes of tumours with differential activities of the three identified disease processes: cell cycle (pink), Hedgehog/Wnt (blue), and Notch (green). The murine cell line we used to derive the transcriptional signature harboured a G12D mutation, whereas patients in the TCGA and ICGC cohorts also showed a variety of KRAS mutation types. However, we did not find any of our subtypes biased towards one particular KRAS mutation type (S7 Fig).
When comparing our disease regulatory processes to the subtypes identified by Bailey et al. [9], we found that the Hedgehog/Wnt process is overrepresented by samples from the squamous subtype (phyper = 1.1x10−11), the cell cycle process by samples from the immunogenic subtype (phyper = 7.8x10−12), and the Notch by samples from the ADEX (p-value = 8.2x10−8) and pancreatic progenitor (phyper = 6.1x10−8) subtypes (S6 Fig).
MR Regulatory Processes Show Different Survival and Mutational Load
In all further analyses, we focused on the two biggest studies in our dataset collection and used the ICGC cohort [9] (n = 242) as a discovery set and the TCGA cohort [40] (n = 178) as an independent validation set. The cohorts are comparable and patients all had their pancreatic cancers resected at early stage disease (typically stage I/II).
To quantify the clinical importance of the different regulatory processes, we compared them to survival data (clinical features of both cohorts are summarized in S2 Table). In discovery and validation cohorts, the Hedgehog/Wnt group has a very poor prognosis, while the Notch group has the best prognosis (Fig 3A) (Cox proportional hazards regression model, Hedeghog/Wnt group HR = 1.73, 95% CI 1.1 to 2.72, p-value = 0.018; Notch group HR = 0.62, 95% CI 0.42 to 0.93, p-value = 0.019; when compared to the cell cycle group and after correcting for gender, age, and tumour stage). Our findings agree with previous observations linking the repression of the Hedgehog pathway to more aggressive pancreatic cancers [9, 41, 42], and this particular subtype also overlapped with the poor prognostic group found in Bailey et al. [9].
Fig. 3. Differences in survival and mutational burden between subtypes. 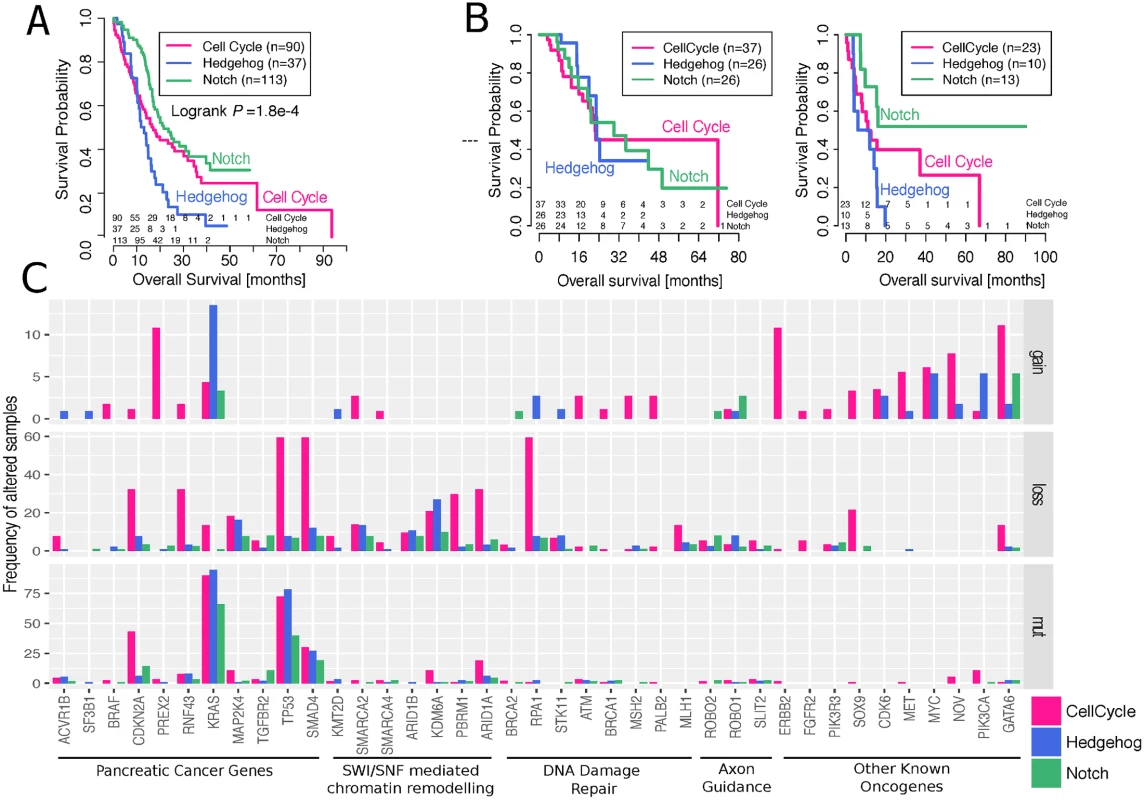
A. Kaplan–Meier survival curves of the different tumour subgroups using the ICGC cohort. Numbers of subjects at risk at the start of each time interval are shown above the x-axis. The groups overall showed significant survival differences (logrank p-value = 1.8e–4). More specifically, Hedeghog/Wnt group HR = 1.73, 95% CI 1.1 to 2.72, coxPH test p-value = 0.018; Notch group HR = 0.62, 95% CI 0.42 to 0.93, coxPH test p-value = 0.019; when compared to the cell cycle group and after correcting for gender, age, and tumour stage. B. Kaplan–Meier survival curves of the different tumour subgroups using the TCGA cohort for subsets of individuals that did or did not receive adjuvant targeted therapy treatment. Numbers of subjects at risk at the start of each time interval are shown above the x-axis. Hedeghog/Wnt group HR = 4.12, 95% CI 1.2 to 13.8, coxPH test p-value = 0.02; when compared to the cell cycle group and after correcting for gender, age, tumour stage, and radiation therapy indicator. C. Mutations in key genes and pathways in pancreatic cancer. The upper and middle panels show the frequency of altered samples by copy number changes (gains refer to amplifications of >5 copies); the bottom panel shows the frequency of altered samples by nonsilent single nucleotide variants, small insertions, or deletions with moderate-to-high biological effect. For consistency with the ICGC cohort, we substratified the TCGA dataset into treated and untreated samples. The untreated cohort demonstrated identical survival stratification to our discovery cohort, with the Notch group exhibiting favourable outcome and Hedgehog/Wnt exhibiting the worst. Although these results reciprocated the findings in the discovery cohort, the p-value was not significant at a threshold of 0.05, which is in part due to the small sample size and the low number of events.
Using a multivariate Cox proportional hazards regression analysis, we noticed that different pharmaceutical treatments had an effect on survival rates (ANOVA p-value = 0.04; after adjusting for gender, age, and tumour stage). We split the cohorts into “treated” and “nontreated” based on the treatment indicator in the TCGA data and observed that the prognosis for the Hedgehog/Wnt group was worse only if the patients are not treated (HR = 4.12 compared to cell cycle group; 95% CI 1.2 to 13.8, coxPH test p-value = 0.02; after correcting for gender, age, tumour stage, and radiation therapy indicator). In the vast majority of cases, gemcitabine, a known chemotherapy agent, was listed as listed as the “drug name” by itself (57%) or in combination with another chemotherapy drug (e.g. oxaliplatin, irinotecan, Abraxane) or one of the other listed drugs (39%), suggesting that chemotherapy was the basis of the “targeted therapy” indicator. However, it was not possible to obtain information about the specifics of the treatment regime, and we also noted that 12 cases annotated as “not treated” in the TCGA data were also listed as receivers of gemcitabine. Thus, our analyses are exploratory and no final conclusions are possible, yet there is some evidence that targeted therapeutics used in the Hedgehog/Wnt group show a pharmacological benefit to this group with a dismal prognosis.
We next investigated the molecular basis for these survival differences and compared the mutational patterns between the three subtypes (Fig 3C). We found that the cell cycle process had a significantly (ANOVA p-value < 0.01) higher mutational burden than the Hedgehog/Wnt and Notch processes—with an average of 9.4% of altered samples in the cell cycle group, compared to 4.6% and 3.2% in the Hedgehog/Wnt and Notch groups, respectively—when considering a panel of 39 key pancreatic cancer genes and pathways [5]. The same pattern was observed in the data from the TCGA cohort (S8 Fig).
MR Regulatory Processes Show Different Immune Activity
To understand how well the patient subtypes were represented in experimental model systems, we used the Broad Institute’s cell line resource [43] and found that all the cell lines shared the characteristics of the cell cycle process but not of the other two processes (S9 Fig). As cell lines are artificially homogeneous cell populations, we hypothesized that Hedgehog and Notch activity are determined by the tumour’s interaction with its microenvironment.
We thus focused on differences in the tumour microenvironment to explain the differences between regulatory processes and used ESTIMATE [32] to infer stromal and immune cell admixture from gene expression data. The samples in the Hedgehog and Notch processes showed a significantly higher stromal infiltration compared to the cell cycle subtype (Fig 4B) on both the discovery and validation cohort. All three subgroups showed significantly varied levels of immune cell enrichment, with Notch being the most immunogenic and cell cycle being the least. Although Hedgehog and cell cycle samples demonstrate considerable stromal infiltration, Hedgehog is significantly less immunogenic in both cohorts (ICGC: p = 1.7e–05, TCGA: p = 7.8e–08; Wilcoxon rank sum test). By contrast, the difference in stromal content between the Hedgehog and Notch classes is considerably subtler (ICGC: p = 0.0016; TCGA p = 0.97; Wilcoxon rank sum test).
Fig. 4. Subtypes show different immune activity. 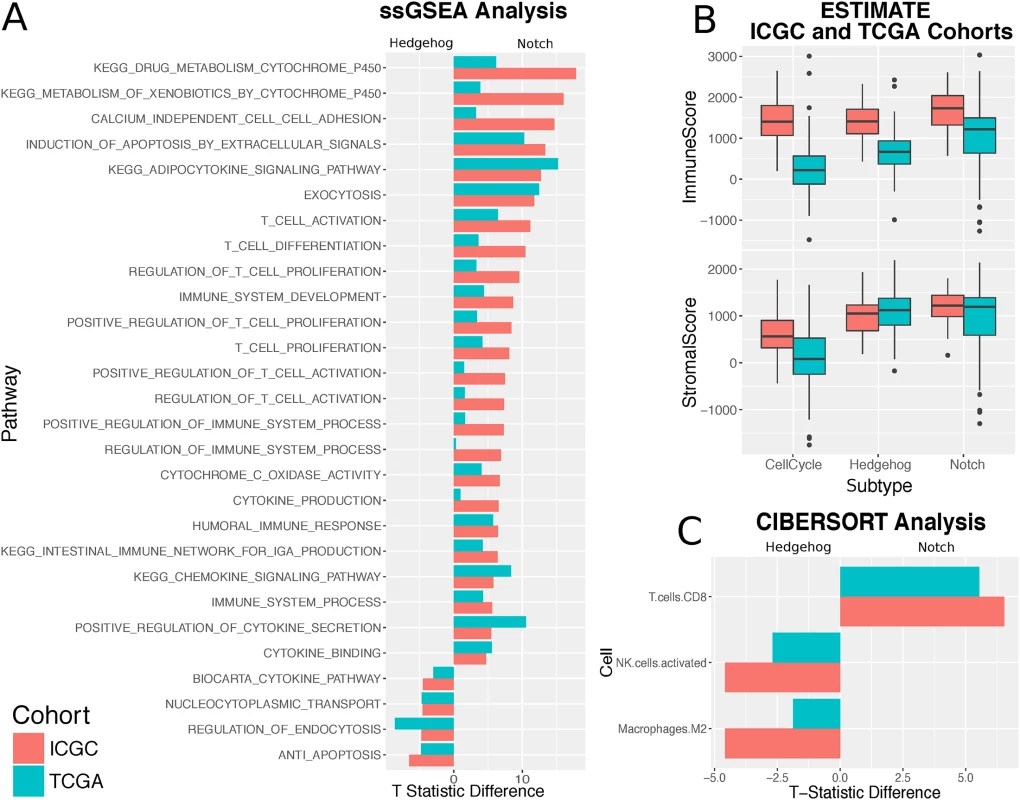
A. Bar plot showing the Pearson partial correlation t-statistic difference between Hedgehog and Notch for ssGSEA pathway enrichment scores significantly associated with one subtype or the other. B. Boxplots from the ESTIMATE analysis showing the variation in the stromal and immune content between the Hedgehog, Notch, and cell cycle subgroups for both the ICGC and TCGA cohorts. C. Bar plot showing the difference in Pearson partial correlation coefficient difference between Hedgehog and Notch for estimated CIBERSORT leukocyte cell fractions significantly associated with one subtype or the other. A negative difference highlights strong association with Hedgehog, whereas a positive value indicates a strong association with Notch. We used complementary strategies to explore the immune content of the three processes. First, we aggregated a collection of 77 immunological pathways from MSigDB. We found significant positive associations between Notch activity and 22 pathways. Of these were enrichment for T cell–related pathways, such as those pertaining to T cell activation, proliferation, and differentiation (Fig 4A). General immune system pathways, including the adaptive immune response and the positive regulation of the immune response, were similarly far more associated with Notch than Hedgehog. No significant associations were observed between T cell–related pathways and Hedgehog activity enrichment across both datasets (S10 Fig).
We then focused on dissecting the heterogeneity of leukocyte populations in the tumour samples using a leukocyte deconvolution method [32]. Both methods use a panel of cell type–specific signatures to identify which cell types are present in a mixed gene expression profile (see Methods). Results between the ICGC and TCGA cohorts correlated well, with both demonstrating the significant prevalence of infiltrating CD8+ T cells in Notch pathway–dominated samples (Fig 4C) and a dominant presence of M2 macrophages in samples exhibiting strong Hedgehog signalling.
Finally, we correlated Notch and Hedgehog activity with the expression of known immune therapy targets [44]: programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T lymphocyte–associated protein 4 (CTLA4), TIGIT, and LAG3. Across both the discovery and validation cohorts, CTLA4, PD-1, and TIGIT showed significant positive correlations with Notch activity, whereas no significant associations were observed with Hedgehog activity (S10 Fig).
Discussion
The first aim of our study was to identify transcription factors determining the transcriptional response to oncogenic KRAS. To address this aim, we used master regulator analysis to combine a murine gene expression signature of oncogenic KRAS with a coexpression network integrating data from 560 patients. We found that the KRAS-specific master regulators represented three main biological processes: Notch, repressed Hedgehog/Wnt, and the cell cycle. The second aim was to explore the extent to which activity of these biological processes had on the development of the disease and patient outcome. We demonstrated that the functional groupings of the transcription factors represented distinct clinical entities of PDAC, with differences in survival, mutational load, and immune activity. These three patient subtypes represent three routes of PDAC development, characterized by three different transcriptional programs.
Our study has several limitations. Although the number of samples studied here is large compared to those of other publications in PDAC, the size of the patient cohorts prevents strong conclusions about effect sizes and clinical impact. By reanalysing mainly published data, our study is constrained by the quantity and quality of the information provided in these studies. For example, mutational profiles were not available for all patients and the clinical information is often incomplete, which impedes the assessment of treatment effects. In addition, we did not have access to tissue sections for the analysed genomic profiles that could have been used to validate hypotheses about immune activity in the subtypes.
The groups we identified in general agreed with previous patient stratifications, with one surprising exception: the cell cycle group overlapped significantly with Bailey et al.’s immunogenic subtype [9], even though in our reanalysis of the data, these patients show little evidence for immune activity. This indicates that more work needs to be done to consolidate different stratification schemes into consensus subtypes of pancreatic cancer.
We found some evidence demonstrating the potential clinical importance of the groups we identified. Using survival data from two independent cohorts, we demonstrated that the Hedgehog process has the worst prognosis and the Notch process has the best prognosis. We demonstrated that the cell cycle process has the largest mutational burden of all the regulatory processes and has a smaller stromal composition. At the same time, the Notch and Hedgehog/Wnt groups showed substantial stromal contributions. We characterized stromal content and found M2 macrophages enriched in the Hedgehog/Wnt process and CD8+ T cells in the Notch process. Furthermore, analysis of checkpoint markers showed that the T cells collated with CTLA, PD-1, and TIGIT. This indicates that the Notch subtype is potentially amenable to immunotherapy. The Hedgehog/Wnt group, even though it has the worst prognosis, is potentially amenable to newer targeted therapies.
In summary, these examples show how the transcriptional signatures of KRAS-specific master regulators could be used in the clinic to stratify patients and guide therapeutic decisions.
Supporting Information
Zdroje
1. CRUK. Pancreatic Cancer Risks and Causes 2014. http://www.cancerresearchuk.org/about-cancer/type/pancreatic-cancer/about/pancreatic-cancer-risks-and-causes.
2. Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1109–13. doi: 10.1038/nature09460 20981101
3. Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988;16(16):7773–82. 3047672
4. Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–6. doi: 10.1126/science.1164368 18772397
5. Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169 25719666
6. Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547 23103869
7. Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor - and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47(10):1168–78. doi: 10.1038/ng.3398 26343385
8. Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–3. doi: 10.1038/nm.2344 21460848
9. Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965 26909576
10. Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. 14706336
11. Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122(2):639–53. doi: 10.1172/JCI59227 22232209
12. di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144(6):1220–9. doi: 10.1053/j.gastro.2013.01.071 23622131
13. Castro MA, de Santiago I, Campbell TM, Vaughn C, Hickey TE, Ross E, et al. Regulators of genetic risk of breast cancer identified by integrative network analysis. Nat Genet. 2016;48(1):12–21. doi: 10.1038/ng.3458 26618344
14. Fletcher MN, Castro MA, Wang X, de Santiago I, O'Reilly M, Chin SF, et al. Master regulators of FGFR2 signalling and breast cancer risk. Nat Commun. 2013;4 : 2464. doi: 10.1038/ncomms3464 24043118
15. Basso K, Margolin AA, Stolovitzky G, Klein U, Dalla-Favera R, Califano A. Reverse engineering of regulatory networks in human B cells. Nat Genet. 2005;37(4):382–90. doi: 10.1038/ng1532 15778709
16. Margolin AA, Nemenman I, Basso K, Wiggins C, Stolovitzky G, Dalla Favera R, et al. ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinformatics. 2006;7 Suppl 1:S7.
17. Schreiber FS, Deramaudt TB, Brunner TB, Boretti MI, Gooch KJ, Stoffers DA, et al. Successful growth and characterization of mouse pancreatic ductal cells: functional properties of the Ki-RAS(G12V) oncogene. Gastroenterology. 2004;127(1):250–60. 15236190
18. Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410(6832):1111–6. doi: 10.1038/35074129 11323676
19. Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–8. doi: 10.1101/gad.943001 11751630
20. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007 25605792
21. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088 23550210
22. Gautier L, Cope L, Bolstad BM, Irizarry RA. affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20(3):307–15. doi: 10.1093/bioinformatics/btg405 14960456
23. Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249 12925520
24. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–27. doi: 10.1093/biostatistics/kxj037 16632515
25. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106 20979621
26. Schafer J, Strimmer K. A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat Appl Genet Mol Biol. 2005;4:Article32. doi: 10.2202/1544-6115.1175 16646851
27. Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24(12):1461–2. doi: 10.1093/bioinformatics/btn209 18441000
28. Stouffer SA, Suchman Edward A., DeVinney Leland C., Star Shirley A., and Williams Robin M. Jr. Studies in Social Psychology in World War II: The American Soldier. Vol. 1, Adjustment During Army Life. Princeton: Princeton University Press.; 1949.
29. Alvarez MJ, Shen Y, Giorgi FM, Lachmann A, Ding BB, Ye BH, et al. Functional characterization of somatic mutations in cancer using network-based inference of protein activity. Nat Genet. 2016;48(8):838–47. doi: 10.1038/ng.3593 27322546
30. Clauset A, Newman ME, Moore C. Finding community structure in very large networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70(6 Pt 2):066111. doi: 10.1103/PhysRevE.70.066111 15697438
31. Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin). 2012;6(2):80–92.
32. Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4 : 2612. doi: 10.1038/ncomms3612 24113773
33. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–40. doi: 10.1093/bioinformatics/btr260 21546393
34. Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–7. doi: 10.1038/nmeth.3337 25822800
35. DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–9. doi: 10.1038/nature10189 21734707
36. Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55(88):2016–27. 19260470
37. Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16(3):259–66. doi: 10.1016/j.ccr.2009.07.016 19732725
38. Donahue TR, Tran LM, Hill R, Li Y, Kovochich A, Calvopina JH, et al. Integrative survival-based molecular profiling of human pancreatic cancer. Clin Cancer Res. 2012;18(5):1352–63. doi: 10.1158/1078-0432.CCR-11-1539 22261810
39. International Genomics Consortium’s (IGC) Expression Project for Oncology (expO). https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE2109
40. The Cancer Genome Atlas Data Portal 2015. http://cancergenome.nih.gov.
41. Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–47. doi: 10.1016/j.ccr.2014.04.021 24856585
42. Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci U S A. 2014;111(30):E3091–100. doi: 10.1073/pnas.1411679111 25024225
43. Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–7. doi: 10.1038/nature11003 22460905
44. Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–84. doi: 10.1038/nrd4591 26228759
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Evaluating Hospital-Based Surveillance for Outbreak Detection in Bangladesh: Analysis of Healthcare Utilization Data
- Effect of a Primary Care Walking Intervention with and without Nurse Support on Physical Activity Levels in 45- to 75-Year-Olds: The edometer nd onsultation valuation (PACE-UP) Cluster Randomised Clinical Trial
- Patient Safety Incidents Involving Sick Children in Primary Care in England and Wales: A Mixed Methods Analysis
- Biomarker-Defined Subsets of Common Diseases: Policy and Economic Implications of Orphan Drug Act Coverage
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Priority-Setting for Novel Drug Regimens to Treat Tuberculosis: An Epidemiologic Model
- Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain–Barré Syndrome: Systematic Review
- Association of Body Mass Index with DNA Methylation and Gene Expression in Blood Cells and Relations to Cardiometabolic Disease: A Mendelian Randomization Approach
- Socioeconomic Inequalities in Body Mass Index across Adulthood: Coordinated Analyses of Individual Participant Data from Three British Birth Cohort Studies Initiated in 1946, 1958 and 1970
- Mosquito-Disseminated Insecticide for Citywide Vector Control and Its Potential to Block Arbovirus Epidemics: Entomological Observations and Modeling Results from Amazonian Brazil
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- Customised and Noncustomised Birth Weight Centiles and Prediction of Stillbirth and Infant Mortality and Morbidity: A Cohort Study of 979,912 Term Singleton Pregnancies in Scotland
- Master Regulators of Oncogenic Response in Pancreatic Cancer: An Integrative Network Biology Analysis
- Sick Children Crying for Help: Fostering Adverse Event Reports
- What Is the Purpose of the Orphan Drug Act?
- Novel Vector Control Approaches: The Future for Prevention of Zika Virus Transmission?
- Artificially Sweetened Beverages and the Response to the Global Obesity Crisis
- Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp)
- Bolstering Community Cooperation in Ebola Resurgence Protocols: Combining Field Blood Draw and Point-of-Care Diagnosis
- Defining Abnormal Fetal Growth and Perinatal Risk: Population or Customized Standards?
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight
- Population Pharmacokinetic Properties of Piperaquine in Falciparum Malaria: An Individual Participant Data Meta-Analysis
- Using Genetic Variation to Explore the Causal Effect of Maternal Pregnancy Adiposity on Future Offspring Adiposity: A Mendelian Randomisation Study
- What Is the Purpose of the Orphan Drug Act?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



