-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial
Collins Iwuji and colleagues report implementation indicators and early health outcomes from the first phase of a cluster-randomized trial of immediate antiretroviral therapy to all HIV-positive individuals in rural KwaZulu-Natal, South Africa.
Published in the journal: . PLoS Med 13(8): e32767. doi:10.1371/journal.pmed.1002107
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002107Summary
Collins Iwuji and colleagues report implementation indicators and early health outcomes from the first phase of a cluster-randomized trial of immediate antiretroviral therapy to all HIV-positive individuals in rural KwaZulu-Natal, South Africa.
Introduction
Although significant gains have been made in the control of the HIV epidemic in many sub-Saharan countries, the annual number of new HIV infections remains unacceptably high [1]. Approximately 6.3 million people were estimated to be living with HIV in South Africa alone in 2013, of whom 3.1 million were on antiretroviral therapy (ART) [2]. Adult HIV prevalence in KwaZulu-Natal province in 2011 was estimated to be as high as 30% in peri-urban communities [3], making this province an ideal setting to evaluate the impact of universal test and treat on HIV incidence.
HIV viral load (VL) in HIV-positive individuals is the dominant determinant of transmission [4]. Effective ART lowers VL and thus substantially decreases the risk of HIV transmission. In heterosexual couples in stable relationships, ART provided to the HIV-positive partner with T cell lymphocyte CD4+ count between 250 and 550 cells/μl reduced transmission to the HIV-negative partner by 96% [5]. Repeat annual population-based HIV surveys in rural KwaZulu-Natal, South Africa, have shown that individual-level HIV acquisition risk decreased by 38% when ART coverage in the surrounding community increased from <10% to 30%–40% under national treatment guidelines (first ART initiation at a CD4 count of ≤200 cells/μl, then at ≤350 cells/μl) [3]. The impact on HIV transmission at the population level of ART initiation in all HIV-positive individuals soon after HIV diagnosis has not yet been evaluated in a trial setting.
Mathematical models have suggested that significant reductions in HIV transmission could be achieved with optimisation of every step of the HIV care cascade—starting with high uptake of regular HIV testing in all adults—and with immediate treatment initiation in those found to be HIV-positive [6]. The Joint United Nations Programme on HIV/AIDS (UNAIDS) has set targets that by 2020, 90% of all people living with HIV will know their HIV status, 90% of all people with diagnosed HIV infection will receive sustained ART, and 90% of all people receiving ART will achieve viral suppression (the 90-90-90 targets) [7]. However, the impact of the HIV care cascade on HIV incidence would additionally depend on the context of the sexual networks (such as heterogeneity, concurrency, and mixing) in which HIV transmission occurs [8–10].
Experience from public health HIV treatment programmes highlights the challenges of reaching high uptake of HIV testing, linkage to care, ART initiation, and long-term treatment adherence [11]. A recent meta-analysis of 28 studies that evaluated several approaches to community-based HIV testing including door-to-door HIV testing amongst 555,267 participants reported an HIV test acceptance rate of 80% (95% CI 76.9%, 83.1%), with higher HIV test acceptance in community-based than in facility-based programmes, although the former identified fewer HIV-positive people and with substantially less advanced disease [12]. There are limited data on repeat HIV testing, but one population-based study in rural Malawi reported repeat HIV testing uptake of 96% amongst participants who tested HIV-negative in a previous survey and were recontacted [13]. Linkage to care and ART initiation present further challenges in the HIV care cascade, with results from a further meta-analysis of sub-Saharan African data showing that for every 100 patients with a positive HIV test, 72 had a CD4 count performed, 40 were deemed ART-eligible by national treatment criteria, and only 25 started ART [14]. However, somewhat more positively, a recent evaluation of self-testing for HIV and linkage to care in Blantyre, Malawi, reported that 56% of individuals who tested HIV-positive linked to care within 12 mo [15]. There are little, if any, data on the acceptability and uptake of immediate ART for HIV prevention in African populations [16,17].
In early 2012, we initiated a cluster-randomised trial in rural KwaZulu-Natal to evaluate whether immediate ART in HIV-positive individuals could significantly reduce HIV incidence at the population level. At the time of implementation, similar trials were still in their planning phase [18,19]; with the complexity of implementing such a large trial and a lack of available data to inform the design and sample size, we opted to start the interventions in a limited number of clusters randomised for the main trial. We were able to evaluate process indicators such as uptake of initial and repeat home-based HIV testing, linkage to care, uptake of ART, and community attitudes and beliefs about HIV, which are important for the success of the main trial as well as for HIV treatment programmes more generally.
Methods
Ethics Statement
The trial was approved by the Biomedical Research Ethics Committee (BFC 104/11) at the University of KwaZulu-Natal and the Medicines Control Council of South Africa. (ClinicalTrials.gov: NCT01509508; South African National Clinical Trials Register: DOH-27-0512-3974). All participants provided written or witnessed thumbprint informed consent.
Study Design
ANRS 12249 TasP is a cluster-randomised trial including 22 clusters (2 × 11) at full implementation. The full trial protocol has been published previously [20]. The protocol underwent some modification in response to changes in South African national ART guidelines and to optimise trial implementation procedures by introducing an active linkage-to-care team in both arms in May 2013 to facilitate linkage to trial clinics for those not linked to care within 3 mo of being referred. These amendments were approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal and the trial data and safety monitoring board.
We here present results on process indicators from the first phase of the trial in ten (2 × 5) clusters; four (2 × 2) of the ten clusters began enrolment on 9 March 2012, and the remaining six (2 × 3) on 22 January 2013. A total of three HIV survey rounds were conducted in the initial four clusters between 9 March 2012 and 31 August 2013, and two in the remaining six clusters between 22 January 2013 and 5 April 2014. Follow-up for all those identified as HIV-positive and receiving care in trial clinics started on 9 March 2012, depending on cluster implementation date, and ended on 22 May 2014.
Trial Setting
This cluster-randomised trial was implemented in the Hlabisa sub-district of the uMkhanyakude district in northern KwaZulu-Natal, South Africa. The area is largely rural, with scattered homesteads and a national road on the boundary, adjacent to the Africa Centre for Population Health and its demographic surveillance area. It is served by the Hlabisa Department of Health HIV treatment and care programme [21].
Study Procedures
The study procedures and instruments have been fully described previously [20,22]. Procedures relevant to the first phase of the trial are summarised below.
Home-based procedures
In each home-based HIV testing and survey round, conducted every 6 mo, homestead (a bounded physical structure usually comprising one household but occasionally two or more households with different household heads) visits took place between 8 : 00 a.m. and 4 : 30 p.m. weekly from Tuesday to Saturday. Where required, separate homestead visits took place between 10 : 00 a.m. and 6 : 30 p.m. from Thursday to Sunday to accommodate adults not contacted during the standard homestead visit times, mainly students and employed individuals.
Individuals were eligible for trial participation if they were aged ≥16 y and resident (defined as spending four or more nights per week) members of a household in the designated cluster. Individuals were ineligible if they did not fulfil the criteria for residency or lacked the mental capacity to give informed consent.
Verbal consent from the homestead owner was obtained before entering any homestead. After explaining the study, each household head was asked to enumerate all eligible household members and complete a household asset form. Eligible individuals present on the day were taken to a private area and consented in writing to respond to a social and sexual behaviour questionnaire and give a finger-prick blood sample, collected on filter paper as dried blood spots (DBSs). An individual was considered a trial participant if they agreed to complete a study questionnaire, although opting out of providing DBSs was accepted. DBS samples collected longitudinally during survey rounds were tested using HIV ELISA in the Africa Centre for Population Health laboratory in Durban for identification of new cases of HIV infection, which is the primary outcome of the main trial. Researchers and study participants were blinded to these longitudinal DBS results.
HIV pretest counselling and a finger-prick rapid HIV test were offered, following a separate written consenting process, to individuals who completed the study questionnaire, using a serial HIV testing algorithm [23]. HIV 1/2 Gold Screening Test (G-Ocean, Hong Kong, China) was used as the first test, for screening, and Alere Determine HIV-1/2 (Alere, Kempton Park, South Africa) was used as the second test, for confirmation of HIV-positive results, in line with the provincial Department of Health (DoH) protocol. This protocol was later amended on 20 September 2012 to Alere Determine HIV-1/2 for screening and First Response HIV-1/HIV-2 test kit (Premier Medical Corporation, Kachigam, India) for confirmation, following changes in the DoH directive.
The result of the rapid HIV test was given to each participant, with counselling as appropriate, and further psychological support was offered for any serious distress observed. Those who newly tested HIV-positive or who self-reported being HIV-positive were referred to the TasP trial clinic in their cluster. Those who self-reported being HIV-negative were documented as having refused to be tested.
Procedures offered to eligible individuals were the same in each repeat survey round, irrespective of their HIV status recorded in the previous round.
TasP trial clinic procedures
HIV-positive participants attending TasP trial clinics were asked to provide written consent to (i) complete clinical history and examination questionnaires and provide blood specimens for VL testing and (ii) receive care as per national guidelines and ART as per cluster allocation. HIV-positive participants comprised those newly diagnosed as HIV-positive and those who self-reported as HIV-positive during home-based survey visits; they could be either ART-naïve or ART-experienced from their previous HIV care provider.
All consenting HIV-positive participants underwent clinical evaluation and a point-of-care CD4 measurement (Alere Pima CD4 test, Alere, Waltham, MA, US). All participants eligible for ART attended adherence and ART literacy sessions and initiated ART within 2 wk of the baseline visit, or sooner if severely immunocompromised. A fixed dose combination of tenofovir/emtricitabine/efavirenz (Atripla) was used for first-line ART, except if a participant’s clinical condition indicated otherwise. Second-line ART was informed by the results of genotypic resistance tests in participants failing first-line ART.
Participants receiving ART underwent monthly clinical evaluation including scheduled safety monitoring blood and HIV VL measurements (Abbott m2000 RealTime System, Abbott Molecular, Des Plaines, IL, US) at the first visit, at 3 and 6 mo after ART initiation, and every 6 mo thereafter. They were also interviewed for clinical adverse events. Unscheduled clinic visits were also allowed for participants with clinical complaints. In the control clusters, patients not yet eligible for ART were invited to return to the study clinic in 4 to 6 mo for pre-ART care, positive prevention services, repeat clinical assessment, and CD4 count measurement. Participants missing a trial clinic appointment were phoned, and, when possible, a new appointment was scheduled. Participants who failed to link to care 3 mo after being referred by the field team were contacted by a trial linkage-to-care team either by phone or through a home visit.
Intervention
In the intervention clusters, HIV-positive individuals were informed during home-based HIV testing that they would be provided ART irrespective of CD4 count and clinical stage. In the control clusters, HIV-positive individuals were informed that ART would be offered according to the criteria of the national South African guidelines: CD4 count ≤ 350 cells/μl, World Health Organization (WHO) clinical stage 3 or 4, or coinfection with multidrug resistant or extensively drug-resistant tuberculosis.
Definition of Variables and Outcomes
S1 Table presents the outcomes measured in phase 1 and presented here, and those that will be measured after trial completion.
HIV prevalence was estimated on the basis of antibody test results from the DBSs collected during the first survey round only. We estimated ART coverage at the start of the trial (proportion of all HIV-positive individuals on ART) among those with positive DBS results (first survey round) using linked information from DoH clinics and pharmacy records (ARTemis and iDART databases). Matching between the three databases was based on first names, last name, date of birth, South African ID number, and cell phone numbers.
Means and medians (interquartile ranges [IQRs]) for age at registration and other demographic characteristics were computed among individuals who completed at least one individual questionnaire at home.
Proportion of individuals contacted and whose HIV status was ascertained was computed per home-based survey round, i.e., an individual eligible in three survey rounds (taking into account round of registration and population exits), fully contacted in two rounds, but accepting an HIV rapid test only in one round will contribute three episodes in the denominator and two episodes in the numerator for estimation of contact, and two episodes in the denominator and one in the numerator for HIV ascertainment. The HIV status of an individual was ascertained if that person accepted an HIV rapid test and obtained a valid result (i.e., invalid/indeterminate results excluded) or if he/she self-reported being HIV-positive.
Rapid HIV test uptake was computed amongst individuals who were contacted, did not self-report being HIV-positive, and accepted a rapid HIV test.
Linkage to care within 6 mo was computed among individuals who were ascertained as HIV-positive at home, not previously in care (in DoH clinics in the study area), and observed at least 6 mo (taking into account population exits and the end date of data collection). Linkage to care was defined as having a first clinic visit either in a DoH clinic in the study area or a TasP clinic and was obtained by individual linkage in the databases as described above.
ART uptake within 3 mo of the first clinic visit was computed in TasP clinics only, among participants not on ART at the first clinic visit, regardless of ART eligibility criteria. ART uptake was further stratified by CD4 count at first visit in the TasP clinics.
Viral suppression was defined as having VL < 400 copies/ml on ART.
We estimated the status within the HIV care cascade (diagnosed, ever on ART, ever virally suppressed during the trial follow-up) of trial participants who were contacted and identified to be HIV-positive (through DBS results and/or HIV rapid test) who linked to TasP or DoH clinics, using linked information from DoH clinics. In order to present a population cascade, we also estimated the number of HIV-infected individuals who had not been reached by the trial field activities by applying the observed HIV prevalence (from DBS results) to the total population of registered individuals. This assumes there was no selection bias.
Attitude indicators were computed (i) among individuals who completed at least one individual questionnaire at home (the first questionnaire was used for individuals who completed several questionnaires over the trial) or (ii) among HIV-positive participants who linked to TasP clinics, at their first visit, if they were not already on ART at this first clinic visit.
Sample Size
The main trial at full implementation was 80% powered to detect an overall 34% reduction in cumulative HIV incidence over 4 y (n = 22,000; 22 clusters), with an incidence of 2.25% per year in the control clusters. The calculation made allowance for 20% loss to follow-up and assumed a coefficient of variation of 0.25 to account for variation between clusters [20]. Assumptions for attaining this incidence reduction were that the level of population contact would need to be 90%, HIV status ascertainment 80%, linkage to care 70%, and baseline HIV prevalence 24%.
Randomisation
Randomisation was performed by the trial statisticians before the start of the trial. 211 local areas were aggregated into 48 clusters. Initial sample size calculation showed that 34 clusters (2 × 17) would be required, and these were randomly allocated to the two arms, control and intervention. Randomisation was carried out within each stratum to derive an equal number of control and intervention communities per stratum. Random number generation and the randomisation procedure were performed in MapInfo version 11.0. The sample size was subsequently amended to 2 × 11 with an increase in the duration of follow-up through a revision of the protocol for the main trial. For this initial phase, only ten (2 × 5) of the 22 clusters were used. To minimise the degree of between-cluster variation, clusters were stratified on the basis of predicted HIV prevalence, extrapolating from HIV surveillance data from the Africa Centre for Population Health’s demographic surveillance area and data from antenatal clinics (six strata).
Statistical Analyses
Process indicators were summarised by arm and described according to key baseline characteristics (sex, age, education level, marital status, and professional status). They were then compared by arm using Pearson’s chi² test with Rao-Scott second-order correction, which is appropriate in the context of cluster sampling [24]. The p-values were computed with a Satterthwaite approximation to the distribution and with denominator degrees of freedom as recommended by Thomas and Rao [25] or with a design-based t-test (taking into account clustering for variance computation).
Results
Registration and Enrolment of Participants
Three consecutive rounds of home-based HIV testing were conducted in four initial clusters (2 × 2) starting 9 March 2012, and two rounds in six additional clusters (2x3) starting 22 January 2013. A total of 12,894 individuals were registered as eligible between 9 March 2012 and 22 May 2014 (Fig 1). During this period, 9,927 (77.0%) were contacted by the fieldworkers at least once, with no difference between arms (77.7% in the intervention arm and 76.4% in the control arm). Of the 9,927 individuals ever contacted, 9,490 (95.6%) agreed to complete a social and sexual behaviour questionnaire at least once, and HIV status was ascertained at least once for 82.3% (3,698/4,496) and 83.5% (4,535/5,431) in the intervention and control arms, respectively (Fig 1); this translates to ascertained HIV status for 63.9% (3,698/5,790) and 63.8% (4,535/7,104) of all registered individuals, respectively.
Fig. 1. Flow diagram of enrolment by trial arm and sex in the ANRS 12249 TasP trial. 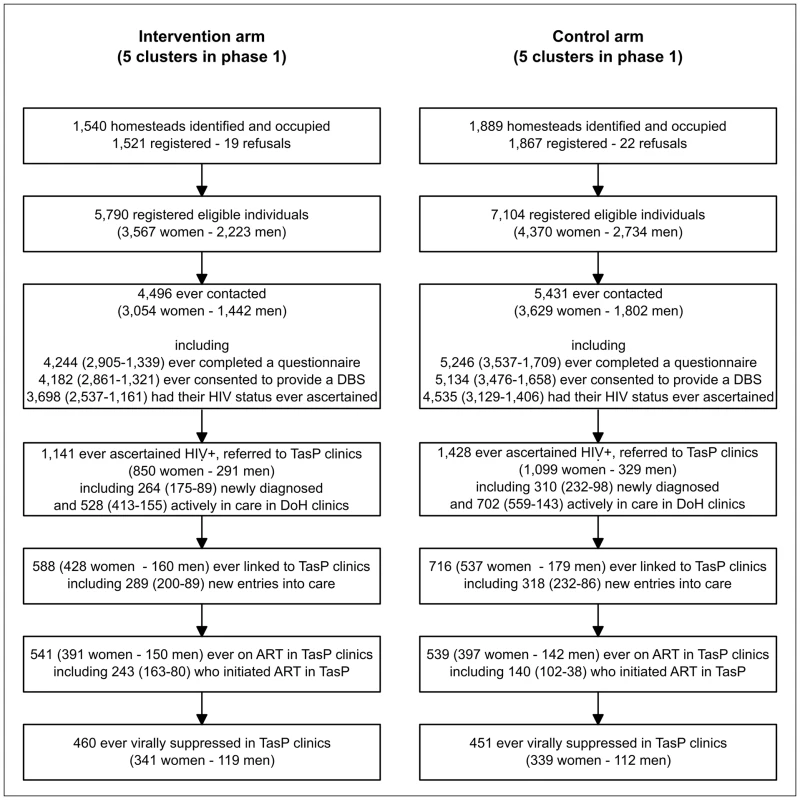
Overall, the HIV prevalence was 30.5% (95% CI 25.0%, 37.0%).
Baseline Characteristics
The median age (IQR) of participants was 32.3 y (22.1–52.4), and the majority were female (67.9%). A third of participants had primary education or less. The majority were never married; very few were formally employed. No difference was observed between arms (Table 1).
Tab. 1. Baseline characteristics of participants in intervention and control arms, first phase of the TasP ANRS 12249 trial, 2012–2014. 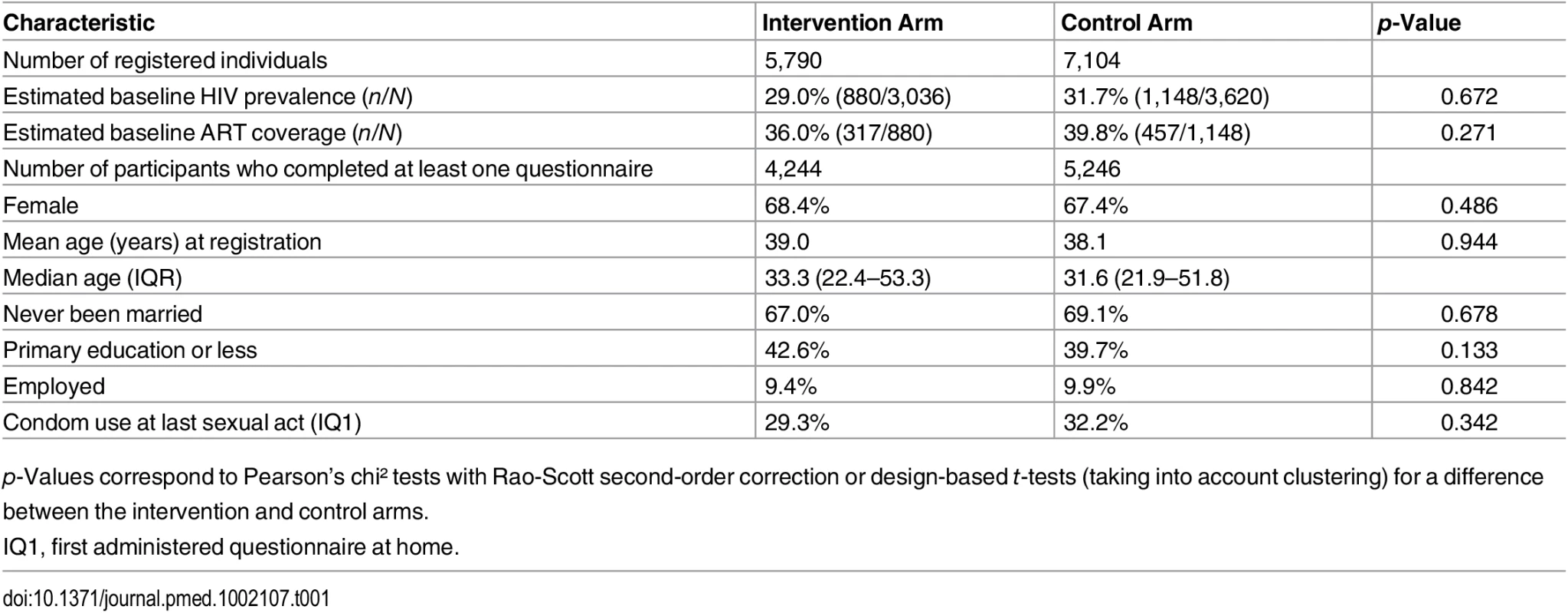
p-Values correspond to Pearson’s chi² tests with Rao-Scott second-order correction or design-based t-tests (taking into account clustering) for a difference between the intervention and control arms. Contact and Uptake of HIV Testing per Round
The proportion of registered individuals contacted per survey round was 66.8%, similar in both arms (p = 0.530) but lower in males than females (53.3% versus 75.2%). Uptake of rapid HIV test at first contact was 73.1% overall, similar in both arms (Table 2). Amongst those contacted, HIV status ascertainment (rapid HIV test uptake plus self-reported HIV-positive) per survey round was 77.6%, also similar between arms (p = 0.676) (Table 2).
Tab. 2. Process indicators by trial arm and by sex, first phase of the ANRS 12249 TasP trial, 2012–2014. 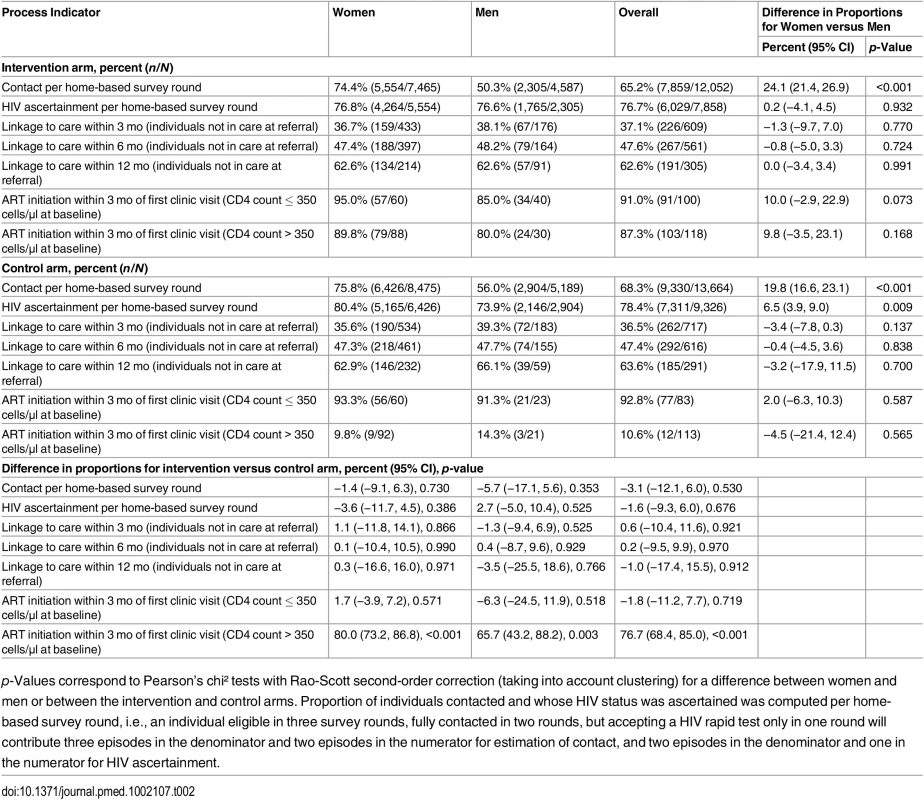
p-Values correspond to Pearson’s chi² tests with Rao-Scott second-order correction (taking into account clustering) for a difference between women and men or between the intervention and control arms. Proportion of individuals contacted and whose HIV status was ascertained was computed per home-based survey round, i.e., an individual eligible in three survey rounds, fully contacted in two rounds, but accepting a HIV rapid test only in one round will contribute three episodes in the denominator and two episodes in the numerator for estimation of contact, and two episodes in the denominator and one in the numerator for HIV ascertainment. Repeat HIV test uptake was 85.3% at second contact in those testing HIV-negative at first contact. Cumulatively in all survey rounds, 2,569 adults were ascertained as HIV-positive (942 tested HIV-positive and 1,627 reported a known HIV-positive status) and referred to TasP clinics in their cluster (Fig 1).
Amongst the 1,694 individuals who were contacted at least once but whose HIV status was never ascertained, 573 (33.8%) did not provide any reason for refusal. Amongst the remaining 1,121 individuals, 545 (48.6%) ever reported that they thought they were HIV-negative, 353 (31.5%) were afraid to test, 96 (8.6%) would test only with their partner, and 323 (28.8%) provided other reasons (overall proportion differs from 100% due to multiple reasons per individual).
Linkage to Care
Of the 2,569 adults ascertained as HIV-positive and referred to TasP clinics, 1,230 were actively engaged with care in DoH clinics at the time of referral (Fig 1). Amongst the remaining 1,339 adults, 1,177 were followed in the trial at least 6 mo after referral (Table 2). Of these, 559 (47.5%) linked to care within 6 mo of referral, with no significant difference between arms (p = 0.970). The corresponding estimate at 12 mo was 63.1% (376/596) overall, again not significantly different between arms (p = 0.912).
Uptake of ART and Retention
In the intervention arm, among participants not already on ART who were followed at least 3 mo in TasP clinics, 103/118 (87.3%) participants with CD4 count > 350 cells/μl initiated ART within 3 mo of the first clinic visit (Table 2). There was no difference between arms in the proportion of treated individuals who achieved viral suppression (448/526, 85.2%, in the intervention arm and 440/518, 84.9%, in the control arm). The median (IQR) duration on ART was 265 d (162–383).
Retention in care in trial clinics at 12 mo was 84.4%, slightly higher in the intervention than in the control arm (86.2% versus 82.5%).
HIV Care Cascade
Among an estimated total of 3,933 (30.5% HIV prevalence × 12,894) HIV-infected individuals ≥16 y living within the trial area, 2,706 (68.8%) were diagnosed (2,569 ever ascertained within TasP plus 137 not ascertained within TasP but ever in care in a DoH clinic), of whom 1,898 (70.1%) were ever in care in a TasP and/or DoH clinic, of whom 1,343 (70.8%) achieved viral suppression (Fig 2). Overall, 34.1% (1,343/3,933) of all HIV-positive individuals were ever virally suppressed.
Fig. 2. Estimated HIV care cascade among all HIV-infected individuals at the population level. 
*The number of non-observed HIV-positive individuals was estimated under the assumption that there was no selection bias, i.e., the observed HIV prevalence of 30.5% was applied to individuals whose HIV status was not observed within TasP. The 2,983 amongst the HIV-infected individuals in column 1 refers to the total number of individuals ascertained as HIV-positive within TasP in both arms of the trial, 2,569 (Fig 1), plus those who declined HIV rapid test but whose DBS HIV ELISA was positive and matched that of 137 individuals on ART within the DoH clinics. Attitude Indicators and Other Observations
Almost all participants in both arms were of the opinion that people should test regularly and agreed that they would start ART as soon as possible if diagnosed HIV-positive, with no difference by arm (Table 3).
Tab. 3. Attitudes towards repeat home-based HIV testing and early treatment by trial arm, first phase of the ANRS 12249 TasP trial, 2012–2014. 
p-Values correspond to Pearson’s chi² tests with Rao-Scott second-order correction (taking into account clustering) for a difference between the intervention and control arms. One female participant newly identified as HIV-positive among the 6,155 individuals who underwent 10,445 HIV rapid tests in the home suffered an acute adjustment reaction with suicidal intent; she was referred to a clinical psychologist for support and care. There were no reports of study-related gender-based violence, partnership dissolution, or breach of confidentiality.
Discussion
This 2-y initial phase of a trial evaluating a treatment as prevention strategy in a rural South African setting confirmed programmatic challenges in reaching optimum numbers of individuals for HIV testing at home during working hours, especially men, hindering HIV status ascertainment. However, among those contacted, uptake of initial and repeat HIV testing was high. Linkage to care in adults newly diagnosed with HIV was slower than expected, but of those who reached the trial clinic, uptake of ART was high regardless of CD4 count, with good viral suppression and retention. These observations are particularly relevant given the most recent WHO guidelines recommending ART be initiated in anyone diagnosed with HIV, irrespective of CD4 cell count [26]. We show a drop-off at each of the first two steps of the HIV care cascade, which would undermine the effectiveness of such a universal testing and treatment policy in reducing HIV transmission.
We were unable to contact one-quarter of the potential target population, especially men; however, home-based HIV testing was effective in ascertaining the HIV status of those contacted. Our results are in line with those from a meta-analysis including 28 studies that showed a pooled 80% uptake of home-based HIV testing [12]. Individuals unaware of their HIV-positive status cannot benefit from ART for their own health, and as they would remain potential transmitters, the population as a whole would not benefit either. Mobile HIV testing has recently been shown to be more efficient than home-based approaches in increasing contact and testing uptake in men [27–29] and in younger individuals and should be considered as a complementary approach in settings such as ours. Concerns about stigma and breach of confidentiality are often cited as reasons for HIV test refusal, but actual harm is rarely reported in published studies [12]. We identified only one serious adverse event following nearly 11,000 home-based tests, which highlights the quality of the pre - and post-test counselling.
First-time clinic engagement was limited, with only 47.5% of HIV-positive participants not already in care attending a trial or DoH clinic within 6 mo of referral, with 63% linkage at 12 mo. Our findings are in line with the findings from a study in Malawi of HIV self-testing and linkage to care [15] as well as those from a systematic review and meta-analysis of 11 sub-Saharan African studies, which reported that only 57% of those diagnosed HIV-positive had been linked to care [30]. The delay in accessing the trial clinics in our study may be associated with earlier HIV identification, in individuals who were asymptomatic, in home-based testing. Furthermore, our study did not show much difference in delay in accessing care between those in the intervention arm (who were told that ART would be provided to all) and those in the control arm (who were told that ART would be provided to those who were treatment-eligible). However, there were some anecdotal reports that fear of stigma may have discouraged rapid linkage to care, as trial clinics provided services only to HIV-positive individuals; ongoing social science work embedded within the trial may shed more light on this issue [22]. Further, about one-fifth of HIV-positive individuals who entered into care reported being unaware of the link between VL and HIV transmission; this highlights the need to incorporate this information into ART literacy and adherence counselling sessions.
The observed uptake of ART once linked to care was high both in treatment-eligible and not-yet-eligible individuals, with 85% of those initiating ART achieving viral suppression (VL < 400 copies/ml) in both arms. Retention was equally high in the two arms in the first year following treatment initiation. In contrast, in a study in urban Soweto, one in five HIV-positive individuals eligible for ART refused to initiate ART [31]. In that study, “feeling healthy” was the commonest reason given for ART refusal, despite a median CD4 count of 110 cells/μl and high rates of tuberculosis. In a qualitative study in Kenya to explore HIV serodiscordant couples’ attitudes toward early initiation of ART, most participants reported interest in initiating ART early, citing individual health benefits and preventing HIV transmission as motivators [16]; with side effects, lifelong adherence, and stigma emerging as potential barriers.
Overall, we estimated that only one-third of all individuals living with HIV in this population were on ART and virally suppressed. However, we were able to link data from DoH clinics only for the trial participants who were contacted and observed HIV-positive (through DBS results and/or ascertainment). We do not have this information for nearly one-quarter of individuals not reached by the trial or who refused to participate, who may be in HIV care at DoH clinics. Hence, the overall proportion of HIV-positive individuals in the population with viral suppression should be considered an underestimate, while the value of 45.0% (1,343/2,983) with viral suppression computed only among observed HIV-positive individuals constitutes an upper estimate. There remains an important gap in reaching the UNAIDS target of 73% (90% of 90% of 90%) of all people living with HIV being virally suppressed.
The 2013 WHO guidelines for ART recommended initiation at CD4 count ≤ 500 cells/μl and immediate ART initiation among specific groups including serodiscordant couples. Following two large randomised clinical trials [32,33] reporting health benefits in individuals initiating ART at higher CD4 counts, the WHO recently concluded that universal testing and treatment should become the standard of care [26]. Many African countries, including South Africa since January 2015, had already adopted the 2013 guidelines. Trials, including ours, currently underway in South Africa, Zambia, Botswana, Uganda, and Kenya that were originally designed to show a decrease in HIV incidence with ART initiated at a CD4 count of ≤350 cells/μl in the control arm have had to adapt to these expanding treatment eligibility criteria [18,19]. It is likely that when the 2015 WHO HIV treatment guidelines are adopted and implemented in these countries, the research focus in these trials may shift to evaluations of programmes that aim to achieve the WHO/UNAIDS 90-90-90 targets (90% of people living with HIV aware of their HIV status, 90% of people diagnosed HIV-positive on ART, 90% of people on ART virally suppressed) by 2020 [7], which our study shows could potentially be challenging. As these changes have not yet been implemented in South Africa, they do not affect the analysis in this paper.
Study limitations include the use of a subset of the original randomisation for the initial phase of the trial, comprising fewer clusters, but this did not seem to affect the distribution of baseline characteristics between the two arms. Although household contact rates were high, we were unable to contact all individuals identified as eligible for the trial, in particular men. Noncontact could potentially be a source of bias, if different between arms. These limitations highlight the challenges of a universal test and treat policy.
In summary, we show that home-based HIV testing was well received in this population, although men were less easily contactable during the day of home visits, and that immediate ART was acceptable, with good viral suppression and retention. However, only about half of the HIV-positive people identified accessed care within 6 mo, with nearly two-thirds by 12 mo; the improvement with time would suggest that people take time in accessing care, rather than refuse to link to care altogether.
These findings inform the now topical debate of how to identify HIV-positive people in the community and to improve the rate of linkage to care, and provide important input in further statistical projections about the burden of HIV and treatment need for populations with high HIV prevalence, such as in sub-Saharan Africa.
Supporting Information
Zdroje
1. Joint United Nations Programme on HIV/AIDS. The gap report. 2014 [cited 11 Jun 2015]. Available: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf.
2. Joint United Nations Programme on HIV/AIDS. How AIDS changed everything. MDG 6 : 15 years, 15 lessons of hope from the AIDS response. 2015 [cited 15 Oct 2015]. Available: http://www.unaids.org/sites/default/files/media_asset/MDG6Report_en.pdf.
3. Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339 : 966–971. doi: 10.1126/science.1228160 23430656
4. Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342 : 921–929. 10738050
5. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365 : 493–505. doi: 10.1056/NEJMoa1105243 21767103
6. Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373 : 48–57. doi: 10.1016/S0140-6736(08)61697-9 19038438
7. Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. 2014 [cited 22 Jan 2015]. Available: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
8. Eaton JW, Johnson LF, Salomon JA, Barnighausen T, Bendavid E, Bershteyn A, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245 22802730
9. Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. AIDS. 2010;24 : 729–735. doi: 10.1097/QAD.0b013e32833433fe 20154580
10. Hontelez JA, Lurie M, Bärnighausen T, Bakker R, Baltussen R, Tanser F, et al. Elimination of HIV in South Africa through expanded access to antiretroviral therapy: a model comparison study. PLoS Med. 2013;10:e1001534. doi: 10.1371/journal.pmed.1001534 24167449
11. Iwuji C, McGrath N, de Oliveira T, Porter K, Pillay D, Fisher M, et al. The art of HIV elimination: past and present science. J AIDS Clin Res. 2015;6 : 525.
12. Suthar AB, Ford N, Bachanas PJ, Wong VJ, Rajan JS, Saltzman AK, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10:e1001496. doi: 10.1371/journal.pmed.1001496 23966838
13. Obare F, Fleming P, Anglewicz P, Thornton R, Martinson F, Kapatuka A, et al. Acceptance of repeat population-based voluntary counselling and testing for HIV in rural Malawi. Sex Transm Infect. 2009;85 : 139–144. doi: 10.1136/sti.2008.030320 18927181
14. Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17 : 1509–1520. doi: 10.1111/j.1365-3156.2012.03089.x 22994151
15. Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. Uptake, accuracy, safety, and linkage into care over two years of promoting annual self-testing for HIV in Blantyre, Malawi: a community-based prospective study. PLoS Med. 2015;12:e1001873. doi: 10.1371/journal.pmed.1001873 26348035
16. Curran K, Ngure K, Shell-Duncan B, Vusha S, Mugo NR, Heffron R, et al. ‘If I am given antiretrovirals I will think I am nearing the grave’: Kenyan HIV serodiscordant couples’ attitudes regarding early initiation of antiretroviral therapy. AIDS. 2014;28 : 227–233. doi: 10.1097/QAD.0000000000000025 24413310
17. Fowler N, Arkell P, Abouyannis M, James C, Roberts L. Attitudes of serodiscordant couples towards antiretroviral-based HIV prevention strategies in Kenya: a qualitative study. AIDS Patient Care STDS. 2015;29 : 33–42. doi: 10.1089/apc.2014.0210 25375792
18. Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, Shanaube K, et al. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials. 2014;15 : 57. doi: 10.1186/1745-6215-15-57 24524229
19. AVAC, Joint United Nations Programme on HIV/AIDS. Antiretroviral treatment for prevention of HIV and tuberculosis: 2013 update on current and planned research efforts. 2014 Apr [cited 5 Feb 2015]. Available: http://www.avac.org/sites/default/files/resource-files/ART%20for%20prevention%20study%20update%20report%20March%202014.pdf.
20. Iwuji CC, Orne-Gliemann J, Tanser F, Boyer S, Lessells RJ, Lert F, et al. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013;14 : 230. doi: 10.1186/1745-6215-14-230 23880306
21. Houlihan CF, Bland RM, Mutevedzi PC, Lessells RJ, Ndirangu J, Thulare H, et al. Cohort profile: Hlabisa HIV treatment and care programme. Int J Epidemiol. 2011;40 : 318–326. doi: 10.1093/ije/dyp402 20154009
22. Orne-Gliemann J, Larmarange J, Boyer S, Iwuji C, McGrath N, Barnighausen T, et al. Addressing social issues in a universal HIV test and treat intervention trial (ANRS 12249 TasP) in South Africa: methods for appraisal. BMC Public Health. 2015;15 : 209. doi: 10.1186/s12889-015-1344-y 25880823
23. World Health Organization. Module 4: HIV testing strategies and algorithms. 2005 [cited 21 Feb 2016]. Available: http://www.who.int/diagnostics_laboratory/documents/guidance/pm_module4.pdf.
24. Rao JNK, Scott AJ. On chi-squared tests for multiway contigency tables with proportions estimated from survey data. Ann Stat. 1984;12 : 46–60.
25. Thomas DR, Rao JNK. Small-sample comparison of level and power for simple goodness-of-fit statistics under cluster sampling. J Am Stat Assoc. 1990;82 : 630–636.
26. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis to ART. 2015 Sep [cited 15 Oct 2015]. Available: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf?ua=1.
27. Maheswaran H, Thulare H, Stanistreet D, Tanser F, Newell ML. Starting a home and mobile HIV testing service in a rural area of South Africa. J Acquir Immune Defic Syndr. 2012;59:e43–e46. doi: 10.1097/QAI.0b013e3182414ed7 22107821
28. Labhardt ND, Motlomelo M, Cerutti B, Pfeiffer K, Kamele M, Hobbins MA, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS Med. 2014;11:e1001768. doi: 10.1371/journal.pmed.1001768 25513807
29. Coates TJ, Kulich M, Celentano DD, Zelaya CE, Chariyalertsak S, Chingono A, et al. Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014;2:e267–e277. doi: 10.1016/S2214-109X(14)70032-4 25103167
30. Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15 : 17383. doi: 10.7448/IAS.15.2.17383 23199799
31. Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25 : 2177–2181. doi: 10.1097/QAD.0b013e32834b6464 21832935
32. INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373 : 795–807. doi: 10.1056/NEJMoa1506816 26192873
33. TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373 : 808–822. doi: 10.1056/NEJMoa1507198 26193126
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Dementia across the Lifespan and around the Globe—Pathophysiology, Prevention, Treatment, and Societal Impact: A Call for Papers
- Progress and Challenges in Scaling Up Laboratory Monitoring of HIV Treatment
- Facility-Based Delivery during the Ebola Virus Disease Epidemic in Rural Liberia: Analysis from a Cross-Sectional, Population-Based Household Survey
- Availability and Use of HIV Monitoring and Early Infant Diagnosis Technologies in WHO Member States in 2011–2013: Analysis of Annual Surveys at the Facility Level
- Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States
- Improving Clinical Risk Stratification at Diagnosis in Primary Prostate Cancer: A Prognostic Modelling Study
- Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 Women of European Descent
- Social Dancing and Incidence of Falls in Older Adults: A Cluster Randomised Controlled Trial
- Genetic and Environmental Risk for Chronic Pain and the Contribution of Risk Variants for Major Depressive Disorder: A Family-Based Mixed-Model Analysis
- Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes
- On Risk Estimation versus Risk Stratification in Early Prostate Cancer
- Make Data Sharing Routine to Prepare for Public Health Emergencies
- Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis
- Concussions and Repercussions
- South Asia as a Reservoir for the Global Spread of Ciprofloxacin-Resistant : A Cross-Sectional Study
- Building from the HIV Response toward Universal Health Coverage
- Transitioning to Country Ownership of HIV Programs in Rwanda
- Accelerating the Uptake and Timing of Antiretroviral Therapy Initiation in Sub-Saharan Africa: An Operations Research Agenda
- Glycemic Control and the Risk of Tuberculosis: A Cohort Study
- Adjuvant Trastuzumab in HER2-Positive Early Breast Cancer by Age and Hormone Receptor Status: A Cost-Utility Analysis
- Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial
- An Audit and Feedback Intervention for Reducing Antibiotic Prescribing in General Dental Practice: The RAPiD Cluster Randomised Controlled Trial
- Multidrug-Resistant Tuberculosis Treatment in North Korea: Is Scale-Up Possible?
- Associations between Mental Health and Ebola-Related Health Behaviors: A Regionally Representative Cross-sectional Survey in Post-conflict Sierra Leone
- Pancreatic Cancer Surgical Resection Margins: Molecular Assessment by Mass Spectrometry Imaging
- Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1–Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda
- Measuring Burden of Unhealthy Behaviours Using a Multivariable Predictive Approach: Life Expectancy Lost in Canada Attributable to Smoking, Alcohol, Physical Inactivity, and Diet
- Comparison of Outcomes before and after Ohio's Law Mandating Use of the FDA-Approved Protocol for Medication Abortion: A Retrospective Cohort Study
- Core Outcomes for Colorectal Cancer Surgery: A Consensus Study
- Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Glycemic Control and the Risk of Tuberculosis: A Cohort Study
- Transitioning to Country Ownership of HIV Programs in Rwanda
- Dementia across the Lifespan and around the Globe—Pathophysiology, Prevention, Treatment, and Societal Impact: A Call for Papers
- Social Dancing and Incidence of Falls in Older Adults: A Cluster Randomised Controlled Trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



