-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaExercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
Moholdt and colleagues show that exercise training in pregnancy reduces incidence of gestational diabetes and blood pressure, but not weight gain.
Published in the journal: . PLoS Med 13(7): e32767. doi:10.1371/journal.pmed.1002079
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002079Summary
Moholdt and colleagues show that exercise training in pregnancy reduces incidence of gestational diabetes and blood pressure, but not weight gain.
Introduction
Maternal obesity is a risk factor for adverse pregnancy outcomes, such as gestational diabetes mellitus (GDM) [1], gestational hypertension, preeclampsia, need for cesarean delivery, and large for gestational age [2–4]. Because the prevalence of overweight and obesity among reproductive-age women is increasing, effective preventive strategies are urgently needed.
Excessive gestational weight gain (GWG) is also associated with negative obstetric outcomes [1,5,6]. The 2009 Institute of Medicine (IOM) guidelines on GWG suggest that underweight women (body mass index [BMI] ≤ 18.5 kg/m2) should gain 12.5–18.0 kg during pregnancy; normal weight women (BMI 18.5–24.9 kg/m2), 11.5–16.0 kg; overweight women (BMI 25.0–29.9 kg/m2), 7.0–11.5 kg; and obese women (BMI ≥ 30.0 kg/m2), 5.0–9.0 kg [7]. Overweight and obese women are about two times more likely than normal weight women to exceed these recommendations [8]; thus, there is a special need to find feasible and effective interventions to reduce GWG in women with a high BMI.
Previous research on clinical effects of lifestyle interventions during pregnancy in overweight/obese women has shown conflicting results [9–14]. Most studies have assessed the combined effect of physical activity and dietary guidance. To our knowledge, there are only three previous randomized controlled trials (RCTs) [14–16] assessing the isolated effects of exercise training in pregnancy on GWG and clinical outcomes in overweight and obese women. These studies found no significant difference in GWG between exercise and control groups. However, one study was limited by a small study sample (n = 12) [14], and one study reported results from only a subgroup [15].
Few studies exist on GDM prevention via exercise training in obese women [17–19], and to our knowledge no previous RCT has shown that GDM can be prevented by exercise training as the sole intervention [14,18,20,21]. However, according to a recent meta-analysis [22], structured physical exercise programs during pregnancy decrease the risk of GDM. Hence, there is still a need to establish the potential effects of exercise training on GDM prevention, and especially so in overweight/obese women.
To address the shortcomings in the research on effective prevention of GWG and of GDM, our aim was to assess whether regular supervised exercise training could reduce GWG and improve clinical outcomes, compared to standard maternity care, in women with a prepregnancy BMI of 28 kg/m2 or more.
Methods
Design and Participants
The study was approved by the Regional Committee for Medical and Health Research Ethics (REK midt 2010/1522) and registered in ClinicalTrials.gov (NCT01243554). The Exercise Training in Pregnancy (ETIP) trial was a single-center, parallel-group RCT of regular exercise training during pregnancy compared to standard maternity care in women with prepregnancy BMI ≥ 28 kg/m2. The study protocol has been published previously [23]. The trial was performed at the Norwegian University of Science and Technology (NTNU) and St. Olavs Hospital, Trondheim University Hospital, in Trondheim, Norway.
We made the following changes to the protocol after trial commencement: body composition was measured using air displacement plethysmography starting 28 June 2011, to improve assessments of body composition. The time limit for completed baseline testing and inclusion into the study was changed from gestational week 16 to gestational week 18 on 15 November 2012, and we changed the inclusion criteria for BMI from ≥30 to ≥28 kg/m2 on 22 March 2013. We changed the time limit for inclusion and the BMI criteria to increase recruitment into the trial. All changes were reported and approved by the Regional Committee for Medical and Health Research Ethics. The procedures followed in the ETIP study were in accordance with ethical standards of research and the Helsinki Declaration.
At recruitment, women received written information, and they signed informed consent on behalf of themselves and their offspring before participation and randomization. Inclusion criteria were prepregnancy BMI ≥ 28 kg/m2, age ≥ 18 y, gestational week < 18, and carrying one singleton live fetus at 11–14 wk ultrasound scan. The participants had to be able to come to St. Olavs Hospital for assessments and exercise classes. Exclusion criteria were high risk for preterm labor, diseases that could interfere with participation, and habitual exercise training (twice or more weekly) in the period before inclusion. Women were recruited through invitations sent along with notices for routine ultrasound scan at the hospital, and additionally through Google advertisements. The women received infant food worth US$65. The participants in this study gave written informed consent to publication of their case details.
Intervention and Outcomes
The exercise group was offered, in addition to standard maternity care, exercise sessions at the hospital three times weekly, from baseline (gestational week 12–18) until delivery. The exercise sessions were supervised by a physical therapist and were in accordance with the recommendations from the American College of Obstetricians and Gynecologists [24]. Each session lasted 60 min and consisted of treadmill walking/jogging for 35 min (endurance training) and resistance training for large muscle groups and the pelvic floor muscles for 25 min. The intensity of the endurance training was set to ~80% of maximal capacity (corresponding to Borg scale 12–15) [25]. The resistance training consisted of squats, push-ups, diagonal lifts on all fours, and oblique abdominal crunches, with three sets of ten repetitions of each exercise separated by a 1-min rest between sets. Participants also did three sets of the “plank exercise” for 30 s. We adjusted the program according to each woman’s strength level. The pelvic floor exercises consisted of three sets of ten repetitions of pulling the pelvic floor up and holding the contraction for 6–8 s.
In addition, the women were asked to follow a 50-min home exercise program at least once weekly (35 min of endurance training and 15 min of strength exercises) and to do daily pelvic floor muscle exercises. We registered adherence to the supervised exercise program, and the participants reported their home exercise in a training diary. The participants received a weight gain curve showing recommended weight gain throughout pregnancy in accordance to 2009 IOM guidelines [7], and were encouraged to compare their own weight gain with this curve. The women were invited to attend one motivational interview session [23], either individually or in a group, during the intervention period.
The control group received ordinary maternity care by their midwife, general practitioner, and/or obstetrician. The Norwegian national directions for standard maternity care among healthy pregnant women at the time the study was conducted included offering of an ultrasound examination by gestational week 18 and providing information about healthy eating and healthy lifestyle [26]. The women in the control group were asked to continue their normal daily activities and were not discouraged from exercising on their own.
All participants underwent the same test protocol at baseline (gestational week 12–18) and at late pregnancy (gestational week 34–37). In addition, the hospital personnel measured the women’s body weight immediately before delivery.
Our primary outcome measure was GWG calculated as the difference between weight at baseline and weight at delivery. Maternal body weight at baseline, in late pregnancy, and before delivery was measured with a calibrated electronic scale (Seca 770, Medema, Norway) to the nearest 0.1 kg, with the participant wearing indoor clothing, without shoes. If the hospital staff did not have time to measure the women’s weight right before delivery, we used women’s self-reported weight at the time of delivery to calculate the outcome measure.
Secondary outcome measures were BMI, body composition, physical activity level, skinfold thickness, blood pressure, various blood tests, incidence of GDM, and incidence of maternal hypertension in late pregnancy. Height was measured at baseline with a wall-mounted Seca 222 stadiometer. BMI was calculated as weight in kilograms divided by the square of height in meters. Systolic and diastolic blood pressure were measured on the right arm after 15 min of supine resting using a CASMED 740 MAXNIBP (CAS Medical Systems). We used the average of three measurements taken at 2-min intervals. Skinfold thickness was measured on the right side of the body at the sites subscapular, biceps, and triceps, using a Harpenden Skinfold Caliper (Holtain). We used the average of three measurements for each site. Body composition was measured using air displacement plethysmography (BOD POD, COSMED). The participant entered the BOD POD wearing only underwear and a swim cap. Physical activity level was measured by a questionnaire where the participants reported their frequency, duration, and intensity of weekly physical activity.
After a 10-h fast, we drew venous blood for fasting plasma glucose and other blood measurements. The participants then drank 75 g of glucose dissolved in 2.5 dl of water, and blood was drawn again after 2 h (120-min plasma glucose). According to the study protocol [23], GDM was to be diagnosed by the 2009 WHO definition: fasting plasma glucose ≥ 7.0 mmol/l and/or 120-min plasma glucose ≥ 7.8 mmol/l [27]. However, in 2013 WHO, in collaboration with the International Association of Diabetes and Pregnancy Study Groups (IADPSG), endorsed adjusted diagnostic criteria for classification of GDM: fasting plasma glucose ≥ 5.1 mmol/l and/or 120-min plasma glucose ≥ 8.5 mmol/l [28]. GDM is therefore reported here by both definitions. Plasma glucose, high-sensitivity C-reactive protein (CRP), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, HbA1c, ferritin, and hemoglobin were measured using a Roche Modular P. We assessed insulin with ELISA (IBL International) using a DS2 ELISA processing system (Dynex Technologies). All assays were performed according to the manufacturer’s instructions. The inter - and intra-assay coefficients of variation were 2.1% and 1.5% for glucose, 3.8% and <1% for high-sensitivity CRP, 2.5% and 0.9% for total cholesterol, 2.8% and 0.8% for HDL cholesterol, 2.4% and 0.8% for LDL cholesterol, 2.9% and 0.9% for triglycerides, and 5.3% and 9.5% for insulin. Homeostatic assessment of insulin resistance (HOMA2-IR) was calculated as [glucose × insulin]/22.5 [29].
Statistical Methods
Sample size was calculated based on prior studies [30,31] using a 6-kg clinically relevant difference in mean weight gain between the exercise and the control group, from baseline to delivery. According to this, a two-sided independent sample t-test with a 5% level of significance, a standard deviation of 10, and a power of 0.90 gave a target study population of 59 in each group. Dropout was estimated at 15%; therefore, we aimed to include 150 women.
After baseline assessments, the participants were randomly allocated 1 : 1 to the intervention or the control group. Allocation was done using a computer random number generator developed and administrated at the Unit for Applied Clinical Research, NTNU. The randomization had varying block sizes, with the first, the smallest, and the largest block defined by the computer technician at the Unit for Applied Clinical Research. The investigators enrolling the patients (K. K. G. and T. M.) got the allocation results on screen and by e-mail after registration of each new participant into the study and did not have the full randomization list available.
Weight measurement at delivery and blood analyses were done by personnel blinded for group allocation. All other assessments and intervention administration were done non-blinded. The statistician conducting the statistical analyses was blinded for group allocation.
The trial and the principal analyses were based on intention to treat. All available data were used at all time points. We also performed, as described in the original protocol, per protocol analyses including only the women in the exercise group who adhered to the exercise protocol [23]. Baseline data were tested for normality and analyzed by an independent sample t-test and by Fisher’s exact test.
The outcome measurements were analyzed in accordance to the treatment arm to which patients were randomized, regardless of nonadherence. The effect of treatment on the primary and secondary outcomes was assessed with mixed linear models for continuous outcomes and mixed logistic models for dichotomous outcomes. For the primary outcome, the effect of time and treatment was taken as a fixed effect having the levels baseline, training late pregnancy, control late pregnancy, training delivery, and control delivery. For the secondary outcomes, the effect of time and treatment was taken as a fixed effect having the levels baseline, training late pregnancy, and control late pregnancy. Due to randomization, no systematic differences between groups at baseline were assumed. To account for repeated measurements, participant ID was included as a random effect. The analyses were performed using R version 2.13.1, Stata version 13.1, and IMB SPSS Statistics 22. All results are given as mean values with 95% confidence intervals, and p-values less than 0.05 were considered significant. We did supplementary analyses of GWG where we adjusted for gestational age at delivery.
Per protocol analyses [23] including only the women in the exercise group who adhered to the exercise protocol were performed on both primary and secondary outcomes. Adherence to the exercise protocol was defined as (1) attending ≥ 42 organized exercise sessions, (2) attending ≥ 28 exercise sessions + performing ≥ 28 home exercise sessions, or (3) performing ≥ 60 home exercise sessions. The exercise had to be ≥50 min of either aerobic or strength training to count as a home session.
Results
Fig 1 outlines the flow of participants during the trial.
Fig. 1. Flow chart of the ETIP study. 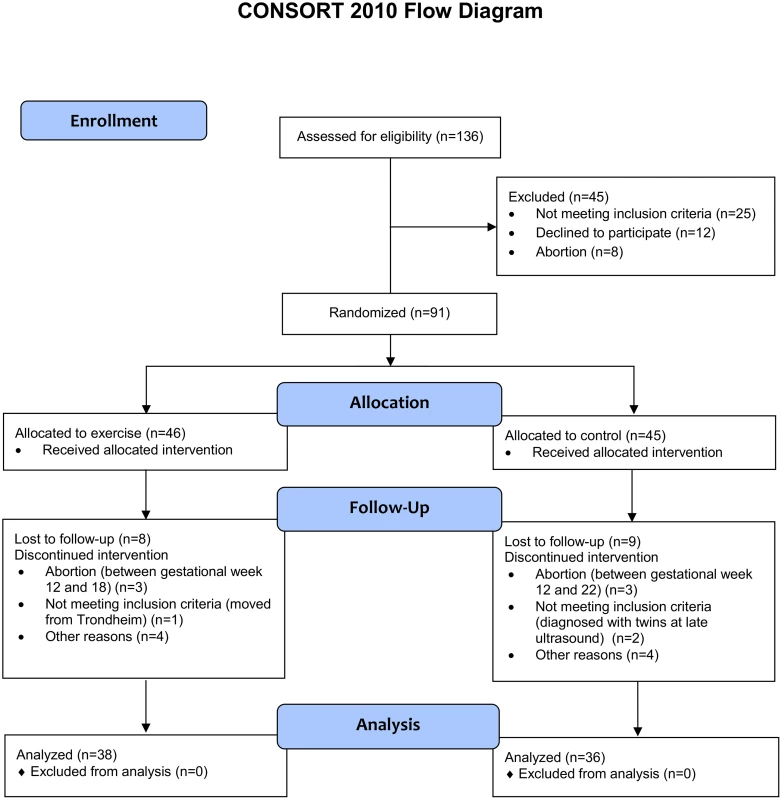
Recruitment started on 20 September 2010 and was continued until 1 March 2015. The final data collection date for the primary outcome measure was 20 June 2015. The aim of our study was to include 150 participants, but enrollment was stopped on 1 March 2015 at 91 randomized participants, due to the prolonged time for inclusion and fewer eligible participants than expected. Table 1 shows the baseline characteristics of the participants.
Tab. 1. Baseline characteristics of all women included in the ETIP study. 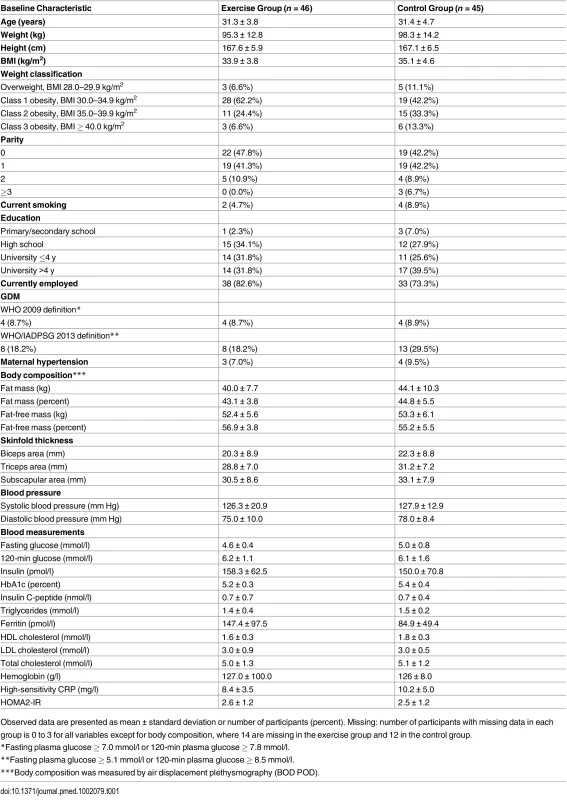
Observed data are presented as mean ± standard deviation or number of participants (percent). Missing: number of participants with missing data in each group is 0 to 3 for all variables except for body composition, where 14 are missing in the exercise group and 12 in the control group. There were no significant differences between groups at baseline, except from mean fasting glucose (4.6 mmol/l in the exercise group, 5.0 mmol/l in the control group; p = 0.02). Table 2 shows the model-based analyses for the continuous primary and secondary outcomes. The mean number of weeks from inclusion to delivery was 23.3 (range 10–28) in the exercise group and 24.7 (range 19–30) in the control group. Mean gestational age was 39.5 wk (range 27–42 wk) in the exercise group and 39.4 wk (range 37–42) in the control group.
Tab. 2. Primary and secondary outcomes in late pregnancy and at delivery. 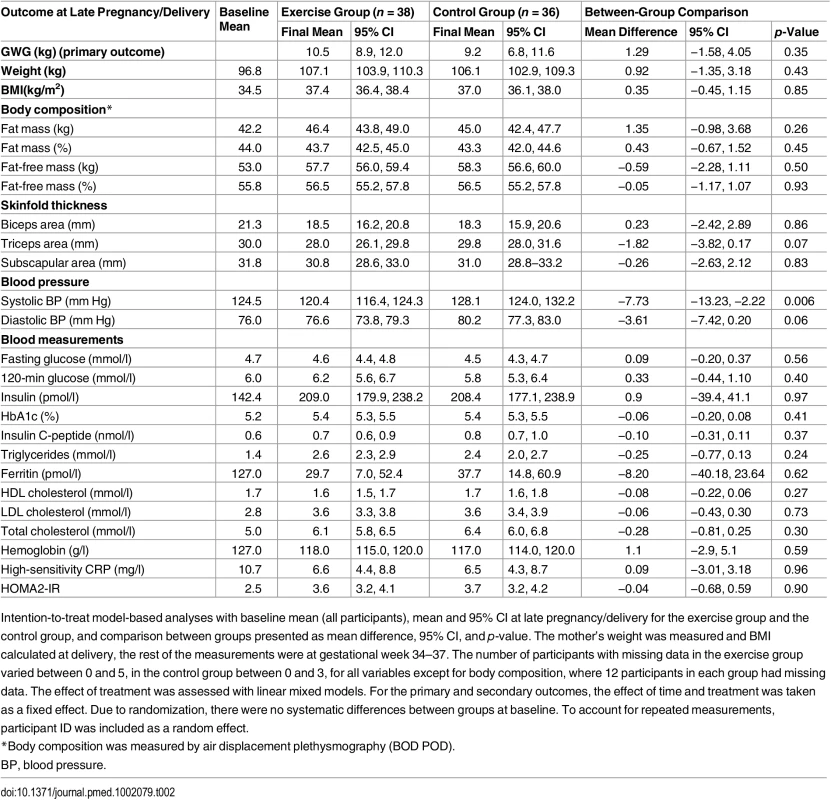
Intention-to-treat model-based analyses with baseline mean (all participants), mean and 95% CI at late pregnancy/delivery for the exercise group and the control group, and comparison between groups presented as mean difference, 95% CI, and p-value. The mother’s weight was measured and BMI calculated at delivery, the rest of the measurements were at gestational week 34–37. The number of participants with missing data in the exercise group varied between 0 and 5, in the control group between 0 and 3, for all variables except for body composition, where 12 participants in each group had missing data. The effect of treatment was assessed with linear mixed models. For the primary and secondary outcomes, the effect of time and treatment was taken as a fixed effect. Due to randomization, there were no systematic differences between groups at baseline. To account for repeated measurements, participant ID was included as a random effect. Gestational Weight Gain
We found no significant differences in GWG between the exercise group and the control group (Table 2). Body weight at delivery was self-reported by five women in the exercise group and four women in the control group. The proportion of women exceeding the IOM guidelines for recommended GWG was similar in the two groups (Table 3). Adjusting for gestational age in the analyses did not affect the GWG comparison between groups significantly (mean difference 0.56, p = 0.67).
Tab. 3. Secondary outcomes in late pregnancy and at delivery. 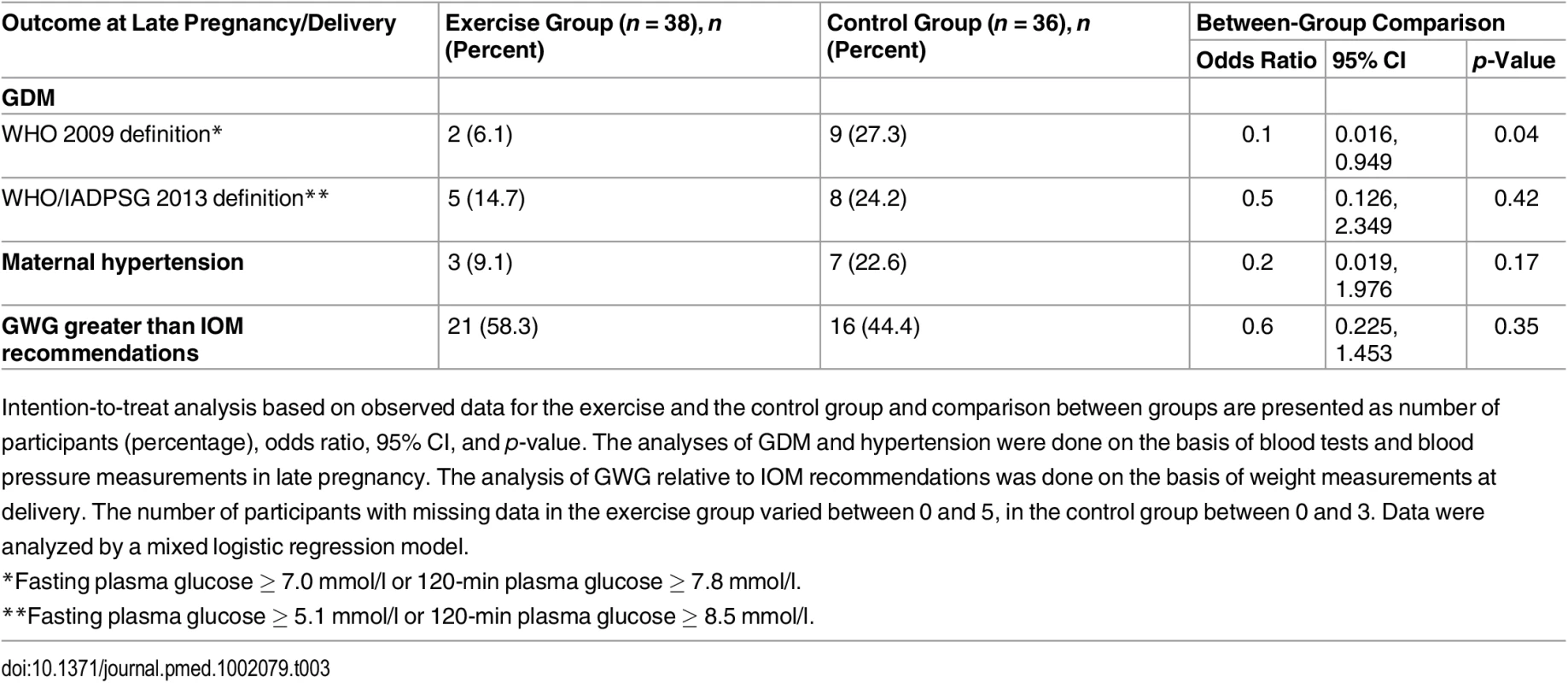
Intention-to-treat analysis based on observed data for the exercise and the control group and comparison between groups are presented as number of participants (percentage), odds ratio, 95% CI, and p-value. The analyses of GDM and hypertension were done on the basis of blood tests and blood pressure measurements in late pregnancy. The analysis of GWG relative to IOM recommendations was done on the basis of weight measurements at delivery. The number of participants with missing data in the exercise group varied between 0 and 5, in the control group between 0 and 3. Data were analyzed by a mixed logistic regression model. Gestational Diabetes Mellitus
In late pregnancy, two women (6.1%) in the exercise group and nine women (27.3%) in the control group had developed GDM according to the WHO 2009 definition [27], with a statistical difference between groups (p = 0.04; Table 3). According to the WHO/IADPSG 2013 definition of GDM [28], there was no significant difference between the groups (Table 3). There was no significant difference in fasting glucose, 120-min glucose, insulin, or HbA1c level between the groups (Table 2).
Blood Pressure and Other Secondary Outcomes
In late pregnancy we found a significantly lower systolic blood pressure (p = 0.006) in the exercise group compared to the control group (Table 2). There were no significant differences in other secondary outcome measures (Tables 2 and 3).
Physical Activity
The proportion of women reporting to be physically active for at least 30 min each day in late pregnancy was equal in the two groups: 61% in the exercise group and 66% in the control group (p = 0.73). The proportion of women reporting regular exercise training in late pregnancy was significantly higher in the exercise than in the control group: 77% and 23%, respectively (p < 0.01).
Per Protocol Analyses
In the exercise group, 50% of the women fulfilled the training intervention as described in the study protocol [23]. In the per protocol analyses, we found no significant difference in weight gain and mean weight at delivery between the per protocol exercise group and the control group (S1 Table). Resting systolic and diastolic blood pressure were significantly lower in the per protocol exercise group (115.7 mm Hg/75.1 mm Hg) compared to the control group (128.1 mm Hg/80.2 mm Hg), with p = 0.001 and p = 0.02, respectively. A tendency toward lower incidence of GDM (5.9% in the per protocol exercise group, 27.3% in the control group, p = 0.11) and maternal hypertension (11.1% in the per protocol exercise group, 21.2% in the control group, p = 0.14) was seen in the per protocol exercise group (S2 Table).
Adverse Events
No adverse events were reported during the exercise training or study assessments (Table 4).
Tab. 4. Abortions, premature deliveries, and other adverse events occurring during follow-up. 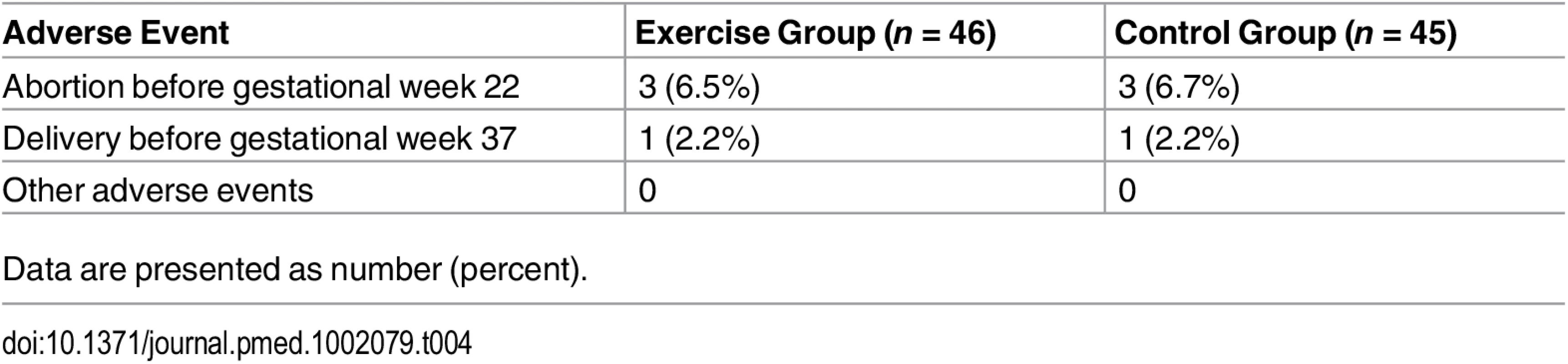
Data are presented as number (percent). Discussion
Main Findings
We found no difference in GWG between women randomized to an exercise training program versus standard maternity care, but found an apparent reduction in the incidence of GDM and lower systolic blood pressure in late pregnancy among the women randomized to the exercise training program. In the per protocol analyses including only the women who had adhered to the exercise program (n = 19), exercise training also seemed to reduce diastolic blood pressure in late pregnancy.
Gestational Weight Gain
Our findings of no difference in GWG and body composition between groups are in line with several other clinical trials on overweight or obese pregnant women [14–16,32–34]. However, a systematic review by Sui and Dodd [20] that included 216 participants (five randomized trials) found that supervised exercise interventions were associated with lower GWG among overweight or obese pregnant women. But the trials included in this systematic review differed with respect to the type and duration of exercise, and a clinically relevant difference in weight gain was not precalculated. Also Barakat et al. [35] and Haakstad and Bø [36] found significantly lower GWG among women who participated in supervised exercise during pregnancy. However, these two studies included women from all weight classes, and their findings might not translate specifically to overweight/obese women. We can only speculate about why there was no difference in GWG between the two groups in the ETIP study. The proportion of women whose self-reported activity level fulfilled the recommended 30 min of daily physical activity in late pregnancy was higher in the exercise group, but some of the women in the control group exercised on their own. Only 50% of the women in the exercise group adhered to the exercise protocol as prescribed a priori [23]. A possible effect of regular exercise during pregnancy may have been missed in our study due to the relatively low adherence to the training protocol. Protocol adherence is a challenge in all exercise studies. We tried to improve adherence by offering motivational talks throughout the intervention period, as well as adjusting the training times so that more women would be able to attend. The low adherence may have been due to pregnancy symptoms such as tiredness and nausea, limited previous experience with exercise training, or difficulties in prioritizing time for exercise. Furthermore, the intervention protocol might have been too comprehensive for these women. Further studies should carefully consider how exercise adherence can be obtained in this population.
Although the exercise training program in our study followed the current recommendations for exercise in pregnancy, it is possible that the exercise frequency and/or intensity of our program were not sufficient to affect the outcome measures related to weight gain. As our study population had a relatively low fitness level, the amount of energy spent during the exercise sessions was rather low (~400 kcal/session) and was probably not sufficient to affect the energy balance significantly. It is also possible that some of the women in the exercise group compensated for energy expenditure during the exercise sessions either by decreasing their physical activity level during the remaining time of the week [37] or by increasing their energy intake [38]. According to three recent meta-analyses [32,39,40], interventions combining physical activity and diet have proven effective in reducing GWG in overweight and obese women. We did not include any dietary advice or intervention in our study, and probably exercise training alone is not sufficient to reduce GWG in this population.
Changes in body composition throughout pregnancy might be an important determinant of glucose metabolism. Few studies have assessed body composition changes after exercise in pregnancy. Our findings of no significant differences between groups in body composition in late pregnancy are in line with a recent RCT on the effects of a 16-wk moderate intensity cycling program in overweight and obese pregnant women [33].
Gestational Diabetes Mellitus
Our finding of an apparently lower incidence of GDM according to the WHO 2009 definition [27] among the women in the exercise group is in line with a recent meta-analysis of 13 RCTs [22] that concluded that structured moderate intensity exercise programs during pregnancy decrease the risk of GDM. However, two previous Cochrane reviews, one on exercise as the sole intervention [19] and one on both diet and exercise interventions [41], concluded that there is no clear GDM risk reduction after exercise training. Nobles et al. [42] randomized 251 women with increased risk of GDM to either exercise training or a comparison health and wellness group and found no reduction in GDM risk after exercise, in line with another previous review [20]. The recently published DALI Lifestyle Pilot study [43] found that women with BMI ≥ 29 kg/m2 randomized to a healthy eating intervention had significantly lower fasting glucose and 2-h insulin concentrations than women in an exercise only group. In contrast to the DALI study, our results indicate that exercise training alone may be sufficient to prevent glucose intolerance in overweight or obese pregnant women. An important difference between the DALI study and ours is that the exercise training was supervised in our study.
Using the WHO/IADPSG 2013 definition [28] of GDM in the ETIP study, the number of GDM cases increased in both groups, and there was no longer a significant difference between the groups. The WHO/IADPSG 2013 definition is mainly based on the HAPO study (2008) [44], which found strong associations between glucose levels below the WHO 2009 diagnostic definitions and adverse outcomes for both mother and child. However, a retrospective cohort study [45] that included 1,892 women diagnosed with GDM according to the WHO/IADPSG 2013 definition found a significantly higher risk for adverse pregnancy outcomes in those who also would be diagnosed as having GDM according to the WHO 2009 definition.
Despite the difference between the exercise and control groups in GDM incidence, we found no differences between the groups at late pregnancy in glucose levels, insulin, or HOMA2-IR. One possible reason for this finding is that women with high risk of GDM may respond differently to exercise training than women with lower risk [46], such that the average glucose and insulin levels are not sufficiently affected to obtain a difference between groups.
Blood Pressure
We found a significantly lower systolic blood pressure among the women in the exercise group in late pregnancy, compared to the women in the control group. Diastolic blood pressure did not differ between groups in late pregnancy in the intention-to-treat analysis, but was significantly lower in the exercise group in the per protocol analysis. High blood pressure in pregnancy is associated with increased risk for preeclampsia [47] and thus is important to prevent. To our knowledge, only one previous RCT [33] has studied the effect of exercise training in pregnancy on exact blood pressure measurements among overweight/obese women. Seneviratne et al. [33] found no effect of exercise training on blood pressure in late pregnancy. Other studies that have assessed the effects of exercise on maternal hypertension risk have assessed hypertension as a dichotomous variable [34,35,39]. Some of these studies found no effect of exercise [17,34], but one study [35] found a reduced incidence of maternal hypertension after exercise training. The latter study included both normal weight, overweight, and obese women. Although fewer women in the exercise group than in the control group had hypertension in late pregnancy in our study, the difference was not statistically significant. Further studies are needed to ascertain whether exercise training can prevent hypertensive pregnancies in overweight/obese women.
Generalizability
The ETIP study had few exclusion criteria, and the participants were representative of Norwegian women with BMI ≥ 28 kg/m2 regarding age, parity, and education. However, participants had to have time available for the testing and training. The exercise group was offered training sessions at day and evening times. It is also possible that the participants volunteering for the ETIP study were extra aware of the possible beneficial effects of exercise training in pregnancy and thus were motivated to participate in our trial.
Clinical Relevance
Obese women have elevated risk of GDM and maternal hypertension; thus, finding effective prevention strategies is highly relevant. The study revealed no adverse events related to moderate physical activity during pregnancy. The effect of exercise training to reduce weight gain may most likely be improved with additional dietary interventions. During the study we experienced difficulties in motivating the women in the exercise group to adhere to the training program, despite supervised training sessions at St. Olavs Hospital, training sessions at different times during the week, and individually adjusted exercises. We think further studies should evaluate how supervised exercise programs for obese women can be implemented in the health care system, as well as how to obtain good adherence to such programs.
Strengths
In our study, exercise training was the only intervention provided. This makes it easier to assess the isolated effects of exercise on pregnancy outcomes. The training program being standardized and supervised makes it easy to reproduce. Furthermore, we had thorough recording of exercise adherence as well as physical activity levels in the two groups. The primary outcome measure (GWG) was assessed by personnel blinded for group allocation. We also regard the assessment of body composition with the gold standard method of air displacement plethysmography as a strength.
Limitations
The main limitation of the trial was the reduced statistical power because we were able to include only 2/3 of the 150 participants estimated in the power calculation. We analyzed 30 different secondary outcomes among a limited number of women, and thereby increased the risk for detecting differences between groups by chance, and making type 1 errors. Furthermore, only 50% of the participants in the exercise group performed the exercise training program per protocol, which makes it more difficult to detect possible effects of the intervention. However, adherence to exercise in the ETIP study was similar to that in most of the comparable clinical studies. Care must be taken in interpreting the results from the per protocol analysis. Such analyses could be selection biased if the reasons influencing compliance with the exercise training program are associated with prognostic factors [48].
Conclusion
In this trial we did not observe a reduction of GWG or an improvement in body composition among overweight/obese women who were offered supervised exercise training during pregnancy. However, exercise training seemed to reduce the incidence of GDM as well as systolic blood pressure in late pregnancy. As exercise adherence is a major challenge in this population, there is a special need to find methods to reduce participant attrition in future studies.
Supporting Information
Zdroje
1. Brunner S, Stecher L, Ziebarth S, Nehring I, Rifas-Shiman SL, Sommer C, et al. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia. 2015;58 : 2229–2237. doi: 10.1007/s00125-015-3686-5 26141788
2. Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001;91 : 436–440. 11236410
3. Martin KE, Grivell RM, Yelland LN, Dodd JM. The influence of maternal BMI and gestational diabetes on pregnancy outcome. Diabetes Res Clin Pract. 2015;108 : 508–513. doi: 10.1016/j.diabres.2014.12.015 25796512
4. Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. 2010;10 : 56. doi: 10.1186/1471-2393-10-56 20849609
5. Oken E, Kleinman KP, Belfort MB, Hammitt JK, Gillman MW. Associations of gestational weight gain with short - and longer-term maternal and child health outcomes. Am J Epidemiol. 2009;170 : 173–180. doi: 10.1093/aje/kwp101 19439579
6. Crane JM, White J, Murphy P, Burrage L, Hutchens D. The effect of gestational weight gain by body mass index on maternal and neonatal outcomes. J Obstet Gynaecol Can. 2009;31 : 28–35. 19208280
7. Rasmussen KM, Abrams B, Bodnar LM, Butte NF, Catalano PM, Maria Siega-Riz A. Recommendations for weight gain during pregnancy in the context of the obesity epidemic. Obstet Gynecol. 2010;116 : 1191–1195. doi: 10.1097/AOG.0b013e3181f60da7 20966705
8. Cedergren M. Effects of gestational weight gain and body mass index on obstetric outcome in Sweden. Int J Gynaecol Obstet. 2006;93 : 269–274. doi: 10.1016/j.ijgo.2006.03.002 16626716
9. Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr. 2010;91 : 373–380. doi: 10.3945/ajcn.2009.28166 19955397
10. Bogaerts AF, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh BR. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes (Lond). 2013;37 : 814–821. doi: 10.1038/ijo.2012.162
11. Harrison CL, Lombard CB, Strauss BJ, Teede HJ. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: a randomized controlled trial. Obesity (Silver Spring). 2013;21 : 904–909. doi: 10.1002/oby.20163
12. Petrella E, Malavolti M, Bertarini V, Pignatti L, Neri I, Battistini NC, et al. Gestational weight gain in overweight and obese women enrolled in a healthy lifestyle and eating habits program. J Matern Fetal Neonatal Med. 2014;27 : 1348–1352. doi: 10.3109/14767058.2013.858318 24175912
13. Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jorgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care. 2011;34 : 2502–2507. doi: 10.2337/dc11-1150 21972411
14. Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab. 2009;35 : 418–421. doi: 10.1016/j.diabet.2009.04.008 19747869
15. Choi J, Fukuoka Y, Lee JH. The effects of physical activity and physical activity plus diet interventions on body weight in overweight or obese women who are pregnant or in postpartum: a systematic review and meta-analysis of randomized controlled trials. Prev Med. 2013;56 : 351–364. doi: 10.1016/j.ypmed.2013.02.021 23480971
16. Seneviratne SN, Jiang Y, Derraik J, McCowan L, Parry GK, Biggs JB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG. 2016;123 : 588–597. doi: 10.1111/1471-0528.13738 26542419
17. Simmons D, Jelsma JG, Galjaard S, Devlieger R, van Assche A, Jans G, et al. Results From a European multicenter randomized trial of physical activity and/or healthy eating to reduce the risk of gestational diabetes mellitus: the DALI Lifestyle Pilot. Diabetes Care. 2015;38 : 1650–1656. doi: 10.2337/dc15-0360 26112044
18. Barakat R, Pelaez M, Montejo R, Luaces M, Zakynthinaki M. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol. 2011;204 : 402e1–7. doi: 10.1016/j.ajog.2011.01.043
19. Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2012;7:CD009021. doi: 10.1002/14651858.CD009021.pub2 22786521
20. Sui Z, Dodd JM. Exercise in obese pregnant women: positive impacts and current perceptions. Int J Womens Health. 2013;5 : 389–398. doi: 10.2147/IJWH.S34042 23861603
21. Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33 : 1457–1459. doi: 10.2337/dc09-2336 20357374
22. Sanabria-Martinez G, Garcia-Hermoso A, Poyatos-Leon R, Alvarez-Bueno C, Sanchez-Lopez M, Martinez-Vizcaino V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG. 2015;122 : 1167–1174. doi: 10.1111/1471-0528.13429 26036300
23. Moholdt TT, Salvesen K, Ingul CB, Vik T, Oken E, Morkved S. Exercise Training in Pregnancy for Obese Women (ETIP): study protocol for a randomised controlled trial. Trials. 2011;12 : 154. doi: 10.1186/1745-6215-12-154 21682869
24. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37 : 6–12. 12547738
25. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2 : 92–98. 5523831
26. Helsedirektoratet. Svangerskapsomsorgen: nasjonal faglig retningslinje for svangerskapsomsorgen. IS-1179. 2005 May 1 [cited 15 May 2016]. Available: https://helsedirektoratet.no/retningslinjer/nasjonal-faglig-retningslinje-for-svangerskapsomsorgen.
27. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33 : 676–682. doi: 10.2337/dc09-1848 20190296
28. World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: World Health Organization; 2013.
29. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28 : 412–419. 3899825
30. Christiansen T, Paulsen SK, Bruun JM, Pedersen SB, Richelsen B. Exercise training versus diet-induced weight-loss on metabolic risk factors and inflammatory markers in obese subjects: a 12-week randomized intervention study. Am J Physiol Endocrinol Metab. 2010;298:E824–E831. doi: 10.1152/ajpendo.00574.2009 20086201
31. Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond). 2008;32 : 495–501. doi: 10.1038/sj.ijo.0803710
32. Agha M, Agha RA, Sandall J. Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: a systematic review and meta-analysis. PLoS ONE. 2014;9:e95132. doi: 10.1371/journal.pone.0095132 24827704
33. Seneviratne SN, Jiang Y, Derraik J, McCowan L, Parry GK, Biggs JB, et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: a randomised controlled trial. BJOG. 2015;123 : 588–597. doi: 10.1111/1471-0528.13738 26542419
34. Ruiz JR, Perales M, Pelaez M, Lopez C, Lucia A, Barakat R. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc. 2013;88 : 1388–1397. doi: 10.1016/j.mayocp.2013.07.020 24290112
35. Barakat R, Pelaez M, Cordero Y, Perales M, Lopez C, Coteron J, et al. Exercise during pregnancy protects against hypertension and macrosomia: randomized clinical trial. Am J Obstet Gynecol. 2015;214 : 649.e1–8. doi: 10.1016/j.ajog.2015.11.039
36. Haakstad LA, Bø K. Effect of regular exercise on prevention of excessive weight gain in pregnancy: a randomised controlled trial. Eur J Contracept Reprod Health Care. 2011;16 : 116–125. doi: 10.3109/13625187.2011.560307 21417561
37. Thivel D, Aucouturier J, Metz L, Morio B, Duche P. Is there spontaneous energy expenditure compensation in response to intensive exercise in obese youth? Pediatr Obes. 2014;9 : 147–154. doi: 10.1111/j.2047-6310.2013.00148.x 23447495
38. Finlayson G, Bryant E, Blundell JE, King NA. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol Behav. 2009;97 : 62–67. doi: 10.1016/j.physbeh.2009.02.002 19419671
39. Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015;6:CD007145. doi: 10.1002/14651858.CD007145.pub3 26068707
40. Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012;10 : 47. doi: 10.1186/1741-7015-10-47 22574949
41. Bain E, Crane M, Tieu J, Han S, Crowther CA, Middleton P. Diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database Syst Rev. 2015;4:CD010443. doi: 10.1002/14651858.CD010443.pub2 25864059
42. Nobles C, Marcus BH, Stanek EJ 3rd, Braun B, Whitcomb BW, Solomon CG, et al. Effect of an exercise intervention on gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2015;125 : 1195–1204. doi: 10.1097/aog.0000000000000738 25932848
43. Simmons D. Prevention of gestational diabetes mellitus: where are we now? Diabetes Obes Metab. 2015;17 : 824–834. doi: 10.1111/dom.12495 25974384
44. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358 : 1991–2002. doi: 10.1056/NEJMoa0707943 18463375
45. Sacks DA, Black MH, Li X, Montoro MN, Lawrence JM. Adverse pregnancy outcomes using the International Association of the Diabetes and Pregnancy Study Groups criteria: glycemic thresholds and associated risks. Obstet Gynecol. 2015;126 : 67–73. doi: 10.1097/aog.0000000000000865 26241258
46. Ruchat SM, Davenport MH, Giroux I, Hillier M, Batada A, Sopper MM, et al. Effect of exercise intensity and duration on capillary glucose responses in pregnant women at low and high risk for gestational diabetes. Diabetes Metab Res Rev. 2012;28 : 669–678. doi: 10.1002/dmrr.2324 22865627
47. Torloni MR, Betran AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obesity Rev. 2009;10 : 194–203. doi: 10.1111/j.1467-789X.2008.00541.x
48. Hernan MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9 : 48–55. doi: 10.1177/1740774511420743 21948059
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- The Clinical and Public Health Challenges of Diabetes Prevention: A Search for Sustainable Solutions
- Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
- Diabetes: A Cinderella Subject We Can’t Afford to Ignore
- Prescribing Exercise and Lifestyle Training for High Risk Women in Pregnancy and Early Post-partum—Is It Worth It?
- Population Approaches to Prevention of Type 2 Diabetes
- First-Year Evaluation of Mexico’s Tax on Nonessential Energy-Dense Foods: An Observational Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
- Leveraging Genetics to Advance Type 2 Diabetes Prevention
- Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
- Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
- Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
- Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients
- Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
- Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women
- Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study
- Engagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Cycling and Diabetes Prevention: Practice-Based Evidence for Public Health Action
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



