-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
In a meta-analysis of observational and randomized studies, Uma Mudaliar and colleagues document the effects on weight and cardiovascular indicators of diet and behavior modification interventions based on the Diabetes Prevention Program.
Published in the journal: . PLoS Med 13(7): e32767. doi:10.1371/journal.pmed.1002095
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002095Summary
In a meta-analysis of observational and randomized studies, Uma Mudaliar and colleagues document the effects on weight and cardiovascular indicators of diet and behavior modification interventions based on the Diabetes Prevention Program.
Introduction
Diabetes currently affects approximately 9.3% of the United States population [1], and by 2050, its prevalence is expected to reach 25% [2]. Adults with diabetes have two to four times higher rates of death from heart disease or stroke, and they have medical expenses that are more than two times higher than those for people without diabetes [3–6]. The total annual economic burden associated with diabetes was US$245 billion in 2012, with US$176 billion incurred as direct medical expenditures [3]. In addition, 86 million US adults (35% of the population) have prediabetes [1], which puts them at over four times the risk of progressing to diabetes compared to those who are normoglycemic [7,8].
Large randomized controlled studies [9–11], including the US Diabetes Prevention Program (DPP) trial, have shown that intensive and structured lifestyle modification interventions in people with impaired glucose tolerance can lower the incidence of diabetes by 30%–58% compared to basic lifestyle advice [10–15]. Although primary prevention of diabetes through lifestyle changes is deemed cost-effective, the first-year cost of delivering the original DPP lifestyle intervention was prohibitive (US$1,399 per participant) [16–18]. In addition, lifestyle and cultural patterns vary significantly, across and even within communities, necessitating tailoring of interventions according to regional and ethnic differences to achieve effectiveness, acceptability, and sustainability [19]. To find acceptable, lower-cost alternatives to the resource-intensive DPP lifestyle interventions, a number of studies tested adaptations of DPP delivery in typical US clinics and communities, but still retained the DPP’s core principles of modest weight loss, calorie-restricted diets, and 150 min of moderate-intensity exercise per week; on average, these DPP lifestyle interventions were associated with meaningful pre-post weight loss of approximately 4% [20]. However, it remains unknown whether these nonresearch lifestyle intervention programs were associated with meaningful changes in glycemic markers (fasting blood glucose [FBG] and glycosylated hemoglobin [HbA1c]), blood pressure (BP), and lipids (high density lipoprotein [HDL] and total cholesterol [TC]). Furthermore, there are no comparisons of how data from these translation or effectiveness studies compare to metabolic changes observed in the DPP efficacy trial itself [20,21]. It also remains unclear whether benefits of lifestyle modification programs for diabetes prevention are equally beneficial across persons with objectively defined prediabetes versus those identified only through risk factors for diabetes (e.g., being overweight and hypertensive), and whether program characteristics (e.g., presence of a maintenance component or type of provider) are associated with better outcomes.
Methods
Study Selection
We systematically searched four electronic databases (MEDLINE, EMBASE, Cochrane Library, and Clinicaltrials.gov) for translation or effectiveness studies that tested delivery adaptations of DPP-lifestyle principles in the US and were published between January 1, 2003, and May 1, 2016. Including studies with a similar exposure (DPP-lifestyle principles), albeit via different delivery modalities, and similar outcome measurements allows for the heterogeneity in these studies to answer questions regarding external validity that the original study could not. Indeed, the focus of this work was to inform wider implementation and scaling of interventions in the US.
The search terms used are listed in S1 Table. We supplemented our searches by hand searching reference lists of included articles and other reviews of this topic [22].
We included studies if they met three eligibility criteria. First, each study needed to evaluate implementation of lifestyle intervention programs based on tested DPP principles in US settings. Second, the studies had to have reported pre - and post-intervention estimates for at least one of the following measures: weight, HbA1c, FBG (venous or capillary), systolic or diastolic BP (SBP, DBP), HDL, or TC. Finally, each eligible study had to include adults (age ≥18) at high risk of developing diabetes. To qualify as “high risk,” the target population could have either of the following criteria:
- 1
A diagnosis of prediabetes [23,24]. This could be a self-report of a previous medical diagnosis, or by blood glucose testing, such as
- a
FBG ≥100 mg/dl (impaired fasting glucose [IFG]) or
- b
2 h post-challenge glucose of 140–199 mg/dl (impaired glucose tolerance [IGT]) or
- c
Random (nonfasting) blood glucose between 110–199 mg/dl) [25]; or
- d
HbA1c of 5.7%–6.4% [24]
- a
OR
- 2
Presence of risk factors for diabetes:
- a
Body mass index (BMI) ≥25 kg/m2 AND one additional risk factor such as previous gestational diabetes (GDM), family history, or minority race/ethnicity such as being Asian American, Hispanic, or non-Hispanic US blacks [25]
- a
OR
- b
An American Diabetes Association diabetes risk score of greater than 5 [26]
This systematic review and meta-analysis was focused on studies in the US that implemented specific principles tested in the DPP. The study needed to specifically state that it was DPP inspired, and have both an exercise and dietary component in its intervention. We excluded studies with children, adolescents, or lifestyle interventions that did not involve combined diet and exercise principles tested in the DPP trial. If participants had polycystic ovarian syndrome, current or recent pregnancy, or tested the use of medications (such as metformin) to prevent diabetes, these studies were excluded. If studies had a majority of prediabetes participants (greater than 50%), we included these studies in order to be able to include all available data.
Abstracts were reviewed independently by two authors (UM and AZ) who used the criteria above to determine study eligibility. All discrepancies were resolved by consensus with a third study author (MKA.) When necessary information was not reported in the study, authors of the original article were contacted for further details.
Data regarding study population characteristics, study designs, characteristics of interventions tested (number of core and maintenance sessions, duration of interventions, and follow up time periods), and on baseline and follow-up values for each outcome (weight, FBG, HbA1c, SBP, DBP, HDL, and TC) were extracted by one author (UM) from each eligible article.
Quality Assessment
The DPP trial findings established that standard health advice alone had a low level of efficacy in reducing diabetes incidence among overweight participants with biochemically confirmed impaired glucose tolerance and elevated fasting glucose [13]. Structured behavioral modification interventions, adherence to rigorous lifestyle principles, and associated weight loss were shown to be effective [27]. Unlike large trials such as the DPP, translation studies tend to be small and often use quasi-experimental designs (e.g., single group pre - and post - intervention or pre-post evaluations with control groups) in which random allocation and blinding are impossible [13]. To assess study quality and facilitate interpretation of the available literature, we applied a scoring system with three criteria, each contributing one point in the criteria adapted from those proposed by Juni et al. [28]. The first criterion assessed whether studies used any steps to minimize attrition bias by using an intention to treat analysis, achieving low attrition rates (≤20%), or by comparing characteristics of completers and noncompleters. The second criterion was to assign higher quality to studies that included a control group (randomized, matched, or unmatched comparison). The third criterion focused on whether the study reported on four or more of the following aspects of translating evidence: describing the process of designing the program, describing the enrollment process, documenting session attendance, reporting costs and/or resource inputs, documenting the training process or qualifications of personnel, or describing the qualitative feedback from participants or providers. The latter was considered an important aspect of study quality given that studies were included based on their ability to provide information about how DPP lifestyle programs were implemented and how successful they were. These quality assessment criteria were chosen because they support study replication and comparison. A study was categorized as “high quality” if it had at least two out of three possible points. Further details are provided in the S3 Table.
Statistical Analysis
Using pooled data, descriptive characteristics of the study populations were calculated as weighted means based on sample size. Since very few of the studies had comparison groups, the intervention arms of controlled studies were treated as pre-post groups and aggregated with the remaining single-group pre-post studies. When studies included multiple intervention arms, each distinct intervention arm was treated as a separate pre-post group. For example, in studies in which a lifestyle intervention was delivered using two different media (in person versus remote), these counted as two separate intervention groups within the same study.
Estimates of pre-post intervention change in each outcome and the corresponding standard errors were obtained or calculated based on the data reported. To standardize the length of follow-up, we focused on the 1 y interval between pre - and post - intervention measures. If a study conducted several follow-up assessments for a given outcome, the reported value at the time point closest to 1 y was used to calculate the change from the baseline. We used random effects meta-analysis techniques to calculate aggregate estimates and measures of dispersion to account for inter-study heterogeneity. All estimates were accompanied by a χ2 test for heterogeneity with a corresponding I2 value.
To explore what program characteristics may have been associated with risk factor changes, we performed stratified analyses according to delivery format (individual or group), intervention delivery personnel (by health professionals, lay community center staff, or electronic media), location of the intervention (clinical or community setting), and inclusion of a maintenance component in the study protocol (yes or no). When there was more than one type of delivery format, the delivery format of the core sessions took precedence. For example, if an article had core sessions in the community and a remote component for maintenance, this was coded as “community.” Stratified analyses were conducted for each outcome, except HbA1c, for which data were insufficient to allow stratification. We also stratified by the method used for risk classification (blood glucose criteria or risk factor criteria to classify as “high-risk”). We tested whether any program characteristics were associated with weight change using Spearman’s rank correlation analyses. Program characteristics of interest, in addition to those listed above, included the mean number and duration of core sessions offered, average participant age and BMI, proportions of males and non-Hispanic whites (NHWs) in the study sample, and attrition. Lastly, we evaluated aggregate outcomes in high versus average quality studies.
When studies included a control arm, we included them in separate analyses of the intervention arms against the control arms to determine what incremental changes in weight and cardiometabolic risks was observed.
All calculations were carried out using MIX 2.0 statistical software [29]. Statistically significant differences were noted based on a conservative definition of non-overlapping 95% confidence intervals. All findings were reported in reference to the 1 y findings reported by the original DPP trial [13].
Results
Study and Participant Characteristics
A total of 44 studies, which included 48 intervention groups, met eligibility for inclusion. Of these, 22 studies were single group pre-post designs [30–51], 3 studies had two intervention arms (each contributing two separate groups for analysis) [52–54], 18 studies had separate control arms [55–72], and 1 study had three arms—two intervention groups and one control [73]. Data on weight change from baseline were available from all intervention groups. There were 21 groups that had pre - and post-intervention data on FBG, 8 had HbA1c measures, 23 had SBP measures, 22 had DBP measures, 14 had HDL measures, and 12 had TC measures. Further details are listed in Fig 1.
Fig. 1. Flow diagram of study search. 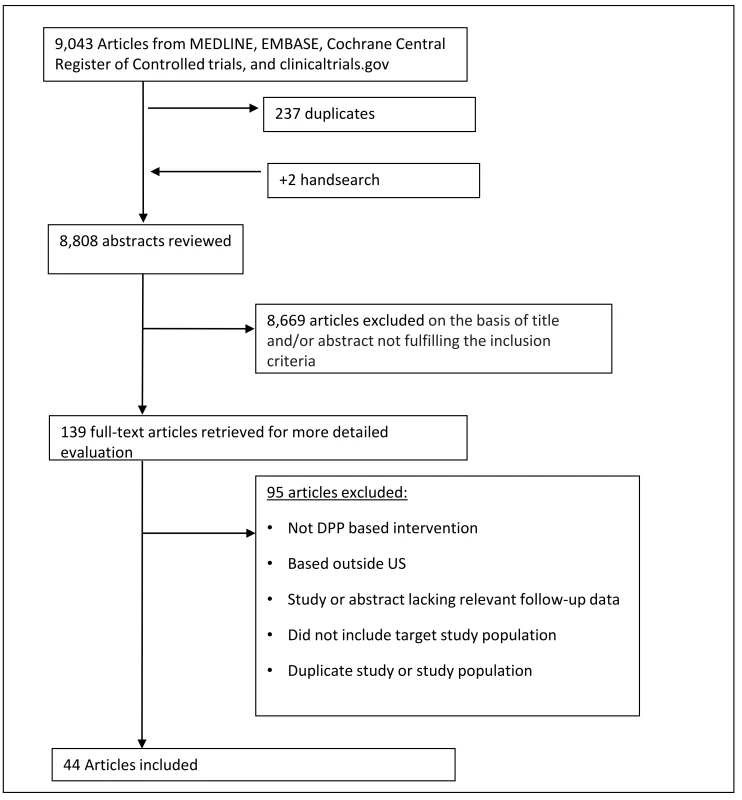
This flow chart describes the number of studies that were involved in each step of the process of study selection, from the initial study search. After the application of inclusion and exclusion criteria, 44 studies met criteria and were included in the final analysis. A total of 8,995 participants were enrolled across all intervention arms. Aggregate participant demographic and clinical characteristics were similar to participants enrolled in the original DPP study (Table 1). At enrollment, participants’ mean age was 50.8 y, mean weight was 99.3 kg, mean BMI was 34.8 kg/m2, 25.2% were male, and 32.9% were non-Hispanic white. Mean baseline levels of cardiovascular risk factors were: 5.9% (HbA1c), 104.6 mg/dl (FBG), 128.7/79.5 mmHg (SBP/DBP), 46.1 mg/dl (HDL), and 183.7 mg/dl (TC). Of the 48 intervention groups, 18 used blood glucose measures to classify risk for diabetes, 22 used the presence of risk factors, and 8 included participants defined as high risk by either of the above criteria.
Tab. 1. Baseline characteristics of trials (aggregate) versus DPP. 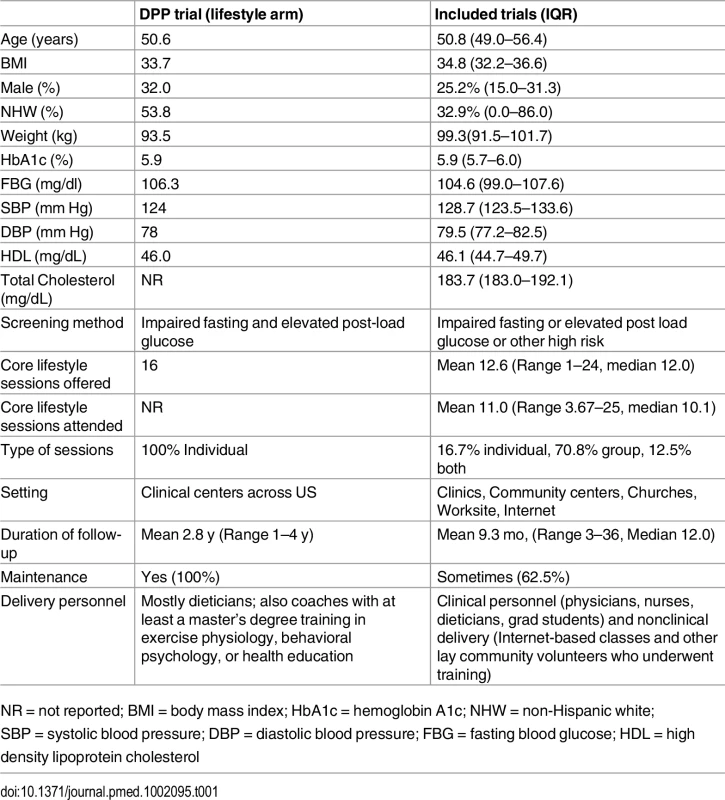
NR = not reported; BMI = body mass index; HbA1c = hemoglobin A1c; NHW = non-Hispanic white; SBP = systolic blood pressure; DBP = diastolic blood pressure; FBG = fasting blood glucose; HDL = high density lipoprotein cholesterol Programs amended the original DPP lifestyle intervention (Table 1) by changing the number or duration of core sessions offered (the DPP offered 16 in-person sessions over 24 wk), conducting group (instead of individual) sessions, modifying the type of lifestyle coach (DPP coaches were qualified dietitians, exercise physiologists, behavioral psychologists, or health educators), and changing or removing the monthly maintenance component where participants met or were contacted for the remainder of the follow-up period to promote continued adherence to healthy lifestyle principles.
The number of core sessions offered in included DPP-lifestyle programs ranged from 1 to 24, with a mean of 12.6 core sessions (median 12.0) offered and 11.0 mean core sessions attended (median 10.1). Thirty interventions incorporated a maintenance component, which varied from emails to intermittent in-person group sessions. Programs with scheduled maintenance ranged from 3–8 monthly sessions as follow-up after the initial core component. Mean study duration was 9.3 mo with a standard deviation (SD) of 5.5 mo and a range from 3 to 15 mo (median 12.0) Across all studies, overall attrition was 23.5% (range: 0.0 to 43.2).
Outcomes
Across all studies, mean absolute pre-post weight change was -3.77 kg (95% CI: -4.55 to 2.99, I2 of 99.06% (95% CI: 98.96, 99.15) (Table 2). Aggregate data from 16 studies that randomly assigned participants to intervention or control groups showed 2.66 kg greater weight loss among intervention participants (-3.25 kg) compared to control participants (-0.59 kg). For further details, see forest plot in S13 Fig.
Fig. 2. Mean weight change of study participants. 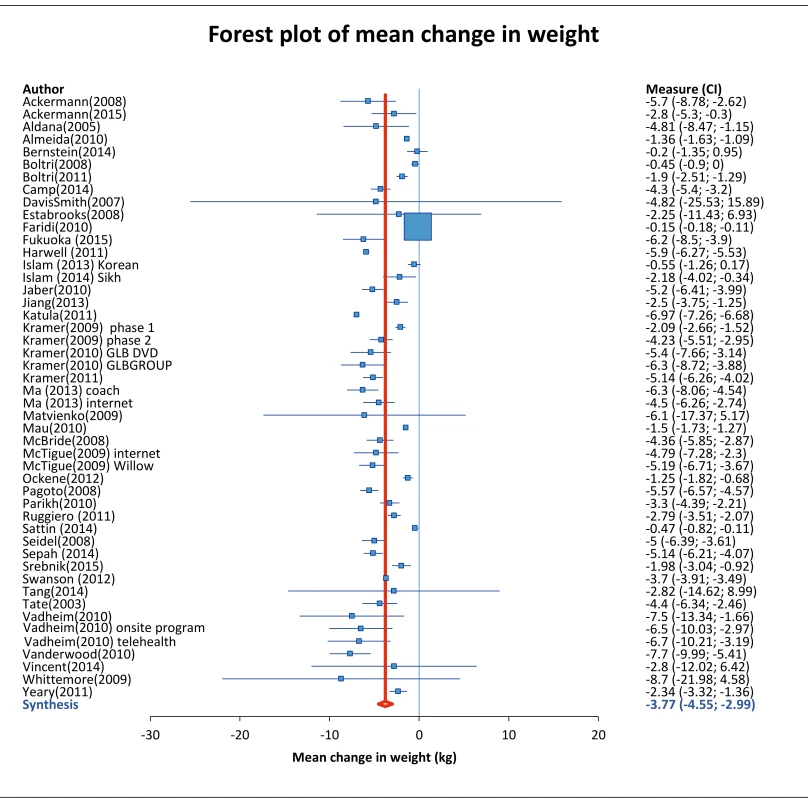
Analysis of all 48 intervention groups which reported weight change from baseline to end of follow-up period. Data was analyzed with Mix 2.0 using random effects method with the weighting of each study proportional to the inverse of the variance. Study weight is indicated by the size of the box, and horizontal lines indicate the 95% confidence interval. The red line indicates the overall weight change, and summary diamond indicates pooled estimate with reported mean (95% CI). Tab. 2. Comparison of outcomes from DPP versus Community Trials. 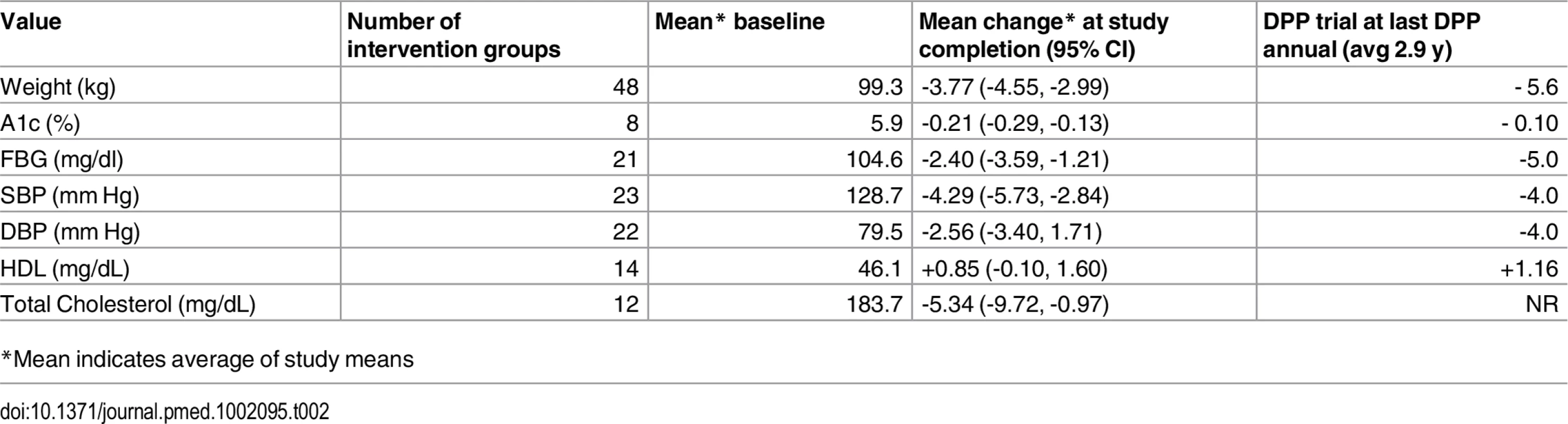
*Mean indicates average of study means Among 8 studies that measured HbA1c, mean pre-post change was -0.21% (95% CI: -0.29; -0.13, I2 82.72% [95% CI: 67.29%, 90.87.]) Among 21 studies reporting FBG, mean change in FBG was -2.40 mg/dl (95% CI: -3.59; -1.21, I2 90.63% [95% CI: 87.08, 93.21]). Among the 23 studies evaluating BP, we observed a mean pre-post change in SBP of -4.29 mmHg (95% CI:-5.73, -2.84, I2 75.40% [95% CI: 61.15, 84.43]) and -2.56 mmHg in DBP (95% CI:-3.40, 1.71, I2 57.96% [95% CI: 29.09, 75.08]). There were 14 and 12 studies which reported on HDL and total cholesterol, respectively. There was an overall pre-post increase in HDL of +0.85 mg/dL (95% CI: -0.10, 1.60, I2 63.12% [95% CI: 34.41, 79.26]) and change in TC of -5.34 mg/dl (95% CI: -9.72, -0.97, I2 56.09% [95% CI: 16.19, 76.99]). There were insufficient data to perform between-group comparisons (intervention versus control) for outcomes other than weight.
Stratified Analyses
Using our conservative definition of non overlapping CIs, studies with a maintenance component had a statistically significantly greater decrease in mean FBG (-3.14 mg/dl) and a greater decrease in weight (-1.66 kg) than intervention programs without a maintenance component (Table 3).
Tab. 3. Outcomes stratified by the presence of maintenance. 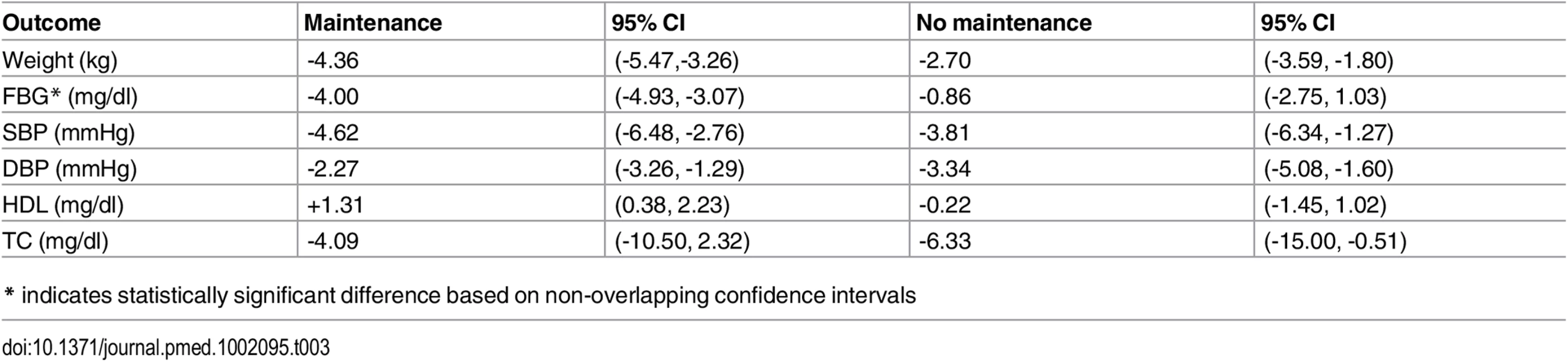
(Supporting forest plot in S9 Fig.) No statistically or clinically significant differences in risk factor changes were observed when comparing studies testing interventions delivered by community workers to studies that employed health professionals, or those that used electronic media (Table 4). Similarly, no outcome differences were noted in studies classifying high-risk for diabetes based on blood glucose testing versus other criteria (Table 5), nor by study quality, (high versus average quality) or setting (clinic, community or remote). (Data shown in S7, S11 and S10 Figs).
Tab. 4. Outcomes stratified by type of provider for delivery of the intervention. 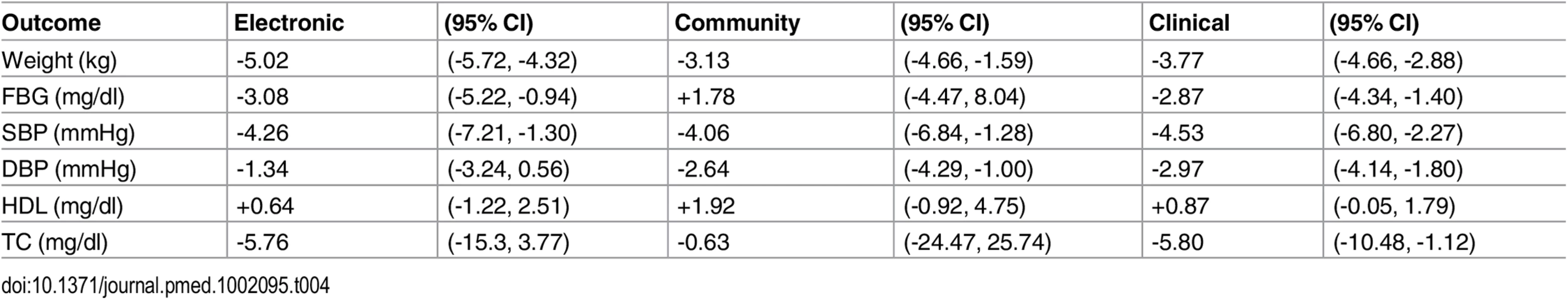
(Supporting forest plot in S8 Fig.) Tab. 5. Outcomes stratified by method used to determine “high risk” status. 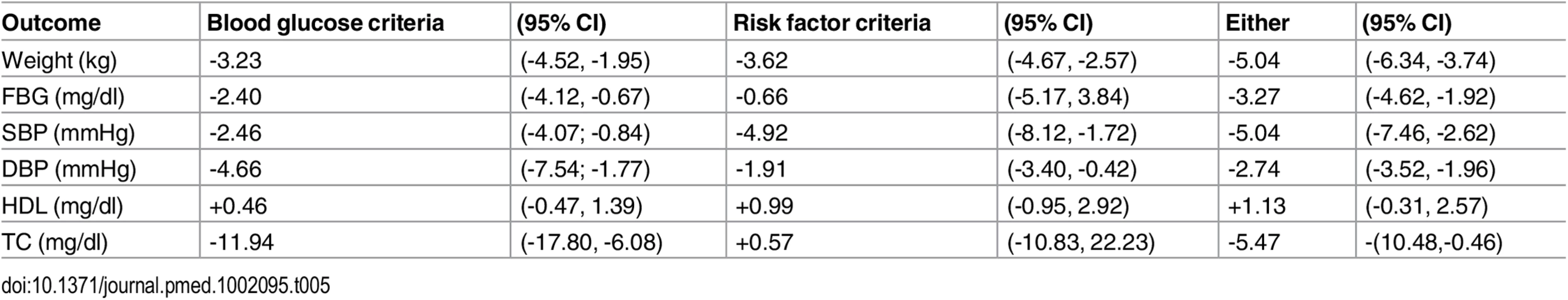
(Supporting forest plots in S7 Fig.) Discussion
This is the first meta-analysis to aggregate both weight and cardiovascular risk factor changes from US community-based studies of DPP-based lifestyle interventions. Characteristics of participants in these studies of DPP lifestyle programs were very similar to those of the original trial participants, but translation study participants had a slightly higher mean starting weight and higher proportion of females [13]. The original DPP participants had a greater mean weight loss at 1 y than the participants in this meta-analysis (6.8 kg versus 3.8 kg), which was likely due to the more resource intensive intervention and individualized support in the trial [13]. However, this weight change was closer to the 4.2 kg weight loss reported in the Finnish Diabetes Prevention Study (DPS) [11]. Studies with adequate control groups showed an additional 1.9 kg weight lost across intervention arms when compared with their respective control arms (3.3 versus 0.6 kg). Indeed, the control groups in these effectiveness studies achieved some benefit from participation, even if only exposed to minimal intervention. Compared to the original DPP, HbA1c, and SBP reductions observed in translation studies were similar; FBG and DBP reductions were somewhat lower than the reductions achieved in the efficacy trial; and comparisons for HDL and TC were not possible.
We noted no difference in cardiometabolic risk factor changes in people with biochemically confirmed prediabetes versus those with diabetes risk factors. That said, progression to diabetes and its complications varies by type of prediabetes. The DPP enrolled patients with both IGT and IFG, who are at approximately three times higher risk of progression to diabetes compared to those with IFG alone [7]. The Finnish DPS, Malmo, and Da Qing studies also included participants with IGT or combined IGT and IFG, who are at higher risk than those with IFG alone [7,9,14]. Meanwhile, the US-based DPP-translation studies primarily used IFG criteria, and none used oral glucose tolerance tests to determine high-risk status. This suggests that participants in these studies had a lower risk profile, which was also reflected in their lower baseline FBG and HbA1c levels. Assuming the participants in this analysis were at a lower baseline risk, the changes in cardiometabolic risks observed were commensurate with the starting risk level, and are therefore still noteworthy. It remains unclear whether the DPP intervention is effective in preventing diabetes among participants with impaired fasting glucoses but normal post-load glucose levels. Also, given ease of testing, HbA1c is now commonly used to diagnose prediabetes, and it is unclear whether DPP results can be extended to this prediabetes population defined by HbA1c.
Our findings are also similar to other recent studies. A Community Guide Review, which evaluated interventions across diverse countries and settings, had similar decreases in FPG with a nonsignificant trend towards decreased blood pressure and cholesterol [22]. The MOVE! program evaluated a ten-module program among 238,000 veterans; high intensity intervention participants achieved 2.7% weight loss at 6 mo compared to a 0.6% weight loss in the low intensity group [13,74]. Our findings were also similar to a systematic review and meta-analysis that pooled 22 studies published before July 2012 that translated diabetes prevention for real-life settings in multiple countries (US, Australia, Europe, and Japan) and had ≥12 mo of follow-up [75]. Since multiple countries were involved, heterogeneous study interventions were benchmarked to Europe-wide diabetes prevention implementation guidelines and showed overall pre-post changes in weight (-2.32 kg), HbA1c (-0.13 mmol/mol), FPG (-0.10 mmol/L [-1.8 mg/dl]), SBP and DBP (-4.30/-4.28 mmHg), HDL (+0.01 mmol/L [+0.39 mg/dl]), and TC (-0.18 mmol/L [-6.96 mg/dl]); importantly, greater adherence to recommendations was associated with larger weight reduction. Our study expands on this work with a larger number of pooled studies and participants, all using a similar core set of intervention principles and comparison to the original DPP Study.
Effective translation of a program depends on multiple components, including referral, uptake, engagement, completion, and post-program sustainability of outcomes in the whole population. In our review, after eligibility criteria was applied, 25.5% of all eligible participants did not enroll; of those who enrolled, there was an additional 23.8% attrition. Rates of attrition also inherently select for those who are the most motivated participants, which biases the results towards effectiveness. This limits the generalizability of our findings, which more accurately apply to those who complete the program.
Implementation of DPP lifestyle programs have been limited by both cost and sustainability of ongoing program participation and risk factor reductions [76]. Most of the programs studied in this analysis provided free testing and intervention supplies but offered few additional incentives to encourage participation. The most common methods used to decrease cost were modifications to the intervention, such as offering the intervention in accessible locations, delivering the intervention through lay providers, and taking advantage of group classes and electronic delivery options. Over 80% of studies tested group interventions, most had fewer mean core sessions compared to DPP, and only 60% offered a maintenance component. Importantly, we noted similar risk factor benefits were achievable in interventions delivered by different providers in both group and individual formats. The similarities in weight loss and secondary outcomes compared to the DPP is encouraging for the ability to make the intervention cost-effective without sacrificing the effectiveness. With options that include group sessions, community-based programs with social support, cultural tailoring, and remote low-cost maintenance such as text messages or phone calls, the interventions allow for scaling to a wider audience.
Reach and sustainability of behavior change interventions remain, as do other challenges of implementing diabetes prevention. The advantages of community-based interventions that were pooled in this study include familiar context, peer support, and convenience to facilitate continued participation. The success of electronic and remote interventions is also encouraging, as these could be distributed nationally with ease. The option of pre-recorded workouts on in-home cable TV illustrates a low-cost method of delivery that does not necessitate travel and is available on demand, in contrast to on-site workout regimens for which participants pay to participate [77]. This preliminary analysis also suggests that programs that implemented a maintenance component after the completion of the core sessions had greater reductions in weight and fasting glucose. The duration and intensity of maintenance that is most effective and the utility after the 1 y mark is largely unknown. Further evaluation of types of maintenance programs following a year-long program would be helpful to understand long-term benefit and sustainability.
Limitations
A key limitation of our analysis was the heterogeneity of the studies included, which is inherent in all meta-analyses. Differences in duration of follow-up (from 1.5 to 36 mo), location of delivery, and other delivery format adaptations of the original DPP program were the most likely sources of heterogeneity. However, as the intervention (DPP-lifestyle program principles) and outcomes were similar, this study adds to the literature by providing external validity and noteworthy pre-post and between group cardiometabolic risk factor changes.
The lack of statistical significance found in most of the stratified analyses is likely due to lack of statistical power, which resulted in large, overlapping confidence intervals, as well as our conservative definition of statistical significance based only on non-overlapping CI’s. As most meta-analyses, our study is confined to the use of previously reported results. We used a more conservative definition of statistical significance by comparing stratum specific results, though this did not allow a more consistent adjustment for confounders. However, a less conservative analytical approach may have found other program characteristics that had “statistically significant” associations with cardiometabolic changes.
Additionally, studies varied significantly in quality. We conducted a sensitivity analysis to evaluate change in weight stratified by study quality (high versus average quality), which showed no significant difference. However, this alone is not expected to account for variation that arises during recruitment, enrollment, and study conduct. The majority of studies in our review used pre-post single group study designs and may be subject to confounding. To address this, we separately examined studies that had control groups and demonstrated that intervention groups achieved larger benefits than control groups.
Conclusion
Delivery of lifestyle programs adhering to DPP principles tested in community and clinical settings achieved similar 1 y decreases in weight, FBG, and HbA1c as the original DPP study, despite the modifications made to lower cost and improve acceptability across various settings. Though unclear if these changes truly translate into reductions in diabetes incidence, prior studies have found decreased incidence to be most closely related to weight loss [13]. Methods to increase uptake and decrease attrition are both needed to enable long-lasting, sustainable lifestyle change in patients with the highest risk of progression to diabetes and its associated complications.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Supporting Information
Zdroje
1. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. In: US Department of Health and Human Services, editor. Atlanta, GA: Centers for Disease Control and Prevention; 2014.
2. Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8 : 29. doi: 10.1186/1478-7954-8-29 20969750
3. Zhuo X, Zhang P, Barker L, Albright A, Thompson TJ, Gregg E. The lifetime cost of diabetes and its implications for diabetes prevention. Diabetes care. 2014;37(9):2557–64. doi: 10.2337/dc13-2484 25147254
4. Zhuo X, Zhang P, Kahn HS, Bardenheier BH, Li R, Gregg EW. Change in Medical Spending Attributable to Diabetes: National Data From 1987 to 2011. Diabetes care. 2015.
5. Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. The New England journal of medicine. 2014;370(16):1514–23. doi: 10.1056/NEJMoa1310799 24738668
6. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. In: US Department of Health and Human Services, editor. Atlanta, GA: Centers for Disease Control and Prevention.; 2011.
7. Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007;78(3):305–12. 17601626
8. Bullard KM, Saydah SH, Imperatore G, Cowie CC, Gregg EW, Geiss LS, et al. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin a1c and fasting plasma glucose: national health and nutrition examination surveys, 1999–2010. Diabetes care. 2013;36(8):2286–93. doi: 10.2337/dc12-2563 23603918
9. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes care. 1997;20(4):537–44. 9096977
10. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–97. 16391903
11. Lindstrom J, Eriksson JG, Valle TT, Aunola S, Cepaitis Z, Hakumaki M, et al. Prevention of diabetes mellitus in subjects with impaired glucose tolerance in the Finnish Diabetes Prevention Study: results from a randomized clinical trial. Journal of the American Society of Nephrology: JASN. 2003;14(7 Suppl 2):S108–13. 12819313
12. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. The New England journal of medicine. 2001;344(18):1343–50. 11333990
13. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England journal of medicine. 2002;346(6):393–403. 11832527
14. Eriksson KF, Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia. 1991;34(12):891–8. 1778354
15. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005;67(2):152–62. 15649575
16. Giraldo MA, Chaudhari LS, Schulz LO. Land-use and land-cover assessment for the study of lifestyle change in a rural Mexican community: the Maycoba project. Int J Health Geogr. 2012;11 : 27. doi: 10.1186/1476-072X-11-27 22776075
17. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes care. 2003;26(9):2518–23. 12941712
18. Hernan WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes care. 2003;26(1):36–47. 12502656
19. Brownson RC, Chriqui JF, Stamatakis KA. Understanding evidence-based public health policy. American journal of public health. 2009;99(9):1576–83. doi: 10.2105/AJPH.2008.156224 19608941
20. Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood). 2012;31(1):67–75.
21. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes care. 1999;22(2):233–40. 10333939
22. Diabetes Prevention and Control: Combined Diet and Physical Activity Promotion Programs to Prevent Type 2 Diabetes Among People at Increased Risk. In: Force CPST, editor. The Community Guide2014.
23. American Diabetes Association. Standard of Medical Care in Diabetes. Diabetes care. 2015;38, 1.
24. American Diabetes Association. Diagnosing Diabetes and Learning about Prediabetes [Website]. http://www.diabetes.org/diabetes-basics/diagnosis/ [updated May 16, 2016; cited 2016 May 2016].
25. Diagnosis and Classification of Diabetes Mellitus. Diabetes care. 2004;27(suppl 1):s5–s10. 14693921
26. American Diabetes Association. Are You at Risk? http://www.diabetes.org/are-you-at-risk/diabetes-risk-test/ [cited 2016 May 18].
27. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes care. 2006;29(9):2102–7. 16936160
28. Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–6. 11440947
29. Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KG. Development and validation of MIX: comprehensive free software for meta-analysis of causal research data. BMC Med Res Methodol. 2006;6 : 50. 17038197
30. Aldana SG, Barlow M, Smith R, Yanowitz FG, Adams T, Loveday L, et al. The diabetes prevention program: a worksite experience. AAOHN J. 2005;53(11):499–505; quiz 6–7. 16309012
31. Boltri JM, Davis-Smith M, Okosun IS, Seale JP, Foster B. Translation of the National Institutes of Health Diabetes Prevention Program in African American churches. J Natl Med Assoc. 2011;103(3):194–202. 21671523
32. Boltri JM, Davis-Smith YM, Seale JP, Shellenberger S, Okosun IS, Cornelius ME. Diabetes prevention in a faith-based setting: results of translational research. J Public Health Manag Pract. 2008;14(1):29–32. 18091037
33. Davis-Smith YM, Boltri JM, Seale JP, Shellenberger S, Blalock T, Tobin B. Implementing a diabetes prevention program in a rural African-American church. J Natl Med Assoc. 2007;99(4):440–6. 17444435
34. Harwell TS, Vanderwood KK, Hall TO, Butcher MK, Helgerson SD. Factors associated with achieving a weight loss goal among participants in an adapted Diabetes Prevention Program. Prim Care Diabetes. 2011;5(2):125–9. doi: 10.1016/j.pcd.2010.12.001 21233033
35. Jaber LA, Pinelli NR, Brown MB, Funnell MM, Anderson R, Hammad A, et al. Feasibility of group lifestyle intervention for diabetes prevention in Arab Americans. Diabetes Res Clin Pract. 2011;91(3):307–15. doi: 10.1016/j.diabres.2010.11.032 21168232
36. Jiang L, Manson SM, Beals J, Henderson WG, Huang H, Acton KJ, et al. Translating the Diabetes Prevention Program into American Indian and Alaska Native communities: results from the Special Diabetes Program for Indians Diabetes Prevention demonstration project. Diabetes care. 2013;36(7):2027–34. doi: 10.2337/dc12-1250 23275375
37. Kramer MK, McWilliams JR, Chen HY, Siminerio LM. A community-based diabetes prevention program: evaluation of the group lifestyle balance program delivered by diabetes educators. Diabetes Educ. 2011;37(5):659–68. doi: 10.1177/0145721711411930 21918204
38. Matvienko OA, Hoehns JD. A lifestyle intervention study in patients with diabetes or impaired glucose tolerance: translation of a research intervention into practice. Journal of the American Board of Family Medicine: JABFM. 2009;22(5):535–43. doi: 10.3122/jabfm.2009.05.090012 19734400
39. Mau MK, Keawe'aimoku Kaholokula J, West MR, Leake A, Efird JT, Rose C, et al. Translating diabetes prevention into native Hawaiian and Pacific Islander communities: the PILI 'Ohana Pilot project. Prog Community Health Partnersh. 2010;4(1):7–16. doi: 10.1353/cpr.0.0111 20364073
40. McBride PE, Einerson JA, Grant H, Sargent C, Underbakke G, Vitcenda M, et al. Putting the Diabetes Prevention Program into practice: a program for weight loss and cardiovascular risk reduction for patients with metabolic syndrome or type 2 diabetes mellitus. J Nutr Health Aging. 2008;12(10):745S–9S. 19043651
41. McTigue KM, Conroy MB, Bigi L, Murphy C, McNeil M. Weight loss through living well: translating an effective lifestyle intervention into clinical practice. Diabetes Educ. 2009;35(2):199–204, 8. doi: 10.1177/0145721709332815 19321806
42. McTigue KM, Conroy MB, Hess R, Bryce CL, Fiorillo AB, Fischer GS, et al. Using the internet to translate an evidence-based lifestyle intervention into practice. Telemed J E Health. 2009;15(9):851–8. doi: 10.1089/tmj.2009.0036 19919191
43. Pagoto SL, Kantor L, Bodenlos JS, Gitkind M, Ma Y. Translating the diabetes prevention program into a hospital-based weight loss program. Health Psychol. 2008;27(1 Suppl):S91–8. doi: 10.1037/0278-6133.27.1.S91 18248110
44. Ruggiero L, Oros S, Choi YK. Community-based translation of the diabetes prevention program's lifestyle intervention in an underserved Latino population. Diabetes Educ. 2011;37(4):564–72. doi: 10.1177/0145721711411107 21690435
45. Seidel MC, Powell RO, Zgibor JC, Siminerio LM, Piatt GA. Translating the Diabetes Prevention Program into an urban medically underserved community: a nonrandomized prospective intervention study. Diabetes care. 2008;31(4):684–9. doi: 10.2337/dc07-1869 18252904
46. Sepah SC, Jiang L, Peters AL. Translating the Diabetes Prevention Program into an Online Social Network: Validation against CDC Standards. Diabetes Educ. 2014;40(4):435–43. 24723130
47. Swanson CM, Bersoux S, Larson MH, Aponte-Furlow RT, Flatten SS, Olsen CL, et al. An outpatient-based clinical program for diabetes prevention: an update. Endocr Pract. 2012;18(2):200–8. doi: 10.4158/EP11226.OR 22068253
48. Vadheim LM, Brewer KA, Kassner DR, Vanderwood KK, Hall TO, Butcher MK, et al. Effectiveness of a lifestyle intervention program among persons at high risk for cardiovascular disease and diabetes in a rural community. J Rural Health. 2010;26(3):266–72. doi: 10.1111/j.1748-0361.2010.00288.x 20633095
49. Vanderwood KK, Hall TO, Harwell TS, Butcher MK, Helgerson SD, Montana Cardiovascular D, et al. Implementing a state-based cardiovascular disease and diabetes prevention program. Diabetes care. 2010;33(12):2543–5. doi: 10.2337/dc10-0862 20805260
50. Yeary KH, Cornell CE, Turner J, Moore P, Bursac Z, Prewitt TE, et al. Feasibility of an evidence-based weight loss intervention for a faith-based, rural, African American population. Prev Chronic Dis. 2011;8(6):A146. 22005639
51. Tang TS, Nwankwo R, Whiten Y, Oney C. Outcomes of a church-based diabetes prevention program delivered by peers: a feasibility study. Diabetes Educ. 2014;40(2):223–30. doi: 10.1177/0145721713520569 24481174
52. Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–11. doi: 10.1016/j.amepre.2009.07.020 19944916
53. Kramer MK, Kriska AM, Venditti EM, Semler LN, Miller RG, McDonald T, et al. A novel approach to diabetes prevention: evaluation of the Group Lifestyle Balance program delivered via DVD. Diabetes Res Clin Pract. 2010;90(3):e60–3. doi: 10.1016/j.diabres.2010.08.013 20863586
54. Vadheim LM, McPherson C, Kassner DR, Vanderwood KK, Hall TO, Butcher MK, et al. Adapted diabetes prevention program lifestyle intervention can be effectively delivered through telehealth. Diabetes Educ. 2010;36(4):651–6. doi: 10.1177/0145721710372811 20534873
55. Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35(4):357–63. doi: 10.1016/j.amepre.2008.06.035 18779029
56. Almeida FA, Shetterly S, Smith-Ray RL, Estabrooks PA. Reach and effectiveness of a weight loss intervention in patients with prediabetes in Colorado. Prev Chronic Dis. 2010;7(5):A103. 20712930
57. Camp A, Tamborlane WV, Kim G, Magenheimer E, Montosa A. Successful implementation of a T2D prevention program in a community health center setting. Diabetes. 2013;62((Camp A.; Tamborlane W.V.; Kim G.; Magenheimer E.; Montosa A.) New Haven, United States):A330.
58. Estabrooks PA, Smith-Ray RL. Piloting a behavioral intervention delivered through interactive voice response telephone messages to promote weight loss in a pre-diabetic population. Patient Educ Couns. 2008;72(1):34–41. doi: 10.1016/j.pec.2008.01.007 18282679
59. Faridi Z, Shuval K, Njike VY, Katz JA, Jennings G, Williams M, et al. Partners reducing effects of diabetes (PREDICT): a diabetes prevention physical activity and dietary intervention through African-American churches. Health Educ Res. 2010;25(2):306–15. doi: 10.1093/her/cyp005 19261690
60. Katula JA, Vitolins MZ, Rosenberger EL, Blackwell CS, Morgan TM, Lawlor MS, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes care [Internet]. 2011; (7):[1451–7 pp.]. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/911/CN-00799911/frame.html.
61. Ockene IS, Tellez TL, Rosal MC, Reed GW, Mordes J, Merriam PA, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. American journal of public health. 2012;102(2):336–42. doi: 10.2105/AJPH.2011.300357 22390448
62. Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. American journal of public health. 2010;100 Suppl 1:S232–9. doi: 10.2105/AJPH.2009.170910 20147680
63. Sattin RW, Dias JK, Williams LB, Joshua T, Marion LN. Effects on weight of a cluster-randomized, controlled trial of a faith-based adaption of the diabetes prevention program within African-American churches. Diabetes. 2014;63((Sattin R.W.; Dias J.K.; Williams L.B.; Joshua T.; Marion L.N.) Augusta, United States):A526–A7.
64. Tate DF, Jackvony EH, Wing RR. Effects of Internet behavioral counseling on weight loss in adults at risk for type 2 diabetes: a randomized trial. JAMA. 2003;289(14):1833–6. 12684363
65. Whittemore R, Melkus G, Wagner J, Dziura J, Northrup V, Grey M. Translating the diabetes prevention program to primary care: a pilot study. Nurs Res. 2009;58(1):2–12. doi: 10.1097/NNR.0b013e31818fcef3 19092550
66. Islam NS, Zanowiak JM, Wyatt LC, Kavathe R, Singh H, Kwon SC, et al. Diabetes prevention in the New York City Sikh Asian Indian community: a pilot study. International journal of environmental research and public health. 2014;11(5):5462–86. doi: 10.3390/ijerph110505462 24852392
67. Vincent D, McEwen MM, Hepworth JT, Stump CS. The effects of a community-based, culturally tailored diabetes prevention intervention for high-risk adults of Mexican descent. Diabetes Educ. 2014;40(2):202–13. doi: 10.1177/0145721714521020 24510942
68. Bernstein AM, Gendy G, Rudd N, Doyle J, Fay S, Moffett K, et al. Management of prediabetes through lifestyle modification in overweight and obese African-American women: the Fitness, Relaxation, and Eating to Stay Healthy (FRESH) randomized controlled trial. Public health. 2014;128(7):674–7. doi: 10.1016/j.puhe.2014.04.005 24996961
69. Fukuoka Y, Gay CL, Joiner KL, Vittinghoff E. A Novel Diabetes Prevention Intervention Using a Mobile App: A Randomized Controlled Trial With Overweight Adults at Risk. Am J Prev Med. 2015;49(2):223–37. doi: 10.1016/j.amepre.2015.01.003 26033349
70. Srebnik D, Chwastiak LA, Russo J, Sylla L. A pilot study of the diabetes prevention program on weight loss for adults at community mental health centers. Psychiatric services. 2015;66(2):200–3. doi: 10.1176/appi.ps.201300576 25642616
71. Ackermann RT, Liss DT, Finch EA, Schmidt KK, Hays LM, Marrero DG, et al. A Randomized Comparative Effectiveness Trial for Preventing Type 2 Diabetes. American journal of public health. 2015;105(11):2328–34. doi: 10.2105/AJPH.2015.302641 26378828
72. Islam NS, Zanowiak JM, Wyatt LC, Chun K, Lee L, Kwon SC, et al. A randomized-controlled, pilot intervention on diabetes prevention and healthy lifestyles in the New York City Korean community. Journal of community health. 2013;38(6):1030–41. doi: 10.1007/s10900-013-9711-z 23813322
73. Ma J, Yank V, Xiao L, Lavori PW, Wilson SR, Rosas LG, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–21. doi: 10.1001/2013.jamainternmed.987 23229846
74. Jackson SL, Long Q, Rhee MK, Olson DE, Tomolo AM, Cunningham SA, et al. Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. The lancet Diabetes & endocrinology. 2015;3(3):173–80.
75. Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes care. 2014;37(4):922–33. doi: 10.2337/dc13-2195 24652723
76. Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes care. 2014;37(4):943–9. doi: 10.2337/dc13-1954 24652724
77. Ackermann RT, Sandy LG, Beauregard T, Coblitz M, Norton KL, Vojta D. A randomized comparative effectiveness trial of using cable television to deliver diabetes prevention programming. Obesity. 2014;22(7):1601–7. doi: 10.1002/oby.20762 24740868
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- The Clinical and Public Health Challenges of Diabetes Prevention: A Search for Sustainable Solutions
- Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
- Diabetes: A Cinderella Subject We Can’t Afford to Ignore
- Prescribing Exercise and Lifestyle Training for High Risk Women in Pregnancy and Early Post-partum—Is It Worth It?
- Population Approaches to Prevention of Type 2 Diabetes
- First-Year Evaluation of Mexico’s Tax on Nonessential Energy-Dense Foods: An Observational Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
- Leveraging Genetics to Advance Type 2 Diabetes Prevention
- Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
- Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
- Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
- Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients
- Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
- Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women
- Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study
- Engagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Cycling and Diabetes Prevention: Practice-Based Evidence for Public Health Action
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



