-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
Using a large prospective cohort, Annalijn Conklin and colleagues investigate the association between dietary diversity and type 2 diabetes risk.
Published in the journal: . PLoS Med 13(7): e32767. doi:10.1371/journal.pmed.1002085
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002085Summary
Using a large prospective cohort, Annalijn Conklin and colleagues investigate the association between dietary diversity and type 2 diabetes risk.
Introduction
Non-communicable diseases present a significant challenge to both high-income and low-income countries, with growing numbers of people experiencing the health and economic burden of one or more chronic conditions [1]. Diet is a key modifiable risk factor for multiple chronic diseases, with poor quality diets being a leading cause of type 2 diabetes (T2D), cardiovascular diseases, hypertension, and certain cancers [2]. It is estimated that diets that do not match nutritional guidelines contribute to 70,000 premature deaths in the United Kingdom [3]. Inadequate consumption of fruits and vegetables in particular is estimated to contribute to 5% of excess mortality globally [2]. Many national and international policies acknowledge the importance of supporting individuals in achieving a healthy balanced diet, and numerous dietary guidelines emphasise the critical role of the consumption of a diet that is varied and includes different foods from different food groups [2,4–7].
Previous aetiological work has tended to examine the association between diet and health by studying individual nutrients, certain food groups, or overall diet quality. Although greater intake of different food subtypes (minor food groups) from each major food group is crucial for nutritional adequacy [8], indices of diet quality rarely include a measure of dietary diversity and none address variety within food groups other than for fruits and vegetables [9,10]. Recent prospective studies in the EPIC cohort indicated that consuming a higher number of different items within the fruit (0–58) and/or vegetable (0–59) food groups was associated with a reduced risk of T2D [11] and certain cancers [12,13], independent of known confounders and quantity of intake. Furthermore, specific subtypes of dairy products are also likely to matter for T2D, specifically low-fat fermented items such as yoghurt [14]. Consumption of a higher number of major food groups has been associated with lower all-cause and cause-specific mortality [15,16]. More recently, however, analysis in a multi-ethnic cohort concluded that a higher number of different food items (between 0 and 120) consumed at least twice a week was not associated with incident T2D [17].
It is possible that a diet that is comprised of all five major food groups could still rely on consumption of a narrow range of foods within each food group. In that sense, it would have overall diversity at the major food group consumption level but would not be varied in terms of different subtypes of foods. Therefore, we aimed to investigate how variation between and within each major food group was related to diabetes risk. We hypothesised that greater diversity across major food groups would be associated with lower T2D incidence and that there would be an independent impact of greater diversity of minor food groups within each major group. A secondary aim was to assess the monetary cost associated with dietary diversity, and we expected a greater cost associated with greater diversity.
Methods
Ethics Statement
A prescribed informed consent statement was signed by all participants in the EPIC-Norfolk study. The study was approved by the Norwich District Health Authority Ethics Committee.
Study Population
The EPIC-Norfolk study is a population-based prospective cohort study that has been described in detail elsewhere [18]. In brief, EPIC-Norfolk included 25,639 participants (55% women) aged 40–79 years (99.7% white) who were recruited from age-sex registers of general practices in a geographically circumscribed area in the East of England, and who attended a clinical assessment at cohort entry (1993–1997). Participants were followed up using an 18-mo postal questionnaire, a second clinical assessment (1998–2000), and a second postal questionnaire (2002–2004). We excluded participants with known diabetes at baseline (n = 855), unknown diabetes status (n = 5), or missing information on potential confounders (n = 1,541), providing a final sample of 23,238 individuals for analysis (S1 Fig).
Case Ascertainment
New T2D cases were ascertained from multiple sources: two follow-up health and lifestyle questionnaires providing self-reported information on doctor-diagnosed diabetes or medications; medications brought to the second clinical exam; and record linkage. Record linkage to external sources included the listing of any EPIC-Norfolk participant in the general practice diabetes register, local hospital diabetes register, hospital admissions data with screening for diabetes-related admissions, and Office of National Statistics mortality data with coding for diabetes. Participants who self-reported a history of diabetes which could not be confirmed against any other sources were not considered as confirmed cases. Follow-up was censored at date of diagnosis of T2D, 31 July 2006, or date of death, whichever came first.
Dietary Diversity Assessment
A semi-quantitative Food Frequency Questionnaire (FFQ) was used to assess habitual dietary intake at baseline, asking respondents to “estimate average food use during the last year” for 130 of the most commonly consumed food and beverage products. The FFQ provided a standard serving size for each product with nine standard response categories, from never or less than once/month to six or more/day [19]. A separate question was concerned with daily intake of milk, with six possible responses from none to more than one pint.
We used raw frequency data to construct a summary score to assess total diet diversity based on a count of five major food groups used in current food guides for eating well: dairy products, fruits, vegetables, grain/cereal products, and meat and alternatives (protein) [8,20,21]. We also constructed additional scores for dairy diversity (milk, cheese, yoghurt), fruit diversity (vitamin A-rich, citrus and berry, other), vegetable diversity (vitamin A-rich, dark green leafy, starchy tubers, other), “meat and alternatives” diversity (flesh meat—red (including processed), organ meat, flesh meat—poultry, fish and seafood, eggs, legumes/beans and nuts and seeds), and grain diversity (whole grains, non-wholegrains). We assigned individual FFQ items to specific subtypes within each major food group based on previous work [8] and United Nation’s Food and Agriculture Organization food group classification guidance [22] (see S1 Table). Similar to other studies [23], items consumed at least twice per week were considered to constitute habitual intake and counted in the relevant food group. A participant scored zero when they reported intakes of an item to be once a week, 1–3 a month, or never/less than once a month. FFQ responses of one pint (0.5683 L) or more than one pint counted as daily milk intake based on dietary guidelines of 3 cups/d (1.249 imperial pint) [24]. Mixed dishes (e.g., soups, quiche) were separated into main components using codebook description of standard recipes [25], and assigned to relevant food groups and subtypes when ingredients contributed at least 10% to the dish’s total weight or were listed among the top five components. For items with unavailable codebook recipes, we used online lists of ingredients for common brands (e.g., Heinz oxtail soup). Each diversity score increased by one when a different food group was consumed; the score increased regardless of the quantity of an item from a given group or the number of possible items from the same group. We also calculated a composite score for diversity of intake of all food group subtypes (0–18).
Covariables based on completed health and lifestyle questionnaires included education level (four categories), UK Registrar General’s occupational social class (six categories), smoking status (three categories) [26], overall physical activity (four categories), and history of myocardial infarction, stroke, or cancer and family history of diabetes (binary). Waist circumference, height, and weight were measured to standard protocol, and body mass index (BMI) calculated as kg/m2.
Diet Cost Estimation
The monetary cost of the reported diets was estimated by linking food price data for individual foods to the EPIC FFQ’s nutrient composition database as described previously [27]. Retail prices for each of the 289 component food items in the FFQ were obtained by using standardized and published price collection methods [28]. In brief, each food and drink item in the FFQ was priced by using MySupermarket.com, a website for comparing supermarket food prices nationwide in the United Kingdom. For each of the 289 items in the FFQ, we selected the lowest, non-sale price from among the five nationwide retailers on the website at that time (June 2012): Tesco, Sainsbury’s, Asda, Waitrose, and Ocado, which together had a 68% market share at that time [29]. For packaged food (including most fresh produce), we selected the middle size of the range of size options or the larger size if only two options were available. As described previously [28,30], prices were adjusted for preparation losses and cooking fraction to yield an adjusted food price of £/100 g edible portion. The addition of this new variable to the EPIC-Norfolk’s food and nutrient database [31] allowed the derivation of dietary cost for each participant. The variable associated with each individual's diet was cost per day (£/d).
Data Analysis
Means with standard deviations and frequencies were used to describe the characteristics of the cohort across three levels of the total diet diversity score (≤3, 4, or 5). Covariance matrices were used to assess the strength of relationships between diversity scores. Multivariable Cox regression analyses were used to examine the relationship between each diversity score and the risk of developing T2D. Hazard ratios (HR) and 95% confidence intervals (95% CI) were estimated using a series of models: model 1 adjusted for age, sex, BMI, and total energy intake (Kcal) (n = 23,912); model 2 additionally adjusted for lifestyle factors (smoking status, alcohol intake (units/week) and physical activity level) plus family history of diabetes (n = 23,705); and model 3 further adjusted for socioeconomic status (education and occupational social class) (n = 23,238). Using model 3, the independent relationship of total diet diversity and T2D was then examined by separately including each specific food group diversity score and by including all five specific food group diversity scores. In addition, the independent relationship of each specific food group diversity score with T2D was examined by including (1) the total diet diversity score, (2) the four other specific food group diversity scores, or (3) the total diet diversity score and all other specific food group diversity scores.
Sensitivity analyses included the total quantity of intake of all items from the relevant food group in model 3 to control for the relationship between the diversity of food groups and the number of foods reported, which is independently associated with nutrient adequacy [32]. Waist circumference, as a marker of central adiposity, was also included in model 3, as it may be an independent risk factor of cardio-metabolic conditions [33]. Vegetable diversity was re-examined after excluding all potato items and, alternatively, restricting to baked and boiled potatoes given the high consumption in the UK of fried potato products, which would contribute to higher fat and energy intakes. Analyses were also repeated after additionally excluding participants with self-reported chronic conditions. We also undertook a sensitivity analysis in the sub-sample of EPIC-Norfolk (n = 10,787) in whom HbA1c was measured at baseline to exclude individuals (n = 262) who had a baseline HbA1c ≥6.5% (or ≥48 mmol/mol), which is indicative of prevalent but undiagnosed diabetes.
Multivariable linear regression was used to assess cross-sectional associations at baseline between each diversity score and diet cost, adjusting for age, sex, and total energy intake (n = 23,238). We used regression coefficients for post-estimation calculation of adjusted means (95% CI). Statistical analyses were conducted using Stata version 13.1.
Results
The average duration of follow up was 10 (±1.5) y, and we identified 892 new cases of T2D over 245,045 person-years of follow up. On average, participants reported consuming 4.7 (0.6) major food groups at least twice or more per week. Very few participants reported consuming foods from two groups (0.45%), one group (0.07%), or none (0.01%); while most reported consuming four (21.29%) or five groups (74.43%) and some consumed only three groups (3.75%). Within the specific food groups, there was more evidence of heterogeneity in reported diets between individuals. A diversity score of zero was observed in 13.4% of participants for dairy products, 7.8% for fruits, and 8.1% for meat (and alternatives). For participants who scored three for total diet diversity, we found that 80% scored zero for dairy, 62% for fruit, 10% for vegetables, 55% for meat, and 9% for grain. And among participants who scored four for total diet diversity, there were 47% scoring zero for dairy, 24% for fruit, 1% for vegetables, 27% for meat and 1% for grain.
Total diet diversity was positively correlated with diversity within each specific food group: dairy (r = 0.52), fruits (r = 0.42), vegetables (r = 0.28), meat and alternatives (r = 0.35), and grains (r = 0.21). The specific food group diversity scores were not correlated with each other, except for the scores for diversity in fruits and vegetables (r = 0.23), and vegetables and meat and alternatives (r = 0.25). Table 1 shows that participants who reported regular consumption of a diet with greater total diet diversity had more favourable socioeconomic and lifestyle profiles.
Tab. 1. Baseline characteristics of total diet diversity in participants in the EPIC-Norfolk study. 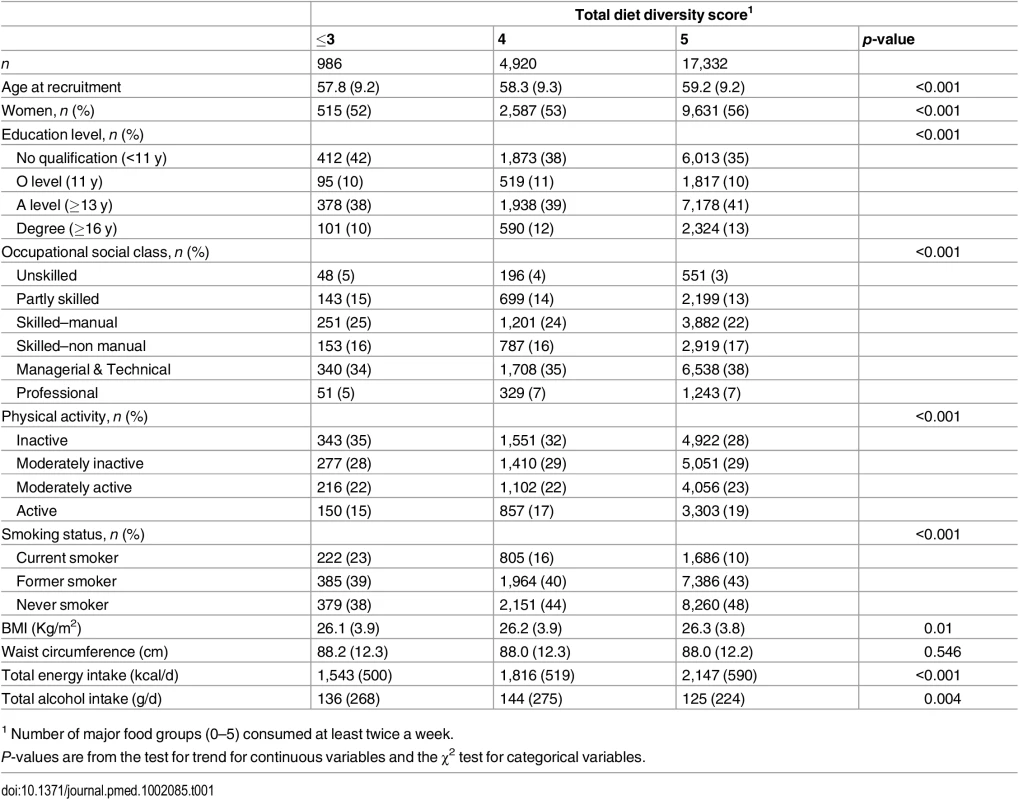
1 Number of major food groups (0–5) consumed at least twice a week. As shown in Table 2, the total diet diversity score and the specific food group diversity scores for dairy products, fruits, and vegetables were each inversely associated with risk of developing T2D (Model 1). Participants who reported meeting the recommendation to consume foods from all five food groups had a 30% lower incidence of T2D (HR 0.70 [0.51, 0.95]), but those consuming only four major food groups did not have a lower risk (HR 0.85 [0.62, 1.18]) compared to those reporting intakes of three or fewer food groups. Similarly, those participants who reported the greatest level of diversity of consumption of dairy products, fruits, or vegetables had a 38% (HR 0.62 [0.47, 0.83]), 35% (HR 0.65 [0.50, 0.84]), and 33% (HR 0.67 [0.52, 0.86]), respectively, lower risk of T2D compared to the individuals with the least variation of subtypes within a specific food group. In the case of these three specific food groups, there was a significant linear trend with the risk of developing diabetes being inversely related to the degree of food group diversity. There was no association with diversity within the meat or grain food groups. Adjustment for family history and lifestyle factors (Model 2) and additionally for socioeconomic status (Model 3) did not appreciably alter the HRs. We also observed a strong inverse association between the summary score for diversity of all food group subtypes and risk of developing type 2 diabetes (p for trend <0.01) (S2 Table).
Tab. 2. Adjusted hazard ratios (95% CI) of incident diabetes for total diet diversity and for diversity within each major food group in the EPIC-Norfolk study. 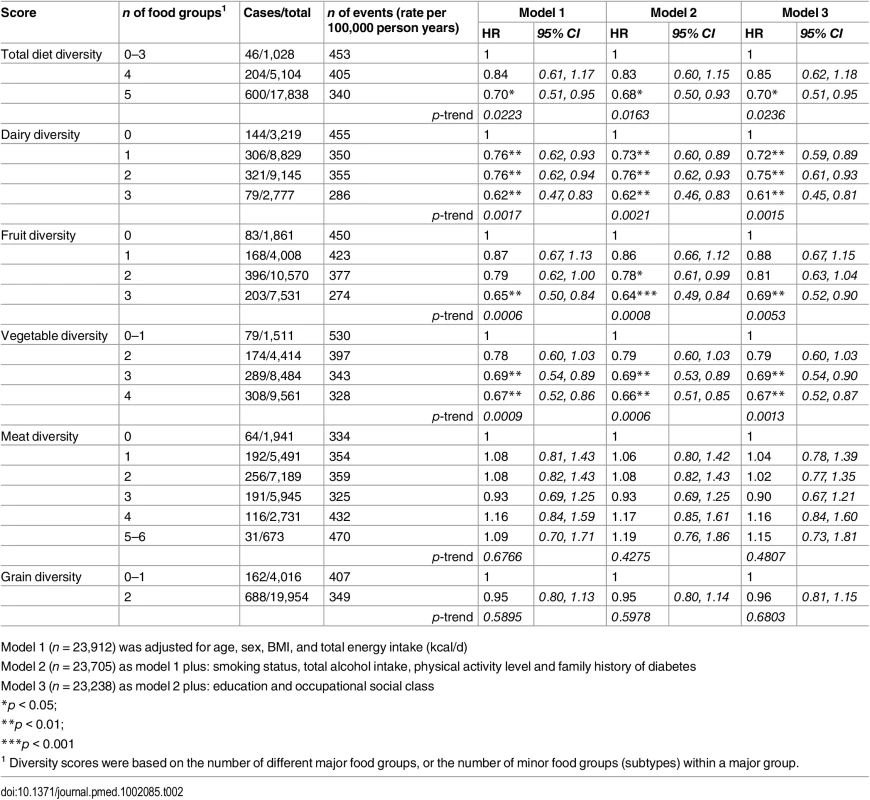
Model 1 (n = 23,912) was adjusted for age, sex, BMI, and total energy intake (kcal/d) After additionally mutually adjusting for all diversity scores within specific food groups, the inverse association of total diet diversity with diabetes risk was attenuated and became non-significant (p = 0.47) (Table 3, Model 6). In analyses adjusting for the association of other specific food group diversity scores, the inverse association of dairy, fruit, and vegetable diversity with T2D remained statistically significant (Table 4, Model 2). However, after accounting for total diet diversity and all other specific food group diversity scores, only dairy and vegetable diversity were significantly independently associated with diabetes risk (Table 4, Model 3).
Tab. 3. Adjusted hazard ratios (95% CI) of incident diabetes for total diet diversity in the EPIC-Norfolk study, independent of diversity within specific food groups. 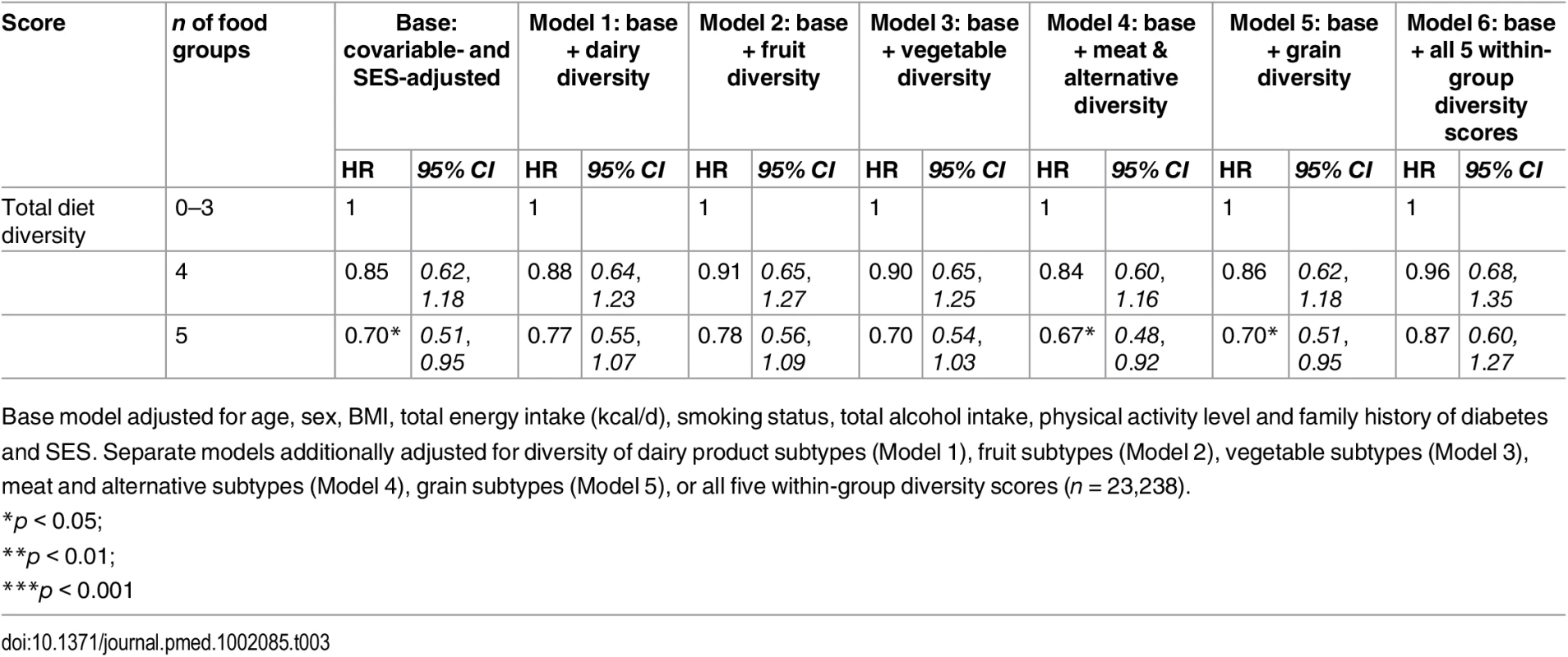
Base model adjusted for age, sex, BMI, total energy intake (kcal/d), smoking status, total alcohol intake, physical activity level and family history of diabetes and SES. Separate models additionally adjusted for diversity of dairy product subtypes (Model 1), fruit subtypes (Model 2), vegetable subtypes (Model 3), meat and alternative subtypes (Model 4), grain subtypes (Model 5), or all five within-group diversity scores (n = 23,238). Tab. 4. Adjusted hazard ratios (95% CI) of incident diabetes for diversity of dairy products, fruits, vegetables, grains, and meat products in the EPIC-Norfolk study, independent of total diet diversity and diversity within other food groups. 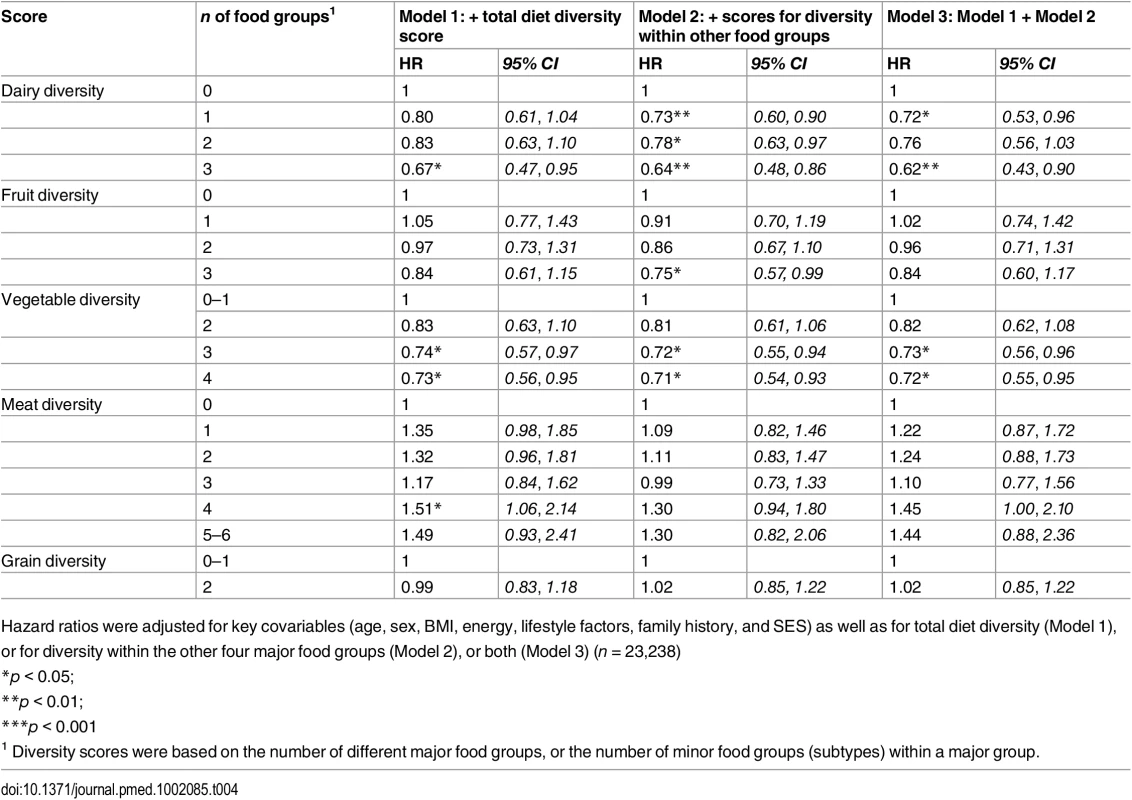
Hazard ratios were adjusted for key covariables (age, sex, BMI, energy, lifestyle factors, family history, and SES) as well as for total diet diversity (Model 1), or for diversity within the other four major food groups (Model 2), or both (Model 3) (n = 23,238) Inclusion of total quantity of all items from a given food group attenuated results for total diet diversity and dairy diversity, although inverse associations were amplified for vegetable diversity and unaffected for fruit diversity. Results were unaffected in sensitivity analyses after including waist circumference or excluding participants with self-reported chronic conditions. After excluding participants with a baseline level of HbA1c ≥ 6.5%, greatest fruit diversity and vegetable diversity showed stronger inverse associations with T2D (HR 0.42 [0.23, 0.75] and HR 0.56 [0.32, 0.97], respectively) as did total diet diversity (HR 0.53 [0.28, 1.00]) (S3 Table). Finally, inverse associations were stronger for total diet diversity and similar for vegetable diversity when we counted only baked and boiled potatoes, or did not count potato items consumed at least twice a week (S4 Table).
The adjusted mean diet cost was 18% higher for participants consuming all five major food groups (£4.15/day [4.14 to 4.16]), and 7% for four major food groups (£3.85/day [3.83 to 3.88]), compared to those reporting limited diversity (£3.53/day [3.48 to 3.59]) (Table 5 and S2 Fig). The comparison for the costs of diversity within the specific food groups suggested differences between extreme categories of 7% (£0.29/day) for dairy diversity (4.25 [4.22, 4.29] versus 3.96 [3.93, 3.99]), 22% (£0.81/day) for fruit diversity (4.43 [4.41, 4.45] versus 3.63 [3.59, 3.67]), 30% (£1.01/day) for vegetable diversity (4.40 [4.38, 4.42] versus 3.39 [3.35, 3.44]), 42% (£1.47/day) for meat (and alternatives) diversity (4.93 [4.87, 5.00] versus 3.48 [3.44, 3.51]), and -1% (£0.06/day) for grain diversity (4.05 [4.04, 4.06] versus 4.11 [4.08, 4.14]) (Table 5 and S2 Fig). The summary score for diversity of all food group subtypes was also associated with a significant added diet cost (p for trend < 0.001) (Table 5).
Tab. 5. Adjusted mean daily diet cost (95% CI) of total diet diversity and of diversity within each major food group in the EPIC-Norfolk study. 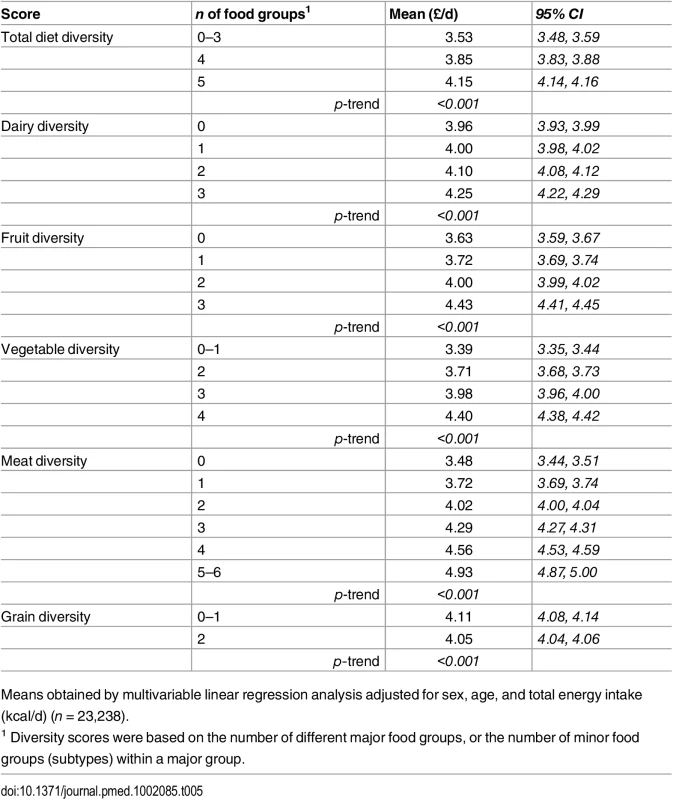
Means obtained by multivariable linear regression analysis adjusted for sex, age, and total energy intake (kcal/d) (n = 23,238). Discussion
This prospective population-based cohort study of 23,238 British adults suggested that individuals who report regular weekly consumption of all five major food groups subsequently had a lower risk of developing type 2 diabetes as did people who consumed diets that were rich in variability within the dairy, fruit, and vegetable food groups. The association of total diet diversity was attenuated after accounting for diversity within the five food groups. However, greater diversity within dairy, fruit, and vegetable food groups remained predictive of diabetes, independent of diversity in each of the other food groups. The cost of a diet that was varied was significantly higher than the cost of one that was the least diverse.
Previous epidemiological studies show that several diet quality indices are associated with 9%–13% reduced risk of T2D [9,10]. However, these studies do not separately examine the role of dietary diversity in relation to T2D. Variety of foods is only considered as a component of a few diet quality indices (e.g., Healthy Eating Index and Dietary Guidelines Index) [9,10]. Studies using diet diaries have shown the risk of T2D is lower by 14%–21% in people reporting higher vegetable intake, particularly green leafy vegetables [11,34], and by 15%–28% with greater reported dairy product intake, specifically yoghurt consumption [14,35]. Previous studies have also examined the broader health impact of total diet diversity, showing higher risk of mortality in people who reported consuming diets with only two food groups or fewer per week when measured by 24 h recall [15,16]. To date, the only published study that examined diversity within specific food groups (fruits and vegetables), showed that people who reported consuming 12 different fruit and vegetable items per week had a 39% lower risk of developing T2D [11]. Despite common advice to consume a varied diet [2,5,7], we are not aware of studies investigating how the number of different food groups and different subtypes within each food group included in a diet are associated with risk of diabetes. Our findings suggest that individuals who meet the recommendation to consume a healthy diet with food items from each of the five food groups had a reduced risk of developing T2D. More notably, our results further showed that people reporting regular consumption of the full range of food subtypes within dairy, fruit, and vegetable food groups also had a reduced risk of T2D.
The biological pathways linking the inverse associations of total diet diversity and diversity within three specific food groups with T2D risk are unclear. A recent study using FFQ data in older adults reports that greater diversity of foods consumed was significantly positively correlated with a more diverse intestinal microbiota, suggesting that dietary diversity influences microbiota composition [36]. Complementary experiments in that study further showed that dietary changes toward lower diversity resulted in losses in the range of different intestinal microbiota and that reduced microbiota diversity was associated with poorer health outcomes [36]. Greater within-group diversity may also have a specific role for health by providing a balance of the multitude of micronutrients, dietary fibre, and other bioactive compounds necessary for maintaining physical functioning [37]. The particular benefits of fruit and vegetable diversity may derive from the inclusion of phytochemicals that are more specific to certain subgroups that individuals with more varied intakes might consume preferentially [38]. For example, greater vegetable diversity may provide individuals with specific subgroups that contain high concentrations of flavonoids and carotenoids, which have known health benefits [39].
While diverse diets may be healthier, they are also more costly [40–42]. Others have reported a difference of 12% in total weekly food expenditure when comparing top and bottom ranges of food variety [43,44]. In the current study, the adjusted mean cost of the whole diet was 18% higher for participants consuming all five food groups compared to those consuming only three or fewer groups. In light of global 5-a-day campaigns emphasising fruit and vegetable variety, it is important for public health efforts to acknowledge that the adoption of diets including all vegetable and all fruit subtypes may be substantially more costly for consumers and may especially exacerbate existing socioeconomic inequalities in diet. Others also note the higher cost of better quality diets [28,45]. Modelling evidence indicates that combining food taxes and subsidies as a multifaceted policy intervention could best support individuals in making healthy food choices so as to prevent chronic conditions and to help reduce health disparities [46,47]. Given the rising price of healthy food groups, there is a need for a comprehensive food pricing strategy to target the increase in the diversity of foods individuals consume, particularly within fruits and vegetables. Further work should investigate how to develop and implement such a policy approach and to evaluate the impact on equity.
The strengths and weaknesses of this study deserve attention. Strengths include a large sample size, prospective study design, thorough assessment of new cases of T2D with self-report information supplemented by external sources, use of established classification of food groups, and comprehensive information on covariables, thereby minimising sources of bias and confounding. In particular, we examined the exposure to different subtypes within each major food group using two approaches (separate within-group scores and a composite score of all food subtypes). The greater magnitude of effect on diabetes incidence and more pronounced diet cost using the composite score further corroborates the primary findings. Another strength of our study was the availability in a subgroup of HbA1c data at baseline, allowing us to confirm that our findings were unaffected by undiagnosed cases of T2D at baseline. However, some potential limitations merit discussion. First, as an observational study, results may be limited by residual confounding or confounding by unmeasured factors. Second, dietary data were based on self-report from FFQ and therefore may be prone to error and bias [19]. In particular, participants who reported diets with limited variety of food groups may have poor completion of the FFQ. Nonetheless, we took an over-inclusive scoring approach, which likely captured diets that had lower levels of diversity. Moreover, FFQ data are suitable for ranking individuals according to habitual intakes and our scoring approach using frequency information avoided the many assumptions used to estimate absolute intakes [19]. However, the diversity scores were limited by the fact that they were based on a simple yes/no for consumption at least twice a week, regardless of the amount consumed, the number of items consumed within a given food group, or the potential healthfulness of an item (e.g., whole-fat versus low-fat milk, lean meats versus red and processed meats, fried fish versus baked fish). In addition, our study did not account for changes in diet diversity and/or changes in other lifestyle factors over follow-up, and our price data were from 2012 because information was not available retrospectively for study baseline. Finally, the EPIC-Norfolk data provides strong external validity and generalisability only to other predominantly European-descended and middle-aged populations.
Conclusion
This large epidemiological study in a population-based cohort is the first to report an association of total diet diversity and diversity within specific food groups with lower risk of diabetes. These findings support current public health recommendations encouraging consumption of all major food groups and also of different types of fruits, vegetables, and dairy products as part of a regular balanced diet. However, the additional cost of greater diversity deserves attention toward a comprehensive food pricing strategy. Future work should investigate how to develop and implement such a policy approach, including the consideration of financial incentives to actively support lower-income groups in achieving a healthy, mixed diet.
Supporting Information
Zdroje
1. World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: WHO, 2009.
2. World Health Organization. Global strategy on diet, physical activity and health. Geneva, Switzerland: WHO Publications; 2004.
3. The Strategy Unit. Food Matters: towards a strategy for the 21st century. London, UK: Cabinet Office, 2008.
4. USDA and DHHS. Nutrition and your health: dietary guidelines for Americans. Washington, DC: US Government Printing Office; 1980.
5. USDA and DHHS. Dietary guidelines for Americans, 7th ed Washington, DC: Government Printing Office, 2010.
6. Department of National Health and Welfare. Canada's Food Guide: handbook. Ottawa, ON.: The Ministry of Health and Welfare, Health Promotion Directorate; 1982.
7. World Cancer Research Fund. 10 ways to prevent cancer [online] http://www.wcrf-uk.org/cancer_prevention/recommendations/index.php: WCRF [accessed on: 17 April 2014].
8. Foote JA, Murphy SP, Wilkens LR, Basiotis PP, Carlson A. Dietary variety increases the probability of nutrient adequacy among adults. The Journal of Nutrition. 2004;134(7):1779–85. 15226469
9. Kant AK. Indexes of overall diet quality: a review. Journal of the American Dietetic Association. 1996;96(8):785–91. doi: 10.1016/s0002-8223(96)00217-9 8683010
10. Waijers P, Feskens E, Ocké MC. A critical review of predefined diet quality scores. British Journal of Nutrition. 2007;97 : 219–31. doi: 10.1017/S0007114507250421 17298689
11. Cooper A, Sharp S, Lentjes M, Luben R, Khaw K, Wareham N, et al. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care. 2012;35(6):1293–300. doi: 10.2337/dc11-2388 22474042
12. Jeurnink SM, Büchner FL, Bueno-de-Mesquita HB, Siersema PD, Boshuizen HC, Numans ME, et al. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European prospective investigation into cancer and nutrition. International Journal of Cancer. 2012;131(6):E963–E73. doi: 10.1002/ijc.27517 22392502
13. Büchner FL, Bueno-de-Mesquita HB, Ros MM, Overvad K, Dahm CC, Hansen L, et al. Variety in fruit and vegetable consumption and the risk of lung cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiology Biomarkers & Prevention. 2010;19(9):2278–86. doi: 10.1158/1055-9965.epi-10-0489
14. O’Connor L, Lentjes MH, Luben R, Khaw K-T, Wareham N, Forouhi N. Dietary dairy product intake and incident type 2 diabetes: a prospective study using dietary data from a 7-day food diary. Diabetologia. 2014;57(5):909–17. doi: 10.1007/s00125-014-3176-1 24510203
15. Kant A, Schatzkin A, Harris T, Ziegler R, Block G. Dietary diversity and subsequent mortality in the First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. The American Journal of Clinical Nutrition. 1993;57(3):434–40. 8382446
16. Kant AK, Schatzkin A, Ziegler RG. Dietary diversity and subsequent cause-specific mortality in the NHANES I epidemiologic follow-up study. Journal of the American College of Nutrition. 1995;14(3):233–8. 8586771
17. de Oliveira Otto M, Padhye N, Bertoni A, Jacobs D, Mozaffarian D. Everything in moderation—dietary diversity and quality, central obesity and risk of diabetes. PLoS ONE. 2015;10(10):e0141341. doi: 10.1371/journal.pone.0141341 26517708
18. Day N, Oakes S, Luben R, Khaw KT, Bingham S, Welch A, et al. EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. British Journal of Cancer. 1999;80 Suppl 1 : 95–103. 10466767
19. Willett W. Nutritional epidemiology. Third edition. Oxford: Oxford University Press; 2013.
20. Department of Health (DH), National Health and Medical Research Council. The Five Food Groups https://www.eatforhealth.gov.au/food-essentials/five-food-groups: Australian Government; [cited 13 May 2016].
21. US Department of Agriculture. Choose my plate http://www.choosemyplate.org [13 May 2016].
22. FAO Nutrition and Consumer Protection Division. Guidelines for measuring household and individual dietary diversity. Rome, Italy: Food and Agriculture Organization of the United Nations, 2011.
23. McNaughton SA, Bates CJ, Mishra GD. Diet quality is associated with all-cause mortality in adults aged 65 years and older. The Journal of Nutrition. 2012;142(2):320–5. doi: 10.3945/jn.111.148692 22190031
24. USDA and DHHS. Dietary guidelines for Americans, 2005. Washington, DC: US Government Printing Office, 2005.
25. Food Standards Agency. McCance and Widdowson's The Composition of Foods, Sixth edition. Cambridge: Royal Society of Chemistry; 2002.
26. Office of Population Censuses and Surveys. General Household Survey. London: HMSO, 1992.
27. Monsivais P, Scarborough P, Lloyd T, Mizdrak A, Luben R, Mulligan AA, et al. Greater accordance with the Dietary Approaches to Stop Hypertension dietary pattern is associated with lower diet-related greenhouse gas production but higher dietary costs in the United Kingdom. The American Journal of Clinical Nutrition. 2015;102(1):138–45. doi: 10.3945/ajcn.114.090639 25926505
28. Monsivais P, Drewnowski A. Lower-energy-density diets are associated with higher monetary costs per kilocalorie and are consumed by women of higher socioeconomic status. Journal of the American Dietetic Association. 2009;109(5):814–22. doi: 10.1016/j.jada.2009.02.002 19394467
29. News BBC. Tesco market share dips below 30% 31 January 2012: [Online] Accessed on: 2 February 2015.
30. Monsivais P, Perrigue M, Adams S, Drewnowski A. Measuring diet cost at the individual level: a comparison of three methods. European Journal of Clinical Nutrition. 2013;67(11):1220–5. doi: 10.1038/ejcn.2013.176 24045791
31. Mulligan AA, Luben RN, Bhaniani A, Parry-Smith DJ, O'Connor L, Khawaja AP, et al. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open. 2014;4(3). doi: 10.1136/bmjopen-2013-004503
32. Krebs-Smith SM, Smiciklas-Wright H, Guthrie HA, Krebs-Smith J. The effects of variety in food choices on dietary quality. Journal of the American Dietetic Association. 1987;87(7):897–903. 3598038
33. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. The American Journal of Clinical Nutrition. 2007;85(5):1197–202. 17490953
34. Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis2010 2010-08-19 23 : 05 : 28.
35. Elwood PC, Pickering JE, Givens DI, Gallacher JE. The Consumption of Milk and Dairy Foods and the Incidence of Vascular Disease and Diabetes: An Overview of the Evidence. Lipids. 2010;45(10):925–39. doi: 10.1007/s11745-010-3412-5 PMC2950929. 20397059
36. Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. doi: 10.1038/nature11319 22797518
37. Bernstein MA, Tucker KL, Ryan ND, O’Neill EF, Clements KM, Nelson ME, et al. Higher dietary variety is associated with better nutritional status in frail elderly people. Journal of the American Dietetic Association. 2002;102(8):1096–104. doi: 10.1016/s0002-8223(02)90246-4 12171454
38. Randall E, Nichaman M, Contant C. Diet diversity and nutrient intake. Journal of the American Dietetic Association. 1985;85(7):830–6. 4008835
39. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Archives of Internal Medicine. 2009;169(7):659–69. doi: 10.1001/archinternmed.2009.38 19364995
40. Lo Y-T, Chang Y-H, Lee M-S, Wahlqvist ML. Health and nutrition economics: diet costs are associated with diet quality. Asia Pacific Journal Of Clinical Nutrition. 2009;18(4):598–604. PubMed Central PMCID: PMC19965354. 19965354
41. Galobardes B, Morabia A, Bernstein MS. Diet and socioeconomic position: does the use of different indicators matter? International Journal of Epidemiology. 2001;30(2):334–40. doi: 10.1093/ije/30.2.334 11369739
42. Giskes K, Turrell G, Patterson C, Newman B. Socioeconomic differences among Australian adults in consumption of fruit and vegetables and intakes of vitamins A, C and folate. Journal of Human Nutrition and Diet. 2002;15(5):375–85. doi: 10.1046/j.1365-277X.2002.00387.x
43. Temple JB. Household factors associated with older Australian's purchasing a varied diet: results from household expenditure data. Nutrition & Dietetics. 2006;63(1):28–35. doi: 10.1111/j.1747-0080.2006.00035.x
44. Lo Y-T, Chang Y-H, Wahlqvist ML, Huang H-B, Lee M-S. Spending on vegetable and fruit consumption could reduce all-cause mortality among older adults. Nutrition Journal. 2012;11(1):113.
45. Rao M, Afshin A, Singh G, Mozaffarian D. Do healthier foods and diet patterns cost more than less healthy options? A systematic review and meta-analysis. BMJ Open. 2013;3(12). doi: 10.1136/bmjopen-2013-004277
46. Sturm R, An R. Obesity and economic environments. CA: A Cancer Journal for Clinicians. 2014;64(5):337–50. doi: 10.3322/caac.21237
47. Mozaffarian D, Rogoff KS, Ludwig DS. The real cost of food: can taxes and subsidies improve public health? JAMA. 2014;312(9):889–90. doi: 10.1001/jama.2014.8232 25182094
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- The Clinical and Public Health Challenges of Diabetes Prevention: A Search for Sustainable Solutions
- Screening for Dysglycemia: Connecting Supply and Demand to Slow Growth in Diabetes Incidence
- Diabetes: A Cinderella Subject We Can’t Afford to Ignore
- Prescribing Exercise and Lifestyle Training for High Risk Women in Pregnancy and Early Post-partum—Is It Worth It?
- Population Approaches to Prevention of Type 2 Diabetes
- First-Year Evaluation of Mexico’s Tax on Nonessential Energy-Dense Foods: An Observational Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
- Leveraging Genetics to Advance Type 2 Diabetes Prevention
- Cardiometabolic Risk Factor Changes Observed in Diabetes Prevention Programs in US Settings: A Systematic Review and Meta-analysis
- Risks and Population Burden of Cardiovascular Diseases Associated with Diabetes in China: A Prospective Study of 0.5 Million Adults
- Dietary Diversity, Diet Cost, and Incidence of Type 2 Diabetes in the United Kingdom: A Prospective Cohort Study
- Detecting Dysglycemia Using the 2015 United States Preventive Services Task Force Screening Criteria: A Cohort Analysis of Community Health Center Patients
- Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial)
- Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women
- Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study
- Engagement, Retention, and Progression to Type 2 Diabetes: A Retrospective Analysis of the Cluster-Randomised "Let's Prevent Diabetes" Trial
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Supported Telemonitoring and Glycemic Control in People with Type 2 Diabetes: The Telescot Diabetes Pragmatic Multicenter Randomized Controlled Trial
- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Cycling and Diabetes Prevention: Practice-Based Evidence for Public Health Action
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mothers after Gestational Diabetes in Australia (MAGDA): A Randomised Controlled Trial of a Postnatal Diabetes Prevention Program
- Consumption of Meals Prepared at Home and Risk of Type 2 Diabetes: An Analysis of Two Prospective Cohort Studies
- Obesity and Life Expectancy with and without Diabetes in Adults Aged 55 Years and Older in the Netherlands: A Prospective Cohort Study
- Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-analysis of Randomised Controlled Feeding Trials
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



