-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCardiovascular and Renal Outcomes of Renin–Angiotensin System Blockade in Adult Patients with Diabetes Mellitus: A Systematic Review with Network Meta-Analyses
In a systematic review with network meta-analyses, Ferrán Catalá-López and colleagues synthesize published and unpublished data from randomized controlled trials of renin-angiotensin system inhibitors.
Published in the journal: . PLoS Med 13(3): e32767. doi:10.1371/journal.pmed.1001971
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001971Summary
In a systematic review with network meta-analyses, Ferrán Catalá-López and colleagues synthesize published and unpublished data from randomized controlled trials of renin-angiotensin system inhibitors.
Introduction
Diabetes mellitus has become one of the most challenging public health problems worldwide, affecting approximately 410 million people [1] and accounting for 1.3 million deaths in 2013, twice as many as in 1990 [2]. Complications of diabetes mellitus, especially cardiovascular and renal sequelae, cause substantial premature death and disability [1–4].
Medications aimed at inhibiting the renin–angiotensin system (RAS) have been used extensively for preventing cardiovascular and renal outcomes in patients with diabetes. Blockade of the RAS is a key therapeutic target because RAS controls circulatory volume and electrolyte balance and is an important regulator of hemodynamic stability. Currently, three classes of drugs that interact with the RAS are used to inhibit the effects of angiotensin II: angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and direct renin (DR) inhibitors. ACE inhibitors block the conversion of angiotensin I into angiotensin II, ARBs selectively inhibit angiotensin II from activating the angiotensin-specific receptor AT1, and DR inhibitors block the conversion of angiotensinogen into angiotensin I. Although all RAS blockers are intended to inhibit the effects of angiotensin II, there are differences that may distinguish them [5].
Most evidence-based guidelines for the management of hypertension and diabetes have generally recommended the use of ACE inhibitors and ARBs in preference to other antihypertensive agents [6–9]. In these guidelines, any particular RAS blocker (ACE inhibitor or ARB) is preferentially recommended as the treatment of choice. However, current guidelines are based on only a small number of randomized trials comparing the effects of RAS blockade specifically in patients with diabetes. Cardiovascular and renal outcomes with RAS blockers for adults with diabetes have been evaluated in large multicenter randomized controlled trials [10–22] and meta-analyses [23–28]. The task of establishing the comparative effectiveness of RAS blockers has been limited by the very complex array of trials that compare treatments. Results of recent meta-analyses have highlighted potential differences in treatment effects between ACE inhibitors and ARBs [23,24]. Traditionally, meta-analyses of RAS blockers have been limited by not including all the valuable information on the most common serious cardiovascular and renal outcomes [23–28], not exploring effects in the subgroup of patients with diabetes [29–37], and, importantly, omitting large trials with direct comparisons of RAS blockers and competing agents in clinically important subgroups [22,38–41]. Determining whether RAS blockers may be different in terms of their relative benefits and safety is a topic of great interest to patients, clinicians, scientists, guideline developers, and policy-makers. Unlike for previous analyses [23–27,42], many more trials, patients, and outcome data are now available for a comprehensive study to address this clinical question.
Given this knowledge gap, we aimed to examine the comparative effects of classes of RAS blockers in terms of cardiovascular and renal outcomes in the treatment of adult patients with diabetes mellitus. We used network meta-analyses to integrate direct and indirect evidence comparing multiple interventions of interest into unified analyses of all available randomized trials that can serve to guide evidence-based decision-making.
Methods
This systematic review was conducted and reported in accordance with the reporting guidance provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (S1 Checklist) [43,44]. We developed a systematic review protocol and registered it with PROSPERO (CRD42014014404). Our methods are briefly described here.
Study Eligibility Criteria
Studies meeting the following selection criteria were included: randomized parallel-group controlled trials of a minimum 1-y duration; participants adults aged 18 y or older with type 1 or 2 diabetes mellitus; reporting at least one of the cardiovascular or renal outcomes of interest as well as the number of participants enrolled in each treatment arm and the number of participants with events in each treatment arm; comparison of RAS blockers in monotherapy and/or combination therapy regimens, including ACE inhibitors, ARBs, and DR inhibitors versus each other or versus placebo or other active antihypertensive treatments (e.g., calcium channel blockers [CCBs], beta blockers [BBs], or diuretics).
Electronic Literature Search
Based on awareness of a large number of existing reviews and meta-analyses, an unlimited primary search for randomized trials was not conducted. In its place, we used a staged approach to study identification, beginning with a systematic search of relevant trials included in systematic reviews available in PubMed/MEDLINE and the Cochrane Database of Systematic Reviews (1 January 2004 to 17 July 2014) and existing meta-analyses of which we were aware (see S1 Text for details of search terms and S2 Text for references of previous reviews). PubMed/MEDLINE was next searched to identify other additional relevant trials published outside the time frames of previous reviews (1 January 2010 to 5 September 2014; reflects content from PubMed searches last conducted on 1 October 2014). We compiled a list of the unique PubMed/MEDLINE identification numbers of all relevant articles, and performed a related articles search. This technique has been shown to be effective in identifying relevant studies [45], increases efficiency in study identification in the presence of an already large evidence base, and is being used as part of an ongoing network meta-analysis research program [46]. Searches were supplemented by manual searches of clinical trial registers (including www.ClinicalTrials.gov) and review of references of relevant papers. Finally, we contacted authors of primary publications and sponsors of trials for missing outcome data or unclear information. Six drug manufacturers (Boehringer Ingelheim for ONTARGET [Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial] [40,41], PRoFESS [Prevention Regimen for Effectively Avoiding Second Strokes] [47], and TRANSCEND [Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects with Cardiovascular Disease] [48,49]; Daiichi Sankio for ROADMAP [Randomized Olmesartan and Diabetes Microalbuminuria Prevention] [50]; Sanofi for IDNT [Irbesartan Diabetic Nephropathy Trial] [16] and IRMA 2 [Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria] [17]; Servier for EUROPA/PERSUADE [Perindopril Substudy in Coronary Artery Disease and Diabetes] [51]; Takeda for DIRECT-Prevent 1 [52,53], DIRECT-Protect 1 [52,53], and DIRECT Protect 2 [53,54]; and Novartis for ALTITUDE [Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints] [22], VALUE [Valsartan Antihypertensive Long-Term Use Evaluation] [38], VALIANT [Valsartan in Acute Myocardial Infarction Trial] [39], Val-HeFT [Valsartan Heart Failure Trial] [55], and ASTRONAUT [Aliskiren Trial on Acute Heart Failure Outcomes] [56]) and four independent investigators (for ALLHAT [Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial] [20], CASE-J [Candesartan Antihypertensive Survival Evaluation in Japan] [57], COLM [Combination of Olmesartan and CCB or Low Dose Diuretics in High Risk Elderly Hypertensive Patients] [58], and OSCAR [Olmesartan and Calcium Antagonists Randomized] [59]) provided additional information about outcome data.
Prespecified Outcome Measures
The prespecified primary endpoints were major cardiovascular outcome (composite endpoint including death from cardiovascular causes, myocardial infarction, and stroke) and progression of renal disease (composite endpoint including doubling of baseline serum creatinine level, end-stage renal disease [ESRD] defined as the need for any dialysis or renal transplantation, and all-cause mortality). We also analyzed all of the component endpoints of these two composite endpoints separately. Secondary endpoints were angina pectoris and hospitalization for heart failure. All outcomes were based on the longest follow-up period available for each included study.
Screening, Data Extraction, and Risk of Bias Assessment
Eligible trials identified from our searching efforts were screened by one reviewer (F. Catalá-López, qualified clinical epidemiologist) and verified independently by two trained reviewers (D. Macías Saint-Gerons and C. de la Fuente-Honrubia, senior pharmacoepidemiologists). Using a predesigned form that was piloted on a small sample (14%) of studies, the same reviewers were also responsible for extraction and verification of data on general participant characteristics (e.g., average age, gender, type and duration of diabetes, level of albuminuria, presence of hypertension, presence of coronary artery disease, mean or median follow-up) and outcome data. The Cochrane risk of bias scale [60]—which considers sequence generation, allocation concealment, blinding, and other aspects of bias—was used to assess each study’s risk of bias. The overall rating of risk of bias for each study was the worst rating for any of the criteria (e.g., if any domain is scored high risk of bias, the study was considered high risk of bias). We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology [61,62] to evaluate the quality of evidence for each outcome (high, moderate, low, or very low quality). The approach we used was consistent with previous reporting of network meta-analyses [63]. Risk of bias assessments and application of GRADE methods were performed by one reviewer (F. Catalá-López) and validated by a second reviewer (D. Macías Saint-Gerons, C. de la Fuente-Honrubia. or M. Ridao). Any discrepancies between reviewers for any of the above steps were discussed until consensus was achieved.
Data Synthesis and Analyses
Our analysis classified RAS blockers used in monotherapy and/or combination as separate treatment nodes irrespective of their doses: ACE inhibitor, ARB, ACE inhibitor combination (e.g., ACE inhibitor plus ARB, ACE inhibitor plus DR inhibitor, ACE inhibitor plus diuretic, ACE inhibitor plus CCB), ARB combination (e.g., ARB plus DR inhibitor, ARB plus diuretic, ARB plus CCB), DR inhibitor combination (e.g., DR inhibitor plus diuretic), and placebo or control (conventional therapy/usual care). In the ASTRONAUT trial [56] (aliskiren versus placebo), more than 95% of the patients with diabetes were receiving a diuretic in both groups; therefore, the diabetes subgroup analysis from this study was regarded as a trial of DR inhibitor plus diuretic versus diuretic. Similarly, the ALTITUDE trial [22] (aliskiren versus placebo) evaluated a strategy of aliskiren in patients with diabetes who were also receiving an ACE inhibitor or an ARB and was therefore regarded as a trial of DR inhibitor plus ACE inhibitor versus DR inhibitor plus ARB versus ACE inhibitor versus ARB.
Whenever possible we used results from intention-to-treat analyses. To calculate direct estimates of treatment effect for each pair of medications, we conducted pairwise random effects meta-analyses. We report the results as odds ratios (ORs) and corresponding 95% confidence intervals (CIs). We evaluated statistical heterogeneity by estimating the variance between studies with Cochran’s Q test and I2 statistic [64,65]. The I2 statistic is the proportion of total variation observed between the trials attributable to differences between trials rather than to sampling error. We considered I2 < 30% as representing low statistical heterogeneity and I2 > 75% as representing high statistical heterogeneity. Patient and study characteristics were also empirically assessed by members of the research team to establish that these were comparable across studies and comparisons. For each outcome, we present graphically the geometry of the treatment network of all comparisons using a network graph [66]. Using a Bayesian framework, we did network meta-analyses for each prespecified outcome and treatment. Network meta-analysis [67,68] allows the integration of data from both direct and indirect evidence, increasing precision while randomization is preserved, but can also be used to estimate comparisons between pairs of treatments that have not been compared in individual studies. When performing a network meta-analysis, we relied on the assumptions of transitivity [69] (i.e., if drug B is superior to drug A, and drug C is superior to drug B, it is assumed that drug C is superior to drug A) and consistency (equivalency of treatment effects from direct and indirect evidence). We used random effects network meta-analysis models as recommended elsewhere, which account for correlations in multi-arm trials [70] and which use vague (noninformative) prior distributions for all treatment effects as well as the between-study variance parameter (S3 Text). We report the results as posterior median ORs with corresponding 95% credibility intervals (CrIs), which are the Bayesian analogue of 95% CIs. The ORs reported are relative effects of multiple RAS blocker regimens. We express these using ACE inhibitor as the reference treatment, because ACE inhibitors were the first class to be developed (with the arrival of captopril and enalapril in the early 1980s) and because these agents are frequently prescribed worldwide. All network meta-analyses were based on a total of 100,000 iterations or more, with a burn-in of 50,000 iterations. We assessed convergence on the basis of Brooks–Gelman–Rubin plots [71]. The consistency of results was qualitatively examined by comparing the results obtained via pairwise meta-analyses versus network meta-analyses. Consistency was also examined by fitting both consistency and inconsistency models [72] for network meta-analysis and comparing the deviance information criterion (DIC) between models, with smaller values indicative of a better fit, and a difference of five or more being considered as important. In general, when both models had a similar fit to the data as indicated by their DIC values, we concluded that there was no evidence of inconsistency. We also used the surface under the cumulative ranking curve (SUCRA) to potentially rank the treatments [73].
A priori sensitivity analyses were conducted to explore potentially important effect modifiers. These included separate analyses that involved exclusion of the following: studies with high risk of bias, small studies with fewer than 100 patients, studies in which >50% patients presented type 1 diabetes, and studies in non-hypertensive patients. Other preplanned analyses were the extension of the primary unadjusted network meta-analysis model to include covariates in meta-regression models [74] that considered adjustments for the following: year of publication, mean age of trial participants, percent of male participants, and control group risk rate. Considering that follow-up times varied between the included trials, we also estimated the rate ratios based on patient-years and corresponding 95% CrIs. All pairwise meta-analyses were conducted using Stata 13 (StataCorp, College Station, Texas, US), while Bayesian network meta-analyses were performed using WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, UK).
Results
Overview of Study Selection and Study Characteristics
We included 71 studies (described in 88 publications) that satisfied our inclusion criteria (PRISMA flowchart is shown in S1 Fig; details of the included studies are shown in S4 Text and S1–S7 Tables), with 103,120 individuals randomly assigned to one of the study treatments. The median duration of follow-up of the set of included studies was 3.2 y (range 1.0–9.0), and the median sample size was 436 individuals (range 30–13,168), with 55 trials having at least 100 participants and 24 trials having more than 1,000. Fifty-four studies (76%) were two-arm trials, 12 (17%) were three-arm trials involving three active agents or two active agents and placebo, and four (6%) were analyzed as multi-arm trials with four different active comparisons or three active agents and placebo. In terms of methodological quality and potential risk of bias (S2 and S3 Tables), 37 trials (52%) had an unclear risk of bias for at least one criterion, 25 trials (35%) had a low risk of bias, and nine trials (13%) had a high risk of bias. Fifty trials (70%) were funded by drug companies.
The characteristics of patients amongst the included studies are described in S4 Table. At baseline, the median age was 60 y, the median percentage of male participants was 61%, and the median duration of diabetes was 10.3 y. Forty-seven studies (63%) targeted type 2 diabetes, (5%) enrolled patients with type 1 diabetes, and ten studies (32%) contained mixed populations with type 1 or type 2 diabetes. In terms of level of albuminuria, 15 trials enrolled patients with macroalbuminuria, 13 enrolled patients with microalbuminuria, seven enrolled patients with normoalbuminuria, and 25 trials enrolled mixed populations. Approximately half of all studies (37 trials) included patients with hypertension at baseline, 15 trials enrolled normotensive participants, and 19 trials enrolled mixed populations. In terms of history of coronary disease, 58% of all studies (41 trials) were rated as unclear or not reported, and 27 trials were rated as having a mixed population (e.g., 5%–75% of participants with coronary disease at baseline).
The structure of the underlying evidence base for each analysis is described in Table 1 and Fig 1. The studies reporting data for each clinical outcome of interest are summarized in Table 1 (and S4–S7 Tables). Figs 1 and S2 show the evidence networks of eligible treatment comparisons for the full network meta-analysis. ACE inhibitors, placebo use or control (conventional therapy/usual care), and ARBs were most investigated (49, 36, and 25 trials, respectively). Treatments of interest in the main analyses were any RAS blocker compared with another RAS blocker or placebo or control. Other antihypertensive control arms (e.g., CCBs, BBs, or diuretics) were included in the evidence networks in order to incorporate additional indirect evidence for the analyses while preserving randomization. The results for these alternative antihypertensive treatments are presented in S8 Table.
Fig. 1. Evidence network of all treatment comparisons for all studies. 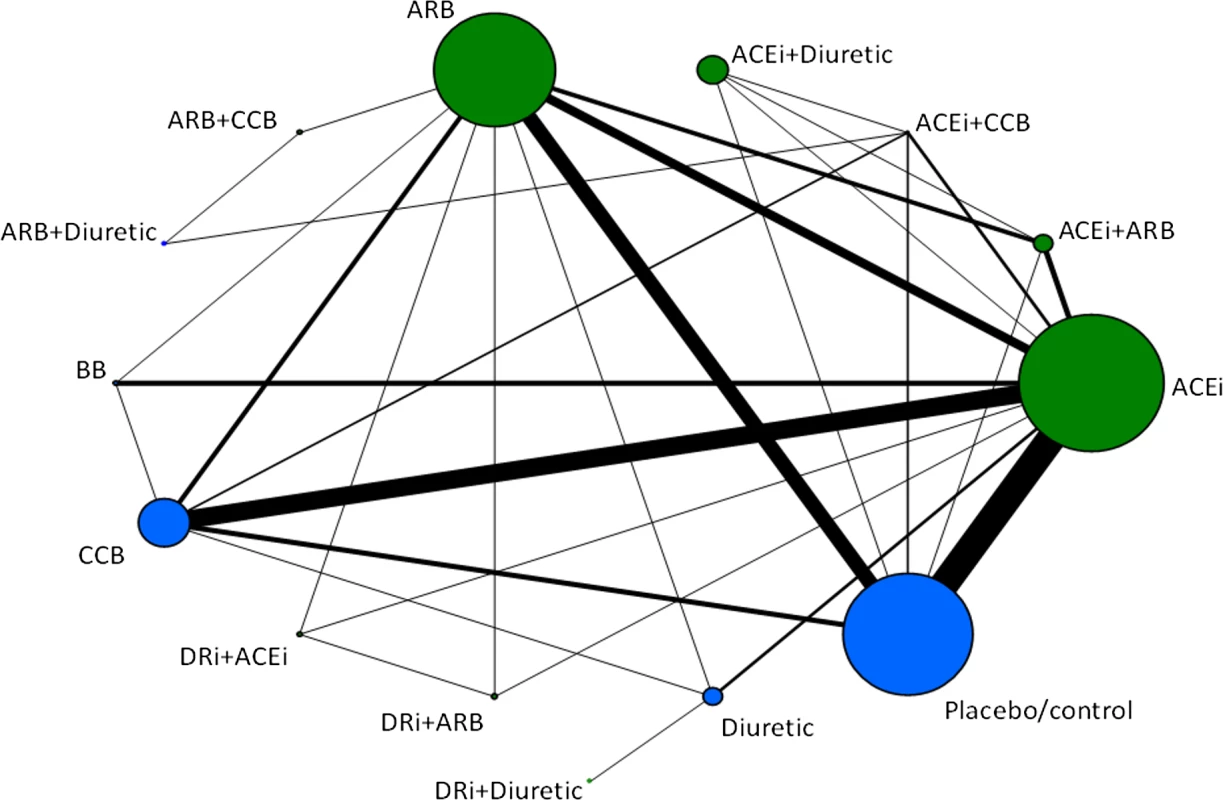
Lines represent direct comparisons within randomized controlled trials. The size of nodes is proportional to the number of randomized participants (sample size), and the width of the lines is proportional to the number of trials comparing each pair of treatments. Nodes in green represent RAS blockers (in monotherapy and/or combination therapies). Nodes in blue represent other control arms included in the evidence networks to preserve randomization. ACEi, ACE inhibitor; DRi, DR inhibitor. Tab. 1. Availability of data for principal analyses. 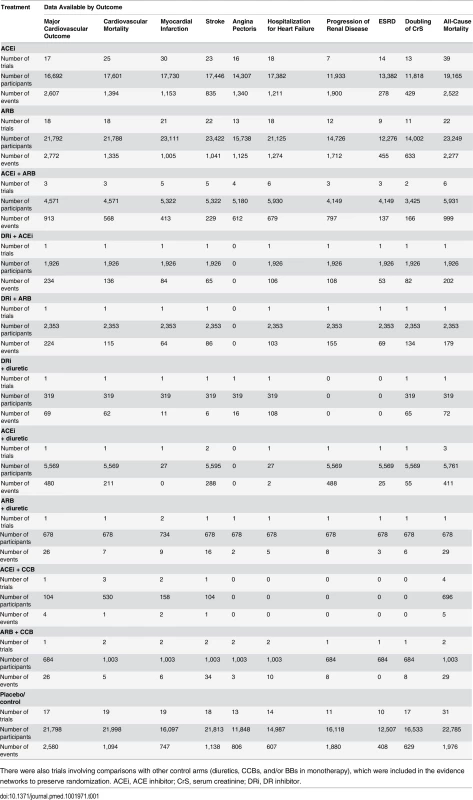
There were also trials involving comparisons with other control arms (diuretics, CCBs, and/or BBs in monotherapy), which were included in the evidence networks to preserve randomization. ACEi, ACE inhibitor; CrS, serum creatinine; DRi, DR inhibitor. Findings from Network Meta-Analysis
Summaries from all network meta-analyses are provided below for each outcome.
Major cardiovascular outcome (composite) was reported in 12,328 of 92,469 patients from a total of 33 studies. With placebo use as the reference, ACE inhibitors (OR 0.87; 95% CrI 0.75–0.99; moderate confidence) significantly reduced the risk of major cardiovascular outcome. Compared with ACE inhibitor as the reference treatment, none of the remaining RAS blockers used in monotherapy or combination were associated with significant risk reductions: ARB (1.02; 95% CrI 0.90–1.18; moderate confidence), ACE inhibitor plus ARB (0.97; 95% CrI 0.79–1.19; moderate confidence), DR inhibitor plus ARB (1.00; 95% CrI 0.73–1.38; low confidence), and DR inhibitor plus ACE inhibitor (1.32; 95% CrI 0.96–1.81; low confidence) (Tables 2, 3, and S8).
Tab. 2. Cardiovascular outcomes: comparisons of random effects pairwise meta-analysis with Bayesian network meta-analysis, including confidence of assessments. 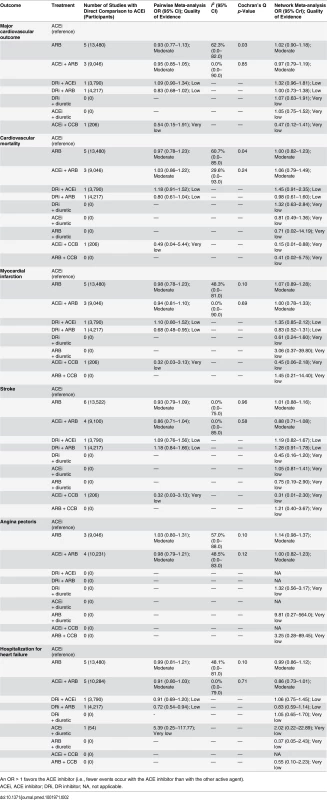
An OR > 1 favors the ACE inhibitor (i.e., fewer events occur with the ACE inhibitor than with the other active agent). Tab. 3. Major cardiovascular outcome (composite endpoint) and all possible treatment comparisons. 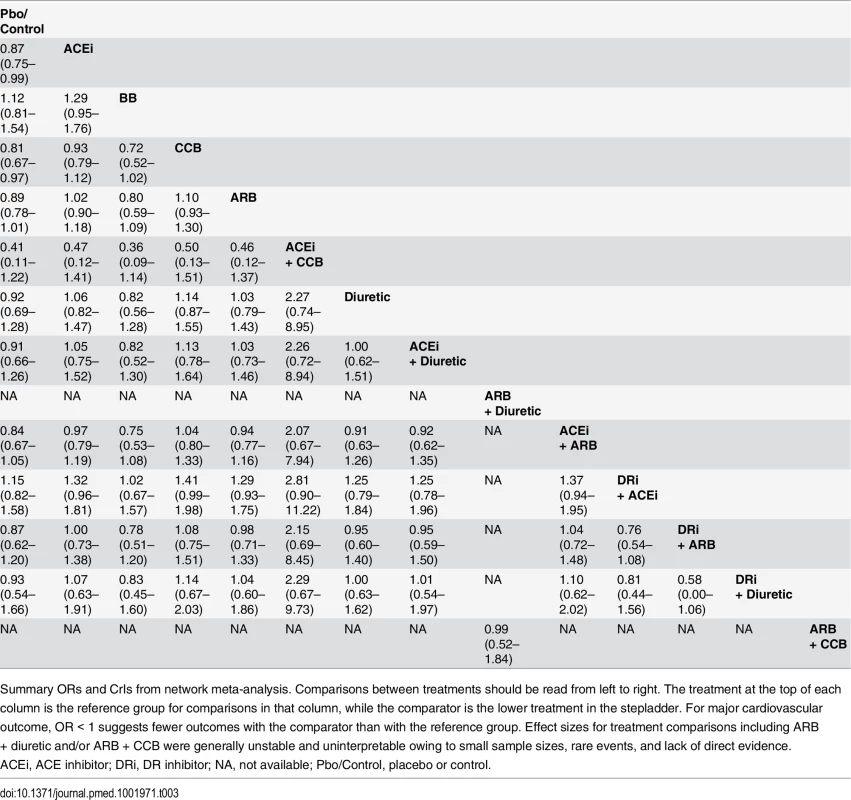
Summary ORs and CrIs from network meta-analysis. Comparisons between treatments should be read from left to right. The treatment at the top of each column is the reference group for comparisons in that column, while the comparator is the lower treatment in the stepladder. For major cardiovascular outcome, OR < 1 suggests fewer outcomes with the comparator than with the reference group. Effect sizes for treatment comparisons including ARB + diuretic and/or ARB + CCB were generally unstable and uninterpretable owing to small sample sizes, rare events, and lack of direct evidence. Progression of renal disease (composite endpoint of ESRD, doubling of serum creatinine, and all-cause mortality) occurred in 9,267 of 69,380 patients from 18 studies with available data. No RAS blocker was found to reduce progression of renal disease compared to placebo. Compared with ACE inhibitor as the reference treatment, no RAS blocker used in monotherapy and/or combination was associated with any significant reduction of progression of renal disease. Compared with ACE inhibitor as the reference, ORs were 1.10 (95% CrI 0.90–1.40; moderate confidence) for ARB, 0.97 (95% CrI 0.72–1.29; moderate confidence) for ACE inhibitor plus ARB, 0.99 (95% CrI 0.65–1.57; low confidence) for DR inhibitor plus ACE inhibitor, and 1.18 (95% CrI 0.78–1.84; low confidence) for DR inhibitor plus ARB (Tables 4, 5, and S8).
Tab. 4. Renal outcomes and all-cause mortality: comparisons of random effects pairwise meta-analysis with Bayesian network meta-analysis, including confidence of assessments. 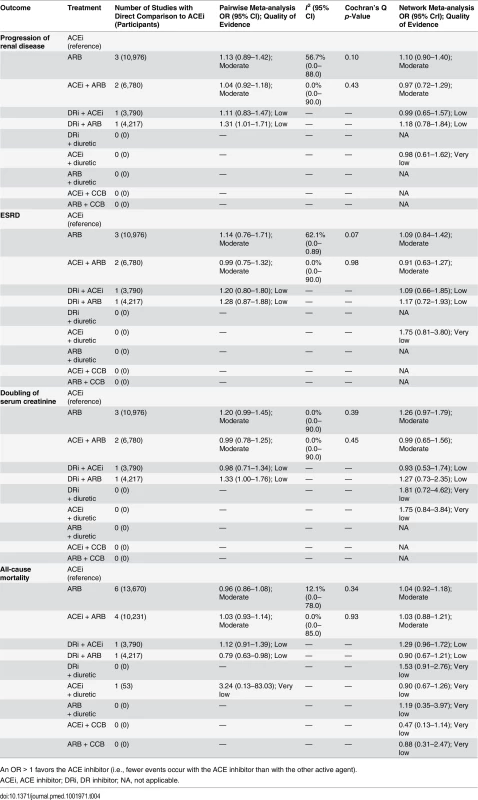
An OR > 1 favors the ACE inhibitor (i.e., fewer events occur with the ACE inhibitor than with the other active agent). Tab. 5. Progression of renal disease (composite outcome) and all possible treatment comparisons. 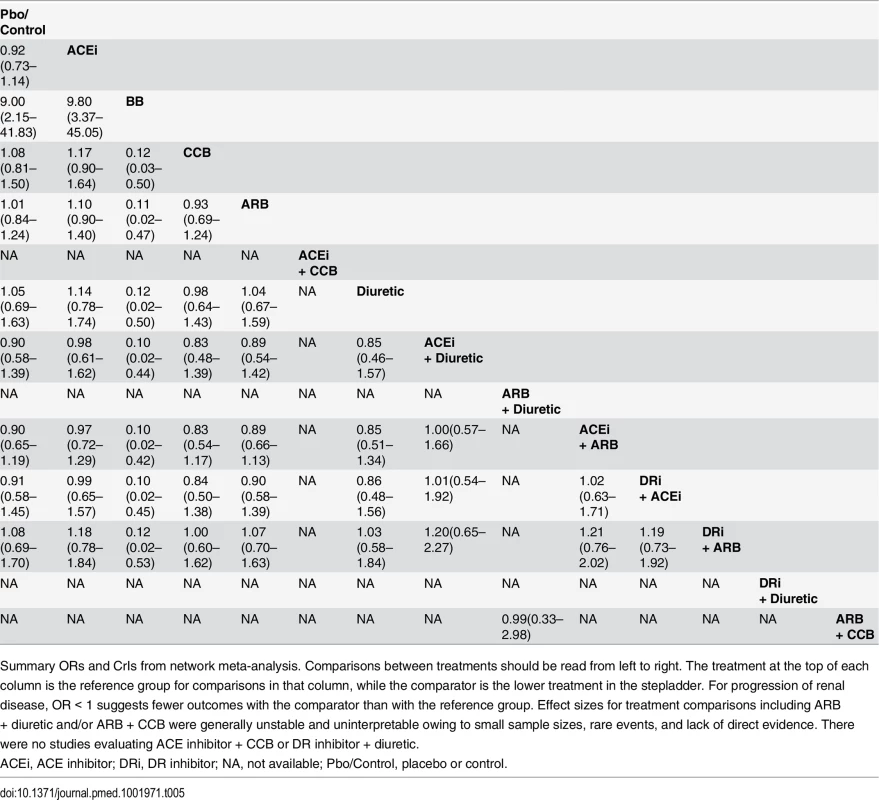
Summary ORs and CrIs from network meta-analysis. Comparisons between treatments should be read from left to right. The treatment at the top of each column is the reference group for comparisons in that column, while the comparator is the lower treatment in the stepladder. For progression of renal disease, OR < 1 suggests fewer outcomes with the comparator than with the reference group. Effect sizes for treatment comparisons including ARB + diuretic and/or ARB + CCB were generally unstable and uninterpretable owing to small sample sizes, rare events, and lack of direct evidence. There were no studies evaluating ACE inhibitor + CCB or DR inhibitor + diuretic. Cardiovascular mortality occurred in 6,166 of 95,060 patients from 41 studies that provided data. With placebo as the reference treatment, ACE inhibitor plus CCB (OR 0.14; 95% CrI 0.01–0.83) appeared to reduce the risk of cardiovascular mortality, though with very low confidence. Compared with ACE inhibitor as the reference treatment (Tables 2 and S8), no RAS blocker (except ACE inhibitor plus CCB) was associated with a significant reduction in cardiovascular mortality: ARB (1.07; 95% CrI 0.88–1.33; moderate confidence), ACE inhibitor plus ARB (1.06; 95% CrI 0.79–1.49; moderate confidence), DR inhibitor plus ARB (0.98; 95% CrI 0.61–1.60; low confidence), and DR inhibitor plus ACE inhibitor (1.45; 95% CrI 0.91–2.35; low confidence).
Myocardial infarction occurred in 4,593 of 84,792 patients from 48 studies with available data. Both ACE inhibitors (OR 0.77; 95% CrI 0.62–0.92; moderate confidence) and ARBs (0.82; 95% CrI 0.67–0.98; moderate confidence) were associated with reduced risk of myocardial infarction compared with placebo. Compared with ACE inhibitor as the reference treatment (Tables 2 and S8), no RAS blocker used in monotherapy and/or combination was associated with a significant reduction in myocardial infarction: ARB (1.07; 95% CrI 0.89–1.28; moderate confidence), ACE inhibitor plus ARB (1.00; 95% CrI 0.78–1.33; moderate confidence), DR inhibitor plus ARB (0.83; 95% CrI 0.52–1.31; low confidence), and DR inhibitor plus ACE inhibitor (1.35; 95% CrI 0.85–2.12; low confidence).
Stroke was observed in 4,591 of 95,155 patients from 42 studies. The combination of ACE inhibitor plus ARB was associated with a reduced risk of stroke compared with placebo (OR 0.80; 95% CrI 0.62–0.98; moderate confidence). Compared with ACE inhibitor as the reference treatment (Tables 2 and S8), no RAS blocker used in monotherapy and/or combination was associated with a significant reduction in the incidence of stroke: ARB (1.01; 95% CrI 0.88–1.16; moderate confidence), ACE inhibitor plus ARB (0.88; 95% CrI 0.71–1.08; moderate confidence). Combinations of DR inhibitor with ACE inhibitor/ARB were potentially associated with an increased risk of stroke: DR inhibitor plus ARB compared with ACE inhibitor plus ARB (1.44; 95% CrI 1.00–2.13; low confidence; S8 Table).
ESRD was reported in 1,786 of 67,316 patients from the 22 studies with available data. ACE inhibitors (OR 0.68; 95% CrI 0.51–0.91; moderate confidence), ARBs (0.74; 95% CrI 0.57–0.97; moderate confidence), and combinations of ACE inhibitor plus ARB (0.62; 95% CrI 0.42–0.90; moderate confidence) were associated with a reduced risk of ESRD compared with placebo. Compared with ACE inhibitor as the reference treatment (Tables 4 and S8), no RAS blocker used in monotherapy and/or combination was associated with a significant reduction in ESRD: ARB (1.09; 95% CrI 0.84–1.42; moderate confidence), ACE inhibitor plus ARB (0.91; 95% CrI 0.63–1.27; moderate confidence), DR inhibitor plus ACE inhibitor (1.09; 95% CrI 0.66–1.85; low confidence), and DR inhibitor plus ARB (1.17; 95% CrI 0.72–1.93; low confidence).
Doubling of serum creatinine level was reported in 2,645 of 67,505 patients from 24 studies. With placebo as the reference treatment, ACE inhibitors (OR 0.70; 95% CrI 0.52–0.91; moderate confidence) were associated with important reductions in the risk of doubling of serum creatinine. Compared with ACE inhibitor as the reference treatment (Tables 4 and S8), no RAS blocker used in monotherapy and/or combination was associated with a significant reduction of doubling of serum creatinine: ARB (1.26; 95% CrI 0.97–1.79; moderate confidence), ACE inhibitor plus ARB (0.99; 95% CrI 0.65–1.56; moderate confidence), DR inhibitor plus ARB (1.27; 95% CrI 0.73–2.35; low confidence), and DR inhibitor plus ACE inhibitor (0.93; 95% CrI 0.53–1.74; low confidence).
Death from any cause was reported in 11,199 of 101,369 patients from 59 studies. Compared with placebo or with ACE inhibitor as the reference treatment (S8 Table), no RAS blocker used in monotherapy and/or combination was associated with a significant reduction in all-cause mortality. Compared with ACE inhibitor as the reference treatment (Tables 4 and S8), the ORs were 1.04 (95% CrI 0.92–1.18; moderate confidence) for ARB, 1.03 (95% CrI 0.88–1.21; moderate confidence) for ACE inhibitor plus ARB, 1.29 (95% CrI 0.96–1.72; low confidence) for DR inhibitor plus ACE inhibitor, and 0.90 (95% CrI 0.67–1.21; low confidence) for DR inhibitor plus ARB.
Angina pectoris was reported in 5,026 of 65,656 patients from 30 studies. Both ACE inhibitors (0.81; 95% CrI 0.65–0.96; moderate confidence) and ARBs (0.76; 95% CrI 0.61–0.98; moderate confidence) were associated with a reduced risk of angina compared with placebo. Compared with ACE inhibitor as the reference treatment (Tables 2 and S8), no RAS blocker used in monotherapy and/or combination was associated with a significant reduction in angina pectoris: ARB (1.14; 95% CrI 0.98–1.37; moderate confidence), ACE inhibitor plus ARB (1.00; 95% CrI 0.82–1.23; moderate confidence), and DR inhibitor plus diuretic (1.32; 95% CrI 0.56–3.17; very low confidence).
Hospitalization for heart failure was reported in 5,272 of 81,373 patients from 33 studies. With placebo as reference treatment, ARB alone or in combination with ACE inhibitor seemed to be associated with a reduced risk of hospitalization for heart failure: 0.86 (95% CrI 0.72–0.99; moderate confidence) for ARB and 0.75 (95% CrI 0.60–0.91; moderate confidence) for ACE inhibitor plus ARB. Because of borderline estimates, ACE inhibitors were potentially associated with a decreased risk of heart failure hospitalization compared with placebo: 0.86 (95% CrI 0.73–1.01; moderate confidence). Compared with ACE inhibitor as the reference treatment (Tables 2 and S8), no RAS blocker used in monotherapy and/or combination was associated with a significant reduction in heart failure hospitalization: ARB (0.99; 95% CrI 0.86–1.12; moderate confidence), ACE inhibitor plus ARB (0.86; 95% CrI 0.73–1.01; moderate confidence), DR inhibitor plus ACE inhibitor (1.06; 95% CrI 0.75–1.45; low confidence), and DR inhibitor plus ARB (0.83; 95% CrI 0.59–1.14; low confidence).
Additional Analyses and Evaluation of Models
A summary of SUCRA values with 95% CrIs by treatment and outcome is reported in S9 Table. Overall, many of these estimates were imprecise and do not allow for firm conclusions to be drawn. The full details of the sensitivity analyses are reported in S10 Table. For each outcome, our findings were robust when we removed studies with high risk of bias, small studies, type 1 diabetes mellitus studies, and studies in normotensive patients. We note that the results were not materially different when we examined the distribution of potential effect modifiers by including covariates in network meta-regression models or when estimating the rate ratios based on patient-years. Because of the small number of studies with available data, we were unable to perform preplanned meta-regression analysis to evaluate the impact of history of coronary disease. There was generally a better trade-off between model fit and complexity when consistency was assumed than when it was not (S11 Table). Potential significant inconsistency was identified in a small number of cases: for stroke (consistency model DIC = 581.58 versus inconsistency model DIC = 589.80) and for angina pectoris (407.85 versus 414.21). Data were double-checked, and we could not identify any important effect modifier that differed across comparisons.
Discussion
In our main analyses, we found no significant differences in the risk of major cardiovascular outcome (composite of cardiovascular death, myocardial infarction, and stroke) between ACE inhibitor and either ARB or the combination of ACE inhibitor plus ARB. For the risk of progression of renal disease (composite of ESRD, doubling of serum creatinine, and all-cause mortality), no significant differences were detected between ACE inhibitor and any of the remaining therapies, such as ARB or the combination of ACE inhibitor plus ARB. Our findings also suggest that no RAS blocker strategy was superior to ACE inhibitor with respect to all-cause mortality, cardiovascular mortality, myocardial infarction, ESRD, or doubling of serum creatinine. Evidence can now be drawn from data for over 103,000 adults with diabetes from 71 randomized trials with at least 1 y of follow-up, and this increased sample size can provide more precise estimates of the cardiovascular and renal effects. We believe it is unlikely that future trials will show clinically relevant advantages of ARBs over ACE inhibitors (and vice versa) in preventing cardiovascular outcomes in adults with diabetes mellitus. For renal outcomes, some may consider, however, the necessity for additional industry-independent trials providing specific comparative data for the renoprotective effects of RAS blockade in patients with diabetic kidney disease. Despite many systematic reviews evaluating RAS blockers [23–37], to the best of our knowledge, this is the most comprehensive published systematic review (and network meta-analysis) of comparative treatment effects associated with the use of RAS blockers. It fully integrates all major cardiorenal outcomes and studies assessing ACE inhibitors, ARBs, and the DR inhibitor aliskiren in adults with diabetes, thereby providing clinicians and patients with an overall appraisal of these therapies. Compared with previous reviews in diabetes mellitus [23–26] (S12–S14 Tables), our analyses incorporate between more than two and five times the number of study participants and events. Our review was prospectively registered in PROSPERO and also adheres to the recently developed PRISMA reporting standards for network meta-analyses (S1 Checklist) [44]. Our main conclusions differed from those reported in recent meta-analyses in adults with diabetes, which claim different treatment effects for classes of RAS blockers [23,24].
Unlike previous reviews, which were limited to analyzing data only from published randomized trials, we were able to include unpublished data from large trials and/or specific data for subgroups of patients with diabetes [20,39–41,47–49]. Our network meta-analyses considered both direct and indirect comparisons of multiple antihypertensive regimens, by including relevant evidence on the comparative effects of RAS blockade. Specifically, the direct comparisons of ACE inhibitors, ARBs, the DR inhibitor aliskiren, and their combinations are largely driven by head-to-head comparative data, including a number of large randomized trials [39–41,55,75]. In 2003, results of the Valsartan in Acute Myocardial Infarction Trial (VALIANT) [39] found no significant differences between an ARB and an ACE inhibitor in all-cause mortality or major cardiovascular events among patients with myocardial infarction complicated by congestive heart failure and/or evidence of left ventricular systolic dysfunction. However, combining an ARB and an ACE inhibitor increased the rate of adverse events without improving survival. Similarly, in 2008, the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) [40,41] showed no differences between an ACE inhibitor and an ARB, alone or in combination, for major cardiovascular and renal events, but highlighted the danger of dual blockade of RAS, reporting an increased risk of acute dialysis and hyperkalemia in patients with vascular disease or high-risk diabetes and who were prescribed an ACE inhibitor and an ARB together. To specifically assess the renal effects of dual therapy, the Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial [75] was conducted in patients with diabetes mellitus and diabetic nephropathy. In line with previous studies [39–41], the VA NEPHRON-D trial showed that dual therapy with an ACE inhibitor and an ARB yielded no significant benefit with respect to the endpoints of renal disease progression, cardiovascular disease, or all-cause mortality, but was associated with an increased risk of adverse events compared with ARB alone.
Our findings reinforce the recommendations of current guidelines in North America and Europe suggesting that ACE inhibitors and ARBs, as preferred antihypertensive therapies, have generally similar effects on cardiovascular and renal outcomes [6–9]. Several points also deserve to be mentioned. Our findings show that dual therapy with an ACE inhibitor and an ARB had no additional beneficial effect on major cardiovascular and renal outcomes when compared to an ACE inhibitor or ARB alone in adults with diabetes. Accordingly, given the lack of consistent benefits on major clinical outcomes and considering the existent evidence [22,30,39–41,75] of adverse effects in terms of hypotension, hyperkalemia, and acute kidney injury, overall recommendations cautioning against the use of combination therapy with an ACE inhibitor and an ARB are still valid. In addition, this review provided relevant evidence to refute the claim raised during the last decade that ARBs may increase the risk of cardiovascular outcomes [76–79]. For example, results of the Randomized Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) trial [50] and the Olmesartan Reducing Incidence of End Stage Renal Disease in Diabetic Nephropathy Trial (ORIENT) [80] showed an increased number of cardiovascular deaths among diabetic patients randomized to the single ARB olmesartan. Regarding cardiovascular safety, the US Food and Drug Administration and the European Medicines Agency conducted safety reviews with inconclusive findings [81,82]. Overall, our network meta-analysis found that ARBs as a class were not associated with an increased risk of cardiovascular risk (major cardiovascular outcome, cardiovascular death, myocardial infarction, stroke, angina pectoris, or hospitalization for heart failure) when compared with ACE inhibitors or placebo. Our evaluation showed favorable effects of ARBs as a class compared with placebo for the risk of myocardial infarction, stroke, and hospitalization for heart failure; potential benefits for decreasing the risk of major cardiovascular outcome and angina pectoris; and neutral effects on cardiovascular mortality and all-cause mortality.
For the DR inhibitor aliskiren, our review identified limited evidence and did not allow us to reach definitive conclusions for any of the cardiovascular and renal outcomes of interest. Most trials currently available in the literature comparing therapy with DR inhibitor have focused on surrogate outcomes and have been underpowered to provide robust estimates of major outcomes and adverse events [5,30]. The Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) [22] trial was stopped prematurely because the addition of the DR inhibitor aliskiren to standard therapy with RAS blockade did not result in a decrease of cardiovascular and renal events and increased the risk of adverse outcomes such as stroke, hyperkalemia, and hypotension. Similarly, the Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT) [56] failed to show that the DR inhibitor was superior to placebo in addition to standard therapy in reducing cardiovascular death or heart failure rehospitalization among patients hospitalized for heart failure with reduced left ventricular ejection fraction. ASTRONAUT was stopped early, and event rates of hyperkalemia, hypotension, and renal impairment/renal failure were found to be higher in the aliskiren group. In ASTRONAUT, all-cause mortality was significantly increased with aliskiren in patients with diabetes but not in those without diabetes. As a consequence of these findings, regulatory authorities recommended new contraindications and warnings for aliskiren-containing medicines in patients with diabetes or kidney problems [83] and required aliskiren investigational treatment to be withdrawn from patients with diabetes in Aliskiren Trial to Minimize Outcomes in Patients with Heart Failure (ATMOSPHERE) [84,85], an ongoing trial of more than 7,000 patients with heart failure comparing aliskiren and the ACE inhibitor enalapril, alone and in combination.
During the performance and reporting of our review, an additional network meta-analysis of antihypertensive medications in diabetes and kidney disease was published and identified. Palmer et al. [42] evaluated blood-pressure-lowering agents in 157 studies comprising 43,256 participants and concluded that no treatment prolonged survival, but ACE inhibitor and ARB treatment, alone or in combination, were the most effective strategies against ESRD. Compared with placebo, they found that the combination of an ACE inhibitor and an ARB seemed to prevent ESRD, did not increase doubling of serum creatinine, and improved albuminuria, at the expense of an increased risk of hyperkalemia and acute kidney injury. Although our review indicates a fairly consistent pattern of findings with Palmer et al. [42] for ESRD and all-cause mortality for ACE inhibitors and ARBs against placebo, our network meta-analysis provided greater insight into the comparative effects of classes of RAS blockers on major cardiovascular and renal outcomes, largely based on expanded long-term trial datasets (considering both unpublished outcomes and events).
There are limitations to be noted regarding our review. First, as in other meta-analyses, there is clinical and methodological heterogeneity in the included trials in terms of different study characteristics and broad group populations according to the original trial designs. These data should be viewed as reflecting real world practice treatment with different RAS blockers, reflecting more closely the heterogeneous case mix of diabetic patients encountered in clinical practice. Second, we used study-level data instead of individual patient data, so the small number of studies limited the sensitivity analyses that could be conducted to account for heterogeneity in the absence of patient-level data. Although meta-regression analyses were performed to evaluate the effect of potential effect modifiers, the results of these analyses may be underpowered. Given the lack of consistently reported data in each trial, we did not adjust our analyses for treatment dose or adherence to therapy. Third, we followed the clinical guidelines approach of formulating our treatment comparisons at the drug-class level. Caution should be used when interpreting findings, considering the fact that not all single ACE inhibitor/ARB agents are represented in the randomized trials that formed the evidence base for our network meta-analyses. Fourth, our study does not consider costs or patient preferences for the different treatment strategies and outcomes. Future research needs to be conducted to explore the comparative cost-effectiveness of RAS blockade. Fifth, we evaluated major cardiovascular and renal outcomes using composite endpoints for a comparative effectiveness assessment. In past decades, the composite endpoints of major cardiovascular outcome and progression of renal disease have become standard outcomes of large randomized trials [13,40,41,75]. The power of analyses may be increased when such composite measures are used as compared with the individual components of these measures, since by grouping numerous types of events into a larger category, the composite endpoint will occur more frequently than any of the individual components. Confidence in composite endpoints depends partly on a belief that similar risk reductions apply to all the components of the composite endpoint. However, it is acknowledged that the use of composite endpoints is frequently complicated because of poor reporting and uncertain clinical relevance in many trials [86,87]. For example, our results showed no benefits of RAS blockers over placebo in the composite endpoint of progression of renal disease (a composite of the onset of ESRD, doubling of the baseline serum creatinine concentration, or death from any cause). ESRD was the only renal outcome for which RAS blockers (ACE inhibitor or ARB alone or in combination) were convincingly beneficial. Indeed, in this instance, ACE inhibitors reduced the incidence of both ESRD and doubling of creatinine. No RAS blocker reduced overall mortality in adults with diabetes. These examples highlight the challenges that clinicians, trialists, and guideline developers face when making decisions on the basis of composite endpoints, particularly when results for each single component of a composite endpoint are not presented separately in published reports. Although we adopted reproducible definitions that will enhance cross-study comparisons, this review was challenged by the heterogeneous reporting of events in studies. None of the trials examined and reported all of the outcomes of interest. It is also important to emphasize the limited ability of randomized controlled trials with a median duration of about 3 y to inform lifelong treatment decisions. Finally, publication bias was not quantitatively assessed, as there were inadequate numbers of included trials with direct comparisons to properly assess a funnel plot or use more advanced regression-based methods.
Conclusions
Using randomized trial data and a novel evidence synthesis approach, our analyses indicate that comparisons of different RAS blockers showed similar effects of ACE inhibitors and ARBs on major cardiovascular and renal outcomes in adults with diabetes mellitus. Compared with monotherapy, the combination of an ACE inhibitor and an ARB failed to provide significant benefits in terms of major outcomes. Clinicians should discuss the balance between benefits, costs, and potential harms with the individual patient with diabetes before starting treatment. These findings are important for helping clinicians, health-care providers, policy-makers, and patients with diabetes make informed decisions regarding treatment selection.
Supporting Information
Zdroje
1. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries for 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386 : 743–800. doi: 10.1016/S0140-6736(15)60692-4 26063472
2. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 385 : 117–171. doi: 10.1016/S0140-6736(14)61682-2 25530442
3. GBD 2013 DALYs and HALE Collaborators, Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386 : 2145–2191. doi: 10.1016/S0140-6736(15)61340-X 26321261
4. Clarke PM, Glasziou P, Patel A, Chalmers J, Woodward M, Harrap SB, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010;7:e1000236. doi: 10.1371/journal.pmed.1000236 20186272
5. Sanders GD, Coeytaux R, Dolor RJ, Hasselblad V, Patel UD, Powers B, et al. Angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor antagonists (ARBs), and direct renin inhibitors for treating essential hypertension: an update. 2011 Jun [cited 25 Jul 2015]. Rockville (Maryland): Agency for Healthcare Research and Quality. Available: http://www.ncbi.nlm.nih.gov/books/NBK61789/.
6. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(Suppl 1):S1–S94.
7. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311 : 507–520. doi: 10.1001/jama.2013.284427 24352797
8. Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34 : 3035–3087. doi: 10.1093/eurheartj/eht108 23996285
9. Dasgupta K, Quinn RR, Zarnke KB, Rabi DM, Ravani P, Daskalopoulou SS, et al. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;30 : 485–501. doi: 10.1016/j.cjca.2014.02.002 24786438
10. UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998;317 : 713–720. 9732338
11. Tatti P, Pahor M, Byington RP, Di Mauro P, Guarisco R, Strollo G, et al. Outcome results of the Fosinopril Versus Amlodipine Cardiovascular Events Randomized Trial (FACET) in patients with hypertension and NIDDM. Diabetes Care. 1998;21 : 597–603. 9571349
12. Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N Engl J Med. 1998;338 : 645–652. 9486993
13. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355 : 253–259. 10675071
14. Lindholm LH, Hansson L, Ekbom T, Dahlöf B, Lanke J, Linjer E, et al. Comparison of antihypertensive treatments in preventing cardiovascular events in elderly diabetic patients: results from the Swedish Trial in Old Patients with Hypertension-2. STOP Hypertension-2 Study Group. J Hypertens. 2000;18 : 1671–1675.
15. Niskanen L, Hedner T, Hansson L, Lanke J, Niklason A, CAPPP Study Group. Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/beta-blocker-based treatment regimen: a subanalysis of the Captopril Prevention Project. Diabetes Care. 2001;24 : 2091–2096. 11723089
16. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345 : 851–860. 11565517
17. Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345 : 870–878. 11565519
18. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345 : 861–869. 11565518
19. Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint Reduction in Hypertension Study (LIFE): a randomised trial against atenolol. Lancet. 2002;359 : 1004–1010. 11937179
20. Whelton PK, Barzilay J, Cushman WC, Davis BR, Iamathi E, Kostis JB, et al. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Arch Intern Med. 2005;165 : 1401–1409. 15983290
21. Patel A, ADVANCE Collaborative Group, MacMahon S, Chalmers J, Neal B, Woodward M, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370 : 829–840. 17765963
22. Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367 : 2204–2213. doi: 10.1056/NEJMoa1208799 23121378
23. Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014;174 : 773–785. doi: 10.1001/jamainternmed.2014.348 24687000
24. Wu HY, Huang JW, Lin HJ, Liao WC, Peng YS, Jung KY, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and Bayesian network meta-analysis. BMJ. 2013;347:f6008. doi: 10.1136/bmj.f6008 24157497
25. Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J. Reno-protective effects of renin-angiotensin system blockade in type 2 diabetic patients: a systematic review and network meta-analysis. Diabetologia. 2012;55 : 566–578. doi: 10.1007/s00125-011-2398-8 22189484
26. Nakao YM, Teramukai S, Tanaka S, Yasuno S, Fujimoto A, Kasahara M, et al. Effects of renin-angiotensin system blockades on cardiovascular outcomes in patients with diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2012;96 : 68–75. doi: 10.1016/j.diabres.2011.11.025 22197527
27. Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366 : 2026–2033. 16338452
28. Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ. 2004;329 : 828. 15459003
29. Xue H, Lu Z, Tang WL, Pang LW, Wang GM, Wong GW, et al. First-line drugs inhibiting the renin angiotensin system versus other first-line antihypertensive drug classes for hypertension. Cochrane Database Syst Rev. 2015;1:CD008170. doi: 10.1002/14651858.CD008170.pub2 25577154
30. Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomized trials. BMJ. 2013;346:f360. doi: 10.1136/bmj.f360 23358488
31. Savarese G, Costanzo P, Cleland JG, Vassallo E, Ruggiero D, Rosano G, et al. A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. J Am Coll Cardiol. 2013;61 : 131–142. doi: 10.1016/j.jacc.2012.10.011 23219304
32. van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad JJ, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33 : 2088–2097. doi: 10.1093/eurheartj/ehs075 22511654
33. Sciarretta S, Palano F, Tocci G, Baldini R, Volpe M. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med. 2011;171 : 384–394. doi: 10.1001/archinternmed.2010.427 21059964
34. Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ. 2011;342:d2234. doi: 10.1136/bmj.d2234 21521728
35. Kuenzli A, Bucher HC, Anand I, Arutiunov G, Kum LC, McKelvie R, et al. Meta-analysis of combined therapy with angiotensin receptor antagonists versus ACE inhibitors alone in patients with heart failure. PLoS ONE. 2010;5:e9946. doi: 10.1371/journal.pone.0009946 20376345
36. Baker WL, Coleman CI, Kluger J, Reinhart KM, Talati R, Quercia R, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors or angiotensin II-receptor blockers for ischemic heart disease. Ann Intern Med. 2009;151 : 861–871. doi: 10.7326/0003-4819-151-12-200912150-00162 20008762
37. Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289 : 2534–2544. 12759325
38. Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363 : 2022–2031. 15207952
39. Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349 : 1893–1906. 14610160
40. ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358 : 1547–1559. doi: 10.1056/NEJMoa0801317 18378520
41. Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372 : 547–553. doi: 10.1016/S0140-6736(08)61236-2 18707986
42. Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385 : 2047–2056. doi: 10.1016/S0140-6736(14)62459-4 26009228
43. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 19621072
44. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162 : 777–784. doi: 10.7326/M14-2385 26030634
45. Sampson M, Shojania KG, McGowan J, Daniel R, Rader T, Iansavichene AE, et al. Surveillance search techniques identified the need to update systematic reviews. J Clin Epidemiol. 2008;61 : 755–762. doi: 10.1016/j.jclinepi.2007.10.003 18586179
46. Hutton B, Tetzlaff J, Yazdi F, Thielman J, Kanji S, Fergusson D, et al. Comparative effectiveness of monotherapies and combination therapies for patients with hypertension: protocol for a systematic review with network meta-analyses. Syst Rev. 2013;2 : 44. doi: 10.1186/2046-4053-2-44 23809864
47. Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359 : 1225–1237. doi: 10.1056/NEJMoa0804593 18753639
48. Telmisartan Randomised Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) Investigators, Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372 : 1174–1183. doi: 10.1016/S0140-6736(08)61242-8 18757085
49. Mann JF, Schmieder RE, Dyal L, McQueen MJ, Schumacher H, Pogue J, et al. Effect of telmisartan on renal outcomes: a randomized trial. Ann Intern Med. 2009;151 : 1–10,W1-W2. 19451556
50. Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364 : 907–917. doi: 10.1056/NEJMoa1007994 21388309
51. Daly CA, Fox KM, Remme WJ, Bertrand ME, Ferrari R, Simoons ML, et al. The effect of perindopril on cardiovascular morbidity and mortality in patients with diabetes in the EUROPA study: results from the PERSUADE substudy. Eur Heart J. 2005;26 : 1369–1378. 15860521
52. Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet. 2008;372 : 1394–1402. doi: 10.1016/S0140-6736(08)61412-9 18823656
53. Bilous R, Chaturvedi N, Sjølie AK, Fuller J, Klein R, Orchard T, et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann Intern Med. 2009;151 : 11–20,W3-W4. 19451554
54. Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomized placebo-controlled trial. Lancet. 2008;372 : 1385–1393. doi: 10.1016/S0140-6736(08)61411-7 18823658
55. Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345 : 1667–1675. 11759645
56. Gheorghiade M, Böhm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309 : 1125–1135. doi: 10.1001/jama.2013.1954 23478743
57. Nakao K, Hirata M, Oba K, Yasuno S, Ueshima K, Fujimoto A, et al. Role of diabetes and obesity in outcomes of the candesartan antihypertensive survival evaluation in Japan (CASE-J) trial. Hypertens Res. 2010;33 : 600–606. doi: 10.1038/hr.2010.38 20379187
58. Ogihara T, Saruta T, Rakugi H, Saito I, Shimamoto K, Matsuoka H, et al. Combinations of olmesartan and a calcium channel blocker or a diuretic in elderly hypertensive patients: a randomized, controlled trial. J Hypertens. 2014;32 : 2054–2063. doi: 10.1097/HJH.0000000000000281 24999799
59. Matsui K, Kim-Mitsuyama S, Ogawa H, Jinnouchi T, Jinnouchi H, Arakawa K, et al. Sex differences in response to angiotensin II receptor blocker-based therapy in elderly, high-risk, hypertensive Japanese patients: a subanalysis of the OSCAR study. Hypertens Res. 2014;37 : 526–532. doi: 10.1038/hr.2014.23 24599010
60. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 22008217
61. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64 : 401–406. doi: 10.1016/j.jclinepi.2010.07.015 21208779
62. Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630 25252733
63. Rochwerg B, Alhazzani W, Sindi A, Heels-Ansdell D, Thabane L, Fox-Robichaud A, et al. Fluid resuscitation in sepsis: a systematic review and network meta-analysis. Ann Intern Med. 2014;161 : 347–355. doi: 10.7326/M14-0178 25047428
64. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10 : 101–129.
65. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327 : 557–560. 12958120
66. Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181 : 488–493. doi: 10.1503/cmaj.081086 19654195
67. Catalá-López F, Tobías A, Cameron C, Moher D, Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: an introduction. Rheumatol Int. 2014;34 : 1489–1496. doi: 10.1007/s00296-014-2994-2 24691560
68. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159 : 130–137. doi: 10.7326/0003-4819-159-2-201307160-00008 23856683
69. Catalá-López F, Hutton B, Moher D. The transitive property across randomized controlled trials: if B is better than A, and C is better than B, will C be better than A? Rev Esp Cardiol. 2014;67 : 597–602. doi: 10.1016/j.rec.2013.11.016 25037537
70. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33 : 607–617. doi: 10.1177/0272989X12458724 23104435
71. Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992;7 : 457–472.
72. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013;33 : 641–656. doi: 10.1177/0272989X12455847 23804508
73. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64 : 163–171. doi: 10.1016/j.jclinepi.2010.03.016 20688472
74. Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity—subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33 : 618–640. doi: 10.1177/0272989X13485157 23804507
75. Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369 : 1892–1903. doi: 10.1056/NEJMoa1303154 24206457
76. Verma S, Strauss M. Angiotensin receptor blockers and myocardial infarction. BMJ. 2004;329 : 1248–1249. 15564232
77. Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114 : 838–854. 16923768
78. Hall AS, Strauss MH. More about the “ARB MI paradox”. Heart. 2007;93 : 1011–1014. 17699164
79. Hobbs FD. Angiotensin receptor blockers and cardiovascular outcomes. BMJ. 2011;342:d2193. doi: 10.1136/bmj.d2193 21527455
80. Imai E, Chan JC, Ito S, Yamasaki T, Kobayashi F, Haneda M, et al. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia. 2011;54 : 2978–2986. doi: 10.1007/s00125-011-2325-z 21993710
81. US Food and Drug Safety Administration. FDA drug safety communication: FDA review of cardiovascular risks for diabetics taking hypertension drug olmesartan not conclusive; label updates required. 2014 Jun 6 [cited 12 Jun 2015]. Available: http://www.fda.gov/Drugs/DrugSafety/ucm402323.htm.
82. European Medicines Agency. Pharmacovigilance Risk Assessment Committee (PRAC). Minutes of the Meeting—29–31 October 2012. 2012 Nov 29 [cited 12 Jun 2015]. Available: http://www.ema.europa.eu/docs/en_GB/document_library/Minutes/2012/12/WC500135712.pdf.
83. European Medicines Agency. European Medicines Agency recommends new contraindications and warnings for aliskiren-containing medicines. 2012 Feb 17 [cited 2 Jun 2015]. Available: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2012/02/news_detail_001446.jsp&mid=WC0b01ac058004d5c1.
84. Krum H, Massie B, Abraham WT, Dickstein K, Kober L, McMurray JJ, et al. Direct renin inhibition in addition to or as an alternative to angiotensin converting enzyme inhibition in patients with chronic systolic heart failure: rationale and design of the Aliskiren Trial to Minimize OutcomeS in Patients with HEart failuRE (ATMOSPHERE) study. Eur J Heart Fail. 2011;13 : 107–114. doi: 10.1093/eurjhf/hfq212 21169387
85. Cleland JG, Clark AL, Costanzo P, Francis DP. Diabetes, aliskiren, and heart failure: let’s bring ASTRONAUT down to earth. Eur Heart J. 2013;34 : 3097–3099. doi: 10.1093/eurheartj/eht366 23999453
86. Montori VM, Permanyer-Miralda G, Ferreira-González I, Busse JW, Pacheco-Huergo V, Bryant D, et al. Validity of composite end points in clinical trials. BMJ. 2005;330 : 594–596. 15761002
87. Ferreira-González I, Busse JW, Heels-Ansdell D, Montori VM, Akl EA, Bryant DM, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ. 2007;334 : 786. 17403713
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Trans-Pacific Partnership Provisions in Intellectual Property, Transparency, and Investment Chapters Threaten Access to Medicines in the US and Elsewhere
- An Uninformative Truth: The Logic of Amarin’s Off-Label Promotion
- Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study
- Pragmatic Trials for Noncommunicable Diseases: Relieving Constraints
- Antibiotic Resistance in India: Drivers and Opportunities for Action
- Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis
- Routine Pediatric Enterovirus 71 Vaccination in China: a Cost-Effectiveness Analysis
- Increased Duration of Paid Maternity Leave Lowers Infant Mortality in Low- and Middle-Income Countries: A Quasi-Experimental Study
- Planned Repeat Cesarean Section at Term and Adverse Childhood Health Outcomes: A Record-Linkage Study
- Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea
- A Novel Brief Therapy for Patients Who Attempt Suicide: A 24-months Follow-Up Randomized Controlled Study of the Attempted Suicide Short Intervention Program (ASSIP)
- Comparison of the Schwartz and CKD-EPI Equations for Estimating Glomerular Filtration Rate in Children, Adolescents, and Adults: A Retrospective Cross-Sectional Study
- Cardiovascular and Renal Outcomes of Renin–Angiotensin System Blockade in Adult Patients with Diabetes Mellitus: A Systematic Review with Network Meta-Analyses
- Length of Stay After Childbirth in 92 Countries and Associated Factors in 30 Low- and Middle-Income Countries: Compilation of Reported Data and a Cross-sectional Analysis from Nationally Representative Surveys
- Transformative Innovations in Reproductive, Maternal, Newborn, and Child Health over the Next 20 Years
- Compassionate and Proactive Interventions by Health Workers in the United Kingdom: A Better Approach to Prevent and Respond to Female Genital Mutilation?
- Translational Research for Tuberculosis Elimination: Priorities, Challenges, and Actions
- The Community As the Patient in Malaria-Endemic Areas: Preempting Drug Resistance with Multiple First-Line Therapies
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea
- Comparison of the Schwartz and CKD-EPI Equations for Estimating Glomerular Filtration Rate in Children, Adolescents, and Adults: A Retrospective Cross-Sectional Study
- Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study
- A Novel Brief Therapy for Patients Who Attempt Suicide: A 24-months Follow-Up Randomized Controlled Study of the Attempted Suicide Short Intervention Program (ASSIP)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



