-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMonitoring Pharmacologically Induced Immunosuppression by Immune Repertoire Sequencing to Detect Acute Allograft Rejection in Heart Transplant Patients: A Proof-of-Concept Diagnostic Accuracy Study
A novel, proof-of-concept, diagnostic test for allograft rejection. The authors use a barcode-sequencing approach to determine the isotypes and clonal composition of circulating B cells in heart transplant patients.
Published in the journal: . PLoS Med 12(10): e32767. doi:10.1371/journal.pmed.1001890
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001890Summary
A novel, proof-of-concept, diagnostic test for allograft rejection. The authors use a barcode-sequencing approach to determine the isotypes and clonal composition of circulating B cells in heart transplant patients.
Introduction
An enduring challenge in immunology is the lack of quantitative measurements of immune strength. Current clinical practice relies on very crude estimates of the activity of the immune system, such as white blood cell counts. In view of the lack of more predictive assays, pharmacological immunosuppressive therapy, e.g., in the context of post-organ-transplant therapy, is guided mainly by dosage and measurement of the concentration of immunosuppressive drugs in blood. In adult transplant recipients, these immunosuppressive drugs typically include induction agents (e.g., lymphocyte-depleting antibodies, such as anti-thymocyte globulin) followed by maintenance with a combination of corticosteroids (prednisone), calcineurin inhibitors (tacrolimus and cyclosporine), and anti-proliferative agents (most commonly mycophenolate mofetil [MMF]). Immunosuppression is therefore achieved by combining several drugs with distinct mechanisms of action. Calcineurin inhibitors, for example, inhibit or deplete T helper and T killer cells, respectively, and consequently reduce T-helper-cell-dependent B cell activation [1,2]. While corticosteroids have a general immunosuppressive effect, MMF specifically inhibits T and B cell division. Consequently, while post-transplant immunosuppressive therapy is primarily aimed at preventing acute rejection events associated with T cell activation, it will also, directly and indirectly, affect the composition of circulating naïve and activated B cells [3,4]. While in some cases therapeutic drug levels (e.g., trough or C0 levels of tacrolimus) can be monitored, these levels are more reflective of toxicity than therapeutic efficacy. Further, this approach does not account for individual differences in the response to immunosuppressive drugs, and frequently gives rise to complications related to over - or under-immunosuppression.
Measuring the activity of the immune system directly would allow a more comprehensive understanding of the net state of the immune system. Here, we tested the hypothesis that immune repertoire sequencing of the B cell antibody heavy chain could provide an accurate and individualized measure of the activity of the adaptive immune response. The antibody heavy chain IGH transcript is unique as its expression changes fundamentally, not only in abundance but also in sequence, when a B cell is activated. B cells can undergo hypermutation and class-switch recombination when activated. Activated B cells express high levels of mutated antibodies of the IgG and IgA isotypes, while naïve B cells express non-mutated IgM antibodies at low levels (Fig 1). A lower abundance of class-switched sequences indicates a lower number of activated B cells and, consequently, lower activity of the adaptive immune system. Indeed, microarray studies have previously demonstrated that immunosuppressed individuals display reduced expression of antibody transcripts in general, and class-switched antibody transcripts in particular [5]. Furthermore, reduced hypermutation in IgM sequences following immunosuppressive therapy has previously been observed [6].
Fig. 1. Monitoring overall immunosuppression. 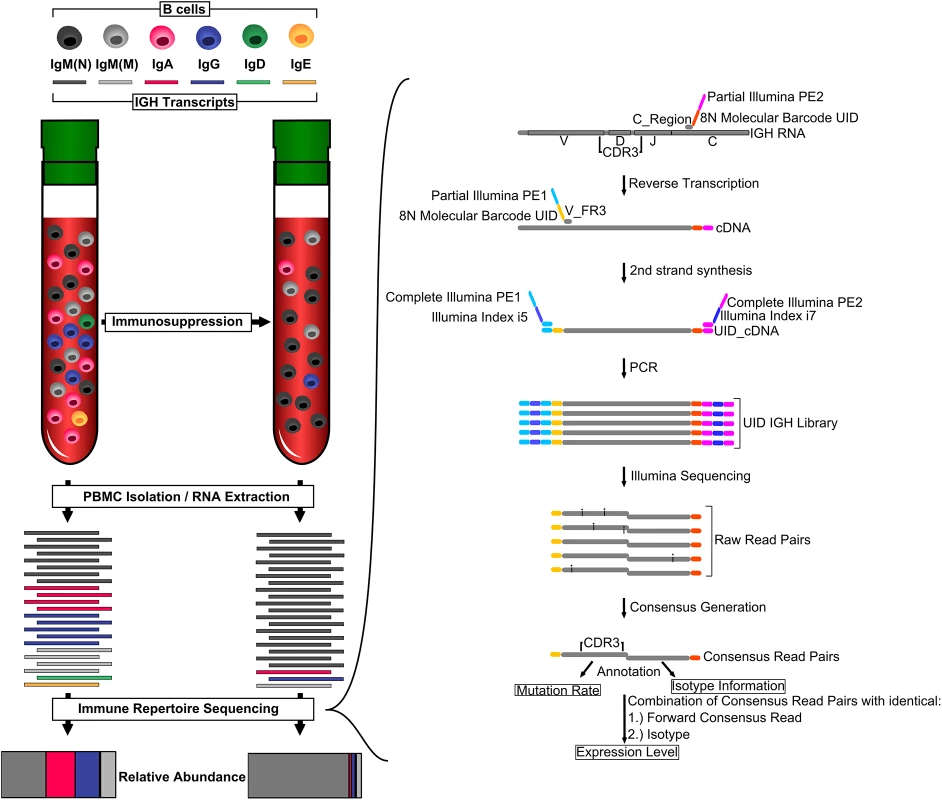
Organ transplant recipients receive immunosuppressive therapy, which usually includes calcineurin inhibitors. These drugs inhibit T cell activation, leading to fewer B cells expressing class-switched and mutated IGH transcripts. We measured overall immunosuppression using immune repertoire sequencing (IGH-Seq). A schematic of the IGH-Seq protocol and data analysis is shown on the right. C_Region, primer pool specific to constant region; CDR3, complementary determining region 3; PBMC, peripheral blood mononuclear cell; UID, unique identifier; V_FR3, primer pool specific to V segments. Immune repertoire sequencing relies on high-throughput sequencers to identify the composition of IGH transcripts in a blood sample, and has been used for various applications, including tracking of minimal residual disease in acute lymphoblastic leukemia, studying age-related changes in the immune system, and identifying the memory B cell response to influenza vaccination [7–11]. In a previous study, we described a molecular-barcoded immune repertoire sequencing protocol that is able to determine the sequence and abundance of IGH transcripts with unprecedented accuracy [7].
Here, we determined whether the immune repertoire sequencing approach enables quantification of loss of immune activity in response to pharmacological immunosuppression. We found that the fraction of antibody sequences related to activated B cells is inversely correlated with the dosage of immunosuppressive drugs, and could be used as a marker of net immunosuppression. We furthermore found that antibody repertoire sequencing detects immune activation during acute rejection events, as diagnosed by cell-free donor-derived DNA (cfdDNA) levels or endomyocardial biopsies [12,13].
Methods
Patient Selection
This work was approved by the Stanford University Institutional Review Board (protocol 17666), and all individuals provided written informed consent.
The specific goal of this proof-of-concept study was to test the clinical utility of immune repertoire sequencing to measure immunosuppression and predict acute rejection after heart transplantation. Participants included in this study were selected from a larger, ongoing prospective cohort study that involves recruitment of de novo pediatric and adult heart transplant candidates at Stanford Medical Center. The original cohort study was designed to determine the utility of cfdDNA for acute rejection surveillance after heart transplantation, and has been described previously [13]. Participant enrollment started in March 2010 and continues to this time, with patient follow-up for 5 y after transplantation. Multi-organ transplant candidates and recipients of repeat heart transplantation are excluded. Participants are recruited while on the waiting list for heart transplantation, and include patients requiring transplantation for a variety of end-stage heart diseases, including congenital heart disease, ischemic cardiomyopathy, dilated cardiomyopathy, valvular heart disease, and other etiologies.
We selected 12 adult transplant recipients from this larger prospective cohort study to represent patients with and without acute rejection events who were otherwise free of severe infections and other immune-related disorders. All buffy coat samples available at the onset of the study were analyzed, resulting in an average of 15 mo of follow-up for the 12 participants. Samples collected after this point were not included in the study. The main outcome measures of this study were the abundance and composition of the circulating B cell repertoire, in order to determine the temporal response of the B cell repertoire to immunosuppression after heart transplantation. We were interested in investigating the temporal dynamics of the B cell repertoire and therefore selected patients for whom a relatively large number of longitudinal samples were available. Among the patients analyzed in this study, six had experienced a rejection-free post-transplant course (patients 1–6; endomyocardial biopsy grade < 2R and cfdDNA below a previously determined threshold of 1% donor DNA), and six patients had experienced a moderate-to-severe rejection event as determined by endomyocardial biopsy grade ≥ 2R or elevated cfdDNA levels (patients 7–12) (S1 Table). We analyzed two additional time courses from patients with atypical post-transplant courses: patient 13 had experienced multiple opportunistic infections, and patient 14 had had a stem cell transplant for amyloid light chain (AL) amyloidosis. A total of 155 samples were analyzed (130 from patients 1–12 and 25 from patients 13 and 14; average of 11 samples per patient).
Post-Transplant Therapeutic Protocol
Post-transplant immunosuppression consisted of methylprednisolone 500 mg administered immediately postoperatively, followed by 125 mg every 8 h for three doses. Anti-thymocyte globulin (rATG) 1 mg/kg was administered on postoperative days 1, 2, and 3. Maintenance immunosuppression consisted of prednisone 20 mg twice daily starting on postoperative day 1 and tapered to <0.1 mg/kg/d by the sixth postoperative month, and tapered further if endomyocardial biopsies showed no evidence of cellular rejection. Tacrolimus was started on postoperative day 1, and dosing was adjusted to maintain a level of 10–15 ng/ml during months 0–6, 7–10 ng/ml during months 6–12, and 5–10 ng/ml thereafter. MMF was started at 1,000 mg twice daily on postoperative day 1, and dose adjustments were made, if required, in response to leukopenia. All heart transplant recipients, except for those in whom both the donor and recipient had no history of prior cytomegalovirus infection (i.e., donor and recipient cytomegalovirus IgG-negative), received anti-viral prophylaxis with valganciclovir 900 mg twice daily for 2 wk, then 900 mg daily until 6 mo post-transplant, followed by 450 mg daily until 12 mo post-transplant, at which point anti-viral prophylaxis was discontinued. Valganciclovir dose reductions were made in the setting of leukopenia. Anti-fungal prophylaxis consisted of itraconazole 300 mg daily for the first 3 mo post-transplant, and prophylaxis against Pneumocystis jiroveci infection consisted of trimethoprim 80 mg/sulfamethoxazole daily. All heart transplant recipients were monitored for acute cellular rejection (ACR) by surveillance endomyocardial biopsies performed at scheduled intervals after transplant: weekly during the first month, biweekly until the third month, monthly until the sixth month, and then at months 9, 12, 16, 20, and 24. Biopsies were graded according to the International Society for Heart and Lung Transplantation 2004 revised grading scale (0, 1R, 2R, 3R). Blood was sampled at the same time points, and when blood sampling and endomyocardial biopsies were performed on the same day, care was taken to ensure that blood was collected prior to the biopsy procedure. A subset of heart transplant recipients also had blood samples collected on post-transplant days 1 and 7. There were no adverse effects of performing biopsies or blood draws.
Whole Blood Processing and Nucleic Acid Extraction
Plasma and buffy coat samples were extracted from whole blood by sequential centrifugation within 3 h of sample collection and stored at −80°C. When required for analysis, plasma samples were thawed, and circulating DNA was immediately extracted from 0.5–1 ml of plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen). RNA was extracted from buffy coat samples using TRIzol Reagent (Life Technologies) followed by RNeasy column (Qiagen) extraction.
cfdDNA Library Preparation
Libraries were generated by a single researcher who was blinded to all clinical data until after sequencing and initial data analyses had occurred. Sequencing libraries were prepared from the purified patient plasma DNA using the NEBNext DNA Library Prep Master Mix Set for Illumina with standard Illumina indexed adapters, or using a microfluidics-based automated library preparation platform (Mondrian SP Ovation SP Ultralow Library System, Nugen). Libraries were characterized using the Agilent 2100 Bioanalyzer (high-sensitivity DNA kit) and quantified by quantitative PCR.
Immune Repertoire Library Preparation
Libraries were generated by two investigators (L. P. and C. V.) who were blinded to all clinical data until after sequencing and initial data analyses had occurred. 0.5–1 μm of total RNA were used as input for library preparation. Reverse transcription used SuperScript III Enzyme (Life Technologies) and primers for all five isotype constant regions containing eight random nucleotides and partial Illumina adapters. Second-strand synthesis was done using Phusion High-Fidelity DNA Polymerase (New England Biolabs) and primers for framing region 3 off all IGH variable (V) segments containing eight random nucleotides and partial Illumina adapters. The combined 16 random nucleotides (eight constant region primers and eight V segment primers) serve as a unique identifier (UID) for each IGH transcript molecule. Double-stranded cDNA was purified two times using AMPure XP beads at a ratio of 1 : 1. Double-stranded cDNA was amplified with Platinum Taq DNA Polymerase High Fidelity (Life Technologies) and two custom Illumina indexing primers completing the Illumina adapters and containing two Illumina index sequences (i5 and i7). Final sequencing libraries were generated by purifying the PCR product using AMPure XP beads at a ratio of 1 : 1 (Fig 1). Primer sequences were described previously [7].
Sequencing and Data Analysis
Immune repertoire libraries were grouped into library pools with up to 50 libraries with compatible Illumina indices and sequenced on the Illumina HiSeq 2000 or MiSeq using 2 × 100 bp or 2 × 150 bp protocols. The sequencing runs yielded between 1 and 10 million raw reads per sample. Raw reads were split into UID groups with unique 16-nucleotide identifiers. Every UID group defined a single original IGH transcript molecule, and consensus reads were built for forward and reverse reads of each UID group, correcting sequencing errors for UID groups with coverage of two or more raw sequencing reads. While this approach does not result in the correction of sequencing errors for UID groups with a coverage of one, it still identifies these groups as originating from a unique molecule, thereby aiding in quantification. Forward consensus reads were annotated by alignments to V segments using BLAST, yielding mutation rate information. Reverse consensus reads were aligned to constant regions using BLAST [14], yielding isotype information. To compare samples, data for each sample were randomly subsampled to 4,000 aligned consensus read pairs. Samples producing fewer than 4,000 aligned consensus reads were discarded. UID groups with identical forward consensus reads (containing complementary determining region 3 sequence information) and isotype were assumed to originate from the same B cell clone and were combined to determine expression levels of each antibody IGH transcript. Quantification and visualization was done using custom scripts based on NumPy/SciPy/Matplotlib/Python [15–18] scripts, which are available in S1 Data and Scripts.
Anellovirus Load
Shotgun DNA sequencing reads generated from the cfdDNA libraries that did not map to the human genome were aligned to a reference of viral, bacterial, and fungal genomes (BLAST). The relative genomic abundance of Anelloviridae virus was calculated with GRAMMy, a tool that utilizes BLAST-derived nucleic acid sequence similarity data to estimate the relative abundance of species [19].
Statistical Analysis
Differences between groups (no evidence of rejection, mild acute rejection diagnosed by endomyocardial biopsy, moderate-to-severe acute rejection diagnosed by endomyocardial biopsy, and rejection diagnosed by elevated cfdDNA) were tested using Mann-Whitney U tests. C-statistic, sensitivity, and specificity were determined using the sklearn.metrics package for Python [20]. Confidence intervals for sensitivity, specificity, positive predictive value, and negative predictive value were calculated according to the efficient-score method described by Wilson and Newcombe [21,22]. Confidence intervals for the c-statistic were determined using bootstrapping. The confidence interval for the Pearson correlation (r) between activated B cell sequence (ABS) level (defined as the ratio of all highly expressed IgA, IgD, IgG, IgE, and mutated IgM sequences to the total number of molecules) and tacrolimus level (Fig 2B) was calculated based on the Fisher r-to-z transformation.
Fig. 2. Using immune repertoire sequencing to measure immunosuppression. 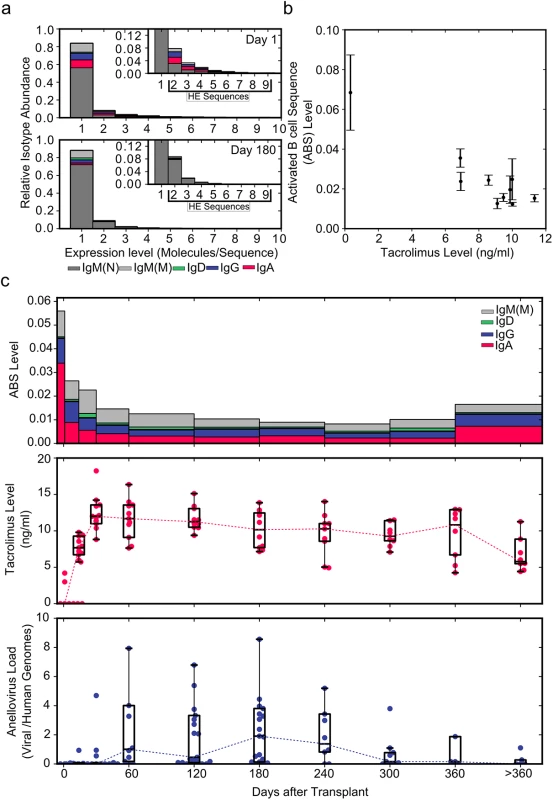
(A) Relative IGH sequence composition at increasing expression levels (molecules/sequence) is shown across all individuals (n = 12) in a histogram for days 1 and 180 post-transplant. Bar colors indicate the contribution of different immunoglobulin isotypes. Inserts show rescaled histograms. Highly expressed (HE) sequences (sequences represented by more than one molecule) are labeled in the insert. (B) ABS level (defined as the ratio of all highly expressed IgA, IgD, IgE, IgG, and mutated IgM sequences to the total number of molecules) as a function of tacrolimus trough level (n = 130) (average ± standard error of the mean). (C) Median ABS level (colors indicate isotype contribution), tacrolimus trough level, and total anellovirus load for all study patients (n = 12) during the first year after transplant. Data Sharing
The shotgun DNA sequence data have been deposited in the Sequence Read Archive (SRP034946). The immune repertoire sequence data have been deposited in the Sequence Read Archive (PRJNA260905).
Results
In this proof-of-concept study, we analyzed 130 blood samples from 12 patients who underwent pharmacological immunosuppression to prevent allograft rejection after heart transplantation. These individuals were a subset of a larger prospective cohort study [13] and were selected to represent patients with and without acute rejection events who were otherwise free of severe infections or other immune-related disorders. Starting on the first day post-transplant, until up to 2 y after transplantation, samples were collected at predetermined time points, with decreasing frequency as the study progressed. Concurrently with immune repertoire measurements (Fig 1), allograft health was monitored using cfdDNA levels and endomyocardial biopsies. We used DNA extracted from the plasma fraction of whole blood samples for the cfdDNA measurements and RNA extracted from the buffy coat for the immune repertoire sequencing protocol. Molecular barcodes were incorporated during reverse transcription of the extracted RNA and second-strand synthesis of the resulting cDNA. The barcoded double-stranded cDNA molecules were amplified as described previously (Fig 1) [7], and sequenced on HiSeq 2000 (2 × 100 bp) or MiSeq (2 × 150 bp) platforms. Expression level, mutation rate, and isotype were determined for each antibody IGH transcript (Fig 1).
Immunosuppression Causes Shift in Isotype Expression
We first determined whether we could measure the effects of immunosuppression on the activity of the immune system using immune repertoire sequencing of the antibody IGH transcript. To this end, we pooled data for all participants in the study and separated antibody IGH transcript sequences by expression level.
Lowly expressed sequences were defined as antibody IGH transcript sequences that were represented by only one molecule and that were likely expressed by naïve or inactive B cells. Highly expressed sequences were defined as antibody IGH transcript sequences that were represented by more than one molecule and that were likely expressed by activated B cells such as plasmablasts [7,23].
We compared all data, including time points with diagnosed rejection events, pooled from the 12 participants of the main study at the beginning of (day 1) and 6 mo into (day 180, 20 d before first rejection event) immunosuppressive therapy. When normalized to the total number of molecules analyzed, the number of highly expressed sequences did not vary substantially between day 1 (2,131 highly expressed sequences/19,845 molecules; ratio 0.107) and day 180 (8,018 highly expressed sequences/79,852 molecules; ratio 0.100). Interestingly, a much larger change was observed in the composition of highly expressed sequences. Highly expressed IgA, IgD, IgE, IgG, and mutated IgM sequences decreased from 1,296/19,845 molecules (ratio 0.065) at day 1 to 847/79,852 molecules (ratio 0.011) by day 180 after the induction of immunosuppression (Fig 2A and 2C). Based on this change we defined the ABS level as this ratio of all highly expressed IgA, IgD, IgE, IgG, and mutated IgM sequences to the total number of molecules. The decrease in ABS level after the induction of immunosuppressive therapy indicated that it is possible to quantify the level of immunosuppression using immune repertoire sequencing.
We found that ABS level decreased rapidly in the first month, and the initial rapid decrease was followed by a slow decline over subsequent months. ABS level approached approximately 15% of the original level after 10 mo, after which the ABS level increased in response to tapering of immunosuppressive therapy. The decrease in ABS level was strongly and negatively correlated with blood tacrolimus concentration (Pearson r = −0.867, 95% CI −0.968 to −0.523, p = 0.0014; Fig 2B and 2C).
By reducing the activity of the immune system, immunosuppressive therapy also alters selective pressure on bacterial and viral populations in the human body. We previously showed that the composition of the viral population, as measured by sequencing of cell-free DNA in plasma, is significantly affected by post-transplant immunosuppressive therapy [12]. In particular, we found that the number of sequences related to the Anelloviridae family of viruses increased dramatically between months 1 and 10 after transplantation. The apparent inverse correlation of anellovirus load and ABS level observed in this work (Fig 2C) further confirmed our observation that ABS level reflects the overall level of immunosuppression.
Elevated ABS Levels Indicate Acute Rejection Events
Next, we examined whether ABS levels are also informative of acute rejection events. We examined ABS level in four patients, two of whom experienced acute rejection as diagnosed by either cfdDNA level (>1% cfdDNA) or endomyocardial biopsy. While the ABS level of individuals without rejection remained at baseline throughout the study period (Fig 3A and 3B), ABS level in individuals with ACR increased dramatically at the time of or prior to ACR, indicating increased activity of the alloimmune response. The ABS levels of individuals increased up to 8-fold (Fig 3C and 3D), with the median ABS level elevated approximately 3-fold. Interestingly, in one example, both cfdDNA and ABS levels were elevated, yet endomyocardial biopsy, graded by a pathologist, indicated no rejection event at this time point (Fig 3D). This discrepancy suggests that ABS level can be used as an additional independent marker of acute rejection when cfdDNA measurements disagree with biopsy results.
Fig. 3. Reduced level of overall immunosuppression correlates with acute rejection events. 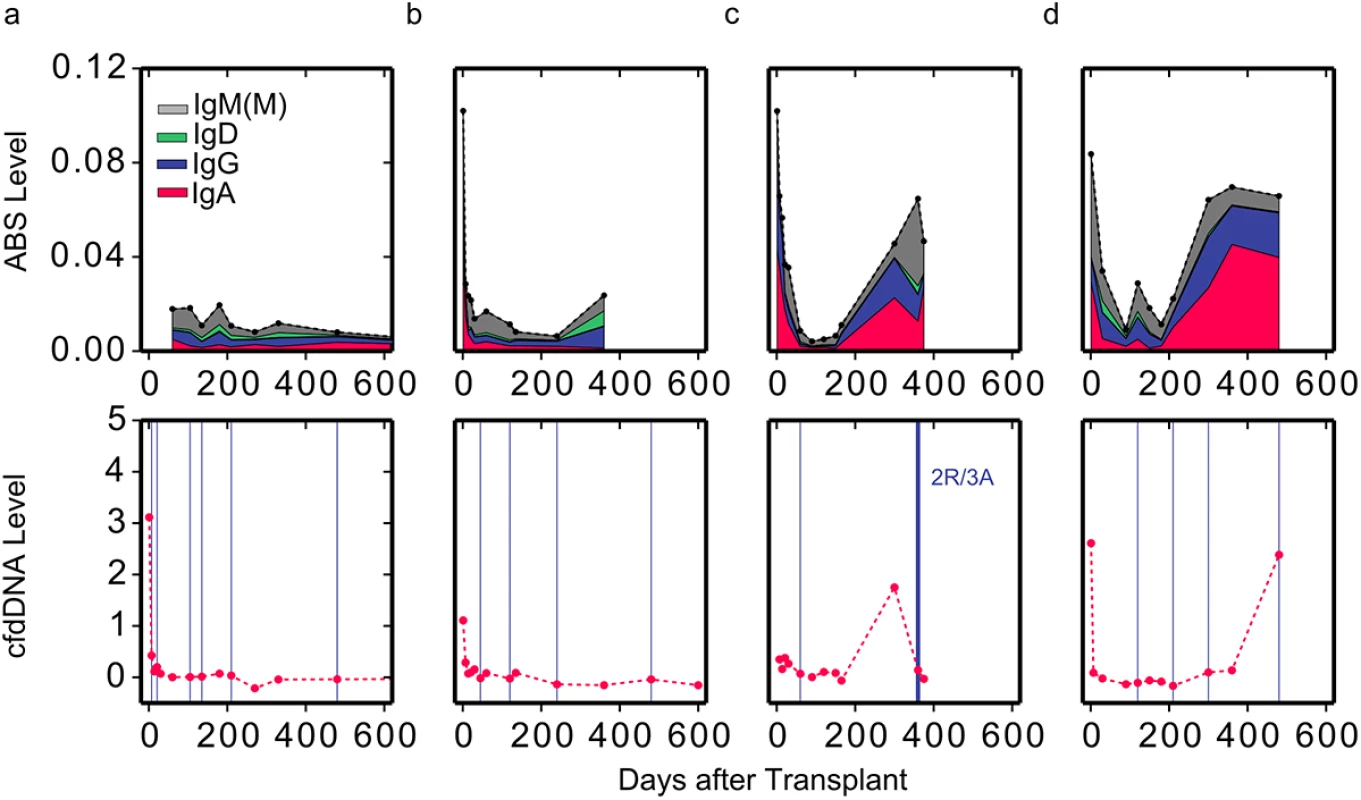
ABS levels are shown for two individuals with (C and D) and two individuals without (A and B) moderate-to-severe acute rejection events (top). Colors indicate isotype contribution. cfdDNA levels are shown for the same individuals (bottom). Biopsy grades are shown as vertical blue lines (bottom). Thin lines indicate mild ACR events (grade 1R), and thick lines indicate moderate-to-severe ACR events (grade ≥ 2R). ABS Levels Correlate Well with Endomyocardial Biopsy Results and cfdDNA Levels
To investigate the performance of the immune repertoire sequencing assay compared to cfdDNA and biopsy measurements, we plotted the ABS levels of all 12 study patients, highlighting acute rejection events as diagnosed by cfdDNA and endomyocardial biopsy (Figs 4A and S1). The induction of immunosuppression reduced ABS level to a baseline level of around 0.02 after about 1 mo (Fig 4A). ABS levels subsequently measured during many acute rejection were elevated above this baseline (Fig 4A).
Fig. 4. ABS levels correlate well with cfdDNA and endomyocardial biopsy results. 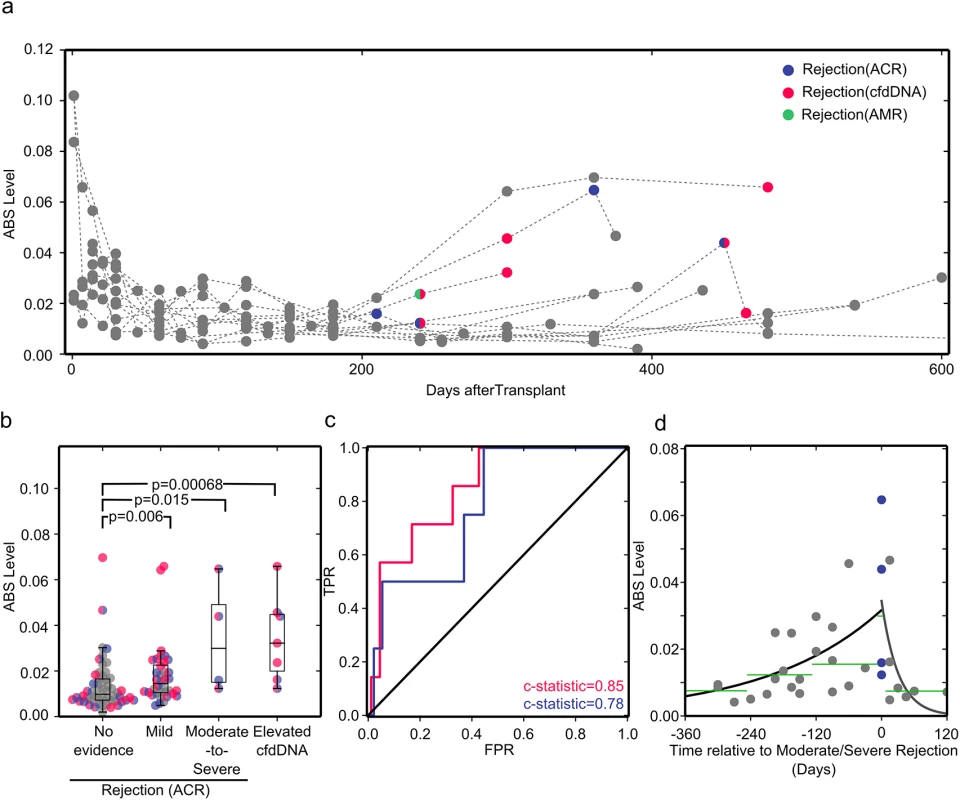
(A) ABS levels plotted for all study participants. Acute rejection events as diagnosed by endomyocardial biopsy (ACR, violet; acute antibody-mediated rejection [AMR], green) or cfdDNA level (pink) are shown in color. (B) Samples are classified by rejection status diagnosed by biopsy as no evidence of rejection, mild ACR (biopsy grade 1R), or moderate-to-severe ACR (biopsy grade ≥ 2R), and by rejection status diagnosed by the cfdDNA assay. Samples are colored as in (A) if at any point the organ recipient experienced a serious rejection event. As biopsy grades are independent of cfdDNA measurements, there is overlap between samples in the elevated cfdDNA and biopsy-proven rejection bins. The groups were compared using a one-sided Mann-Whitney U test, which has high efficiency for both normally and non-normally distributed datasets. (C) Receiver operating characteristic (ROC) curves for ABS levels compared to cfdDNA (pink) and endomyocardial biopsy (violet) results. FPR, false rejection rate; TPR, true positive rate. (D) ABS levels measured before and after rejection diagnosis. The black line shows the single-exponent fit of ABS levels measured prior to rejection, y = a × (exp[b × t]), with best fit values (least squares) of a = 0.0317 and b = 0.00468. The gray line shows the single-exponent fit of ABS levels after diagnosis and during treatment of rejection: y = a × (exp[b× t]), with best fit values (least squares) of a = 0.0344 and b = −0.0324. Green lines show the median ABS levels in the covered time periods. For a systematic analysis, we grouped samples according to biopsy and cfdDNA results. While all endomyocardial biopsies were tested for ACR, only some biopsies were also tested for antibody-mediated rejection, based on clinical parameters, thereby preventing systematic analysis for this type of acute rejection. In the cohort studied, antibody-mediated rejection was clinically reported at only one time point (Fig 4A). Therefore, we separated samples into no evidence of rejection (negative cfdDNA and biopsy grade 0), mild ACR (biopsy grade 1R), moderate-to-severe ACR (biopsy grade ≥ 2R), and rejection diagnosed by cfdDNA alone (“elevated cfdDNA”). We found that all subgroups with acute rejection had significantly different ABS levels compared to the non-rejection subgroup. The samples corresponding to acute rejection detected through cfdDNA measurement alone had the most significant p-value, followed by the samples corresponding to moderate-to-severe rejection (Fig 4B). We determined the area under the ROC curve for different ABS value thresholds, and found very good concordance between ABS levels and measurements of cfdDNA (c-statistic = 0.85 [95% CI 0.7 to 0.97], sensitivity 71.4% [95% CI 30.3% to 94.9%], specificity 82.0% [95% CI 72.1% to 89.1%], threshold 0.023) and between ABS levels and biopsy grades (c-statistic = 0.78 [95% CI 0.54 to 0.99]) (Fig 4C). When compared to cfdDNA as the noninvasive gold standard, the ABS assay achieved a positive predictive value of 0.24 (95% CI 0.09 to 0.48) and a negative predictive value of 0.97 (95% CI 0.9 to 1). The low positive predictive value can be partly explained by elevated ABS levels at time points preceding elevated cfdDNA levels. While these time points are categorized as false positives, they can provide valuable information about the patient’s immune activity prior to acute allograft rejection events.
To investigate the potential for early diagnosis, we analyzed the temporal dynamics of ABS level prior to a biopsy-proven moderate-to-severe rejection event (Fig 4D). A gradual and significant increase in ABS level is observed leading up to rejection episodes. Compared to the baseline ABS level (>8 mo before rejection), the median ABS level increased 2-fold within the 4-mo time window prior to rejection events (p = 0.021, Mann-Whitney U test). We furthermore investigated the response of the antibody repertoire to treatment of acute rejection (Fig 4D). Here, successful rejection treatment gave rise to a rapid decrease in ABS level, reaching a level comparable to the baseline level as early as 1 mo after initiation of treatment. Together, these data highlight the potential for early diagnosis and the use of these measurements to evaluate the efficacy of rejection therapy.
ABS Level Correlates with Infections, Drug Therapy, and B Cell Disorders
We next sought to examine the effect of opportunistic infections and B cell disorders on antibody repertoire sequencing measurements. To this end, we tracked ABS level in 25 samples from two additional heart transplant recipients. The first patient experienced several infections diagnosed both before and after transplantation, including cytomegalovirus, Epstein-Barr virus, hepatitis B virus, varicella zoster virus, staphylococcus, parainfluenza 3, and Mycobacterium avium. Interestingly, ABS level was elevated throughout the course of the study in this individual (Fig 5B) compared to a representative non-rejection individual free of infectious complications (Fig 5A). The second patient had AL amyloidosis, a B cell dyscrasia characterized by excessive antibody light chain production. End-stage cardiomyopathy related to AL amyloidosis was the indication for transplantation in this patient, who received several additional immunosuppressive and chemotherapeutic agents post-transplant in order to treat this disorder (Fig 5C). Two events over the course of his treatment highlight the unique aspects of this case: (1) this patient received an autologous stem cell transplant 6 mo after the heart transplant surgery, and (2) he was repeatedly treated with the drug filgrastim (granulocyte colony stimulating factor) to increase the number of hematopoietic stem cells and granulocytes in his blood prior to and after bone marrow transplantation. We detected a 6-fold increase in this patient’s ABS level following filgrastim administration (Fig 5C). The steep increase in ABS level that we observed is in agreement with a previously reported increase in the number of activated B cells following filgrastim treatment [24,25]. This patient continued to produce an excess of antibody light chains, and a more than 10-fold increase in ABS abundance was detected in the year following bone marrow transplantation, based entirely on an expansion of IgA sequences, despite treatment with immunosuppressive agents. While amyloidosis is generally considered to be caused by a single aberrant B cell clone, this measured increase in IgA was polyclonal (S2 Fig), suggesting a possible polyclonal component of the disorder. These two cases illustrate the potential of immune repertoire sequencing to monitor infectious complications, immunosuppressive drug treatment, and B cell disorders.
Fig. 5. Reduced net immunosuppression correlates with acute rejection. 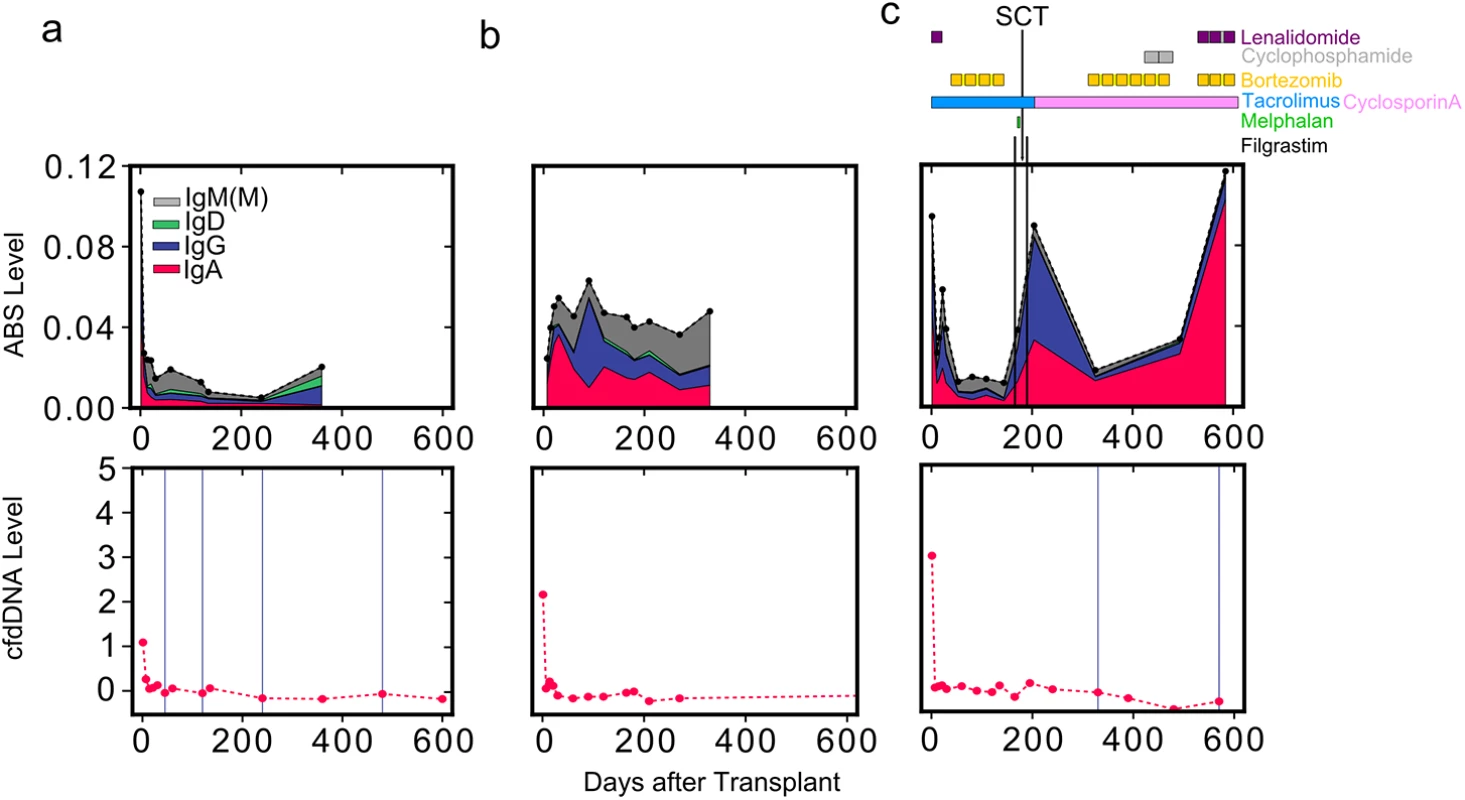
ABS level is shown for three individuals: (A) a heart transplant recipient without moderate-to-severe rejection events, (B) a heart transplant recipient with infectious complications, and (C) a heart transplant recipient with AL amyloidosis. For the patient with AL amyloidosis (C), the approximate schedule of drug treatment is indicated at the top of the panel. Colors indicate isotype contribution. cfdDNA level is shown for the same individuals (bottom). Biopsy grades are shown as vertical blue lines (bottom): thin lines indicate mild rejection events (grade 1R), and thick lines indicate moderate-to-severe rejection events (grade ≥ 2R). SCT, stem cell transplant. Discussion
Heart transplantation is considered the best therapeutic option for the treatment of eligible patients with end-stage heart disease [26]. After transplantation, patient management focuses on the difficult task of achieving a balance between over - and under-immunosuppression. The immune repertoire sequencing assay presented here has the potential to measure the overall level of immunosuppression and to detect episodes of acute rejection after heart transplantation. Yet, further validation of the assay by a prospective study is needed, as this proof-of-concept study included a relatively small number of patients, and the patients included in this study were selected based on their rejection status and the availability of a high number of longitudinal samples.
Samples were collected longitudinally from the heart transplant recipients in this study, and immune repertoire measurements were compared to biopsy grades, tacrolimus levels, and the level of cfdDNA, as determined by shotgun sequencing. This longitudinal sampling enabled us to closely monitor the dynamics of immunosuppression. Measurements of cfdDNA and biopsies performed in parallel to the immune repertoire assay made it possible to compare our assay to both a noninvasive independent assay (cfdDNA) as well as the invasive diagnostic gold standard for acute organ rejection (endomyocardial biopsy). This approach provided us with an unprecedented view of the immune response to pharmacological immunosuppression, and how it escapes sufficient suppression, resulting in acute rejection.
Several commercially available assays aim to accomplish these goals [27,28] but have shown poor predictive accuracy [29,30]. The ImmuKnow (Cylex) assay measures the ability of T cells to be activated in vitro. The AlloMap assay (CareDx) uses microarray analysis to measure the expression levels of 11 genes that change in response to immunosuppression. It provides a numerical score that is affected by the composition and activation state of the various cell populations sampled. While this score is easy to interpret from a clinical perspective, the biological basis for the score is highly convoluted, and the score does not provide useful information outside of adult heart transplantation. In contrast, the immune repertoire sequencing assay that we developed queries the highly informative and well-understood IGH locus. After activation, B cells express IGH transcripts in a way that is both quantitatively (higher expression levels) and qualitatively (hypermutation and isotype class switch recombination) distinct from that of non-activated B cells. B cell activation requires activated T cells and takes several days to complete. Therefore, by quantifying the number of unique IGH sequences expressed by activated B cells (ABSs), our measurement integrates the level of immune activation over an extended period of time and has better test performance characteristics for detecting rejection events diagnosed by biopsy than the AlloMap assay (c-statistic ABS = 0.78 versus c-statistic AlloMap = 0.72; data from [31]).
The immune repertoire assay presented here is very efficient in terms of both sample preparation and sequencing: in this study we implemented a dual indexing strategy that allowed us to multiplex more than 200 samples on a single HiSeq 2000 flow-cell lane, thereby reducing the cost per sample—including sample preparation—to approximately US$40. The assay furthermore provides a value based on internal controls that does not rely on external normalization, and can be performed on small and low-quality samples. In addition to our main focus on immunosuppression and immune activation via rejection, we examined the dynamics of the B cell repertoire for two patients with unusual post-transplant courses: one patient with severe infectious complications and one patient with a B cell disorder. These case studies indicate the potential of immune repertoire sequencing in the management of a diverse set of post-transplant complications and also illustrate the potential value of combining immune repertoire sequencing with other assays, such as the measurement of cfdDNA in plasma, in order to discriminate between possible causes of immune activation.
Prospective studies are required to validate this assay in a larger patient population, to compare immune repertoire sequencing assay results to other noninvasive screening tests for heart transplant complications (cfdDNA, AlloMap), and to investigate the utility of this assay in the setting of other solid organ transplants (e.g., lung, kidney, and liver transplantation). Finally, studies that use immune repertoire sequencing results to tailor immunosuppression in individual patients may enable a “personalized medicine” approach to achieving adequate suppression of the alloimmune response, while avoiding the myriad long-term sequelae of over-immunosuppression.
Supporting Information
Zdroje
1. Plosker GL, Foster RH. Tacrolimus: a further update of its pharmacology and therapeutic use in the management of organ transplantation. Drugs. 2000;59 : 323–389. 10730553
2. Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66 : 807–815. 1715244
3. Heidt S, Roelen DL, Eijsink C, Eikmans M, Van Kooten C, Claas FHJ, et al. Calcineurin inhibitors affect B cell antibody responses indirectly by interfering with T cell help. Clin Exp Immunol. 2010;159 : 199–207. doi: 10.1111/j.1365-2249.2009.04051.x 19922499
4. Perez-Andres M, Paiva B, Nieto WG, Caraux A, Schmitz A, Almeida J, et al. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78 (Suppl 1):S47–S60. doi: 10.1002/cyto.b.20547 20839338
5. Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120 : 1836–1847. doi: 10.1172/JCI39933 20501946
6. Glanville J, Kuo TC, von Budingen H-C, Guey L, Berka J, Sundar PD, et al. Naive antibody gene-segment frequencies are heritable and unaltered by chronic lymphocyte ablation. Proc Natl Acad Sci U S A. 2011;108 : 20066–20071. doi: 10.1073/pnas.1107498108 22123975
7. Vollmers C, Sit RV, Weinstein JA, Dekker CL, Quake SR. Genetic measurement of memory B-cell recall using antibody repertoire sequencing. Proc Natl Acad Sci U S A. 2013;110 : 13463–13468. doi: 10.1073/pnas.1312146110 23898164
8. Boyd SD, Marshall EL, Merker JD, Maniar JM, Zhang LN, Sahaf B, et al. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med. 2009;1 : 12ra23. 20161664
9. Georgiou G, Ippolito GC, Beausang J, Busse CE, Wardemann H, Quake SR. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat Biotechnol. 2014;32 : 158–168. doi: 10.1038/nbt.2782 24441474
10. Jiang N, He J, Weinstein JA, Penland L, Sasaki S, He X-S, et al. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5 : 171ra19. doi: 10.1126/scitranslmed.3004794 23390249
11. Logan AC, Gao H, Wang C, Sahaf B, Jones CD, Marshall EL, et al. High-throughput VDJ sequencing for quantification of minimal residual disease in chronic lymphocytic leukemia and immune reconstitution assessment. Proc Natl Acad Sci U S A. 2011 : 108 : 21194–21199. doi: 10.1073/pnas.1118357109 22160699
12. De Vlaminck I, Khush KK, Strehl C, Kohli B, Luikart H, Neff NF, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155 : 1178–1187. doi: 10.1016/j.cell.2013.10.034 24267896
13. De Vlaminck I, Valantine HA, Snyder TM, Strehl C, Cohen G, Luikart H, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6 : 241ra77. doi: 10.1126/scitranslmed.3007803 24944192
14. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215 : 403–410. 2231712
15. Hunter JD. Matplotlib: a 2D graphics environment. Comput Sci Eng. 2007;9 : 90–95.
16. SciPy Developers. SciPy [computer program]. http://www.scipy.org/. Accessed 10 September 2015.
17. Oliphant TE. Python for scientific computing. Comput Sci Eng. 2007;9 : 10–20.
18. Van der Walt S, Colbert SC, Varoquaux G. The NumPy array: a structure for efficient numerical computation. Comput Sci Eng. 2011;13 : 22–30.
19. Xia LC, Cram JA, Chen T, Fuhrman JA, Sun F. Accurate genome relative abundance estimation based on shotgun metagenomic reads. PLoS ONE. 2011;6:e27992. doi: 10.1371/journal.pone.0027992 22162995
20. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12 : 2825–2830.
21. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22 : 209.
22. Julious SA. Two-sided confidence intervals for the single proportion: comparison of seven methods by Robert G. Newcombe, Statistics in Medicine 1998; 17 : 857–872. Stat Med. 2005;24 : 3383–3384. 16206245
23. Nair N, Newell EW, Vollmers C, Quake SR, Morton JM, Davis MM, et al. High-dimensional immune profiling of total and rotavirus VP6-specific intestinal and circulating B cells by mass cytometry. Mucosal Immunol. 2015 Apr 22. 25899688
24. Tayebi H, Lapierre V, Saas P, Lienard A, Sutton L, Milpied N, et al. Enhanced activation of B cells in a granulocyte colony-stimulating factor-mobilized peripheral blood stem cell graft. Br J Haematol. 2001;114 : 698–700. 11553000
25. Jensen GS, Poppema S, Mant MJ, Pilarski LM. Transition in CD45 isoform expression during differentiation of normal and abnormal B cells. Int Immunol. 1989;1 : 229–236. 2535062
26. Hoffman FM. Outcomes and complications after heart transplantation: a review. J Cardiovasc Nurs. 2005;20:S31–S42. 16160582
27. Giese T, Zeier M, Meuer S. Analysis of NFAT-regulated gene expression in vivo: a novel perspective for optimal individualized doses of calcineurin inhibitors. Nephrol Dial Transplant. 2004;19 (Suppl 4):iv55–iv60. 15240851
28. Wieland E, Olbricht CJ, Süsal C, Gurragchaa P, Böhler T, Israeli M, et al. Biomarkers as a tool for management of immunosuppression in transplant patients. Ther Drug Monit. 2010;32 : 560–572. doi: 10.1097/FTD.0b013e3181efb3d2 20814352
29. Rossano JW, Denfield SW, Kim JJ, Price JF, Jefferies JL, Decker JA, et al. Assessment of the Cylex ImmuKnow cell function assay in pediatric heart transplant patients. J Hear Lung Transplant. 2009;28 : 26–31.
30. Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362 : 1890–1900. doi: 10.1056/NEJMoa0912965 20413602
31. Deng MC, Eisen HJ, Mehra MR, Billingham M, Marboe CC, Berry G, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6 : 150–160. 16433769
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 10- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Léčba bolesti u seniorů
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Transparency in Reporting Observational Studies: Reflections after a Year
- Using Qualitative Evidence in Decision Making for Health and Social Interventions: An Approach to Assess Confidence in Findings from Qualitative Evidence Syntheses (GRADE-CERQual)
- The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement
- New Clinical Decision Instruments Can and Should Reduce Radiation Exposure
- Monitoring Mortality in Forced Migrants—Can Bayesian Methods Help Us to Do Better with the (Little) Data We Have?
- Continuity in Drinking Water Supply
- Water Supply Interruptions and Suspected Cholera Incidence: A Time-Series Regression in the Democratic Republic of the Congo
- Individual Participant Data (IPD) Meta-analyses of Diagnostic and Prognostic Modeling Studies: Guidance on Their Use
- Derivation and Validation of Two Decision Instruments for Selective Chest CT in Blunt Trauma: A Multicenter Prospective Observational Study (NEXUS Chest CT)
- Effect of Health Risk Assessment and Counselling on Health Behaviour and Survival in Older People: A Pragmatic Randomised Trial
- P2RX7 Purinoceptor: A Therapeutic Target for Ameliorating the Symptoms of Duchenne Muscular Dystrophy
- Monitoring Pharmacologically Induced Immunosuppression by Immune Repertoire Sequencing to Detect Acute Allograft Rejection in Heart Transplant Patients: A Proof-of-Concept Diagnostic Accuracy Study
- Strategies for Understanding and Reducing the and Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model
- Upgrading a Piped Water Supply from Intermittent to Continuous Delivery and Association with Waterborne Illness: A Matched Cohort Study in Urban India
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effect of Health Risk Assessment and Counselling on Health Behaviour and Survival in Older People: A Pragmatic Randomised Trial
- Using Qualitative Evidence in Decision Making for Health and Social Interventions: An Approach to Assess Confidence in Findings from Qualitative Evidence Syntheses (GRADE-CERQual)
- Monitoring Pharmacologically Induced Immunosuppression by Immune Repertoire Sequencing to Detect Acute Allograft Rejection in Heart Transplant Patients: A Proof-of-Concept Diagnostic Accuracy Study
- The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



