-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaP2RX7 Purinoceptor: A Therapeutic Target for Ameliorating the Symptoms of Duchenne Muscular Dystrophy
Dariusz Gorecki and colleagues investigate the effect of P2RX7 ablation on muscle, brain, and bone pathology in a DMD model mouse.
Published in the journal: . PLoS Med 12(10): e32767. doi:10.1371/journal.pmed.1001888
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001888Summary
Dariusz Gorecki and colleagues investigate the effect of P2RX7 ablation on muscle, brain, and bone pathology in a DMD model mouse.
Introduction
Duchenne muscular dystrophy (DMD) results in loss of dystrophin, which disrupts structural scaffolds for dystrophin-associated proteins (DAPs) as well as specific signaling processes, causing progressive muscle loss with sterile inflammation [1]. Symptoms also include cognitive and behavioral impairment [2] and bone structure abnormalities [3], both irrespective of the functional muscle impairment. This symptom diversity illustrates the importance of DMD gene expression in various cells.
Molecular approaches aimed at restoration of dystrophin hold some promise, but achieving the 15%–20% level of expression required to fully protect muscle fibers [4] in all crucial muscle groups remains a challenge. Moreover, muscle targeting would not tackle non-muscle symptoms. Therefore, alternative strategies should be investigated, and treatments aimed at alterations downstream from the absence of dystrophin have shown therapeutic promise [5]. Clearly, targeting signaling pathways using pharmacological agents is currently more achievable than restoration of structural proteins via molecular approaches.
We and others have demonstrated that DMD mutations impact on the control of ATP signaling and have identified P2RX7 up-regulation as being responsible for the death of human DMD lymphoblasts and muscles in the mdx mouse model of DMD [6–11]. Analyzing the consequences of P2RX7 activation, we discovered a novel mechanism of autophagic cell death, and pharmacological blockade or genetic ablation of P2RX7 proved protective against the ATP-induced death of dystrophic muscles [12].
P2RX7 belongs to a family of cell membrane ATP-gated ion channels. Unlike some other purinoceptors, full activation of P2RX7 requires high levels of extracellular ATP (eATP), which occur in inflammatory conditions [13]. P2RX7 was originally identified on macrophages and lymphocytes as a sensor of eATP released from damaged cells (one of the damage/danger-associated molecular patterns [DAMPs]) and was considered an activator of the “danger mode” of the immune response [14].
Levels of cytoplasmic ATP in skeletal muscles are particularly high, so there is a potential for 5–10 mM eATP at the dystrophic cell membrane [10]. Furthermore, the DAP α-sarcoglycan is a muscle-specific ATP hydrolase responsible for 25% of eATP degradation [15]. In DMD, α-sarcoglycan is lost from the sarcolemma [1], which raises eATP levels, creating an environment consistent with increased activation of P2RX7.
Crucially, P2RX7 expression and function are increased in many diverse pathologies such as rheumatoid arthritis, graft-versus-host disease, transplant rejection, neuro-inflammation, pain [16], and limb-girdle muscular dystrophy type 2B—another muscular dystrophy with an inflammatory component [8]. As P2RX7 upregulation is present in both DMD [6] and mdx mice [9], over-activation of P2RX7 could contribute to damage both directly by causing the death of dystrophic muscles [12] and indirectly by stimulating harmful inflammatory responses.
We bred mdx mice with Pfizer or Glaxo P2RX7 knockout mice and developed two lines of dystrophic mice (Fig 1A). One line (Pf-mdx/P2X7−/−) lacked functional receptors [17]. The second (G-mdx/P2X7−/−) was an isoform knockout, in which the main (P2RX7a) variant was knocked out but the low-expression P2RX7k variant escaped inactivation [18]. Therefore, the latter served as an additional control. These models were used to establish the impact of P2RX7 ablation on the dystrophic pathology in muscle, brain, and bone and to assess its suitability as a therapeutic target.
Fig. 1. Generation and characterization of mdx/P2X7−/− mice. 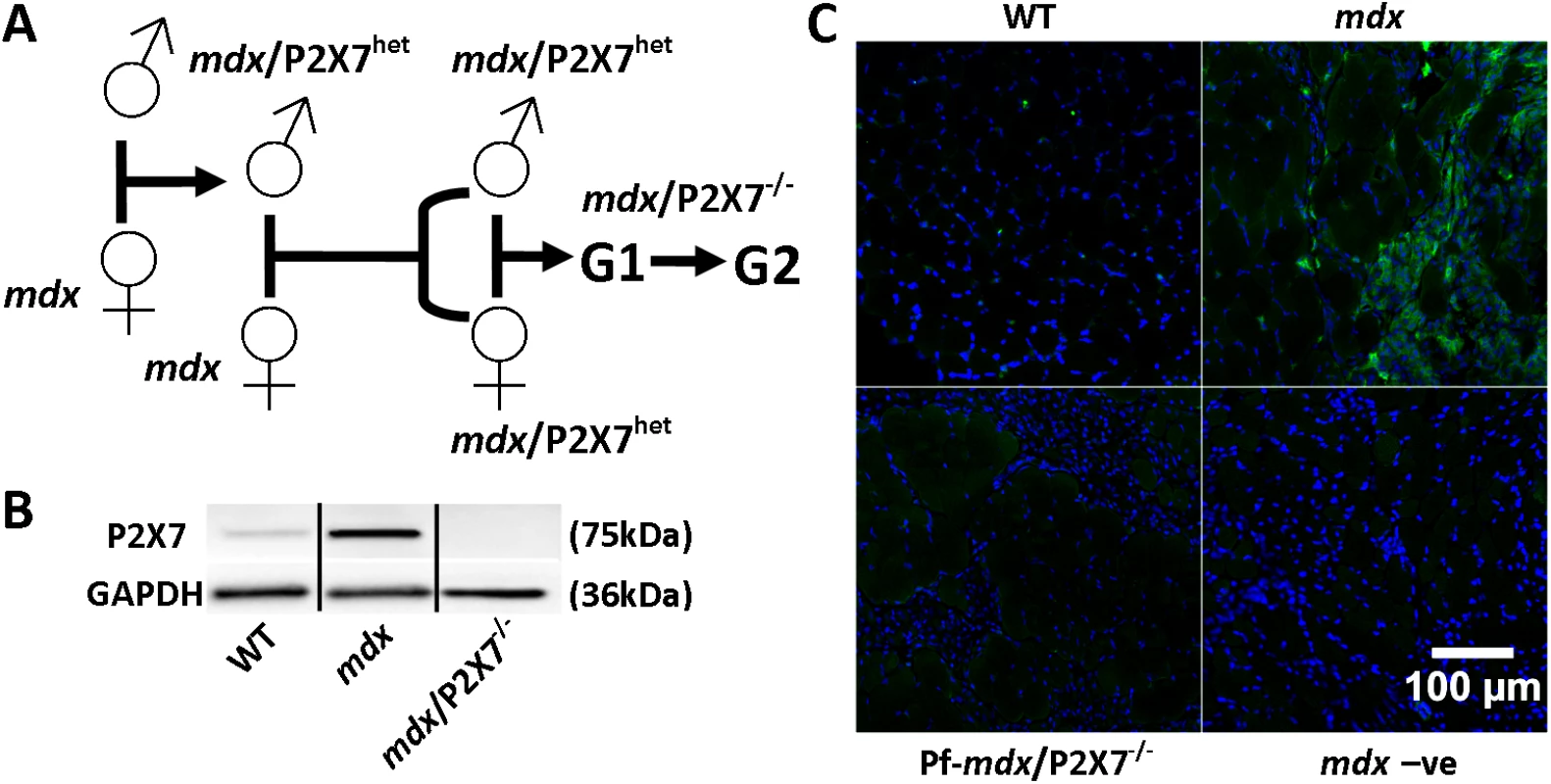
(A) Schematics of mouse breeding. (B) Representative Western blots showing increased expression of P2RX7 in 4-wk-old mdx compared to wild-type (WT) gastrocnemius and its absence in mdx/P2X7−/−. Use of separate Western blots is indicated by solid black lines. (C) Micrographs of P2RX7 immunofluorescence localization (green signal) in 4-wk-old tibialis anterior (TA) muscle from WT, mdx, and Pf-mdx/P2X7−/− mice showing expression in areas rich with infiltrating cells, and negative control using no primary antibody and with a blue signal denoting nuclear counterstaining. Methods
All animal experiments were performed in accordance with approvals from the Institutional Ethical Review Board, University of Portsmouth, and the Home Office, UK (70/7479), and with the recommendations of Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010, L276/33 (20.10.2010). The protocols (see below and S1 Checklist) were approved by the French Local Ethical Committee of Normandy (CENOMEXA; approval numbers N/14-04-04-16, N/15-04-04-17, and N/16-04-04-18) and were conducted in an authorized animal facility (authorization number C-76-540-2), under the supervision of authorized investigators (J. C. do Rego; authorization number 76.A.29 from the Ministère de l’Agriculture et de la Pêche, France). Investigators were blinded to the sample group allocation, where possible.
Generation of Double-Mutant Mice
Animals were central to this project, as there is no in vitro system allowing testing of a disease involving muscle damage and inflammatory processes, which are, in addition, interdependent. In a consensus opinion of experts, the mdx mouse is currently the most appropriate preclinical model to test treatment efficacy for DMD (http://www.treat-nmd.eu/research/preclinical/dmd-sops/).
Pfizer and Glaxo P2RX7 knockout male mice [19,20] were crossed with mdx C57Bl/10ScSn-Dmdmdx/J female mice (Harlan Laboratories). Male F1 mice were sequentially intercrossed with mdx females to generate double-mutant mice. The F3 generation showed the expected Mendelian distribution of genotypes, all of them on an mdx background. The P2X7−/− and mdx genotypes were identified by PCR using ear biopsy genomic DNA. The genotype was confirmed by Western blotting of muscle samples, using anti-dystrophin and anti-P2RX7 antibodies (listed below).
To mimic the clinically relevant situation, analyses were performed in male mice (unbiased by gender). At 4 wk, mdx mice show muscle degeneration and regeneration and an inflammatory cell infiltration pattern akin to the human pathology. The diaphragm is the mdx muscle that undergoes progressive degeneration, which is particularly noticeable at older ages [21]. In DMD patients, those who survive to the third decade present with cardiomyopathy and heart failure [22]. Therefore, diaphragms and hearts were studied at 20 mo to investigate the late-stage effects of receptor ablation.
Antagonist Administration
Starting at 2 wk of age, mdx mice were treated daily by intraperitoneal injection for 4 wk with 125 mg/kg body weight Coomassie Brilliant Blue G 250 (CBB) (Sigma-Aldrich) or for 4 wk with 8.4 mg/kg body weight oxidized ATP (ox-ATP) (Tocris Bioscience) or for 2 wk with 50 mg/kg body weight A-438079 (Tocris Bioscience). Dosage was based on previous studies [9,23,24]. Age-matched control mice received the same volume of sterile saline (for CBB and ox-ATP) or saline with 20% v/v DMSO solution (for A-438079).
PCR Analysis
PCRs for the identification of P2RX7, WT, and mutant alleles were performed using the following primers: Pfizer exon-13 WT (Fv 5′-TGGACTTCTCCGACCTGTCT; Rv 5′-TGGCATAGCACCTGTAAGCA) and neomycin cassette KO (Fv 5′-CTTGGGTGGAGAGGCTATTC; Rv 5′-AGGTGAGATGACAGGAGATC), and Glaxo exon-1 WT (Fv 5′-TGCCCATCTTCTGAACAC; Rv 5′-CTTCCTCTTACTGTTTCCTCCC) and LacZ KO variant (Fv 5′ - TGCCCATCTTCTGAACAC; Rv 5′-GCAAGGCGATTAAGTTGGG). The mdx mutation was identified using ARMS (amplification refractory mutation system) primers as previously described [25]. PCR conditions were as follows: denaturing at 94°C for 3 min; 35–40 cycles of 94°C for 30–40 s, 55–59°C annealing for 30–40 s, and elongation at 72°C for 1 min per kilobase of expected PCR product length; followed by a final 10-min elongation step at 72°C.
Quantitative PCR Analysis
Total RNA was extracted using the RNeasy Kit (Qiagen) per manufacturer’s instructions, and 1 μg of RNA was used for cDNA synthesis. 25–50 ng of the cDNA was used to perform expression analyses with Taqman on the StepOnePlus System (Applied Biosystems) or using the ViiA 7 Real-Time PCR System (Life Technology) and using SYBR Green on the LightCycler 1536 (Roche). All data were analyzed using Qbase+ software (Biogazelle). Quantitative PCR (qPCR) primers used for the analysis were purchased from Applied Biosystems or Primer Design. A set of 12 candidate reference genes (Primer Design) was tested, and the most stably expressed genes were determined using the geNorm tool of the Qbase+ software. These were used as reference to establish individual gene expression values (2−ΔΔCT). Four to five biological replicates (individual mice) for each genotype were analyzed in duplicate, and all samples were run on the same plate to avoid inter-run variation and calibration. qPCR analyses were performed in Portsmouth, Rouen, and Pittsburgh, and results for overlapping transcripts were cross-referenced between collaborating centers.
RNA-Seq Analysis
RNA was extracted as described above from TA muscles isolated from four individual mice per genotype. RNA quality was confirmed using Bioanalyzer (Agilent) per manufacturer’s instructions, and RNA-Seq was performed by a commercial service provider, Source BioScience. Raw FASTQ files were preprocessed by quality and adapter trimming and mapping to mouse genome (mm10 genome assembly, UCSC Genome Browser), and expression quantification was performed using the Tuxedo suite (TopHat and Cufflinks, [http://www.nature.com/nprot/journal/v7/n3/full/nprot.2012.016.html]). Genes of interest were selected to match the Qiagen mouse fibrosis PCR array (http://www.sabiosciences.com/rt_pcr_product/HTML/PAMM-120A.html). The resulting gene-level expression table for relevant RNAs is available as S2 Table.
Antibodies
The following antibodies were used: CD4–14–0041, CD8–14–0081, and Ly6G—14–5931, all rat monoclonal (eBioscience); IL1B—8689, rabbit polyclonal (Cell Signaling Technology); F4/80—ab74383 and collagen type-Ia—ab34710, both rabbit polyclonal (Abcam); CD68—MCA1957GA and CD206—MCA2235GA, both rat monoclonal (AbD Serotec); CD163—sc-33560, rabbit polyclonal (Santa Cruz Biotechnology); dystrophin—MANDRA1, mouse monoclonal, and myogenin—FD5, rat monoclonal (Developmental Studies Hybridoma Bank); LC3II—L7543 and actin—A2066, both rabbit polyclonal, and GAPDH—G9545 (Sigma-Aldrich); collagen type-IV—AB769, goat polyclonal (Chemicon); P2X7–177 003, rabbit polyclonal (Synaptic Systems); β-tubulin—IMG-5810A, rabbit polyclonal (Imgenex); dystrophin—2166, rabbit polyclonal, was a kind gift from D. J. Blake, Cardiff University.
Western Blotting
Total proteins from frozen tissues were extracted by crushing samples in liquid nitrogen, with further homogenization in extraction buffer: 1× Lysis-M, 1× protease inhibitor cocktail, 2× phosphatase inhibitor cocktail (all Roche), 2 mM sodium orthovanadate (Sigma-Aldrich). All samples were centrifuged (800g for 3 min at 4°C), and protein concentrations were determined using the Bicinchoninic Acid Kit (Sigma-Aldrich). 20–40 μg of protein was mixed with Laemmli buffer at a 1 : 1 v/v ratio with 5% v/v β-mercaptoethanol, heated for 5 min at 95°C, and chilled on ice. Samples were separated on 6%–12% w/v SDS-polyacrylamide gels and electroblotted onto Hybond C membranes (Amersham). Blots were blocked in 5% w/v nonfat milk powder in 1× Tris buffered saline with Tween 20 (TBST) (0.01% v/v Tween 20; Sigma-Aldrich) for 1 h prior to probing with a primary antibody diluted in the same blocking buffer (overnight at 4°C or for 2 h at room temperature), then blots were washed (3×) with 1× TBST for 10 min and incubated with the appropriate horseradish-peroxidase-conjugated secondary antibody (Sigma-Aldrich) for 45 min. Specific protein bands were visualized using luminol-based substrates (Uptilight US, Cheshire Sciences), and images were obtained using a G:BOX Chemi XT16 system (Syngene). β-actin, GAPDH, and tubulin were used as protein-loading controls. All densitometric analyses of specific protein bands were made using exposure times within the linear range and the integrated density measurement function of ImageJ software [26,27].
Immunolocalization
Frozen muscle was transferred to a cryostat chamber and allowed to equilibrate to −20°C. Cryosections 5 - to 10-μm thick were then cut from the middle third of the sample and collected on poly-L-lysine (0.5 mg/ml)–coated glass slides. Sections were allowed to air dry for several hours. Samples were fixed in a 2%–4% w/v paraformaldehyde solution in TBST for 15 min at 4°C, followed by two washes in TBST. The primary antibody incubation in TBST containing 10% v/v serum was applied for 2 h at room temperature or overnight at 4°C. Three 5-min TBST washes were applied before secondary antibody incubation in TBST and 2% v/v serum containing Hoechst fluorescent nuclear counterstain for 1 h at room temperature. Sections were finally washed three times for 10 min before mounting in FluorSave (Merk Millipore) fluorescence mounting medium. Either entire cross-sections through the mid-portion of TA muscles were captured in their entirety using a zoom microscope (Axiozoom V.16, Zeiss), or whole cross-sections were made of montaged 20× magnification fields of view. For quantification of immunofluorescent cells, a semi-automated (unbiased) method using a thresholding macro designed in ImageJ was used. Numbers were then expressed per unit of area. For diaphragms, counts per unit of area for each animal were derived by averaging the counts from five fields of view encompassing a significant portion of each diaphragm cross-section. Counts were also made using the threshold and analyze particles functions of ImageJ. Enumeration of dystrophin revertant fibers (the dystrophin-expressing fibers arising due to spontaneous exon skipping occurring in myogenic cells) was achieved using the cell counter plugin of ImageJ [26,27] applied to whole cross-sections of diaphragm. Revertant fibers were reported as number per square millimeter. For the quantification of IgG permeability into muscle fibers, the entire cross-section was analyzed. Manually delineated IgG-positive (compromised) fluorescent areas were compared (percent of total area of the muscle cross-section).
Morphometric Analysis
Muscle fiber size and central nucleation were visualized by collagen type-IV and Hoechst immunofluorescence staining of frozen muscle sections. Individual microscope fields of view were montaged using ImageJ to present whole cross-sections through the muscle. Image analysis was performed on these composite images using Fiji and ImageJ open-source software (US National Institutes of Health). A macro was developed to sequentially (i) subtract background components to minimize noise that could interfere with further analysis; (ii) apply band-pass thresholds to separate color channels; (iii) dilate borders to close inconsistent gaps; (iv) skeletonize these borders; (v) apply a convolution filter to translate pixels uniformly for border detection; (v) generate a mask of the muscle fiber borders using the analyze particles function, simultaneously eliminating stray “non-border” signals; and (vi) overlay threshold-delimited nuclei over the border mask, before another analyze particles command was used to measure morphometric variables including “area” and “minimum Feret diameter.” Fiji image processing steps and macro construction were partially derived from published standard operating procedures [28] (http://treat-nmd.eu/research/preclinical/dmd-sops/). An average of 3,243 muscle fibers per 4-wk-old TA were measured: the mdx TA had an average of 3,161 fibers and the Pf-mdx/P2X7−/− TA had an average of 3,325 fibers. In the 20-mo-old TA analysis, an average of 4,639 muscle fibers were measured per cross-section: mdx TA had an average of 4,493 fibers and Pf-mdx/P2X7−/− TA had an average of 4,786 fibers. There was no difference in the number of muscle fibers between cross-sections from mdx and Pf-mdx/P2X7−/− TA (t-test, t = 0.51, df = 7, p = 0.624). Fiber number per unit of tissue area is presented. The step-by-step protocols developed by us and used in this study are shown as video available at http://youtu.be/GZVaRQYgGQU (morphometric analysis using Fiji) and http://youtu.be/oxyM7r7VYp0 (central nucleation count from the analyze particles output).
Trichrome Staining
The trichrome method employed here is the TREAT-NMD-recommended protocol (http://treat-nmd.eu/research/preclinical/dmd-sops/). Briefly, 10-μm-thick cryosections were fixed in 4% w/v PFA and 0.1 M PBS followed by Bouin’s fixative, stained with Biebrich scarlet-acid fuchsin solution (Sigma-HT151, Sigma-Aldrich) for 20 min, washed in water, and incubated in a phosphotungstic/phosphomolybdic acid solution (5% w/v phosphotungstic acid, 5% w/v phosphomolybdic acid, DDH2O) for 2 × 3 min, directly before incubation in aniline blue solution (2.5% w/v aniline blue, 2% v/v acetic acid, DDH2O) for 7 min. Optionally, sections were washed in water and incubated in glacial acetic acid solution (1% v/v; DDH2O) for 1 min. Finally, sections were washed, dehydrated, and mounted, and the entire sections were visualized using bright-field Axiozoom V.16 (Zeiss); positive (blue) areas are expressed as percent of the total area of each muscle cross-section.
Serum Creatine Kinase Levels
Sera were prepared and creatine kinase (CK) levels were analyzed using the Creatine Kinase Enzymatic Assay Kit (3460–07, BioScientific) or the Creatine Kinase Activity Assay Kit (Mak116-1kt, Sigma-Aldrich), according to manufacturers’ instructions.
Bone Morphometric Analyses (6 mo)
Bones were removed, cleared of soft tissues, and analyzed by micro-computed tomography (μCT) as described previously [3]. Briefly, μCT 40 (Scanco Medical) was used to assess trabecular bone volume fraction (bone volume/total volume [BV/TV]), micro-architecture in the metaphyseal region of the tibia, and cortical geometry at the mid-tibia. For trabecular bone, BV/TV (percent), trabecular thickness, trabecular number (the number of plates per millimeter of length), and trabecular separation (Tb.Sp) (micrometers) were assessed on 100 contiguous μCT slides, starting 100 slides below the growth plate.
Bone Morphometric Analyses (4 wk)
Mice tibias were imaged by means of μCT (XT H 225, X-Tek Systems), and a complete data acquisition was performed (V = 50–55 kV, I = 95–110 μA, voxel size = 6–8 μm, rotational step = 0.19°/360°, acquisition time = 90 min). The regions of interest used for the morphometric assessment were selected to include both the cancellous and cortical bones in the proximal tibias and the cortical bones in the mid-shaft of the tibias, similarly to the methodology used in [3]. Image processing was performed using the seeded region growing (SRG) technique (ImageJ) to automate segmentation of the bone tissue from the background. The morphometric parameters measured were bone volume fraction (BV/TV), trabecular bone thickness, and Tb.Sp.
Force Measurements in Diaphragms Ex Vivo
Whole diaphragms were excised from 4-mo-old WT, mdx, Pf-mdx/P2X7−/−, and G-mdx/P2X7−/− mice and placed into Krebs-Ringer solution (NaCl 118 mM, KCl 4.7 mM, NaHCO3 24.88 mM, KH2PO4 1.18 mM, glucose 11.1 mM, MgSO4 0.82 mM, CaCl2 2.52 mM), taking care not to handle the muscle wherever possible. Sutures were then tied to two points on either side of a segment of attached rib, which were then attached to an immobile plastic clamp. A centrally derived triangular section of the diaphragm was used for testing. Contractile force was translated along another suture, tied to the central tendon apex of the approximately equilateral triangular section of muscle. This suture was, in turn, attached to a mechanical force transducer (ADInstruments), amplifier, and data acquisition setup. Excitation of muscle was achieved via local field potentials through platinum electrodes in oxygenated (95% O2, 5% CO2) Krebs-Ringer solution, at a constantly maintained temperature of 37°C.
Each diaphragm was stretched in very small increments from an initial resting state to establish the optimal excitation-to-force generation length. Diagnostic testing also confirmed that the voltage twitch stimulus of 140 V (2-ms length) was adequate to elicit a maximal twitch response. To achieve a maximal isometric tetanic force response, diaphragm sections were subjected to a 140 V (2 ms) stimulus train at 100-Hz frequency for 0.5–1 s. The test regime involved collecting six twitch responses, followed by six tetanic trains, with a 2-min rest period between each. Maximal twitch and tetanic response were taken from the tallest of the respective force traces. All forces were normalized to muscle wet weight and expressed as Newtons per gram of tissue (N/g). This testing strategy [29] followed the TREAT-NMD standard operating procedures for the use of experimental animals (http://treat-nmd.eu/research/preclinical/dmd-sops/).
Running Wheel Activity
Locomotor activity was assessed using cages equipped with voluntary running wheels (Intellibio), placed in a dimly lit, quiet, and ventilated room, at a temperature of 22 ± 1°C, under a 12 h light/12 h dark cycle (light on between 7 : 00 a.m. and 7 : 00 p.m.). Mice were individually housed in cages. The recording apparatus was connected to a computer to process the data. Animals had 24-h access to the wheels, and total distance travelled was measured.
Grip Strength Test
In this and all other in vivo tests, investigators were blinded with respect to the sample group allocation.
The grip strength test was performed to assess muscular strength and forepaw grasping reflex. The mouse was held by the tail and slowly approached to a metallic grid (6 × 6 cm) connected to a force sensor (Bioseb). Once the animal gripped the grid by its forelimbs, a gentle horizontal traction was applied to the tail until the animal let the grid go. The maximal force was recorded over two trials with a 1-min inter-trial interval. Strength was estimated by the mean of both trials.
Elevated Zero Maze Test
Anxiogenic activity was assessed in the elevated zero maze test, a pharmacologically validated assay of anxiety in rodents, based on the natural aversion of mice to elevated and open spaces. The apparatus consisted of an infrared-transmitting, ring-shaped, black Plexiglas platform (outer diameter 45 cm, width 6 cm), placed 60 cm above ground level in a dimly illuminated room, divided equally into four quadrants with two opposing open quadrants and two opposing closed quadrants (surrounded by a 27-cm wall from the surface of the maze). Each mouse, previously isolated for 15 min before the experiment in a small individual cage, was placed at the end of an open section, with its head facing a closed quadrant. The time spent and the distance travelled in open and closed quadrants during a 5-min period were recorded using the EthoVision XT 9.0 automated image analysis system (Noldus Information Technologies).
Rotarod Test
The Rotamex-5 (Columbus Instruments) was used for the evaluation of motor coordination. Mice were placed onto the rod of the apparatus at 0 rpm to allow them to balance and then rotation was increased by about 0.4 rpm/s, to a maximum of 17 rpm. The time period for which the mice were able to maintain their balance on the bar was recorded automatically using photobeam break technology and the instrument’s software.
Parallel Rod Floor Test
This test was performed using the parallel rod floor apparatus (Stoelting Europe) as described previously [30]. Briefly, the instrument floor consisted of a series of parallel steel rods, a stainless steel base plate with an acrylic border raised 1 cm above the base plate, and a clear acrylic box with no bottom and a removable lid. Locomotor activity and number of foot slips were recorded during two consecutive periods of 5 min. A slip was detected by the AnyMaze automated analysis system as a paw touched the base plate, completing a circuit.
Novel Object Recognition Test
Experiments were undertaken using a Plexiglas open field box (40 × 40 cm) with grey walls (30 cm high) and a white floor under constant room temperature (23 ± 1°C) and homogeneous dim illumination (open field center: 40 lux). To test the memory retention of each mouse at three distinct retention intervals, three sets of three objects of different shapes and color were used, which were either glass, plastic, or metal. The procedure started with a 5-d habituation period [31]. The object recognition test started 2 d after the end of habituation. Mice were first submitted to a single acquisition trial, where they were exposed to two new identical objects for 10 min. Memory retention was tested at 10 min, 24 h, or 48 h. Each animal was thus submitted to three successive acquisition/retention phases, following a sequence of retention delays that was counterbalanced among individuals. During these test phases, one of the familiar objects was replaced by a novel object, with a different shape and color. During the 5-min testing, exploration of an object was defined as pointing the nose to the object and/or touching the object with the nose. The total time spent with each object and number of times each object was explored were recorded and scored using fully automated EthoVision XT 10.0 video tracking software (Noldus Information Technologies). Data are presented as the discrimination index (time or frequency exploring novel object × 100/total object exploration time or frequency).
Statistical Analysis
As detailed in the figure legends, Student’s t unpaired test was used for comparisons between two data groups (P2X7 protein; CD4, CD8, and Ly6G/tubulin mRNA levels; CD68-positive and revertant fiber counts; CD11b and P2X4 protein; in vivo effects of P2X7 antagonists). All other statistical analyses were for three or more data groups and employed ANOVA with Tukey’s post hoc test. In order to account for possible heteroscedasticity of data, the Anderson-Darling normality test was applied to data that yielded p < 0.05 from ANOVA, and permutation analysis was applied using Treeperm v1.6 script in R Studio v3.2.2. With the exception of serum CK levels in Pf-mdx/P2X7−/− mice and F4/80, Foxp3, and IL12 levels in dystrophic muscle (to which Mann-Whitney U and Kruskal-Wallis H tests were applied), Anderson-Darling tests yielded p > 0.05, and, unless otherwise stated, permutation analysis also yielded p < 0.05. A p-value of <0.05 was considered statistically significant, and the values are reported as follows in figures: *p < 0.05, **p < 0.005, ***p < 0.001.
Results
P2RX7 Ablation Improves mdx Mouse Muscle Structure and Function
To mimic the clinically relevant situation, we analyzed the effects of P2RX7 ablation in 4-wk-old male mice. At this age, mdx limb muscles showed significantly increased expression of P2RX7 (Fig 1B and 1C) and the typical degeneration and regeneration pattern akin to human pathology. Gross histological features of mdx and mdx/P2X7−/− muscles were similar, and the fraction of muscle fibers with centralized nuclei was not significantly different. However, using a semi-automated measurement of the minimum Feret diameter of muscle cross-sections, we found a significantly increased diameter of centrally nucleated (C/N) fibers (p < 0.001) in the Pf-mdx/P2X7−/− TA (Fig 2A and 2B), and such an increase is a feature observed in less-severe phenotypes.
Fig. 2. P2RX7 ablation reduces mdx mouse muscle pathology. 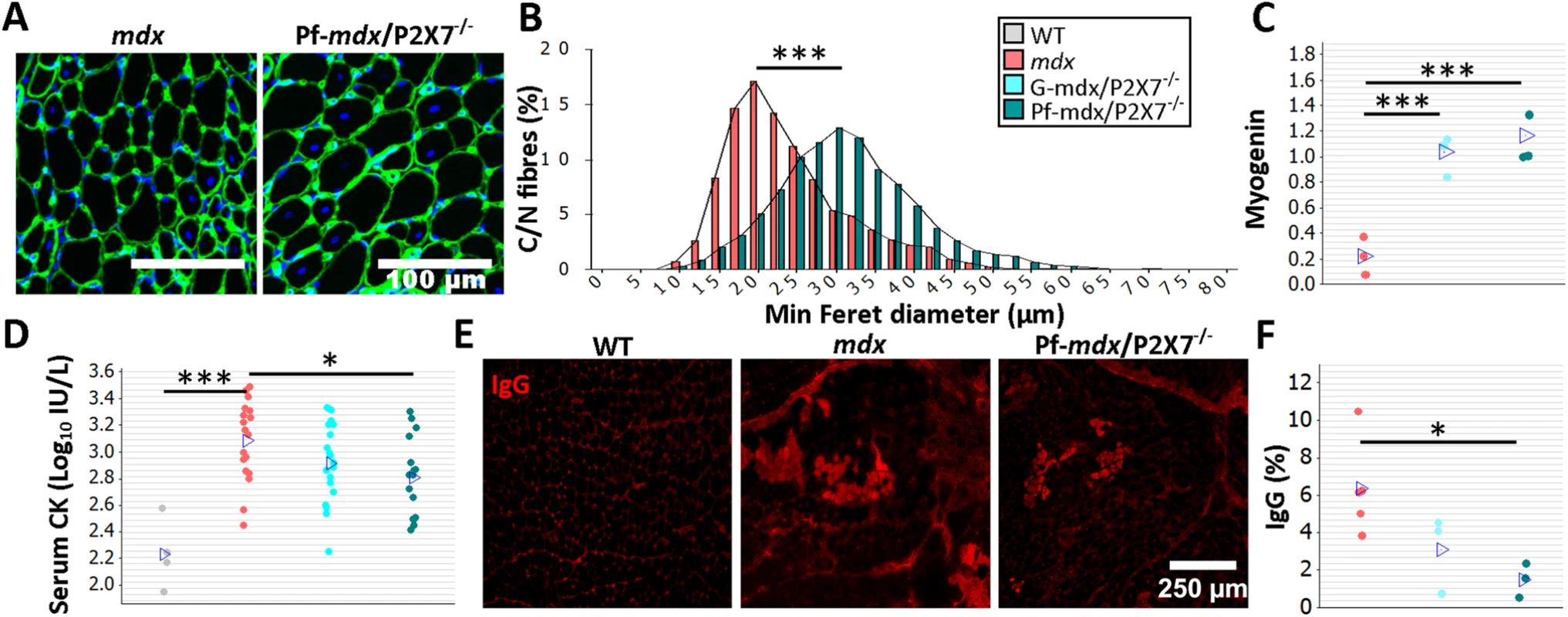
The color-coding legend applies to all graphs in the figure. (A) Collagen type-IV (green) and nuclei (blue) immunofluorescence with an accompanying frequency histogram (B) of the minimum Feret diameter of C/N fibers from 4-wk-old mdx and Pf-mdx/P2X7−/− mice showing the right shift in TA muscle fiber size corresponding with the greater average Feret diameter of Pf-mdx/P2X7−/− fibers (t-test, t = 6.99, df = 6, p < 0.001). (C) Elevated myogenin levels (average Western blot values) in both Pf-mdx/P2X7−/− and G-mdx/P2X7−/− muscle (ANOVA, F = 33.38, df = 2, n = 4, 3, 4, p < 0.001; Tukey’s test, G-mdx/P2X7−/− versus mdx, p < 0.001; G-mdx/P2X7−/− versus Pf-mdx/P2X7−/−, p = 0.516; Pf-mdx/P2X7−/− versus mdx, p < 0.001) and (D) significantly lower average serum CK levels in Pf-mdx/P2X7−/− compared to mdx muscle (Mann-Whitney U test, W = 388, n = 18, 16, p = 0.012; permutation analysis, F = 7.07, p = 0.013; log10 serum CK ANOVA, F = 3.76, df = 2, n = 19, 18, 16, p = 0.030; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.025). (E) Example immunofluorescence micrographs of IgG penetration into muscle fibers and (F) chart showing reduced average IgG influx into Pf-mdx/P2X7−/− muscle (ANOVA, F = 5.52, df = 2, n = 3, 5, 3, p = 0.031; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.032). *p < 0.05, ***p < 0.001. While mRNA levels of MyoD were similarly elevated in all dystrophic mice (S1 Table), transcript and protein levels of myogenin were higher in both mdx/P2X7−/− strains compared to mdx (Fig 2C; G-mdx/P2X7−/− versus mdx, p < 0.001; G-mdx/P2X7−/− versus Pf-mdx/P2X7−/−, p = 0.516; Pf-mdx/P2X7−/− versus mdx, p < 0.001), suggesting improved muscle regeneration.
Analyses of serum CK revealed significantly lower levels in 4-wk-old Pf-mdx/P2X7−/− than in mdx mice (Fig 2D; untransformed Pf-mdx/P2X7−/− versus mdx data, p = 0.013; log10 serum CK, p = 0.025), indicative of less sarcolemma damage and therefore less leakage of this intracellular muscle enzyme. This was confirmed by decreased sarcolemma permeability to blood-born IgG molecules [32] in mdx/P2X7−/− muscle fibers (Fig 2E and 2F; Pf-mdx/P2X7−/− versus mdx, p = 0.032).
Moreover, the significantly reduced autophagy in 4-wk-old mdx/P2X7−/− muscles in vivo (Fig 3A and 3B), indicated by decreased LC3II (Pf-mdx/P2X7−/− versus mdx, p = 0.008), corresponded with protective effects of P2RX7 ablation against ATP-induced autophagic muscle death, previously shown in vitro [12].
Fig. 3. P2RX7 ablation improves mdx mouse muscles. 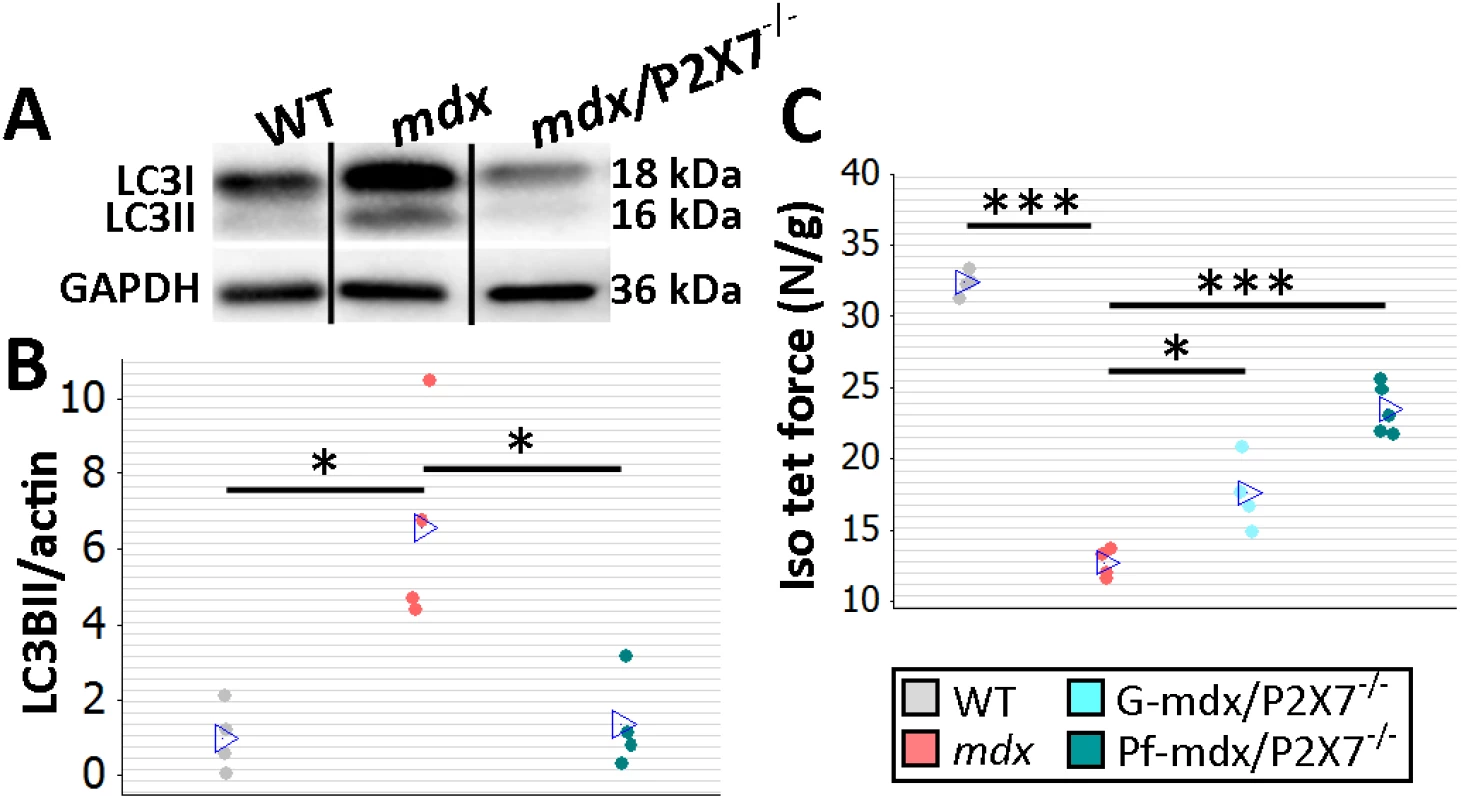
(A) Autophagy induction (LC3I to LC3II shift in representative Western blots) found in mdx muscles is blocked in Pf-mdx/P2X7−/− muscles, with average values shown in (B) (ANOVA, F = 11.57, df = 2, n = 4, p = 0.003; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.008). Note: use of separate Western blots is indicated by solid black lines. (C) Greater average diaphragm isometric tetanic forces at 4 mo in both Pf-mdx/P2X7−/− and G-mdx/P2X7−/− compared to mdx mice (ANOVA, F = 37.97, df = 2, n = 4, 4, 5, p < 0.001; Tukey’s test, G-mdx/P2X7−/− versus mdx, p = 0.010; Pf-mdx/P2X7−/− versus mdx, p < 0.001). *p < 0.05, ***p < 0.001. Finally, we compared muscle force in diaphragm organ bath preparations: isometric tetanic forces generated by 4-mo-old dystrophic diaphragms with ablated P2RX7 were significantly (approx. 30%–50%) greater (Pf-mdx/P2X7−/− versus mdx, p < 0.001) than those generated by mdx muscles (Fig 3C).
Collectively, these data indicate that P2RX7 ablation resulted in significant amelioration of muscle pathology.
Analyses of G-mdx/P2X7−/− mice, which retain the low-expression P2RX7k isoform [18], also in skeletal muscles [9], showed increased tetanic forces (Fig 3C; G-mdx/P2X7−/− versus mdx, p = 0.010). Myogenin levels were elevated compared to mdx and in line with Pf-mdx/P2X7−/− (Fig 2C; G-mdx/P2X7−/− versus mdx, p < 0.001), while CK levels (Fig 2D; G-mdx/P2X7−/− versus mdx, p = 0.111) and sarcolemma permeability to IgG, albeit lower, were not statistically significantly different. The intermediate effects seen in G-mdx/P2X7−/− as compared to Pf-mdx/P2X7−/− mice confirmed the association between decreased P2RX7 expression and the phenotypic improvements. Moreover, we analyzed F1 Pf-mdx/P2X7+/− males. These heterozygous mice had intermediate levels of muscle P2RX7 expression (S1A Fig), exactly as shown before in immune cells [33]. Again, this intermediate level corresponded with the intermediate Feret diameter values in these mice (S1B Fig). F1 and G-mdx/P2X7−/− data also confirmed that the improvements found in double-mutants are not due to the genetic background variation resulting from mdx-P2X7−/− breeding, as this would have had an impact in all mouse lines.
P2RX7 Ablation Reduces the Inflammatory Signature of Dystrophic Muscles
Chronic inflammation is an important pathological feature in DMD and mdx [34], and corticosteroids have beneficial, albeit limited, effects in both [35]. Macrophages, along with T-lymphocytes and neutrophils, make up the bulk of immune cell infiltrations, and M1 pro-inflammatory macrophages dominate and add to the damage. However, M2 macrophages are also present and essential for muscle regeneration [36]. Therefore, a non-selective attenuation of immune cell functions carries the risk of interfering with regenerative processes.
Given P2RX7’s role in induction of inflammation and expression in most immune cells, including in macrophages [14,19], we studied the effects of receptor ablation on the inflammatory signature of dystrophic muscles. To identify major changes, we used inflammatory pathways qPCR panel array analysis (S1 Table) followed by immunodetection. As expected, P2RX7 co-localized with macrophage markers, and the CD163 immunofluorescence associated with the M2 macrophages [36] was present in a subset of CD68-positive cells infiltrating mdx muscles (Fig 4A and 4B).
Fig. 4. P2RX7 ablation in mdx mice reduces macrophage infiltration. 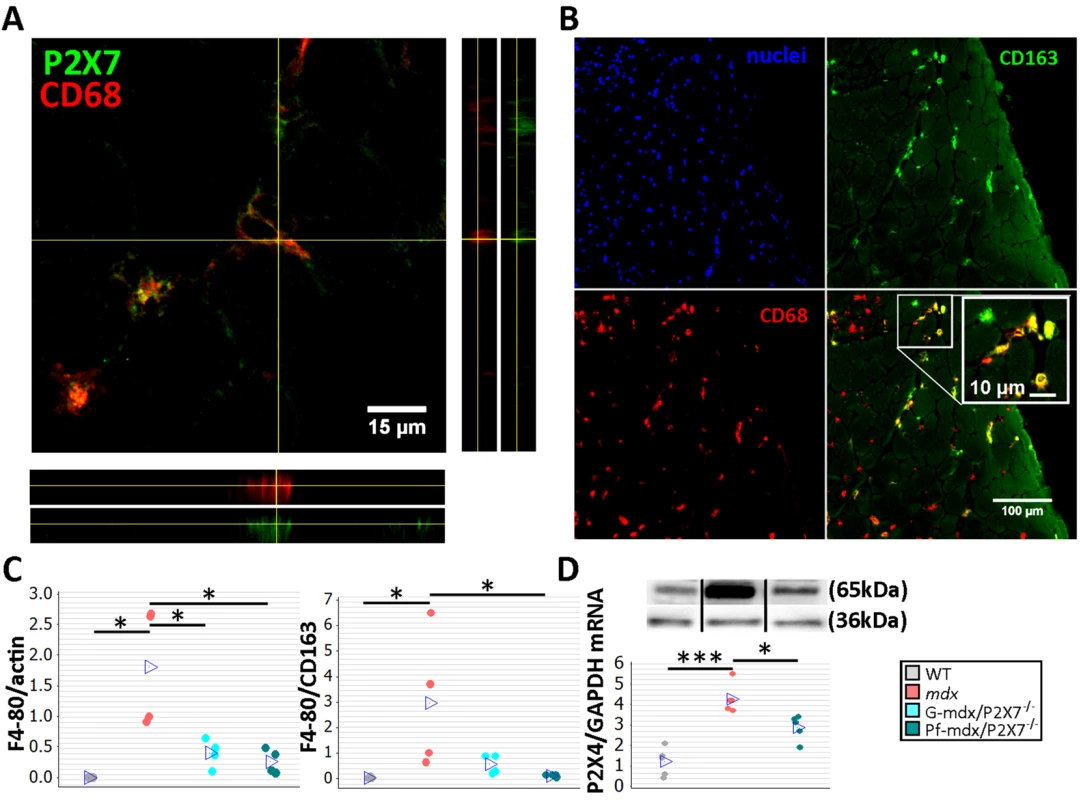
(A) Immunofluorescence co-localization of CD68 macrophage marker with P2RX7. To confirm spatial co-localization, side and bottom panels show overlapping z-plane images. (B) M2 macrophage marker (CD163) co-localization with a subset of CD68-positive cells within the inflammatory infiltrate regions in mdx muscle. Blue signal identifies cell nuclei. (C) F4/80 macrophage marker levels shown as normalized average Western blot values (left) and F4/80 relative to CD163 (right). Significantly less F4/80 was found in Pf-mdx/P2X7−/− and G-mdx/P2X7−/− muscles compared to mdx muscles (Kruskal-Wallis H test, H = 7.73, df = 2, n = 4, p = 0.021; Mann-Whitney U test, Pf-mdx/P2X7−/− versus mdx, W = 26.0, n = 4, p = 0.030; G-mdx/P2X7−/− versus mdx, W = 26.0, n = 4, p = 0.030), while the ratio of F4/80 level to CD163 level, denoting M1/M2 macrophage ratio, was significantly reduced in Pf-mdx/P2X7−/− muscles (Kruskal-Wallis H test, H = 8.77, df = 2, n = 4, p = 0.012; Mann-Whitney U test, W = 26.0, n = 4, p = 0.030). (D) A representative Western blot (top) illustrating decreased P2RX4 protein levels, and corresponding qPCR data (bottom) showing decreased expression of P2RX4 mRNA in Pf-mdx/P2X7−/− gastrocnemius compared to mdx gastrocnemius (ANOVA, F = 25.96, df = 2, n = 5, p < 0.001; Tukey’s test, mdx versus C57, p < 0.001; Pf-mdx/P2X7−/− versus mdx, p = 0.017; Pf-mdx/P2X7−/− versus WT, p = 0.005). *p < 0.05, ***p < 0.001. Use of separate Western blots is indicated by solid black lines. Comparisons of immune cells in mdx and mdx/P2X7−/− muscles at 4 wk showed that F4/80 pan-macrophage marker levels were lower in both mdx/P2X7−/− strains (Fig 4C, left panel; Pf-mdx/P2X7−/− versus mdx, p = 0.030; G-mdx/P2X7−/− versus mdx, p = 0.030), and the F4/80 to CD163 (pro-inflammatory to pro-regenerative) marker ratio was also significantly reduced in Pf-mdx/P2X7−/− compared to mdx mice (Fig 4C, right panel; p = 0.030). P2RX4 expression was significantly lower in Pf-mdx/P2X7−/− muscles (Fig 4D; mdx versus WT, p < 0.001; Pf-mdx/P2X7−/− versus mdx, p = 0.017; Pf-mdx/P2X7−/− versus WT, p = 0.005), which agreed with the presence of this receptor in infiltrating macrophages [37] and thus further confirmed reduced macrophage loads. This result also excluded potential compensatory overexpression of this receptor in the absence of P2RX7.
The CD4 and CD8 cell numbers were not significantly affected by P2RX7 ablation (Fig 5A; p > 0.05), but a significant increase in Foxp3 and IL12α expression in mdx/P2X7−/− muscles (Fig 5B; Pf-mdx/P2X7−/− versus mdx: Foxp3, p = 0.022, and IL12α, p = 0.001; see also S1 Table) indicated a shift towards Treg cells, known to suppress immune responses [38]. This result showed that P2RX7 depletion ameliorates tissue inflammation by promoting Treg cell functions [39] also in dystrophic muscles.
Fig. 5. P2RX7 ablation in mdx mice reduces the inflammatory signature. 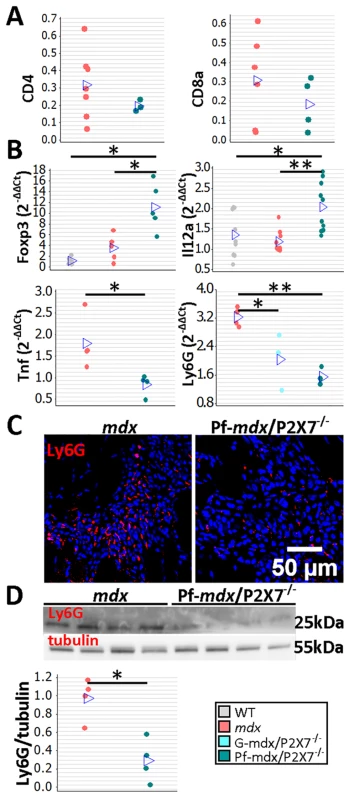
(A) Enumeration of CD4- and CD8-positive cells in TA muscle from mdx and Pf-mdx/P2X7−/− mice showed no statistical difference in these cell numbers between the two genotypes (CD4 t-test, t = 0.97, df = 7, p = 0.366; CD8 t-test, t = 0.95, df = 8, p = 0.372). This finding corresponded with CD4 and CD8 qPCR data (S1 Table). (B) Selected results of qPCR gene expression analyses using inflammatory panels: relative expression levels (2−ΔΔCT) in muscle-derived mRNAs from mdx and mdx/P2X7−/− mice demonstrate significant differences in expression levels of Foxp3 (Kruskal-Wallis H test, H = 10.26, df = 2, n = 5, p = 0.006; Mann-Whitney U test, Pf-mdx/P2X7−/− versus mdx, W = 16.0, n = 5, p = 0.022), IL12α (Kruskal-Wallis H test, H = 11.08, df = 2, n = 9, 10, 10, p = 0.004; Mann-Whitney U test, Pf-mdx/P2X7−/− versus mdx, W = 61.0, n = 10, p = 0.001), TNFα (t-test, t = 2.86, df = 6, p = 0.029), and Ly6G (ANOVA, F = 14.68, df = 2, n = 3, 4, 4, p = 0.002; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.002) transcripts in mdx/P2X7−/− mice compared to mdx. Lower expression of Ly6G mRNA corresponded with a lower number of Ly6G immunopositive neutrophils (red signal in Ly6G immunolocalization micrograph) (C) and significantly lower Ly6G protein levels (D) in Pf-mdx/P2X7−/− compared to mdx muscles (t-test, t = 4.14, df = 6, p = 0.006). Blue signal—Hoechst nuclear counterstaining. *p < 0.05, **p < 0.005. Together, these data indicate a significantly reduced inflammatory profile in 4-wk-old Pf-mdx/P2X7−/− muscles. Moreover, the decreased TNFα expression (Fig 5B; p = 0.029; S1 Table) agreed with its production being dependent on P2RX7 function [19] but was also an important result given that pharmacological interference with this inflammatory mediator has been shown to reduce DMD pathogenesis [40]. The significantly reduced neutrophil marker Ly6G mRNA expression found in Pf-mdx/P2X7−/− muscles (Fig 5B; Pf-mdx/P2X7−/− versus mdx, p = 0.002; S1 Table) was confirmed by both immunolocalization (Fig 5C) and Western blotting (Fig 5D; p = 0.006). Clearly, in agreement with recent studies [41], P2RX7 ablation has a widespread impact on inflammatory cell migration, and this correlates with significant improvements in dystrophic muscles.
Dystrophic Muscles Present an Early Fibrotic Signature, Which Is Reduced by P2RX7 Ablation
Unlike in DMD, the mdx mouse shows little fibrosis in limb muscles. Surprisingly, combined RNA-Seq and qPCR analyses of 4-wk-old leg muscle samples revealed a clear pro-fibrotic gene expression signature at this early disease stage (Fig 6).
Fig. 6. Expression of genes associated with fibrosis as measured by RNA-Seq. 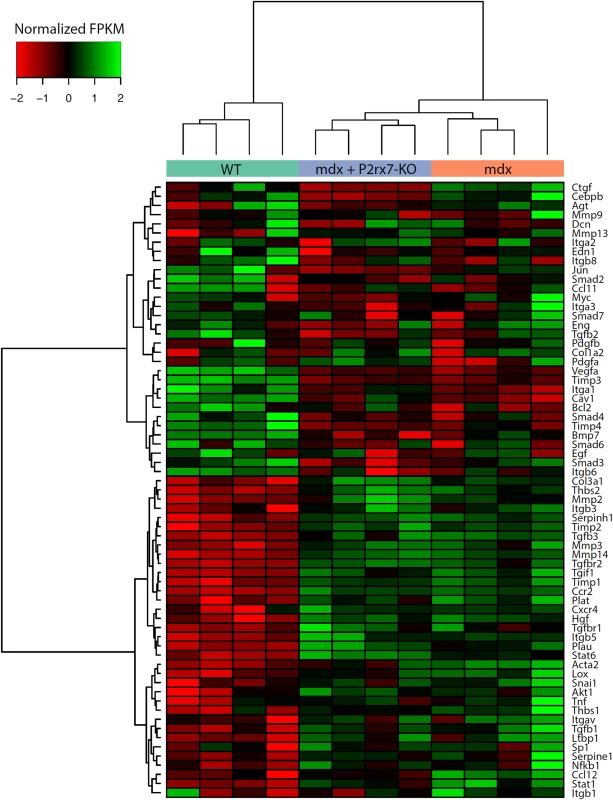
Fragments per kilobase per million fragments mapped (FPKM) values for each sample were obtained using Cuffnorm (Tuxedo suite), log-transformed, and normalized to zero mean and unit standard deviation (rows with missing expression values were removed). Hierarchical clustering was performed using the Ward’s method [42]. KO, knockout. The expression pattern of genes involved in the regulation of fibrosis showed significant up-regulation of pro-fibrotic and down-regulation of anti-fibrotic genes (S2 Table; S2 Fig). Importantly, P2RX7 ablation reduced this early fibrotic signature (Figs 6 and S2), with many genes expressed at levels resembling those in WT muscles (for specific p-values see S2 Table). This gene-level expression analysis not only uncovered an early, pro-fibrotic environment in mdx muscles but also confirmed that the widespread effects of P2RX7 ablation include lessening of this phenotype, which is one of the critically important abnormalities of the dystrophic pathology.
P2RX7 Ablation Produces Long-Term Improvements in Skeletal and Cardiac Muscles
To assess the long-term effects of P2RX7 ablation, we tested 20-mo-old limb, diaphragm, and cardiac muscles [21]. As expected at this stage, the leukocyte (CD11b-positive cell) numbers were low and were not different between Pf-mdx/P2X7−/− and mdx TA muscles (Fig 7A; p > 0.05).
Fig. 7. P2RX7 ablation reduces inflammation and fibrosis in 20-mo-old tibialis anterior muscles. 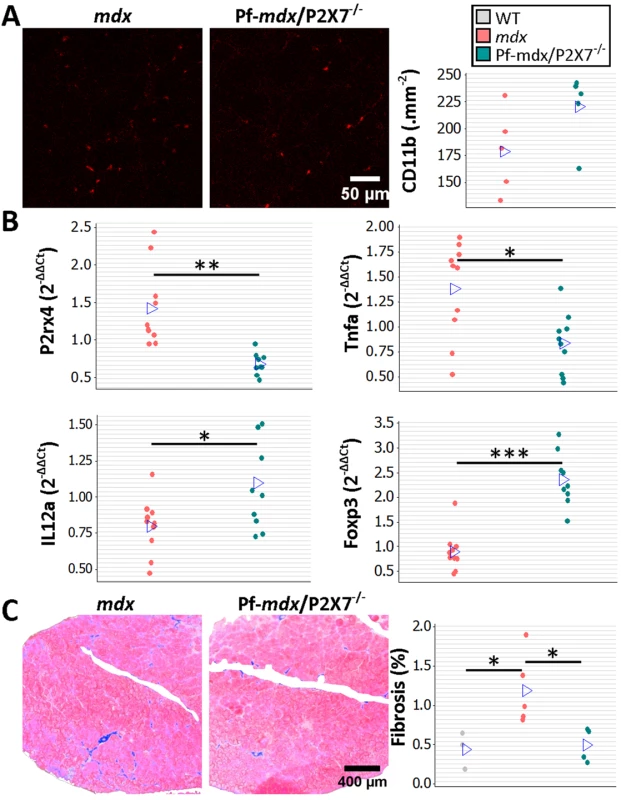
(A) Representative immunofluorescence micrographs (left) and enumeration of CD11b-expressing cells in 20-mo-old TA showing no significant difference in the numbers of infiltrating leukocytes in Pf-mdx/P2X7−/− compared to mdx muscles (t-test, t = 1.82, df = 8, p = 0.107). (B) qPCR gene expression analyses: relative expression levels (2−ΔΔCT) in muscle-derived mRNAs from mdx and mdx/P2X7−/− TA demonstrate significant decreases in P2RX4 (t-test, t = 4.04, df = 17, p = 0.001) and TNFα (t-test, t = 3.07, df = 18, p = 0.006), with concomitant increases in expression levels of IL12α (t-test, t = 2.56, df = 18, p = 0.020) and Foxp3 (t-test, t = 6.8, df = 17, p < 0.001) in Pf-mdx/P2X7−/− compared to mdx muscles. (C) Representative images of trichrome staining (left) and trichrome average intensities in 20-mo-old TA muscles demonstrating a significant decrease in fibrosis in Pf-mdx/P2X7−/− compared to mdx mice (ANOVA, F = 6.18, df = 2, n = 3, 5, 4, p = 0.020; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.038). *p < 0.05, **p < 0.005, ***p < 0.001. However, qPCR analyses showed significantly lower expression of P2RX4 (p = 0.001) and TNFα (p = 0.007) genes and significantly increased Foxp3 (p < 0.001) and IL12α (p = 0.020) expression levels in mdx/P2X7−/− TA samples (Fig 7B). These findings agreed with the results in 4-wk-old samples and demonstrated that P2RX7 ablation has a long-term anti-inflammatory effect in dystrophic muscles. Moreover, the statistically significant reduction in trichrome staining in 20-mo-old Pf-mdx/P2X7−/− TA (Fig 7C; Pf-mdx/P2X7−/− versus mdx, p = 0.038) confirmed reduced muscle fibrosis across ages. P2RX7 expression was detectable in mdx muscles at this age (Fig 8A), and the reduced autophagy in 20-mo-old mdx/P2X7−/− muscles (Fig 8A; p = 0.004) corresponded with the results obtained in 4-wk-old samples and extended our earlier observation of the protective effect of P2RX7 ablation against ATP-induced autophagic muscle death [12].
Fig. 8. P2RX7 ablation reduces the pathology in 20-mo-old tibialis anterior muscles. 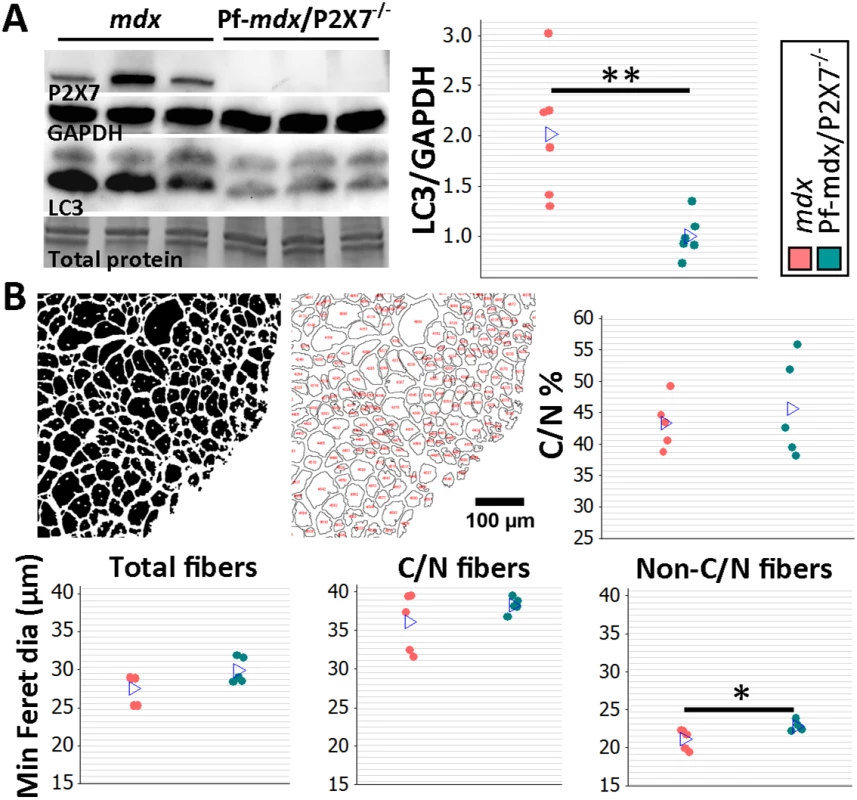
(A) Representative Western blots (left) confirming P2RX7 protein expression in 20-mo-old TA and illustrating decreased autophagy (LC3I to LC3II shift), with the results in the graph showing lower levels of LC3II relative to GAPDH in TA from 20-mo-old Pf-mdx/P2X7−/− compared to mdx mice (t-test, t = 3.74, df = 10, p = 0.004). Total protein staining is shown to illustrate the equal protein loading. (B) Representative ImageJ output masks from morphometric analyses of TA fibers (left) and graphs showing representative results (right and bottom). While no differences were found in the proportion of C/N fibers or the average minimum Feret diameter of total or C/N fibers (t-test, t = 0.57, df = 8, p = 0.586; t = 2.04, df = 8, p = 0.076; t = 1.25, df = 8, p = 0.248, respectively), there was a significant increase in the minimum Feret diameter of non-C/N fibers in mdx/P2X7−/− compared to mdx muscles (t-test, t = 2.58, df = 8, p = 0.032). *p < 0.05, **p < 0.005. Finally, there was no difference in the percentage of C/N fibers (p > 0.05), and measurements of the average minimum Feret diameter showed no differences in total (p > 0.05) and C/N (p > 0.05) fibers, while there was a significant increase in Feret diameter (p = 0.032) in non-C/N fibers from 20-mo-old Pf-mdx/P2X7−/− TA (Fig 8B).
Notably, diaphragm is the mdx muscle that shows mild pathology at early stages and undergoes progressive degeneration that becomes prominent from week 15 [21]. The numbers of CD11b+ and CD68+ inflammatory cells were significantly lower in 20-mo-old Pf-mdx/P2X7−/− diaphragms (Fig 9A; CD11b+, p = 0.015; CD68+, p = 0.009), confirming that the long-term reduction of the pro-inflammatory phenotype also occurs in this progressively damaged muscle.
Fig. 9. P2RX7 ablation continues to reduce dystrophic pathology in 20-mo-old diaphragm and heart. 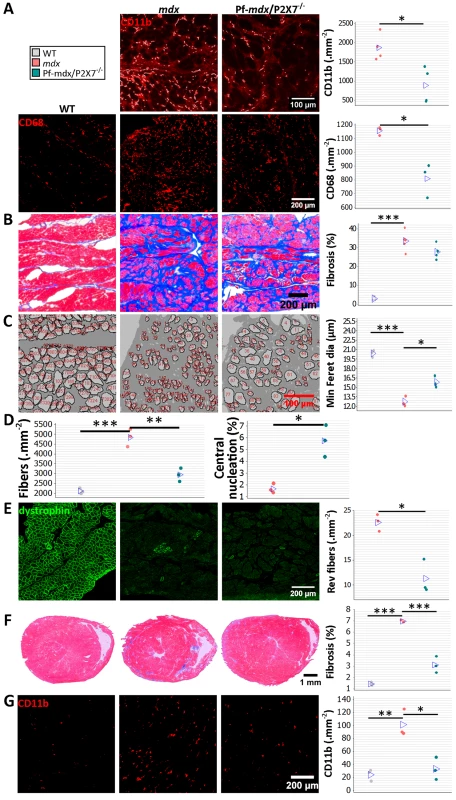
(A) Representative immunofluorescence micrographs (left) and enumeration of CD11b- and CD68-expressing cells in 20-mo-old diaphragms showing reduced numbers of infiltrating leukocytes (CD11b t-test, t = 3.68, df = 6, p = 0.015) and macrophages (CD68+ t-test, t = 4.73, df = 4, p = 0.009) in Pf-mdx/P2X7−/− compared to mdx diaphragms. (B) Trichrome staining (left) and its average intensity in 20-mo-old diaphragms demonstrating no increase in fibrosis in Pf-mdx/P2X7−/− over mdx mice (ANOVA, F = 60.32, df = 2, n = 5, 5, 3, p < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.095; permutation analysis, Pf-mdx/P2X7−/− versus mdx, F = 4.47, p = 0.095). (C) Representative ImageJ output masks from morphometric analyses of diaphragm fibers (left) demonstrating the increased average minimum Feret diameter in mdx/P2X7−/− compared to mdx diaphragms (ANOVA, F = 75.17, df = 2, n = 3, p < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.006; permutation analysis, Pf-mdx/P2X7−/− versus mdx, F = 20.01, p = 0.099). (D) Graphs showing a lower total number of diaphragm fibers per unit area in Pf-mdx/P2X7−/− versus mdx mice (left; ANOVA, F = 52.77, df = 2, n = 3, p < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.001; Pf-mdx/P2X7−/− versus WT, p = 0.051) and the increased proportion of C/N fibers (right; t-test, t = 5, df = 4, p = 0.008). (E) Dystrophin immunofluorescence in representative transverse sections of 20-mo-old diaphragms showing the typical staining (green signal) in dystrophin-positive muscles and clusters of revertant dystrophin-positive fibers in dystrophic samples. The data show significantly fewer revertant fibers in 20-mo-old Pf-mdx/P2X7−/− than in mdx diaphragms (t-test, t = 5.12, df = 4, p = 0.007). (F) Representative trichrome staining (left) of whole hearts from 20-mo-old mice showing a significant decrease in cardiac muscle damage (histological lesions) and average area of fibrosis (blue signal in trichrome staining) in Pf-mdx/P2X7−/− versus mdx mice (ANOVA, F = 166.29, df = 2, n = 4, 3, 3, p < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p < 0.001). (G) Representative examples of CD11b+ leukocyte marker staining (left; red immunofluorescence) and infiltrating cell counts demonstrating fewer infiltrations in Pf-mdx/P2X7−/− compared to mdx hearts (ANOVA, F = 19.65, df = 2, n = 3, p = 0.002; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.005). *p < 0.05, **p < 0.005, ***p < 0.001. Similar levels of connective tissue in trichrome staining (Fig 9B; Pf-mdx/P2X7−/− versus mdx, p = 0.095) showed that the altered profile of cells infiltrating double-mutant muscles did not alter diaphragm fibrosis [36]. There was, however, a statistically significant increase in muscle fiber Feret diameter (Fig 9C; Pf-mdx/P2X7−/− versus mdx, p = 0.006), concomitant with a lower total number of diaphragm fibers per unit area (Fig 9D; Pf-mdx/P2X7−/− versus mdx, p = 0.001; Pf-mdx/P2X7−/− versus WT, p = 0.051), while the percentage of C/N fibers was lower in mdx than in Pf-mdx/P2X7−/− diaphragms (Fig 9D, p = 0.008). Revertant fibers were present in a lower number in Pf-mdx/P2X7−/− than mdx diaphragms (Fig 9E; p = 0.007). Dystrophin-expressing revertant fibers arise due to spontaneous exon skipping. These rare events take place only in proliferating precursor cells, which are activated by muscle degeneration/regeneration. Therefore, in experimental paradigms not affecting splicing of the dystrophin transcript, fewer revertant fibers have been used as an indicator that fewer degeneration/regeneration cycles occurred over the muscle lifetime [43,44].
DMD patients who survive to the third decade present with cardiomyopathy, and heart failure becomes a common cause of death [22]. We analyzed hearts from 20-mo-old mice and found significantly decreased cardiac muscle fibrosis (Pf-mdx/P2X7−/− versus mdx, p < 0.001), measured as area occupied by connective tissue in trichrome staining and structural damage (Fig 9F), coinciding with fewer CD11b-positive leukocytes infiltrating Pf-mdx/P2X7−/− compared to mdx hearts (Fig 9G; Pf-mdx/P2X7−/− versus mdx, p = 0.005).
P2RX7 Ablation Improves mdx Muscle Strength and Endurance In Vivo and Reduces Cognitive and Behavioral Abnormalities
To analyze the effects of receptor ablation on muscle function in vivo, groups of 8 - to 12-wk-old WT, mdx, and Pf-mdx/P2X7−/− male mice followed a functional test regime consisting of forelimb grip strength, rotarod, voluntary wheel activity, and parallel rod floor running. Significant improvements in grip strength (Fig 10A; p = 0.012) and muscle endurance (Fig 10B; p = 0.016) were observed in mdx/P2X7−/− compared to mdx mice. A lack of changes in latency time to fall off the rotarod and in performance in the parallel rod floor tests (Fig 10C, run time and speed; Fig 10D, activations; all p > 0.05 for mdx/P2X7−/− versus mdx) excluded differences in motor coordination between these strains.
Fig. 10. P2RX7 ablation improves mdx muscle strength and endurance and object recognition memory and decreases anxiety in vivo. 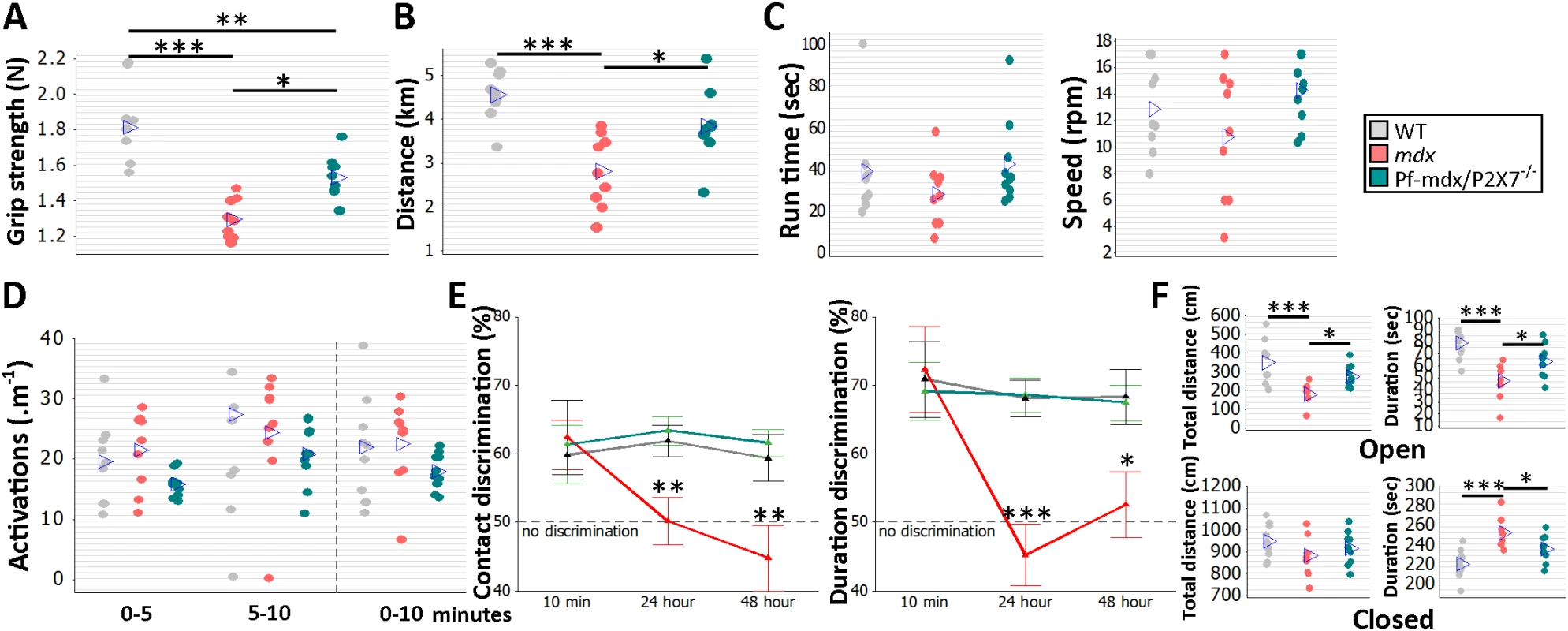
Forelimb grip strength (A) and voluntary wheel run distance (B) were significantly greater in Pf-mdx/P2X7−/− compared to mdx mice (grip strength ANOVA, F = 22.99, df = 2, n = 9, 9, 10, p < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.0118; run distance ANOVA, F = 12.73, df = 2, n = 9, 9, 10, p < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.016). The rotarod test (C) showed no difference for total average run time and average speed (run time ANOVA, F = 1.23, df = 2, n = 9, 9, 10, p = 0.310; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.300; speed ANOVA, F = 2.23, df = 2, n = 9, 9, 10, p = 0.129; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.109), and the parallel rod floor test (D) showed no significant differences in the average number of activations over several run time-spans between WT, mdx, and Pf-mdx/P2X7−/− mice (ANOVA, F = 2.6, 0.62, 1.34; df = 2; n = 9, 9, 10; p = 0.094, 0.545, 0.212; at 0–5, 5–10, and 0–10 min, respectively). In the object recognition test (E) there was no significant difference between genotypes at 10-min retention delay, but at 24 h and 48 h, both duration and contact discrimination were significantly different from the 50% chance level for the Pf-mdx/P2X7−/− mice, but not for mdx mice. Memory retention in Pf-mdx/P2X7−/− mice was equal to that in WT mice, while mdx mice performed at a lower level than WT (contact discrimination 10 min, 24 h, 48 h ANOVA; F = 0.08, 7.37, 6.83; df = 2; n = 10, 9, 10; p = 0.922, 0.003, 0.004; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.984, 0.004, 0.006; Pf-mdx/P2X7−/− versus WT, p = 0.970, 0.908, 0.890; mdx versus WT, p = 0.916, 0.012, 0.017; duration discrimination 10 min, 24 h, 48 h ANOVA; F = 0.09, 16.17, 5.1; df = 2; n = 10, 9, 10; p = 0.913, < 0.001, = 0.013; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.905, < 0.001, = 0.031; Pf-mdx/P2X7−/− versus WT, p = 0.969, 0.994, 0.985; mdx versus WT, p = 0.980, < 0.001, = 0.021,). (F) In the elevated zero maze anxiety test, both the duration and the distance travelled by Pf-mdx/P2X7−/− mice within the open arm of the maze were greater than those of mdx mice, and mdx mice performed at a lower level than WT (distance and duration ANOVA, F = 10.51, 11.76; df = 2; n = 10, 9, 10; p < 0.001, < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.045, 0.047; Pf-mdx/P2X7−/− versus WT, p = 0.109, 0.060; mdx versus WT, p < 0.001, < 0.001). In the closed arm (F, bottom), there was no difference in distance travelled, but Pf-mdx/P2X7−/− mice spent less time in this arm than mdx mice, and mdx mice more time than WT (distance and duration ANOVA, F = 1.42, 11.76; df = 2; n = 10, 9, 10; p = 0.259, < 0.001; Tukey’s test, Pf-mdx/P2X7−/− versus mdx, p = 0.687, 0.047; Pf-mdx/P2X7−/− versus WT, p = 0.659, 0.060; mdx versus WT, p = 0.230, < 0.001). *p < 0.05, **p < 0.005, ***p < 0.001. Cognitive and behavioral impairment is a well-defined feature of DMD: the overall IQ of DMD patients is one standard deviation below the mean of the unaffected population [2]. Distinct dystrophin isoforms are expressed in neurons and glia [45], and P2RX7 is recognized as a gatekeeper of inflammatory processes between these two cell types [46]. Treatments targeting the adult mdx brain produced some improvement [47], proving that at least some abnormalities are reversible. WT, mdx, and Pf-mdx/P2X7−/− mice were therefore compared in a series of behavioral tests to identify the effect(s) of receptor ablation. Consistent with previous studies [48], mdx mice showed impaired long-term recognition memory and enhanced emotional reactivity (Fig 10E; contact discrimination 10 min, 24 h, 48 h; p = 0.916, 0.012, 0.017; duration discrimination 10 min, 24 h, 48 h; p = 0.980, < 0.001, = 0.021; Fig 10F; open-arm distance and duration; p < 0.001, < 0.001; closed-arm distance and duration, p = 0.230, < 0.001). Importantly, ablation of P2RX7 in mdx mice resulted in a significant improvement in performance in the novel object recognition memory test (Fig 10E; contact discrimination 10 min, 24 h, 48 h Tukey’s test; Pf-mdx/P2X7−/− versus mdx, p = 0.984, 0.004, 0.006; Pf-mdx/P2X7−/− versus WT, p = 0.970, 0.908, 0.890; duration discrimination Tukey’s test 10 min, 24 h, 48 h; Pf-mdx/P2X7−/− versus mdx, p = 0.905, < 0.001, = 0.031; Pf-mdx/P2X7−/− versus WT, p = 0.969, 0.994, 0.985) and in reduced anxiety as measured in the elevated zero maze test (Fig 10F; open-arm distance and duration Tukey’s test; Pf-mdx/P2X7−/− versus mdx, p = 0.0450, 0.047; Pf-mdx/P2X7−/− versus WT, p = 0.109, 0.060; closed-arm distance and duration Tukey’s test; Pf-mdx/P2X7−/− versus mdx, p = 0.687, 0.047; Pf-mdx/P2X7−/− versus WT, p = 0.659, 0.060). These tests are not affected by motor activity or muscle strength and therefore reflect the positive impact of P2RX7 ablation on the dystrophic central nervous system (CNS).
P2RX7 Ablation Reduces Bone Loss in Dystrophic Mice
Bone structure abnormalities in DMD patients and mdx mice were previously believed to have musculoskeletal origins. However, rather than being an effect of asymmetric loss of muscle force, these have recently been confirmed as an independent disease manifestation [3]. μCT bone morphometry analyses (Figs 11A and S3) in WT, mdx, and mdx/P2X7−/− mice revealed reduced bone mass in mdx mice as young as 4 wk, i.e., before any muscle loss that could cause bone structure alterations, and confirmed significant bone abnormalities at 6 mo (Fig 11B; 4-wk BV/TV, p = 0.029; 6-mo BV/TV, p = 0.014; 6-mo Tb.Sp, p = 0.008).
Fig. 11. P2RX7 ablation reduces mdx bone loss. 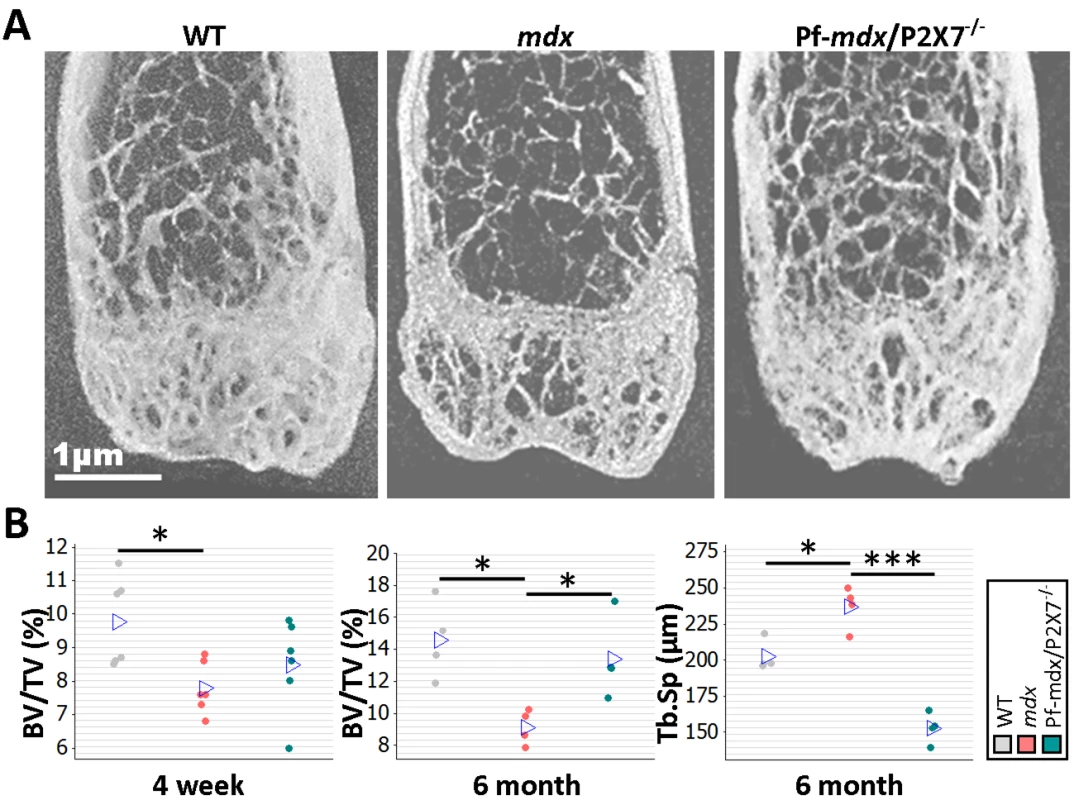
(A) Representative μ-CT images of distal femurs from 6-mo-old mice. (B) μ-CT morphometry in 4-wk-old proximal tibiae and 6-mo-old femurs. As early as age 4 wk, the BV/TV ratio in mdx mice was reduced, and it remained altered at 6 mo. But P2RX7 ablation significantly improved BV/TV and Tb.Sp parameters in older mdx/P2X7−/− mice compared to older mdx mice (4-wk BV/TV ANOVA, F = 4.29, df = 2, n = 6, p = 0.034; Tukey’s test, mdx versus WT, p = 0.029; 6-mo BV/TV ANOVA, F = 7.29, df = 2, n = 4, p = 0.013; Tukey’s test, mdx versus WT, p = 0.014; Pf-mdx/P2X7−/− versus mdx, p = 0.046; 6-mo Tb.Sp ANOVA, F = 48.69, df = 2, n = 4, p < 0.001; Tukey’s test, mdx versus WT, p = 0.008; Pf-mdx/P2X7−/− versus mdx, p < 0.001.). *p < 0.05, ***p < 0.001. In contrast, in 6-mo-old Pf-mdx/P2X7−/− mice, we found significant improvements in BV/TV (p = 0.046) and in Tb.Sp (p < 0.001) compared to mdx mice (Fig 11B). These data demonstrate that P2RX7 ablation improves the dystrophic bone phenotype.
P2RX7 Antagonist Treatment Reduces Dystrophic Pathology
In a preliminary study to determine whether treatment with P2RX7 antagonists can ameliorate dystrophic symptoms in vivo, 2-wk-old mdx mice were injected intraperitoneally with CBB or ox-ATP for 4 wk. CBB treatment reduced CK levels (Fig 12A; p = 0.030), which correlates with mdx/P2X7−/− results presented here and also with previous data showing a reduced number of degeneration/regeneration cycles following CBB administration in 6-mo-old mdx mice [9].
Fig. 12. Short-term P2RX7 antagonist administration reduces severity of muscle pathology. 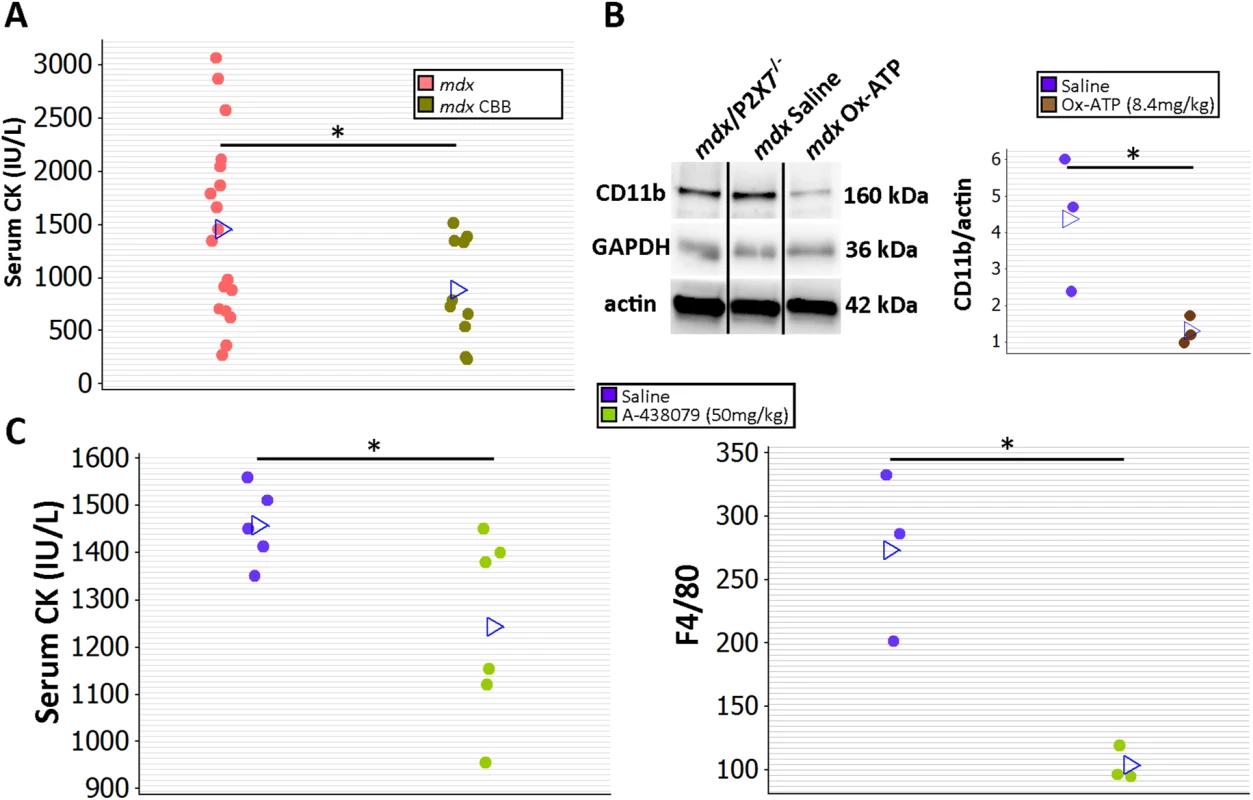
(A) Comparison of serum CK levels in mdx mice (control) and mdx mice injected with the P2RX7 antagonist CBB. Note the high variability of CK levels in the dystrophic sera. Daily administration of CBB over the 4-wk period reduced CK levels (t-test, t = 2.3, df = 26, p = 0.030), in line with the effects of P2RX7 ablation in 4-wk-old Pf-mdx/P2X7−/− mice. (B) Representative Western blots (left) and average value plots (right) demonstrating significantly decreased levels of the CD11b leukocyte marker in gastrocnemius muscles of mdx mice injected with ox-ATP compared to mdx saline-injected controls (t-test, t = 2.84, df = 4, p = 0.047). Use of separate Western blots is indicated by solid black lines. (C) Comparisons of serum CK levels (left) and F4/80+ macrophage loads in mdx mice showing significant decreases (CK t-test, t = 2.26, df = 9, p = 0.050; F4/80 t-test, t = 4.34, df = 4, n = 3, p = 0.012) following 14 daily administrations of the competitive P2RX7 antagonist A-438079. *p < 0.05. Moreover, ox-ATP treatment decreased muscle expression of CD11b, denoting reduced inflammatory cell infiltrations (Fig 12B; p = 0.047). Finally, daily administration of 50 mg/kg of the more selective, competitive P2RX7 antagonist A-438079 for only 2 wk [49] decreased CK levels (p = 0.050) and significantly reduced the number of infiltrating (F4/80 positive) macrophages (Fig 12C; p = 0.012), again in agreement with improvements found in mdx/P2X7−/−.
Discussion
The data we present here demonstrate that P2RX7 ablation in the most widely used animal model of DMD produced significant improvements in key functional and molecular disease parameters in dystrophic leg muscles at 4 wk and in legs, diaphragms, and hearts at 20 mo, i.e., at all the stages where the model reproduces the DMD pathology [21]. In addition to the alleviation of muscle disease, decreased inflammation and reduced non-muscle symptoms in CNS and bone were also clearly evident. This wide therapeutic impact reflects convergence of P2RX7 ablation on multiple pathological mechanisms (summarized in Fig 13), thus offering a promising new approach to treating this debilitating disease (see below).
Fig. 13. P2X7 purinoceptor involvement in the dystrophic pathology. 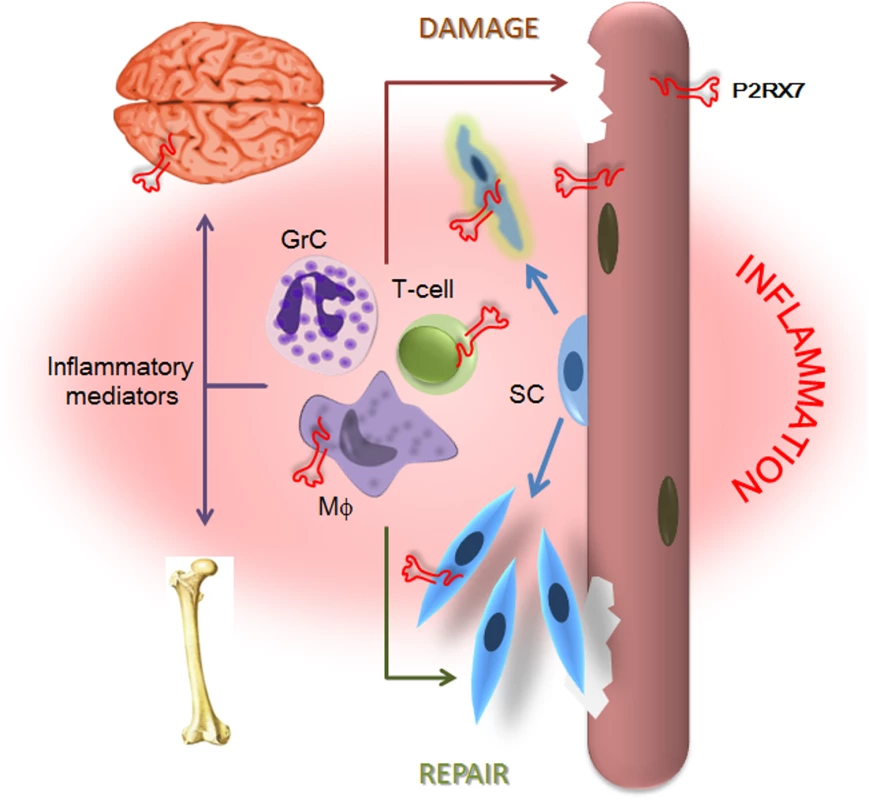
Absence of dystrophin and resulting loss of the DAP complex lead to myofiber damage. Degenerating/dying muscle releases large quantities of DAMPs, including ATP, which trigger chronic inflammation. P2RX7 activation on dystrophic myofibers exacerbates injury by promoting intracellular Ca2+ build-up and autophagic cell death. Infiltrating macrophages (Mφ), T-cells, and granulocytes (GrC) cause further myofiber damage, while chronically elevated levels of inflammatory mediators disturb normal brain and bone functions. Chronic inflammation also reduces repair by altering satellite cell (SC) activation and muscle precursor cell differentiation, while high eATP levels combined with P2RX7 overexpression contribute to their death and thus reduce muscle regeneration further still. The partial effects of P2RX7 ablation observed in G-mdx/P2X7−/− mice (which retained one of the P2RX7 isoforms) and in heterozygous mdx/P2X7+/− (with intermediate P2RX7 levels) suggest that even incomplete inhibition of this receptor would produce some therapeutic effect. Indeed, treatment with broad purinoceptor antagonists has shown some impact in the mdx model of DMD (reviewed in [13]), as did our short-term administration of P2RX7 antagonists (Fig 12). Importantly, the purinoceptor antagonists used here, CBB and ox-ATP, here have slow association rates and do not competitively block agonist activation [50]. We are currently testing new-generation, increased affinity competitive antagonists [51], which are supposed to be significantly more effective, and one such a compound showed some positive effects just after 2 wk of administration (Fig 12).
Regarding the mechanisms by which P2RX7 ablation produced muscle recovery, these involved both direct effects in dystrophic muscle cells showing over-activation of this receptor [7–11] and reduced inflammation [13,34,52]. Regarding the latter, dystrophin-deficient muscles chronically release high levels of ATP together with other DAMPs, and the resulting immune cell infiltrations contribute to disease progression [34,36]. Targeting innate immunity was shown to slow disease progression [53] and therefore may become a new therapeutic strategy to treat DMD. Deletion of P2RX7, with its unique capability to respond to high eATP levels and to activate inflammation through release of pro-inflammatory cytokines [14], is bound to make a significant impact. Indeed, in mdx/P2X7−/− mice, we found an overall decrease in the muscle inflammatory signature and reduced numbers of infiltrating leukocytes. Moreover, we also discovered increased Foxp3 and IL12α mRNA levels in both 4-wk-old and 20-mo-old muscles, indicating a shift in T-cell responses: Foxp3 transcription factor is required for Treg cell development and function, while IL12α is highly expressed by mouse Foxp3+ Treg cells but not by effector CD4+ T cells [38]. Treg cells modulate the immune response to maintain tolerance to self-antigens, and since peripheral tolerance breakdown has been demonstrated in both DMD and mdx [53–56], this result is very encouraging.
In turn, high eATP acting on P2RX7, which is overexpressed in dystrophic muscle cells [7–9], could activate a number of mechanisms, including ERK phosphorylation and ion channel opening, producing activation of signaling cascades. Treatment with apyrase, an ATP-degrading enzyme, reduced intracellular Ca2+ levels in mdx fibers [57], thus confirming that purinoceptors contribute to the deregulated Ca2+ homeostasis in dystrophic muscles. Therefore, P2RX7 ablation or inhibition would eliminate Ca2+ influx occurring via this receptor and also triggering secondary modulation of other Ca2+ channels, abnormal functions of which have been described in mdx myofibers [58,59].
In addition, we have shown recently that the P2RX7 large pore formation (occurring at high eATP levels) leads to the autophagic death of dystrophic muscle cells [12], which contributes both to fiber loss and to exhaustion of the pool of muscle-resident stem cells required for repair and regeneration [43,60]. Indeed, P2RX7 ablation reduced autophagy in both 4-wk-old and 20-mo-old mdx TA. Further studies are needed to clarify the puzzling shift between positive and negative roles for autophagy described in young and old dystrophic muscles, respectively [61].
The improved heart, leg, and diaphragm phenotype in 20-mo-old mdx/P2X7−/− muscles demonstrates that similar mechanisms operate in both skeletal and cardiac muscles and remain active in muscles undergoing continuous, severe degeneration/regeneration akin to human disease. Interestingly, we have confirmed the previous observation [62] that a higher level of fibrosis in diaphragms may coincide with a lower proportion of C/N myofibers (Fig 9). Central nuclei denote muscle regeneration and thus are considered an indicator of muscle pathogenesis. However, as demonstrated in both studies, the proportion of C/N fibers alone is not an accurate marker of muscle degeneration. It has been suggested that such myofibers may be more resistant to mechanical stress [63], which, in turn, could contribute to the differences in the pathology observed in diaphragm compared to limb muscles. The presence of fewer revertant fibers has been used as an indicator that a muscle has undergone fewer degeneration/regeneration cycles [43,44]. Revertant fibers arise due to rare, spontaneous exon skipping events, which take place only in proliferating precursor cells activated by muscle degeneration. Therefore, in experimental paradigms not inducing dystrophin exon skipping, such as described here, revertant fibers are a good indicator of muscles’ degeneration/regeneration history.
The cardiac improvement observed when P2RX7 was blocked is also of clinical importance because, with prolonged patient survival (due to advances in general care), heart failure becomes a more common cause of death in DMD.
Targeting P2RX7 is, to our knowledge, the first clinically relevant treatment for DMD cognitive dysfunction. This is important because severe cognitive impairment occurs in one-third of DMD patients. Absence of dystrophin causes a rearrangement of the precise spatiotemporal pattern of synaptic transmission [64,65]. Re-expression of dystrophin in mdx brains results in some improvement [48], indicating that at least partial correction of the cognitive phenotype can be achieved, even in adult brains. However, gene targeting into the CNS is inherently difficult, and therefore currently tested therapies do not address this aspect of the pathology. In contrast, treatments downstream from the primary deficit offer a better chance of success by exploiting more accessible targets. It is currently not known whether dystrophin absence causes P2RX7 abnormalities in brain cells similar to those identified in muscles and DMD lymphoblasts [6]. In fact, it is not unequivocal which brain cells express P2RX7 [66]. Therefore, we are investigating the dystrophic CNS to establish whether it is possible to attribute this improvement directly to P2RX7 ablation in specific cells. An alternative explanation is that the leaky blood–brain barrier found in dystrophic brains [67] causes inflammatory mediators to affect brain functions [68], in which case, the decreased chronic inflammation in mdx/P2X7−/− mice would be what results in improved brain performance. Whatever the mechanism, our findings suggest that DMD-associated cognitive impairment could be treated with administration of P2RX7 antagonists. Such drugs, including brain-permeable ones, are undergoing trials for other diseases [69].
P2RX7 plays significantly different roles in bone physiology and in disease states [70]. Importantly, ablation of P2RX7 in our model did not exacerbate but rather improved the dystrophic bone phenotype. As bone abnormalities in mdx mice have been linked to chronic inflammation [71], the reduced inflammatory signature in mdx/P2X7−/− muscles may also translate into reduced bone loss in these mice.
A therapeutic strategy that can improve both muscle and non-muscle abnormalities would be a significant development: bone deformities contribute to the physical disability and death of DMD patients. In turn, the brain is the second major site of dystrophin gene expression, and cognitive and behavioral impairments associated with DMD add to the very severe burden of this disease, affecting the quality of life of patients and their families.
The molecular mechanism leading to the P2RX7 abnormality in dystrophinopathy found both in muscle [7–9] and non-muscle cells [6] is unknown. Is this a structural defect related to the scaffolding function of dystrophin or a regulatory defect at the gene or the transcript level? Nonetheless, normal DMD gene expression is essential for normal functioning of various cells, and DMD mutations have detrimental effects evident already at early stages of the disease [72–74]. These findings may lead to a rethink of DMD pathogenesis and treatment.
In summary, loss of dystrophin disrupts many downstream processes. Recent examples have illustrated that such processes may offer good targets for therapeutic interventions that are not constrained by the causative mutation [5,40,52]. Moreover, such approaches may be effective not only in protecting muscle cells but also with respect to inflammation, cognitive impairment, and bone abnormalities, which all make substantial contributions to the DMD pathology.
Our data show that selective ablation or blockade of P2RX7 ameliorates the mdx dystrophic process both short and long term and does not cause detectable side effects in this DMD mouse model. Given that specific P2RX7 antagonists have been in human trials for other conditions [75], these could be readily repurposed for treatment of this lethal disease. Furthermore, nucleoside reverse transcriptase inhibitors (e.g., zidovudine) have been shown to block P2RX7 activation in a number of disease models [76]. These drugs, with an extensive pharmacological and safety record for human use, including in children, appear ready for trials in DMD.
Supporting Information
Zdroje
1. Davies KE, Nowak KJ. Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol. 2006;7 : 762–773. 16971897
2. Snow WM, Anderson JE, Jakobson LS. Neuropsychological and neurobehavioral functioning in Duchenne muscular dystrophy: a review. Neurosci Biobehav Rev. 2013;37 : 743–752. doi: 10.1016/j.neubiorev.2013.03.016 23545331
3. Rufo A, Del Fattore A, Capulli M, Carvello F, De Pasquale L, Ferrari S, et al. Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res. 2011;26 : 1891–1903. doi: 10.1002/jbmr.410 21509823
4. van Putten M, Hulsker M, Nadarajah VD, van Heiningen SH, van Huizen E, van Iterson M, et al. The effects of low levels of dystrophin on mouse muscle function and pathology. PLoS ONE. 2012;7:e31937. doi: 10.1371/journal.pone.0031937 22359642
5. Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC. Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol. 2012;228 : 77–87. doi: 10.1002/path.4054 22653783
6. Ferrari D, Munerati M, Melchiorri L, Hanau S, di Virgilio F, Baricordi OR. Responses to extracellular ATP of lymphoblastoid cell lines from Duchenne muscular dystrophy patients. Am J Physiol. 1994;267:C886–C892. 7524344
7. Yeung D, Zablocki K, Lien CF, Jiang T, Arkle S, Brutkowski W, et al. Increased susceptibility to ATP via alteration of P2X receptor function in dystrophic mdx mouse muscle cells. FASEB J. 2006;20 : 610–620. 16581969
8. Rawat R, Cohen TV, Ampong B, Francia D, Henriques-Pons A, Hoffman EP, et al. Inflammasome up-regulation and activation in dysferlin-deficient skeletal muscle. Am J Pathol. 2010;176 : 2891–2900. doi: 10.2353/ajpath.2010.090058 20413686
9. Young CN, Brutkowski W, Lien CF, Arkle S, Lochmuller H, Zablocki K, et al. P2X7 purinoceptor alterations in dystrophic mdx mouse muscles: relationship to pathology and potential target for treatment. J Cell Mol Med. 2012;16 : 1026–1037. doi: 10.1111/j.1582-4934.2011.01397.x 21794079
10. Valladares D, Almarza G, Contreras A, Pavez M, Buvinic S, Jaimovich E, et al. Electrical stimuli are anti-apoptotic in skeletal muscle via extracellular ATP. Alteration of this signal in Mdx mice is a likely cause of dystrophy. PLoS ONE. 2013;8:e75340. doi: 10.1371/journal.pone.0075340 24282497
11. Young CNJ, Sinadinos A, Gorecki DC. P2X receptor signaling in skeletal muscle health and disease. Wiley Interdiscip Rev Membr Transp Signal. 2013;2 : 265–274.
12. Young C, Sinadinos A, Lefebvre A, Chan P, Arkle S, Vaudry D, et al. A novel mechanism of autophagic cell death in dystrophic muscle regulated by P2RX7 receptor large-pore formation and HSP90. Autophagy. 2015;11 : 113–130. doi: 10.4161/15548627.2014.994402 25700737
13. Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509 : 310–317. doi: 10.1038/nature13085 24828189
14. Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28 : 465–472. 17692395
15. Sandona D, Gastaldello S, Martinello T, Betto R. Characterization of the ATP-hydrolysing activity of alpha-sarcoglycan. Biochem J. 2004;381 : 105–112. 15032752
16. North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83 : 759–769. doi: 10.1124/mol.112.083758 23253448
17. Masin M, Young C, Lim K, Barnes SJ, Xu XJ, Marschall V, et al. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: re-evaluation of P2X7 knockouts. Br J Pharmacol. 2012;165 : 978–993. doi: 10.1111/j.1476-5381.2011.01624.x 21838754
18. Nicke A, Kuan YH, Masin M, Rettinger J, Marquez-Klaka B, Bender O, et al. A functional P2X7 splice variant with an alternative transmembrane domain 1 escapes gene inactivation in P2X7 knock-out mice. J Biol Chem. 2009;284 : 25813–25822. doi: 10.1074/jbc.M109.033134 19546214
19. Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114 : 386–396. 15777864
20. Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276 : 125–132. 11016935
21. Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31 : 1–19. doi: 10.1016/j.nbd.2008.03.008 18499465
22. McNally EM. New approaches in the therapy of cardiomyopathy in muscular dystrophy. Annu Rev Med. 2007;58 : 75–88. 17217326
23. Gourine AV, Poputnikov DM, Zhernosek N, Melenchuk EV, Gerstberger R, Spyer KM, et al. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br J Pharmacol. 2005;146 : 139–145. 15965498
24. Ardissone V, Radaelli E, Zaratin P, Ardizzone M, Ladel C, Gattorno M, et al. Pharmacologic P2X purinergic receptor antagonism in the treatment of collagen-induced arthritis. Arthritis Rheum. 2011;63 : 3323–3332. doi: 10.1002/art.30556 21792831
25. Amalfitano A, Chamberlain JS. The mdx-amplification-resistant mutation system assay, a simple and rapid polymerase chain reaction-based detection of the mdx allele. Muscle Nerve. 1996;19 : 1549–1553. 8941268
26. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9 : 676–682. doi: 10.1038/nmeth.2019 22743772
27. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9 : 671–675. 22930834
28. Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord. 2004;14 : 675–682. 15351425
29. Gosselin LE, Barkley JE, Spencer MJ, McCormick KM, Farkas GA. Ventilatory dysfunction in mdx mice: impact of tumor necrosis factor-alpha deletion. Muscle Nerve. 2003;28 : 336–343. 12929194
30. Kamens HM, Crabbe JC. The parallel rod floor test: a measure of ataxia in mice. Nat Protoc. 2007;2 : 277–281. 17406586
31. Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, et al. Object recognition test in mice. Nat Protoc. 2013;8 : 2531–2537. doi: 10.1038/nprot.2013.155 24263092
32. Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139 : 375–385. 9334342
33. Chen YG, Scheuplein F, Driver JP, Hewes AA, Reifsnyder PC, Leiter EH, et al. Testing the role of P2X7 receptors in the development of type 1 diabetes in nonobese diabetic mice. J Immunol. 2011;186 : 4278–4284. doi: 10.4049/jimmunol.1003733 21357538
34. Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, et al. A chronic inflammatory response dominates the skeletal muscle molecular signature in dystrophin-deficient mdx mice. Hum Mol Genet. 2002;11 : 263–272. 11823445
35. Huynh T, Uaesoontrachoon K, Quinn JL, Tatem KS, Heier CR, Van Der Meulen JH, et al. Selective modulation through the glucocorticoid receptor ameliorates muscle pathology in mdx mice. J Pathol. 2013;231 : 223–235. doi: 10.1002/path.4231 23794417
36. Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18 : 482–496. doi: 10.1093/hmg/ddn376 18996917
37. Yeung D, Kharidia R, Brown SC, Gorecki DC. Enhanced expression of the P2X4 receptor in Duchenne muscular dystrophy correlates with macrophage invasion. Neurobiol Dis. 2004;15 : 212–220. 15006691
38. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450 : 566–569. 18033300
39. Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. doi: 10.1126/scisignal.2001270 21364186
40. Grounds MD, Torrisi J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18 : 676–682. 15054089
41. Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136 : 448–458. doi: 10.1111/j.1365-2567.2012.03601.x 22564028
42. Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58 : 236–244.
43. Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell. 2010;143 : 1059–1071. doi: 10.1016/j.cell.2010.11.039 21145579
44. Pigozzo SR, Da Re L, Romualdi C, Mazzara PG, Galletta E, Fletcher S, et al. Revertant fibers in the mdx murine model of Duchenne muscular dystrophy: an age - and muscle-related reappraisal. PLoS ONE. 2013;8:e72147. doi: 10.1371/journal.pone.0072147 24015212
45. Gorecki DC, Monaco AP, Derry JM, Walker AP, Barnard EA, Barnard PJ. Expression of four alternative dystrophin transcripts in brain regions regulated by different promoters. Hum Mol Genet. 1992;1 : 505–510. 1307251
46. Browne SE. When too much ATP is a bad thing: a pivotal role for P2X7 receptors in motor neuron degeneration. J Neurochem. 2013;126 : 301–304. doi: 10.1111/jnc.12321 23773137
47. Vaillend C, Perronnet C, Ros C, Gruszczynski C, Goyenvalle A, Laroche S, et al. Rescue of a dystrophin-like protein by exon skipping in vivo restores GABAA-receptor clustering in the hippocampus of the mdx mouse. Mol Ther. 2010;18 : 1683–1688. doi: 10.1038/mt.2010.134 20588257
48. Vaillend C, Billard JM, Laroche S. Impaired long-term spatial and recognition memory and enhanced CA1 hippocampal LTP in the dystrophin-deficient Dmd(mdx) mouse. Neurobiol Dis. 2004;17 : 10–20. 15350961
49. McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, et al. P2X7-related modulation of pathological nociception in rats. J Neurosci. 2007;146 : 1817–1828.
50. Michel AD, Chambers LJ, Walter DS. Negative and positive allosteric modulators of the P2X(7) receptor. Br J Pharmacol. 2008;153 : 737–750. 18071294
51. Donnelly-Roberts DL, Jarvis MF. Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol. 2007;151 : 571–579. 17471177
52. Vidal B, Ardite E, Suelves M, Ruiz-Bonilla V, Janue A, Flick MJ, et al. Amelioration of Duchenne muscular dystrophy in mdx mice by elimination of matrix-associated fibrin-driven inflammation coupled to the alphaMbeta2 leukocyte integrin receptor. Hum Mol Genet. 2012;21 : 1989–2004. doi: 10.1093/hmg/dds012 22381526
53. Giordano C, Mojumdar K, Liang F, Lemaire C, Li T, Richardson J, et al. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum Mol Genet. 2014;24 : 2147–2162. doi: 10.1093/hmg/ddu735 25552658
54. Gussoni E, Pavlath GK, Miller RG, Panzara MA, Powell M, Blau HM, et al. Specific T cell receptor gene rearrangements at the site of muscle degeneration in Duchenne muscular dystrophy. J Immunol. 1994;153 : 4798–4805. 7963545
55. Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98 : 235–243. 11161980
56. Camirand G, Stephan L, Rousseau J, Sackett MK, Caron NJ, Mills P, et al. Central tolerance to myogenic cell transplants does not include muscle neoantigens. Transplantation. 2008;85 : 1791–1801. doi: 10.1097/TP.0b013e31817726bc 18580473
57. Altamirano F, Valladares D, Henriquez-Olguin C, Casas M, Lopez JR, Allen PD, et al. Nifedipine treatment reduces resting calcium concentration, oxidative and apoptotic gene expression, and improves muscle function in dystrophic mdx mice. PLoS ONE. 2013;8:e81222. doi: 10.1371/journal.pone.0081222 24349043
58. Friedrich O, Both M, Gillis JM, Chamberlain JS, Fink RH. Mini-dystrophin restores L-type calcium currents in skeletal muscle of transgenic mdx mice. J Physiol. 2004;555 : 251–265. 14594987
59. Vandebrouck A, Ducret T, Basset O, Sebille S, Raymond G, Ruegg U, et al. Regulation of store-operated calcium entries and mitochondrial uptake by minidystrophin expression in cultured myotubes. FASEB J. 2006;20 : 136–138. 16254044
60. Martinello T, Baldoin MC, Morbiato L, Paganin M, Tarricone E, Schiavo G, et al. Extracellular ATP signaling during differentiation of C2C12 skeletal muscle cells: role in proliferation. Mol Cell Biochem. 2011;351 : 183–196. doi: 10.1007/s11010-011-0726-4 21308481
61. Hindi SM, Sato S, Choi Y, Kumar A. Distinct roles of TRAF6 at early and late stages of muscle pathology in the mdx model of Duchenne muscular dystrophy. Hum Mol Genet. 2014;23 : 1492–1505. doi: 10.1093/hmg/ddt536 24163132
62. Gutpell KM, Hrinivich WT, Hoffman LM. Skeletal muscle fibrosis in the mdx/utrn+/ - mouse validates its suitability as a murine model of Duchenne muscular dystrophy. PLoS ONE. 2015;10:e0117306. doi: 10.1371/journal.pone.0117306 25607927
63. Narita S, Yorifuji H. Centrally nucleated fibers (CNFs) compensate the fragility of myofibers in mdx mouse. Neuroreport. 1999;10 : 3233–3235. 10574566
64. Knuesel I, Mastrocola M, Zuellig RA, Bornhauser B, Schaub MC, Fritschy JM. Short communication: altered synaptic clustering of GABAA receptors in mice lacking dystrophin (mdx mice). Eur J Neurosci. 1999;11 : 4457–4462. 10594673
65. Krasowska E, Zablocki K, Gorecki DC, Swinny JD. Aberrant location of inhibitory synaptic marker proteins in the hippocampus of dystrophin-deficient mice: implications for cognitive impairment in duchenne muscular dystrophy. PLoS ONE. 2014;9:e108364. doi: 10.1371/journal.pone.0108364 25260053
66. Sim JA, Young MT, Sung HY, North RA, Surprenant A. Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci. 2004;24 : 6307–6314. 15254086
67. Nico B, Paola Nicchia G, Frigeri A, Corsi P, Mangieri D, Ribatti D, et al. Altered blood-brain barrier development in dystrophic MDX mice. Neuroscience. 2004;125 : 921–935. 15120852
68. Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120 : 277–286. doi: 10.1007/s00401-010-0722-x 20644946
69. Bhattacharya A, Wang Q, Ao H, Shoblock JR, Lord B, Aluisio L, et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol. 2013;170 : 624–640. doi: 10.1111/bph.12314 23889535
70. Kvist TM, Schwarz P, Jorgensen NR. The P2X7 receptor: a key player in immune-mediated bone loss? ScientificWorldJournal. 2014;2014 : 954530. doi: 10.1155/2014/954530 24574936
71. Abou-Khalil R, Yang F, Mortreux M, Lieu S, Yu YY, Wurmser M, et al. Delayed bone regeneration is linked to chronic inflammation in murine muscular dystrophy. J Bone Miner Res. 2014;29 : 304–315. doi: 10.1002/jbmr.2038 23857747
72. Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn. 2006;235 : 203–212. 16258933
73. Ghahramani Seno MM, Graham IR, Athanasopoulos T, Trollet C, Pohlschmidt M, Crompton MR, et al. RNAi-mediated knockdown of dystrophin expression in adult mice does not lead to overt muscular dystrophy pathology. Hum Mol Genet. 2008;17 : 2622–2632. doi: 10.1093/hmg/ddn162 18511456
74. Merrick D, Stadler LK, Larner D, Smith J. Muscular dystrophy begins early in embryonic development deriving from stem cell loss and disrupted skeletal muscle formation. Dis Model Mech. 2009;2 : 374–388. doi: 10.1242/dmm.001008 19535499
75. Arulkumaran N, Unwin RJ, Tam FWK. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Inv Drug. 2011;20 : 897–915.
76. Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y, et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science. 2014;346 : 1000–1003. doi: 10.1126/science.1261754 25414314
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 10- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Transparency in Reporting Observational Studies: Reflections after a Year
- Using Qualitative Evidence in Decision Making for Health and Social Interventions: An Approach to Assess Confidence in Findings from Qualitative Evidence Syntheses (GRADE-CERQual)
- The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement
- New Clinical Decision Instruments Can and Should Reduce Radiation Exposure
- Monitoring Mortality in Forced Migrants—Can Bayesian Methods Help Us to Do Better with the (Little) Data We Have?
- Continuity in Drinking Water Supply
- Water Supply Interruptions and Suspected Cholera Incidence: A Time-Series Regression in the Democratic Republic of the Congo
- Individual Participant Data (IPD) Meta-analyses of Diagnostic and Prognostic Modeling Studies: Guidance on Their Use
- Derivation and Validation of Two Decision Instruments for Selective Chest CT in Blunt Trauma: A Multicenter Prospective Observational Study (NEXUS Chest CT)
- Effect of Health Risk Assessment and Counselling on Health Behaviour and Survival in Older People: A Pragmatic Randomised Trial
- P2RX7 Purinoceptor: A Therapeutic Target for Ameliorating the Symptoms of Duchenne Muscular Dystrophy
- Monitoring Pharmacologically Induced Immunosuppression by Immune Repertoire Sequencing to Detect Acute Allograft Rejection in Heart Transplant Patients: A Proof-of-Concept Diagnostic Accuracy Study
- Strategies for Understanding and Reducing the and Hypnozoite Reservoir in Papua New Guinean Children: A Randomised Placebo-Controlled Trial and Mathematical Model
- Upgrading a Piped Water Supply from Intermittent to Continuous Delivery and Association with Waterborne Illness: A Matched Cohort Study in Urban India
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effect of Health Risk Assessment and Counselling on Health Behaviour and Survival in Older People: A Pragmatic Randomised Trial
- Using Qualitative Evidence in Decision Making for Health and Social Interventions: An Approach to Assess Confidence in Findings from Qualitative Evidence Syntheses (GRADE-CERQual)
- Monitoring Pharmacologically Induced Immunosuppression by Immune Repertoire Sequencing to Detect Acute Allograft Rejection in Heart Transplant Patients: A Proof-of-Concept Diagnostic Accuracy Study
- The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



