-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSetting Research Priorities for Preconception Care in Low- and Middle-Income Countries: Aiming to Reduce Maternal and Child Mortality and Morbidity
article has not abstract
Published in the journal: . PLoS Med 10(9): e32767. doi:10.1371/journal.pmed.1001508
Category: Guidelines and Guidance
doi: https://doi.org/10.1371/journal.pmed.1001508Summary
article has not abstract
#tab:1#
Introduction
Preconception care means providing care before pregnancy is established. Women and couples of reproductive age are generally unaware of the effects that their own health conditions and health-related behaviors may have on the fetus during pregnancy. Although antenatal care is set in the maternal, newborn, and child health (MNCH) continuum [1], it neglects the most critical time of embryonic development, which often occurs before a woman even knows she is pregnant [2]. The evidence increasingly points to earlier care before pregnancy to improve women's health, and better pregnancy outcomes for the mother and newborn [3]–[5].
Preconception care may be defined as “any intervention provided to women and couples of childbearing age, regardless of pregnancy status or desire, before pregnancy, to improve health outcomes for women, newborns and children” [3] or “a set of interventions that aim to identify and modify biomedical, behavioral, and social risks to a woman's health or pregnancy outcome through prevention and management” [4]. For instance, education and awareness about nutritional anemia and congenital malformations can increase receptiveness to and uptake of iron and folic acid supplementation even before pregnancy. The specific aim of preconception care is to improve pregnancy outcomes for mothers and newborns, by optimizing health before a possible pregnancy occurs. Under strict terms, the preconception period may be defined as a minimum of three menstrual cycles prior to the initiation of sexual intercourse, the intent of which is to achieve a wanted and viable pregnancy. An exact “preconception period” has not been standardized by the evidence base; however, since many pregnancies are unplanned, and time to conception for couples varies greatly. We propose that the preconception period be defined as a minimum of one year prior to the initiation of any unprotected sexual intercourse that could possibly result in a pregnancy, reflecting the broader scope of preconception care that extends to adolescents and all women and couples of reproductive age.
A systematic review [3] established that there are currently three levels of evidence within the area of preconception care. For some preconception interventions, such as folic acid supplementation to prevent neural tube defects, the evidence base is strong [6], yet even in developed countries less than half of all women regularly consume folic acid supplements around the time of conception [7]. In other areas, such as intervals between pregnancies, the data shows significant risk in terms of excess maternal deaths, higher rates of prematurity and stillbirths, with short inter-pregnancy intervals 8,9; however, strategies to optimize birth spacing and increase contraceptive uptake are lacking [10]. Finally in women's health, violence against girls and women; unsafe abortions; alcohol and tobacco use; and harmful environmental exposures require further substantiation of magnitude of pre-pregnancy risk, and proof that prevention and management as part of preconception care will have greater impact than prenatal care alone.
Preconception care has the potential to positively impact 208 million pregnancies worldwide each year [11]. Unfortunately, many adolescent girls and women in low - and middle-income countries (LMICs), which have the highest burden of maternal and childhood mortality (map of global infant mortality [World Bank 2011] http://data.worldbank.org/indicator/SP.DYN.IMRT.IN/countries?display=map and map of maternal mortality worldwide [WHO 2010] http://gamapserver.who.int/gho/interactive_charts/mdg5_mm/atlas.html) [12],[13], do not receive the benefits of these interventions, either because they lack access to care or because it is not routinely offered to them before pregnancy. Critical appraisal of the literature review in light of the current global MNCH picture suggests that the greatest benefit would be in these resource-poor countries, and emphasizes the need for implementation strategies and increasing coverage of existing cost-effective preconception interventions.
Although present-day funding for global health is previously unparalleled [14] and a substantial proportion of maternal and child deaths in LMICs are preventable with existing interventions [15]–[17], progress in reducing these deaths is far too slow. Perhaps one contributing factor is the bias that remains in health care and research investment—for example, worldwide 7.6 million children died in 2010, equivalent to global deaths due to cancer and slightly higher than deaths due to heart disease [18],[19], yet funding favors breakthrough research for cancer and heart disease, which have high media interest, while implementation research and delivery for maternal and child health is sidelined. The persisting high mortality for mothers and children in LMICs [20],[21], with its repercussions on global MNCH and overall population health and development, represents a continuing failure and challenge. We assembled a group of maternal and child health professionals whose specific goal was to identify and prioritize evidence-based, equitable research investment opportunities for development and increased delivery of effective preconception interventions in LMIC, with the intent of reducing maternal, fetal, newborn, and childhood mortality and severe morbidity.
Methods
The Child Health and Nutrition Research Initiative (CHNRI) methodology for research priority-setting was proposed to inform those who develop research policy and/or invest in health research with the aim to impact population health and improve equity in health care [22]–[24]. The process uses a systematic and transparent approach involving an array of health professionals to enlist a wide spectrum of research options relating to a certain health topic and context. Research options are generated in a structured way, using four basic domains: description (epidemiology), discovery (new interventions), development (improving existing interventions), and delivery (health policy and implementation). A priori criteria relevant to the topic are used to score competing research questions in all four domains and then order them in terms of potential influence on health and equity (conceptual framework shown in Figure 1; further details published in previous CHNRI exercises) [25]–[27].
Fig. 1. Conceptual framework. 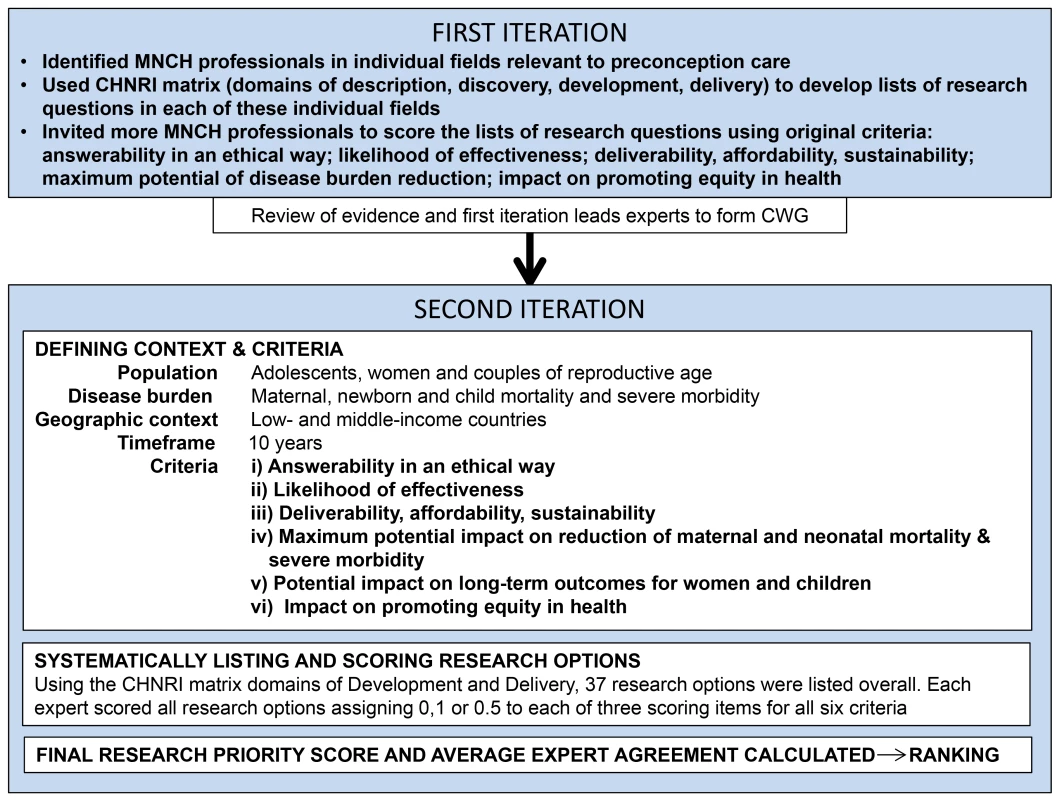
Overview of the key steps in this adaptation of the CHNRI methodology. A notable difference from previous CHNRI exercises was the application of the methodology in two iterations. Following an initial consultation coordinated by the Harvard School of Public Health, Aga Khan University, and the Bill & Melinda Gates Foundation, a list of potential experts was drawn up by the investigators in individual fields related to preconception care (such as nutrition, mental health, and infectious diseases) as identified by the initial review. The leads were approached to draw up a list of key questions based on their assessment of research gaps and identify additional subject experts to score the lists of research questions using the original CHNRI criteria: answerability in an ethical way; likelihood of effectiveness; deliverability, affordability, and sustainability; maximum potential impact on burden reduction; and impact on promoting greater equity in health [22]–[24]. Forty-eight of 130 approached completed the scoring (the scored lists may be accessed by contacting the authors), and were geographically representative mainly of Asia, North America, and South America. Many found criterion 4 “maximum potential of disease burden reduction” difficult to estimate, since preconception care to improve maternal and neonatal outcomes is a relatively new avenue. There were also discrepancies in the number and scope of research questions, as well as number of scorers, for each field.
At a meeting of leading international MNCH experts, the potential risk factors and interventions (Box 1) ascertained from the AKU team's systematic review of the literature on preconception care [3 were presented, along with a synopsis of the first iteration. These experts agreed to form a core working group (CWG) to proceed with a second iteration of the CHNRI process for preconception care in a more holistic manner.
#tab:2#
The CHNRI methodology involves four stages (Table S1 provides more information about the modified process, limitations, and validation) [27]:
(1) Defining Context and Criteria
An open group discussion was held during the CWG meeting to define the context and criteria, with modifications being incorporated until all CWG members were satisfied. Over 90% of all maternal and child deaths occur in LMICs, particularly South Asia and sub-Saharan Africa [28]. Scaling up preconception care directed at adolescents, women, and couples of reproductive age in such high-burden, resource-poor regions could hasten the decline in global maternal and childhood mortality and severe morbidity through development and delivery of effective interventions. This became the context and target population for the research priority-setting exercise, with a timeline for development and delivery of interventions within 10 years. The CWG emphasized the importance of local or regional MNCH priorities, and underscored that investment in cross-cutting interventions would have greater benefits in improving MNCH outcomes [22],[24].
The criteria were modified to reflect the context and subject of preconception care, with criterion (iv) being amended to “maximum potential impact on reduction of maternal and neonatal mortality and severe morbidity,” and addition of the criterion “potential impact on long-term outcomes for women and children.”
(2) Expert Input-Listing and Scoring Competing Research Options
Experts were invited to participate on the basis of their record in maternal and child health research relevant to preconception care. There was an overlap of 11 experts who were involved in both iterations. A serious attempt was made to be inclusive of experts with varied areas of expertise and from different countries (Table S2), with the addition of more experts from Europe and Africa in the second iteration. Experts received a summary of findings with effect sizes from the primary review, along with previously published CHNRI exercises on childhood mortality and stillbirths to help them understand the background literature and priority-setting process. Experts used the CHNRI matrix (Table 1) to develop an extensive list of research options (more expansive research ideas) and research questions (narrower in focus) in preconception care.
Tab. 1. CHNRI matrix. 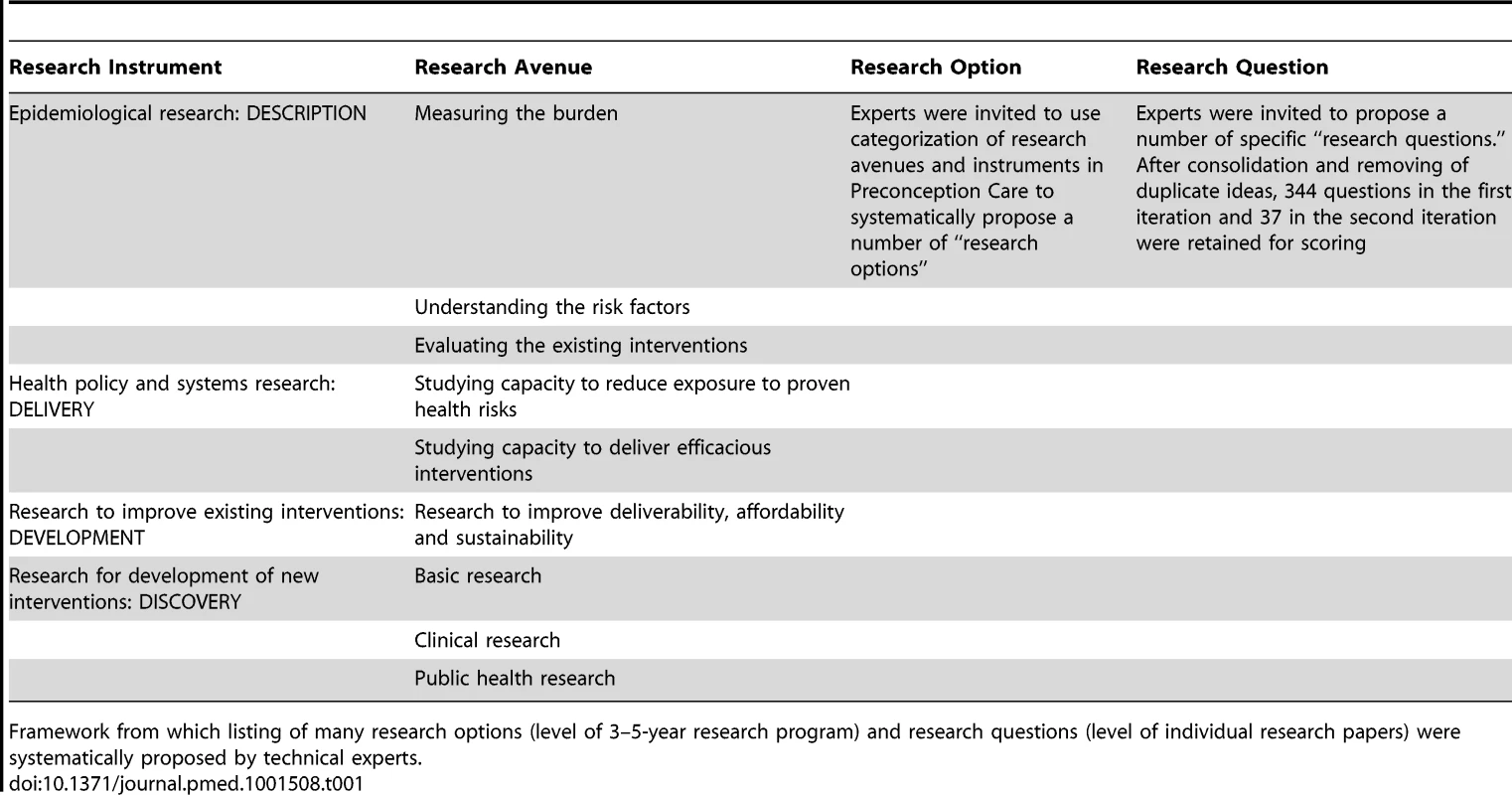
Framework from which listing of many research options (level of 3–5-year research program) and research questions (level of individual research papers) were systematically proposed by technical experts. In the second iteration, the CWG advised that the timelines and criteria for the domains “description” and “discovery” would be inconsistent with those of “development” and “delivery,” and would therefore be ranked inaccurately. A consensus was reached to focus on development and delivery to enable preconception care services to reach women of reproductive age, which would accelerate maternal and child mortality and morbidity reduction in LMICs. Each expert in the CWG presented further information in one area of preconception care most relevant to their own experience and suggested more research options. Short group discussions were held at the end of each session to seek clarification, air dissenting opinions, and outline the most important research options in that area. The chairperson for each half-day session was tasked with drawing out all opinions and promoting clarity, and another member was assigned to document research options and the group's suggestions for emphasis or caveats therein. The lists of research questions from the first iteration were compressed to highlight important gaps, yet still represent the range of research possibilities in preconception care. The final list of research questions was reviewed by the whole CWG at the end of the meeting to ensure that they were framed correctly and comprehensively to allow scoring. The CWG also attempted to ensure that the phrasing of the research questions was not rigid, so for example given the option “What approaches work to increase the use of effective contraception…” research could assess contraceptive counseling to educate and empower women to plan their pregnancies in different settings, or women with and without their husbands, and so forth. Each expert scored the new list, assigning a score to each of 37 research questions in the domains of development and delivery using the six modified criteria (final criteria questions are shown in Box 2, the actual scoring sheet used by experts is shown in Table S3). 24 of 30 scorers participated in the second iteration, which was acceptable as it has been modeled that the CHNRI exercise reaches saturation with 20–25 scorers.
#tab:4#
The first iteration was conducted entirely via email from March to June 2011. The research options for the second iteration were developed during an expert meeting in July 2011, and the scoring was completed via email by September 2011.
(3) Weighting criteria based on input from societal stakeholders
The scoring criteria may be perceived with varying importance based on the perspective of different stakeholders. For example, parents who experienced a stillbirth may rate mortality reduction higher than a donor organization who may value answerability, or a public health official most concerned with deliverability. For previous CHNRI exercises, a range of stakeholders were polled to weight the criteria [29]; however, the CWG decided not to assign weights for this exercise. Rather, the final rankings are based on the average merit of each research option across all scoring criteria and expert perspectives.
(4) Computing Research Priority Scores (RPSs) and Average Expert Agreement (AEA).
Overall RPS was calculated as the mean of scores for the six criteria [23] according to the formula:
AEA was calculated for each research question as the average proportion of scorers that gave the most common answer while scoring that question: (Where q is 1 of 18 criteria questions that experts used to evaluate competing research options).Results
Table 2 shows the top research questions (those with RPS>80), of 381 in total (344 questions in the first iteration and 37 questions in the second). Table S4 shows the final scores and ranking of the research questions from the more robust second iteration. The RPS indicates the perceived likelihood that each research option will meet the chosen priority-setting criteria. In the areas of development and delivery of existing interventions, the highest-ranked research option seeks to address the gap in coverage of nutritional interventions, such as supplementation, through integration with other programs. The specified context (expectation of medium term impact in LMIC) allowed research to identify obstacles to delivery of interventions, and research to optimize the use of those interventions, to receive high scores. Priority areas identified were adolescent health, chronic conditions, infectious diseases and immunization, contraception, improving the supply chain for preconception commodities, and public health approaches to reduce exposure to environmental pollutants with adverse MNCH effects.
Tab. 2. The top 12 research questions. 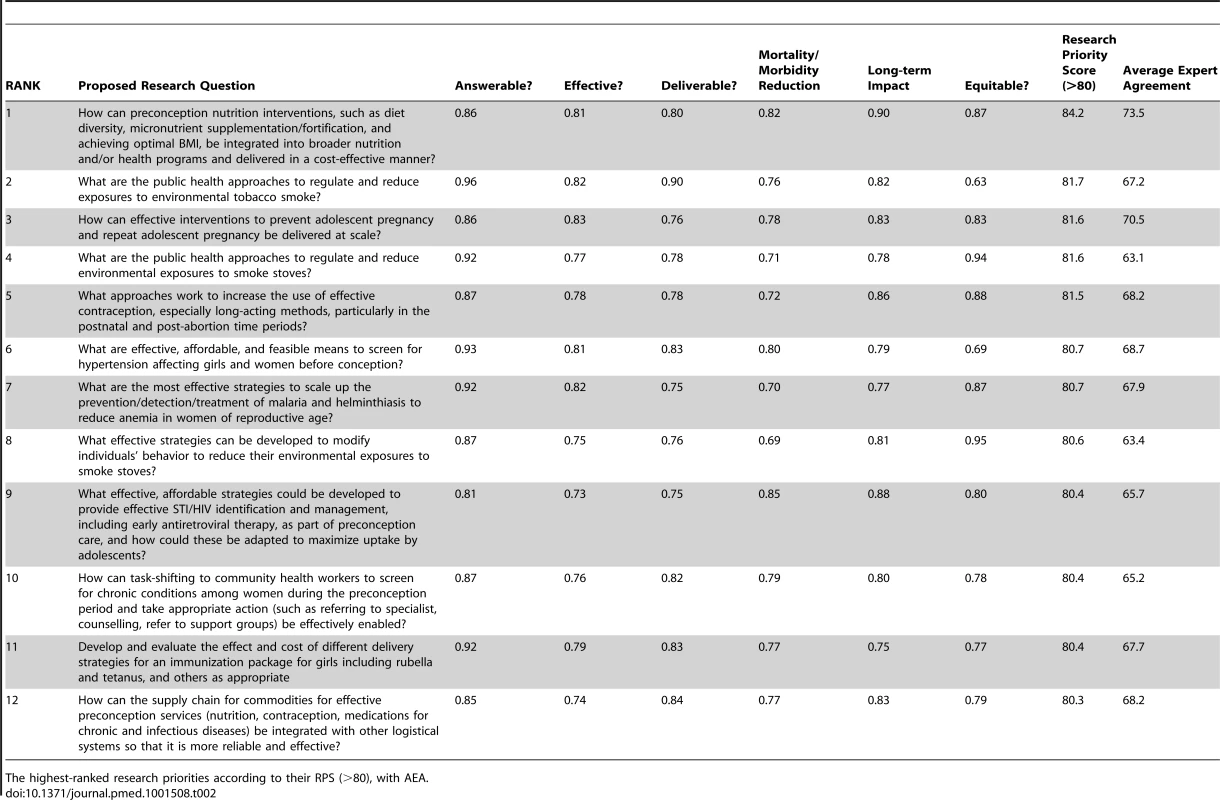
The highest-ranked research priorities according to their RPS (>80), with AEA. Three central issues were consistently emphasized, with experts advocating for integration of preconception interventions with other programs and systems; moving beyond the health care setting with task-shifting to community health workers (CHWs); and maximizing uptake of preconception services by adolescents. Addressing these fundamental issues enables interventions to be delivered affordably and sustainably on a wider scale, yet they are rarely considered by research funding agencies.
Although discovery research options were excluded (see “Input from technical experts” above), questions suggested the need to develop simple, cost-effective methods to screen for, diagnose, and treat health conditions that have negative consequences particularly for women of reproductive age. The research questions with the lowest scores reflected interventions for which there is little evidence of effect, such as strategies to promote women's mental health; reduce coerced sex and intimate partner violence; reduce genetic disease risk in the community; and prevent or treat substance use among women and couples of reproductive age. Utilizing information technologies to improve demand for preconception services was also ranked low because of uncertainty about its potential to reduce mortality and severe morbidity.
Discrimination between levels of agreement among scorers on the prioritization of research questions was achieved by calculating AEA (Tables 2 and S4). AEA scores ranged from 60.5% to 84.2%, indicating the proportion of scorers that gave the most common score to an average criteria question for a specific research option. In general, the questions with high AEA were also those that achieved high RPS. Greater points of contention were research options that would require individual behavior modification to improve pre-pregnancy health, or a shift in cultural norms (e.g., economic incentives to increase demand for services, involving men in preconception health, and addressing consanguinity).
The actual scores marked for all research questions by individual experts and calculations for AEA are presented in Table S5. The results exposed how research questions can be prioritized in completely different ways, depending on the criterion used. Box 3 shows the three highest scoring questions for each of the six priority-setting criteria. The most answerable research relates to developing public health approaches to reduce environmental tobacco smoke exposure. This option was also the highest ranked question for deliverability, and scored highly on effectiveness and equity. Other answerable research possibilities were development of diabetes screening tools, and evaluating the feasibility of information technology as an aid for front-line health workers in continuity of care. The ideas most likely to be effective were scaling up interventions to prevent pregnancy in adolescence and anemia in women of reproductive age. The strongest opportunities to improve delivery were assessing the use of cellphones and other information technologies to increase demand for, and promote provision of, preconception care services. The greatest impact on maternal and newborn mortality and morbidity was assigned to developing strategies to identify and manage sexually transmitted infections (STIs)/HIV in the preconception population; deciding upon an essential package of preconception interventions for all girls and women; and studying how best to integrate nutrition interventions into broader initiatives. It was agreed that two of these same research questions would also have the greatest impact on long-term outcomes for women and children. Engaging CHWs in primary preventive and promotive mental health services was another option thought to impact long-term outcomes. Research that would maximally contribute to improving equity was evaluating behavioral and public health strategies to reduce exposure to smoke stoves. It was notably recommended that improved quality and accessibility of post-abortion care services at all tiers of the health system would also improve health equity for women of reproductive age.
Discussion
This research priority-setting focused on development and delivery of existing interventions during the preconception period in LMICs. The latter has been recognized as a critically important entry point to influence optimal health, nutrition, and birth preparedness in LMICs [30]. The research questions that received the highest scores therefore highlighted the need to develop strategies to increase coverage of basic interventions such as improving nutrition; reproductive planning for adolescents; contraception; prevention, detection and treatment of chronic conditions that affect maternal health; immunization, diagnosis, and treatment of infectious diseases; and reducing harmful environmental smoke exposures. The highest priorities also advocated for a systems-based approach to increase preconception care services in LMICs including integration with other programs; task-shifting to CHWs; improving supply chains for preconception care commodities; partnerships with media and information technology; maximizing demand for and uptake of preconception interventions, especially by adolescents.
The CHNRI methodology aims to ensure that those research options with evidence of true potential impact in the chosen context receive commensurate support from the global health community. The simple, structured scoring method means that those research options that meet most criteria and achieve high expert consensus are ranked highly. Moreover, for each individual research question it exposes strengths and weaknesses through estimations collected from numerous technical experts from various backgrounds. Although the CHNRI process attempts to achieve fairness and greater accuracy in research priority-setting, there are limitations to this method (Table S1). First, the list of research questions developed is not exhaustive and therefore cannot include all possible sound research ideas. Since our primary review focused on health-related interventions in the preconception period, social sector interventions were not emphasized even though these indirectly promote health and wellbeing. In this exercise, education or improving women's literacy was not suggested as a discrete research option although it is an essential means to achieve preconception care and better maternal health. However, this is a major component of other research options that were elaborated, notably adolescent health and pregnancy prevention programs, community-based platforms that target maternal and newborn health in rural areas; and utilizing media and information technology to reach adolescent girls and women with preconception care information and services. Second, the method of expert selection may be seen as the initiator of the process tending to invite other like-minded experts or experts known to them to participate. In this exercise, the initiator invited only the first participants, and then asked them to invite other MNCH experts representing their area of expertise and other geographical regions. In the first iteration, three invited experts were unable to participate in developing research options and 82 were unable to score, with experts mostly commonly citing time constraints as the reason for non-participation. Dissension among experts was reflected in the AEA score, and rearranging research options by this score did not make a significant difference in their priority order since most options with high RPS (>80) also had high AEA (>65). Third, in this application of the CHNRI method, no stakeholder weighting was performed, hence the results are reflective of MNCH experts but do not consider other values that stakeholders might have.
This objective representation of research priorities may be used to guide research policy that is likely to have an impact on the health of women and couples of reproductive age, as well as their young children; and eventually make both research and health care more equitable.
The United Nations Millennium Development Goals (MDGs) aimed to reduce childhood mortality and improve maternal health. While significant progress towards these targets has been achieved, it is recognized that progress in reducing newborn deaths is slow [12] and major challenges remain in reducing maternal mortality [28]. Improving birth preparedness and the health of the mother is a critical step in achieving these targets and has received relatively less attention. The list of research ideas put forth may not seem novel or innovative. Many are already recognized gaps in MNCH. However since research in underlying determinants of health, health policy, and systems, or applied health are rarely appreciated by researchers and investors, this exercise draws new attention to these long-standing concerns. Addressing these issues in LMIC is crucial to build on our success in improving MNCH as we move forward after 2015, the deadline to meet the MDGs
New interventions and strategies, strengthened health systems, quality services, and equity in coverage are needed to confront infectious diseases, chronic conditions, unsafe abortions, and undernutrition, prematurity, and stillbirths, which still threaten maternal and child survival. It is imperative that preconception care is seen as an earlier opportunity, not just for family planning or to reduce maternal and neonatal mortality, but also to improve long-term outcomes for adolescent girls, women, and children. Adolescent health and reproductive health must increasingly be considered as crucial stages in the continuum of care. Health research investment and policy should be pursued in a more balanced way, promoting increased access and delivery of an essential package of preconception interventions. This exercise has led to a concerted global effort led by the World Health Organization to tackle the challenges of reaching girls and women with preconception care services. Reaching a consensus on what constitutes such a package of preconception interventions in LMICs, and investing in implementation research to ensure maximum coverage and uptake should be the next step. Within LMICs, different regions and individual countries may need to further prioritize their MNCH policies and research investment according to their specific causes of maternal and newborn mortality and morbidity and feasibility of scaling up certain interventions.
Supporting Information
Zdroje
1. World Health Organization (2010) Package of interventions for family planning, safe abortion care, maternal, newborn and child health. Geneva: WHO.
2. AtrashH (2006) Preconception care for improving perinatal outcomes: the time to act. Maternal Child Health J 10 : 3–11.
3. Bhutta Z, Dean S, Imam A, Lassi Z (2011) A systematic review of preconception risks and interventions. Karachi: The Aga Khan University. Available: http://mother-childlink.tghn.org/site_media/media/articles/Preconception_Report.pdf. Accessed 3 August 2013.
4. CDC (2006) JohnsonK, PosnerSF, BiermannJ, CorderoJF, et al. (2006) CDC/ATSDR preconception care work group; select panel on preconception care. Recommendations to improve preconception health and health care United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep 55 : 23.
5. JackB, AtrashH (2008) Preconception health and health care: the clinical content of preconception care. Am J Obstet Genecol 199: S257–S395.
6. De-RegilLM, Fernandez-GaxiolaAC, DowswellT, Pena-RosasJP (2010) Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database Syst Rev 10: CD007950.
7. RayJG, VermeulenMJ, MeierC, WyattPR (2004) Risk of congenital anomalies detected during antenatal serum screening in women with pregestational diabetes. QJM 97 : 651–653.
8. Conde-AgudeloA, BelizánJM (2000) Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ 321 : 1255.
9. Conde-AgudeloA, Rosas-BermúdezA, Kafury-GoetaAC (2006) Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA 295 : 1809.
10. YeakeyMP, MuntiferingCJ, RamachandranDV, MyintYM, CreangaAA, et al. (2009) How contraceptive use affects birth intervals: results of a literature review. Studies in Family Planning 40 : 205–214.
11. SinghS, SedghG, HussainR (2010) Unintended pregnancy: worldwide levels, trends, and outcomes. Studies in Family Planning 41 : 241–250.
12. LiuL, JohnsonHL, CousensS, PerinJ, ScottS, et al. (2012) Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379 : 9–15.
13. WHO (2012) Trends in maternal mortality: 1990 to 2010. Available: http://www.unfpa.org/webdav/site/global/shared/documents/publications/2012/Trends_in_maternal_mortality_A4-1.pdf. Geneva: WHO.
14. De Francisco A, Matlin S (2006) Monitoring financial flows for health research. Geneva: Global Forum for Health research.
15. DarmstadtGL, BhuttaZA, CousensS, AdamT, WalkerN, et al. (2005) Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet 365 : 977–988.
16. KerberKJ, de Graft-JohnsonJE, BhuttaZA, OkongP, StarrsA, et al. (2007) Continuum of care for maternal, newborn, and child health: from slogan to service delivery. Lancet 370 : 1358–1369.
17. The Partnership for Maternal Newborn & Child Health (PMNCH) (2011) A global review of the key interventions related to reproductive, maternal, newborn and child health (RMNCH). Geneva: PMNCH.
18. World Health Organization (2011) Cardiovascular diseases: factsheet number 317. Geneva: WHO.
19. World Health Organization (2012) Cancer: Factsheet No 297. Geneva: WHO.
20. HoganMC, ForemanKJ, NaghaviM, AhnSY, WangM, et al. (2010) Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 375 : 1609–1623.
21. RajaratnamJK, MarcusJR, FlaxmanAD, WangH, Levin-RectorA, et al. (2010) Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millennium Development Goal 4. Lancet 375 : 1988–2008.
22. RudanI, ChopraM, KapiririL, GibsonJ, LansangMA, et al. (2008) Setting priorities in global child health research investments: universal challenges and conceptual framework. Croat Med J 49 : 307–317.
23. RudanI, GibsonJ, KapiririL, LansangMA, HyderAA, et al. (2007) Setting priorities in global child health research investments: assessment of principles and practice. Croat Med J 48 : 595.
24. RudanI, GibsonJL, AmeratungaS, El ArifeenS, BhuttaZA, et al. (2008) Setting priorities in global child health research investments: guidelines for implementation of the CHNRI method. Croat Med J 49 : 720.
25. LawnJE, BahlR, BergstromS, BhuttaZA, DarmstadtGL, et al. (2011) Setting research priorities to reduce almost one million deaths from birth asphyxia by 2015. PLoS Med 8: e1000389 doi:10.1371/journal.pmed.1000389
26. RudanI, El ArifeenS, BhuttaZA, BlackRE, BrooksA, et al. (2011) Setting Research Priorities to Reduce Global Mortality from Childhood Pneumonia by 2015. PLoS Med 8: e1001099 doi:10.1371/journal.pmed.1001099
27. RudanI, El ArifeenS, BlackRE, CampbellH (2007) Childhood pneumonia and diarrhoea: setting our priorities right. Lancet Infect Dis 7 : 56–61.
28. BhuttaZA, ChopraM, AxelsonH, BermanP, BoermaT, et al. (2010) Countdown to 2015 decade report (2000–2010): taking stock of maternal, newborn, and child survival. Lancet 375 : 2032–2044.
29. KapiririL, TomlinsonM, ChopraM, El ArifeenS, BlackRE, et al. (2007) Setting priorities in global child health research investments: addressing values of stakeholders. Croat Med J 48 : 618.
30. BhuttaZA, DasJK, RizviA, et al. (2013) Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet In press.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 9- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa
- Translating Cochrane Reviews to Ensure that Healthcare Decision-Making is Informed by High-Quality Research Evidence
- Physician Emigration from Sub-Saharan Africa to the United States: Analysis of the 2011 AMA Physician Masterfile
- Acupuncture and Counselling for Depression in Primary Care: A Randomised Controlled Trial
- Serotype-Specific Changes in Invasive Pneumococcal Disease after Pneumococcal Conjugate Vaccine Introduction: A Pooled Analysis of Multiple Surveillance Sites
- Risk Factors and Outcomes for Late Presentation for HIV-Positive Persons in Europe: Results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE)
- Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis
- Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China
- Feasibility of Mass Vaccination Campaign with Oral Cholera Vaccines in Response to an Outbreak in Guinea
- Preconception Care in Low- and Middle-Income Countries: New Opportunities and a New Metric
- How to Stir Up Trouble…while Riding a Rollercoaster
- Health Workforce Brain Drain: From Denouncing the Challenge to Solving the Problem
- Association of the ANRS-12126 Male Circumcision Project with HIV Levels among Men in a South African Township: Evaluation of Effectiveness using Cross-sectional Surveys
- Setting Research Priorities for Preconception Care in Low- and Middle-Income Countries: Aiming to Reduce Maternal and Child Mortality and Morbidity
- Transnational Tobacco Company Interests in Smokeless Tobacco in Europe: Analysis of Internal Industry Documents and Contemporary Industry Materials
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China
- Translating Cochrane Reviews to Ensure that Healthcare Decision-Making is Informed by High-Quality Research Evidence
- Physician Emigration from Sub-Saharan Africa to the United States: Analysis of the 2011 AMA Physician Masterfile
- Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





