-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation of the ANRS-12126 Male Circumcision Project with HIV Levels among Men in a South African Township: Evaluation of Effectiveness using Cross-sectional Surveys
Background:
Randomized controlled trials have shown that voluntary medical male circumcision (VMMC) reduces HIV infection by 50% to 60% in sub-Saharan African populations; however, little is known about the population-level effect of adult male circumcision (MC) as an HIV prevention method. We assessed the effectiveness of VMMC roll-out on the levels of HIV in the South African township of Orange Farm where the first randomized controlled trial (RCT) to test the effect of VMMC on HIV acquisition was conducted in 2002–2005.Methods and Findings:
The Bophelo Pele project is a community-based campaign against HIV, which includes the roll-out of free VMMC. A baseline cross-sectional biomedical survey was conducted in 2007–2008 among a random sample of 1,998 men aged 15 to 49 (survey response rate 80.7%). In 2010–2011, we conducted a follow-up random survey among 3,338 men aged 15 to 49 (survey response rate 79.6%) to evaluate the project. Participants were interviewed, blood samples were collected and tested for HIV and recent HIV infection (using the BED HIV incidence assay), and MC status was assessed through a clinical examination. Data were analyzed using multivariate and propensity statistical methods.
Owing to the VMMCs performed in the context of the RCT and the Bophelo Pele project, the prevalence rate of adult MC increased from 0.12 (95% CI 0.10–0.14) to 0.53 (95% CI 0.51–0.55). Without these VMMCs, the HIV prevalence rate in 2010–2011 would have been 19% (95% CI 12%–26%) higher (0.147 instead of 0.123).
When comparing circumcised and uncircumcised men, no association of MC status with sexual behavior was detected. Among circumcised and uncircumcised men, the proportion consistently using condoms with non-spousal partners in the past 12 months was 44.0% (95% CI 41.7%–46.5%) versus 45.4% (95% CI 42.2%–48.6%) with weighted prevalence rate ratio (wPRR) = 0.94 (95% CI 0.85–1.03). The proportion having two or more non-spousal partners was 50.4% (95% CI 47.9%–52.9%) versus 44.2% (95% CI 41.3%–46.9%) with wPRR = 1.03 (95% CI 0.95–1.10).
We found a reduction of BED-estimated HIV incidence rate ranging from 57% (95% CI 29%–76%) to 61% (95% CI 14%–83%) among circumcised men in comparison with uncircumcised men.Conclusions:
Findings suggest that the roll-out of VMMC in Orange Farm is associated with a significant reduction of HIV levels in the community. The main limitation of the study is that it was not randomized and cannot prove a causal association. The roll-out of VMMC among adults in sub-Saharan Africa should be an international priority and needs to be accelerated to effectively combat the spread of HIV.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(9): e32767. doi:10.1371/journal.pmed.1001509
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001509Summary
Background:
Randomized controlled trials have shown that voluntary medical male circumcision (VMMC) reduces HIV infection by 50% to 60% in sub-Saharan African populations; however, little is known about the population-level effect of adult male circumcision (MC) as an HIV prevention method. We assessed the effectiveness of VMMC roll-out on the levels of HIV in the South African township of Orange Farm where the first randomized controlled trial (RCT) to test the effect of VMMC on HIV acquisition was conducted in 2002–2005.Methods and Findings:
The Bophelo Pele project is a community-based campaign against HIV, which includes the roll-out of free VMMC. A baseline cross-sectional biomedical survey was conducted in 2007–2008 among a random sample of 1,998 men aged 15 to 49 (survey response rate 80.7%). In 2010–2011, we conducted a follow-up random survey among 3,338 men aged 15 to 49 (survey response rate 79.6%) to evaluate the project. Participants were interviewed, blood samples were collected and tested for HIV and recent HIV infection (using the BED HIV incidence assay), and MC status was assessed through a clinical examination. Data were analyzed using multivariate and propensity statistical methods.
Owing to the VMMCs performed in the context of the RCT and the Bophelo Pele project, the prevalence rate of adult MC increased from 0.12 (95% CI 0.10–0.14) to 0.53 (95% CI 0.51–0.55). Without these VMMCs, the HIV prevalence rate in 2010–2011 would have been 19% (95% CI 12%–26%) higher (0.147 instead of 0.123).
When comparing circumcised and uncircumcised men, no association of MC status with sexual behavior was detected. Among circumcised and uncircumcised men, the proportion consistently using condoms with non-spousal partners in the past 12 months was 44.0% (95% CI 41.7%–46.5%) versus 45.4% (95% CI 42.2%–48.6%) with weighted prevalence rate ratio (wPRR) = 0.94 (95% CI 0.85–1.03). The proportion having two or more non-spousal partners was 50.4% (95% CI 47.9%–52.9%) versus 44.2% (95% CI 41.3%–46.9%) with wPRR = 1.03 (95% CI 0.95–1.10).
We found a reduction of BED-estimated HIV incidence rate ranging from 57% (95% CI 29%–76%) to 61% (95% CI 14%–83%) among circumcised men in comparison with uncircumcised men.Conclusions:
Findings suggest that the roll-out of VMMC in Orange Farm is associated with a significant reduction of HIV levels in the community. The main limitation of the study is that it was not randomized and cannot prove a causal association. The roll-out of VMMC among adults in sub-Saharan Africa should be an international priority and needs to be accelerated to effectively combat the spread of HIV.
Please see later in the article for the Editors' SummaryIntroduction
According to the 2012 Joint United Nations Programme on HIV/AIDS (UNAIDS) Report on the global AIDS epidemic [1], sub-Saharan Africa continues to bear a disproportionate share of the global HIV burden. The regions of eastern and southern Africa remain the areas most heavily affected by HIV/AIDS. In particular, South Africa, with an estimated 5.6 (5.3–5.9) million HIV-infected people in 2011, continues to have the world's largest epidemic. The vast majority of newly HIV-infected individuals from sub-Saharan Africa acquire the virus during unprotected heterosexual intercourse.
In this context, preventing the heterosexual transmission of HIV among adults, especially young adults, is a public health priority. From the beginning of the HIV/AIDS epidemic, several randomized controlled trials (RCTs) were conducted to test a number of behavioral and biomedical prevention strategies. The efficacy of voluntary medical adult male circumcision (VMMC) in reducing male HIV acquisition by 50% to 60% in sub-Saharan African populations has been demonstrated in three RCTs published in 2005 [2] and in 2007 [3],[4]. VMMC is to date one of the most promising interventions to curb the spread of HIV in these regions, demonstrated to be acceptable in traditionally non-circumcising African communities [5], and expected to be significantly life - and cost-saving in terms of averted HIV infections and related medical costs [6]–[10]. Since 2007, it is recommended by UNAIDS and the World Health Organization (WHO) as an important, complementary strategy to fight HIV in these settings.
However, to our knowledge, the effectiveness of VMMC roll-out in reducing the spread of HIV among adults has not yet been published in scientific journals. Such evidence is of major public health importance because it will mobilize the international community in support of VMMC roll-out programs in sub-Saharan Africa. This demonstration can be achieved by demonstrating that the overall reduction in HIV prevalence and incidence rates among all adult men attributable to a VMMC campaign is sizeable. The a priori conditions being that VMMC uptake is substantial and that its protective effect on HIV acquisition is not compensated by an increase in risky sexual behavior.
The primary objectives of this study were to assess the association of VMMC roll-out in a South African community with (a) VMMC uptake, (b) risky sexual behavior, and (c) the levels of HIV prevalence and incidence rates among adult men.
Methods
Ethics Committee Approval
Ethical clearance for both surveys was granted by the Human Research Ethics Committee (Medical) of the University of the Witwatersrand on May 8, 2007 (protocol study number M070367).
Study Setting
The study was conducted in the township of Orange Farm, located in Gauteng province, South Africa. The township has an estimated population of 110,000 adults. The HIV epidemic in the province is among the most severe in the world, with a prevalence rate estimated at 0.30 among antenatal women in 2010 [11].
ANRS Project
The ANRS (French Agency for AIDS and Viral Hepatitis Research) project consists of an RCT and a community roll-out. The RCT (ANRS 1265) was the first study to test the effect of VMMC on HIV acquisition and was conducted in this community in 2002–2005 [2]. About 2,700 VMMCs were performed among volunteers aged 18 to 24 y in that period. The roll-out, also known as the “Bophelo Pele” ANRS-12126 project, was implemented in early 2008. This roll-out is a comprehensive community-based HIV prevention intervention offering free VMMC services to all men aged 15 and older living in Orange Farm. Through the roll-out, about 18 000 adult VMMCs were performed between 2008 and 2010. This ongoing roll-out has been described elsewhere in detail [12]. In brief, project activities include community mobilization and outreach, using communication approaches aimed at both men and women, and incorporating broader HIV prevention strategies. Free VMMC is offered at the project's main center, which has been designed for low-income settings according to UNAIDS/WHO operational guidelines [13]. Prior to surgery, participants receive group education on VMMC, individual HIV risk reduction counseling, treatment of symptomatic sexually transmitted infections (STI), and they are offered HIV testing.
Baseline Survey
A cross-sectional survey was conducted at baseline among a random sample of 1,998 men between November 2007 and April 2008, after the RCT, and before the roll-out became fully operational, to collect baseline data. This survey was used to assess MC prevalence before the VMMC roll-out. A first random sample of households was selected from Statistics South Africa Enumerator Area aerial photographs. All men aged 15 to 49 y, who had slept in the selected households the night before the investigative team's visit, were eligible for inclusion. A second random sample of households was selected and volunteers underwent the same procedures, except that only men aged 16 to 29 were included. Voluntary, written informed consent was obtained, in addition to parental consent for those aged under 18. Each participant was interviewed at the study site using an anonymous structured standardized questionnaire adapted from an instrument designed by UNAIDS [14]. The following background characteristics were collected: age group, ethnic group, religion, having at least one child, occupation, alcohol consumption, education level, having ever been married, and, if relevant, date and place of MC. The sexual behavior characteristics collected were: age at first sexual intercourse, number of lifetime sexual partners, self-reported consistent condom use with non-spousal partners in the last 12 months, and number of non-spousal partners in the last 12 months. In addition to variables listed above, intention to become circumcised was collected among uncircumcised men. Each interview was followed by an individual HIV and STI counseling session during which confidential HIV testing was offered using rapid tests. Participants underwent a clinical examination performed by a trained male nurse during which their clinical MC status (presence or absence of foreskin) was assessed. Participants with symptomatic STIs were treated free of charge at the study site or at local health facilities according to the national STI syndromic management treatment guidelines. Individuals testing HIV positive were offered an immediate CD4 count at the study site. Antiretroviral (ARV) treatment was arranged, in collaboration with the health facilities in the community as per national guidelines. The household response rate was 3,258/3,390 (96.1%), the individual response was 2,000/2,383 (83.9%), and the combined response rate was 80.7%.
Follow-up Survey
This survey, designed to evaluate the Bophelo Pele project, was conducted between October 2010 and June 2011, independently from the baseline survey, 3 y after the beginning of the roll-out. For this survey, a first random sample of households was selected from Statistics South Africa Enumerator Area aerial photographs. All men aged 15 to 49, who had slept in the selected households the night before the investigative team's visit, were eligible for inclusion. Voluntary, written informed consent was required, in addition to parental consent for those aged under 18. Each participant was interviewed at the study site using an anonymous structured standardized questionnaire adapted from an instrument designed by UNAIDS [14]. Participants were encouraged to undergo HIV testing, which was provided at the study site using rapid tests. Participants with symptomatic STIs were treated free of charge at the study site or at local health facilities according to the national STI syndromic management treatment guidelines. Individuals testing HIV positive were offered an immediate CD4 count at the study site. ARV treatment was arranged, in collaboration with the health facilities in the community as per national guidelines. A second random sample of households was selected and volunteers underwent the same procedures, except that only men aged 18 to 33 were included. A total of 3,338 volunteers aged 15 to 49 were recruited. The household response rate was 7,701/8,022 (96.0%), the individual response was 3,334/4,021 (82.9%), and the combined response rate was 79.6%.
Laboratory Procedures
Each participant was invited to supply a venous blood sample (8 ml) for HIV testing. Samples were collected in plasma preparation tubes and centrifuged. Samples were tested within 6 months following collection. A screening test (GenscreenTM HIV1/2 version 2, Bio-Rad) was performed on all plasma samples. For reactive samples, a confirmatory test was run (VironostikaTM HIV Uni - Form II plus O, bioMérieux, Boxtel). If the sample reacted positively for both assays, a second confirmatory test was conducted (Murex HIV-1.2.O, Murex Biotech Ltd.). Plasma samples testing positive for HIV were also tested for the presence of ARV drugs currently in use in South Africa (lamivudine, stavudine, zidovudine, nevirapine, efavirenz, ritonavir, lopinavir, atazanavir, emtricitabine, tenofovir) using Ultra Performance Liquid Chromatography coupled with Tandem Mass Spectrometry according to a slightly modified previously published method [15].
In addition, plasma samples testing positive for HIV were also tested using the BED HIV incidence assay (HIV-1 Calypte Incidence BED EIA [BED]; Calypte Biomedical Corporation). Since the first detuned enzyme immunoassay to detect recent HIV seroconversion was described in 1,998 [16], there has been great interest in the application of laboratory methods to measure HIV incidence rates from cross-sectional samples [17]. Currently, the most widely used incidence assay is the BED HIV-1 Capture EIA (BED) assay [18]. The BED assay detects levels of anti-HIV IgG relative to total IgG and is based on the observation that the ratio of anti-HIV IgG to total IgG increases with time after HIV infection. If a confirmed HIV-1 positive specimen is reactive on the standard sensitive EIA and has a normalized optical density lower than a given cut-off value of the BED assay, it is considered recently infected. However, two of the current challenges in using HIV incidence assays to characterize HIV incidence rates are (a) knowledge of the BED window period, defined as the time interval following HIV infection during which individuals are characterized by the assay as recently infected, that is, the optical density is lower than the pre-set cut-off value, and (b) misclassifications. The main source of misclassifications is the number of HIV-infected persons falsely identified as recent seroconverters, which depends on the proportion of HIV-positive participants whose infection duration exceeds the BED window period. Different cut-off values and correction methods were used. See Text S1.
Outcomes
We assessed six outcomes of interest. From the clinical examination data, we calculated the adult MC prevalence rate. From the questionnaire data, we calculated two sexual behavior outcomes that could potentially change among those having undergone MC surgery: prevalence rate of men consistently using condoms with non-spousal partners in the last 12 months, and prevalence rate of men having two or more non-spousal partners in the last 12 months. From the serological data, we calculated the prevalence rate of ARV detection, the HIV prevalence rate, and the BED HIV incidence rate. The level of HIV among men was investigated by comparing these two latter rates between circumcised and uncircumcised men, and by calculating the increase in HIV prevalence rate that would have been expected without the VMMCs performed by the ANRS project.
Statistical Analyses
Covariates and standardization
The basic covariates were the following: age group in deciles, ethnic group (Sotho, Zulu, other), religion (Christian, no religion, other), having at least one child (yes, no), occupation (employed, unemployed, other), age at first sexual intercourse (age 15 or older, before age 15), alcohol consumption (less than once a week, once a week or more), education level (not at school and grade 12 not completed, at school and grade 12 not completed, grade 12 completed), and ever having been married (yes, no). Other sexual behavior covariates were number of lifetime partners, coded as a continuous variable and as a discrete variable (less than 5, 5 or more), number of non-spousal partnerships in the past 12 months (less than 2, 2 or more), consistent condom use with non-spousal partners in the last 12 months (yes, no). Results were standardized on the age-structure observed in 2010 using weighting coefficients calculated for each age group.
Baseline MC prevalence rate
In the baseline survey, circumcised men were either circumcised during the RCT or in another context. We calculated the MC prevalence rate which would have been expected in 2007–2008 if the men who were circumcised during the RCT had remained uncircumcised. We called this rate the baseline MC prevalence rate. We assumed that this proportion would have remained constant over time in the period 2008–2011 without the ANRS project.
MC uptake
We defined MC uptake as the proportion of the population that was circumcised at the time of the follow-up survey when excluding the proportion that were not circumcised in the ANRS project context (baseline MC prevalence rate).
BED HIV incidence rates
To calculate BED HIV incidence rates, we used the follow-up survey data and cut-off values of 0.80 and 1.51, which correspond in this population to BED window periods of about 6 and 12 months, respectively [19], with and without correction for misclassifications. Two correction methods were used [20],[21]. In these calculations, we excluded those testing positive for at least one ARV, because they were not likely to have been infected with HIV within the preceding 12 months.
Risk factor analysis of MC status
Using the follow-up survey data, we analyzed the association of background and sexual behavior characteristics with MC status by estimating prevalence rate ratios (PRR) using bivariate and multivariate general linear models (log-binomial and Poisson regression).
Comparison of outcomes between circumcised and uncircumcised men
To compare HIV prevalence and incidence rates between circumcised and uncircumcised men, we calculated HIV PRR and HIV incidence rate ratios (IRR) using general linear models and the follow-up survey data. As participants were not randomized, we observed that men accepting the intervention had different risk profiles than those who remained uncircumcised. To take into account this selection bias, these regressions were weighted by the inverse of the estimation of the propensity score [22] to obtain weighted PRR (wPRR) and IRR (wIRR). Each circumcised participant was weighted by the inverse of the propensity score, and each uncircumcised participant was weighted by the inverse of one minus the propensity score. This score is the probability of being circumcised and was estimated using a logistic regression from the following set of basic covariates: background characteristics and age at first sexual intercourse younger than 15 y. These basic covariates are not altered by the intervention and are potentially associated with MC status as well as HIV prevalence and incidence rates. To assess the association of possible sexual behavior changes with the intervention, we added the other sexual behavior characteristics to the regression model.
Association of VMMC roll-out with HIV levels
Using the follow-up survey data, we estimated what would have been the HIV prevalence rate among circumcised men if they had not been circumcised, by calculating the HIV prevalence rate among uncircumcised men after weighing each uncircumcised man by p/(1−p), with p being his propensity score [23]. This allowed us to calculate what the HIV prevalence and incidence rates would have been, averaged on all men in 2010–2011, assuming that the MC prevalence rate had remained at its baseline level.
Other details on the statistical analyses are provided in Text S1. Statistical analyses were performed using R version 2.14.1. All the confidence intervals are 95% intervals and were estimated along with p-values by bootstrap based on at least 2,000 replications.
Results
Baseline Data and MC Prevalence Rate
The baseline survey participants' background characteristics are described in Table 1. During the baseline survey, MC prevalence rate was 0.17 (95% CI 0.15–0.19). The baseline MC prevalence rate, corresponding, as described in the methods, to the MC prevalence rate expected if the men who were circumcised during the RCT had remained uncircumcised, was estimated to be 0.12 (95% CI 0.10–0.14).
Tab. 1. Characteristics of the sample surveyed in 2007–2008 (baseline survey). 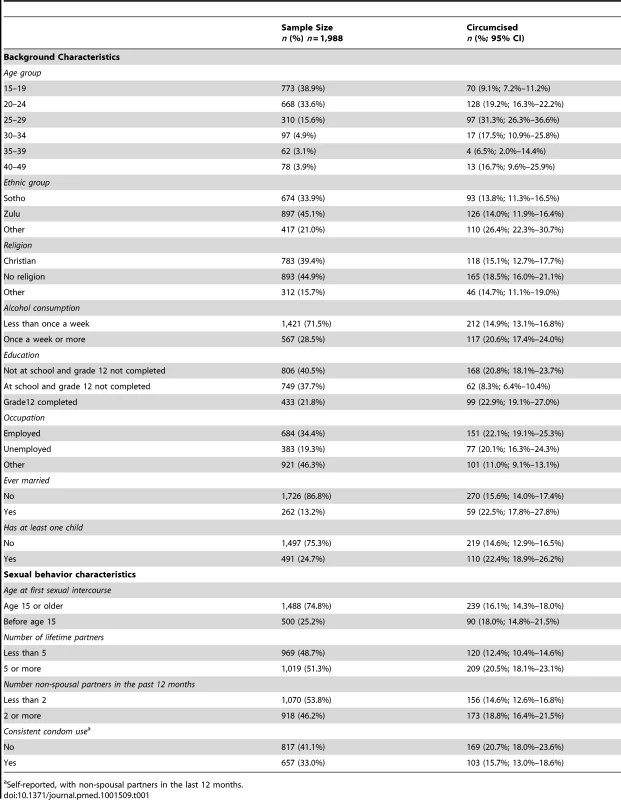
Self-reported, with non-spousal partners in the last 12 months. MC Prevalence Rate
In 2010–2011, at the time of the follow-up survey, MC prevalence rate was 1,771/3,338 (0.53; 95% CI 0.51–0.55) among adults. In the 15 - to 29-y age group, MC prevalence rate was 1,630/2,810 (0.58; 95% CI 0.56–0.60). Among men who had been circumcised during the roll-out, 817/892 (91.5%; 95% CI 89.8%–93.3%) reported having been circumcised by the ANRS-12126 project.
VMMC Uptake
In 2007–2008, baseline MC prevalence rate was 237/1,988 (0.12; 95% CI 0.10–0.14). From this estimate, we calculated that following the VMMCs performed during the ANRS project, the adult VMMC uptake among uncircumcised men was 46.7% (95% CI 44.3%–49.0%). VMMC uptake was 52.6% (95% CI 50.3%–54.7%) in the 15 - to 24-y age group. Figure 1 represents the MC prevalence rate by age group before and after the ANRS project.
Fig. 1. Male circumcision prevalence rates by age group before and after the ANRS project in the community of Orange Farm (South Africa). 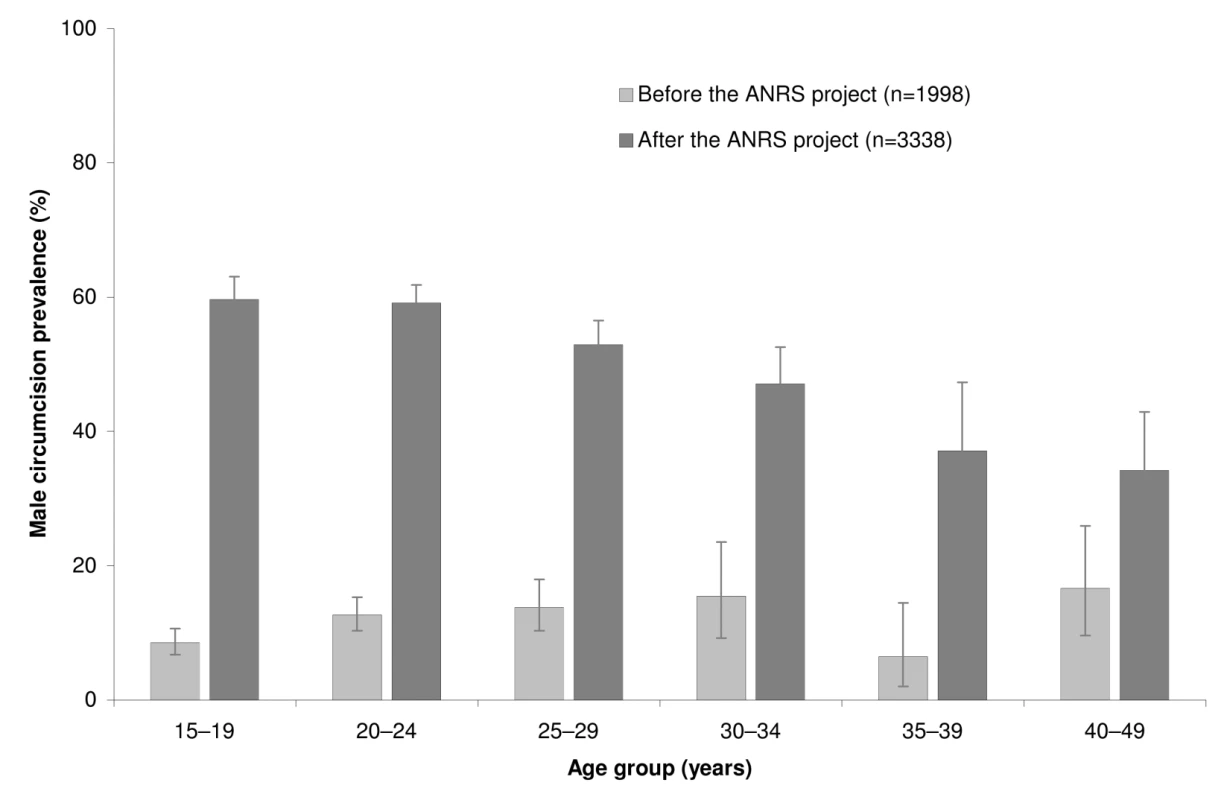
The error bars represent the 95% confidence intervals. Description of the Follow-up Survey Sample and Comparison by MC Status
The follow-up survey participants' background characteristics are described in Table 2. Among them, 169/3,338 (5.1%) had participated in the baseline survey. The age distribution of the Orange Farm male population is reported in Figure S1. We compared 1,848 circumcised men with 1,490 uncircumcised men. Circumcised men (mean age in years = 24.5; 95% CI 24.0–25.0) were younger (p<0.001) than uncircumcised men (mean age in years = 27.3; 95% CI 26.7–28.0). Their comparison in terms of background and sexual behavior characteristics is reported in Table 2. When controlling for age, circumcised men were less likely to be Zulu (a traditionally non-circumcising group), more likely to be in school, more educated, and less likely to be married. In the multivariate analysis, the same factors, apart from marital status, remained statistically significant.
Tab. 2. Characteristics of the sample surveyed in 2010–11 (follow-up survey) and association with male circumcision status. 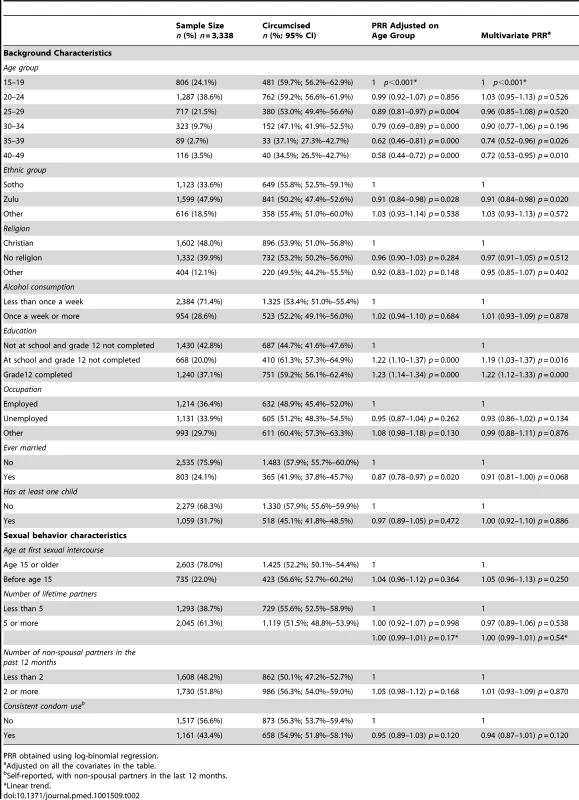
PRR obtained using log-binomial regression. Sexual Behavior and MC Status
No significant association between MC status and sexual behavior characteristics was identified after weighting. Among circumcised and uncircumcised men, the proportion consistently using condoms with non-spousal partners in the past 12 months was 615/1,399 (44.0%; 95% CI 41.7%–46.5%) versus 505/1,113 (45.4%; 95% CI 42.2%–48.6%) with wPRR = 0.94 (95% CI 0.85–1.03). The proportion having two or more non-spousal partners was 893/1,771 (50.4%; 95% CI 47.9%–52.9%) versus 692/1,567 (44.2%; 95% CI 41.3%–46.9%) with wPRR = 1.03 (95% CI 0.95–1.10). The details are provided in Table 3.
Tab. 3. Variations among men of key outcomes between the baseline and the follow-up survey, and by circumcision status in the follow-up survey. 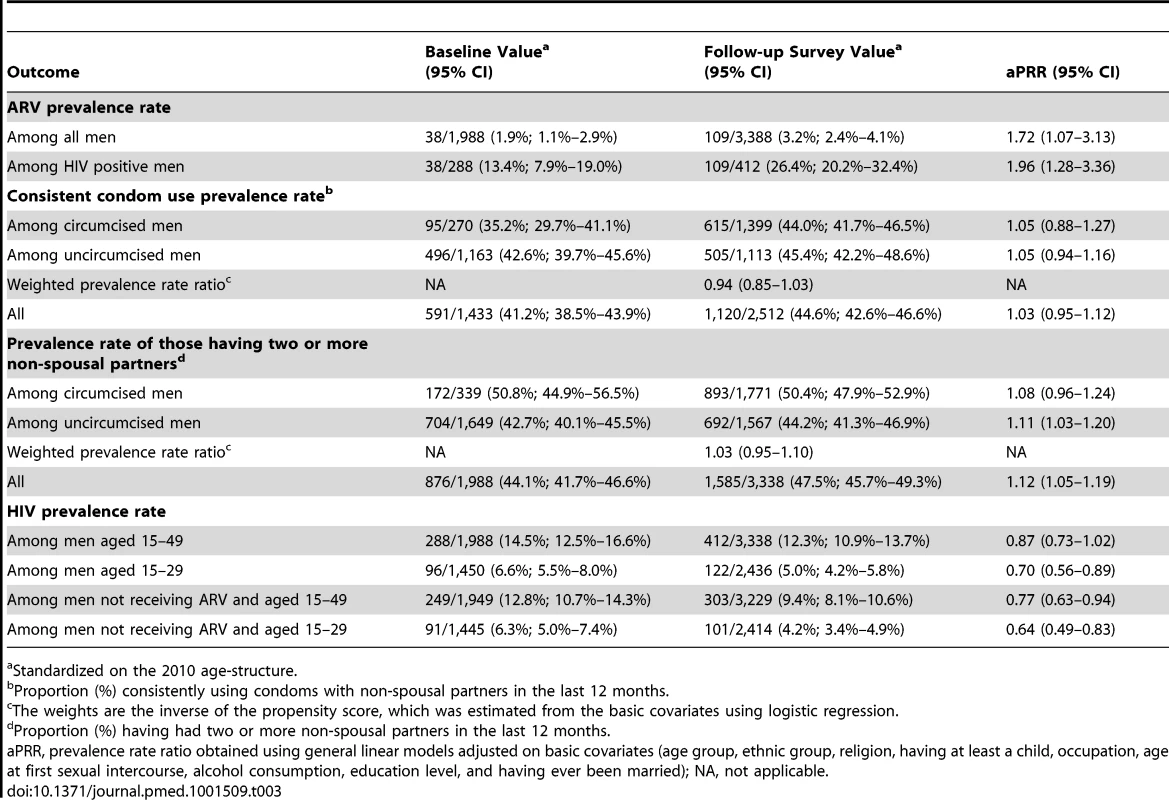
Standardized on the 2010 age-structure. HIV and ARV Prevalence Rate by MC Status at Follow-up
Overall HIV prevalence rate was 412/3,338 (0.12; 95% CI 0.11–0.14). ARV prevalence rate was 109/3,388 (0.032; 95% CI 0.024–0.041) and reached 109/412 (0.26; 95% CI 0.20–0.32) among those tested positive for HIV. The proportion of HIV positive men taking ARVs among circumcised and uncircumcised men was similar: 31/117 (26.8%) versus 77/295 (26.2%) with PRR adjusted on age = 1.08 95% CI 0.63–1.68; p = 0.74. Figure 2 illustrates HIV prevalence rate by age in 5-y age-group increments. HIV prevalence rate was 295/1,567 (0.19; 95% CI 0.16–0.21) among uncircumcised men and 117/1,771 (0.066; 95% CI 0.053–0.081) among circumcised men. As indicated in Table 4, a propensity analysis showed that HIV prevalence rate was lower among circumcised men, with wPRR = 0.52 (95% CI 0.41–0.67), corresponding to a reduction of 48% (95% CI 33%–59%). When controlling for the additional sexual behavior covariates, the wPRR was similar: 0.50 (95% CI 0.39–0.64).
Fig. 2. HIV prevalence rates by age group and circumcision status (n = 3,338). 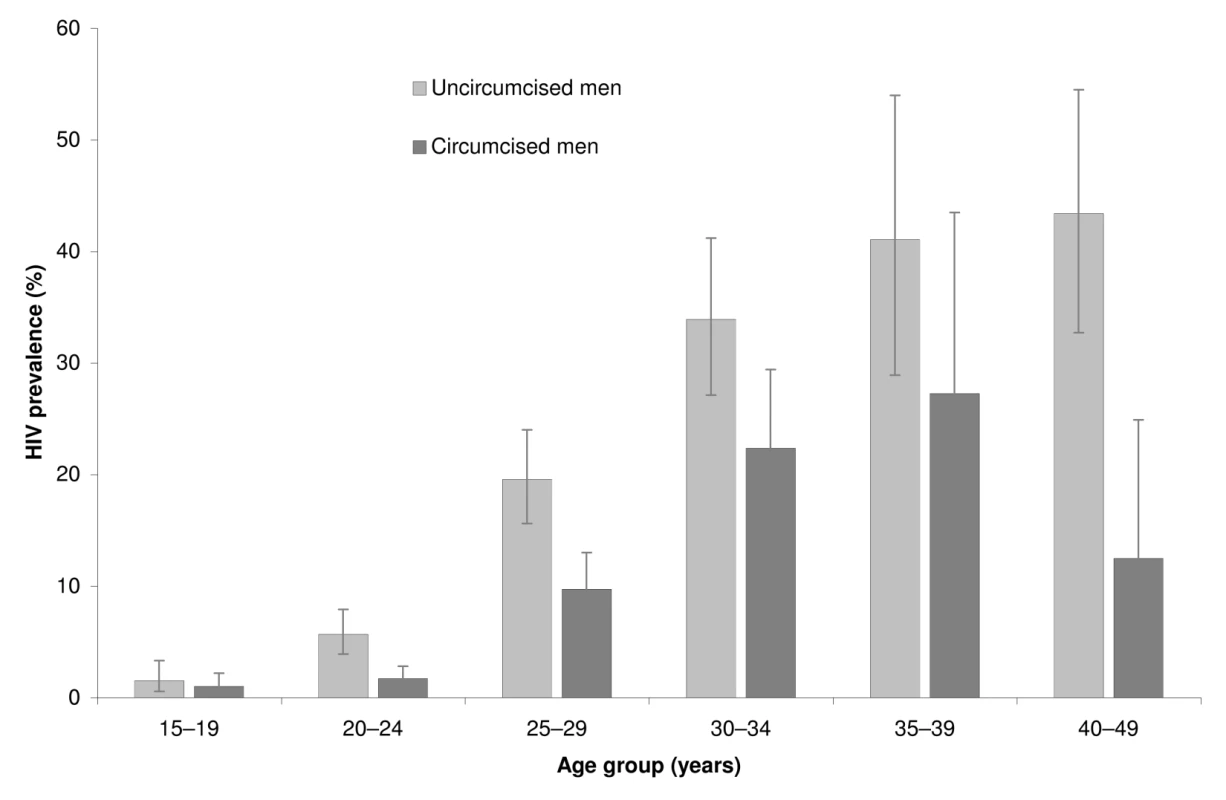
The error bars represent the 95% confidence intervals. Tab. 4. HIV prevalence among circumcised and uncircumcised men in the follow-up survey. 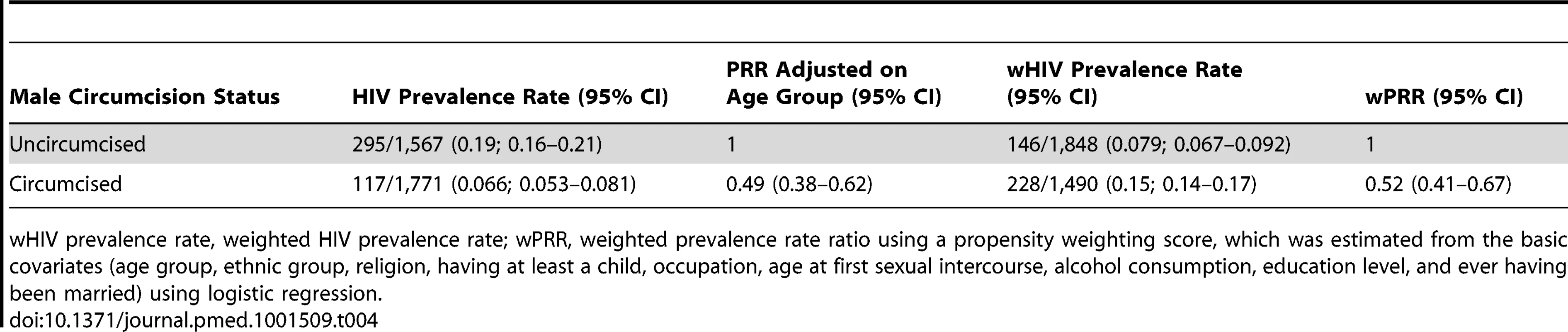
wHIV prevalence rate, weighted HIV prevalence rate; wPRR, weighted prevalence rate ratio using a propensity weighting score, which was estimated from the basic covariates (age group, ethnic group, religion, having at least a child, occupation, age at first sexual intercourse, alcohol consumption, education level, and ever having been married) using logistic regression. HIV Prevalence Rate by Intention to Become Circumcised at Baseline
Using the baseline data, among uncircumcised men, when controlling for basic covariates, we could not detect a difference in HIV prevalence rate among those intending to become circumcised in comparison to other men (adjusted PRR = 1.01; 95% CI 0.74–1.40).
Association of the ANRS Project VMMCs with HIV Prevalence Rate at Follow-up
Without the VMMCs performed during the ANRS project, HIV prevalence rate would have been 0.147 (95% CI 0.129–0.164) at the time of the follow-up survey instead of 0.123 (95% CI 0.109–0.138). It follows that without these VMMCs, HIV prevalence rate in 2010–2011 would have been 19% higher (95% CI 12%–26%) among men aged 15 to 49 y. It would have been 28% higher (95% CI 16%–42%) among men aged 15 to 29 y. Among men not receiving ARV, the relative increases would have been 22.0% (95% CI 13.9%–30.7%) for the 15 to 49 age group and 28.5% (95% CI 15.1%–43.0%) for the 15 to 29 age group.
BED HIV Incidence Rate at Follow-up
The results obtained when using the BED HIV incidence assay are provided in Table 5. This table shows that the estimations of wIRR between circumcised and uncircumcised men remained fairly stable when varying cut-off values and corrections methods, with estimated wIRRs ranging from 0.39 to 0.43, and 95% CI from a minimum lower boundary of 0.15 to a maximum upper boundary of 0.82. These values correspond to a reduction of BED HIV incidence rate ranging from 57%–61% with 95% confidence intervals of 29% to 76% and 14% to 83%, respectively. As shown in Table 5, these results were similar when controlling for sexual behavior characteristics. Other results are provided in Text S1.
Tab. 5. HIV incidence rates and rate ratios obtained in 2010–2011 with the BED incidence assay for selected cut-off values, with and without corrections for misclassifications. 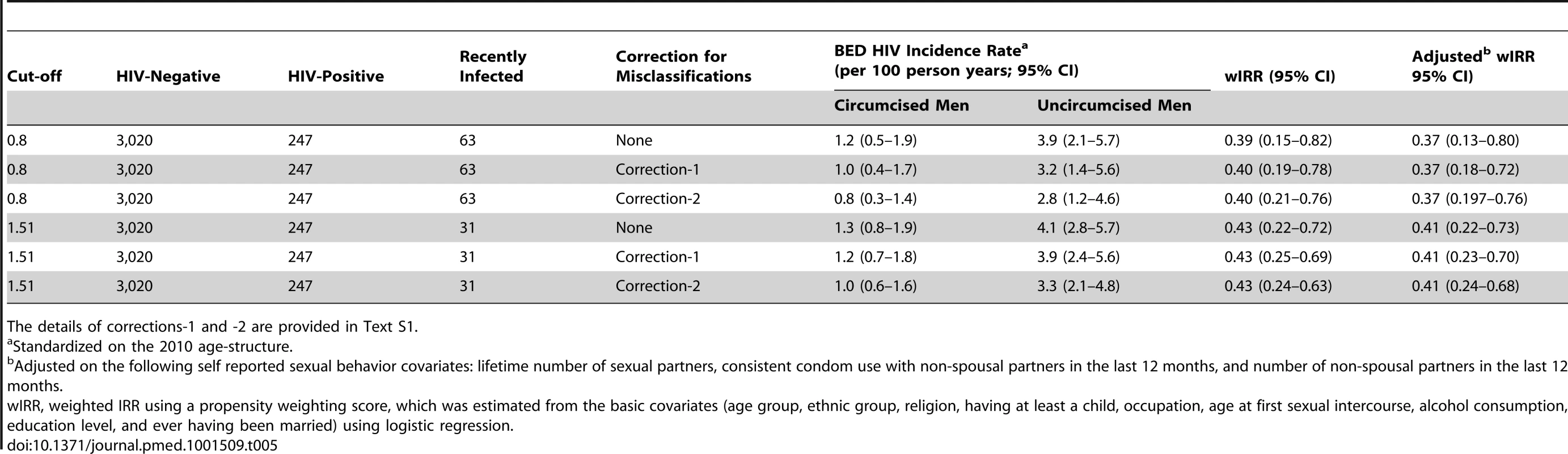
The details of corrections-1 and -2 are provided in Text S1. Variation over Time
Table 3 indicates that ARV prevalence rate increased over time. This table also indicates that no variation in consistent condom use with non-spousal partners was detected. In contrast, we detected a small increase over time of the number of non-spousal partners in the last 12 months. This table shows an overall decrease in HIV prevalence rate when excluding men on ARV, and among men aged 15 to 29.
Discussion
This study has shown that the roll-out of free VMMC can lead to a substantial uptake in just a few years, especially among young men, in an African community where MC is not a social norm. Furthermore, we could not detect any evidence of sexual behavior differences between circumcised and uncircumcised men. Lastly, the roll-out of VMMC in this community was associated with a reduction in the prevalence and incidence of HIV among circumcised men in comparison with uncircumcised men, and we estimated that without the project, HIV prevalence averaged on all adult men would have been significantly higher.
This effectiveness study has some limitations. Firstly, this study used a quasi-experimental design. It was not randomized and cannot prove a causal association. Secondly, HIV incidence was not measured in the context of a cohort study, which is the gold standard. We chose another design in order to be able to extrapolate HIV incidence among a random, cross sectional sample of men to the entire male population of the community. The possible selection bias associated with cohort studies followed up for many years may undermine extrapolating results from the sample analysis. Thirdly, we used an incidence assay to estimate IRRs, which has limitations, as described below. Lastly, the propensity score methods used can only reduce the selection bias associated with the observed covariates. We cannot exclude the possibility that there were also some unobserved confounding factors. However, the fact that those intending to become circumcised are not more aware of their HIV status [24] and not more infected with HIV, as found here, is reassuring. One additional limitation is that this study was conducted without the support of a national adult VMMC campaign. As a result, the VMMC uptake obtained may have been lower than what would have been observed if such campaign was in effect, since it would likely have reinforced our local communication campaign and encouraged VMMC uptake in the community.
Observing a lower HIV prevalence rate among circumcised men in comparison with uncircumcised men is not surprising. It is the natural consequence of (a) the established causal relationship between adult VMMC and the reduction of male HIV acquisition demonstrated in the three RCTs [2]–[4] and (b) the absence of differences in sexual behavior between circumcised and uncircumcised men, which could have reduced, if not annulled, the protective effect of MC. The reduced HIV incidence rate among circumcised men caused a reduction in HIV prevalence rate.
The absence of a statistical association between MC status and sexual behavior is encouraging and suggests that the so-called “risk compensation” or “behavioral disinhibition” (i.e., increased risky sexual behavior following adult MC) is either too small to be detectable even in large samples, or simply does not exist. The association of MC with no or minor sexual behavior changes had already been suggested but only in the context of the three MC RCTs and post-trial follow-up studies [2],[25],[26] and is consistent with the findings of a study conducted in the Orange Farm community, which showed that willingness to become circumcised was not associated with sexual behavior characteristics [24].
The BED assay provides more or less precise estimations of HIV incidence rates because it is nearly impossible to know precisely the factor by which the assay overestimates or underestimates HIV incidence rates. However, estimations of HIV IRRs are likely to be more precise, especially when calculating ratios of incidence rates measured at the same time among subgroups of the same population having the same gender and of approximately the same age [19]. This was clearly observed in our study with a rather stable estimation of HIV IRRs obtained when varying cut-off values and types of correction. It is therefore not surprising that we found an association of MC with HIV incidence rates similar to what was observed in the RCT conducted in the same community and elsewhere.
Six years after the international recommendation to include adult VMMC as a complementary HIV prevention method among adults in sub-Saharan Africa, about three million adult VMMCs have been performed. This represents less than 10% of the 35 million adult VMMC that are needed to effectively reduce the spread of the epidemic [10]. Our experience in Orange Farm shows that an uptake of about 50% can be obtained in about 3 y. However, this is contingent upon the implementation of an intensive promotion campaign incorporating broader HIV prevention methods, community involvement and support, a dedicated project staff, and the availability of quality adult MC surgeries optimizing cost, time, and personnel to increase accessibility. The current campaign in Orange Farm has been conducted while funded by private institutions. The success of this intervention, and the fact that VMMC roll-out among adults is a short-term task, shows that the involvement of private structures should be encouraged.
This study pleads for the changing of norms and practices regarding MC in southern and eastern Africa, where some ethnic groups, such as the Zulus, were circumcised in the past and have only recently (at the beginning of the 19th century) abandoned this cultural practice for military reasons under the leadership of Dingiswayo [27]. One possibility to promote this change is to encourage neonatal MC, similarly to countries where MC is the norm, and where the procedure is performed at an early age, usually before puberty.
Because adult VMMC has been shown to reduce the acquisition not only of HIV but also of herpes simplex virus 2 (HSV-2) and human papillomavirus (HPV) [28]–[30], the next step should be to confirm that these results are reproduced in similar phase 4 studies. It will also be important to demonstrate that women, as expected, do indirectly benefit from the roll-out of adult VMMC through the reduction of their exposure to HIV, because of the overall reduction in HIV prevalence rate among men. This finding is especially important because there is a potential increase of male-to-female HIV transmission during the healing period following MC surgery if the abstinence period is not observed [31], even if it is unlikely that this effect will undermine the benefits of adult VMMC roll-out [32].
This study suggests that the roll-out of adult VMMC is associated with a reduction in HIV in a sub-Saharan community where MC is not a social norm. Along with studies demonstrating the acceptability of adult VMMC in traditionally non-circumcising communities in sub-Saharan Africa [5], it gives hope that the epidemic can be reduced in settings where most men are uncircumcised. However, the demonstration that VMMC roll-out can indirectly lead to a reduction of HIV acquisition among women and uncircumcised men needs to be undertaken. The main implication of this study is that the current roll-out of adult VMMC—endorsed by UNAIDS and WHO, and supported by international agencies such as PEPFAR, the Global Fund, and by donors like the Bill and Melinda Gates Foundation—should be accelerated.
Supporting Information
Zdroje
1. UNAIDS (2012) UNAIDS Report on the global AIDS epidemic. Geneva: WHO.
2. AuvertB, TaljaardD, LagardeE, Sobngwi-TambekouJ, SittaR, et al. (2005) Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2: e298 doi:10.1371/journal.pmed.0020298
3. BaileyRC, MosesS, ParkerCB, AgotK, MacleanI, et al. (2007) Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369 : 643–656.
4. GrayRH, KigoziG, SerwaddaD, MakumbiF, WatyaS, et al. (2007) Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369 : 657–666.
5. WestercampN, BaileyRC (2006) Acceptability of male circumcision for prevention of HIV/AIDS in Sub-Saharan Africa: a review. AIDS Behav 11 : 341–355.
6. NagelkerkeNJ, MosesS, de VlasSJ, BaileyRC (2007) Modelling the public health impact of male circumcision for HIV prevention in high prevalence areas in Africa. BMC Infect Dis 7 : 16.
7. UNAIDS-WHO-SACEMA (2009) Expert group on modelling the impact and cost of male circumcision for HIV prevention: male circumcision for HIV prevention in high HIV prevalence settings: what can mathematical modelling contribute to informed decision making? PLoS Med 6: e1000109 doi:10.1371/journal.pmed.1000109
8. WhiteRG, GlynnJR, OrrothKK, FreemanEE, BakkerR, et al. (2008) Male circumcision for HIV prevention in sub-Saharan Africa: who, what and when? AIDS 22 : 1841–1850.
9. WilliamsBG, Lloyd-SmithJO, GouwsE, HankinsC, GetzWM, et al. (2006) The potential impact of male circumcision on HIV in Sub-Saharan Africa. PLoS Med 3: e262 doi:10.1371/journal.pmed.0030262
10. AuvertB, MarseilleE, KorenrompEL, Lloyd-SmithJ, SittaR, et al. (2008) Estimating the resources needed and savings anticipated from roll-out of adult male circumcision in Sub-Saharan Africa. PLoS One 3: e2679 doi:10.1371/journal.pone.0002679
11. (2011) The 2010 National antenatal sentinel HIV and syphilis prevalence survey in South Africa. Department of Health, Pretoria, South Africa. 50–53 p.
12. LissoubaP, TaljaardD, RechD, DoyleS, ShabanguD, et al. (2010) A model for the roll-out of comprehensive adult male circumcision services in African low-income settings of high HIV incidence: the ANRS 12126 Bophelo Pele Project. PLoS Med 7: e1000309 doi:10.1371/journal.pmed.1000309
13. WHO-UNAIDS-JHPIEGO (2008) Manual for male circumcision under local anaesthesia. Version 2.5C. Geneva: WHO-UNAIDS.
14. UNAIDS (1998) Looking deeper into the HIV epidemic: a questionnaire for tracing sexual networks. In Best practice collection, Key materiel 98/27. Geneva: UNAIDS. 1–24.
15. JungBH, RezkNL, BridgesAS, CorbettAH, KashubaAD (2007) Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 21 : 1095–1104.
16. JanssenRS, SattenGA, StramerSL, RawalBD, O'BrienTR, et al. (1998) New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280 : 42–48.
17. GuyR, GoldJ, CallejaJM, KimAA, ParekhB, et al. (2009) Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect Dis 9 : 747–759.
18. ParekhBS, KennedyMS, DobbsT, PauCP, ByersR, et al. (2002) Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses 18 : 295–307.
19. FiammaA, LissoubaP, OliverA, SinghB, LaeyendeckerO, et al. (2010) Can HIV incidence testing be used for evaluating HIV intervention programs? A reanalysis of the Orange Farm male circumcision trial. BMC Infectious Diseases 10 : 137.
20. McWalterTA, WelteA (2009) A comparison of biomarker based incidence estimators. PLoS One 4: e7368 doi:10.1371/journal.pone.0007368
21. McDougalJS, ParekhBS, PetersonML, BransonBM, DobbsT, et al. (2006) Comparison of HIV type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res Hum Retroviruses 22 : 945–952.
22. RosenbaumPR, RubinDB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70 : 41–55.
23. SatoT, MatsuyamaY (2003) Marginal structural models as a tool for standardization. Epidemiology 14 : 680–686.
24. LissoubaP, TaljaardD, RechD, Dermaux-MsimangV, LegeaiC, et al. (2011) Adult male circumcision as an intervention against HIV: An operational study of uptake in a South African community (ANRS 12126). BMC Infect Dis 11 : 253.
25. AgotKE, KiarieJN, NguyenHQ, OdhiamboJO, OnyangoTM, et al. (2007) Male circumcision in Siaya and Bondo Districts, Kenya: prospective cohort study to assess behavioral disinhibition following circumcision. J Acquir Immune Defic Syndr 44 : 66–70.
26. MattsonCL, CampbellRT, BaileyRC, AgotK, Ndinya-AcholaJO, et al. (2008) Risk compensation is not associated with male circumcision in Kisumu, Kenya: a multi-faceted assessment of men enrolled in a randomized controlled trial. PLoS ONE 3: e2443 doi:10.1371/journal.pone.0002443
27. Ngwane Z (1997) Zulu. Bond G, editor. New-York: Rosen Publishing Group. pp. 38.
28. MahianeSG, LegeaiC, TaljaardD, LatoucheA, PurenA, et al. (2009) Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. AIDS 23 : 377–383.
29. AuvertB, Sobngwi-TambekouJ, CutlerE, NieuwoudtM, LissoubaP, et al. (2009) Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: Results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis 199 : 14–19.
30. TobianAA, SerwaddaD, QuinnTC, KigoziG, GravittPE, et al. (2009) Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 360 : 1298–1309.
31. WawerMJ, MakumbiF, KigoziG, SerwaddaD, WatyaS, et al. (2009) Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet 374 : 229–237.
32. HallettTB, SinghK, SmithJA, WhiteRG, Abu-RaddadLJ, et al. (2008) Understanding the impact of male circumcision interventions on the spread of HIV in southern Africa. PLoS One 3: e2212 doi:10.1371/journal.pone.0002212
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 9- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa
- Translating Cochrane Reviews to Ensure that Healthcare Decision-Making is Informed by High-Quality Research Evidence
- Physician Emigration from Sub-Saharan Africa to the United States: Analysis of the 2011 AMA Physician Masterfile
- Acupuncture and Counselling for Depression in Primary Care: A Randomised Controlled Trial
- Serotype-Specific Changes in Invasive Pneumococcal Disease after Pneumococcal Conjugate Vaccine Introduction: A Pooled Analysis of Multiple Surveillance Sites
- Risk Factors and Outcomes for Late Presentation for HIV-Positive Persons in Europe: Results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE)
- Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis
- Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China
- Feasibility of Mass Vaccination Campaign with Oral Cholera Vaccines in Response to an Outbreak in Guinea
- Preconception Care in Low- and Middle-Income Countries: New Opportunities and a New Metric
- How to Stir Up Trouble…while Riding a Rollercoaster
- Health Workforce Brain Drain: From Denouncing the Challenge to Solving the Problem
- Association of the ANRS-12126 Male Circumcision Project with HIV Levels among Men in a South African Township: Evaluation of Effectiveness using Cross-sectional Surveys
- Setting Research Priorities for Preconception Care in Low- and Middle-Income Countries: Aiming to Reduce Maternal and Child Mortality and Morbidity
- Transnational Tobacco Company Interests in Smokeless Tobacco in Europe: Analysis of Internal Industry Documents and Contemporary Industry Materials
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmarket Surveillance of Medical Devices: A Comparison of Strategies in the US, EU, Japan, and China
- Translating Cochrane Reviews to Ensure that Healthcare Decision-Making is Informed by High-Quality Research Evidence
- Physician Emigration from Sub-Saharan Africa to the United States: Analysis of the 2011 AMA Physician Masterfile
- Current and Former Smoking and Risk for Venous Thromboembolism: A Systematic Review and Meta-Analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



