-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaChallenges for HIV Pre-Exposure Prophylaxis among Men Who Have Sex with Men in the United States
article has not abstract
Published in the journal: . PLoS Med 9(8): e32767. doi:10.1371/journal.pmed.1001286
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.1001286Summary
article has not abstract
Summary Points
-
Pre-exposure prophylaxis (PrEP) with anti-retroviral (ARV) medications is partially efficacious for preventing HIV infection among men who have sex with men (MSM) and heterosexuals.
-
As PrEP becomes available and prescribed for use among MSM a better understanding of willingness to use PrEP and avoidance of condom use are needed so that behavioral programs and counseling may be enhanced for maximum benefit.
-
Targeted messaging will be needed about ARV prophylaxis for various at risk populations, but the general message should be that condoms continue to be the most effective way to prevent HIV transmission through sex and that PrEP is an additional biomedical intervention.
-
As new effective biomedical intervention methods, such as PrEP, become available language about “protected” and “unprotected” sex, which used to exclusively mean condom use, will need to adapt.
Pre-exposure prophylaxis (PrEP) to prevent HIV infection with anti-retroviral (ARV) medications was found to be partially efficacious among men who have sex with men (MSM) [1] and heterosexuals [2],[3]. Other studies have provided information about potential uptake of PrEP among MSM, including factors associated with use and sharing of HIV medications before [4] and after [5] ARV efficacy was known. In a study of high-risk, substance-using MSM in four United States cities conducted prior to the release of efficacy trial results, black and Latino (versus white) MSM were more willing to use a less effective PrEP product in order to avoid condom use [6]; further, high-risk MSM with less education reported more non-prescribed, pre-efficacy ARV use (by HIV-negative men) and sharing of ARVs with sex partners (by HIV-positive men) to prevent HIV infection [4]. In an Internet study of US MSM immediately following release of the efficacy trial results among MSM, black and Latino (versus white) MSM were more willing to use PrEP after efficacy was known [5].
Colleagues [7]–[9] have identified important challenges relating to the implementation of PrEP, including specialist and generalist physician willingness to prescribe PrEP based on trial results [10]. These issues will become even more prominent due to the recent recommendation of the US Food and Drug Administration (FDA) Advisory Committee to approve emtricitabine/tenofovir disoproxil fumarate (TDF/FTC or Truvada) for use as PrEP among sexually active adult men and women. This essay addresses PrEP implementation challenges for MSM and their communities in the US.
Challenges
PrEP implementation among MSM poses several challenges, including (a) understanding of PrEP use and use preferences; (b) PrEP implementation costs to individuals and the health care system, and the associated epidemiological impact; (c) effective messaging about PrEP to various MSM-related audiences; and (d) implications of PrEP on the dialogue and language for research and PrEP use in practice.
Understanding PrEP Use and Preferences among MSM
Prior to known efficacy, ARV use and sharing for HIV prophylaxis among MSM was minor [11]. In a large sample of HIV-negative substance-using MSM [4] in four US cities (Chicago, Los Angeles, New York, San Diego), a group at substantial HIV risk, only 2% reported PrEP and 4% post-exposure prophylaxis (PEP) use at a time preceding known efficacy of ARV use for prophylaxis of HIV infection. Among HIV-positive MSM in the study, 2.5% and 4% reported sharing ARV drugs with partners for PrEP and PEP, respectively. In a separate analysis of the data and focusing on self-reported efficacy level needed in order to forego condom use, substantial proportions of the HIV-negative men [6] were willing to have anal sex without a condom while taking PrEP (28% for receptive and 51% for insertive), given an efficacy level at or below the range of efficacy trial findings among MSM (i.e., 44% efficacy overall for oral PrEP, up to a 73% efficacy with 90% adherence in the iPrEX trial [1]). In an Internet study of US MSM conducted in early 2011, soon after efficacy trial results among MSM were announced [5], 83% of the HIV-negative men reported that they were likely to use an oral PrEP product at 44% efficacy, the overall efficacy level found in the trial [1]. The demand for PrEP could be relatively high, depending on access, eligibility, and cost coverage. Racial/ethnic minority MSM may be particularly willing to use PrEP, and could especially benefit from its use, given extremely high HIV prevalence and incidence levels among black and Latino MSM populations [9]. As ARVs become increasingly available by prescription for HIV-negative and HIV-positive MSM, non-prescribed use of ARVs by HIV-negative men and sharing by men with HIV may increase.
More surveillance and assessment is needed to monitor and better understand, among other issues, reasons for differential willingness to use PrEP and avoidance of condom use among MSM—so that behavioral programs and counseling may be enhanced for maximum benefit for both ARV prophylaxis and condom use. Ongoing community-level behavioral and clinical research is needed to determine the prevalence and effectiveness of intermittent dosing; behavioral risk substitution for condom use, including use of seroadaptive behaviors (e.g., HIV serosorting, behavioral positioning as a lower risk insertive versus receptive partner); preferences about PrEP formulation (e.g., oral, topical); provider knowledge, perceptions, and clinical behavior; and PrEP costs and access through different service provision settings.
PrEP should be prescribed and clinically monitored by a health care professional to ensure appropriate use and effectiveness ([12]; US Centers for Disease Control and Prevention [CDC] full guidelines forthcoming), which includes medication adherence and regular HIV testing. Trial data indicate that good adherence is critical for high PrEP efficacy [1] and innovative approaches to promote PrEP adherence that are easily implemented in clinical and community settings are needed [13], including alternatives to oral administration of medication (e.g., rectal gel). Concerns about acquisition and transmission of drug-resistant HIV strains have also been raised [13], particularly if PrEP is being applied after HIV infection but before detectable infection, when a more rigorous ARV treatment should be employed. Further, monitoring is needed to capture both prescribed and non-prescribed use of ARV for HIV prophylaxis to better understand community-level utilization of PrEP and PEP, and to address the clinical, behavioral, and epidemiological implications of ARV use to prevent HIV transmission in MSM populations.
PrEP Costs and the Related Epidemiological Impact
Issues of cost to the individual and the health care system are central to PrEP implementation [14],[15]. There continue to be waiting lists for ARV treatment in some communities due to limited funding; large segments of HIV-negative persons will not have insurance or the direct payment capability to pay for PrEP. Drug manufacturers, insurance companies, and federal agencies must work to close gaps in access, particularly among resource-poor MSM with limited access to HIV prevention and health care.
ARV coverage for HIV-negative and -positive individuals has serious implications for HIV epidemiology. Mathematical models generally focus on efficacy, coverage, and inadvertent taking of PrEP by newly HIV-infected persons before diagnosis, and have demonstrated that ARV resistance could be a concern if PrEP is not monitored for HIV infection systematically [14],[15]. Others have assessed potential cost-effectiveness of PrEP among high-risk groups such as MSM [16]; however, more cost analysis and epidemiological modeling is needed to determine implementation scenarios for optimal health benefit.
PrEP Information and Messaging for Various MSM-Related Audiences
Targeted educational messages will be needed about ARV prophylaxis for various populations (Table 1). The general message should be that condoms continue to be the most effective way to prevent HIV transmission and acquisition through sex and that PrEP is an additional biomedical intervention that can provide protection from HIV infection. Multi-level prevention messages will need to be developed in order to maximize overall protective effects addressing key audiences of HIV-negative MSM highly affected by HIV (e.g., black and Latino MSM), HIV-negative MSM not using other prevention methods (e.g., condoms), HIV-positive MSM on ARVs (because they might consider sharing ARVs), and health care providers. For MSM and their providers, clear information and guidelines are needed about PrEP efficacy, risks of side effects and other PrEP use complications, the need for ongoing HIV testing and PrEP monitoring by providers, and the importance of adherence to prescribed PrEP [7]–[9]. Critical distinctions between PrEP and PEP and the appropriate use of each should also be addressed. At broader MSM community levels and for providers, information regarding for whom PrEP is most suitable should be provided. Specific PrEP guidelines are being developed by CDC and interim guidelines currently exist [10]; however, determination of the appropriateness of PrEP will ultimately be a mutual decision made between the patient and provider. For all of the audiences noted above, information on the dangers of sharing PrEP with others should address the risk of medication ineffectiveness and complications when PrEP is not directly prescribed and monitored by a qualified health care provider [7],[8]. Lastly, nonjudgmental support from providers is needed for HIV-negative MSM on PrEP, and provider services to promote adherence and appropriate monitoring are needed for all MSM receiving ARVs.
Tab. 1. Types of targeted PrEP messaging needed for MSM and health care providers by subgroup. 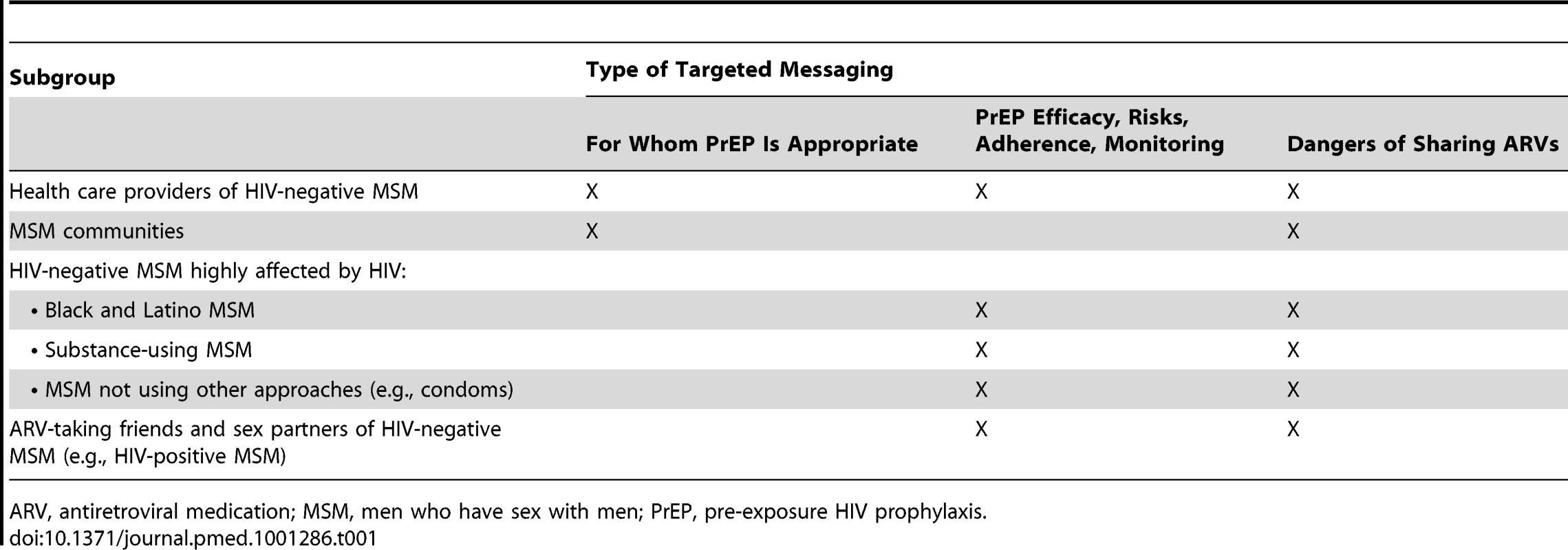
ARV, antiretroviral medication; MSM, men who have sex with men; PrEP, pre-exposure HIV prophylaxis. Dialogue and Language Issues Associated with PrEP
As we transition from an era of one effective biomedical intervention method (i.e., condoms) to an era of multiple efficacious interventions (i.e., condoms, ARV prophylaxis, and ARV treatment as prevention [17]), language about “protected” and “unprotected” sex will need to adapt. Protection from HIV infection previously meant condom use; however, protection in the future could also include oral PrEP, PEP, topical agents, or other products. This is also an opportune time to have a more explicit discussion about the efficacy and effectiveness of both methods. The research literature suggests that condoms are generally in the range of 80%–87% effective in reducing HIV transmission in studies of vaginal use among heterosexuals [14],[15]. Given efficacy trial results among MSM, PrEP can be 44% efficacious among MSM and 73% efficacious with high (90%) adherence. However, other host and partner factors are critically important in the equation (e.g., viral load, immune system functioning, number and intensity of exposures, severity of PrEP side effects). Taken together, condom use continues to be strongly recommended for the prevention of sexual transmission of HIV for all MSM, and PrEP may provide additional protection for some very high-risk MSM, determined jointly by MSM and their health care providers. However, when researchers and practitioners discuss protective action and its effect on HIV transmission, we must be clear in assessing condom use, and PrEP uptake and adherence. For example, MSM studies will need to assess separately for sexual event-level condom use and adherence and PrEP use and adherence, and include or control for each other in analysis.
Language becomes complicated when capturing PrEP and PEP—and the potential use of both by some men—in practice. Taking ARVs for prophylaxis before sexual exposure is considered PrEP. However, if a high-risk HIV-negative person who has recently engaged in sexual risk behavior is prescribed and is adherent to PrEP, but still becomes infected with HIV, PrEP becomes a suboptimal form of treatment. Over time, success of ARV prophylaxis may be due to administration before and/or after sexual exposures. The choice of approach—PrEP as a continuous approach, or PEP as an episodic approach—is contingent upon close monitoring and open discussions between providers and patients. As PrEP and PEP use become more common in the future, distinguishing between PrEP and PEP could become complex for individuals, and thus for research based on behavioral self-report. Detailed assessments will be needed to measure the complexity of ARV use over time.
Next Steps
Although PrEP will likely be inaccessible to many US MSM in the near future because of prohibitive cost to individuals and the health care system, some HIV-negative MSM may be prescribed ARVs and other MSM may inappropriately obtain ARVs from friends or sex partners to prevent HIV infection. Over time, access to PrEP could become a reality for many MSM, particularly in high-income countries, as availability increases and costs decrease. Given the emergence of this prophylaxis option, public health officials and providers are challenged to address community-level monitoring of prescribed and non-prescribed PrEP use in addition to condom use; develop effective multiple messages about condom use and PrEP for MSM and for their health care providers; disseminate information about the hazards of sharing ARV medications; and develop language about HIV prevention and risk reduction that has historically focused almost exclusively on condom use for sexually active MSM. As other efficacious HIV prevention interventions become available (e.g., topical antiviral products) [17], lessons learned from PrEP implementation can be applied to roll out of those approaches as well.
Zdroje
1. GrantRM, LamaJR, AndersonPL, McMahanV, LiuAY, et al. (2010) Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Eng J Med 363 : 2587–2599.
2. Thigpen MC, Kebaabetswe PM, Smith DK, Segolodi TM, Soud FA, et al.. (2011) Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study [abstract WELBC01]. IAS Conference on HIV Pathogenesis,Treatment, and Prevention; July 2011; Rome, Italy.
3. University of Washington, International Clinical Research Center (2011) Pivotal study finds that HIV medications are highly effective as prophylaxis against HIV infection in men and women in Africa [press release]. 13 July 2011. Available: http://depts.washington.edu/uwicrc/research/studies/files/PrEP_PressRelease-UW_13Jul2011.pdf. Accessed 1 March 2012.
4. ManserghG, KoblinBA, ColfaxGN, McKirnanDJ, FloresSA, et al. (2010) Preefficacy use and sharing of antiretroviral medications to prevent sexually-transmitted HIV infection among US men who have sex with men. J Acquir Immune Defic Syndr 55 (2) e14–e16.
5. Sullivan P, Liu A, Fuchs J, Irby R, Tarver R, et al.. (2011) Awareness of and intention to use PrEP: a post-iPrEX survey of US MSM. National HIV Prevention Conference; August 2011; Atlanta.
6. KoblinBA, ManserghG, FryeV, TieuHV, HooverDR, et al. (2011) Condom use decision making in the context of hypothetical pre-exposure prophylaxis efficacy among substance-using men who have sex with men: Project MIX. J Acquir Immune Defic Syndr 58 (3) 319–327.
7. BuchbinderSP, LiuA (2011) Pre-exposure prophylaxis and the promise of combination prevention approaches. AIDS Behav Supp 1: S72–S79.
8. MyersGM, MayerKH (2011) Oral preexposure anti-HIV prophylaxis for high-risk U.S. populations: current considerations in light of new findings. AIDS Patient Care STDs 25 : 63–71.
9. UnderhillK, OperarioD, MimiagaMJ, SkeerMR, MayerKH (2010) Implementation science of pre-exposure prophylaxis: preparing for public use. Curr HIV/AIDS Rep 7 : 210–219.
10. Mayer K, Mimiaga M, White J, Krakower D Vanderwarker R (2011) Knowledge, attitudes, and beliefs of Massachusetts physicians regarding topical and oral antiretroviral chemoprophylaxis. Conference on Retroviral and Opportunistic Infections; February 2011; Boston. Session 195, abstract 1000.
11. MimiagaMJ, CaseP, JohnsonCV, SafrenSA, MayerKH (2009) Preexposure antiretroviral prophylaxis attitudes in high-risk Boston area men who report having sex with men: limited knowledge and experience but potential for increased utilization after education. J Acquir Immune Defic Syndr 50 : 77–83.
12. US Centers for Disease Control and Prevention (2011) Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. Morbid Mortal Weekly Rep 60 : 65–68 Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6003a1.htm?s_cid=mm6003a1_w. Accessed 1 March 2012.
13. AmicoKR (2011) Standard of care for antiretroviral therapy adherence and retention in care from the perspective of care providers attending the 5th International Conference on HIV Treatment Adherence. J Int Assoc Physicians AIDS Care (Chic) 10 (5) 291–296.
14. AbbasUL, HoodG, WetzelAW, MellorsJW (2011) Factors influencing the emergence and spread of HIV drug resistance arising from rollout of antiretroviral pre-exposure prophylaxis (PrEP). PLoS ONE 6 (4) e18165 doi:10.1371/journal.pone.0018165.
15. SupervieV, BarrettM, KahnJS, MusakaG, MoetiTL, et al. (2011) Modeling dynamic interactions between PrEP interventions and treatment programs: predicting HIV transmission and resistance. Sci Rep 1 : 185.
16. PaltielAD, FreedbergKA, ScottCA, SchackmanBR, LosinaE, et al. (2009) HIV preexposure prophylaxis in the United States: Impact on lifetime infection risk, clinical outcomes, and cost effectiveness. Clin Infect Dis 48 : 806–815.
17. CohenMS, ChenYQ, McCauleyM, GambleT, HosseinipourMC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Eng J Med 365 (6) 493–505.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Multidrug Resistant Pulmonary Tuberculosis Treatment Regimens and Patient Outcomes: An Individual Patient Data Meta-analysis of 9,153 Patients
- Challenges for HIV Pre-Exposure Prophylaxis among Men Who Have Sex with Men in the United States
- What Is the Relationship of Medical Humanitarian Organisations with Mining and Other Extractive Industries?
- Conflict of Interest Reporting by Authors Involved in Promotion of Off-Label Drug Use: An Analysis of Journal Disclosures
- Child Mortality Estimation: A Global Overview of Infant and Child Mortality Age Patterns in Light of New Empirical Data
- What Is the Optimal First Line Antiretroviral Therapy in Resource-Limited Settings?
- Educating Enough Competent Health Professionals: Advancing Educational Innovation at Muhimbili University of Health and Allied Sciences, Tanzania
- Developing and Costing Local Strategies to Improve Maternal and Child Health: The Investment Case Framework
- Child Mortality Estimation: Methods Used to Adjust for Bias due to AIDS in Estimating Trends in Under-Five Mortality
- Child Mortality Estimation: Appropriate Time Periods for Child Mortality Estimates from Full Birth Histories
- Child Mortality Estimation: Accelerated Progress in Reducing Global Child Mortality, 1990–2010
- Why We Need Urban Health Equity Indicators: Integrating Science, Policy, and Community
- Legal Disputes over Duties to Disclose Treatment Risks to Patients: A Review of Negligence Claims and Complaints in Australia
- An Alternative Framework for Analyzing Financial Protection in Health
- Child Mortality Estimation: A Comparison of UN IGME and IHME Estimates of Levels and Trends in Under-Five Mortality Rates and Deaths
- The Role of HIV-Related Stigma in Utilization of Skilled Childbirth Services in Rural Kenya: A Prospective Mixed-Methods Study
- Neonatal Mortality Risk Associated with Preterm Birth in East Africa, Adjusted by Weight for Gestational Age: Individual Participant Level Meta-Analysis
- Efficacy and Safety of Three Antiretroviral Regimens for Initial Treatment of HIV-1: A Randomized Clinical Trial in Diverse Multinational Settings
- The Air That We Breathe: Addressing the Risks of Global Urbanization on Health
- Effects of Intensive Blood Pressure Lowering on Cardiovascular and Renal Outcomes: A Systematic Review and Meta-Analysis
- Child Mortality Estimation: Estimating Sex Differences in Childhood Mortality since the 1970s
- Feasibility, Yield, and Cost of Active Tuberculosis Case Finding Linked to a Mobile HIV Service in Cape Town, South Africa: A Cross-sectional Study
- Diagnosing Severe Falciparum Malaria in Parasitaemic African Children: A Prospective Evaluation of Plasma HRP2 Measurement
- Child Mortality Estimation: Consistency of Under-Five Mortality Rate Estimates Using Full Birth Histories and Summary Birth Histories
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Feasibility, Yield, and Cost of Active Tuberculosis Case Finding Linked to a Mobile HIV Service in Cape Town, South Africa: A Cross-sectional Study
- Multidrug Resistant Pulmonary Tuberculosis Treatment Regimens and Patient Outcomes: An Individual Patient Data Meta-analysis of 9,153 Patients
- Child Mortality Estimation: A Global Overview of Infant and Child Mortality Age Patterns in Light of New Empirical Data
- What Is the Optimal First Line Antiretroviral Therapy in Resource-Limited Settings?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



