-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEcology: A Prerequisite for Malaria Elimination and Eradication
article has not abstract
Published in the journal: . PLoS Med 7(8): e32767. doi:10.1371/journal.pmed.1000303
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1000303Summary
article has not abstract
Summary Points
-
Existing front-line vector control measures, such as insecticide-treated nets and residual sprays, cannot break the transmission cycle of Plasmodium falciparum in the most intensely endemic parts of Africa and the Pacific
-
The goal of malaria eradication will require urgent strategic investment into understanding the ecology and evolution of the mosquito vectors that transmit malaria
-
Priority areas will include understanding aspects of the mosquito life cycle beyond the blood feeding processes which directly mediate malaria transmission
-
Global commitment to malaria eradication necessitates a corresponding long-term commitment to vector ecology
Introduction
The Global Malaria Eradication Program, launched in the middle of the last century, over-promised and under-delivered [1]. Decades of pessimism followed, during which malariologists were afraid to even mention the goal of this program by name [2]. The term eradication was often nervously referred to as “the E-word” by a disillusioned community that had learned from bitter experience that optimistic forecasts [3] had been based on an oversimplified view of transmission ecology [4]. Eradication of malaria remains beyond our grasp today, but is nevertheless firmly back on the global health agenda as a long-term target [5].
Ecological Obstacles to Eradication with Existing Interventions
By definition, eradication of human malaria parasites globally [5] requires that intervention options are available that can eliminate transmission anywhere in the world. Leading vector control technologies such as insecticide-treated nets (ITNs) and indoor residual spraying (IRS) can suppress transmission by one or even two orders of magnitude [4],[6] and dramatically alleviate disease burden [7],[8]. Nevertheless, these measures alone are not sufficient to eliminate transmission in large tracts of tropical Africa where the entomological inoculation rate (EIR), the most direct measure of human exposure, can exceed a thousand infectious bites per person per year [9],[10]. Expressed in terms of the parasite's reproductive number, this means that if the local parasite population were entirely eliminated by mass drug administration, for example, a single infected person moving into the area could give rise to as many as ten thousand new infections and readily re-establish stable transmission [10]. Under such conditions, simulations predict that even 100% coverage of an entire population with ITNs exhibiting near-ideal properties will fail to push the EIR below the threshold required for local elimination [11]. Although massive benefits of increasing ITN and IRS coverage have been achieved in many parts of equatorial Africa, elimination has remained elusive except for regions on the edge of stable transmission in Kenya, Tanzania and The Gambia (e.g. [12]–[14]). Evidence from the previous malaria eradication drive [4],[15] and contemporary initiatives [8],[16],[17] indicate that transmission remains robust in areas where it has been historically high. We argue here that a failure to appreciate the biological complexities that allow vector populations to resist or evade interventions has substantially impeded control efforts. In particular, we identify seven ecologically imposed obstacles that have limited the effectiveness of vector control, and must be tackled in order to move from control to eradication (Box 1).
Box 1. Ecological Obstacles to Vector Control
(1) Variation in mosquito behaviour
All front-line vector control methods used in Africa today (e.g., ITNs, IRS) are based on the stereotyped view that vectors bite and rest primarily inside houses. This assumption is based on the early characterization of Anopheles gambiae and An. funestus behaviours of feeding and resting almost exclusively indoors [49]. However, even these endophilic species feed outside to some degree, and may do so increasingly in response to domestic interventions [21],[22]. Crucially, many other primary vectors do not conform to this traditional model and often bite outdoors [49] (e.g., An. arabiensis, which dominates transmission in much of Africa [50]). Variation in feeding behaviour within vector species may have a genetic basis [51], which raises the possibility that vector control measures could select for genotypes which are least likely to encounter the intervention. Even when vectors are highly endophilic [6],[29], the application of insecticides in and around houses has fundamental limitations, because exhaustive coverage of all resting sites with IRS [22], or all humans with ITNs [6] is not possible in practice.
(2) Insecticide resistance
The ability of vectors to evolve diverse resistance mechanisms to insecticides has been well documented [20]. Resistance to all major classes of insecticides used against malaria vectors has now been recorded in Africa [52]. Recent evidence from dengue mosquito vectors indicates that permethrin resistance can increase by more than 100-fold in vector populations within just 7–8 y [53]. The capacity of vectors to develop resistance so rapidly will undoubtedly pose a major obstacle to malaria control based exclusively on insecticides.
(3) Behavioural avoidance
The emergence of new vector behavioural phenotypes is a less-recognized phenomenon than insecticide resistance, but it has the potential to similarly diminish the effectiveness of current interventions. Documented examples of adaptable vector behaviours that could impact interventions such as ITNs and IRS include changes in host-species preferences [27], and feeding outdoors or in the early evening when people are not protected by their houses or bed nets [21],[22]. During the last malaria eradication drive, several accounts of mosquitoes shifting from feeding inside to outside, and from humans to animals, were reported in response to insecticide use indoors [54]. Whether these behavioural shifts were a consequence of phenotypic plasticity or evolutionary change within vector populations is unknown. Regardless of the mechanism, such behavioural plasticity limits contact between vectors and insecticides, thus diminishing the effectiveness of the interventions that use them [21].
(4) Vector biodiversity
Over 30 different primary vectors dominate transmission in various parts of the world [55]. Many of these are part of species complexes and are represented by several genetically distinct chromosomal and molecular forms within a species that have distinct ecological and behavioural niches [51]. In addition to this complexity within primary vectors, low levels of transmission are frequently maintained by a myriad of behaviourally and ecologically diverse secondary vectors. Although these species are routinely ignored, even the small fraction of transmission they generate may be sufficient to sustain Plasmodium spp. in human populations [56]. The diversity of vector species increases outside of Africa, and presents a huge challenge to conventional methods of vector control [57]. Furthermore, vectors currently viewed as “secondary” may expand to dominate residual transmission and act as de facto primary vectors following the successful implementation of interventions aimed at current priority vector species [58].
(5) Competitive and food web interactions
Mosquito vectors are embedded within ecological communities where they act as predators, prey, and competitors. Consequently the reduction of one target vector may trigger a cascade of ecological effects that could impede or enhance transmission by another. Studies have reported that suppression of one vector species through habitat change or control was followed by an increase in another. Notable examples include the apparent replacement of An. funestus by An. rivolurum [59], and An. parensis [60] in areas of east Africa following house spraying. These changes were attributed to a reduction in interspecific competition caused by the intervention that allowed these secondary vectors to move into the niche formerly occupied by An. funestus.
Virtually nothing is known about the role of vectors in regulating, or being regulated by, their prey or predators. Consequently the potential use of biological control to manage vector populations is vastly underexplored. Perhaps the best-described biological regulator of vector populations is themselves. Several studies have shown that traits such as the larval development, fecundity, survival, and population growth rates of mosquito vectors is negatively correlated with their population size [61]–[63]. This density dependence means that as vector populations fall in response to interventions, the individual vectorial capacity of the remaining survivors may be significantly greater than that of the average mosquito pre-intervention. Consequently vector populations may become increasingly difficult to suppress as their abundance moves towards zero. Complementary approaches may therefore be required to eliminate residual transmission by vector populations which have been reduced far below their carrying capacity by interventions.
(6) Dispersal and mating behaviour
Knowledge of mosquito dispersal range is essential for accurate predictions of the optimal spatial implementation of more conventional control methods such as ITNs, IRS, and larviciding [64]–[66], and of the rate of spread of resistance genes. Unfortunately, direct observations of the dispersal ability of malaria vectors have been made in only a limited subset of vector species, environments, and experimental conditions, and few generalities can be made for this behaviour. Additionally, the control of vector populations and/or their disease transmission ability through the release of genetically modified or sterile males will depend on both the dispersal ability of released individuals and their ability to successfully compete for wild females. Efforts to ensure the reproductive success of such males are hampered by large knowledge gaps in our understanding of the environmental, genetic, and phenotypic determinants of male mating competitiveness, survival, and dispersal ability under natural conditions [30].
(7) Environmental change
Climate and environmental change are driving the expansion of numerous vector species and the intensification of pathogen transmission in many locations [67]. Specific examples include deforestation, which has prompted an increase in the human-biting rate of formerly zoophilic vectors in several parts of the tropics and the instigation of new malaria epidemics [68],[69]. Historical and forecasted rises in temperature have also been implicated in the spread of malaria into new habitats and regions [70]. Mitigating against the detrimental impacts of environmental change on malaria transmission will be particularly difficult when public health goals conflict with economic development. For example, following the elimination of malaria in the Demerara River Estuary of Guiana (by DDT spraying), the human population grew rapidly and land use activities switched from livestock herding to more profitable rice farming [25]. The removal of livestock from the landscape, however, caused the formerly zoophilic An. aquasalis to switch its feeding from livestock to humans. This change initiated the return of transmission into the area after 16 years of absence [25]. Irrigation and dam construction have also been linked to an increase in malaria risk, although the nature of the effect varies substantially between epidemiological, entomological, and socioeconomic settings [71]. While environmental changes to enable poverty reduction are essential to economic development and infectious disease control, sustaining malaria eradication will require a clearer mechanistic understanding of the impacts of both vector control and concurrent changes in natural resource management and land use activities.
Thinking Outside the House
The ecological hurdles detailed in Box 1 imply that there exists a fundamental limit to the degree of control that can be achieved with ITNs or IRS. In most settings, achieving elimination will require interventions which target mosquitoes outside of human habitations. Existing and new interventions must be combined into integrated packages [18],[19] that control mosquitoes at multiple points in the continuum from egg to adult, by targeting the key environmental resources upon which they rely to complete their life cycle: aquatic larval habitat, mates, sugar sources, blood hosts, and resting sites (Figure 1). With the exception of blood, very little is known about how mosquitoes use these resources or how to manipulate them so that malaria transmission is interrupted. We conclude that a better understanding of all aspects of vector ecology will inevitably yield numerous new and mutually complementary targets for integrated vector control. Ecology is therefore a prerequisite to eradication or elimination, and will be essential to sustaining success in the long term.
Fig. 1. Life cycle components of malaria vector mosquitoes and corresponding examples of targets for novel intervention strategies. 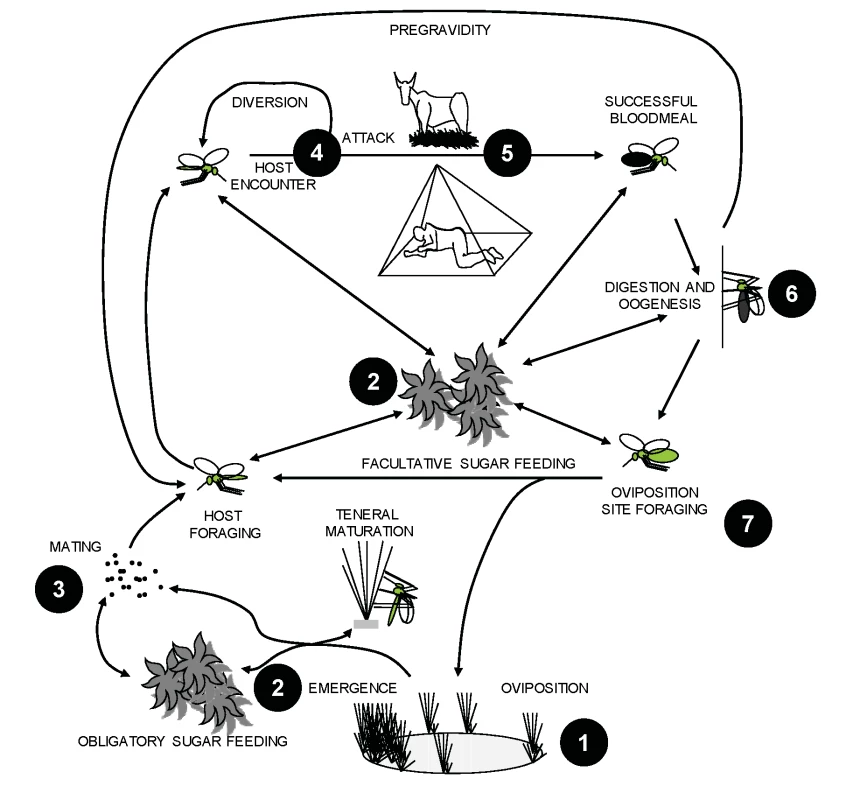
(1) Environmental management [39] and larvicide application by direct means [32],[33] or by autodissemination via adults [37]; (2) insecticide application to natural sugar sources [35], toxic sugar baits [36], and paratransgenic bacteria [40]; (3) pheromone trapping [41] and release of genetically modified or sterile males [30],[42]; (4) spatial and contact repellents [43] that work both indoors and outdoors and physical barriers to prevent mosquito entry into houses [44]; (5) zooprophylaxis [29], insecticide-treated cattle [45], and odour-baited traps [46]; (6) adult contamination with biological [47] and chemical [37] agents which may be autodisseminated; and (7) environmental management of water resources for adult vector control through increased mortality cost of foraging for oviposition sites [48]. Historically, vector biologists have focused primarily on evaluating specific control interventions and less on fundamental studies of vector ecology. Now that the gap between currently achievable levels of control and the ultimate goal of eradication is becoming clear, new intervention options for integrated vector management [18],[19] are urgently needed. Strategic investment in vector ecology will thus be an essential enabling step towards malaria eradication. The ways in which vectors utilize resources vary from one environmental setting to another, so a clear understanding of such ecological processes is essential for identifying intervention strategies which work within a range of settings. Such demonstration of ecological generalisability, as well as scalability in the context of available human resource capacities, will be essential for ensuring the success of developing country control programs. Furthermore, the long-term effectiveness of any control strategy will depend on whether vectors respond to the evolutionary selection pressure created by interventions. For example, mosquitoes may respond by phenotypic plasticity, or by evolving traits such as insecticide resistance [20] or behavioural avoidance [21],[22]. Numerous examples of such phenotypic and genetic changes have been documented in response to previous control efforts (e.g., Box 1) and are likely to influence the sustainability of future eradication attempts. Consequently, understanding the likelihood of and rate at which such evolutionary changes can occur is vital for mitigating any detrimental epidemiological consequences they may bring. Finally, the applicability of any vector control strategy will depend on the dynamic human component of vector ecology, particularly the political, social, and economic factors that determine land and water use within afflicted communities. Knowledge of the quantitative relationship between these behaviours and malaria hazard, vulnerability, and risk is vital [23],[24]. Ultimately both human and vector populations will readjust their distribution and behaviour in response to changing patterns of ecological resources, sometimes with catastrophic effects for the maintenance of control efforts (e.g. [25]). While vector control cannot and should not come at the expense of impeding economic development which could promote a wider strengthening of health care and protective measures, all efforts should be made to identify and mitigate any potential conflicts between land use and vector control before they arise.
So Where Do We Stand Right Now?
Our understanding of the ecology of mosquitoes that transmit malaria lags decades behind that of agricultural pests, endangered species, and model organisms. The reasons are multifaceted [26],[27], and disincentives include the lack of ecological representation and thus support on the funding panels of biomedical donors, limited training opportunities in fundamental ecology for medical entomologists, and the necessary ethical restrictions upon the types of experimental manipulations that are widely used to gain valuable insights into the population, community, and ecosystem dynamics of other insects [28] which do not transmit pathogens to humans. Examples of procedures which can yield crucial scientific information, but which are increasingly difficult to justify ethically include human landing catches [21] (because of the exposure risks they entail) and mark–recapture studies of mosquito demography and dispersal (because of community concerns about the re-release of potentially infectious mosquitoes that could instead have been killed). Evaluation of the potential use of alternative animal hosts to divert mosquitoes from biting humans also poses potential risks; theoretical simulations indicate there are plausible scenarios under which this may increase transmission by increasing blood availability and vector survival [29].
The paucity of national funding schemes for ecological research within impoverished malarious countries, and limited access to relevant overseas funding, have restricted the conversion of indigenous talent into an adequate expertise base. Furthermore, the primary focus of malaria control on developing countries with limited infrastructure or research capacity may deter the engagement of ecologists from the developed world who have a myriad of more convenient, accessible, and tractable organisms at their disposal. Until the inherent challenges of working in these more demanding settings is recognized and valued by mainstream ecology, researchers may have little incentive to build their careers in this area. Sponsors of fundamental biological research have typically undersupported vector ecology, on the basis of the assumption that public health and medical donors with often substantially larger budgets will fill this gap. Unfortunately this has rarely been the case in practice because donors generally prioritize applied research focusing on the development and delivery of interventions which have more obvious and immediate potential benefits [26].
Most funding for ecological studies of malaria vectors in recent years has been driven by the needs of specific biotechnological interventions [30],[31] rather than by the pursuit of basic ecological knowledge [26]. While this emphasis on applied research is clearly justified and understandable, benefits accrued will be short-lived unless such funding is matched by investment in the fundamental science that will provide new solutions to deal with resistance to current interventions and go beyond currently achievable levels of control to bring elimination realistically within reach.
As a result of these various funding deficiencies, huge knowledge gaps exist in relation to most components of the mosquito life cycle that occur outside of houses, including larval growth and sugar feeding, oviposition, and adult dispersal (Figure 1) [26],[31]. Even the development of delivery systems for the historically successful and recently rejuvenated strategy of physically eliminating or applying insecticides to larval habitats [32],[33] is severely limited by a paucity of suitable field survey methods and large-scale studies of aquatic-stage ecology [34]. An excellent example of what is possible with solid ecological observation and a little imagination comes from the deserts of Israel where dramatic reductions of malaria vector density around oases and cisterns, which may be comparable with dry-season refugia in Africa, were achieved using low-technology toxic sugar baits which are as lethal to mosquitoes as contact with an ITN [35],[36]. Similarly, results of a recent study of the effectiveness of exploiting resting and oviposition behaviours in Aedes aegypti, the primary vector of Dengue, to distribute insecticides to their own larval habitats [37] are encouraging. Nevertheless, the fact that no field estimates were available for any of the parameters of the coverage amplification model used to explain this success [37] highlights the knowledge gaps that may impede the use of this method against malaria vectors. Although further research into the behaviours that predispose vectors to such novel interventions is obviously attractive, a more integrated and holistic approach [26] is also required to maximize the value of ecological research as a means to identify additional strategies for controlling, eliminating, and eradicating malaria transmission.
Making It Happen
The overarching strategic priority for increased investment should therefore be to improve the quantitative understanding of mosquito life history, fitness, genetics, and behavioural processes as determinants of their population stability and malaria transmission intensity. Support should be directed towards delivering key outcomes, without which malaria eradication is difficult to envisage (Box 2). As such, ecology should—like other basic disciplines such as molecular biology and bioinformatics—be considered an enabling science essential for defining the target product profiles of completely new control technologies and delivery systems. To achieve these outcomes and make malaria eradication a realistic ambition, we propose key areas for specific strategic investment (Box 3).
Box 2. Key Outcomes of Enhanced Investment in Malaria Vector and Transmission Ecology
(1) Identification of specific vulnerabilities of vector and sporogonic-stage parasite populations that can be prioritized for intervention development and delivery
(2) Estimation of threshold values of vector population parameters required to achieve pathogen elimination
(3) Quantification of the strength of selection pressures imposed on vectors by particular interventions so that the likelihood and rate at which physiological and behavioural resistance traits emerge can be predicted and managed
(4) Avoidance of the mistakes of the previous eradication drive through biologically realistic understanding of the scale of the challenge
(5) Estimation of achievable endpoints for single and multiple interventions and synergies and redundancies associated with particular combinations
(6) Stimulation of creative, “blue skies” scientific investigation resulting in identification of unforeseen novel intervention targets and strategies
Box 3. Key Areas for Specific Strategic Investment in Ecological Research to Enable Malaria Eradication
(1) Development of new field measurement tools for surveying diverse primary and secondary vector populations and the environmental conditions and resources they rely upon through all phases of their life cycles
(2) Establishment of comprehensive, long-term data collection systems spanning individual to landscape scales from diverse and representative field sites
(3) Creation and maintenance of public data repositories with standardized, simplified data storage formats for mosquito ecology data combined with policies and incentive systems that facilitate data sharing and synergy between laboratory - and field-based investigators
(4) Application of cutting-edge mathematical modelling approaches to understand vector populations dynamics, pathogen transmission, and optimal intervention strategies
(5) Development and application of enclosed, pathogen-free, semi-field mesocosms in which vector populations can be experimentally manipulated [38]
6) Exploitation of the perturbations of vector populations and parasite transmission processes resulting from ongoing scale-up of existing intervention measures so that the population dynamics, behavioural specialization, and competitive relationships between mosquito species can be lucidly understood
(7) Engagement and recruitment of leading theoretical and empirical ecologists into malaria vector research, control, and capacity strengthening
Direct research investment will be required to develop new field measurement tools, establish a network of longitudinal population monitoring sites with complementary semi-field facilities [38], apply advanced modelling approaches, and promote career and skills development of endemic-country scientists in both public health and vector ecology. Beyond these obvious needs, additional incentives are required to engage expert ecologists from more knowledge-rich fields into malaria vector ecology. While ecology-oriented funding agencies have a vital role in facilitating the reinvigoration of vector biology, the bulk of the required financing will ultimately have to come from the health sector funders and policy makers who have prioritized malaria eradication and committed themselves to the long, hard road towards this worthy but distant goal.
Zdroje
1. NajeraJA
2001 Malaria control: achievements, problems and strategies. Parasitologia 43 1 89
2. RobertsL
EnserinkM
2007 Did they really say…eradication? Science 318 1544 1545
3. MacDonaldG
1957 The epidemiology and control of malaria. London Oxford University Press
4. MolineauxL
GramicciaG
1980 The Garki Project. Geneva World Health Organisation 311
5. FeachemR
SabotO
2008 A new global malaria eradication strategy. Lancet 371 1633 1635
6. KilleenGF
SmithTA
FergusonHM
AbdullaS
MshindaH
2007 Preventing childhood malaria in Africa by protecting adults from mosquitoes with insecticide-treated nets. PLoS Med 4 e229 doi:10.1371/journal.pmed.0040229
7. LengelerC
2004 Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev: CD000363. doi:10.1002/14651858.CD000363.pub2
8. SharpBL
KleinschmidtI
StreatE
MaharajR
BarnesKI
2007 Seven years of regional malaria control collaboration-Mozambique, South Africa, and Swaziland. Am J Trop Med Hyg 76 42 47
9. SmithDL
DushoffJ
SnowRW
HaySI
2005 The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature 438 492 495
10. SmithDL
McKenzieFE
SnowRW
HaySI
2007 Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol 5 e42 doi:10.1371/journal.pbio.0050042
11. KilleenGF
TamiA
KihondaJ
OkumuFO
KotasME
2007 Cost-sharing strategies combining targeted public subsidies with private-sector delivery achieve high bednet coverage and reduced malaria transmission in Kilombero Valley, southern Tanzania. BMC Infect Dis 7 121
12. CeesaySJ
Casals-PascualC
ErskineJ
AnyaSE
DuahNO
2008 Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 372 1545 1554
13. O'MearaWP
BejonP
MwangiTW
OkiroEA
PeshuN
2008 Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet 372 1555 1562
14. BhattaraiA
AliA
KachurSP
MartenssonA
AbbasAK
2007 Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Africa. PLoS Med 4 1784 doi:10.1371/journal.pmed.0040309
15. KouznetsovRL
1977 Malaria control by application of indoor spraying of residual insecticides in tropical Africa and its impact on community health. Tropical Doctor 7 81 93
16. FeganGW
NoorAM
AkhwaleWS
CousensS
SnowRW
2007 Effect of expanded insecticide-treated bednet coverage on child survival in rural Kenya: a longitudinal study. Lancet 370 1035 1039
17. HawleyWA
ter KuileFO
SteketeeRS
NahlenBL
TerlouwDJ
2003 Implications of the western Kenya permethrin-treated bed net study for policy, program implementation, and future research. Am J Trop Med Hyg 68 168 173
18. TownsonH
NathanR
ZaimM
GuilletP
MangaL
2005 Exploiting the potential of vector control for disease prevention. Bull World Health Organ 83 942 947
19. WHO 2004 Global Strategic Framework for Integrated Vector Management. Geneva World Health Organization 15
20. Kelly-HopeL
RansonH
HemingwayJ
2008 Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis 8 387 389
21. GovellaNJ
OkumuFO
KilleenGF
2010 Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg 82 415 419
22. PatesH
CurtisC
2005 Mosquito behavior and vector control. Annu Rev Entomol 50 53 70
23. BatesI
FentonC
GruberJ
LallooD
Medina LaraA
2004 Vulnerability to malaria, tuberculosis and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect Dis 4 267 277
24. BatesI
FentonC
GruberJ
LallooD
Medina LaraA
2004 Vulnerability to malaria, tuberculosis and HIV/AIDS infection and disease. Part 2: determinants operating at environmental and institutional level. Lancet Infect Dis 4 368 375
25. GiglioliG
1963 Ecological change as a factor in renewed malaria transmission in an eradicated area. Bull World Health Organ 29 131 145
26. FishD
2008 Vector-borne diseases: Understanding the environmental, human health and ecological connections. Washington, D.C. Institute of Medicine 12
27. LyimoIN
FergusonHM
2009 Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol 25 189 196
28. OdumEP
1984 The mesocosm. BioScience 34 558 562
29. SaulA
2003 Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malar J 2 32
30. FergusonHM
JohnB
Ng'habiK
KnolsBG
2005 Redressing the sex imbalance in knowledge of vector biology. Trends Ecol Evol 20 202 209
31. MshindaH
KilleenGF
MukabanaRW
MathengeE
MboeraLEG
2004 Development of genetically modified mosquitoes in Africa. Lancet Infect Dis 4 264 265
32. FillingerU
NdegwaB
GithekoA
LindsaySW
2009 Integrated malaria vector control with microbial larvicides and insecticide treated nets in the western Kenyan highlands: a controlled trial. Bull World Health Organ 87 655 665
33. GeissbuhlerY
KannadyK
ChakiPP
EmidiB
GovellaNJ
2009 Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS One 4 e5107 doi:10.1371/journal.pone.0005107
34. GuW
UtzingerJ
NovakRJ
2008 Habitat-based larval interventions: a new perspective for malaria control. Am J Trop Med Hyg 78 2 6
35. MüllerG
SchleinY
2006 Sugar-questing mosquitoes in arid areas gather on scarce blossoms that can be used for control. Int J Parasitol 36 1077 1080
36. MüllerG
SchleinY
2008 Efficacy of toxic sugar baits against cistern-dwelling Anopheles claviger. Trans R Soc Trop Med Hyg 102 480 484
37. DevineGJ
PereaEZ
KilleenGF
StancilJD
ClarkSJ
2009 Autodissemination of an insecticide by adult mosquitoes drammatically amplifies lethal coverage of their aquatic habitats. Proc Natl Acad Sci U S A 106 11530 11534
38. FergusonHM
Ng'habiKR
WalderT
KadungulaD
MooreSJ
2008 Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar J 7 158
39. KeiserJ
SingerBH
UtzingerJ
2005 Reducing the burden of malaria in different eco-epidemiological settings with environmental management: a systematic review. Lancet Infect Dis 5 695 708
40. RiehleMA
Jacobs-LorenaM
2005 Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochem Mol Biol 35 699 707
41. Rodriguez-SaonaCR
PolkDF
BarryJD
2009 Optimization of pheromone deployment for effective mating disruption of oriental beetle (Coleoptera: Scarabaeidae) in commercial blueberries. J Econ Entomol 102 659 669
42. ScottTW
TakkenW
KnolsBGJ
BoeteC
2002 Ecology of genetically modified mosquitoes. Science 298 117 119
43. GriecoJP
AcheeNL
ChareonviriyaphapT
SuwonkerdW
ChauhanK
2007 A new classification system for the actions of IRS chemicals traditionally used for malaria control. PLoS One 2 e716 doi:10.1371/journal.pone.0000716
44. KirbyMJ
AmehD
BottomleyC
GreenC
JawaraM
2009 Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet 374 998 1009
45. RowlandM
DurraniN
KenwardM
MohammedN
UrahmanH
2001 Control of malaria in Pakistan by applying deltamethrin insecticide to cattle: a community-randomised trial. Lancet 357 1837 1841
46. OkumuFO
KilleenGF
OgomaSB
BiswaroL
SmallegangeRC
2010 Development and field evaluation of a mosquito lure that is more attractive than humans. PLoS One 5 e8591 doi:10.1371/journal.pone.0008951
47. ScholteEJ
Ng'habiK
KihondaJ
TakkenW
PaaijmansKP
2005 An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308 1641 1642
48. GuW
RegensJL
BeierJC
NovakRJ
2006 Source reduction of mosquito larval habitats has unexpected consequences on malaria transmission. Proc Natl Acad Sci U S A 103 17560 17563
49. GilliesMT
DeMeillonB
1968 The Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region). Johannesburg South African Institute for Medical Research
50. TiradosI
CostantiniC
GibsonG
TorrSJ
2006 Blood-feeding behaviour of the malarial mosquito Anopheles arabiensis: implications for vector control. Med Vet Entomol 20 425 437
51. ColuzziM
SabatiniA
PetrarcaV
DidecoMA
1979 Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex Trans Roy Soc Trop Med Hyg 73 483 497
52. RansonH
AbdallahH
BadoloA
GuelbeogoWM
Kerah-HinzoumbeC
2009 Insecticide resistance in Anopheles gambiae: data from the first year of a multi-country study highlight the extent of the problem. Malar J 8 299
53. GarciaGP
FloresAE
Fernandez-SalasI
Saavedra-RodriguezK
Reyes-SolisG
2009 Recent rapid rise of a permethrin knock down resistance allele in Aedes aegypti in Mexico. PLoS Negl Trop Dis 3 e531 doi:10.1371/journal.pntd.0000531
54. Garrett-JonesC
BorehamP
PantCP
1980 Feeding habits of anophelines (Diptera: Culicidae) in 1971-1978, with reference to the human blood index: a review. Bull Entomol Res 70 165 185
55. KiszewskiA
MellingerA
SpielmanA
MalaneyP
SachsSE
2004 A global index representing the stabililty of malaria transmission. Am J Trop Med Hyg 70 486 498
56. BeierJC
KilleenGF
GithureJ
1999 Short report: Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg 61 109 113
57. TrungHD
BortelWV
SochanthaT
KeokenchanhK
BriëtOJ
2005 Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health 10 251 262
58. BayohMN
MathiasDK
OdiereMR
MutukuFM
KamauL
2010 Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J 9 62
59. GilliesMT
SmithA
1960 Effect of a residual house-spraying campagn on species balance in the Anopheles funestus group: The replacement of Anopheles gambiae Giles with Anopheles rivulorum Leeson. Bull Entomol Res 51 248 252
60. GilliesMT
FurlongM
1964 An investigation into the behaviour of Anopheles parensis Gillies at Malindi on the coast of Kenya. Bull Entomol Res 55 1 16
61. YangGJ
BrookBW
WhelanPI
ClelandS
BradshawCJA
2008 Endogenous and exogenous factors controlling temporal abundance patterns of tropical mosquitoes. Ecol Appl 18 2028 2040
62. BriegelH
1990 Fecundity, metabolism, and body size in Anopheles (Diptera, Culicidae), vectors of malaria. J Med Entomol 27 839 850
63. LyimoEO
TakkenW
KoellaJC
1992 Effect of rearing temperature and larval density on larval survival, age at pupation and adult size of Anopheles gambiae. Entomol Exp Appl 63 265 271
64. KilleenGF
KnolsBG
GuW
2003 Taking malaria transmission out of the bottle: implications of mosquito dispersal for vector-control interventions. Lancet Infect Dis 3 297 303
65. GuW
NovakRJ
2009 Predicting the impact of insecticide-treated bednets on malaria transmission: the devil is in the detail. Malar J 8 256
66. ZhouG
GithekoAK
MinakawaN
YanG
2010 Community-wide benefits of targeted indoor residual spray for malaria control in the western Kenya highland. Malar J 9 67
67. PatzJA
OlsonSH
UejioCK
GibbsHK
2008 Disease emergence from global climate and land use change. Med Clin North Am 92 1473 1491
68. VittorAY
GilmanRH
TielschJ
GlassG
ShieldsT
2006 The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg 74 3 11
69. Cox-SinghJ
SinghB
2008 Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol 24 406 410
70. PatzJA
OlsonSH
2006 Malaria risk and temperature: influences from global climate change and local land use practices. Proc Natl Acad Sci U S A 103 5829 5834
71. KeiserJ
De CastroMC
MalteseMF
BosR
TannerM
2005 Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am J Trop Med Hyg 72 392 406
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 8- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Léčba bolesti u seniorů
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Reducing Malaria Transmission in Africa: A Model-Based Evaluation of Intervention Strategies
- Quantifying the Impoverishing Effects of Purchasing Medicines: A Cross-Country Comparison of the Affordability of Medicines in the Developing World
- Physical Activity Attenuates the Genetic Predisposition to Obesity in 20,000 Men and Women from EPIC-Norfolk Prospective Population Study
- Using Touchscreen Electronic Medical Record Systems to Support and Monitor National Scale-Up of Antiretroviral Therapy in Malawi
- Social Relationships Are Key to Health, and to Health Policy
- Assessing Strategy and Equity in the Elimination of Malaria
- Challenges in Developing Evidence-Based Recommendations Using the GRADE Approach: The Case of Mental, Neurological, and Substance Use Disorders
- Will Cardiovascular Disease Prevention Widen Health Inequalities?
- Moving from Data on Deaths to Public Health Policy in Agincourt, South Africa: Approaches to Analysing and Understanding Verbal Autopsy Findings
- Rapid Scaling Up of Insecticide-Treated Bed Net Coverage in Africa and Its Relationship with Development Assistance for Health: A Systematic Synthesis of Supply, Distribution, and Household Survey Data
- Ecology: A Prerequisite for Malaria Elimination and Eradication
- An Intervention to Reduce HIV Risk Behavior of Substance-Using Men Who Have Sex with Men: A Two-Group Randomized Trial with a Nonrandomized Third Group
- Impact of Community-Based Maternal Health Workers on Coverage of Essential Maternal Health Interventions among Internally Displaced Communities in Eastern Burma: The MOM Project
- The Costs and Underappreciated Consequences of Research Misconduct: A Case Study
- The Effect of Raltegravir Intensification on Low-level Residual Viremia in HIV-Infected Patients on Antiretroviral Therapy: A Randomized Controlled Trial
- Harnessing Health IT for Improved Cardiovascular Risk Management
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Using Touchscreen Electronic Medical Record Systems to Support and Monitor National Scale-Up of Antiretroviral Therapy in Malawi
- Physical Activity Attenuates the Genetic Predisposition to Obesity in 20,000 Men and Women from EPIC-Norfolk Prospective Population Study
- Challenges in Developing Evidence-Based Recommendations Using the GRADE Approach: The Case of Mental, Neurological, and Substance Use Disorders
- Reducing Malaria Transmission in Africa: A Model-Based Evaluation of Intervention Strategies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



