-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDoes Seasonal Influenza Vaccination Increase the Risk of Illness with the 2009 A/H1N1 Pandemic Virus?
article has not abstract
Published in the journal: . PLoS Med 7(4): e32767. doi:10.1371/journal.pmed.1000259
Category: Perspective
doi: https://doi.org/10.1371/journal.pmed.1000259Summary
article has not abstract
Linked Research Article
This Perspective discusses the following study published in PLoS Medicine:
Skowronski DM, De Serres G, Crocroft N, Janjua NZ, Boulianne N, et al. (2010) Association between the 2008–09 Seasonal Influenza Vaccine and Pandemic H1N1 Illness during Spring–Summer 2009: Four Observational Studies from Canada. PLoS Med 7(4): e1000258. doi:10.1371/journal.pmed.1000258
In three case-control studies and a household transmission cohort, Danuta Skowronski and colleagues find an association between prior seasonal flu vaccination and increased risk of 2009 pandemic H1N1 flu.
Background
As the novel pandemic influenza A (H1N1) (pH1N1) virus spread around the world in late spring 2009 with a well-matched pandemic vaccine not immediately available, the question of partial protection afforded by seasonal influenza vaccine arose. Coverage of the seasonal influenza vaccine had reached 30%–40% in the general population in 2008–09 in the US and Canada, following recent expansion of vaccine recommendations.
Serology studies demonstrated a lack of cross-reactive antibody to the novel virus in vaccinated and unvaccinated people under 60 years of age, suggesting that there would be no protection against pandemic influenza from natural immunity or seasonal vaccination [1]. By contrast, about one third of seniors over 60 y had cross-reactive antibodies [1], perhaps due to childhood exposure to antigenically similar A/H1N1 viruses. As a result, the mean age of pandemic cases and deaths was younger than that of interpandemic seasons [2], a signature age shift also experienced in three historical influenza pandemics [3].
Unexpected Findings in a Sentinel Surveillance System
The spring 2009 pandemic wave was the perfect opportunity to address the association between seasonal trivalent inactivated influenza vaccine (TIV) and risk of pandemic illness. In this issue of PLoS Medicine, Danuta Skowronski and colleagues report the unexpected results of a series of Canadian epidemiological studies suggesting a counterproductive effect of the vaccine [4]. The findings are based on Canada's unique near-real-time sentinel system for monitoring influenza vaccine effectiveness. Patients with influenza-like illness who presented to a network of participating physicians were tested for influenza virus by RT-PCR, and information on demographics, clinical outcomes, and vaccine status was collected. In this sentinel system, vaccine effectiveness may be measured by comparing vaccination status among influenza-positive “case” patients with influenza-negative “control” patients. This approach has produced accurate measures of vaccine effectiveness for TIV in the past, with estimates of protection in healthy adults higher when the vaccine is well-matched with circulating influenza strains and lower for mismatched seasons [5]. The sentinel system was expanded to continue during April to July 2009, as the pH1N1 virus defied influenza seasonality and rapidly became dominant over seasonal influenza viruses in Canada.
Additional Analyses and Proposed Biological Mechanisms
The Canadian sentinel study showed that receipt of TIV in the previous season (autumn 2008) appeared to increase the risk of pH1N1 illness by 1.03 - to 2.74-fold, even after adjustment for comorbidities, age, and geography [4]. The investigators were prudent and conducted multiple sensitivity analyses to attempt to explain their perplexing findings. Importantly, TIV remained protective against seasonal influenza viruses circulating in April through May 2009, with an effectiveness estimated at 56% (41%–67%), suggesting that the system had not suddenly become flawed. TIV appeared as a risk factor in people under 50 y, but not in seniors—although senior estimates were imprecise due to lower rates of pandemic illness in that age group. Interestingly, if vaccine were truly a risk factor in younger adults, seniors may have fared better because their immune response to vaccination is less rigorous [6].
Because of the potential public health seriousness of the findings, complementary observational studies were launched in Ontario and Quebec, based on hospital and community cases and controls. These studies confirmed TIV as a risk factor for 2009 pH1N1 illness, but were somewhat reassuring in that TIV did not increase severity of disease. Finally, a household study in Quebec did not show a convincing difference in secondary attack rates by vaccination status, although the statistical power was rather limited.
The authors proposed several biological mechanisms to explain why seasonal vaccination may increase the risk of pandemic illness [4]. One mechanism involves lack of heterosubtypic immunity in recipients of TIV, as heterobsutypic immunity may be generated through T cell responses during natural infection with seasonal influenza viruses, but not through vaccination. This explanation remains hypothetical, as biological evidence of heterosubtypic immunity in humans is scarce despite circumstantial evidence from past pandemics [7],[8]. Other proposed mechanisms were original antigenic sin and antibody-dependent enhancement, whereby TIV may induce high antibody titers to seasonal influenza viruses, which may cross-react with pH1N1 without neutralizing it, and counteract development of a robust antibody response to pandemic influenza infection. However, the evidence that antibody response in human populations depends on the sequence of past influenza infections remains debated. Overall, full characterization of baseline pre-pandemic immune profiles of recipients of inactivated and live-attenuated seasonal influenza vaccines and of unvaccinated individuals of various ages, would be highly informative to basic science and public health. Hopefully, such key studies can still be conducted in part by analysis of stored blood bank sera.
Potential Biases and Findings from Other Countries
The Canadian authors quickly found themselves at odds with expert review committees who were not convinced by the data and largely dismissed the findings as due to confounding bias—a fair criticism of observational studies. To their credit, the authors had thoroughly assessed potential biases in their article [4], in particular relative to the selection of controls and differences in health care–seeking behavior, and repeated the study in different Canadian provinces. They also provided a full description of their study population and carefully compared vaccine coverage and prevalence of comorbidities in controls with national or province-level age-specific estimates—the best one can do short of a randomized study. In parallel, profound bias in observational studies of vaccine effectiveness does exist, as was amply documented in several cohort studies overestimating the mortality benefits of seasonal influenza vaccination in seniors [9].
Given the uncertainty associated with observational studies, we believe it would be premature to conclude that TIV increased the risk of 2009 pandemic illness, especially in light of six other contemporaneous observational studies in civilian populations that have produced highly conflicting results (see Table 1 for details on study design, population sampled, and results) [10]–[15]. We note the large spread of vaccine effectiveness estimates in those studies; indeed, four of the studies set in the US and Australia did not show any association [12]–[15], whereas two Mexican studies suggested a protective effect of 35%–73% [10],[11]. The most recent Canadian study in this issue of PLoS Medicine [4] is clearly at odds with these results, with an estimated average negative effectiveness of −68% based on their Sentinel system. Only one study, set in the US military population, potentially corroborated the findings of the Canadian study [16].
Tab. 1. Comparison of observational studies evaluating the effectiveness of seasonal influenza vaccination to prevent 2009 pA/H1N1 morbidity in civilian populations. 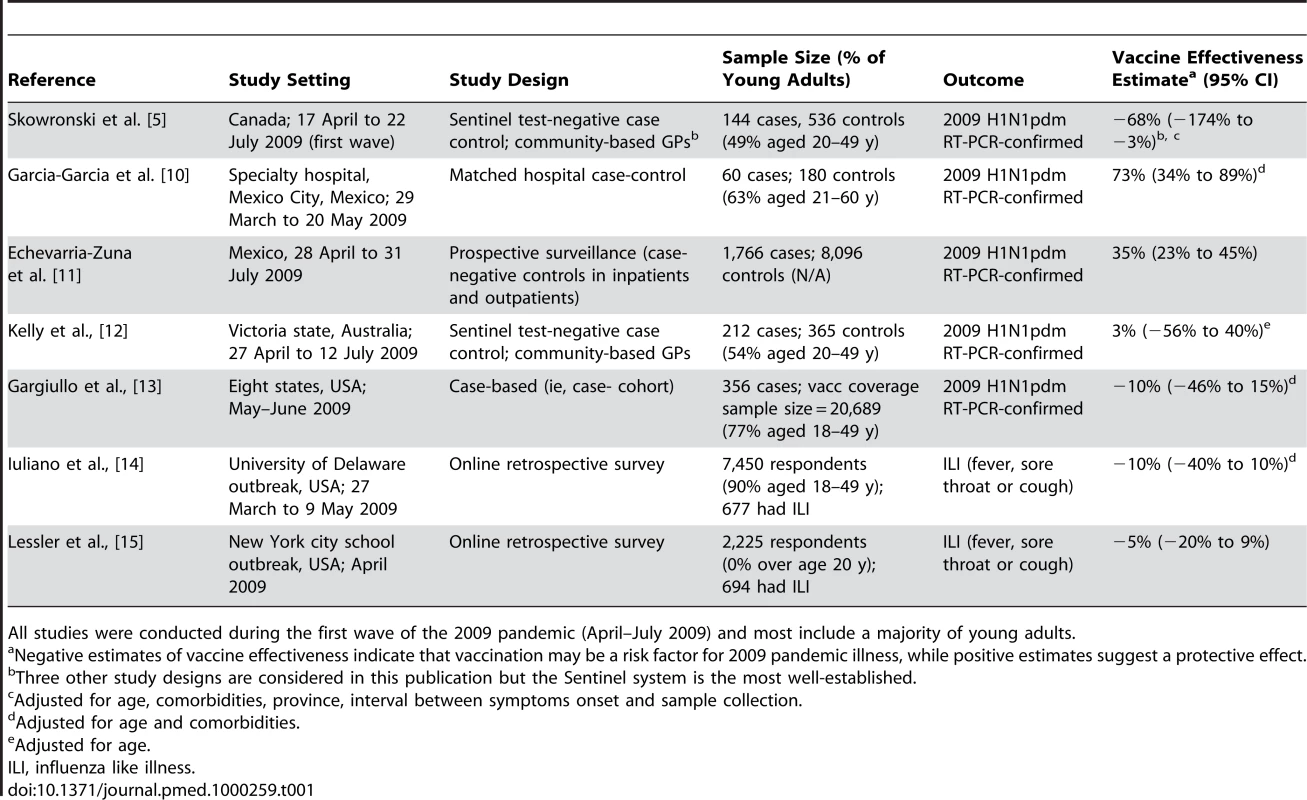
All studies were conducted during the first wave of the 2009 pandemic (April–July 2009) and most include a majority of young adults. All studies, including [4], are potentially prone to bias due to lack of randomization. Perhaps the more extreme Canadian, US, and Mexican studies were deeply biased, or perhaps the population experiences were truly different due to their vaccination histories and past influenza exposure. Given the sudden spread of the pandemic virus, it would have been extremely difficult to design a prospective (randomized) trial to evaluate TIV effectiveness—and such a study is now forever complicated by pandemic vaccination efforts.
Policy Implications and a Way Forward
The putative association between seasonal vaccination and 2009 pH1N1 illness remains an open question, given the conflicting evidence from available research. Canadian health authorities debated whether to postpone seasonal vaccination in the autumn of 2009 until after a second pandemic wave had occurred, but decided to follow normal vaccine recommendations instead, in part because of uncertainty about a resurgence of seasonal influenza viruses during the 2009–10 season [17]. This illustrates the difficulty of making policy decisions in the midst of a public health crisis, when officials must rely on limited and possibly biased evidence from observational data, even in the best possible scenario of a well-established sentinel monitoring system already in place.
What happens next? Given the timeliness of the Canadian sentinel system, data on the association between seasonal TIV and risk of pH1N1 illness during the autumn 2009 pandemic wave will become available very soon, and will be crucial in confirming or refuting the earlier Canadian results. In addition, evidence may be gained from disease patterns during the autumn 2009 pandemic wave in other countries and from immunological studies characterizing the baseline immunological status of vaccinated and unvaccinated populations. Overall, this perplexing experience should teach us how to best react to disparate and conflicting studies and prepare us for the next public health crisis, so that we can better manage future alerts for unexpected risk factors.
Zdroje
1. HancockK
VeguillaV
LuX
ZhongW
ButlerEN
2009 Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 361 1945 1952
2. ChowellG
BertozziSM
ColcheroMA
Lopez-GatellH
Alpuche-ArandaC
2009 Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 361 674 679
3. MillerMA
ViboudC
BalinskaM
SimonsenL
2009 The signature features of influenza pandemics–implications for policy. N Engl J Med 360 2595 2598
4. SkowronskiD
De SerresG
CrowcroftN
al.E
2010 Association between the 2008–09 Seasonal Influenza Vaccine and Pandemic H1N1 Illness during Spring–Summer 2009: Four Observational Studies from Canada. PLoS Med 7 e1000258 doi:10.1371/journal.pmed.1000258
5. SkowronskiDM
De SerresG
DickinsonJ
PetricM
MakA
2009 Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis 199 168 179
6. GoodwinK
ViboudC
SimonsenL
2006 Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 24 1159 696
7. FergusonNM
GalvaniAP
BushRM
2003 Ecological and immunological determinants of influenza evolution. Nature 422 428 433
8. EpsteinSL
2006 Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: An experiment of nature. J Infect Dis 193 49 53
9. SimonsenL
TaylorRJ
ViboudC
MillerMA
JacksonLA
2007 Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis 7 658 666
10. Garcia-GarciaL
Valdespino-GomezJL
Lazcano-PonceE
Jimenez-CoronaA
Higuera-IglesiasA
2009 Partial protection of seasonal trivalent inactivated vaccine against novel pandemic influenza A/H1N1 2009: Case-control study in Mexico City. BMJ 339 b3928
11. Echevarria-ZunoS
Mejia-ArangureJM
Mar-ObesoAJ
Grajales-MunizC
Robles-PerezE
2009 Infection and death from influenza A H1N1 virus in Mexico: A retrospective analysis. Lancet 374 2072 2079
12. KellyH
GrantK
2009 Interim analysis of pandemic influenza (H1N1) 2009 in Australia: Surveillance trends, age of infection and effectiveness of seasonal vaccination. Euro Surveill 14
13. 2009 Effectiveness of 2008–09 trivalent influenza vaccine against 2009 pandemic influenza A (H1N1) - United States, May–June 2009. MMWR Morb Mortal Wkly Rep 58 1241 1245
14. IulianoAD
ReedC
GuhA
DesaiM
DeeDL
2009 Notes from the field: Outbreak of 2009 pandemic influenza A (H1N1) virus at a large public university in Delaware, April–May 2009. Clin Infect Dis 49 1811 1820
15. LesslerJ
ReichNG
CummingsDA
NairHP
JordanHT
2009 Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med 361 2628 2636
16. Crum-CianfloneNF
BlairPJ
FaixD
ArnoldJ
EcholsS
ShermanSS
Clinical and epidemiologic characteristics of an outbreak of novel H1N1 (swine origin) influenza A virus among United States military beneficiaries. CID 2009; 49 1801 10
17. Public Health Agency of Canada October 2009 Statement on Seasonal Trivalent Inactivated Influenza Vaccine (TIV) for 2009–2010. Available: http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/09vol35/acs-dcc-6/index-eng.php. Accessed 25 February 2010
Štítky
Interní lékařství
Článek Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility AnalysisČlánek The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility Analysis
- The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study
- Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages
- Association between the 2008–09 Seasonal Influenza Vaccine and Pandemic H1N1 Illness during Spring–Summer 2009: Four Observational Studies from Canada
- China's Engagement with Global Health Diplomacy: Was SARS a Watershed?
- Laboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN)
- Mortality Measurement Matters: Improving Data Collection and Estimation Methods for Child and Adult Mortality
- Brazil and the Framework Convention on Tobacco Control: Global Health Diplomacy as Soft Power
- Health Diplomacy and the Enduring Relevance of Foreign Policy Interests
- Measuring Under-Five Mortality: Validation of New Low-Cost Methods
- What Can We Conclude from Death Registration? Improved Methods for Evaluating Completeness
- Improving Newborn Survival in Low-Income Countries: Community-Based Approaches and Lessons from South Asia
- Does Seasonal Influenza Vaccination Increase the Risk of Illness with the 2009 A/H1N1 Pandemic Virus?
- Measuring Adult Mortality Using Sibling Survival: A New Analytical Method and New Results for 44 Countries, 1974–2006
- Early Emergence of Ethnic Differences in Type 2 Diabetes Precursors in the UK: The Child Heart and Health Study in England (CHASE Study)
- Comparative Effectiveness Research: Challenges for Medical Journals
- Alternative Strategies to Reduce Maternal Mortality in India: A Cost-Effectiveness Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages
- Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility Analysis
- China's Engagement with Global Health Diplomacy: Was SARS a Watershed?
- Laboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



