-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAlternative Strategies to Reduce Maternal Mortality in India: A Cost-Effectiveness Analysis
Background:
Approximately one-quarter of all pregnancy - and delivery-related maternal deaths worldwide occur in India. Taking into account the costs, feasibility, and operational complexity of alternative interventions, we estimate the clinical and population-level benefits associated with strategies to improve the safety of pregnancy and childbirth in India.Methods and Findings:
Country - and region-specific data were synthesized using a computer-based model that simulates the natural history of pregnancy (both planned and unintended) and pregnancy - and childbirth-associated complications in individual women; and considers delivery location, attendant, and facility level. Model outcomes included clinical events, population measures, costs, and cost-effectiveness ratios. Separate models were adapted to urban and rural India using survey-based data (e.g., unmet need for birth spacing/limiting, facility births, skilled birth attendants). Model validation compared projected maternal indicators with empiric data. Strategies consisted of improving coverage of effective interventions that could be provided individually or packaged as integrated services, could reduce the incidence of a complication or its case fatality rate, and could include improved logistics such as reliable transport to an appropriate referral facility as well as recognition of referral need and quality of care. Increasing family planning was the most effective individual intervention to reduce pregnancy-related mortality. If over the next 5 y the unmet need for spacing and limiting births was met, more than 150,000 maternal deaths would be prevented; more than US$1 billion saved; and at least one of every two abortion-related deaths averted. Still, reductions in maternal mortality reached a threshold (∼23%–35%) without including strategies that ensured reliable access to intrapartum and emergency obstetrical care (EmOC). An integrated and stepwise approach was identified that would ultimately prevent four of five maternal deaths; this approach coupled stepwise improvements in family planning and safe abortion with consecutively implemented strategies that incrementally increased skilled attendants, improved antenatal/postpartum care, shifted births away from home, and improved recognition of referral need, transport, and availability/quality of EmOC. The strategies in this approach ranged from being cost-saving to having incremental cost-effectiveness ratios less than US$500 per year of life saved (YLS), well below India's per capita gross domestic product (GDP), a common benchmark for cost-effectiveness.Conclusions:
Early intensive efforts to improve family planning and control of fertility choices and to provide safe abortion, accompanied by a paced systematic and stepwise effort to scale up capacity for integrated maternal health services over several years, is as cost-effective as childhood immunization or treatment of malaria, tuberculosis, or HIV. In just 5 y, more than 150,000 maternal deaths would be averted through increasing contraception rates to meet women's needs for spacing and limiting births; nearly US$1.5 billion would be saved by coupling safe abortion to aggressive family planning efforts; and with stepwise investments to improve access to pregnancy-related health services and to high-quality facility-based intrapartum care, more than 75% of maternal deaths could be prevented. If accomplished over the next decade, the lives of more than one million women would be saved.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(4): e32767. doi:10.1371/journal.pmed.1000264
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000264Summary
Background:
Approximately one-quarter of all pregnancy - and delivery-related maternal deaths worldwide occur in India. Taking into account the costs, feasibility, and operational complexity of alternative interventions, we estimate the clinical and population-level benefits associated with strategies to improve the safety of pregnancy and childbirth in India.Methods and Findings:
Country - and region-specific data were synthesized using a computer-based model that simulates the natural history of pregnancy (both planned and unintended) and pregnancy - and childbirth-associated complications in individual women; and considers delivery location, attendant, and facility level. Model outcomes included clinical events, population measures, costs, and cost-effectiveness ratios. Separate models were adapted to urban and rural India using survey-based data (e.g., unmet need for birth spacing/limiting, facility births, skilled birth attendants). Model validation compared projected maternal indicators with empiric data. Strategies consisted of improving coverage of effective interventions that could be provided individually or packaged as integrated services, could reduce the incidence of a complication or its case fatality rate, and could include improved logistics such as reliable transport to an appropriate referral facility as well as recognition of referral need and quality of care. Increasing family planning was the most effective individual intervention to reduce pregnancy-related mortality. If over the next 5 y the unmet need for spacing and limiting births was met, more than 150,000 maternal deaths would be prevented; more than US$1 billion saved; and at least one of every two abortion-related deaths averted. Still, reductions in maternal mortality reached a threshold (∼23%–35%) without including strategies that ensured reliable access to intrapartum and emergency obstetrical care (EmOC). An integrated and stepwise approach was identified that would ultimately prevent four of five maternal deaths; this approach coupled stepwise improvements in family planning and safe abortion with consecutively implemented strategies that incrementally increased skilled attendants, improved antenatal/postpartum care, shifted births away from home, and improved recognition of referral need, transport, and availability/quality of EmOC. The strategies in this approach ranged from being cost-saving to having incremental cost-effectiveness ratios less than US$500 per year of life saved (YLS), well below India's per capita gross domestic product (GDP), a common benchmark for cost-effectiveness.Conclusions:
Early intensive efforts to improve family planning and control of fertility choices and to provide safe abortion, accompanied by a paced systematic and stepwise effort to scale up capacity for integrated maternal health services over several years, is as cost-effective as childhood immunization or treatment of malaria, tuberculosis, or HIV. In just 5 y, more than 150,000 maternal deaths would be averted through increasing contraception rates to meet women's needs for spacing and limiting births; nearly US$1.5 billion would be saved by coupling safe abortion to aggressive family planning efforts; and with stepwise investments to improve access to pregnancy-related health services and to high-quality facility-based intrapartum care, more than 75% of maternal deaths could be prevented. If accomplished over the next decade, the lives of more than one million women would be saved.
: Please see later in the article for the Editors' SummaryIntroduction
Approximately one-quarter of all pregnancy - and delivery-related maternal deaths worldwide occur in India, which has the highest burden of maternal mortality for any single country [1],[2]. Although the inclusion of maternal mortality reduction in the United Nations' Millennium Development Goals (MDGs) reflects the importance of improving maternal health as a key mechanism in reducing poverty and promoting social and economic growth, global progress has been suboptimal [2]–[4]. Several factors may be changing the landscape for maternal health in India in particular [5]. These factors include more information on maternal mortality measures [6],[7], an increasing number of studies evaluating interventions [8], renewed determination on the part of the maternal health and public health communities [9], and, most importantly, the emergence of maternal mortality reduction as a clear priority on the Indian national political agenda [5],[10]–[12].
Despite consensus on the need for universal access to high-quality intrapartum and emergency obstetrical care (EmOC), uncertainties remain about how to adapt “ideal recommendations” to specific situations [8],[13]. The need for an adequate supply of skilled providers, functional referral and transport, and well-equipped facilities for EmOC will prove a formidable barrier in the near-term for countries with weak health systems, as well as for states with inadequate health delivery infrastructure and for communities in predominantly rural areas [13]. This challenge will be particularly relevant for India, with its largely rural population, and striking disparities between states. For example, while Kerala reports a maternal mortality ratio (MMR) of fewer than 100 maternal deaths per 100,000 live births, rural Uttar Pradesh and Rajasthan report MMRs of more than 400 [14],[15].
To most effectively leverage India's national commitment to reducing maternal mortality, identifying evidence-based strategies that consider the local context is imperative. Our analysis is motivated by questions that include: What are the fundamental drivers of the effectiveness, cost-effectiveness, and affordability of a package of interventions to reduce maternal mortality? Because adequate facilities, health infrastructure, and skilled human resources will not be readily available in all settings, can we provide interim guidance to policy makers? While no single empirical study can provide clear answers to these questions, a modeling approach within a decision-analytic framework can extend empiric information by extrapolating outcomes beyond the time horizon of a single study, can facilitate synthesis of multiple data sources in an internally consistent and epidemiologically plausible way [16], and may be adapted to specific settings so that existing infrastructure, resources, and political realities can be considered.
Previous model-based studies have provided important insights into the potential high public health value of reducing maternal deaths, however, many of these have not considered the full range of interventions to reduce maternal mortality, such as family planning, safe abortion, and intrapartum care [7],[17],[18]. Some have only focused on single interventions [19]–[21], others have not included costs [22], and recent analyses that did assess multiple strategies did not explicitly model critical barriers to life-saving referral, such as recognition of referral need and accessible transport [23],[24]. Taking into account the costs, feasibility, and operational complexity of alternative interventions, we extend this body of work to estimate the clinical and population-level benefits associated with a comprehensive set of strategies to improve the safety of pregnancy and childbirth in India.
Methods
Analytic Overview
The best available data were synthesized using a computer-based model that simulates the natural history of pregnancy and relevant comorbidities, aggregates individual outcomes to the population level, and reflects setting-specific epidemiology. Separate models were adapted to urban and rural India using data on antenatal care, family planning, facility births, and skilled birth attendants (SBAs), and information about access to transport, referral facilities, and quality of care. Model outcomes include clinical events (e.g., pregnancies, live births, maternal complications), measures of maternal mortality (e.g., MMR, proportionate mortality ratio [i.e., proportion of deaths that are pregnancy-related among women aged 15–45 y], and lifetime risk of maternal death), population outcomes (e.g., life expectancy), and economic costs.
We evaluated alternative approaches to reducing maternal mortality in settings in India that differ according to underlying maternal risk, health, and socioeconomic status. Interventions can be provided individually or packaged into integrated services. Following standard recommendations for economic evaluation [25], strategies are first ranked in terms of increasing costs and benefits; those that are less effective and more costly than an alternative strategy are considered inefficient, and those that cost less than the status quo are considered “cost saving.” For all other strategies, we calculate an incremental cost-effectiveness ratio, defined as the additional cost of a specific strategy divided by its additional clinical benefit, compared with the next least expensive strategy. We considered interventions with cost-effectiveness ratios of less than the per capita gross domestic product (GDP) (US$1,068) to be very cost-effective as suggested by the Commission on Macroeconomics and Health. Sensitivity analyses are conducted to assess the impact of parameter uncertainty.
The Model
The computer-based Global Maternal Health Policy Model simulates the natural history of pregnancy (both planned and unintended) and pregnancy - and childbirth-associated complications (Figure 1). This model defines health states to reflect important characteristics that affect prognosis, quality of life, and resource use. The time horizon incorporates a woman's lifetime and is divided into equal time increments during which women transition from one health state to another. Nonpregnant girls enter the model and in each time period may become pregnant depending on age, use of contraception, and clinical history (Figure 1, upper panel). Once pregnant, women have a chance of spontaneous abortion (i.e., miscarriage), induced abortion, or continued pregnancy. A proportion of induced abortions will be unsafe (i.e., surgical or medical abortion conducted by untrained personnel). Labor and delivery may be associated with a direct complication of pregnancy (e.g., hypertensive disorders of pregnancy, obstructed labor, hemorrhage, sepsis). Case fatality rates are conditional on the type and severity of complication (e.g., moderate sepsis requiring antibiotics versus severe hemorrhage requiring blood transfusion) and underlying comorbidity (e.g., anemia). Nonfatal complications include neurological sequelae, rectovaginal fistula, severe anemia, and infertility (Figure 1, upper panel). In addition to death from maternal complications, women face an annual risk of death from age-specific all-cause mortality.
Fig. 1. Schematic of the model. 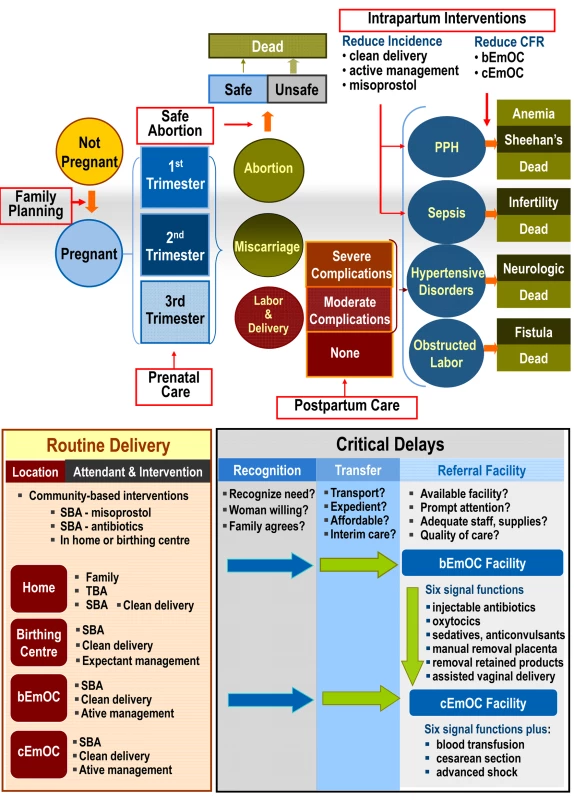
Upper panel: Model simulates the natural history of pregnancy (both planned and unintended) and pregnancy- and childbirth-associated complications. Case fatality rates for complications depend on severity and comorbidity. General intervention categories (open red boxes) include family planning for spacing or limiting births, antenatal or prenatal care (and treatment of anemia), safe abortion, intrapartum care (e.g., active management of labor), basic and comprehensive EmOC, and postpartum care. Interventions can reduce the incidence or severity of a complication or can reduce the case fatality rate through appropriate treatment. Lower panel: Model reflects the intervention pathway during labor and delivery, including location (home, birthing or health center, bEmOC, cEmOC), attendant (family member, traditional birth attendant [TBA], or SBA), and three potential barriers to effective treatment in the event of a complication, including recognition of referral need, transfer (e.g., transport), and timely quality care in an appropriate EmOC facility. Management of labor and delivery depends on attendant (e.g., SBA, clean delivery) and site (e.g., expectant management in birthing center, active management in EmOC facility), as does access to specific levels of treatment (e.g., blood transfusion only available in cEmOC). Strategies in the model to reduce maternal mortality consist of improving coverage of effective interventions, which may be provided individually or packaged as integrated services. In addition to family planning, antenatal care (i.e., prenatal care) and treatment of anemia, safe abortion, and postpartum care, the model includes both intrapartum interventions that reduce the incidence of a complication (e.g., misoprostol for postpartum hemorrhage [PPH], clean delivery for sepsis), as well as those that reduce the case fatality rate through appropriate management in a referral facility (Figure 1, upper panel).
The effectiveness of interventions to either reduce the incidence of complications or to reduce case fatality rates associated with complications depends, in part, on access to specific services (e.g., trained SBA) and to specific levels of facilities (e.g., comprehensive EmOC [cEmOC] with capacity for blood transfusion). Accordingly, the ultimate impact of interventions depends on several setting-specific factors. These include delivery site, presence of birth attendant, quality and type of referral facility, as well as successful referral when necessary. The model therefore explicitly considers the location of delivery, type of assistance, access to basic or comprehensive obstetrical care, and the ability to overcome a series of barriers around the timing of delivery (e.g., recognition of referral need, reliable transport, timely treatment at an appropriate referral facility); these factors collectively determine the health services a woman can access and the specific interventions that would be included (Figure 1, lower panel).
Delivery setting is differentiated by provider (e.g., family member, traditional birth attendant [TBA], or SBA) and by site (e.g., home versus facility). Facility levels are categorized as (1) birthing centers or health centers, which cannot provide all services necessary to qualify as a basic emergency obstetrical care (bEmOC) facility, but are staffed with SBA who provide expectant management of labor and more reliable referral when necessary than with delivery at home; (2) facilities with bEmOC capacity (e.g., first referral units); and (3) facilities with cEmOC capacity (e.g., district hospitals) [26],[27]. Facilities capable of bEmOC are assumed to be capable of administering injectable antibiotics, oxytocics, and sedatives or anticonvulsants, and performing manual removal of placenta, removal of retained products, and assisted vaginal delivery. Facilities capable of cEmOC also are able to provide blood transfusion, cesarean section, and management of advanced shock.
This model also allows us to evaluate phased approaches that involve scaling up access to services over time; the stepwise investments in infrastructure required to assure high-quality intrapartum care are designated as “upgrades.” In addition to reducing unmet need for family planning and unsafe abortion, four consecutively implemented strategies increased skilled attendants, improved antenatal/postpartum care, incrementally shifted births away from home, and improved the availability and quality of EmOC. For women delivering at home or in birthing centers, these “upgrades” also improved recognition of referral need, access to transport, and expedient referral to an appropriate facility (Figure 2).
Fig. 2. Stepwise improvements in scaling up maternal services. 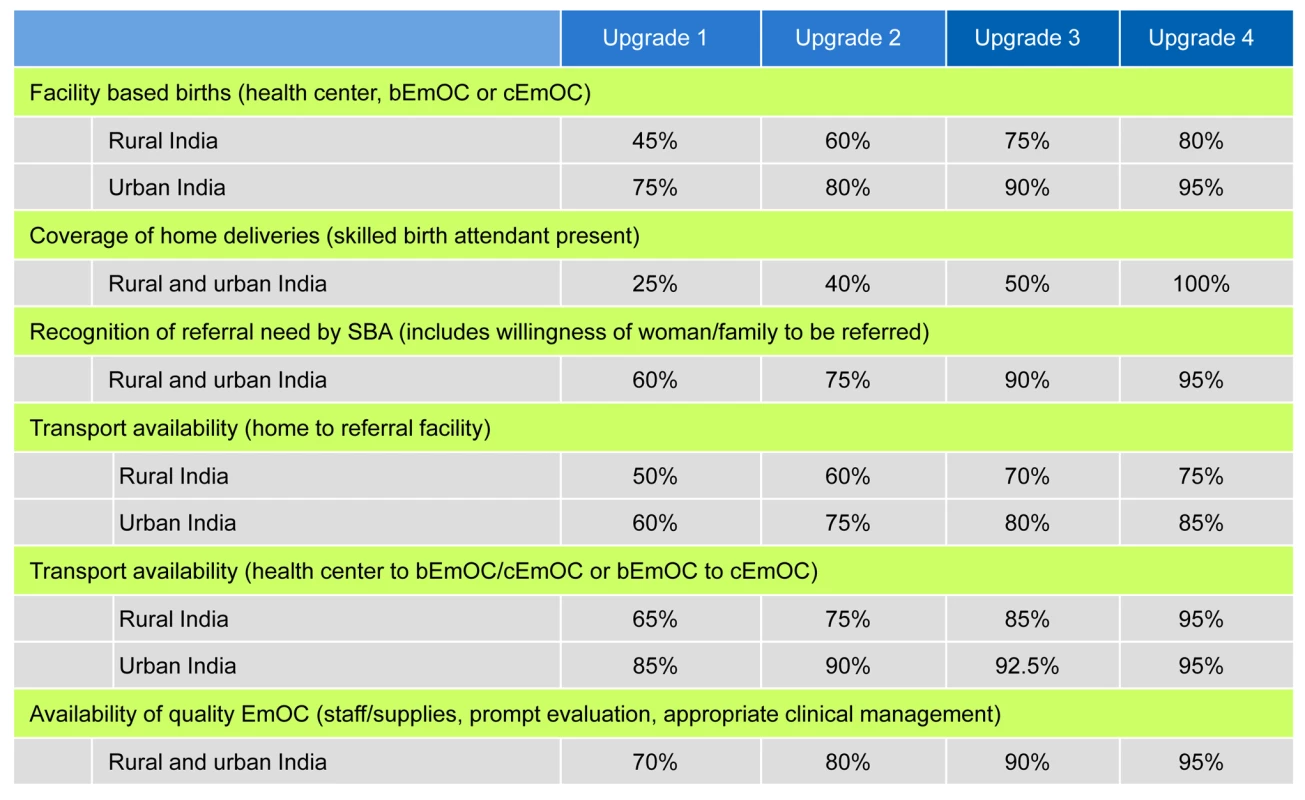
Four strategies that scale up access to critical maternal health services in consecutive phases are designated as upgrade 1, upgrade 2, upgrade 3, and upgrade 4. Shown are the percent increases in facility-based delivery, SBAs, recognition of referral need (by SBA at birthing/health center), transport (to appropriate referral facility), and availability/quality of EmOC (including adequate staff/supplies, appropriate clinical treatment, immediate attention), for rural and urban India. Shifts from home births assume a 70% shift to health centers/birthing centers and a 30% shift to EmOC; for routine births in EmOC, we assume 90% bEmOC and 10% cEmOC. Alternatives evaluated in sensitivity analysis (Results and Text S1). All models were built using TreeAge Pro 2008 (TreeAge Software Inc.) and analyzed using IBM/Lenovo Dual-Core VT Pro Desktop computers running Microsoft Windows XP, using Microsoft Excel 2007 and Visual Basic for Applications 6.5 (Microsoft Corp.). We used Monte Carlo simulation to generate the number of per woman events such as pregnancies, live births, facility-based births, and maternal complications.
Data
Selected parameters and assumptions used in the model are provided in Tables 1 and 2 [7],[14],[15],[17],[22],[23],[28]–[73]. Additional details are provided in Text S1. Initial estimates of incidence and case fatality rates associated with pregnancy-related complications were obtained from published data, and a plausible range for sensitivity analysis was based on review of the literature. Case fatality rates were adjusted based on complication severity (e.g., life-threatening complications requiring cEmOC) and underlying severity of anemia (Table 1; Text S1). The effectiveness of interventions to reduce the incidence of complications (e.g., active management of labor) was estimated from published studies using methods detailed in Text S1. The effectiveness of interventions to reduce case fatality rates was from published studies and assumed treatment in an appropriate facility; a wide plausible range was used for sensitivity analyses (Table 1; Text S1). Data on facility births, SBAs, family planning for spacing or limiting births, and antenatal care were from country-specific surveys [28].
Tab. 1. Selected model parameters: Incidence and mortality of pregnancy and delivery-related complications, and impact of interventions. 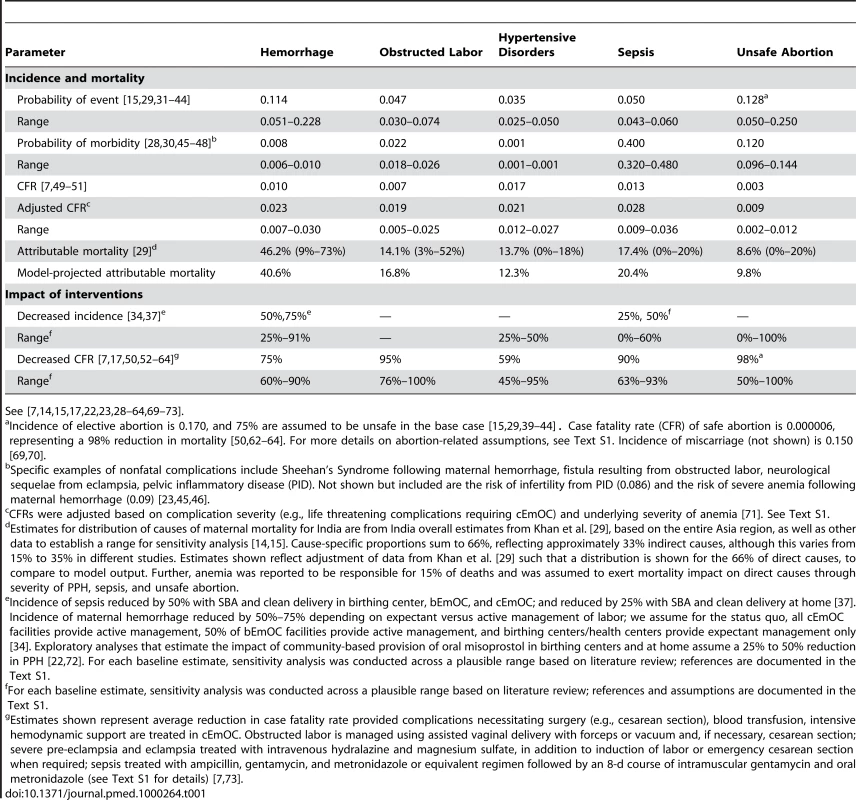
See [7],[14],[15],[17],[22],[23],[28]–[64],[69]–[73]. Tab. 2. Selected model parameters and assumptions: Coverage of interventions and maternal health indicators by setting. 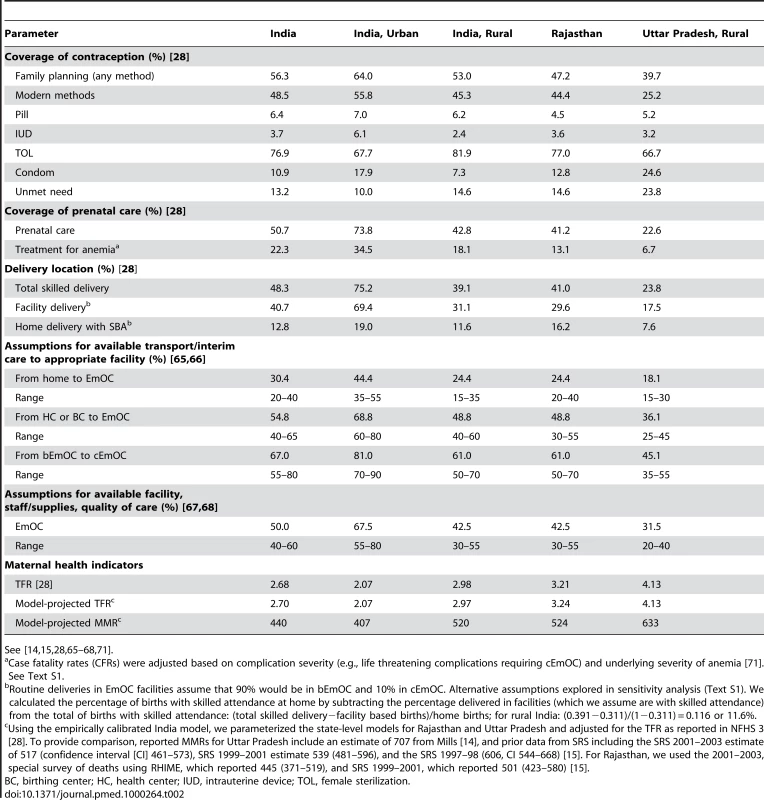
See [14],[15],[28],[65]–[68],[71]. For women delivering at home or in a birthing center, the probability of successful referral depended on overcoming three potential barriers [74]: (1) delay in recognizing referral need; (2) delay in transfer to referral facility (means of transport and interim care en route); (3) delay in receiving appropriate care at the appropriate EmOC facility such as inadequate staffing and supplies, inexpedient attention (e.g., delay to collect fees), and/or non-evidence-based or substandard care. Assumptions about the latter two delays were based on survey data (e.g., National Family Health Survey [NFHS-3] [28],[75], District Level Household Survey [DLHS] [76], and Facility Survey [26]), state-level facility surveys [77],[78], government reports [27], and published studies (Text S1) [14],[15],[79]–[83].
Model performance was assessed by comparing the distribution of direct causes of maternal mortality, life expectancy, proportionate mortality ratio, MMR, and total fertility rate (TFR) to empiric data [2],[14],[15],[28],[29],[32],[38],[84]–[87] in rural and urban India. Model validation was assessed by using state-specific data from Rajasthan and Uttar Pradesh as model inputs, and comparing model-projected indicators of maternal mortality with survey-reported outcomes [14],[15]. In addition, a secondary analysis assessing all strategies evaluated in the base case was conducted in Uttar Pradesh. Details of this process are included in Text S1.
Costs
Selected costs used in the model are provided in Table 3 [18],[25],[80],[88]–[94]. Details are provided in Text S1. With the exception of facility costs, salaries, and transport costs, resource requirements to deliver interventions and the costs of maternal complications were estimated from the United Nations Population Fund's (UNFPA) Reproductive Health Costing Tools Model (RHCTM) [88]. The RHCTM uses an ingredients approach to estimate direct costs (including drugs, supplies, and personnel requirements) of 45 reproductive health interventions, as well as investments required for scale-up. We obtained personnel costs (salaries) and facility costs from public access country-specific databases [25],[89], and drugs and supply costs from the UNICEF Supply Catalogue and Management Sciences for Health (MSH) International Drug Price Indicator Guide [90],[91]. We leveraged public access sources and published studies to inform assumptions about the financial requirements for improving transport and scaling up facility - and human-resource capacity [7],[30],[80],[92],[93]. We assessed the face validity of model input values and established a plausible range for sensitivity analysis by comparing estimates to those in published studies (Text S1). All costs were converted to 2006 US$.
Tab. 3. Selected model input costs. 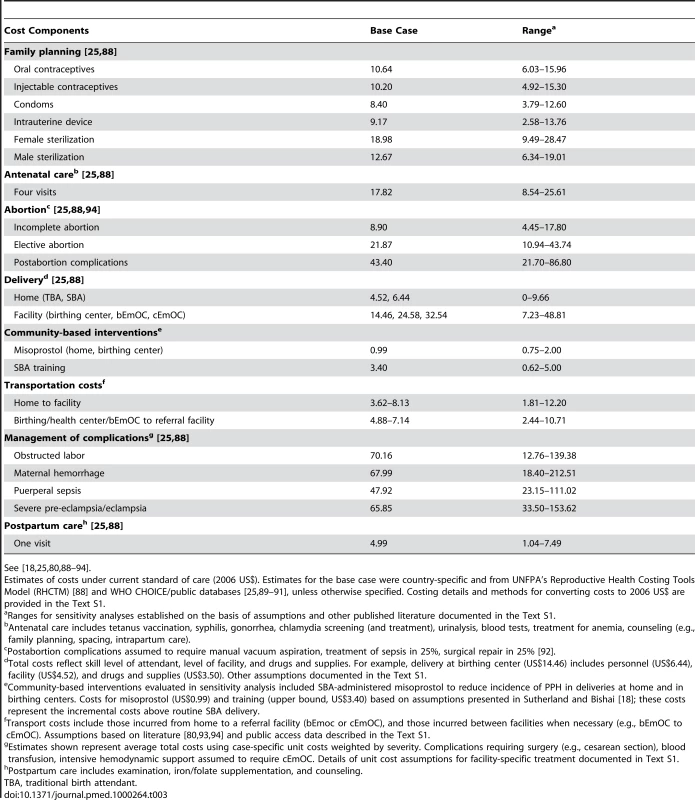
See [18],[25],[80],[88]–[94]. Results
Model Validation
Model-estimated life expectancy for a 15-y-old female was 55 y compared to the World Health Organization's (WHO) estimate of 55.1 y [85]. The distribution of maternal deaths by cause closely approximated published regional estimates (Table 1) [29]. Model-generated estimates of TFR and MMR for India, stratified for rural and urban status, closely approximated survey-reported values [1],[2],[28],[38], as did the state-specific models for Rajasthan and Uttar Pradesh (Table 2) [14],[15]. Model predicted deaths for 2005, taking into account direct and indirect causes of maternal mortality, were 117,657, compared to 117,000 estimated by UNICEF, WHO, and UNFPA [86],[87].
Enhanced Family Planning and Safe Abortion
Increased family planning to reduce the unmet need (for spacing and limiting births) by amounts ranging from 25% to 100%, reduced maternal deaths by amounts ranging from 7.0% to 28.1% in rural India and 5.8% to 23.5% in urban India (Table 4). In rural India, eliminating the unmet need for family planning decreased the TFR from 2.97 to 2.14, the proportion of deaths that are pregnancy related from 16.4% to 12.3%, and the lifetime risk of maternal death from 1 in 65 to 1 in 90. In rural India alone, the cost savings for a single birth cohort of 15-y-old girls (2010) that would be expected to accrue over their reproductive lifespans ranged from US$111.4 million to US$448.2 million. Reducing the unmet need, coupled with provision of safe abortion, provided synergistic benefits and saved additional costs (Table 4). Results were similar in urban India, although the amount of deaths averted and costs saved were smaller, reflecting both the lower initial TFR and the smaller population size (Table 4).
Tab. 4. Health and economic outcomes of family planning to reduce the unmet need for limiting and spacing births, and safe abortion, in rural and urban India. 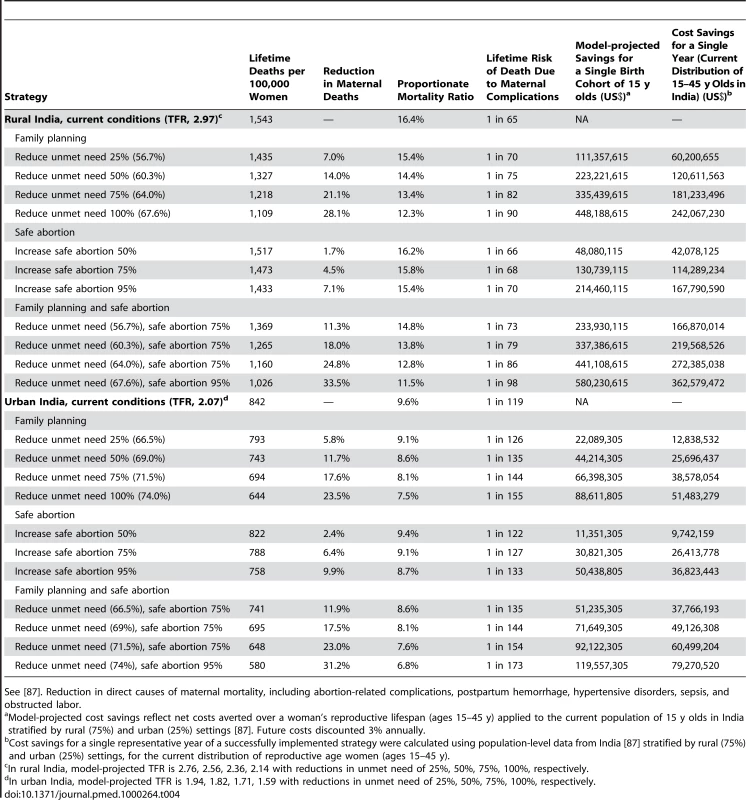
See [87]. Reduction in direct causes of maternal mortality, including abortion-related complications, postpartum hemorrhage, hypertensive disorders, sepsis, and obstructed labor. Increased family planning to reduce the unmet need also reduced the number of deaths attributable to unsafe abortion (Figure 3). For example, in rural India increasing contraceptive rates to 67.6% cut abortion-related deaths by more than 50%—even with no change in rates of unsafe abortion. Adding improved access to safe abortion and postabortion care for three out of four women pursuing elective termination of pregnancy prevented an additional 22% to 50% of abortion-related deaths, depending on the underlying level of unmet need; similarly, the additional cost savings ranged from 22% more, to more than double the savings expected from family planning alone.
Fig. 3. Averted deaths with family planning and safe abortion. 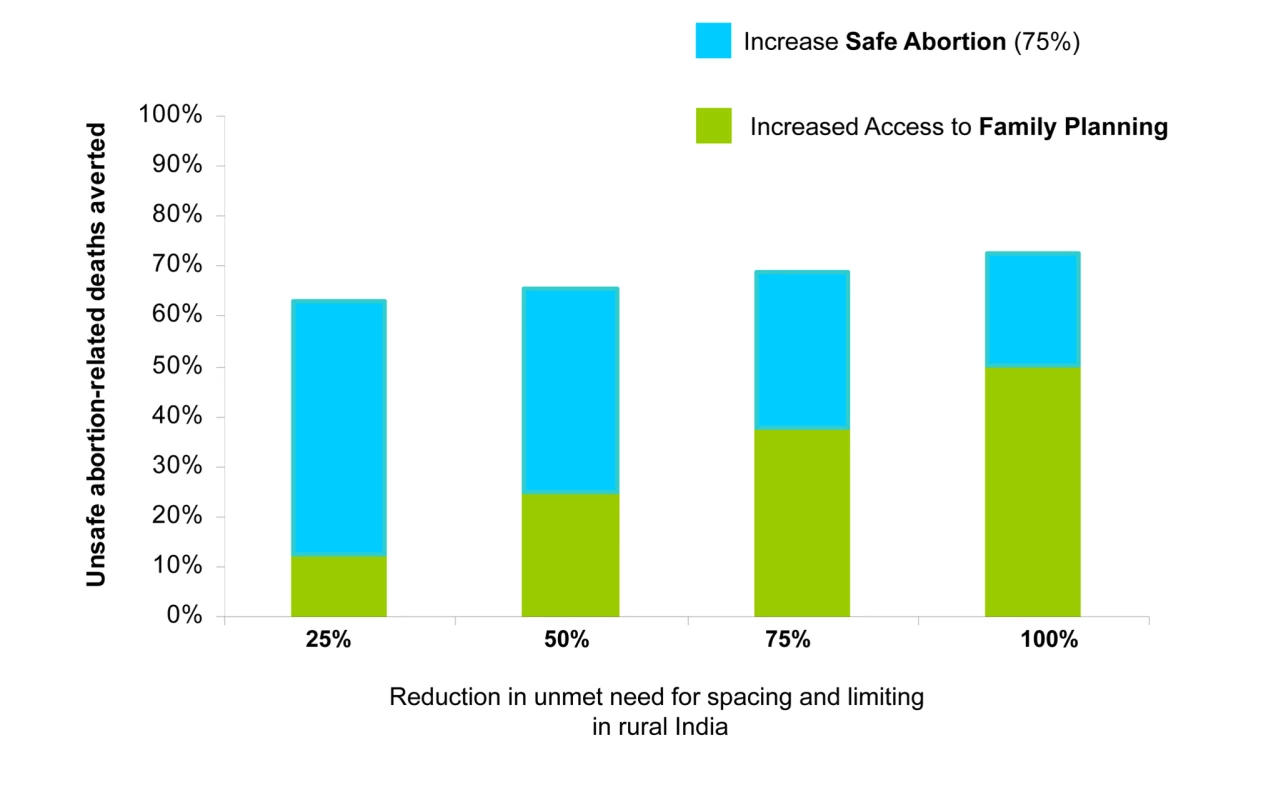
Averted deaths attributable to unsafe abortion in rural India by addressing need for family planning (green shading) and providing 75% safe abortion (blue shading). Magnitude of additional averted abortion-related deaths with improved access to safe abortion depends on the amount of unmet need for contraception. Interventions Packaged as Integrated Services
Our results suggest that reaching the MDG 5 goal of a 75% reduction in maternal mortality would require investments targeting the intrapartum period, in addition to family planning and safer abortion. Without these additional strategies, the model predicts a ceiling on the level of maternal mortality reduction achievable, ranging from 32% in urban India to 34% in rural India.
Table 5 shows the health and economic outcomes associated with interventions packaged as integrated services; these included phased approaches that scale up access to intrapartum services over time in rural (upper section) and urban India (lower section), coupled with incremental improvements in family planning and safe abortion. The four stepwise “upgrades” incorporated improvements in available SBAs for home births, recognition of referral need, transport, and availability/quality of EmOC, as well as shifts from home - to facility-based delivery.
Tab. 5. Health and economic outcomes of integrated packages of services: intrapartum care, family planning, and safe abortion. 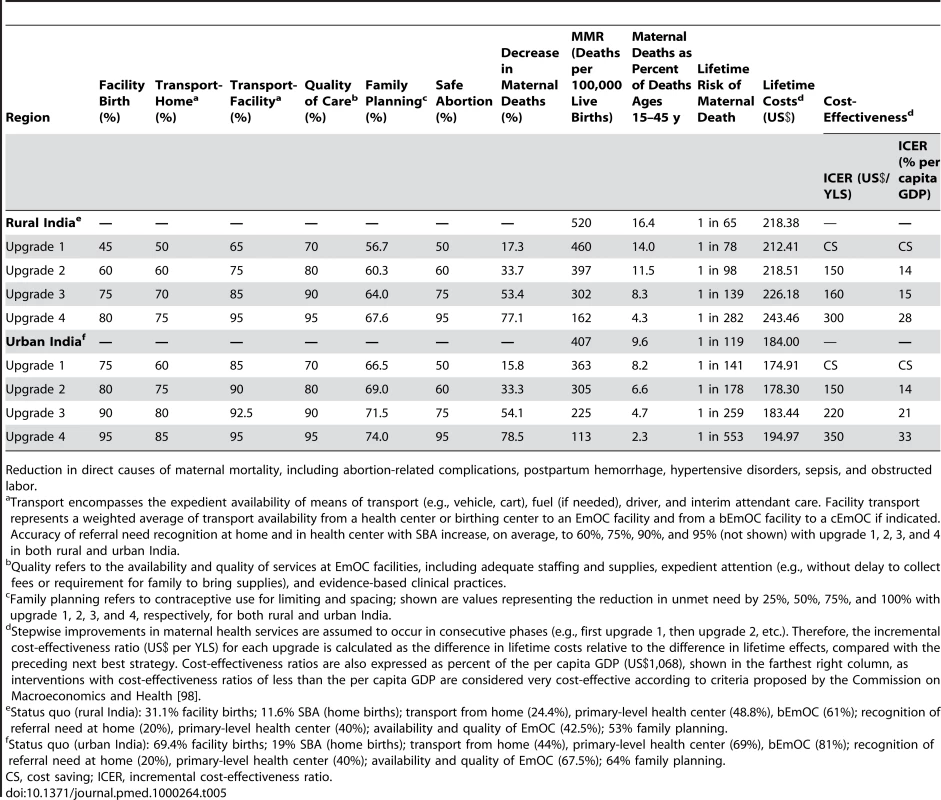
Reduction in direct causes of maternal mortality, including abortion-related complications, postpartum hemorrhage, hypertensive disorders, sepsis, and obstructed labor. Compared to the status quo in rural India (upper section, Table 5), our model predicted that integrated strategies coupling family planning and safe abortion with four consecutive “upgrades” would be expected to reduce maternal deaths by 17.3% to 77.1%, the MMR to less than 200, proportionate mortality ratio by 14% to 4%, and lifetime risk of maternal mortality from one in 78 to one in 282. Compared to the status quo in urban India (Table 4, lower section), similar reductions in maternal deaths were predicted; although the number of absolute lives saved would be lower, the MMR, lifetime risk, and proportionate mortality ratio would be expected to decline to 113, one in 553, and 2.3%, respectively, with the most intensive strategy (upgrade 4).
Because the stepwise improvements in each component of the integrated package (intrapartum care, family planning, and safe abortion) were assumed to occur in consecutive phases, the incremental cost-effectiveness ratio for each “upgrade” strategy was calculated as the difference in costs relative to the difference in effects, compared with the preceding next best strategy. While the initial strategy was cost saving in both urban and rural India, incremental cost-effectiveness ratios ranged from US$150 to US$300 per YLS in rural India and from US$150 to US$350 per YLS in urban India. Cost-effectiveness ratios are also expressed as percent of the per capita GDP (US$1,068). Even the most intensive and effective strategic package was well below 50% of the per capita GDP.
In contrast to these integrated strategies, implementing only the stepwise intrapartum care upgrades—without family planning and safe abortion—was less effective and less cost-effective. The incremental cost-effectiveness ratios ranged from US$490–US$1,060 in rural India and US$200–US$990 per YLS in urban India (Text S1).
Integrated Safe Motherhood Interventions in Rural Uttar Pradesh
Figure 4 (upper panel) displays the health outcomes associated with stepwise approaches to improve maternal health in rural Uttar Pradesh. The vertical axis (from bottom to top) shows outcomes associated with increased access to family planning and safe abortion; the horizontal axis (from left to right) displays outcomes associated with investments in high-quality health-center–based intrapartum care (e.g., facility, attendance, referral, transport, EmOC). In Figure 4 (upper panel) the cell located in the far left lower corner represents current conditions in rural Uttar Pradesh. Each of the other cells represents a unique strategy; the reduction in maternal deaths expected with each strategy, relative to current conditions, is shown. For example, a strategy that reduced the unmet need by 75%, increased safe abortion to 60%, and implemented improvements to intrapartum care consistent with upgrade 3, reduced maternal deaths by 57%.
Fig. 4. Health and economic outcomes in rural Uttar Pradesh. 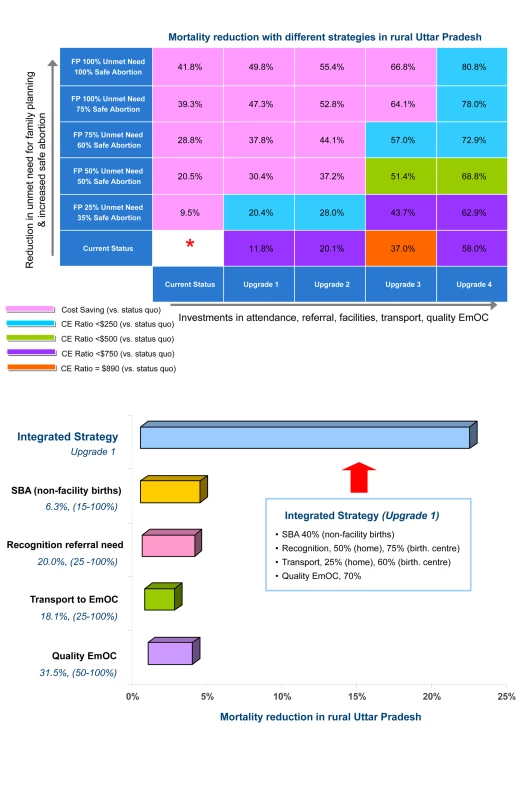
Upper panel. Reduction in maternal deaths and cost-effectiveness with stepwise approaches to improve maternal health in rural Uttar Pradesh. The vertical axis (from bottom to top) shows outcomes associated with increased access to family planning and safe abortion. The horizontal axis (from left to right) displays outcomes associated with investments in high-quality health-center–based intrapartum care, which involved stepwise improvements in SBAs, recognition of referral need, and antenatal/postpartum care, incrementally shifted births away from home, and improved transport, availability, and quality of EmOC. Each cell represents a unique strategy; the reduction in maternal deaths shown is relative to current conditions (far lower left corner). Shading reflects cost-effectiveness ratios, compared to status quo (pink, cost saving; blue, <US0/YLS; green, <US0/YLS; purple, <US0/YLS; orange, US0/YLS). See text for details. Lower panel. Sensitivity analysis depicting the impact of improving only one component of intrapartum care services in rural Uttar Pradesh. Base case and improvements for each component are shown in numbers below the component name (e.g., quality of EmOC is 31.5% in the base case, improvements of 50%–100% were assessed). In settings where most deliveries occur at home, linkage of services in multiple domains is a critical determinant of reduction in maternal deaths. Even large improvements in skilled birth attendants for nonfacility births (yellow bar), recognition of referral need (pink bar), transport (green bar), or quality of EmOC (purple bar) fail to reduce mortality more than 2%–5% if other interdependent components are not improved as well. In contrast, even modest improvements in all four components, as shown in upgrade 1 (blue bar), reduce mortality by 22%. Cost-effectiveness of the integrated strategy ranged from cost saving to US0 per YLS compared with US0 to US,900 per YLS for single unlinked improvements. In Figure 4 (upper panel) each cell is also color-coded to reflect the cost-effectiveness profile associated with the particular strategy. Strategies that only employed family planning and safe abortion (vertical axis, from bottom to top) were generally cost saving, but reduced mortality by a maximum of 40%. Strategies that only invested in intrapartum care improvements (horizontal axis, left to right) were generally associated with the highest cost-effectiveness ratios (i.e., least attractive), reflecting the higher costs required for infrastructure improvements. An overarching strategic approach that moves along the diagonal, from the lower left corner to the upper right corner, was most effective and cost-effective; the cost savings from enhanced family planning and safe abortion offset the resources required to improve intrapartum care.
Sensitivity Analyses
For deliveries at home and in birthing centers in rural Uttar Pradesh, removing only one “delay” in accessing EmOC had minimal impact (<5%) on lowering maternal mortality and was not cost-effective (e.g., US$700–US$4,900 per YLS) (Figure 4, lower panel). In contrast, an integrated strategy that made modest improvements in all components (e.g., SBA, referral, transport, and quality) reduced mortality by 22%. Cost-effectiveness of an integrated strategy ranged from cost saving to US$170 per YLS (Text S1).
Universal antenatal care by itself averted fewer than 2% of maternal deaths; however, if enhanced antenatal care increased the probability of either facility-based delivery or SBA-attended birth (linked with accurate referral and transport) from 31% to 60%, health benefits increased 5-fold (Text S1).
As a greater proportion of routine deliveries shifted from home to facilities, we assumed 70% would shift to birthing centers or health centers staffed by SBA and 30% to facilities with full EmOC capacity. Although the differential benefits of routine delivery in birthing/health centers versus bEmOC was dependent on expedient transfer from a center to referral EmOC if needed, provided this was assured, both approaches were cost-effective. In contrast, when we varied assumptions about the proportion of routine deliveries in cEmOC versus bEmOC, cost-effectiveness results changed drastically; as routine deliveries shifted to cEmOC, the incremental cost-effectiveness ratios became much less attractive, ranging from US$8,300 to US$27,000 per YLS.
Table 6 shows the potential incremental benefits and cost-effectiveness of adjunctive community-based SBA-administered misoprostol for births at home and birthing centers/health centers in rural India. For all four “upgrade” strategies, additional lives could be saved; depending on the phase of improvements in intrapartum care, an additional 7%–13% of maternal deaths were prevented. Cost-savings for a single birth cohort of 15-y-old girls (2010) expected to accrue over their reproductive lifespan (age 15–45 y) ranged from US$128 million to US$190 million.
Tab. 6. Incremental benefits of community-based misoprostol in rural India. 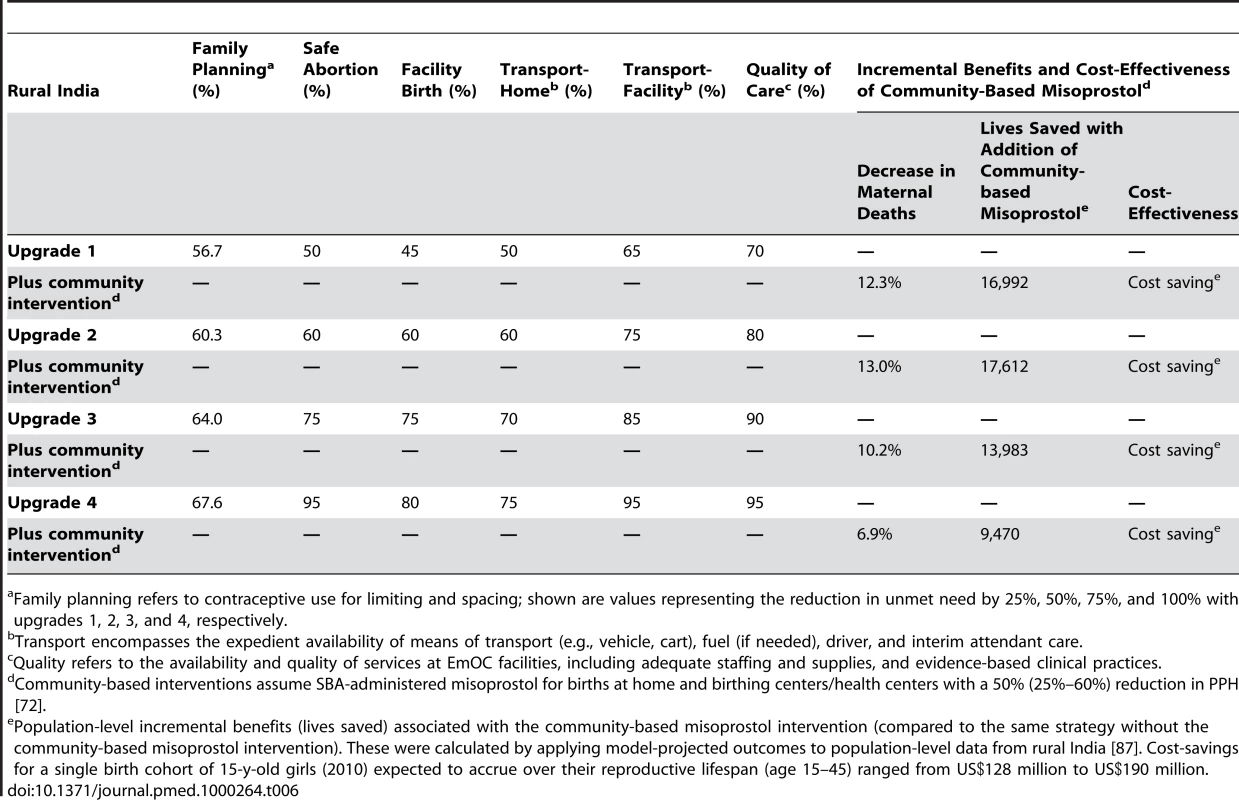
Family planning refers to contraceptive use for limiting and spacing; shown are values representing the reduction in unmet need by 25%, 50%, 75%, and 100% with upgrades 1, 2, 3, and 4, respectively. Discussion
We have identified several strategic options that would cost-effectively reduce maternal mortality in both rural and urban India. Our principal findings are that early intensive efforts to improve family planning and provide safe abortion, accompanied by a systematic stepwise effort to scale up intrapartum and EmOC, could reduce maternal mortality by 75%. Despite the inherent uncertainty in data and assumptions used in the analysis, four critical themes emerge as robust.
First, increasing effective family planning is the most effective individual intervention to reduce pregnancy-related mortality. If the unmet need was met in rural and urban India by 2012, our results imply that the lives of 168,000 women would be saved by the end of 2015. The cost savings over that time period would exceed US$1 billion. Because strategies to increase contraceptive options for limiting and spacing do not require the same level of infrastructure as improving intrapartum care, targeting these strategies toward rural areas with high TFRs is a promising way to initiate equitable improvements in maternal health.
Second, two distinct—yet synergistic—approaches, family planning and safe abortion, can reduce deaths from unsafe abortion. Enhanced access to family planning by itself reduces demand for elective abortion and consequently reduces deaths attributable to unsafe abortion. In fact, reducing the unmet need for contraception can prevent one of every two abortion-related deaths. Furthermore, just a fraction of the cost savings from family planning would fully fund an intervention to provide safe abortion and postabortion care.
Third, despite the substantial health and economic benefits associated with family planning and safe abortion, there is a threshold above which further reductions in mortality are impossible. MDG 5 will therefore not be achievable without involving integrated interventions that ensure reliable access to high-quality intrapartum and EmOC. These interventions could be implemented, however, in a staged, scale-up fashion.
While formidable effort and financial investment would be required to scale up maternal health services over time, we identified a number of phased approaches that would ultimately prevent four out of five maternal deaths. Coupled with stepwise improvements in family planning and safe abortion, these approaches incrementally shifted home births to birthing centers or facilities with EmOC, and improved both access to SBAs as well as accurate recognition of referral need, transport, and availability/quality of EmOC. Successful implementation of these strategies would be expected to dramatically reduce the MMR, proportionate mortality ratio, and lifetime risk of maternal death.
Fourth, despite the possible variation in pace associated with scaling up maternal health services in India, systematic and consecutive phases will be cost-effective. Our results showed that—when coupled with family planning and safe abortion—both early initial strategies and late intensive strategies resulted in cost-effectiveness ratios that were just a fraction of India's per capita GDP; these would unarguably be considered very cost-effective [95]–[98].
Although our general findings are consistent with earlier suggestions that interventions to reduce maternal mortality are good public health investments [7],[17], our analytic approach also allowed us to identify more and less efficient ways to achieve this. In this regard, we highlight four robust insights: (1) In settings with limited infrastructure, investing in “intermediate” facilities (e.g., birthing centers) is very cost-effective, provided there is reliable referral capacity and transport to an appropriate EmOC facility if necessary; (2) A strategy of routine hospital-based delivery (i.e., cEmOC) is not cost-effective; (3) A community-based strategy that includes SBA-administered oral misoprostol in homes and birthing centers is likely to be cost-effective. Although alone it cannot substitute for reliable intrapartum care and EmOC, if added to a long-term plan that increases facility-based intrapartum care, it will save additional lives and will reduce costs; (4) Because settings with the highest TFRs and worst maternal health indicators also tend to be those with the greatest need for enhanced health delivery infrastructure, early consistent commitments to provide family planning and safe abortion reduce the total resources required. To place the synergistic benefits of enhanced family planning and safe abortion in context, the magnitude of cost savings from eliminating unmet need and ensuring access to safe abortion is approximately 25% of the required 10-y investment estimated by Johns et al. for scaling up maternal services in India [30].
We may have underestimated both effectiveness and cost-effectiveness by excluding effects of certain indirect indicators and interventions. Although we purposely focused on maternal mortality, if we included neonatal health and survival, for example, most strategies would be even more cost-effective due to associations between place of birth and presence of a skilled attendant, with neonatal and maternal deaths [99],[100]. With the exception of anemia, we focused on direct causes of maternal mortality; a priority for future analyses is to include interventions to reduce the indirect causes of pregnancy-related mortality. The analysis would be strengthened by availability of indicators that reflect safe motherhood externalities including measures of enhanced household well-being, increased school attendance, decreased numbers of orphans, and reduced impoverishment resulting from catastrophic expenses [94],[99].
Other limitations in our analysis stem from its inherent reliance on high-quality data about maternal mortality specifically. While our calibration of setting-specific models allows us to better represent within-country differences in baseline risk, coverage, and capacity than previous studies, high-quality empiric evidence for the effectiveness of comprehensive strategies to reduce maternal mortality and morbidity is often either lacking or inconsistent. More studies quantifying the benefits of community-level interventions on preventing maternal morbidity remain a priority [100]. The additional costs that we assumed would be required to scale up interventions and build infrastructure are, at best, gross estimates. That being said, our assumption of 2 - to 3-fold increases in the per woman costs to reflect the additional resources required to improve capacity is consistent with those implied by recent analyses assessing global resource needs for maternal health [30],[93].
Shiffman and Smith [101] have described the importance of framing priority public health issues in a manner that resonates with both the “internal” community and other “external” decision makers. With regard to the internal community, the strategies we identified as most effective support three crucial elements already recognized as essential to achieve MDG 5: family planning and control of fertility choices, provision of safe abortion, and assurance that all women have access to intrapartum care and EmOC. Our results reinforce this message, and extend it by quantifying the cost savings of family planning and safe abortion, and identifying efficient and cost-effective approaches to scaling-up capacity for integrated maternal health services.
With regard to the external community, we have tried to provide a range of outcomes that can be used to create effective “take home” messages for different target audiences. For example, in only 5 y, more than 150,000 lives could be saved just from increasing contraception rates by a few percentage points; nearly US$1.5 billion could be saved by adding safe abortion to family planning efforts; and finally, with stepwise investments to provide facility-based intrapartum care, the majority of maternal deaths could be prevented. In the next decade, this accomplishment would save the lives of 1 million Indian women.
Finally, by placing and prioritizing safe motherhood in the context of other global health priorities [101],[102], our results can also be effectively framed for policymakers who must allocate limited resources, by providing comparative and contextual information about the relative benefits and cost-effectiveness of investments in maternal health measured against other public health priorities. One of the robust findings of our analysis, for example, is that there are integrated strategies that involve improvements in family planning, safe abortion, and intrapartum care that are equally or more cost-effective or attractive than childhood immunization or treatment of malaria, tuberculosis, or HIV [95].
The Indian government has initiated several policies to improve maternal health [5], particularly in rural areas [27],[103], and efforts to both implement and evaluate new strategies are ongoing [83],[94],[104]–[106]. Although our analysis is intended to catalyze actionable steps, we recognize that decisions in India about the choice of strategies and rate of stepwise investments to reduce maternal mortality will be a function not only of cost-effectiveness and affordability, but also of political will and local circumstances. Identifying approaches that can be tailored to local situations, but that rely on firm core principles and are cost-effective, holds considerable promise as a way to mobilize further political support and convince stakeholders that MDG 5 is within reach.
In particular, it is clear from our analysis that an initial focus on family planning, especially in rural poor areas, will significantly prevent pregnancy-related deaths, reduce deaths from unsafe abortion, and save resources. Providing universal access to safe abortion will further augment these benefits. The cost savings from these two strategies will partially offset the resources required to invest in the necessary infrastructure that would assure every woman access to high-quality intrapartum care and EmOC. While MDG 5 is unlikely to be met without assuring access to health-center–based intrapartum care, implementation of a phased stepwise approach, designed to reach this goal while reflecting the current realities and most feasible initial approaches in different settings, is absolutely within reach.
Supporting Information
Zdroje
1. World Health Organization (WHO) 2007 Maternal mortality in 2005: estimates developed by WHO, UNICEF, UNFPA, and The World Bank. Available: http://www.who.int/whosis/mme_2005.pdf. Accessed 19 August 2009
2. HillK
ThomasK
AbouZahrC
WalkerN
SayL
2007 Estimates of maternal mortality worldwide between 1990 and 2005: an assessment of available data. Lancet 370 1311 1319
3. United Nations (UN) Millennium Development Goals: Goal 5 (MDG 5). Improve maternal health. Available: http://www.un.org/millenniumgoals/maternal.shtml. Accessed 31 July 2009
4. World Health Organization (WHO) 2005 Millennium Development Goals and Health–India. Chapter III: Goal 5: improve maternal health. Geneva: WHO. Available: http://www.whoindia.org/LinkFiles/MDG_Chapter-03.pdf. Accessed 31 July 2009
5. ShiffmanJ
VedR
2007 The state of political priority for safe motherhood in India. BJOG 114 785 90
6. RonsmansC
GrahamWJ
on behalf of The Lancet Maternal Survival Series steering group 2006 Maternal mortality: who, when, where and why. Lancet 368 1189 1200
7. GrahamWJ
CairnsJ
BhattacharyaS
BulloughCHW
QuayyumZ
2006 Chapter 26: Maternal and perinatal conditions.
JamisonDT
BremanJG
MeashamAR
AlleyneG
ClaesonM
Disease control priorities in developing countries. 2nd edition New York Oxford University Press 499 530 Available: http://www.dcp2.org/pubs/DCP/26/. Accessed 23 August 2009
8. CampbellOM
GrahamWJ
Lancet Maternal Survival Series steering group 2006 Strategies for reducing maternal mortality: getting on with what works. Lancet 368 1284 99
9. RosenfieldA
MaineD
FreedmanL
2006 Meeting MDG-5: an impossible dream? Lancet 368 1133 1135
10. Government of India 2005 Reproductive and Child Health Programme Document (RCH II–Document 2): the principles and evidence base for State RCH II Programme Implementation Plans (PIPs). New Delhi, India: Government of India. Available: http://www.whoindia.org/LinkFiles/Child_Health_in_India_PIP_Doc_Chapter01.pdf. Accessed 21 August 2009
11. Government of India, Ministry of Health and Family Welfare, Department of Family Welfare 2005 National rural healthcare mission. New Delhi, India: Government of India. Available: http://www.mohfw.nic.in/NRHM/Documents/NRHM%20Mission%20Document.pdf. Accessed 21 August 2009
12. ShiffmanJ
2007 Generating political priority for maternal mortality reduction in 5 developing countries. Am J Public Health 97 796 803
13. CostelloA
AzadK
BarnettS
2006 An alternative strategy to reduce maternal mortality. Lancet 368 1477 1479
14. MillsS
BosE
LuleE
RamanaGNV
BulataoR
2007 Obstetric care in poor settings in Ghana, India, and Kenya. Washington (D.C.): The World Bank. Available: http://www-wds.worldbank.org/servlet/main?menuPK=64187510&pagePK=64193027&piPK=64187937&theSitePK=523679&entityID=000310607_20080123112201. Accessed 27 July 2009
15. Registrar General, India 2006 Maternal mortality in India: 1997–2003; trends, causes and risk factors. New Delhi, India. Available: http://www.mp.gov.in/health/Maternal_Mortality_in_India_1997-2003.pdf. Accessed 22 August 2009
16. GoldieSJ
Goldhaber-FiebertJD
GarnettGP
2006 Public health policy for cervical cancer prevention: role of decision science, economic evaluation, and mathematical modeling. Vaccine 24 S155 S163
17. AdamT
LimSS
MehtaS
BhuttaZA
FogstadH
2005 Cost effectiveness analysis of strategies for maternal and neonatal health in developing countries. BMJ 331 1107
18. SutherlandT
BishaiD
2009 Cost-effectiveness of misoprostol and prenatal iron supplementation as maternal mortality interventions in home births in rural India. Int J Gynaecol Obstet 104 189 193
19. LevineR
LangerA
BirdsallN
MathenyG
WrightM
2006 Contraception. Disease control priorities in developing countries. 2nd edition New York Oxford University Press 1075 1090 Available at: http://www.dcp2.org/pubs/DCP
20. Terris-PrestholtF
Watson-JonesD
MugeyeK
KumaranayakeL
NdekiL
2003 Is antenatal syphilis screening still cost effective in sub-Saharan Africa. Sex Transm Infect 79 375 381
21. NakhaeeN
MirahmadizadehAR
GorjiHA
MohammadiM
2002 Assessing the cost-effectiveness of contraceptive methods in Shiraz, Islamic Republic of Iran. East Mediterr Health J 8 55 63
22. PagelC
LewyckaS
ColbournT
MwansamboC
MequidT
2009 Estimation of potential effects of improved community-based drug provision, to augment health-facility strengthening, on maternal mortality due to post-partum haemorrhage and sepsis in sub-Saharan Africa: an equity-effectiveness model. Lancet 374 1441 1448
23. HuD
BertozziSM
GakidouE
SweetS
GoldieSJ
2007 The costs, benefits, and cost-effectiveness of interventions to reduce maternal morbidity and mortality in Mexico. PLoS ONE 2 e750 doi:10.1371/journal.pone.0000750
24. PrataN
SreenivasA
GreigF
WalshJ
PottsM
2010 Setting priorities for safe motherhood interventions in resource-scarce settings. Health Policy 94 1 13
25. World Health Organization (WHO) CHOICE: choosing interventions that are cost-effective. Available: http://www.who.int/choice/en/. Accessed 12 July 2007
26. International Institute for Population Sciences (IIPS) 2005 Reproductive and child health project. Facility survey. DLHS-2, 2003. Available: http://www.rchiips.org/pdf/rch2/National_Facility_Report_RCH-II.pdf. Accessed 1 September 2009
27. Government of India 2008 Bulletin on rural health statistics in India. Rural health system in India. Available: http://www.mohfw.nic.in/Bulletin%20on%20RHS%20-%20March,%202008%20-%20PDF%20Version/Rural%20Health%20Care%20System%20in%20India.pdf. Accessed 1 September 2009
28. International Institute for Population Sciences (IIPS) and ORC Macro 2007 National Family Health Survey (NFHS-3), 2005–06: India. Mumbai: IIPS. Available: http://www.nfhsindia.org/nfhs3_national_report.html. Accessed 23 August 2009
29. KhanKS
WojdylaD
SayL
GulmezogluAM
Van LookPF
2006 WHO analysis of causes of maternal death: a systematic review. Lancet 367 1066 1074
30. JohnsB
SigurbjornsdottirK
FogstadH
ZupanJ
2007 Estimated global resources needed to attain universal coverage of maternal and newborn health services. Bull World Health Organ 85 256 263
31. World Health Organization (WHO) 2002 Estimates of DALYs by sex, cause and WHO mortality sub-region, estimates for 2001. Geneva: WHO. Available: http://www.who.int/healthinfo/global_burden_disease/estimates_regional_2001/en/index.html. Accessed 23 August 2009
32. World Health Organization (WHO) 2007 World Health Statistics. Available: http://www.who.int/healthinfo/statistics/gbdwhoregionincidence2002.xls. Accessed 7 May 2009
33. UNICEF 2004 State of the world's children. Available: http://www.unicef.org/sowc/archive/ENGLISH/The%20State%20of%20the%20World%27s%20Children%202004.pdf. Accessed 7 May 2009
34. DoleaC
AbouZahrC
SteinC
2003 Global burden of maternal hemorrhage in the year 2000. Geneva, Switzerland: World Health Organization. Available: http://www.who.int/healthinfo/statistics/bod_maternalhaemorrhage.pdf. Accessed 23 August 2009
35. DoleaC
AbouZahrC
2003 Global burden of obstructed labor in the year 2000. Geneva, Switzerland: World Health Organization. Available: http://www.who.int/healthinfo/statistics/bod_obstructedlabour.pdf. Accessed 23 August 2009
36. DoleaC
AbouZahrC
2003 Global burden of hypertensive disorders of pregnancy in the year 2000. Geneva, Switzerland: World Health Organization. Available: http://www.who.int/healthinfo/statistics/bod_hypertensivedisordersofpregnancy.pdf. Accessed 23 August 2009
37. DoleaC
SteinC
2003 Global burden of maternal sepsis in the year 2000. Geneva, Switzerland: World Health Organization. Available: http://www.who.int/healthinfo/statistics/bod_maternalsepsis.pdf. Accessed 23 August 2009
38. AbouZahrC
WardlawT
2004 Maternal mortality in 2000: estimates developed by WHO, UNICEF and UNFPA. Geneva: World Health Organization. Available: http://www.who.int/reproductivehealth/publications/monitoring/9241562706/en/index.html. Accessed 23 August 2009
39. HenshawSK
SinghS
HaasT
1999 The incidence of abortion worldwide. Int Fam Plann Persp 25 S30 S38
40. ShahI
AhmanE
2004 Age patterns of unsafe abortion in developing country regions. Reprod Health Matters 12 S9 S17
41. BererM
2004 National laws and unsafe abortion: the parameters of change. Reprod Health Matters 12 1 8
42. The Alan Guttmacher Institute (AGI) 2006 Preventing unsafe abortion and its consequences: priorities for research and action. New York: Alan Guttmacher Institute. Available: http://www.guttmacher.org/pubs/2006/07/10/PreventingUnsafeAbortion.pdf. Accessed 24 August 2009
43. The Alan Guttmacher Institute (AGI) 2007 Facts on induced abortion worldwide: worldwide incidence and trends. Available: http://www.guttmacher.org/pubs/fb_IAW.html. Accessed 18 August 2009
44. LuleE
SinghS
ChowdurySA
2007 Fertility regulation behaviors and their costs: contraception and unintended pregnancies in Africa and Eastern Europe & Central Asia. Washington (D.C.): World Bank. Available: http://siteresources.worldbank.org/HEALTHNUTRITIONANDPOPULATION/Resources/281627-1095698140167/FertilityRegulationsFinal.pdf. Accessed 24 August 2009
45. MurrayCJL
LopezAD
1996 Estimating causes of death: new methods and global and regional applications for 1990.
MurrayCJL
LopezAD
The global burden of disease, vol. 1 of Global burden of disease and injury series Cambridge Harvard University Press 117 200
46. MurrayCJL
LopezAD
1998 Health dimensions of sex and reproduction. Cambridge Harvard School of Public Health
47. AhmanE
DoleaC
ShahI
2006 The global burden of unsafe abortion in the year 2000, Geneva: World Health Organization, Available: http://www.who.int/healthinfo/statistics/bod_abortions.pdf. Accessed 4 August 2008
48. SinghS
2006 Hospital admissions resulting from unsafe abortion: estimates from 13 developing countries. Lancet 368 1887 1892
49. BiswasAB
DasDK
MisraR
RoyRN
GhoshD
2005 Availability and use of emergency obstetric care services in four districts of West Bengal, India. J Health Popul Nutr 23 266 274
50. AhmanE
ShahI
2007 Unsafe abortion: global and regional estimates of unsafe abortion and associated mortality in 2003 (5th edn). Geneva: World Health Organization. Available: http://www.who.int/reproductivehealth/publications/unsafe_abortion/9789241596121/en/index.html. Accessed 24 August 2009
51. AhmanE
ShahI
2004 Unsafe abortion: global and regional estimates of unsafe abortion and associated mortality in 2000 (4th edn). Geneva: World Health Organization. Available: http://www.who.int/reproductivehealth/publications/unsafe_abortion/9241591803/en/index.html. Accessed 24 August 2009
52. GulmezogluAM
VillarJ
NgocNT
PiaggioG
CarroliG
2001 WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 358 689 695
53. JohansonRB
MenonBK
2000 Vacuum extraction versus forceps for assisted vaginal delivery. Cochrane Database Syst Rev 2 CD000224
54. HofmeyrGJ
KulierR
2000 External cephalic version for breech presentation at term. Cochrane Database Syst Rev 2 CD000083
55. HofmeyrGJ
HannahME
2003 Planned Caesarean section for term breech delivery. Cochrane Database Syst Rev 3 CD000166
56. SchuitemakerN
van RoosmalenJ
DekkerG
Van DongenP
van GeijnH
1997 Maternal mortality after cesarean section in The Netherlands. Acta Obstet Gynecol Scand 76 332 334
57. Magpie Trial Collaborative Group 2002 Do women with pre-eclampsia and their babies benefit from magnesium sulphate? The Magpie Trial: a randomised placebo controlled trial. Lancet 359 1877 1890
58. DuleyL
GülmezogluA
Henderson-SmartD
2003 Magnesium sulphate and other anticonvulsants for women with eclampsia. Cochrane Database Syst Rev 2 CD000025
59. CrowtherC
HillerJ
DoyleL
2002 Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev 4 CD001060
60. FrenchLM
2003 Prevention and treatment of postpartum endometritis. Curr Womens Health Rep 4 274 279
61. FrenchLM
SmaillFM
2004 Antibiotic regimens for endometritis after delivery. Cochrane Database Syst Rev 4 CD001067
62. KooninLM
SmithJC
RamickM
1993 Abortion surveillance–United States, 1990. MMWR CDC Surveill Summ 42 29 57
63. Institute of Medicine (IOM) 1975 Legalized abortion and the public health; report of a study by a committee of the Institute of Medicine. Washington (D.C.) National Academy of Sciences
64. GrimesDA
2005 Risks of mifepristone abortion in context. Contraception 71 161
65. World Bank 2002 India's transport sector: the challenges ahead. Vol 1. Washington (D.C.): World Bank Group. Available: http://www-wds.worldbank.org/external/default/main?pagePK=64193027&piPK=64187937&theSitePK=523679&menuPK=64187510&searchMenuPK=64187283&theSitePK=523679&entityID=000094946_02070604022321&searchMenuPK=64187283&theSitePK=523679. Accessed 23 August 2009
66. PMGSY (Pradhan Mantri Gram Sadak Yojana) 2006 Briefing book. Available: http://pmgsy.nic.in/. Accessed 27 July 27 2009
67. KatrakH
2008 Measuring the shortage of medical practitioners in rural and urban areas in developing countries: a simple framework and simulation exercise with data from India. Int J Health Plann Manage 23 93 105
68. MaS
SoodN
2008 A comparison of the health systems in China and India. Rand Corporation. Available: http://www.rand.org/pubs/occasional_papers/2008/RAND_OP212.pdf. Accessed 27 July 2009
69. HarlapS
ShionoPH
RamcharanS
1980 A life table of spontaneous abortions and the effects of age, parity and other variables.
PorterIH
HookEB
Human embryonic and fetal death New York Academic Press 145 158
70. MenkenJ
RahmanMO
2006 Chapter 3: Reproductive health.
MersonMH
BlackRE
MillsAJ
International public health: diseases, programs, systems, and policies, 2nd ed Sudsbury (Massachusetts) Jones and Bartlett Publishers 71 125
71. BrabinBJ
HakimiM
PelletierD
2001 An analysis of anemia and pregnancy-related maternal mortality. J Nutr 131 604S 614S
72. HofmeyrGJ
GulmezogluAM
2008 Misoprostol for the prevention and treatment of pospartum hemorrhage. Best Practice & Research Clinical Obstet and Gynaec 22 1025 1041
73. Cahuana-HurtadoL
Sosa-RubiS
BertozziS
2004 The application of the mother baby package reproductive health costing spreadsheet in Morelos: National Institute of Public Health, Division of Health Economics and Policy, Mexico
74. ThaddeusS
MaineD
1994 Too far to walk: maternal mortality in context. Soc Sci Med 38 1091 1110
75. International Institute for Population Sciences (IIPS) and ORC Macro 2007 National Family Health Survey (NFHS-3), 2005–06: India. Maternal health, Chapter 8. Mumbai: IIPS. 191–222 Available: http://www.nfhsindia.org/NFHS-3%20Data/VOL-1/india_volume_I_chapter_8_corrected_for_website_17oct08.pdf. Accessed 23 August 2009
76. International Institute for Population Sciences (IIPS) 2006 Reproductive and Child Health Project. District Level Household Survey. DLHS-2. 2002–2004. Available: http://www.rchiips.org/pdf/rch2/National_Report_RCH-II.pdf. Accessed 1 September 2009
77. International Institute for Population Sciences (IIPS) 2007 Fact sheet: Uttar Pradesh. Reproductive and Child Health Project. District Level Household and Facility Survey. DLHS-3. Available: http://www.rchiips.org/pdf/rch3/state/Uttar-Pradesh.pdf. Accessed 1 September 2009
78. International Institute for Population Sciences (IIPS) 2007 Fact sheet: Rajasthan. Reproductive and Child Health Project. District Level Household and Facility Survey. DLHS-3. Available: http://www.rchiips.org/pdf/rch3/state/Rajsthan.pdf. Accessed 1 September 2009
79. VoraKS
MavalankarDV
RamaniKV
UpadhyayaM
SharmaB
2009 Maternal health situation in India: a case study. J Health Popul Nutr 27 184 201
80. IyengarSD
IyengarK
GuptaV
2009 Maternal health: a case study of Rajasthan. J Health Popul Nutr 27 271 292
81. IyengarSD
IyengarK
SuhalkaV
AgarwalK
2009 Comparison of domiciliary and institutional delivery-care practices in Rural Rajasthan, India. J Health Popul Nutr 27 303 312
82. IyengarK
IyengarSD
SuhalkaV
DashoraK
2009 Pregnancy-related deaths in rural Rajasthan, India: exploring causes, context, and care-seeking through verbal autopsy. J Health Popul Nutr 27 293 302
83. MavalankarDV
VoraKS
RamaniKV
RamanP
SharmaB
2009 Maternal health in Gujarat, India: a case study. J Health Popul Nutr 27 235 248
84. AMDD Working Group on Indicators 2002 Program note: using UN process indicators to assess needs in emergency obstetric services: Bhutan, Cameroon and Rajasthan, India. Int J Gynaecol Obstet 77 277 284
85. World Health Organization (WHO) 2006 World Health Report: working together for health. Geneva: World Health Organization. Available: http://www.who.int/whr/2006/en/index.html. Accessed 23 August 2009
86. UNICEF, WHO, UNFPA 2007 Maternal mortality declining in middle-income countries; women still die in pregnancy and childbirth in low-income countries. UNICEF, WHO, UNFPA Joint Press Release. Available: http://www.unicef.org/media/media_41208.html. Accessed 27 July 2009
87. United Nations (UN), Department of Economic and Social Affairs, Population Division 2007 World Population Prospects: the 2006 revision. CD-ROM edition - extended dataset in Excel and ASCII formats. New York United Nations
88. UNFPA 2007 Reproductive Health Costing Tools Model. New York: UNFPA. Available: http://www.who.int/pmnch/topics/economics/costing_tools/en/index15.html. Accessed 27 July 2009
89. International Labour Organization (ILO) Laborsta database. Available: http://laborsta.ilo.org/. Accessed 27 July 2009
90. UNICEF Supply Division. Available: http://www.supply.unicef.dk/catalogue/. Accessed 27 July 2009
91. Management Sciences for Health (MSH). International Drug Price Indicator Guide (IDPIG). Available: http://erc.msh.org/mainpage.cfm?file=1.0.htm&id=1&temptitle=Introduction&module=DMP&language=English. Accessed 27 July 2009
92. DuggalR
2004 The political economy of abortion in India: cost and expenditure patterns. Reprod Health Matters 12 S130 S137
93. BorghiJ
EnsorT
SomanathanA
LissnerC
MillsA
on behalf of The Lancet Maternal Survival Series steering group 2006 Mobilising financial resources for maternal health. Lancet 368 1457 1465
94. BhatR
MavalankarDV
SinghPV
SinghN
2009 Maternal healthcare financing: Gujarat's Chiranjeevi Scheme and its beneficiaries. J Health Popul Nutr 27 249 258
95. JamisonDT
BremanJG
MeashamAR
AlleyneG
ClaesonM
2006 Disease control priorities in developing countries. 2nd edition. Washington (D.C.): The International Bank for Reconstruction and Development/The World Bank. Available: http://www.dcp2.org. Accessed 4 May 2009
96. LaxminarayanR
ChowJ
Shahid–SallesSA
2006 Intervention cost–effectiveness: overview of main messages. Disease control priorities in developing countries. 2nd edition. 35–86. New York: Oxford University Press. Available: http://www.dcp2.org/pubs/DCP/2/FullText. Accessed 4 May 2009
97. BrenzelL
WolfsonLJ
Fox-RushbyJ
MillerM
HalseyNA
2006 Vaccine–preventable diseases. Disease control priorities in developing countries. 2nd edition. 389-412. New York: Oxford University Press. Available: http://www.dcp2.org/pubs/DCP/20/FullText. Accessed 4 May 2009
98. World Health Organization (WHO) 2001 Macroeconomics and health: investing in health for economic development: report of the Commission on Macroeconomics and Health. Geneva World Health Organization
99. EkmanB
PathmanathanI
LiljestrandJ
2008 Integrating health interventions for women, newborn babies, and children: a framework for action. Lancet 372 990 1000
100. BangRA
BangAT
ReddyMH
DeshmukhMD
BaituleSB
2004 Maternal morbidity during labour and the puerperium in rural homes and the need for medical attention: A prospective observational study in Gadchiroli, India. BJOG 111 231 238
101. ShiffmanJ
SmithS
2007 Generation of political priority for global health initiatives: a framework and case study of maternal mortality. Lancet 370 1370 1379
102. FilippiV
RonsmansC
CampbellOM
GrahamWJ
MillsA
2006 Maternal health in poor countries: the broader context and a call for action. Lancet 368 1535 1541
103. National Rural Health Mission 2005–2012 Mission document. Available: http://india.gov.in/outerwin.php?id=http://mohfw.nic.in/NRHM/NRHM%28National%20Rural%20Health%20Mission%29.htm. Accessed 1 September 2009
104. PadmanabanP
RamanPS
MavalankarDV
2009 Innovations and challenges in reducing maternal mortality in Tamil Nadu, India. J Health Popul Nutr 27 202 219
105. PrakasammaM
2009 Maternal mortality-reduction programme in Andhra Pradesh. J Health Popul Nutr 27 220 234
106. McPakeB
KoblinskyM
2009 Improving maternal survival in South Asia-what can we learn from case studies? J Health Popul Nutr 27 93 107
Štítky
Interní lékařství
Článek Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility AnalysisČlánek The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 4- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility Analysis
- The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study
- Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages
- Association between the 2008–09 Seasonal Influenza Vaccine and Pandemic H1N1 Illness during Spring–Summer 2009: Four Observational Studies from Canada
- China's Engagement with Global Health Diplomacy: Was SARS a Watershed?
- Laboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN)
- Mortality Measurement Matters: Improving Data Collection and Estimation Methods for Child and Adult Mortality
- Brazil and the Framework Convention on Tobacco Control: Global Health Diplomacy as Soft Power
- Health Diplomacy and the Enduring Relevance of Foreign Policy Interests
- Measuring Under-Five Mortality: Validation of New Low-Cost Methods
- What Can We Conclude from Death Registration? Improved Methods for Evaluating Completeness
- Improving Newborn Survival in Low-Income Countries: Community-Based Approaches and Lessons from South Asia
- Does Seasonal Influenza Vaccination Increase the Risk of Illness with the 2009 A/H1N1 Pandemic Virus?
- Measuring Adult Mortality Using Sibling Survival: A New Analytical Method and New Results for 44 Countries, 1974–2006
- Early Emergence of Ethnic Differences in Type 2 Diabetes Precursors in the UK: The Child Heart and Health Study in England (CHASE Study)
- Comparative Effectiveness Research: Challenges for Medical Journals
- Alternative Strategies to Reduce Maternal Mortality in India: A Cost-Effectiveness Analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages
- Economic Appraisal of Ontario's Universal Influenza Immunization Program: A Cost-Utility Analysis
- China's Engagement with Global Health Diplomacy: Was SARS a Watershed?
- Laboratory Capacity Building in Asia for Infectious Disease Research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN)
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



