-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
Approximately 10% of reproductive aged women are considered infertile. While great strides have been made in assisted reproductive technologies, overall success rates, especially considering the cost, remain low. Studies indicate that due to its sequential nature, nearly 75% of pregnancy failures are due to defects that occur very early in gestation. Therefore, understanding the physiological changes that occur in the endometrium during this period and how those changes are regulated is of paramount importance if we are to improve our ability to address female reproductive health concerns. We investigated a family of growth factor receptors and identified one that critically regulates the growth and survival of the endometrium in response to the implanting embryo. Furthermore, we used unbiased approaches to identify which signaling pathways and genetic networks are activated downstream of this receptor to execute each of the processes necessary for a successful pregnancy. Understanding the mechanisms and genetic networks with which pregnancy is regulated is a prerequisite to the development of effective pharmaceutical therapeutics.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004451
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004451Summary
Approximately 10% of reproductive aged women are considered infertile. While great strides have been made in assisted reproductive technologies, overall success rates, especially considering the cost, remain low. Studies indicate that due to its sequential nature, nearly 75% of pregnancy failures are due to defects that occur very early in gestation. Therefore, understanding the physiological changes that occur in the endometrium during this period and how those changes are regulated is of paramount importance if we are to improve our ability to address female reproductive health concerns. We investigated a family of growth factor receptors and identified one that critically regulates the growth and survival of the endometrium in response to the implanting embryo. Furthermore, we used unbiased approaches to identify which signaling pathways and genetic networks are activated downstream of this receptor to execute each of the processes necessary for a successful pregnancy. Understanding the mechanisms and genetic networks with which pregnancy is regulated is a prerequisite to the development of effective pharmaceutical therapeutics.
Introduction
Human reproduction is remarkably inefficient with a mere 30% of human chorionic gonadotropin (hCG) positive pregnancies resulting in live birth [1]–[3]. Furthermore, an estimated 9% of couples are considered infertile [4]. Pregnancy is a complex, sequential series of events including fertilization, blastocyst attachment and implantation, uterine decidualization, placentation, and parturition (reviewed in [5]). Defects or a failure in any one process results in a ripple effect of detrimental consequences and potentially pregnancy demise [6]. Detailed studies assessing at which stage of pregnancy failures occur indicate that approximately 30% are lost preimplantation, primarily due to genetic defects in the embryo [7]–[9]. Another 30% of pregnancies are lost in the immediate few weeks following implantation [7], and there is a remaining 9–12% risk for women under 35 years of age to miscarry between 6–12 weeks [1]. Moreover, 1–2% of couples, nearly one-third of which experience clinical depression, suffer from 3 or more consecutive pregnancy losses [10]. When women with recurrent pregnancy loss finally do maintain a pregnancy, they are at increased risk for many adverse obstetric outcomes including preterm birth, placenta previa and low birth weight [11]. While some phenomena like embryonic chromosomal abnormalities have been identified, 50–75% of couples with recurrent pregnancy loss show no identified causes [12].
Furthering the understanding of the mechanisms regulating early pregnancy is of paramount importance. The preeminent factors governing female reproductive health are the steroid hormones progesterone and estrogen. While estrogen is known for its mitogenic effects and temporal influence on the window of receptivity [13], progesterone is the predominant hormone of pregnancy (reviewed in [14]). The use of transgenic and knockout animals has done much to advance our understanding of the molecular mediators of pregnancy, including those regulated by progesterone. Particularly compelling was the identification of a signaling axis in which the progesterone receptor (Pgr)-mediated induction of indian hedgehog (Ihh) leads to the stromal induction of the chicken ovalbumin upstream promoter-transcription factor (Nr2f2; COUP-TFII), as mice lacking any of these genes are infertile [15]–[17]. Interestingly, previous works indicate that epidermal growth factor (EGF) signaling is downstream of this signaling axis [16], [18].
The spatiotemporal expression of EGF family members during the implantation period has led to the longstanding interest in the role of growth factor signaling in reproduction [19]–[21]. Of particular interest is the heparin-binding epidermal growth factor-like growth factor (HB-EGF, Hegf1), due to its expression in the luminal epithelium surrounding blastocysts at the time of attachment and the effects it has on blastocysts [22], [23]. Furthermore, blastocysts express HB-EGF, and it has been shown to influence uterine decidualization [24]. However, maternal ablation of HB-EGF expression did not render mice infertile, rather these females exhibited a deferred implantation window and reduced litter size. The lack of complete infertility led to the speculation that this phenotype was due to compensation by elevated expression of the ligand amphiregulin (Areg) [25]. These results, coupled with the fact that triple knockout females lacking expression of Areg, Egf and Tgfa [26] are fertile, together suggest that the ligands may act in a redundant fashion and led to the hypothesis that the receptors are the rate-limiting factor.
There are four members of the ERBB family of receptor tyrosine kinases: the epidermal growth factor receptor (Egfr/Erbb1) and v-erb-b2 erythroblastic leukemia viral oncogene homolog 2–4 (Her2, Erbb3 and Erbb4, respectively). Whole animal knockouts of ERBB receptors results in embryonic lethality, demonstrating the critical role in physiology these receptors play [27]–[32]. Erbb1-3 are expressed in the mouse endometrium while Erbb4 localizes predominantly to the muscular myometrium and has been shown to not be critical for fertility [33]–[36]. In the present study, we examined the role of Erbb1-3 in the endometrium using loss-of-function approaches in mice and primary human endometrial stromal cells and determined that that EGFR critically regulates endometrial function.
Results
Egfr ablation results in severe subfertility
To circumvent the embryonic lethality exhibited by whole body ERBB knockout animals, mice carrying floxed alleles [37]–[39] were crossed with the PgrCre mouse model [40], effectively ablating Erbb expression in the uterus (Figure 1). To determine the impact that Erbb ablation has on female fertility, control (Pgr+/+Erbbf/f; f/f) and knockout females (PgrCre/+Erbbf/f; d/d) were mated with wild type males of proven fertility in a 6-month fertility trial. Uterine ablation of Egfr (Erbb1) had a major impact on endometrial function, causing infertility in three of seven females tested, while four of the seven females produced only 10 pups over a six month period. In contrast, ablation of either Her2 (Erbb2) or Erbb3 resulted in only a modest impairment in the average litter size (Table 1). Given that of the Erbb receptors expressed in the endometrium, ablation of Egfr had the most significant impact on fertility, we chose to focus our investigation on this receptor.
Fig. 1. Egfr is effectively ablated in the murine uterus. 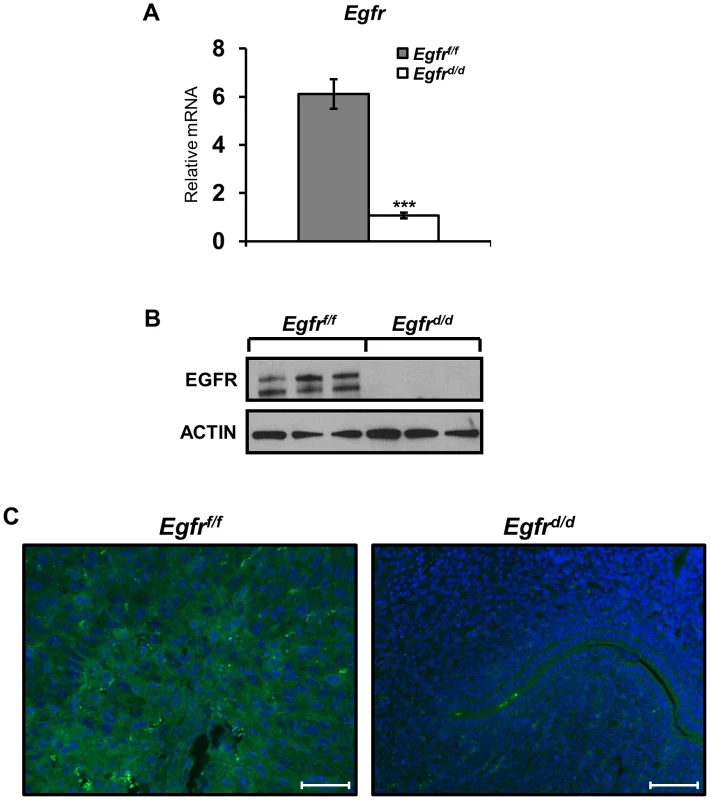
Successful generation of an Egfr conditional knockout (Egfrd/d) using the PgrCre mouse model as determined by (A) qPCR; average +/− SEM. ***-p<0.001, (B) western blot and (C) immunofluorescence. Scale bars 50 µm. Tab. 1. Egfr (Erbb1) is the predominate endometrial Erbb receptor of female fertility. 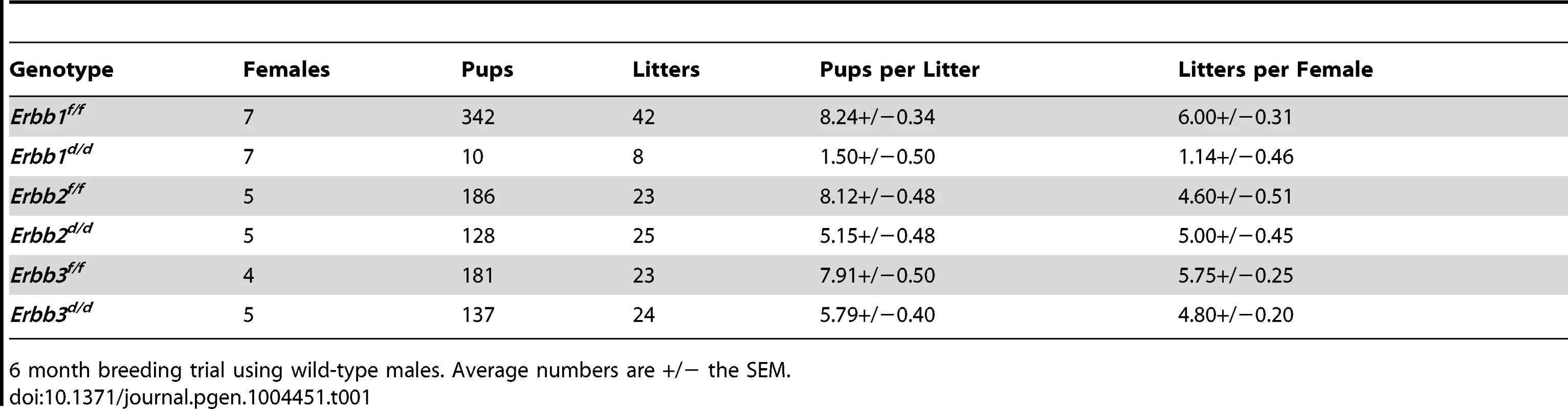
6 month breeding trial using wild-type males. Average numbers are +/− the SEM. No overt defects in ovarian or oviduct function using the PgrCre to ablate Egfr expression
The PgrCre not only excises alleles in the uterus, but is also active in the oviduct and during a brief window in the corpus luteum [40]. Prior to investigating the loss of Egfr in the endometrium, the role of Egfr in ovarian, luteal and oviductal function was interrogated. To examine the ability to produce oocytes, 3-week old females were stimulated with gonadotropins and mated with wild-type males. At day 0.5 of pregnancy, ova were recovered from the oviduct and no differences in the number present were observed between genotypes (Figure S1A). To assay ovulation, fertilization and blastocyst transport through the oviduct, naturally cycling adult females were mated with wild-type males and blastocysts were flushed from the uterus. Though a trend toward a lower number of uterine blastocysts was observed in Egfrd/d females, the difference was not statistically significant (Figure S1B). Importantly, following flushing of the uterus, the oviducts were dissected to examine whether any blastocysts failed to be transported to the uterus and none were observed. Luteal function was examined by measuring the serum concentration of progesterone at day 5.5 of pregnancy, and no differences were observed (Figure S1C). Together, these data suggest that ovarian function is largely intact and the subfertility observed in Egfrd/d females is due to uterine defects.
Egfr ablation does not inhibit the acute response to hormones
We next sought to determine how the loss of Egfr impaired female fertility. It is well known that the uterus is a steroid hormone responsive organ, and the actions of estrogen (E2) and progesterone (P4) are absolutely critical for the establishment of a successful pregnancy (reviewed in [14]). Many previous reports have identified a potential link between EGF and E2 action [41], [42]. To determine whether the impairment in fertility observed in Egfrd/d females was due to deficient E2 action, ovariectomized mice were given three daily injections of E2 and the E2-dependant induction of uterine wet weight and target gene expression were assessed. Surprisingly, there was no difference in uterine weight gain or in E2 target gene expression between Egfrf/f and Egfrd/d females (Figure 2A, B). Next, we sought to determine whether the observed subfertility was due to defects in progesterone action. To address this possibility, ovariectomized mice were treated with a single dose of P4 for 6 h and target gene expression was measured by qPCR. As expected, expression of acute P4 target genes Ihh, Il13ra2, Cyp26a1 and Areg was induced after ligand exposure (Figure 2C). The ability of Egfrd/d uteri to induce expression of Ihh, Il13ra2, and Areg was comparable to that of controls. The expression of Cyp26a1 trended toward being reduced, but this difference was not statistically significant.
Fig. 2. Ablation of Egfr does not impair acute hormone responsiveness. 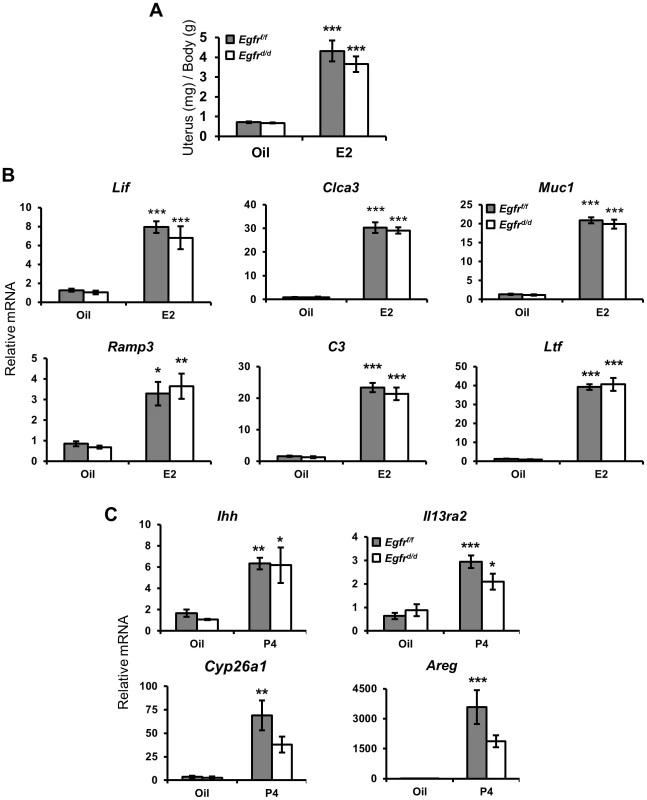
Mice were ovariectomized and treated with: (A,B) estrogen for 3 d or (C) progesterone for 6 h. (A) Measurement of the induction of uterine wet weight in response to estrogen. qPCR measurement of hormone target genes after (B) 3 d of estrogen or (C) 6 h of progesterone. Average +/− SEM. *-p<0.05; **-p<0.01; ***-p<0.001. Egfrd/d females exhibit normal expression of preimplantation markers
Pregnancy requires the coordinated orchestration of P4 and E2 action, and this first becomes evident in the preimplantation period when the uterus undergoes a switch from predominantly E2-mediated physiology to P4-mediated action. Two days prior to blastocyst attachment, the uterine epithelium is proliferating under the influence of E2. Following ovulation and the luteal production of P4 one day prior to attachment, proliferation ceases in the epithelium while it is induced in the stroma. Exogenous hormones may be given to mice to mimic this period free of ovarian or embryonic influences [43]. With this regimen, Egfrf/f mice exhibit the characteristic induction of epithelial proliferation with E2 (Figure 3B) as well as the inhibition of epithelial proliferation and concomitant induction of stromal proliferation after P4 treatment as measured by BrdU incorporation (Figure 3C). Under the same hormone paradigm, the response of Egfrd/d mice did not differ from controls (Figure 3E, F). To further investigate the preimplantation period, and because Egfr has been identified to be involved in IHH signaling during the preimplantation window, we also measured the expression level of several genes in this signaling axis. No significant differences in the expression level of Ihh, Ptch, Gli1 or COUP-TFII were found between Egfrf/f and Egfrd/d mice (Figure 3H).
Fig. 3. Ablation of Egfr does not alter delayed pregnancy proliferation or preimplantation markers. 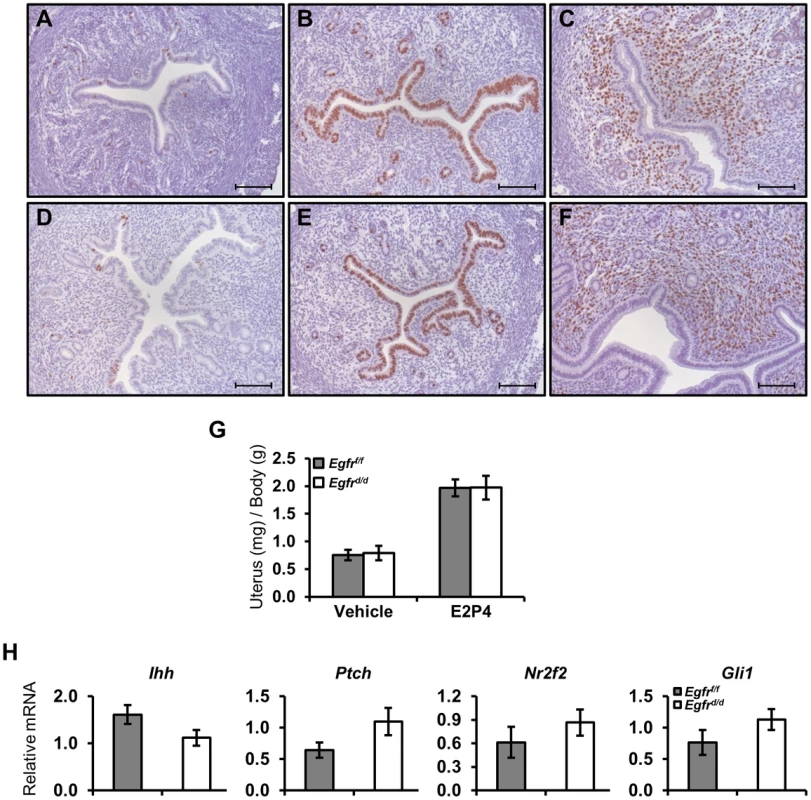
Mice were ovariectomized and treated with exogenous hormones to mimic various stages of preimplantation biology. (A–F) Measurement of proliferation by BrdU immunohistochemistry in (A–C) Egfrf/f or (D–F) Egfrd/d mice primed with estrogen and then treated with (A,D) vehicle, (B,E) estrogen or (C,F) estrogen (50 ng) and progesterone for 15 hours. Scale bars: 100 µm. (G,H) Mice were treated with 3 days of estrogen (100 ng), given 2 days rest and then treated for 2 days with estrogen (6.7 ng) and progesterone (1 mg) to mimic day 3.5 of pregnancy. (G) Measurement of uterine wet weights after oil or hormone treatment. (H) qPCR measurement of known preimplantation regulators. Early pregnancy failure and implantation site demise is a key factor in female Egfrd/d subfertility
With no apparent defects during the preimplantation period, we next chose to investigate a potential role for Egfr during blastocyst implantation. To address this, control and conditional knockout mice were mated with wild-type males. The morning a vaginal plug was observed was considered day 0.5 of pregnancy, and mice were sacrificed at various time points thereafter. Beginning at day 5.5, one day after blastocyst attachment, implantation sites and decidual balls were readily observable in Egfrf/f females (Figure 4A). Likewise, implantation sites were visible in Egfrd/d females (Figure 4D), indicating that the uterus is at least permissive and capable of blastocyst attachment and implantation. However, the average number of observed implantation sites (Figure 4G) and the size of those observed was reduced (Figure 4H). We next asked whether the differences observed at day 5.5 were simply due to a delay in the timing of pregnancy, as occurs in the previously mentioned Hb-egfd/d model, or if the attenuated implantation reaction would have a ripple effect and manifest as a more severe phenotype later in pregnancy. At day 6.5 of pregnancy, obvious increases in implantation site size can be observed in Egfrf/f mice (Figure 4B). However, implantation sites in Egfrd/d mice fail to show a significant increase in diameter (Figure 4E). Furthermore, intrauterine hemorrhaging indicative of embryo resorption is readily apparent in Egfrd/d mice. Continued observation of pregnancy progression to day 9.5 reveals that while the Egfrf/f implantation sites develop normally (Figure 4C), those in Egfrd/d females fail to recover from the early impairments in implantation and continue to be reabsorbed (Figure 4F).
Fig. 4. Egfr ablation causes blastocyst implantation site demise. 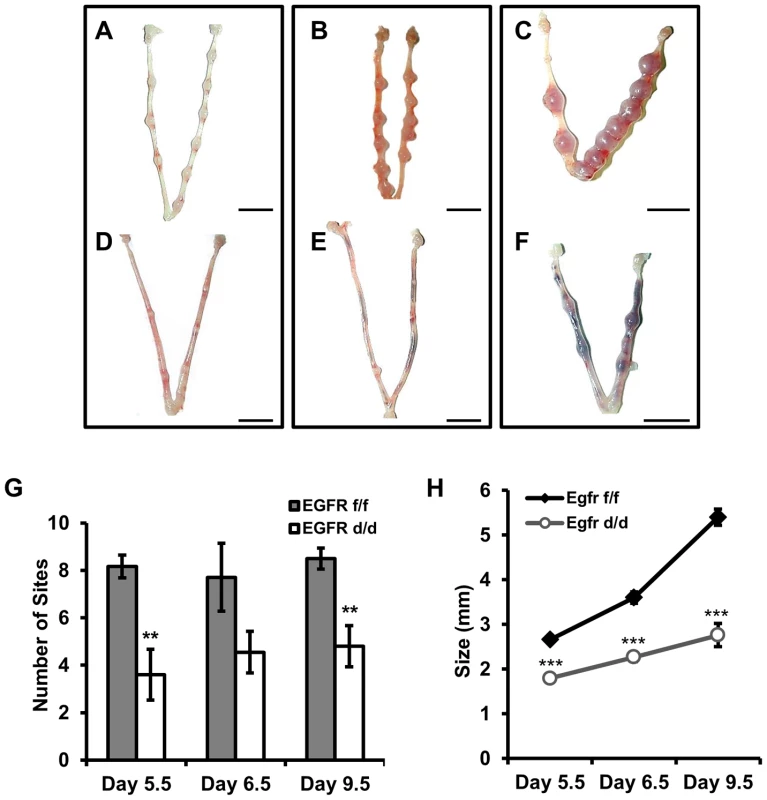
(A–F) Gross morphology of pregnant uteri. (A–C) Egfrf/f and (D–F) Egfrd/d females were mated with wild-type males. The morning observance of a vaginal plug was considered day 0.5 of pregnancy. Pregnancy was assessed at (A,D) d5.5, (B,E) d6.5 and (C,F) d9.5. Scale bars: 1 cm. (G) Average number of implantation sites observed. (H) Average lateral diameter of implantation sites. Numbers represent average +/− the SEM. **-p<0.01; ***-p<0.001. Implantation site demise is due to a failure in the maintenance and progression of decidualization
The attachment of the blastocyst to the uterine epithelium and subsequent invasion into the adjacent stroma triggers the proliferation and differentiation of said cells in a process termed decidualization (reviewed in [5]). To investigate the cause of implantation failure, we assessed the ability of Egfrd/d mice to mount a decidual response following exogenous hormone treatment and mechanical deciduogenic stimulus. One day after stimulation (analogous to day 5.5 of natural pregnancy), the hallmark induction of biomass and increased size of the stimulated horn is apparent in Egfrf/f mice (Figure 5A). Similar to the findings during implantation, Egfrd/d uteri do exhibit a response to deciduogenic stimuli, however, one that is dampened. Also similar to implantation, while the control uteri continue to increase in size two days after stimulation (analogous to day 6.5 of natural pregnancy), Egfrd/d uterine horns fail to progress, and they regress to a point where stimulated horns are indistinguishable from those that were left unstimulated (Figure 5B). When the decidual response five days after stimulation (analogous to day 9.5 of pregnancy) was assayed, the difference between Egfrf/f and Egfrd/d mice is greater than 10-fold (Figure 5C,D), which is in contrast to the lack of detectable differences between Erbb2 or Erbb3 mice and their respective controls during decidualization (Figure S2).
Fig. 5. Egfr ablation results in major defects in decidualization. 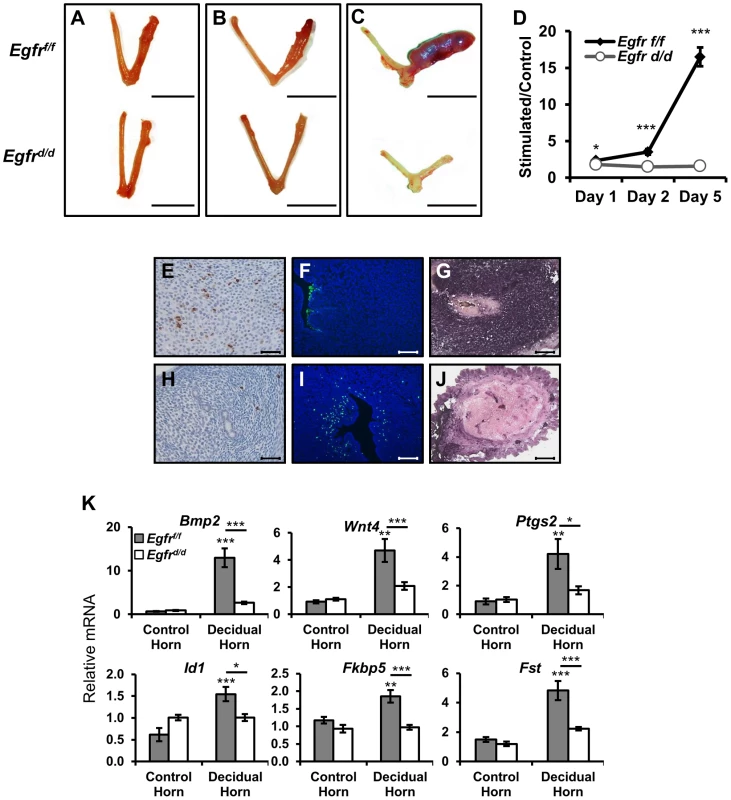
Ovariectomized mice were treated with exogenous hormones and a deciduogenic stimulus was administered to one uterine horn. Gross uterine morphology of uteri (A) 1 day, (B) 2 days, or (C) 5 days after deciduogenic stimulus. Scale bar: 1 cm. (D) Wet weight ratio of stimulated uterine horn relative to unstimulated horn. Histological analysis of day 2 stimulated uterine cross-sections comparing (E–G) Egfrf/f and (H–J) Egfrd/d. (E,H) Proliferative marker phospho-histone H3, (F,I) apoptosis via TUNEL assay, and (G,J) differentiation via alkaline phosphatase staining. Scale bars: 100 µm (E,H,F,I) and 50 µm (G,J). (K) Measurement of the induction of mRNA expression of known decidual regulators on day 1 uteri by quantitative real-time PCR. Average +/− SEM. *-p<0.05; **-p<0.01; ***-p<0.001. n.s. - not significant. As mentioned previously, decidualization is a complex process that begins with a wave of proliferation that eventually leads to differentiation. To determine whether defects in any of these cellular functions may be contributing to the decidualization phenotype, we used immunohistochemistry to compare tissue cross-sections of stimulated uterine horns. When day two stimulated sections were stained using an antibody against the mitotic marker phospho-histone H3, positive immunoreactivity was observed in the nuclei of many Egfrf/f stromal cells (Figure 5E). Strikingly, cross-sections from Egfrd/d uteri exhibit very few immunopositive cells (Figure 5H). Because it appeared as if though Egfrd/d mice were able to initiate decidualization but failed to maintain it, we next asked if the observed decrease in proliferation was coupled with increased apoptosis. While the uterine epithelium of sections from control mice were positive for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), a process known to aid blastocyst invasion, the subepithelial stroma was negative (Figure 5F). Conversely, no staining was observed in the epithelium of Egfrd/d sections while many of the subepithelial stromal cells did stain positively (Figure 5I). Differentiation of decidualizing cells follows the wave of proliferation. To determine whether defects in proliferation prevented advancement of decidualization to differentiation or if the cells that were able to initiate a response were then able to successfully differentiate, alkaline phosphatase staining was performed. Robust, pervasive dark purple staining is detected in the stroma of decidualizing control mice (Figure 5G), which is largely absent in the knockout sections (Figure 5J).
Previous work has identified important molecular mediators and markers of decidualization, including Bmp2 [44], [45], Wnt4 [46], and their effectors. The expression of these genes was measured in both unstimulated and stimulated uterine horns of Egfrf/f and Egfrd/d mice. As expected, the expression of Bmp2, Wnt4, Ptgs2, Id1, Fkbp5 and Fst show robust induction in the stimulated uterine horn of Egfrf/f mice. When examining the expression of these genes in Egfrd/d mice, no significant differences were found between stimulated and unstimulated horns (Figure 5K). Together, these results demonstrate that pregnancy demise in the absence of Egfr is due to multiple failures during decidualization.
Microarray analysis reveals that BMP2 and WNT4 are major mediators of EGFR action
To gain further insight into the concatenation of these genes and which cellular processes are affected in their respective absence, microarray analysis was conducted comparing gene signatures between each knockout and their respective controls (Egfrd/d vs Egfrf/f; Wnt4d/d vs Wnt4f/f) using day 1 decidual uteri. Additionally, published microarray data comparing Bmp2d/d uteri to controls under identical conditions was used for comparison [45]. Using uniform gene calling (see methods), a total of 3,529 genes were altered in the absence of Egfr, 1,414 genes in the absence of Bmp2 and 1,305 genes in the absence of Wnt4 (Table 2). More significantly, when the gene signatures of each respective model were compared, significant overlap was observed, with 80.7% and 65.8% of genes regulated by Bmp2 and Wnt4, respectively, also being regulated by Egfr. However, a large number of genes (54.5%) regulated by Egfr are unique and not altered in the absence of either Bmp2 or Wnt4 (Figure 6A). Together, these data implicate a hierarchy in which EGFR signaling acts genetically upstream, while BMP2 and WNT4 act as downstream mediators.
Fig. 6. Decidual microarrays reveal BMP2 and WNT as EGFR mediators. 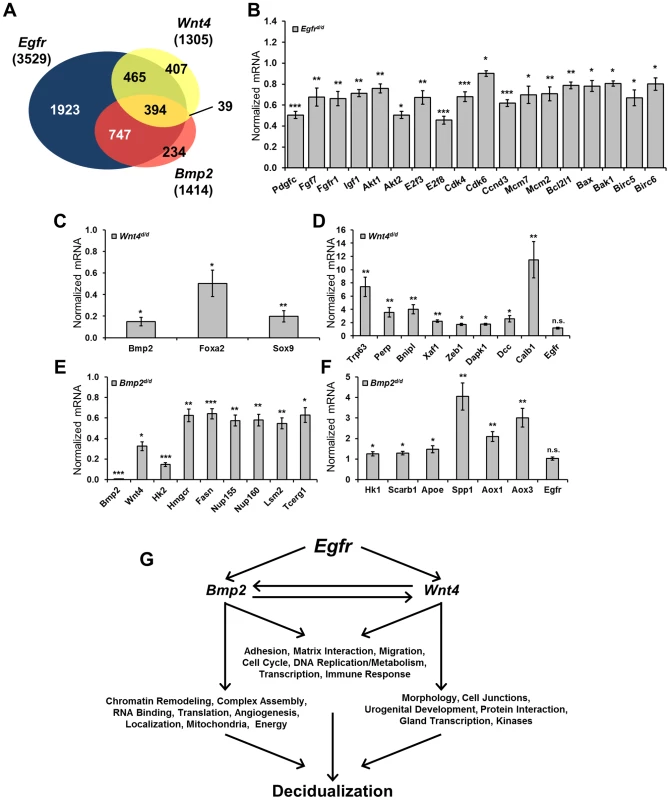
(A) Venn diagram comparing gene expression signatures from Egfr, Bmp2 and Wnt4 conditional knockout day 1 decidual uteri. (B–F) Quantitative real-time PCR validation of microarray results using independent samples, normalized to control expression. (B) Validation of genes altered in the absence of Egfr. (C) Validation of genes with reduced expression and (D) elevated expression in Wnt4 uteri. (E) Validation of genes with reduced expression and (F) elevated expression in the absence of Bmp2. (G) Model depicting which cellular processes and gene ontologies are deregulated in the respective mouse models as determined by DAVID analysis. Tab. 2. Summary of microarray gene expression changes. 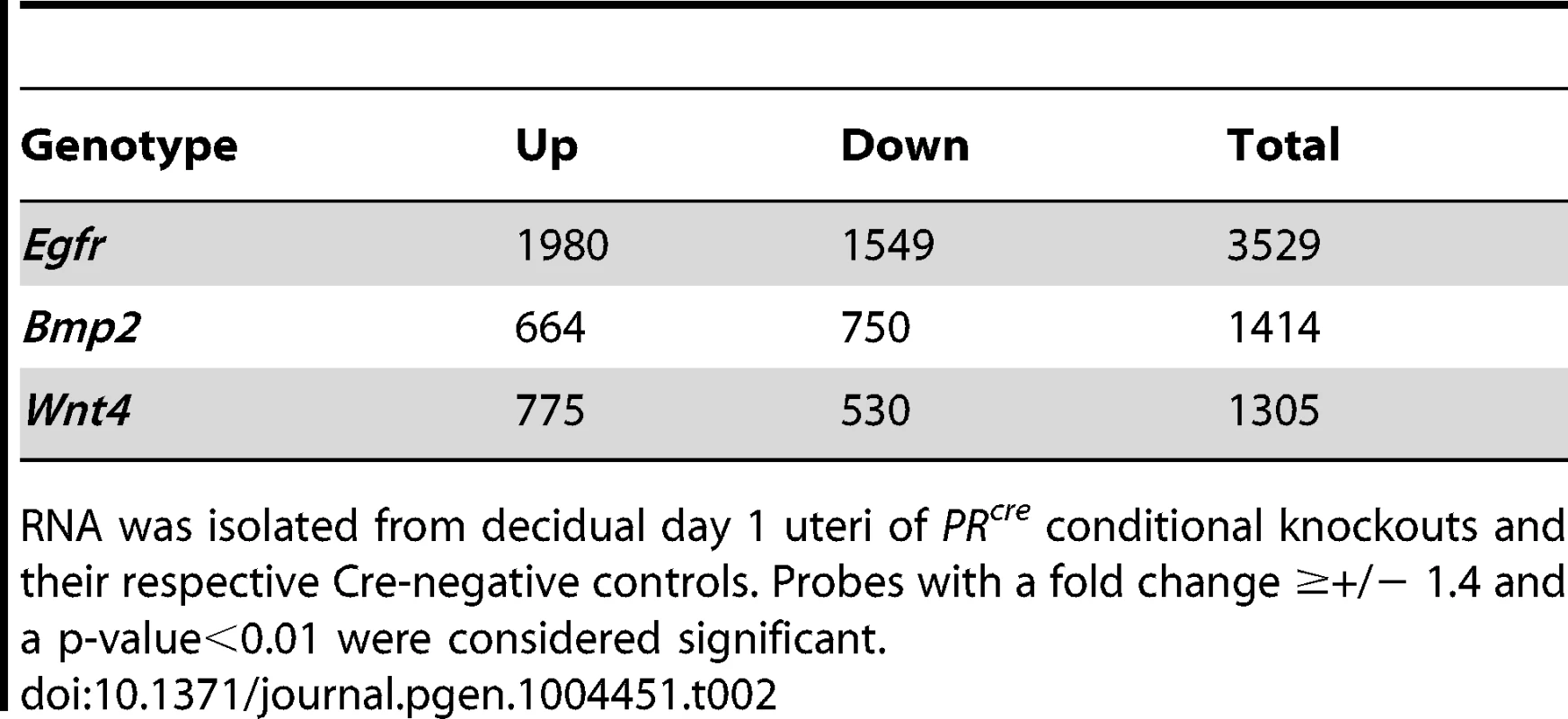
RNA was isolated from decidual day 1 uteri of PRcre conditional knockouts and their respective Cre-negative controls. Probes with a fold change ≥+/− 1.4 and a p-value<0.01 were considered significant. We next used the Database for Annotation, Visualization and Integrated Discovery (DAVID) to determine which gene ontologies and cellular functions were most affected in each model [47], [48]. Analysis on the EGFR gene signature revealed a strong enrichment in cell division, chromatin modification and kinase signaling. Validation of the microarray results was achieved by qPCR of representative genes using independent samples (Figure 6B). Of the 1,305 genes significantly altered in the absence of Wnt4, there was a strong enrichment in developmental processes, chondrogenesis and tube development. Genes that were upregulated (Figure 6C) as well as those with reduced induction (Figure 6D) were validated by qPCR. Interestingly, when the genes in common between the EGFR and WNT4 signatures were analyzed, enrichments in morphogenesis, cell junctions, urogenital development and ion channels were found. Although analysis on the Bmp2 array has been previously published [45], the fact that Bmp2 was one of the most misregulated genes in the absence of Egfr and the gene signatures have extensive overlap led us to investigate which processes were regulated by both genes. Enrichments in chromatin remodeling, metabolism, nucleic acid processing and molecular localization were found, and candidate genes from these processes were validated by qPCR using samples from Bmp2d/d uteri (Figure 6E, F). When functional analysis was conducted on the 394 genes that were altered in all three microarrays, enrichments for adhesion and matrix interaction, cell cycle, DNA replication and metabolism were found. Importantly, while Bmp2 and Wnt4 were misregulated in the absence of Egfr (and each in its reciprocal array), Egfr is not altered in their absence (Summarized in Figure 6G).
EGFR regulates human endometrial stromal cell decidualization
Primary human endometrial stromal cells (HESC) can be isolated, cultured in vitro and treated with a hormone cocktail of estrogen, progestin and cAMP (EPC) to induce decidualization [49]. To test whether EGFR is important for human decidualization, we combined this system with a siRNA loss of function approach. HESC transfected with a non-targeting pool of control siRNA and treated with EPC show robust induction of classic decidual markers IGFBP1 and PRL. Conversely, in cells transfected with EGFR siRNA, the induction of these markers is markedly impaired (Figure 7A). In addition to changes in gene expression, it is known that stromal fibroblasts undergo a transformation to an epithelioid, cobblestone morphology (reviewed in [50]). While a single representative image can sometimes effectively illustrate the difference between two groups of cells, they are often subjective. To address this, changes in cell morphology were quantified using a high-content, automated imaging system. Cells were transfected with siRNA, treated with EPC and then fixed at various times. Whole cells and nuclear fractions were imaged using CellMask Blue and DAPI, respectively, while antibodies recognizing IGFBP1 and EGFR were used for immunofluorescence. In cells transfected with non-targeting siRNA, large nuclei (Figure 7B), a rounded, cobblestone cell morphology (Figure 7C) and high levels of IGFBP1 (Figure 7D) and EGFR (Figure 7E) are observed. Strikingly, when EGFR expression is significantly attenuated (Figure 7I, L), cells maintain a spindle-like, fibroblast morphology (Figure 7G) and exhibit only basal levels of IGFBP1 expression (Figure 7H). Following segmentation and quantification (see methods), significant increases in cell size (Figure 7J) and IGFBP1 expression (Figure 7K) can be quantified over time, while no significant differences were observed between treated and untreated HESCs lacking EGFR.
Fig. 7. EGFR attenuation inhibits human endometrial stromal cell (HESC) decidualization. 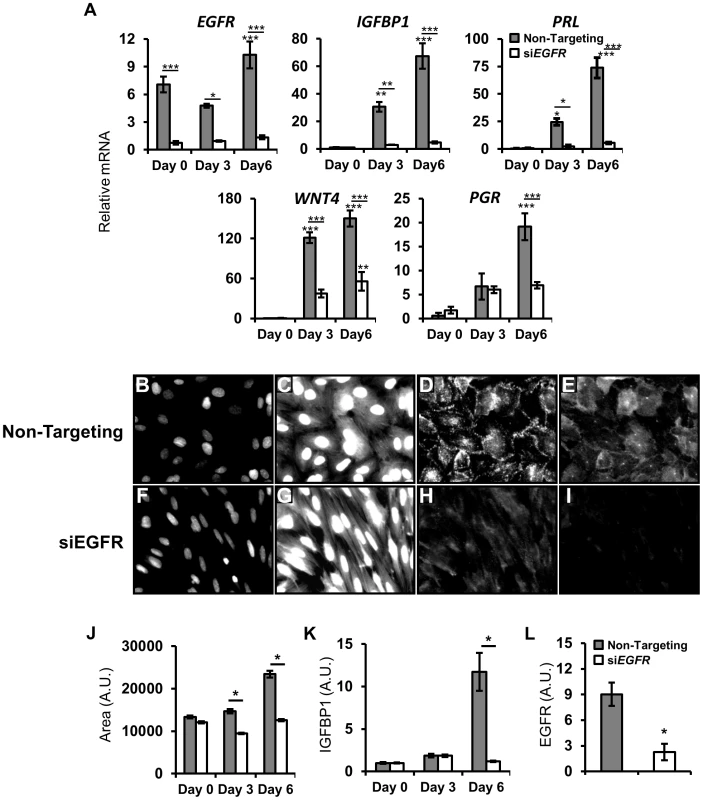
HESC were transfected with non-targeting or EGFR siRNA. After 48 h, HESC were treated with deciduogenic hormones and assessed at day 0, day 3 and day 6. (A) qPCR analysis of known decidual markers and regulators. (B–L) Day 6 automated quantitative image-based analysis of the effects of siEGFR on decidualization. (B–I) Immunofluorescent images of HESC transfected with (B–E) non-targeting siRNA or (F–I) EGFR siRNA after 6 days of deciduogenic hormones. (B,F) DAPI staining of nuclei (470 nm, low exposure). (C,G) Staining of cell surfaces with CellMask Blue stain (470 nm, high exposure). (D,H) Immunolableing of decidual marker IGFBP1. (E,I) EGFR immunolabeling. (J–L) Quantification of siEGFR and decidual changes. (J) Measurement of cell size. (K) Induction of IGFBP1 expression. (L) Day 6 expression levels of EGFR. Average +/− SEM. *-p<0.05; **-p<0.01; ***-p<0.001. Direct and decidual-dependent kinome alterations following EGFR attenuation in HESC
The receptor tyrosine kinase EGFR is capable of activating a plethora of signaling cascades, and thus it is likely that the mechanisms by which the aforementioned impairments in decidualization and pregnancy are affected involve phosphorylation. To investigate which phosphorylation events may be altered in the absence of EGFR, we utilized phospho-kinase antibody arrays. HESC were treated with either HB-EGF for 30 minutes (Figure 8A) or EPC for 3 days (Figure 8B) to examine the role of EGFR signaling both acutely and throughout decidualization. Widespread alterations in many phosphorylation events were evident in cells with attenuated EGFR expression. Of the 44 different substrates in the array (Table S1), 11 were altered (absolute fold >1.5) only following HB-EGF treatment, 12 were altered only following EPC treatment, and 11 were altered after both HB-EGF and EPC treatment (Figure 8A–C).
Fig. 8. Direct and decidual-dependent kinome alterations following EGFR attenuation in HESC by kinase antibody array. 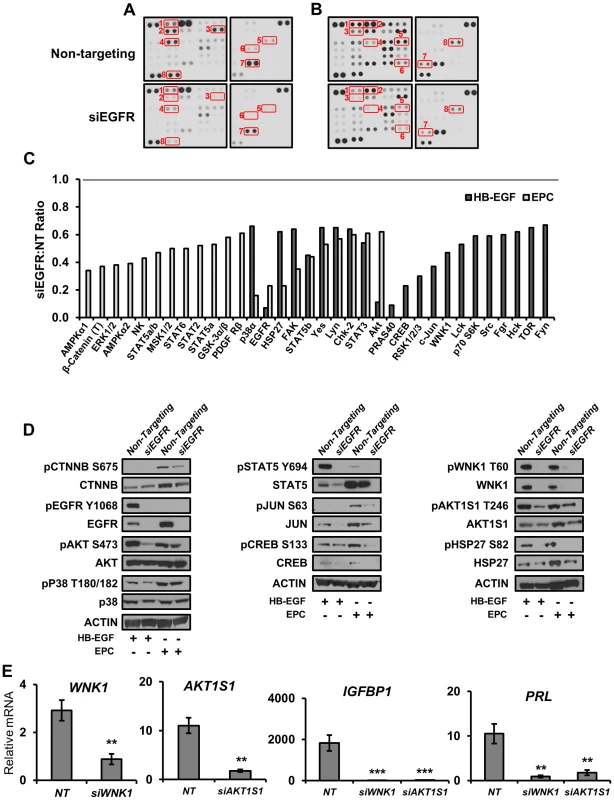
(A–C) HESC were transfected with non-targeting or EGFR siRNA and treated with (A) HB-EGF for 30 mins or (B) deciduogenic hormones for 72 h to determine the effect on 44 different kinase substrates via kinase antibody arrays. (C) Relative (siEGFR vs non-targeting siRNA) quantification of antibodies exhibiting an absolute fold change >1.5. (T) indicates an antibody recognizing total proteins; all others are phosphorylation specific. (D) Validation of kinase array results by western blot using independent samples. (E) HESC were transfected with non-targeting (NT), WNK1, or AKT1S1 siRNA, treated with deciduogenic hormones for 6 d and the expression of decidual markers was measured by qPCR. Numbers represent average +/− the SEM. **-p<0.01; ***-p<0.001. To corroborate the results of the kinase arrays as well investigate whether the absence of EGFR was affecting the expression or phosphorylation of the identified kinases, western blots with independent samples were performed (Figure 8D). Utilizing this approach, we were able to not only confirm that there are widespread alterations in the total amount of phosphorylated kinases present in cellular extracts, but we also identified changes in the expression of several proteins that was dependent on either treatment type or EGFR status. For example, intuitively, the observed decrease in pEGFR is predominantly due to the siRNA-induced depletion of total protein, but it was also observed that in comparison to HB-EGF treatment, EGFR expression is induced 2.5-fold during decidualization. Another example is cJUN, which is also induced during decidualization. Although phosphorylation of cJUN is not detected in samples treated with HB-EGF, total protein expression is virtually lost in the absence of EGFR. During decidualization, total cJUN is reduced by approximately 50% and the protein that remains is phosphorylated at approximately 50% the rate observed in cells with EGFR expression, culminating in an ultimate 75% reduction in phosphorylated cJUN (Figure 8D). Importantly, β-catenin, a downstream effector of WNT signaling, follows a similar pattern; it is induced during decidualization, though to a lesser extent with hypophosphorylation in the absence of EGFR. Additionally, we investigated the function of two members identified in the array that have not yet been reported in the endometrium, WNK1 and AKT1S1 (PRAS40), due to their role as signaling intermediaries. Interestingly, the reduction in expression of either member by siRNA resulted in a significant attenuation in the expression of decidual markers IGFBP1 and PRL (Figure 8E). These results identify and provide valuable insight into the complex signaling networks important to endometrial function.
Discussion
Erbb signaling, predominantly via EGFR, plays a vital role in early pregnancy
In this report, we hypothesized that the receptors may be the rate limiting factor of Erbb signaling in the regulation of female fertility. We show that EGFR, the only Erbb receptor expressed in the endometrium possessing both functional ligand binding and active kinase activity, plays a critical role in fertility. Modest reductions in litter size observed in the absence of uterine Her2 or Erbb3 indicates that they may facilitate EGFR signaling as dimerizing partners but do not play a critical role in pregnancy individually. Growth factors bear their namesake due to the ability to promote growth, and evidence of a relationship between they and the mitogenic hormone estrogen has been well documented [27], [51], [52]. Given the demonstrated relationship between EGF signaling and estrogen, one might intuitively predict that the fertility defects observed in Egfrd/d mice are due to impairments in estrogen action. Surprisingly, neither epithelial proliferation nor estrogen target gene expression was affected. Taken together, these results suggest that although one of the functions of EGFR may be the activation of the estrogen receptor, estrogen action itself does not require the EGF-receptor.
We did, however, observe significant defects in blastocyst implantation. The observation of implantation sites in the Egfrd/d uterus one day after implantation indicates that the Egfr is not necessary for the recognition of and initial response to the blastocyst, and its absence is still permissive of attachment and invasion. Although a reaction is initiated, it is one that is diminutive and abbreviated, and this may explain the reduction in the number of macroscopically observable implantation sites. We observed EGFR expression in the stromal compartment during this period, and the predominant ligand, HB-EGF, is first expressed in the luminal epithelium at the time of attachment but can then be subsequently observed in proliferating stromal cells [22]. Thus it is intuitive that the primary uterine phenotypes would be in stromal cell function at the site of blastocyst invasion. Defects during the periimplantation period are especially clinically relevant as 30% of human pregnancies are lost during the immediate weeks following attachment [7]. Pregnancy demise due to defects during implantation is a phenomenon that is recapitulated herein when the small implantation sites in Egfrd/d uteri fail to progress and intrauterine hemorrhaging and fetal resorption ensue.
Egfr plays a pivotal role in the amplification of decidualization
The decidua plays a critical role in regulating trophoblast invasion, modulating the local immune response, protection from reactive oxygen species and development of the placenta. Consequently, defects in decidualization ripple throughout pregnancy, manifesting in adverse obstetric outcomes such as those in placental position (placenta accreta, placenta previa) and sufficiency (preeclampsia and intrauterine growth restriction), miscarriage, recurrent pregnancy loss, and infertility (reviewed in [6]). Examining the cause of implantation site demise in Egfrd/d uteri, we observed that EGFR plays a critical role in the amplification and maintenance of decidualization. In results complementing natural pregnancy, an initial response to deciduogenic stimuli did occur, albeit to a lesser extent. However, as the decidua continued to expand in control mice two days after stimulation, proliferation was nearly absent in the stimulated horns of knockout uteri, which had actually begun to regress due to increased apoptosis.
To investigate the global impact on gene expression caused by the loss of Egfr, we conducted microarrays one day after deciduogenic stimuli and identified over 3,000 misregulated genes in the absence of Egfr. Bioinformatics analysis revealed widespread alterations including genes involved in: growth factor and kinase signaling (Pdgfc, Fgf7, Fgrfr1, Igf1, Akt1, Akt2), the cell cycle (E2f3, E2f8, Cdk4, Cdk6, Ccnd3), DNA replication (Mcm2, Mcm7) and cell survival (Bcl2l1, Bax, Bak1, Birc5, Birc6). The gene networks identified here are in accordance with the phenotype observed where deficient growth signals prevent the advancement of the cell cycle and DNA replication, resulting in a concomitant loss in cell survival. Additionally, we observed significant alterations to two factors previously reported to play a key role in decidualization, Bmp2 and Wnt4.
Of the many BMP ligands and receptors, Bmp2, Bmpr2 and Acvr1 have been previously shown to play important roles in endometrial function, with both early implantation (Bmp2 and Acvr1) and mid-gestational (Bmpr2) defects, some reminiscent of human intrauterine growth restriction [45], [53], [54]. Of the many members of the WNT signaling family expressed during implantation [55], several (Wnt4, Wnt6, Sfrp4, Dact1) were found to be misregulated in the absence of Bmp2 [45]. Wnt4 is the gene that is most abundantly expressed in the decidua, and its ablation resulted in subfertility due to decreased stromal cell survival and differentiation. Interestingly, the induction of Bmp2 during decidualization was markedly impaired in the absence of Wnt4, leading the authors to propose that Bmp2 and Wnt4 function in a mutually activating relationship [46]. Together, these results suggest that the three genes may regulate decidualization in a complex network. To elucidate this relationship, we conducted a microarray on Wnt4d/d day 1 decidua and compared it to both our Egfr microarray and a published Bmp2 decidual array.
Gene ontology and functional clustering revealed strong enrichments in developmental processes in the 1,305 genes that were altered in the absence of Wnt4. These results were fitting since WNT signaling plays a well-documented role in uterine development and differentiation [46], [56]–[59]. Interestingly, when we investigated the relationship between Egfr, Wnt4 and Bmp2 by comparing all three microarrays, Egfr appeared to be the predominant regulator, not only encompassing 80.7% of the Bmp2 gene signature and 65.8% of the Wnt4 signature, but also affecting an additional 1,923 unique genes. Furthermore, while Bmp2 and Wnt4 were altered in all three models, Egfr was not affected in the absence of either Bmp2 or Wnt4, further suggesting a hierarchy in which Bmp2 and Wnt4 are mediators of Egfr action.
We were also able to identify distinct differences between the enriched functions within the Egfr-Bmp2 and Egfr-Wnt4 common genes. The 747 genes regulated by Egfr and Bmp2 exhibit enrichment in general cell functions (RNA trafficking [Nup155, Nup160], RNA processing [Lsm2], transcription elongation [Tcerg1]), and metabolism (cholesterol and lipids [Apoe, Hmgcr, Cyp39a1, Fasn, Scarb1], glucose [Hk1, Hk2] and respiration [Aox1, Aox3])). The 465 genes regulated by Egfr and Wnt4 display an enrichment in cell fate and differentiation with processes such as morphogenesis and urogenital development (Foxa2, Zeb1, Sox9, Trp63), cell interaction (Perp, Trp63) and cell survival/death (Dapk1, Dcc, Xaf1, Bnipl) being enriched. By comparing three different mouse models, this analysis provides valuable insight into how multiple signaling networks converge in the execution of decidualization and thereby the achievement of a successful pregnancy.
Attenuation of EGFR causes extensive alterations to kinase signaling, preventing HESC decidualization
Treatment of cultured HESCs with a hormone cocktail of estrogen, progestin and cyclic AMP (EPC) induces in vitro decidualization, the hallmarks of which are the induction of IGFBP1, PRL and transformation from a spindle-like fibroblast to an epithelioid, cobblestone-like morphology [60]. Using molecular and novel image-based approaches, we demonstrated that EGFR is critical for human decidualization. Interestingly, while basal and early decidual expression of PGR was unaffected by siEGFR, the amplification of PGR mRNA late during decidualization was blunted. The decidual induction of PGR makes it challenging to determine whether EGFR signaling regulates PGR directly or if its attenuation is due to the lack of decidualization. It has been reported that predominant EGFR ligands during pregnancy, Hbegf and Areg, as well as Egfr itself, are PGR target genes [21], [25], [61]. An attractive conjecture would be that a feed-forward amplification loop exists in which PGR induces EGFR signaling that in turn feeds back to maintain PGR activation. Such a model would serve as an exquisite sensor of progesterone activity, linking the health of the pregnancy to endometrial function.
Enzymes, particularly kinases, are some of the most commonly targeted proteins for therapeutic manipulation (reviewed in [62]). To better understand which signaling pathways EGFR might be acting through in HESC, we examined the activation of acute targets 30 minutes after growth factor treatment as well as those that are regulated during decidualization after 3 days of hormones. Using phosphokinase antibody arrays and western blot confirmation, we identified a wide array of kinome alterations in the absence of EGFR.
Significant impairments in the rapid activation of members of the SRC cascade (SRC, FAK, FYN, HCK, LCK, LYN, YES), known to be important during HESC decidualization [63], were evident in the absence of EGFR. Additionally, AKT/mTOR signaling (AKT, WNK1, PRAS40, MTOR, P70S6K) as well as MAPK and downstream effectors (P38a, RSK1/2/3, HSP27, JUN, CREB) were inhibited following HB-EGF treatment. While some of these same signaling members (AKT, GSK3b, ERK1/2, P38a, PDGFR, FAK, LYN, YES) were also affected following decidualization, pathways showing more dramatic alteration in the hormone milieu include STAT signaling (STAT2, STAT3, STAT5a/b, STAT6), the response to cellular stress (AMPKa1, AMPKa2, HSP27, MSK1/2), and importantly CTNNB, a downstream WNT effector. The interconnected nature and cross talk that exists between most signaling pathways makes the establishment of definitive causal relationships difficult, but it is clear that the absence of EGFR causes widespread havoc on intracellular signaling and thus prevents the feed-forward amplification of decidualization.
Additionally, we demonstrate that inhibition of EGFR signaling intermediaries AKT1S1 or WNK1 also impairs HESC decidualization. The identification of a role for WNK1 in decidualization is particularly intriguing. WNK1 is a member of the WNK subfamily of kinases that is most prominently known to play important roles in the regulation of ion transporters and electrolyte homeostasis. Mutations in WNK1 were discovered to cause a familial form of hypertension known as Gordon's syndrome, characterized by increased renal salt reabsorption [64]. Additionally, WNK1 has been shown to regulate the epithelial sodium channel ENaC [65], an interesting finding considering endometrial ENaC has recently been shown to be critical for blastocyst implantation and is markedly reduced in women who experience an unsuccessful in vitro fertilization cycle [66]. Although we are the first to show that WNK1 is activated downstream of EGFR signaling in endometrial cells, additional evidence of this relationship exists in that WNK1 was shown to be required for the EGF-dependent activation of ERK5 in kidney cells [67]. While the WNK family is most similar to the MEKK family of kinases, they are atypical in that they do not fit in any of the seven major kinase families [68]. Of the 518 different protein kinases identified in the human genome, 428 of them utilize a highly conserved lysine in subdomain II [69]. In contrast, the WNK family bears their namesake (With No Lysine [K]) due to the absence of this important residue [68], [70]. The atypical catalytic domain of WNK proteins not only confers unique substrate binding but also represents an attractive target for the development of specific small molecules. For example, while the acute nature of targeted periimplantation growth factor supplementation in women with a history of pregnancy complications would likely limit the potential for adverse side effects, EGFR is expressed in many tissues and is well known to play oncogenic roles in the brain, lung, breast, pancreas and colon. Development of pharmaceuticals targeting the atypical kinase domain of WNK1 could potentially confer not only target protein specificity but also reduce non-uterine effects.
Summary
Our present findings demonstrate that the coordinate actions of EGFR are critical for the successful progression of early pregnancy. The serial, progressive nature of pregnancy prescribes that deficiencies in early events generate ripple effects, potentially leading to pregnancy termination [6]. Thus, identification and modulation of members involved during this period is of paramount importance in the improvement of female reproductive health. We show here that EGFR is active during this window, triggering a multitude of signaling and transcriptional events, and thus it establishes the cellular context necessary for the continued progression of pregnancy. Several studies have implicated a potential a role of EGFR signaling in human pregnancy. A maternal polymorphism in the 5′ untranslated region of the EGF locus was associated with intrauterine growth restriction (IUGR) [71]. Similarly, decreased expression and activation of EGFR is associated with low birth weight and IUGR [72]–[74]. Elucidation of the EGFR signaling network also has implications beyond reproductive health as it is overexpressed in approximately 50% of endometrial tumors and is significantly associated with decreased survival in patients with Type II endometrial cancer [75], [76]. Furthermore, expression of negative regulators of EGFR signaling, TOB1 and MIG6, has been found to be reduced in women with endometriosis [77], [78]. Insight gained herein will aid in the development and advancement in our ability to treat women suffering from reproductive dysfunction.
Methods
Mice and human specimens
Floxed Erbb mice were crossed with the progesterone receptor cre mouse model to generate conditional knockout models. All mice were maintained under pathogen-free conditions. Animal handling and surgery was performed according to the NIH Guide for the Care and Use of Laboratory Animals. All procedures and protocols were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Typical cohorts consisted of 5–10 mice unless specified otherwise.
Endometrial samples were obtained from the proliferative phase of normally cycling women after written informed consent, under an approved protocol by the Institutional Review Board of Baylor College of Medicine. Histologically normal endometrial tissue samples were obtained by biopsy from subjects between 18 and 45 years of age who had no history of uterine disease. Samples were collected at the room temperature and transported to the laboratory in HBSS containing 1% antibiotic-antimycotic (Life Technologies, Carlsbad, CA, USA) on ice and processed for the endometrial stromal cell isolation as described below.
Fertility study and murine hormone treatments
Conditional knockout and respective control females were paired with wild-type males of proven fertility (1∶1) at approximately 8 weeks of age. Fertility was assessed by monitoring litter size and frequency over a six month period. Stimulation of ovulation was induced in 3-week old female mice (N = 3 f/f, 5 d/d) by administering 5 IU of pregnant mare's serum gonadotropin intraperitoneally (i.p.; VWR Scientific), followed by 5 IU human chorionic gonadotropin (i.p.; Pregnyl, Organon International) 48 hours later. Females were placed with wild-type males and 24 hours later ova were flushed from the oviducts and counted. Natural ovulation, fertilization, and oviduct transport were measured in naturally cycling adult females mated to wild-type males. At day 3.5 of pregnancy, uteri were excised and flushed and the blastocysts recovered were counted. Serum progesterone at day 5.5 of pregnancy was measured following isolation by centrifugation using blood collected at the time of sacrifice and serum separator tubes (BD). The serum was sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for analysis of P4 by radioimmunoassay. Blastocyst implantation was measured by mating 8 week old females with wild-type males. The morning a vaginal plug was observed was considered 0.5 days post coitus (day 0.5 of pregnancy). Mice were sacrificed at day 5.5 (N = 6 f/f, 5 d/d), 6.5 (N = 7 f/f, 4 d/d) or 9.5 (N = 4 f/f, 5 d/d) of pregnancy. Uteri were excised, imaged and implantation site diameter was measured using a vernier caliper. For all experiments involving exogenous hormones, females were ovariectomized between 6–8 weeks of age and given 10–14 days to recover and purge endogenous hormones. Hormones were dissolved in sesame oil and administered via 0.1 ml subcutaneous injection. The effects of short term hormone were measured by either three daily injections of 100 ng estradiol (E2) or a single 1 mg progesterone (P4) injection. Mice were sacrificed 6 h after the final injection; uteri were weighed and flash frozen. For preimplantation gene expression studies, mice (N = 6 f/f, 4 d/d) were given 3 d of 100 ng E2 followed with 2 d of rest and then a combination of 6.7 ng E2 with 1 mg P4 for 2 days. Mice were sacrificed 6 h after the final injection, uteri were weighed and flash frozen. For the investigation of preimplantation proliferation using the delayed pregnancy model [43], all mice were given 2 days of 100 ng E2 followed by 2 days of rest. At this point, mice were divided into three cohorts (N = 4 per genotype per treatment): vehicle (4 days vehicle [oil] injections), E2 (3 days of vehicle, 1 injection of 50 ng E2) or EP (3 days of 1 mg P4, 1 injection of 50 ng E2 and 1 mg P4). BrdU (GE Healthcare) was injected intraperitoneally 13 hours after the final hormone injection and mice were sacrificed 2 hours later. The artificial induction of decidualization was achieved using previously described methods [79]. Briefly, following 3 days of 100 ng E2, 2 days of rest and 3 days of 6.7 ng E2 and 1 mg P4, mice were given a deciduogenic stimulus of either 50 ul intrauterine sesame oil injection or mechanically via a scratch with a burred needle. Mice were then given daily injections of the combined E2 and P4 until the day of sacrifice (1 day, 2 days or 5 days after stimulus), 6 hours after the final injection. Uteri were imaged, each horn weighed and then flash frozen.
Histological and immunohistochemical staining
At the time of sacrifice, a mid-portion of uterus was fixed in 4% (v/v) PFA overnight followed by thorough washing with 70% ethanol. Tissues were dehydrated in a graded ethanol series and embedded in paraffin. Sections were cut (5 µm) and mounted on silane-coated slides. Tissues were deparaffinized, rehydrated and boiled in citrate buffer for antigen retrieval. Sections were preincubated in 10% normal goat serum (NGS) in PBS (pH 7.5) for 1–3 hours at room temperature. Tissue sections were incubated overnight at 4°C with primary antibodies specific to phospho-histone H3 (Millipore, 1∶2000). After washing with PBS, sections were incubated with biotin-labeled secondary antibody (Vector, 5 µl/ml) for 1 hour. Sections treated for immunohistochemistry were then incubated with VECTASTAIN ABC solution (Vector Labs) for 30 minutes followed by peroxidase substrate (Vector Labs) for signal development. Immunoreactivity was detected using the DAB substrate kit (Vector) and sections were counterstained with hematoxylin. The TUNEL assay was performed using the Roche in situ cell death detection kit (Roche Applied Science) according to manufacturer's instructions. Alkaline phosphatase staining was performed as previously described [46]. Briefly, 16 µm sections were incubated with a 100 mM Tris buffer (pH 9.5) containing 5-bromo-4-chloro-3-indolyl phosphate and nitro blue tetrazolium chloride (Roche Applied Science). Nuclear Fast Red (Vector) was used for counterstaining.
Western blot analysis
Protein was extracted using lysis buffer (50 mM Tris pH 7.4, 50 mM KCl, 6 mM EDTA, 1% NP-40) with PhosSTOP phosphatase and Complete protease inhibitors (Roche). Protein was separated by electrophoresis using NuPAGE 4–12% Bis-Tris Gels in MOPS buffer (Life Technologies). Proteins were transferred to a PVDF membrane (Millipore) in transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol; Biorad). Membranes were blocked in 5% nonfat dry milk which was dissolved in tris-buffered saline/tween solution (TBS/T; 20 mM Tris, 150 mM NaCl, pH 7.6, and 0.1% tween-20) and then incubated with primary antibodies overnight at 4°C with gentle rocking. The membranes were incubated with secondary antibody for 1 hour, washed with TBS/T and developed with ECL prime reagents (GE Healthcare).
Quantitative real-time PCR
Total RNA was extracted using Trizol reagent (Life Technologies), according to the manufacturer's directions. One microgram of the RNA was reverse transcribed into cDNA with M-MLV (Life Technologies). Expression levels of mRNA were measured by TaqMan Universal PCR Master Mix (Applied Biosystems) or FastStart Universal SYBR Green Master mix (Roche) using the ABI 7500 and QuantStudio 12K Flex Real-Time PCR Systems, according to the manufacturer's instructions. Commercially validated Taqman real-time probes and primers were purchased from Applied Biosystems. The sequences of primers used in SYBR qPCR are listed in Table S2. Relative mRNA levels of transcript were calculated by the 2−ΔΔC method, normalized to the endogenous reference (18S), and plotted as mean ± SEM.
Microarray analysis
Total RNA was isolated using RNeasy kits (Qiagen) from day 1 decidual uteri (see above) according to manufacturer's instructions and three groups of two were pooled for each condition. The integrity of all RNA samples were tested with the Bioanalyzer 2100 (Agilent Technologies). Microarrays were performed by the Genomic and RNA Profiling Core of Baylor College of Medicine. Samples were labeled using the standard Affymetrix linear amplification protocol using the 3′ IVT Express Kit. 250 ng of total RNA was reverse transcribed to produce double-stranded cDNA. The cDNA product was used as a template for the vitro transcription reaction, producing biotin-labeled cRNA. The labeled cRNA was tested for integrity on the Agilent Bioanalyzer and quantified using the NanoDrop ND-1000 spectrophotometer. 15.0 µg of the labeled cRNA was fragmented and re-checked for concentration and size. Hybridization cocktails containing Affymetrix spike-in controls and 15.0 ug of each fragmented, labeled cRNA were loaded onto Affymetrix GeneChip Mouse 430 2.0 arrays. The arrays were hybridized for 16 hours at 45°C with rotation at 60 rpm in the Affymetrix GeneChip Hybridization Oven 640. The arrays were washed and stained with a streptavidin, R-phycoerythrin conjugate stain using the Affymetrix GeneChip Fluidics Station 450. Signal amplification was done using biotinylated anti-streptavidin. The stained arrays were scanned on the Affymetrix GeneChip Scanner 3000. The images were analyzed and quality control metrics recorded using Affymetrix Command Console software version 3.0.0. Microarray CEL files were analyzed using dChip (www.dchip.org, PM-MM model, Quantile normalization). Two-side t-test and fold changes were used to define differentially expressed probes. To obtain a non-redundant list of significantly altered genes, multiple probe sets for a given gene (when present) were averaged. Genes with an absolute fold change ≥1.4 and a p-≤0.01 were considered for further analysis.
Isolation, siRNA transfection, and hormone treatment of human endometrial stromal cells
Endometrial stromal cells were isolated from tissue biopsies as previously described [80] with slight modifications. In brief, tissue samples were washed with DMEM-F12 (Life Technologies) containing 1% antibiotic-antimycotic and minced into small pieces of <1 mm3. The tissues were then incubated for 1.5 hours at 37°C in DMEM-F12 containing 0.25% (w/v) collagenase and 0.05% DNase I (Sigma-Aldrich). After enzymatic digestion, stromal cells were separated from epithelial aggregates using a 40 µm nylon cell strainer (BD-Biosciences). The filtrates were washed twice, and plated in DMEM-F12 media containing 10% fetal bovine serum and 1% antibiotic-antimycotic. Endometrial stromal cells were passaged two to three times before the experiments. Endometrial stromal cells were seeded in 6-well plates at a concentration of 1.5×105 cells/ml, allowed to adhere and attain a confluency of approximately 60%. Cells were then transfected with non-targeting or Erbb siRNA (Thermo Scientific) using Lipofectamine 2000 or RNAiMAX (Life Technologies) according to manufacturer's protocol. All decidual experiments were conducted in opti-MEM media (Life Technologies) containing 2% charcoal-stripped fetal bovine serum and 1% antibiotic-antimycotic. Stromal cultures were treated with decidual hormones as described previously [49]. Decidualization was induced by treatment with 1 µM MPA (Sigma-Aldrich), 10 nM E2 (Sigma-Aldrich), and 50–100 µM dibutyryl cAMP (Sigma-Aldrich) every 48 hours.
HESC immunofluorescence
HESC were grown on acid-etched glass coverslips (12-mm diameter) before transfection and decidualization. Subsequent to treatments, cells were washed once with PBS and fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 30 minutes. Excess fixative was quenched with freshly prepared sodium borohydride (NaBH4; 1 mg/ml). After quenching, cells were washed with TBS three times. Next, fixed cells were permeablized with 0.5% Triton X-100 for 30 minutes and washed with TBS three times. Non-specific antibody binding was blocked by 5% milk/TBS-T for 1 hour at room temperature. Primary antibodies (IGFBP1, 1∶200, Santa Cruz sc-6072; EGFR, 1∶200, Cell Signaling 2646) were diluted in blocking buffer and incubated overnight at 4°C. The following day, cells were washed with TBS and incubated with Alexa Fluor-conjugated secondary antibodies (Life Technologies, 1∶2000) in blocking buffer for 1 hour at room temperature. Then, cells were washed with TBS and post-fixed in 4% PFA for 10 minutes. Cells were washed and excess fixative quenched again in NaBH4 for 10 minutes. Finally, cells were treated with DAPI (1∶1000) for 20 minutes and HCS CellMask Blue (Life Technologies; 1∶10,000) for 1 hour at room temperature. Coverslips were rinsed quickly in water and mounted (SlowFade, Life Technologies) onto slides. Coverslips were imaged immediately.
High throughput microscopy and image processing
Cells were imaged using the Cell Lab IC-100 Image Cytometer (IC-100; Beckman Coulter) equipped with a 40X/0.90NA objective. The imaging camera (Hamamatsu Orca ER) was set to capture 8 bit images at 2×2 binning with at least 4 images captured per field (DAPI, CellMask Blue, IGFBP1, EGFR). In general, >49 images were captured per coverslip. Images were analyzed using custom algorithms developed with the Pipeline Pilot (v8.0) software platform (Accelrys) in a similar workflow as previously described [81]–[83]. After background subtraction, nuclear and cell masks were generated using a combination of non-linear least squares and watershed-from-markers image manipulations of the DAPI images. Cell populations were filtered to discard events with cell aggregates, mitotic cells, apoptotic cells, cellular debris, or poor segmentation. Applied gates were based upon nuclear area, nuclear circularity, and cell size/nucleus ratio. All events with whole cell masks bordering the edge of the image were additionally eliminated from analysis. Post-analysis measurements were exported to spreadsheet software (Microsoft Excel) for further analysis.
Assessment of kinase activity by Proteome Profiler Human Phospho-Kinase array kits
HESC were transfected with siRNA as mentioned above. Cells were split into two cohorts 24 hours after siRNA transfection. One cohort was serum-starved for 24 hours and then treated with decidual media (see above; containing 100 µM cAMP and 5% charcoal-stripped FBS). Treatment media was changed after 35.5 hours and cells were harvested 30 minutes later. The other cohort was double serum starved, starving for 12 hours overnight, refeeding with 10% serum for 12 hours, and then serum-starved again overnight. Cells were then treated with DMEM/F12 (Life Technologies) containing 100 ng/ml rhHB-EGF (R&D systems) for 30 minutes. Lysates were prepared using the supplied buffer. Proteome Profiler Human Phospho-Kinase array kits (R&D Systems) were used according to manufacturer's instructions to measure (in duplicate) changes to 43 different kinases and 2 related total proteins. A total of 500 µg of protein was used for each cohort. A total of 15 different x-ray exposures, ranging from 15 seconds to 15 minutes, were used to capture array signal. Signal intensity was quantified using ImageJ (NIH) and was normalized to the reference spots. Exposures with the greatest dynamic range for each pair of antibody spots were used to determine the fold change in signal intensity. Antibodies with an absolute intensity difference greater than 1.5 were chosen for representation.
Statistical analysis
For comparisons involving two groups, unpaired two-tailed student's t-tests were performed. For comparisons involving three or more groups, a one-way ANOVA followed by Tukey's post hoc multiple-range test was conducted.
Supporting Information
Zdroje
1. WilcoxAJ, WeinbergCR, O'ConnorJF, BairdDD, SchlattererJP, et al. (1988) Incidence of early loss of pregnancy. N Engl J Med 319 : 189–194.
2. ZinamanMJ, CleggED, BrownCC, O'ConnorJ, SelevanSG (1996) Estimates of human fertility and pregnancy loss. Fertil Steril 65 : 503–509.
3. WangX, ChenC, WangL, ChenD, GuangW, et al. (2003) Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril 79 : 577–584.
4. BoivinJ, BuntingL, CollinsJA, NygrenKG (2007) International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod 22 : 1506–1512.
5. WangH, DeySK (2006) Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7 : 185–199.
6. ChaJ, SunX, DeySK (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat Med 18 : 1754–1767.
7. MacklonNS, GeraedtsJP, FauserBC (2002) Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update 8 : 333–343.
8. StephensonMD, AwartaniKA, RobinsonWP (2002) Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod 17 : 446–451.
9. PhilippT, PhilippK, ReinerA, BeerF, KalousekDK (2003) Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod 18 : 1724–1732.
10. RaiR, ReganL (2006) Recurrent miscarriage. Lancet 368 : 601–611.
11. JauniauxE, FarquharsonRG, ChristiansenOB, ExaltoN (2006) Evidence-based guidelines for the investigation and medical treatment of recurrent miscarriage. Hum Reprod 21 : 2216–2222.
12. Practice Committee of the American Society for Reproductive M (2012) Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 98 : 1103–1111.
13. MaWG, SongH, DasSK, PariaBC, DeySK (2003) Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A 100 : 2963–2968.
14. LargeMJ, DeMayoFJ (2012) The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol 358 : 155–165.
15. LydonJP, DeMayoFJ, FunkCR, ManiSK, HughesAR, et al. (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9 : 2266–2278.
16. LeeK, JeongJ, KwakI, YuCT, LanskeB, et al. (2006) Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet 38 : 1204–1209.
17. KuriharaI, LeeDK, PetitFG, JeongJ, LeeK, et al. (2007) COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3: e102.
18. FrancoHL, LeeKY, BroaddusRR, WhiteLD, LanskeB, et al. (2010) Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod 82 : 783–790.
19. DasSK, DasN, WangJ, LimH, SchryverB, et al. (1997) Expression of betacellulin and epiregulin genes in the mouse uterus temporally by the blastocyst solely at the site of its apposition is coincident with the “window” of implantation. Dev Biol 190 : 178–190.
20. TamadaH, DasSK, AndrewsGK, DeySK (1991) Cell-type-specific expression of transforming growth factor-alpha in the mouse uterus during the peri-implantation period. Biol Reprod 45 : 365–372.
21. DasSK, ChakrabortyI, PariaBC, WangXN, PlowmanG, et al. (1995) Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 9 : 691–705.
22. DasSK, WangXN, PariaBC, DammD, AbrahamJA, et al. (1994) Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120 : 1071–1083.
23. RaabG, KoverK, PariaBC, DeySK, EzzellRM, et al. (1996) Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development 122 : 637–645.
24. TanY, LiM, CoxS, DavisMK, TawfikO, et al. (2004) HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol 265 : 181–195.
25. XieH, WangH, TranguchS, IwamotoR, MekadaE, et al. (2007) Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A 104 : 18315–18320.
26. LuettekeNC, QiuTH, FentonSE, TroyerKL, RiedelRF, et al. (1999) Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 126 : 2739–2750.
27. ThreadgillDW, DlugoszAA, HansenLA, TennenbaumT, LichtiU, et al. (1995) Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269 : 230–234.
28. LeeKF, SimonH, ChenH, BatesB, HungMC, et al. (1995) Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature 378 : 394–398.
29. RiethmacherD, Sonnenberg-RiethmacherE, BrinkmannV, YamaaiT, LewinGR, et al. (1997) Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature 389 : 725–730.
30. GassmannM, CasagrandaF, OrioliD, SimonH, LaiC, et al. (1995) Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 378 : 390–394.
31. SibiliaM, WagnerEF (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269 : 234–238.
32. MiettinenPJ, BergerJE, MenesesJ, PhungY, PedersenRA, et al. (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376 : 337–341.
33. DasSK, TsukamuraH, PariaBC, AndrewsGK, DeySK (1994) Differential expression of epidermal growth factor receptor (EGF-R) gene and regulation of EGF-R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology 134 : 971–981.
34. LimH, DeySK, DasSK (1997) Differential expression of the erbB2 gene in the periimplantation mouse uterus: potential mediator of signaling by epidermal growth factor-like growth factors. Endocrinology 138 : 1328–1337.
35. LimH, DasSK, DeySK (1998) erbB genes in the mouse uterus: cell-specific signaling by epidermal growth factor (EGF) family of growth factors during implantation. Dev Biol 204 : 97–110.
36. TidcombeH, Jackson-FisherA, MathersK, SternDF, GassmannM, et al. (2003) Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci U S A 100 : 8281–8286.
37. LeeTC, ThreadgillDW (2009) Generation and validation of mice carrying a conditional allele of the epidermal growth factor receptor. Genesis 47 : 85–92.
38. CroneSA, ZhaoYY, FanL, GuY, MinamisawaS, et al. (2002) ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8 : 459–465.
39. LeeD, YuM, LeeE, KimH, YangY, et al. (2009) Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. J Clin Invest 119 : 2702–2713.
40. SoyalSM, MukherjeeA, LeeKY, LiJ, LiH, et al. (2005) Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41 : 58–66.
41. NelsonKG, TakahashiT, BossertNL, WalmerDK, McLachlanJA (1991) Epidermal growth factor replaces estrogen in the stimulation of female genital-tract growth and differentiation. Proc Natl Acad Sci U S A 88 : 21–25.
42. MellorSJ, ThomasEJ (1995) Interactions between oestradiol and epidermal growth factor in endometrial stromal proliferation and differentiation. J Reprod Fertil 104 : 157–164.
43. TongW, PollardJW (1999) Progesterone inhibits estrogen-induced cyclin D1 and cdk4 nuclear translocation, cyclin E - and cyclin A-cdk2 kinase activation, and cell proliferation in uterine epithelial cells in mice. Mol Cell Biol 19 : 2251–2264.
44. LiQ, KannanA, WangW, DemayoFJ, TaylorRN, et al. (2007) Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282 : 31725–31732.
45. LeeKY, JeongJW, WangJ, MaL, MartinJF, et al. (2007) Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27 : 5468–5478.
46. FrancoHL, DaiD, LeeKY, RubelCA, RoopD, et al. (2011) WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25 : 1176–1187.
47. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 : 44–57.
48. Huang daW, ShermanBT, LempickiRA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37 : 1–13.
49. KesslerCA, SchroederJK, BrarAK, HandwergerS (2006) Transcription factor ETS1 is critical for human uterine decidualization. Mol Hum Reprod 12 : 71–76.
50. GellersenB, BrosensIA, BrosensJJ (2007) Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med 25 : 445–453.
51. HomYK, YoungP, WiesenJF, MiettinenPJ, DerynckR, et al. (1998) Uterine and vaginal organ growth requires epidermal growth factor receptor signaling from stroma. Endocrinology 139 : 913–921.
52. Ignar-TrowbridgeDM, NelsonKG, BidwellMC, CurtisSW, WashburnTF, et al. (1992) Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A 89 : 4658–4662.
53. ClementiC, TripuraniSK, LargeMJ, EdsonMA, CreightonCJ, et al. (2013) Activin-Like Kinase 2 Functions in Peri-implantation Uterine Signaling in Mice and Humans. PLoS Genet 9: e1003863.
54. NagashimaT, LiQ, ClementiC, LydonJP, DeMayoFJ, et al. (2013) BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest 123 : 2539–2550.
55. HayashiK, EriksonDW, TilfordSA, BanyBM, MacleanJA2nd, et al. (2009) Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod 80 : 989–1000.
56. JeongJW, LeeHS, FrancoHL, BroaddusRR, TaketoMM, et al. (2009) beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 28 : 31–40.
57. MericskayM, KitajewskiJ, SassoonD (2004) Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 131 : 2061–2072.
58. MillerC, SassoonDA (1998) Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125 : 3201–3211.
59. VainioS, HeikkilaM, KispertA, ChinN, McMahonAP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397 : 405–409.
60. RichardsRG, BrarAK, FrankGR, HartmanSM, JikiharaH (1995) Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod 52 : 609–615.
61. RubelCA, LanzRB, KommaganiR, FrancoHL, LydonJP, et al. (2012) Research resource: Genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol 26 : 1428–1442.
62. CohenP (2002) Protein kinases–the major drug targets of the twenty-first century? Nat Rev Drug Discov 1 : 309–315.
63. MaruyamaT, YoshimuraY, YodoiJ, SabeH (1999) Activation of c-Src kinase is associated with in vitro decidualization of human endometrial stromal cells. Endocrinology 140 : 2632–2636.
64. GambaG (2005) Role of WNK kinases in regulating tubular salt and potassium transport and in the development of hypertension. Am J Physiol Renal Physiol 288: F245–252.
65. XuBE, StippecS, ChuPY, LazrakA, LiXJ, et al. (2005) WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci U S A 102 : 10315–10320.
66. RuanYC, GuoJH, LiuX, ZhangR, TsangLL, et al. (2012) Activation of the epithelial Na+ channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat Med 18 : 1112–1117.
67. XuBE, StippecS, LenertzL, LeeBH, ZhangW, et al. (2004) WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J Biol Chem 279 : 7826–7831.
68. VerissimoF, JordanP (2001) WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 20 : 5562–5569.
69. ManningG, WhyteDB, MartinezR, HunterT, SudarsanamS (2002) The protein kinase complement of the human genome. Science 298 : 1912–1934.
70. XuB, EnglishJM, WilsbacherJL, StippecS, GoldsmithEJ, et al. (2000) WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275 : 16795–16801.
71. DissanayakeVH, TowerC, BroderickA, StockerLJ, SeneviratneHR, et al. (2007) Polymorphism in the epidermal growth factor gene is associated with birthweight in Sinhalese and white Western Europeans. Mol Hum Reprod 13 : 425–429.
72. FujitaY, KurachiH, MorishigeK, AmemiyaK, TerakawaN, et al. (1991) Decrease in epidermal growth factor receptor and its messenger ribonucleic acid levels in intrauterine growth-retarded and diabetes mellitus-complicated pregnancies. J Clin Endocrinol Metab 72 : 1340–1345.
73. FondacciC, AlsatE, GabrielR, BlotP, NessmannC, et al. (1994) Alterations of human placental epidermal growth factor receptor in intrauterine growth retardation. J Clin Invest 93 : 1149–1155.
74. GabrielR, AlsatE, Evain-BrionD (1994) Alteration of epidermal growth factor receptor in placental membranes of smokers: relationship with intrauterine growth retardation. Am J Obstet Gynecol 170 : 1238–1243.
75. BrysM, SemczukA, RechbergerT, KrajewskaWM (2007) Expression of erbB-1 and erbB-2 genes in normal and pathological human endometrium. Oncol Rep 18 : 261–265.
76. KonecnyGE, SantosL, WinterhoffB, HatmalM, KeeneyGL, et al. (2009) HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer 100 : 89–95.
77. LebovicDI, BaldocchiRA, MuellerMD, TaylorRN (2002) Altered expression of a cell-cycle suppressor gene, Tob-1, in endometriotic cells by cDNA array analyses. Fertil Steril 78 : 849–854.
78. BurneyRO, TalbiS, HamiltonAE, VoKC, NyegaardM, et al. (2007) Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148 : 3814–3826.
79. FinnCA, MartinL (1972) Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod 7 : 82–86.
80. MarkoffE, ZeitlerP, PelegS, HandwergerS (1983) Characterization of the synthesis and release of prolactin by an enriched fraction of human decidual cells. J Clin Endocrinol Metab 56 : 962–968.
81. HartigSM, HeB, NewbergJY, OchsnerSA, LooseDS, et al. (2012) Feed-forward inhibition of androgen receptor activity by glucocorticoid action in human adipocytes. Chem Biol 19 : 1126–1141.
82. HartigSM, NewbergJY, BoltMJ, SzafranAT, MarcelliM, et al. (2011) Automated microscopy and image analysis for androgen receptor function. Methods Mol Biol 776 : 313–331.
83. SzafranAT, SzwarcM, MarcelliM, ManciniMA (2008) Androgen receptor functional analyses by high throughput imaging: determination of ligand, cell cycle, and mutation-specific effects. PLoS One 3: e3605.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



