-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
The migratory path of DTCs determines the shape of the C. elegans gonad. How the spatiotemporal migration pattern is regulated is not clear. We identified a conserved transcription factor BLMP-1 as a central component of a gene regulatory circuit required for the spatiotemporal control of DTC migration. BLMP-1 levels regulate the timing of the DTC dorsal turn, as high levels delay the turn and low levels result in an early turn. We identify and characterize upstream regulators that control BLMP-1 levels. These regulators function in two ways, i.e. by destabilization of BLMP-1 through ubiquitin-mediated proteolysis and by transcriptional repression of the blmp-1 gene to down-regulate BLMP-1. Interestingly, blmp-1 also negatively controls these regulators. Our data suggest that a dietary signal input acts together with a double-negative feedback loop to switch DTCs from the “blmp-1-on” to the “blmp-1-off” state, promoting their dorsal turn. Furthermore, we show that some protein interactions in the circuit are conserved in C. elegans and humans. Our work defines a novel function of the conserved blmp-1 gene in the temporal control of cell migration, and establishes a gene regulatory circuit that integrates the temporal and spatial inputs to direct cell migration during organogenesis.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004428
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004428Summary
The migratory path of DTCs determines the shape of the C. elegans gonad. How the spatiotemporal migration pattern is regulated is not clear. We identified a conserved transcription factor BLMP-1 as a central component of a gene regulatory circuit required for the spatiotemporal control of DTC migration. BLMP-1 levels regulate the timing of the DTC dorsal turn, as high levels delay the turn and low levels result in an early turn. We identify and characterize upstream regulators that control BLMP-1 levels. These regulators function in two ways, i.e. by destabilization of BLMP-1 through ubiquitin-mediated proteolysis and by transcriptional repression of the blmp-1 gene to down-regulate BLMP-1. Interestingly, blmp-1 also negatively controls these regulators. Our data suggest that a dietary signal input acts together with a double-negative feedback loop to switch DTCs from the “blmp-1-on” to the “blmp-1-off” state, promoting their dorsal turn. Furthermore, we show that some protein interactions in the circuit are conserved in C. elegans and humans. Our work defines a novel function of the conserved blmp-1 gene in the temporal control of cell migration, and establishes a gene regulatory circuit that integrates the temporal and spatial inputs to direct cell migration during organogenesis.
Introduction
Cell migration is important for organogenesis and development of animals. Numerous extracellular guidance cues and receptors for the spatial control of cell migration have been identified and characterized [1]. However, little is known about the temporal regulation of cell migration and how the spatial and temporal signals are coordinated to generate a specific and reproducible pattern of cell migration during development.
The bilobed gonad of C. elegans hermaphrodites develops from a four-cell primordium positioned in the ventral midbody [2]. The shape of the two symmetrical U-shaped gonadal arms is determined by the migratory paths of the two distal tip cells (DTC), leader cells found at the tip of each arm [3]. The DTCs undergo three sequential phases of migration and re-orient twice during the three larval developmental stages, thus providing a paradigm for the study of the spatio-temporal regulation of cell migration in vivo [2], [3]. In phase I during the L2 and early L3 stages, the anterior and posterior DTCs move centrifugally along the ventral body wall muscles towards the head or tail, respectively (Figure 1A). In phase II, they turn 90 degrees and move from the ventral to the dorsal muscles, then, during phase III, they again turn 90 degrees and move centripetally along the dorsal body wall muscles and halt in the mid-body. Both orthogonal turns occur during the late L3 stage. The timing of these turns is regulated by a redundant function of the heterochronic genes daf-12, dre-1, and lin-29 [4]. A single mutation in any of these three genes has no effect on DTC migration, but mutation of 2 or 3 of the genes delays the L3-specific DTC migration pattern, which fails to take place even in L4 or the adult. lin-29, daf-12, and dre-1 encode, respectively, a zinc-finger transcription factor, a steroid hormone receptor similar to the vertebrate vitamin D and liver-X receptor, and an F-Box protein of an SCF ubiquitin ligase complex [4]–[6], indicating that a complex mechanism, involving steroid hormone signaling, gene transcription, and protein degradation, is responsible for the temporal control of the dorsal turn. However, how these three genes function to do so is unclear.
Fig. 1. DTC migration defects of blmp-1 mutants. 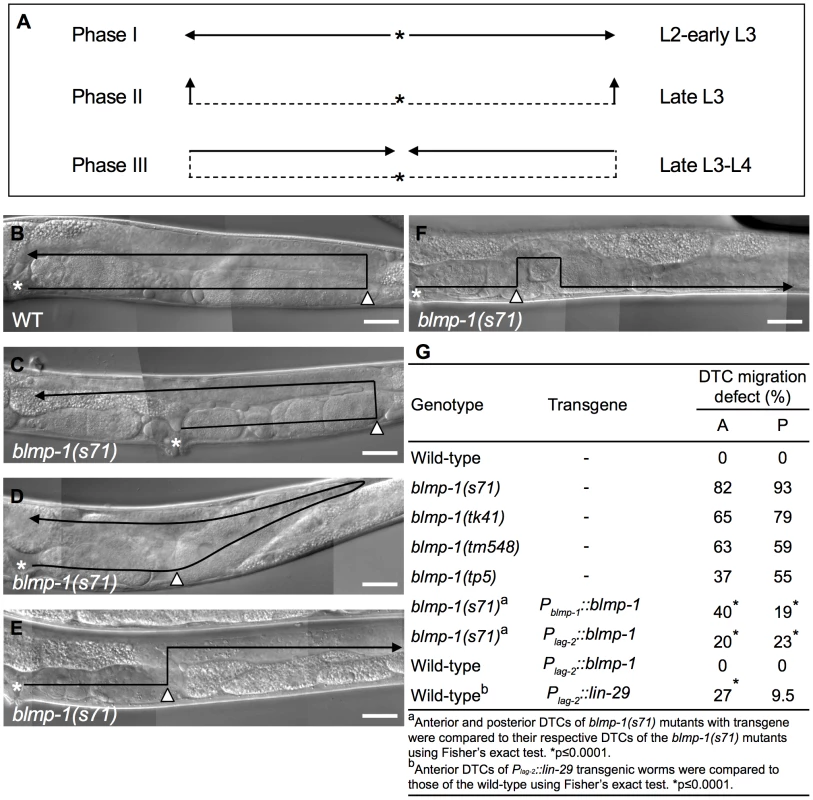
(A) Schematic diagram showing the path and direction of DTC migration in phases I, II, and III. The developmental stage in which each migration phase occurs is indicated on the right. The solid line and arrow show, respectively, the path and direction of each migration phase. (B–F) DIC images of adult wild-type (B) and blmp-1(s71) (C–F) gonads. The black line shows the migratory path, starting from the asterisk, the DTC is indicated by the black arrowhead, and the white arrowhead indicates the position at which the DTC initiated dorsal phase II migration. In (C–F), the DTC had a shorter phase I migratory path (distance between the asterisk and white arrowhead) than the wild-type DTC (B); in addition, the DTC in (C) had a longer phase III migration path, that in (D) had a slanted phase II migration path (see text for details), and that in (E) moved in the opposite direction during phase III migration. (F) The DTC had a similar migratory trajectory to that shown in (E), except that it failed to stay on the dorsal side during phase III migration. The gonadal arm shown in B–E is posterior, while that in F is anterior, as the defect was only observed in this arm, as shown in Table S1. The picture in F was flipped 180 degrees so that its migration trajectory could be easily compared to those in B–E. Scale bar 40 µm. (G) Percentage of the indicated blmp-1 mutants and transgenic worms with a DTC migration defect (shown in Figure 1C–F). A: anterior DTC, P: posterior DTC. At least 50 worms were scored for each genotype. The dorsal migration of DTCs is regulated by the guidance receptors UNC-5 and UNC-40 (a homolog of Deleted in Colorectal Cancer) [7]–[9], which drive DTCs to move away from the ventrally localized UNC-6 to the dorsal side [10], [11]. Dorsally localized UNC-129/TGF-β also promotes DTC dorsal migration through UNC-5 and UNC-40 receptors [12]. Mutations in these genes disrupt the ventral-to-dorsal migration of DTCs. unc-40 appears to be transcribed in the DTCs throughout their migration [7], whereas unc-5 is transcriptionally up-regulated at the time when the dorsal turn is initiated [13]. Precocious expression of unc-5 cDNA at the time when DTCs have not yet turned dorsalward induces unc-6-dependent precocious dorsal migration [13]. These data show that unc-5 is both necessary and sufficient for DTC dorsal migration and that the increase in unc-5 transcription is responsible, at least in part, for the initiation of DTC dorsal migration [8], [9], [13]. However, how unc-5 is temporally regulated to direct dorsal migration precisely at the late L3 stage is unclear.
In this study, we performed genetic screening for mutants defective in DTC migration and isolated blmp-1 mutants. Our results showed that blmp-1 is a heterochronic gene that acts with daf-12, dre-1, and lin-29 in a regulatory circuit to control the correct timing of DTC migration. We also showed that this regulatory circuit is, at least in part, conserved in C. elegans and humans.
Results
blmp-1 mutants show defective DTC migration
To identify genes that are important for the spatiotemporal regulation of DTC migration, we performed genetic screening for mutants defective in DTC migration, as described in the Materials and Methods, and isolated alleles tp5 and tk41. A genetic complementation test showed that tp5 and tk41 were allelic, and that either allele failed to complement the previously identified mutation dpy-24(s71) (dpy stands for dumpy, shorter than wild-type) [14].
We mapped dpy-24(s71) to chromosome I, near stp124, by sequence tag site (STS) mapping [15] (Figure S1A). Three factor mapping using unc-40 and unc-75 and subsequent SNP mapping positioned dpy-24(s71) within the region between cosmids F45H11 and F37D6. Cosmids covering this region were microinjected into the dpy-24(s71) mutant, and cosmid F25D7 rescued the DTC migration defect (Figure S1B). The genomic DNA fragments corresponding to the 5 predicted open reading frames of F25D7 were individually amplified by long PCR and tested for their ability to rescue the dpy-24(s71) mutant and only F25D7.3, which contained a single blmp-1 gene, had a rescue effect (Figure S1B). In addition, F25D7.3 RNA interference (RNAi) phenocopied the dpy-24(s71) mutant (Table 1). These results demonstrated that F25D7.3 corresponded to the blmp-1 gene (named for its sequence similarity to mouse Blimp-1, see below) [16].
Tab. 1. Genetic interactions between blmp-1 and the heterochronic genes lin-29, dre-1, and daf-12 in the timing of the DTC dorsal turn. 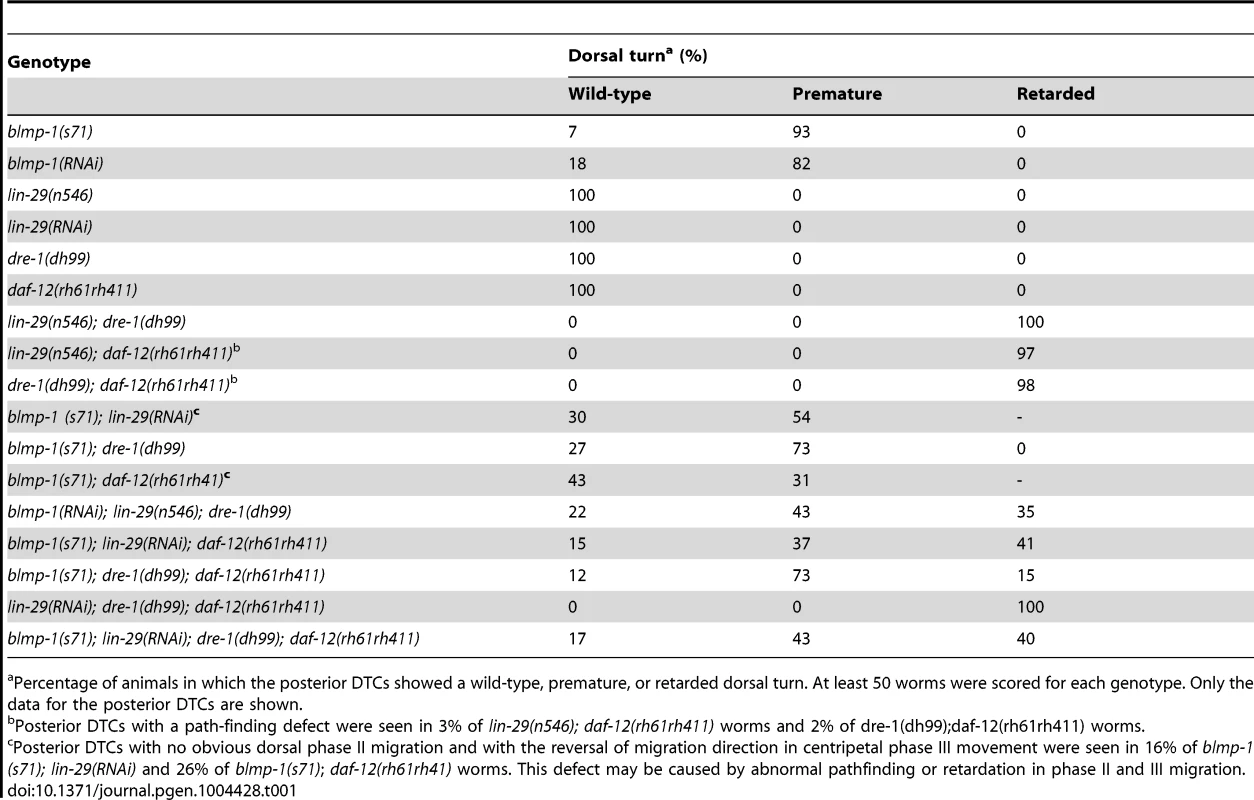
Percentage of animals in which the posterior DTCs showed a wild-type, premature, or retarded dorsal turn. At least 50 worms were scored for each genotype. Only the data for the posterior DTCs are shown. The blmp-1 gene encodes a protein with 27% identity to mouse B lymphocyte-induced maturation protein 1 (Blimp-1) and 26% identity to human positive regulatory domain I-binding factor (PRDI-BF1) (Figure S2). Both Blimp-1 and PRDI-BF1 are thought to act predominantly as transcription repressors and are essential for the terminal differentiation of B and T cells [17], [18]. As shown in Figure 2, BLMP-1, like Blimp-1 and PRDI-BF1, is predicted to contain a positive regulatory (PR) domain, a nuclear localization signal (NLS), and five Kruppel-type [(Cys)2-(His)2] zinc fingers. The zinc fingers of both Blimp-1 and PRDI-BF1 have been shown to bind to target DNA and are essential for their transcriptional repression activities [19]–[21].
Fig. 2. Gene structure of blmp-1. 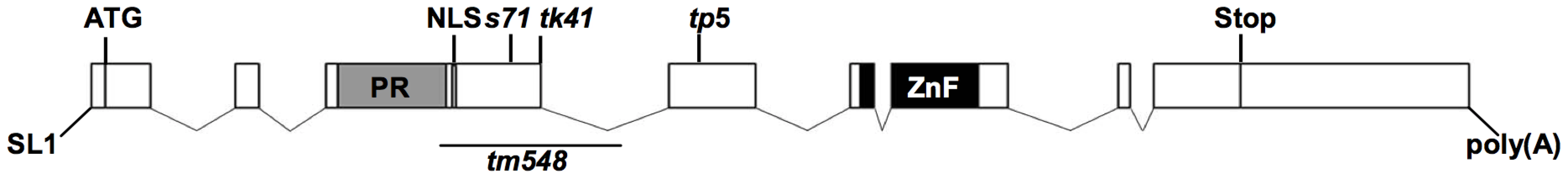
Gene structure of blmp-1 deduced from genomic and cDNA sequences. The boxes indicate exons. The regions encoding the PRDI-BF1-RIZ1 homologous region (PR) domain, nuclear localization signal (NLS), and zinc finger motifs are marked, as is the trans spliced leader SL1. The positions of the blmp-1 mutant alleles, including the region corresponding to the tm548 deletion, are indicated. Alleles s71, tk41, and tp5 have, respectively, a non-sense mutation in codon 281, 381, or 434, and are predicted to encode truncated proteins without zinc fingers (Figure 2 and S2). The deletion allele tm548, which was isolated by a reverse genetic approach (National Bioresource Project), has an 810 bp deletion, removing part of exon 3 and part of intron 3 (Figure 2 and S2) and may result in a truncated BLMP-1 protein containing the first 254 amino acids of BLMP-1 and 17 amino acids encoded by the third intron.
DTCs in blmp-1 mutants undergo a precocious dorsal turn
The four blmp-1 mutants, s71, tk41, tp5, and tm548, were found to have a similar set of defects, including a DTC migration abnormality (shown for blmp-1(s71) in Figure 1), a weak dumpy phenotype (shown for blmp-1(s71) in Figure S3A), and a partially penetrant embryonic lethality (shown for all four in Figure S3B). Wild-type blmp-1 genomic DNA rescued the dumpy phenotype and embryonic lethality of the blmp-1(s71) mutant (Figure S3B), showing that these defects were caused by the loss of blmp-1.
The DTC migration patterns of these mutants were varied, but shared the common feature that the mutant DTCs had a shorter centrifugal phase I migration path and executed the dorsal turn at a point closer to the mid-body than wild-type DTCs (Figure 1B–G, Table S1), suggesting either slower movement and/or precocious initiation of the dorsal turn. However, we timed the movement of the DTCs during phase I migration and found that the blmp-1 mutant DTCs did not migrate significantly slower than the wild-type DTCs (Table S2). In addition, some DTCs migrated obliquely with respect to the dorsal-ventral axis until they reached the dorsal muscle (Figure 1D); such a migratory route is probably due to the simultaneous execution of centrifugal phase I and dorsal phase II migrations, support for a precocious execution of the dorsal turn at the time when phase I migration normally occurs.
To examine whether the abnormal DTC migration pattern of the blmp-1 mutants was indeed caused by a precocious dorsal turn, we performed a time-course analysis of DTC migration in the wild-type and blmp-1(s71) mutant, using the division stages of the vulval precursor cell P6.p as temporal developmental markers, as described previously [22]. P6.p is generated in mid L1, undergoes three rounds of cell division during L3, and gives rise to eight descendants that constitute the vulva [23]. Figure 3A shows representative DIC images of wild-type (a, b, e, f) and blmp-1(s71) (c, d g, h) posterior gonadal arms in early L3 (top panels) and late L3 (bottom panels). Figure 3B shows that, in the wild-type, the DTCs in more than 90% of worms underwent ventral-to-dorsal migration at the four-P6.p cell stage and the DTCs in none made a dorsal turn before P6.p cell division, whereas, in the s71 mutant, the anterior DTCs in 36% of worms and the posterior DTCs in 66% of worms had turned dorsalward before P6.p divided. These results demonstrate that loss of blmp-1 causes a precocious initiation of DTC dorsal migration (early L3, rather than late L3) and that blmp-1 functions to prevent a precocious DTC dorsal turn.
Fig. 3. DTCs in the blmp-1 mutant undergo a precocious dorsal turn. 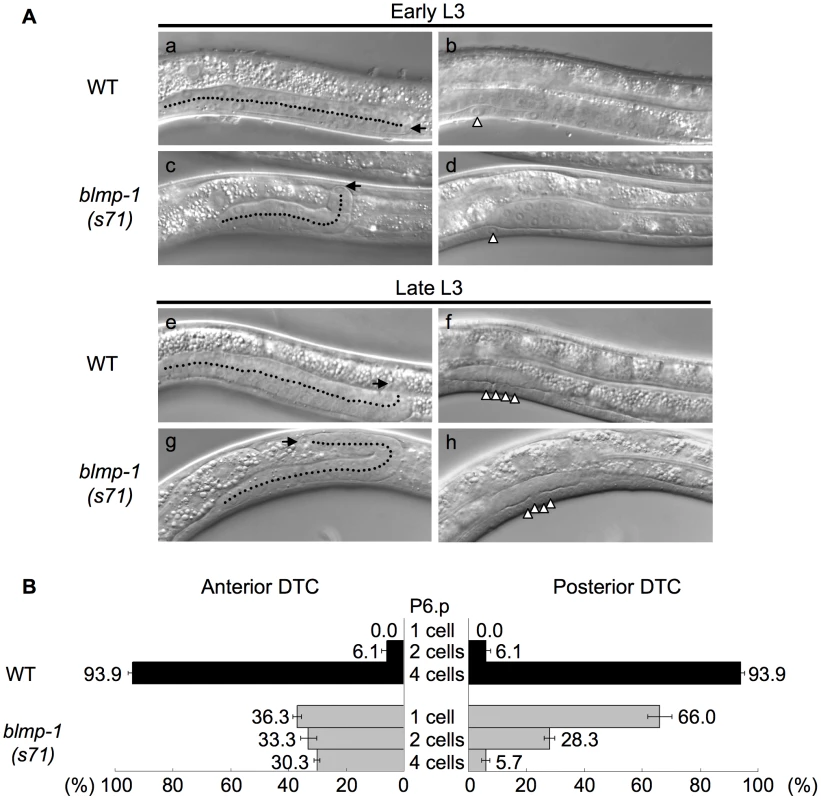
(A) DIC images of wild-type (WT) and blmp-1(s71) posterior gonadal arms during the early L3 (top panels) and late L3 (bottom panels) stages. (a–d) In early L3, when the worms were in the one-P6.p-cell stage (b, d), the wild-type DTC (a) was still in phase I migration, while the blmp-1 DTC (c) had already made a dorsal turn. (e–h) In late L3, when the worms were in the four-P6.p-cell stage (f, h), the wild-type DTC (e) had just begun the dorsal turn, while the blmp-1 DTC (g) had already completed the dorsal phase II migration and undergone centripetal phase III migration. In the left panel, the arrow and dotted line indicate, respectively, the DTC and its migratory path. In the right panel, the arrowhead indicates the P6.p division stage of the same worm shown in the left panel. (B) The percentage of worms with anterior (left) and posterior (right) DTCs that initiated the phase II dorsal turn at the indicated division stage of the P6.p cell (shown in the center) in wild-type (black bars) and blmp-1(s71) (gray bars) worms. At least 33 worms were examined for each genotype. BLMP-1 is present in the DTC before the dorsal turn
To determine the localization of BLMP-1 and explore its function, we raised polyclonal antibodies against bacterially expressed recombinant BLMP-1 (Materials and Methods) and used the affinity-purified antibodies to stain whole-mount animals. The results showed that BLMP-1 was localized in the nucleus and was detected in hypodermal, vulval, and intestinal cells (Figure 4A), as well as DTCs (Figure 4B). We next stained the mutant embryos with the blmp-1 mutations (s71, tm548, tp5 and tk71), which are loss-of-function recessive alleles by genetic tests (Materials and Methods). Little or no signals were detected in these mutant embryos as shown in representative images in Figure 4C, demonstrating the specificity of the antibodies. Notably, BLMP-1 was seen in DTCs prior to the mid L3 larval stage (two P6.p-descent cells), but not during, or after, mid L3 stage, after the DTCs had undergone the dorsal turn (Figure 4B). This result and the precocious dorsal turn phenotype of the blmp-1 mutant support a model in which BLMP-1 functions in DTCs before mid L3 stage to prevent these cells from undergoing a precocious dorsal turn.
Fig. 4. BLMP-1 expression pattern and its regulation. 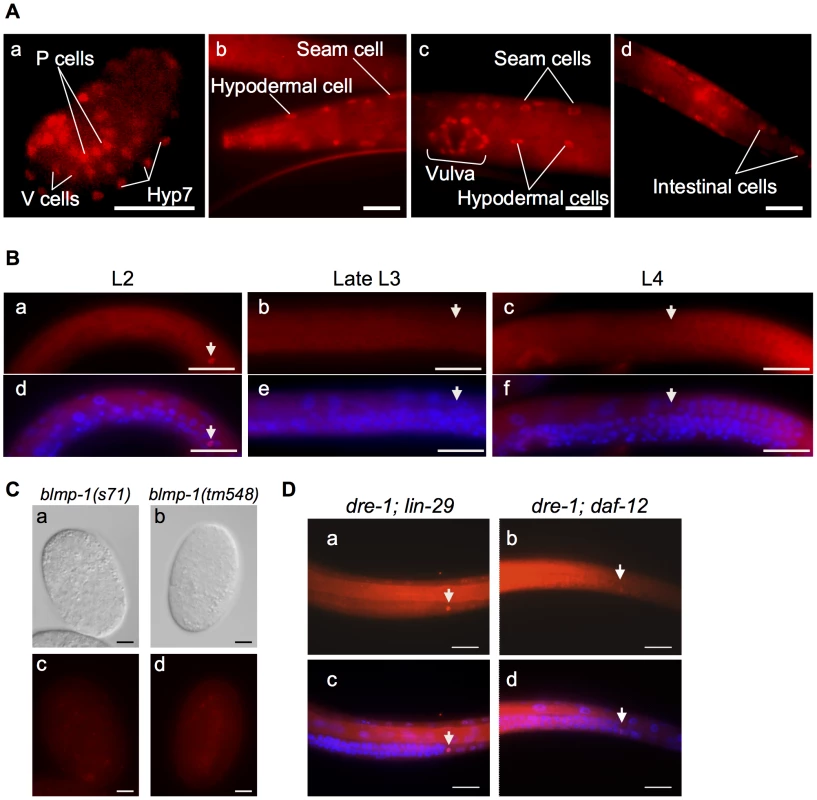
(A) Representative images of a wild-type embryo (a) or larva (b–d) stained with anti-BLMP-1 antibodies. (a) In the 1.5-fold embryo, BLMP-1 is detected in Hyp7, V cells, and P cell precursors. (b–d) In the larva, BLMP-1 is detected in hypodermal and seam cells (b and c), vulval cells (c), and posterior intestinal cells (d). Scale bar 40 µm. (B) BLMP-1 levels in wild-type DTCs at different larval stages revealed by immunostaining with anti-BLMP-1 antibody (a–c) or together with DAPI staining (d–f). (a) BLMP-1 is detected during centrifugal phase I migration in L2. (b, c) No BLMP-1 is detected during dorsal phase II migration in late L3 (b) or during centripetal phase III migration in L4 (c). (d, e, f) The same worms as those in, respectively, a, b, or c stained with DAPI to label nuclei and to examine the developmental stage of the gonad. Scale bar 20 µm. The arrowheads indicate DTCs. (C) The DIC (a, b) and immunostaining (c, d) images of blmp-1(s71) (a, c) and blmp-1(tm548) (b,d) embryos stained by anti-BLMP-1 antibodies. Scale bar 10 µm. (D) Presence of BLMP-1 at the L4 stage in the DTCs of the double mutants lin-29;dre-1 (a) and dre-1;daf-12 (b), as revealed by immunostaining with anti-BLMP-1 antibodies. (c, d) The same worms as those in a and b, respectively, stained with DAPI. Scale bar 20 µm. The localization of BLMP-1 in DTCs supports the model that BLMP-1 controls the timing of DTC dorsal migration in a cell-autonomous fashion. We then tested whether expression of blmp-1 cDNA in DTCs under the control of the lag-2 promoter Plag-2 is sufficient to rescue the DTC migration defect of the blmp-1 mutant. Plag-2 drives gene expression in DTCs, but not in body wall muscle or hypodermis, the structures on which DTCs migrate [24]. In blmp-1 mutants carrying the Plag-2 blmp-1 transgene, the percentage of worms with abnormal anterior or posterior DTC migration was reduced, respectively, from 82% to 20% and from 93% to 23% (Figure 1G), similar levels to those seen when blmp-1 cDNA was expressed under the control of the blmp-1 endogenous promoter Pblmp-1 (40% or 19% for the anterior and posterior DTC, respectively; Figure 1G). This result further supports a cell-autonomous role of blmp-1 in DTC migration.
blmp-1 represses unc-5 transcription to prevent the DTCs from dorsalward turning
Next, we tested whether blmp-1 may prevent dorsalward turning of DTCs by regulating a dorsal-ventral guidance system at the early larval stage. The dorsal migration of DTCs is regulated by the guidance receptors UNC-5 and UNC-40 (a homolog of Deleted in Colorectal Cancer) [7]–[9], which drive DTCs to move away from the ventrally localized UNC-6 [10]–[11]. The observations that blmp-1, unc-5 and unc-40 function cell-autonomously in the control of DTC migration [7], [9] (Figure 1G) suggest that blmp-1 may prevent DTC precocious dorsalward turning by regulating unc-5 and/or unc-40. unc-40 appears to be transcribed in the DTCs throughout their migration [7], whereas unc-5 is transcriptionally up-regulated at the time when the dorsal turn is initiated [13]. Using the transgene Punc-5(1 kb) gfp, in which an approximately 1 kb sequence upstream of the unc-5 coding sequence was used to drive the GFP reporter, we confirmed this unc-5 expression pattern, i.e. unc-5 was expressed during and after, but not before, the DTC dorsal turn (Figure S4). Interestingly, this temporal pattern of unc-5 transcription is complementary to that of BLMP-1 expression in DTCs, as BLMP-1 was present in DTCs only before the dorsal turn (Figure 4B). This raised the possibility that BLMP-1 inhibits unc-5 transcription and thus prevents DTCs from turning dorsalward. If this were the case, the correct timing of the disappearance of BLMP-1 from DTCs would alleviate this transcriptional inhibition and allow unc-5 transcription and the DTC dorsal turn. We tested this hypothesis by altering the temporal expression pattern of blmp-1 and examining their effects on unc-5 transcription.
At the early L3 stage when the P6.p cell has not yet divided, no wild-type worms contained DTCs that had turned dorsalward or showed any expression of the Punc-5(1 kb)::gfp transgene (Figure 5Ba), whereas, in some blmp-1(s71) mutants, DTCs at the same stage had performed the dorsal turn and displayed precocious unc-5 transcription (Figure 5Bb). Thus, loss of blmp-1 causes precocious unc-5 expression, which coincides with the precocious dorsalward turning of DTCs.
Fig. 5. Effects of heterochronic mutations on the transcription of unc-5 and lin-29 in DTCs. 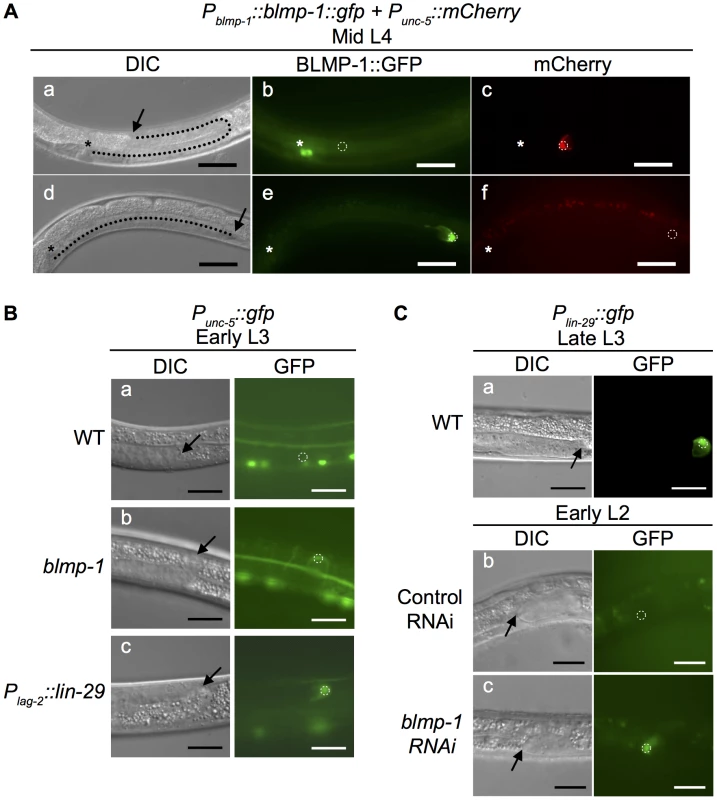
(A) Persistent BLMP-1 expression in DTCs suppresses unc-5 transcription during late larval development. DIC, GFP, and mCherry images at the mid L4 stage of two worms carrying the Pblmp-1::bmp-1::gfp and Punc-5(4.6 kb)::mCherry transgenes. (a–c) A representative worm in which BLMP-1 was normally down-regulated. The DTC has undergone a dorsal turn (a) and does not express BLMP-1::GFP (b), but expresses unc-5. (d–f) A representative worm in which BLMP-1 was still expressed. The DTC has failed to turn dorsalward and is moving centrifugally (d), shows persistent BLMP-1::GFP expression (e), and does not express unc-5 (f). The arrows indicate DTCs and the asterisks the developing vulva. Scale bar 40 µm. (B) The blmp-1 mutation causes precocious unc-5 transcription through precocious expression of lin-29. (a–c) DIC and GFP images of the posterior DTC at the early L3 stage in a wild-type worm (a), a blmp-1(s71) worm (b), and a Plag-2::lin-29-expressing worm (c). All worms carried a Punc-5(4.6 kb)::gfp transgene. Scale bar 20 µm. (C) DIC and GFP images of transgenic animals carrying Plin-29::gfp (a) during late L3 stage. The transgenic animal carrying Plin-29::gfp treated with control vector RNAi (b) or blmp-1 RNAi (c) during the early L2 stage. The arrows indicate DTCs. Scale bars 20 µm. Next, we tested whether the blmp-1 precocious dorsal turn phenotype required unc-5 by examining and comparing the DTC dorsal migration patterns of the blmp-1 and unc-5 single mutants and the blmp-1; unc-5 double mutant. Approximately 48% anterior DTCs and 83% posterior DTCs failed to turn dorsalward in the unc-5(e53) mutant (Table S3). No precocious dorsal turn was observed in the blmp-1 (s71); unc-5 double mutant, showing that the unc-5(e53) mutation blocked the precocious dorsal turn phenotype of the blmp-1 (s71) mutant and that the blmp-1 precocious dorsal turn phenotype required unc-5.
Constitutive expression of blmp-1 delays dorsal turn and inhibits unc-5 expression
Because BLMP-1 is present in the DTC before the dorsal turn, we examined the significance of blmp-1 down-regulation and its effect on the initiation of DTC dorsalward turning. To this end, we overexpressed blmp-1 using the transgene Pblmp-1::blmp-1::gfp, in which the fusion protein BLMP-1::GFP was expressed under the control of the promoter Pblmp-1. In the resulting transgenic line, 10% of transgenic worms had DTCs that displayed normal BLMP-1::GFP down-regulation, underwent a normal dorsal turn (Figure 5A, a, b), whereas 90% of transgenic worms showed persistent BLMP-1::GFP expression, even at the L4 stage, showing that BLMP-1::GFP was not appropriately down-regulated in these worms, and these worms showed no sign of dorsal turn (Figure 5A, d, e). This retarded turn phenotype was in contrast to the precocious dorsal migration phenotype caused by loss of blmp-1 (Figures 1C–G). Collectively, these results show that the BLMP-1 level is important regulation for DTC dorsal migration and that the timely disappearance of BLMP-1 allows the DTCs to turn dorsalward, hence switching their migration phase from centrifugal phase I migration to ventral-to-dorsal dorsal phase II migration.
To test whether blmp-1 overexpression might repress unc-5 expression in DTCs and thus resulted in the retarded turning phenotype, we expressed the transgene Pblmp-1::blmp-1::gfp in worms carrying the Punc-5(1 kb)::mCherry reporter. In the resulting transgenic line, 10% of transgenic worms had DTCs that displayed normal BLMP-1::GFP down-regulation, underwent a normal dorsal turn, and showed Punc-5(1 kb)::mCherry expression (Figure 5A, a–c), whereas 90% of transgenic worms showed persistent BLMP-1::GFP expression, and these worms showed no sign of dorsal turn or unc-5 expression (Figure 5A, d–f). Thus, constitutive blmp-1 expression in the late larval stage represses unc-5 transcription and blocks the DTC dorsal turn. These results confirmed the causal relationship of the reciprocal patterns of BLMP-1 expression and unc-5 transcription in DTCs and support the model in which BLMP-1 inhibits unc-5 transcription and thus prevents DTCs from turning dorsalward during early larval development.
Reciprocal suppression of blmp-1 and either daf-12, dre-1, or lin-29 in the control of the timing of DTC dorsal migration
The heterochronic genes daf-12, dre-1, and lin-29 function redundantly to specify the temporal identity of DTCs and prevent DTCs from undergoing retarded ventral-to-dorsal migration [4]. We tested the genetic interaction of blmp-1 with daf-12, dre-1, and lin-29 in the temporal control of DTC dorsal turn. For daf-12, we used the null allele rh61rh411. Because complete loss of dre-1 or blmp-1 caused lethality [4], [25] and the lin-29(n546); blmp-1(s71) double mutant was very sick and could not be maintained as homozygote, we used viable alleles and/or RNAi for dre-1, blmp-1 and lin-29 to analyze the genetic interactions of these genes. The precocious phenotype of the blmp-1(s71) mutant was partially suppressed by a single mutation of lin-29, dre-1, or daf-12 or combined mutations of any 2 or all 3 (Table 1). For example, 93% of blmp-1(s71) mutants had a precocious DTC migration defect, whereas 54% of the blmp-1(s71); lin-29(RNAi) double mutants, 73% of the blmp-1(s71); dre-1(dh99) double mutants, and 31% of the blmp-1(s71);daf-12(rh61rh41) double mutants displayed a precocious turn defect. This suggests that the blmp-1 precocious phenotype may require the activity of lin-29, dre-1, or daf-12, and that blmp-1 may function upstream of, or in parallel with, lin-29, dre-1, and daf-12 to prevent a precocious DTC dorsal turn during early larval development. Conversely, the blmp-1 mutation also partially suppressed the retarded dorsal migration of the double or triple mutants of lin-29, dre-1, and daf-12 (Table 1). For example, 98% of the dre-1(dh99); daf-12(rh61rh411) double mutants showed retarded DTC migration, whereas 12%, 73%, and 15% of the blmp-1(71); dre-1(dh99); daf-12(rh61rh411) triple mutants showed, respectively, wild-type, precocious, or retarded DTC migration. This suggests that the retarded phenotype of the lin-29, dre-1 and daf-12 double and triple mutants may require blmp-1 activity and that lin-29, dre-1, and daf-12 may function upstream of, or in parallel with, blmp-1 to prevent a delay in the DTC dorsal turn during the late larval stage. Thus, two distinct regulatory hierarchies in these heterochronic genes are likely employed to specify the temporal identities of DTCs at the early and late larval stages, and the switch from the “blmp-1-on” state to the “blmp-1-off” state during developmental progression may determine the timing of the DTC dorsal turn.
BLMP-1 transcriptionally represses lin-29 to prevent DTCs undergoing a precocious dorsal turn
The observation that mutation of daf-12, dre-1, or lin-29 partially suppressed the blmp-1-related precocious DTC dorsal migration defect (Table 1) prompted us to examine whether blmp-1 negatively regulated the transcription of daf-12, dre-1, or lin-29. Using chromatin immunoprecipitation coupled with high-throughput DNA sequencing (ChIP-seq), the modENCODE Consortium has shown that BLMP-1 binds to the upstream sequence of lin-29, but not that of daf-12 or dre-1, at the early larval stage [26]. This suggested that BLMP-1 might negatively regulate lin-29 expression. To test this possibility, we analyzed and compared lin-29 transcription in the wild-type and blmp-1 mutants using the transcriptional reporter Plin-29::gfp, in which gfp was expressed under the control of the lin-29 promoter Plin-29 (Figure 5Ca). We confirm that the transcription of lin-29 starts at approximately mid L3 stage and continues during L4 [4], [27]. We found that knockdown of blmp-1 using RNAi resulted in precocious expression of Plin-29::gfp at the L2 stage (Figure 5Cb,c), showing that blmp-1 represses lin-29 transcription at the L2 stage.
To investigate the significance of this blmp-1-mediated lin-29 down-regulation during DTC phase I migration in L2, we precociously expressed lin-29 under the control of the Plag-2 promoter using the Plag-2::lin-29 transgene. In the wild-type animals carrying this transgene, 27% and 9.5% of the anterior or posterior DTCs, respectively, underwent a precocious dorsal turn (Figure 1G). This result shows the functional importance of blmp-1-mediated repression of lin-29 transcription in preventing precocious DTC dorsal migration during early larval development.
The heterochronic genes daf-12, dre-1, and lin-29 act redundantly to down-regulate BLMP-1 levels in the mid and late L3 stages
The observation that the constitutive expression of blmp-1 in DTCs throughout larval development prevented them from performing the dorsal turn (Figure 5A) highlights the importance of BLMP-1 down-regulation in promoting the dorsal turn. Two observations suggested that daf-12, lin-29, and dre-1 might be responsible for BLMP-1 down-regulation. First, like worms constitutively expressing blmp-1 (Figure 5Ad–f), the double and triple mutants of daf-12, lin-29, and dre-1 had a retarded DTC migration phenotype (Table 1). Second, loss of blmp-1 partially suppressed the retarded phenotype of the double and triple mutants (Table 1), showing that the defect in the double and triple mutants required blmp-1 activity. We therefore examined BLMP-1 levels in mutants defective in daf-12, lin-29 and/or dre-1 using immunostaining with anti-BLMP-1 antibody.
As mentioned above, in the wild-type control, BLMP-1 was not detected in DTCs at the L4 stage (Figure 4B) and a similar result was observed in the daf-12, dre-1, and lin-29 single mutants. In contrast, persistent BLMP-1 expression in L4 stage was seen in DTCs from the double mutants dre-1(dh99); lin-29(n546) (100% of 30 worms scored), dre-1(dh99); daf-12(rh61rh411) (20% of 60 scored), and lin-29(RNAi); daf-12(rh61rh411) (∼5% of 106 scored) (examples shown in Figure 4D). The intensity of the persistent BLMP-1 signal was much weaker in the lin-29(RNAi); daf-12(rh61rh411) double mutant than in the other double mutants and bleached too quickly to be photographed by our imaging system. The daf-12(rh61rh411) allele is molecular null, while lin-29(n546) and dre-1(dh99) are partial loss-of-function [4], [5]. These results suggest that DRE-1 acts together with transcription factor LIN-29 or, to a lesser extent, with transcription factor DAF-12 for efficient down-regulation of BLMP-1 and correct timing of the control of DTC dorsal migration.
LIN-29 and DAF-12 repress blmp-1 transcription to promote the DTC dorsal turn
To determine how BLMP-1 was down-regulated by lin-29, daf-29, and dre-1, we used two gfp reporters in an in vivo expression assay to examine whether blmp-1 expression was repressed at the transcriptional level by transcription factors LIN-29 and DAF-12 and at the post-transcriptional level by the F-Box protein DRE-1. We first generated a transcriptional reporter Pblmp-1::dgfp (Figure 6A), in which dGFP (destabilized GFP) was controlled by the Pblmp-1 promoter. dGFP contains a PEST sequence and has a shorter half-life than normal GFP [28] and therefore allows sensitive detection of promoter activity. In animals carrying the integrated Pblmp-1::dgfp transgene, the DTCs of 75.6% of worms expressed GFP in the early and mid L3 stages, while only 4.3% expressed GFP in the late L3 and L4 stages (Figure 6B and 6C), suggesting that blmp-1 transcription is strongly repressed during, and after, late L3 stage.
Fig. 6. blmp-1 is down-regulated at the transcriptional level by lin-29 and daf-12. 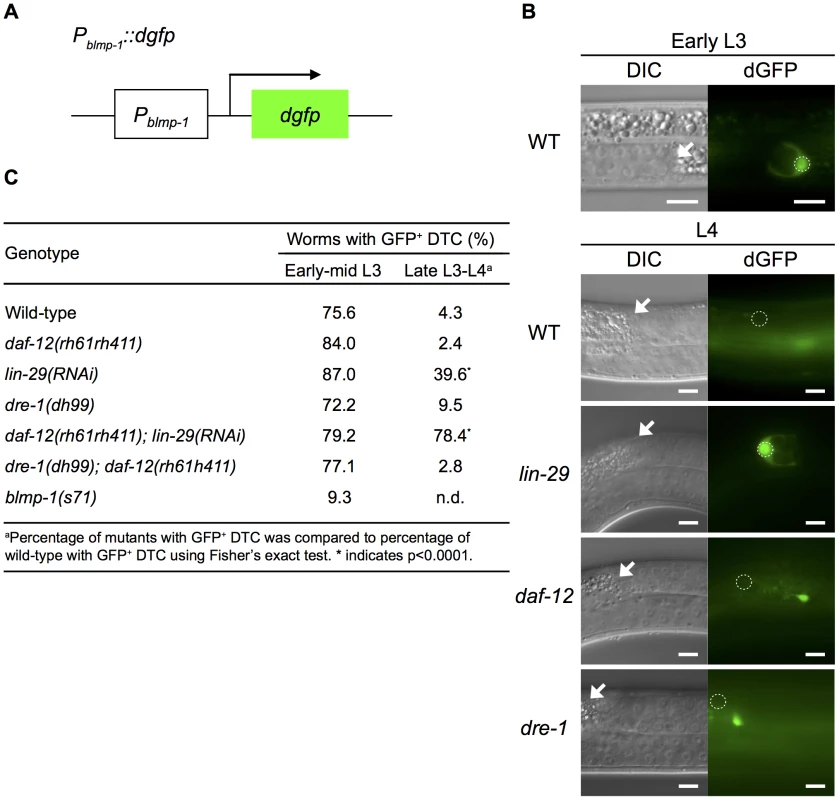
(A) Schematic diagram of the Pblmp-1::dgfp transcriptional reporter. (B) DIC (left) and dGFP (right) images of worms carrying the Pblmp-1::dgfp reporter in the indicated genotypes during the early L3 stage (upper panels) or L4 stage (lower panels). Scale bar 10 µm. (C) Percentages of worms with the posterior DTC expressing the Pblmp-1::dgfp transgene (GFP+ DTC) at the indicated developmental stages. More than 20 worms were examined for each genotype at each developmental stage. Next, we tested whether daf-12 or lin-29 was responsible for blmp-1 transcriptional repression by examining Pblmp-1::dgfp transgene expression in the absence of daf-12 and/or lin-29. As shown in Figure 6C, in early and mid L3, loss of either daf-12 or lin-29 did not affect the percentage of worms with DTCs expressing dgfp. However, during the late L3 and L4 stages, loss of lin-29, but not daf-12, increased the percentage of worms with DTCs expressing dGFP in late L3 and L4. For example, only 4.3% or 2.4% of wild-type or daf-12 mutant worms, respectively, had DTCs expressing dGFP, whereas 39.6% of lin-29(RNAi) worms had dGFP-expressing DTCs. This result shows a differential requirement for lin-29 (stronger) and daf-12 (weaker) for blmp-1 transcriptional repression during, and after, the late L3 stage (Figure 6C). In addition, 78.4% of the daf-12(rh61rh411); lin-29(RNAi) double mutants had DTCs expressing dGFP (Figure 6C). These results suggest that daf-12 plays a non-essential, but auxiliary, role in the repression of blmp-1 transcription.
We also tested the involvement of dre-1 in blmp-1 transcriptional repression. No effect on the Pblmp-1::dgfp transcription level was observed when the dre-1(dh99) mutation was introduced into the wild-type or the daf-12mutant (Figure 6C). Thus, dre-1 probably plays no role in blmp-1 transcriptional repression. Interestingly, blmp-1 seems to be required for its own expression. During early and mid L3 stages, approximately 75% of the wild-type worms had DTCs expressing dGFP from the Pblmp-1::dgfp transgene, but only 9% of the blmp-1(s71) worms had DTCs expressing dGFP.
DRE-1 functions in an SCF ubiquitin ligase complex to regulate BLMP-1 levels
Previous genetic and biochemical data have shown that the F-Box protein DRE-1 functions in an SCF ubiquitin ligase complex, which contains CUL-1 (a cullin scaffold protein), SKP-1 (a SKP1-like adaptor that binds to the F-Box protein), and RBX-1 (an RBX ring finger that bridges to the E2 ubiquitin conjugating enzyme), and is important for the temporal control of somatic and gonad development [4] and the timing of tail spike cell death [29]. This raised the possibility that DRE-1 may regulate BLMP-1 stability through ubiquitin-mediated proteolysis. Like dre-1 RNAi, RNAi for skp-1, rbx-1, or cul-1 caused a retarded DTC migration phenotype in the daf-12(rh61rh411) single mutant [4] (Table S4), consistent with a model in which DRE-1 targets protein(s) for proteolysis in an SCF ubiquitin ligase complex during the temporal regulation of the DTC dorsal turn.
Using the transgene Plag-2::gfp::blmp-1, in which BLMP-1 was tagged with GFP and expressed under the control of the Plag-2 promoter (Figure 7A), we next examined whether DRE-1 destabilized BLMP-1. Two independent transgenic lines carrying an extrachromosomal transgene array were generated. In line 1, approximately 27% of worms expressed GFP in DTCs during early L3 and the percentage decreased to 16.9% during mid L3 to L4, showing approximately 38% down-regulation, and a similar level of down-regulation (35.5%) was observed in line 2 (Figure 7Aa,b and C). In contrast, no down-regulation was observed in the control line carrying the integrated transgene qIs56[Plag-2::gfp], in which gfp was expressed under the control of the Plag-2 promoter (Figure 7Ba,b and C). These results show that BLMP-1 levels drop significantly when entering the mid L3 stage. Although the difference may be due to differences in sensitivity of the gfp reporters, given the previous result that blmp-1 transcriptional repression was detected in late, but not mid, L3 stage, this suggests that BLMP-1 degradation occurs approximately 3 hours earlier than transcriptional repression.
Fig. 7. The stability of the GFP::BLMP-1 fusion protein is negatively regulated by dre-1. 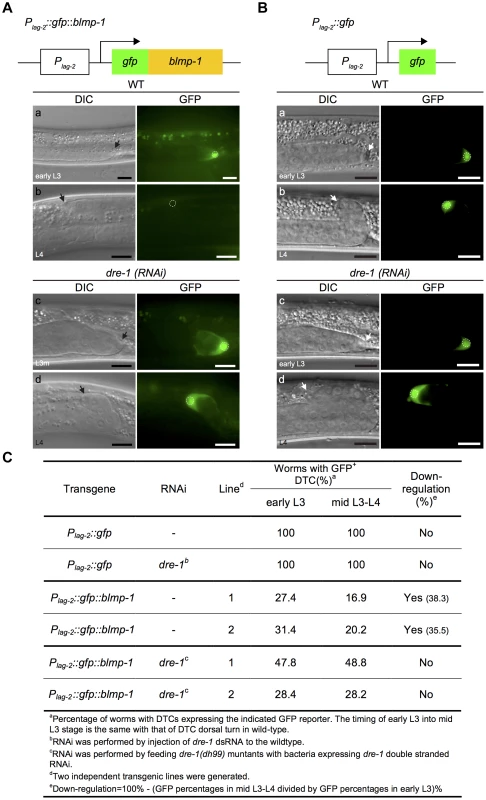
(A) Schematic diagram of the Plag-2::gfp::blmp-1 construct. (a–d) DIC (left) and GFP (right) images of wild-type (WT) and dre-1(RNAi) worms carrying the Plag-2::gfp::blmp-1 transgene during early L3 stage (top panels) or L4 stage (bottom panels). (B) Schematic diagram of the Plag-2::gfp construct. (a–d) DIC (left) and GFP (right) images of wild-type (WT; top panels) and dre-1(RNAi) (bottom panels) worms carrying the Plag-2::gfp transgene during early L3 stage (top) or L4 stage (bottom). L3m: L3 molt. In both A and B, the scale bars are 10 µm, the arrows indicate DTCs, and the DTC nuclei are circled in the GFP images. (C) Percentage of worms with DTCs expressing the indicated GFP reporter (GFP+ DTCs) in the transgenic animals at the indicated larva stage. More than 20 animals were examined in each genotype at each larval stage. Next, we examined whether dre-1 was required for this decrease in GFP::BLMP-1 fusion protein levels. In two independent transgenic lines of the dre-1 mutant, the percentage of worms with DTCs expressing GFP::BLMP-1 during the early L3 stage was similar to that seen during the mid L3-to-L4 stage (Figure 7Ac,d, and C), showing that dre-1 is required for GFP::BLMP-1 degradation. Since loss of dre-1 completely blocked the decrease in GFP::BLMP-1 levels, it is unlikely that any other gene acts in a redundant fashion with dre-1 to regulate BLMP-1 stability.
DRE-1 and BLMP-1 physically interact, as do their mammalian orthologs FBXO11 and PRDI-BF1
The F-box protein of an SCF ligase complex binds to substrates and targets them for ubiquitin-mediated proteolysis [30]. To test the idea that BLMP-1 might be the target of DRE-1 in the SCFDRE-1 ligase complex, we examined whether DRE-1 and BLMP-1 physically interacted by co-immunoprecipitation in mammalian cell cultures. When Myc-tagged dre-1 and HA-tagged blmp-1 were transfected into HEK293T cells, then lysates were subjected to immunoprecipitation with anti-Myc antibody and Western blotting with anti-HA antibody, BLMP-1 was co-immunoprecipitated with DRE-1, while no signal was seen in the singly transfected controls (Figure 8A). The reciprocal experiment using immunoprecipitation with anti-HA antibody and Western blotting with anti-Myc antibody showed that DRE-1 was co-immunoprecipitated with BLMP-1 (Figure 8B). These results demonstrate that DRE-1 binds to BLMP-1 in HEK293T cells.
Fig. 8. BLMP-1 and DRE-1 are co-immunoprecipitated in human cell cultures, as are their human orthologs PRDI-BF1 and FBXO11. 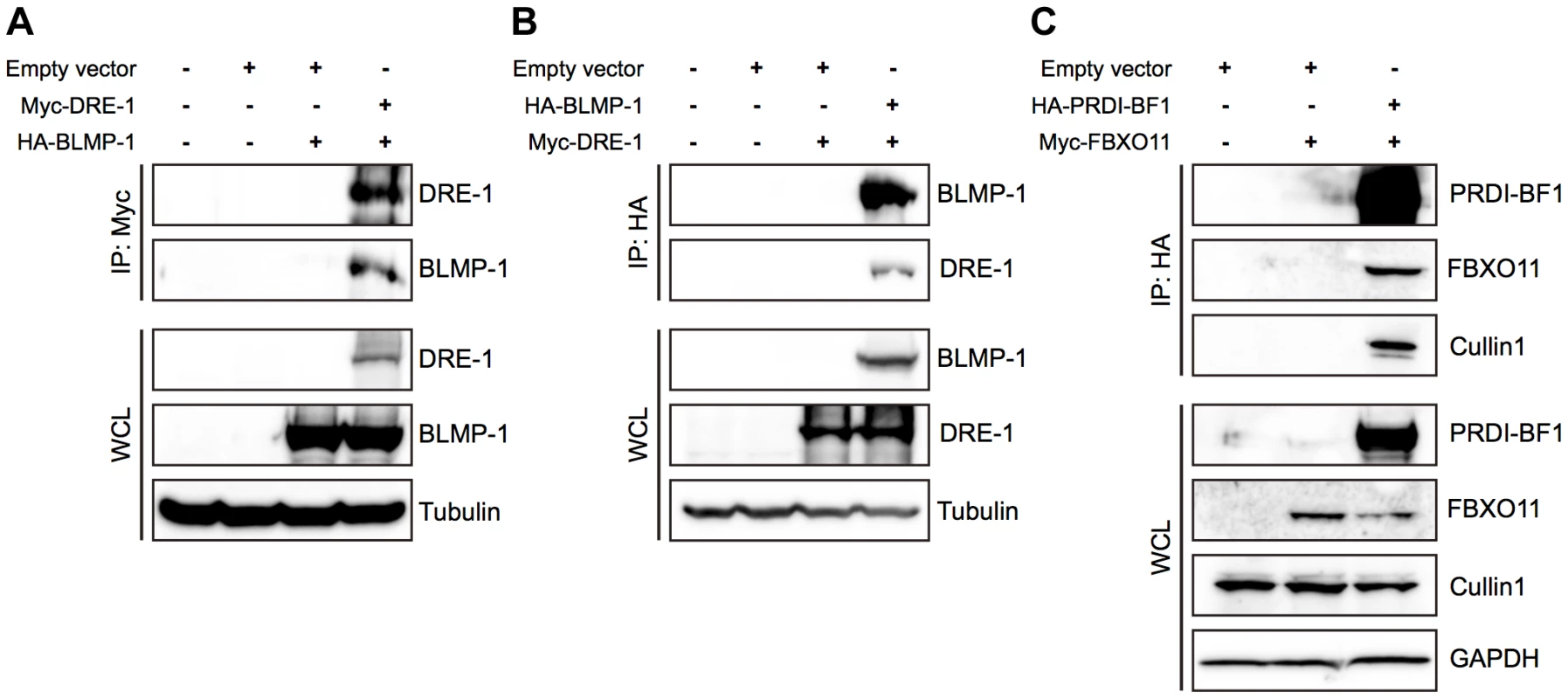
HEK293T cells were transfected with the indicated plasmids. Expression of the indicated plasmids in the whole cell lysate (WCL) is shown in the bottom panels, with tubulin or GAPDH as the loading control. (A, B) Co-immunoprecipitation of C. elegans Myc-DRE-1 and HA-BLMP-1. (A) Myc-DRE-1 was immunoprecipitated with anti-Myc antibodies and Western blots were probed with anti-HA antibodies to detect HA-BLMP-1 and with anti-Myc antibodies to detect Myc-DRE-1. (B) HA-BLMP-1 was immunoprecipitated with anti-HA antibodies and Western blots were probed with anti-Myc antibodies to detect Myc-DRE-1 and with anti-HA antibodies to detect HA-BLMP-1. (C) Co-immunoprecipitation of C. elegans Myc-DRE-1 and HA-BLMP-1. HA-PRDI-BF1 was immunoprecipitated with anti-HA antibodies and Western blots were probed with anti-Myc antibodies to detect Myc-FBXO11 and with anti-cullin 1 antibodies to detect cullin 1. We next examined whether FBXO11 and PRDI-BF1, the respective human orthologs of DRE-1 and BLMP-1, also associate in HEK293T cells and found that Myc-tagged FBXO11 was co-immunoprecipitated with HA-tagged PRDI-BF1 in lysate of cells co-transfected with both, but not in lysate of cells transfected with only Myc-tagged FBXO11 (Figure 8C). This result shows association of FBXO11 and PRDI-BF1 in human cell cultures. In addition, HA-tagged PRDI-BF1 also pulled down endogenous CUL1 in co-immunoprecipitation experiments in HEK293T cells (Figure 8C), demonstrating the association of FBXO11, BLMP-1, and CUL1 in an SCF complex and suggesting that FBXO11 may regulate PRDI-BF1 stability through ubiquitin-mediated proteolysis. These results demonstrate a conserved interaction between DRE-1/FBXO11 and BLMP-1/PRDI-BF1 in both humans and C. elegans. The regulation of BLMP-1/PRDI-BF1 stability by DRE-1/FBXO11 in an SCF ubiquitin ligase complex may also be conserved in evolution.
Discussion
blmp-1 acts in a heterochronic pathway to regulate the timing of the DTC dorsal turn
In this study, we identified a conserved transcription factor, BLMP-1, as an essential component of the heterochronic hierarchy during gonad development, and provided evidence that BLMP-1 levels are critical for the timing of DTC dorsal migration during gonadogenesis. BLMP-1 was present in DTCs only before the dorsal turn and disappeared when the DTC was about to make the dorsal turn. Loss of blmp-1 resulted in precocious unc-5 transcription and DTC dorsal migration, whereas constitutive expression of blmp-1 delayed both events. These data show that blmp-1 controls the temporal identity of DTCs by preventing them from undergoing a precocious dorsal turn. In addition, our results provide a molecular mechanism by which daf-12, dre-1, and lin-29 promote the correct timing of DTC dorsal turn by timely down-regulation of blmp-1. In this model, lin-29 and daf-12 repress blmp-1 transcription, which abolishes the synthesis of blmp-1 mRNA, while dre-1 mediates BLMP-1 degradation. Together, these two negative regulatory systems result in the efficient elimination of blmp-1 activity, and thus alleviate the repression of the DTC dorsal turn.
daf-12, dre-1, and lin-29 function in parallel to reduce BLMP-1 levels prior to the DTC dorsal turn
Interestingly, daf-12, dre-1, and lin-29 appeared to contribute to BLMP-1 down-regulation to different extents. For example, in the dre-1; lin-29, dre-1; daf-12, and lin-29; daf-12 double mutants, 100%, 20%, or 5% of worms, respectively, showed persistent BLMP-1 expression beyond late L3 in the immunostaining experiment. This suggests that, of these three genes, loss of dre-1 has the strongest effect on BLMP-1 down-regulation and loss of daf-12 has the weakest effect. Using transcriptional and translational gfp reporters, it appeared that the DRE-1-dependent protein degradation process occurred at the mid L3 stage, slightly earlier than the transcriptional repression mediated by lin-29 and daf-12 (late L3 stage), although this difference could be due to different sensitivities of the gfp reporters. The notion that lin-29 is more significant than daf-12 in the repression of blmp-1 was further supported by the observation that, using the Pblmp-1::dgfp reporter, loss of lin-29 increased blmp-1 transcription in the late L3 to L4 stages, but loss of daf-12 had no detectable effect on blmp-1 expression (Figure 6C). Further experiments are necessary to test whether LIN-29 may inhibit blmp-1 transcription by directly binding to the blmp-1 genomic sequence.
It is intriguing that only 20% of the dre-1; daf-12 double mutants had DTCs with detectable persistent BLMP-1, whereas 98% of these double mutants showed the retarded phenotype (Table 1). It is possible that, in the mutant, BLMP-1 persists at a low level beyond the detection limit of our system, but sufficient to block lin-29 transcription and therefore abolish the DTC dorsal turn in the absence of daf-12. On the other hand, the retarded DTC migration phenotype of the lin-29; daf-12 double mutant may not be caused solely by persistent BLMP-1 at the undetectable low level, but also by the loss of both transcription activators lin-29 and daf-12, which function in a redundant fashion in the transcriptional activation of unc-5 (see below and Figure S5).
As shown in Figure 5 A, BLMP-1::GFP in worms carrying Pblmp-1::blmp-1::gfp was present and persisted beyond late L3 stage and the transgenic worms frequently showed a retarded DTC migration defect. A similar result was observed in worms carrying Plag-2::blmp-1::gfp (Huang and Wu, unpublished results). Interestingly, wild-type worms carrying Plag-2::blmp-1 had normal DTC migration (Figure 1G), suggesting that BLMP-1 in the transgenic worms was properly degraded beyond L3 stage. These data and the observation that GFP::BLMP-1 expressed from the transgene Plag-2::gfp::blmp-1 was degraded suggest that GFP tag at N-terminus or C-terminus make a difference in GFP fusion BLMP-1 degradation.
Regulation of unc-5 transcription
Using ChIP-seq, the modENCODE Consortium showed that BLMP-1 binds to the upstream sequence of unc-5 in vivo [26], suggesting that it might inhibit unc-5 expression by binding to the regulatory region of the gene. In addition, using the Punc-5(4.6 kb)::gfp transgene, we found that inactivation of either daf-12 or lin-29 slightly reduced unc-5 transcription, while loss of both genes completely abolished it (Figure S5). These results are consistent with the notion that the transcription factors DAF-12 and LIN-29 act together in a redundant fashion to activate unc-5 transcription and promote the DTC dorsal turn. The consensus DAF-12 binding sequence can also be identified in the unc-5 promoter (T.F. Huang and Y.C. Wu, unpublished results), raising the possibility that DAF-12 may directly bind to unc-5 for its transcriptional activation.
A double-negative feedback loop involving blmp-1 and lin-29 contributes to the DTC dorsal turn
The DTC dorsal turn can be characterized by a specific transition in which the DTC moves irreversibly from phase I centrifugal migration into phase II dorsal migration. Phase I centrifugal migration occurs in the “blmp-1-on” state (high BLMP-1 and low UNC-5 levels) and phase II dorsal migration occurs in the“blmp-1-off” state (low BLMP-1 and high UNC-5 levels). A double-negative feedback loop, in which two genes mutually repress each other directly or indirectly, is commonly utilized to generate switch-like bistable responses during the progression of cellular and developmental processes [31]. Our data suggest that lin-29 and blmp-1 act in a double negative feedback loop that helps maintain DTCs in one of the bistable “blmp-1-on” and “blmp-1-off” states. On the basis of our results, we propose a molecular model for the switch-like process of the DTC dorsal turn. In L2 and early L3, BLMP-1 is expressed and represses lin-29 transcription (Figure 9). The double-negative feedback loop keeps DTCs in the “blmp-1-on” state, thus repressing DTC dorsal migration. In late L2, the hormones known as dafachronic acids (DA) are generated from dietary cholesterol and bind to the ligand binding domain of DAF-12 [32]. The DAF-12-DA complex promotes the L2-to-L3 transition [32], but is insufficient to repress blmp-1 transcription. During early to mid L3, dre-1 expression is initiated [4], which reduces BLMP-1 levels, probably through ubiquitin-mediated proteolysis. The decrease in BLMP-1 levels shifts the steady state toward low BLMP-1 levels and high LIN-29 levels (blmp-1 off). In late L3, accumulation of LIN-29 locks the DTCs in the “blmp-1-off” state through the negative feedback loop, thus allowing DTCs to switch to dorsal migration. This gene regulatory circuit integrates the temporal and spatial signals and coordinates with overall development of the organism to direct DTC dorsal migration during organogenesis.
Fig. 9. Spatiotemporal regulation of DTC migration. 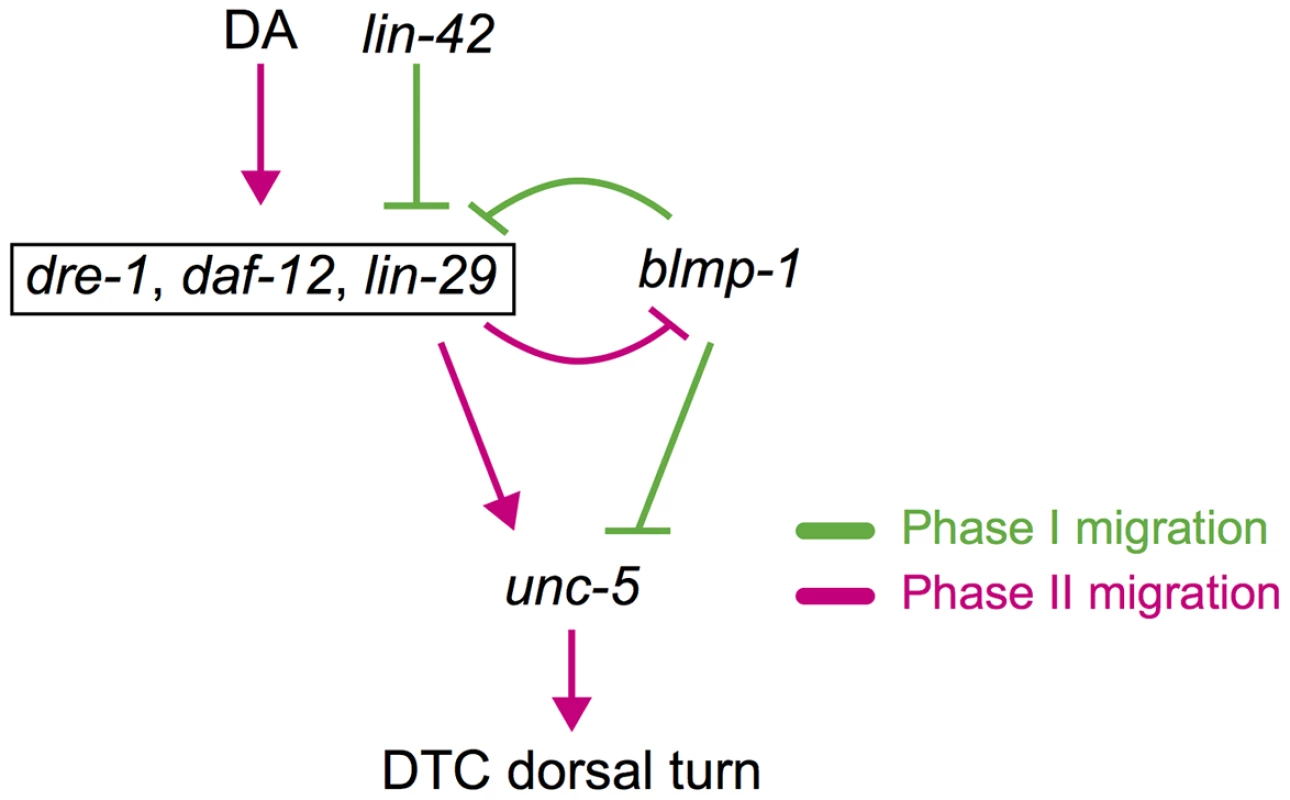
blmp-1 and lin-42 negatively regulate the timing of the DTC dorsal turn, whereas daf-12, dre-1, and lin-29 function in a redundant fashion to positively regulate the turn. Although daf-12 is activated by DAs (dafachronic acids) and lin-42 inhibits daf-12 and lin-29, they are boxed together for convenience. A double-negative feedback loop between blmp-1 and lin-29 may contribute to the switch-like turning process of DTCs (see text for detail). Gene interactions that occur in phase I migration are shown in green and those that occur in phase II migration are shown in red. Expression of lin-29 is negatively regulated by lin-42 [33]. LIN-42 is similar to the Period (Per) family of circadian rhythm proteins and functions as a member of the heterochronic pathway, regulating temporal cell identities [33], [34]. However, how lin-42 regulates the expression of lin-29 is not clear. lin-42 also genetically interacts with daf-12. During development, when conditions are unfavorable, due to starvation, crowding or high temperature, daf-12 promotes diapauses and formation of duaer larvae [5], [35], [36]. Previous studies show that daf-12 represses expression of lin-42 during dauer formation [37] and that lin-42 antagonizes the dauer-inducing signal from ligand-free DAF-12 [38]. However, whether daf-12 and lin-42 also regulate each other in the timing of DTC dorsalward turn during gonadaogenesis needs to be examined in the future.
blmp-1 is important for DTC migration along the A-P axis
In addition to ventral-to-dorsal migration, blmp-1 affects DTC migration along the AP axis. As shown in Figure 1D, some DTCs migrated obliquely with respect to the dorsal-ventral axis in blmp-1 mutants, suggesting simultaneous execution of centrifugal phase I and dorsal phase II movement. This result implies that the mutual exclusion of dorsalward and centrifugal migration in wild type animals was impaired in the blmp-1 mutants. Moreover, as shown in Figure 1E and 1F and Table S1, the reversal of migration direction was frequently observed in the centripetal phase III migration of blmp-1 mutants. Expression of unc-5 by the lag-2 promoter from transgene Plag-2::unc-5 resulted in DTC migration defects, including the reversal of the migration direction during the centripetal phase III migration (Huang, T.F. and Wu, Y.C., unpublished results), similar to those seen in blmp-1 mutants (Table S1). Further experiments are necessary to test whether the phase III DTC migration defect of blmp-1 mutants may be caused by an accumulative high level of unc-5 in the DTCs.
blmp-1 is essential for multiple cellular and developmental processes
Previous studies have shown that blmp-1 mutants have a dumpy phenotype, a weak cuticle sensitive to oxidative stress, and defective adult alae, showing that blmp-1 is important for normal body length, oxidative stress resistance, and alae formation [39]. In addition, blmp-1 is important for pharynx and [40] male tail morphogenesis [41]. Under Nomarski optics, we confirmed the blmp-1 alae defect, as 40% of blmp-1(s71) mutants had incomplete alae and the rest had no alae in the adult stage (Figure S3C). The alae are continuous ridged cuticular structures synthesized by the lateral epidermal seam cells at the larval to adult (L/A) transition and serve as a specific marker of adult fate. The seam cells undergo asymmetric cell divisions at each larval stage and exit the cell cycle at the L/A transition. The blmp-1(s71) mutant was found to have a normal number of seam cells at the late L4 and early adult stage (Figure S3D), indicating that seam cell division was normal. These results and the abnormal alae phenotype suggest that blmp-1 is essential for the adult, but not the larval, fate of the epidermal seam cells.
The dre-1 mutant shows precocious formation of adult alae [4]. We therefore tested the genetic interaction between dre-1 and blmp-1 in the seam cell heterochronic hierarchy. About 8% of dre-1(dh99) mutants had adult alae at the early L4 stage (Figure S3D), confirming a role of dre-1 in preventing the adult fate of the seam cell [4]. At the adult stage, 35% of dre-1(dh99) mutants had incomplete alae (Figure S3Cc and D). Interestingly, the blmp-1; dre-1 double mutant had a weaker defect in alae formation than the blmp-1 single mutant, as 23% of double mutants and 60% of blmp-1 single mutants had no alae (Figure S3C and D). This result shows that dre-1 partially suppresses the blmp-1 alae abnormality and, therefore, blmp-1 may genetically act upstream of, or in parallel to, dre-1 in the specification of the adult seam cell fate, at least in terms of alae formation.
blmp-1(tm548 or s71) mutants have a weak uncoordinated movement phenotype [39]. Although blmp-1 expression has been previously observed in neurons using reporter transgene assays [26], we failed to detect BLMP-1 in neurons. It is possible that our anti-BLMP-1antibody system was not sensitive enough to detect a low amount of protein in neurons. Alternatively, the neuronal expression of blmp-1 revealed by the transgene may be ectopic and not reflect the endogenous expression pattern.
Like the blmp-1 mutations, lin-42 RNAi causes both a precocious DTC dorsal turn [33] and precocious lin-29 expression in L2, one stage earlier than in the wild-type [33]. However, the lin-42(RNAi) and blmp-1 mutations induce precocious dorsal migration at different larval stages, i.e. in L2 for lin-42(RNAi) [33] and early L3 in the case of blmp-1 (Figure 3). Similar to loss of blmp-1, precocious expression of lin-29 under the control of the lag-2 promoter caused the DTCs to undergo a precocious dorsal turn in early L3, but not L2. These results suggest that lin-42 regulates most, if not all, of the genes necessary for the DTC dorsal turn, while blmp-1 regulates the temporal expression of only a subset, including lin-29.
The conserved interactions between BLMP-1/PRDI-BF1 and DRE-1/FBXO11
DRE-1 has two human orthologs FBXO10 and FBXO11, which are localized in the cytoplasm and nucleus, respectively [29]. FBXO10 and DRE-1 mediate, respectively, the degradation of anti-apoptotic protein Bcl2 or CED-9 to promote the death of a cell [29]. Recently, FBXO 11 has been shown to target the pro-oncogene product BCL6 for degradation and to be inactivated in diffuse large B cell lymphomas [42]. BCL6, a zinc finger transcription factor, regulates the transcription of a variety of genes involved in B cell development, differentiation, and activation [43], [44] and is overexpressed in the majority of patients with aggressive diffuse large B cell lymphoma [45]. Using the Clustal Omega multiple sequence alignment website http://www.ebi.ac.uk/Tools/msa/clustalo/, we found that Bcl6 shares sequence similarity with BLMP-1 (20%) and PRDI-BF1 (24%). These observations and our result showing that DRE-1/FBXO11 and BLMP-1/PRDI-BF1 are associated in human cell cultures indicate that DRE-1, FBXO10 and FBXO11 target different proteins for degradation in different cellular or developmental contexts, i.e. DRE-1 targets BLMP-1 and CED-9, FBXO10 targets Bcl2 and FBXO11 targets PRDF-BF1 and Bcl6. It will be interesting to determine how target specification is regulated.
Materials and Methods
Strains
C. elegans strains were cultured at 20°C on NGM agar inoculated with E. coli OP50, as described previously [46]. The N2 Bristol strain was used as the reference wild-type strain. The mutations used were as follows: LGI, blmp-1(s71, tk41, tm548, tp5), lin-29(n546); LGIII, unc-119(ed3); LGV, dre-1(dh99); LGX daf-12(rh61rh411). The blmp-1(tm548) mutant was generated and provided by the National Bioresource Project in Japan. Strains CB4856 and RW7000 were used for single-nucleotide polymorphism (SNP) mapping [15], [47]. Strain OS1841 carrying the transgene Plin-29::GFP was kindly provided by S. Shaham [48].
Genetics
To test whether the tk41, tm548, s71 or tp5 mutation is recessive or dominant, homozygous mutant hermaphrodites were crossed to wild-type males carrying the integrated sur-5::gfp transgene. The cross-progeny hermaphrodites carrying the sur-5::gfp transgene were scored for the dumpy (Dpy) and DTC migration phenotypes using Nomarski microscopy. In these crosses, all heterozygous cross-progeny hermaphrodites were wild-type, showing that these mutations are recessive.
In complementation tests, the tk41, tm548 or tp5 homozygous hermaphrodites were crossed to s71 males carrying the sur-5::gfp transgene, the cross-progeny hermaphrodites with the transgene were scored for the Dpy and DTC migration phenotypes using Nomarski microscopy. In these crosses, all of the mutations tested failed to complement s71.
We positioned blmp-1(s71) on the basis of the Dpy phenotype within the region between snpF45H11 and snpY106g6h, which correspond to cosmids F45H11 and F37D6, respectively, using three-factor mapping with unc-40 and unc-75 and subsequent SNP mapping as previously described [47]. The following cross demonstrates that blmp-1 is located between unc-40 and snpY106g6h: from a blmp-1(s71)unc-75/++ (CB4856) hermaphrodite 37/37 Dpy non-Unc recombinant progeny segregated neither snpY106g6h nor snpF59C6, and 6/6 nonDpy Unc recombinant progeny segregated snpY106g6h and snpF59C6. From an unc-40 blmp-1 (s71)/++(CB4856) hermaphrodite 85/91 nonDpy Unc recombinant progeny segregated snpY106g6h and snpF59C6, 1/91 recombinant progeny segregated snpY106g6h and 5/91 recombinant progeny segregated neither snpY106g6h nor snpF59C6. The following cross demonstrates that blmp-1 is located right of snpF45H11: from an unc-40 blmp-1(s71)/CB4856 hermaphrodite 3/171 Dpy nonUnc recombinant progeny segregated snpF45H11.
Genetic screen
We performed genetic screening for mutants defective in DTC migration under a dissecting microscope, as described in Nishiwaki, 1999, and isolated the mutation tk41. In an independent screen for mutants with a DTC migration defect using Nomarski microscopy, we isolated the mutation tp5.
Analysis of the DTC migration phenotype
The DTC migratory patterns of the wild-type and mutants were determined by observing the shape of the gonadal arms in the adult stage. Worms were mounted on a 4% agar pad containing 20 mM NaN3 and observed on a microscope with Nomarski optics. For the time course analysis of DTC migration, wild-type and blmp-1(s71) mutants at the indicated time points were collected and scored under the Nomarski microscope.
DNA construct
To obtain the 5′ end of blmp-1 cDNA, the first three exons were amplified by RT-PCR using a forward primer corresponding to the SL1 sequence and the reverse primer blmp-1_exon_3/r (see Table S5 for detailed information). The PCR product was fused with the blmp-1 cDNA fragment from the yk487b7 clone by fusion PCR [49], and the resulting product was cloned into the vector pGEM-T Easy (Promega) to generate pYW687, containing the full-length blmp-1 cDNA.
To construct Plag-2::blmp-1 (pYW798), blmp-1 cDNA was PCR-amplified from pYW687 using primers DPY-24-KpnI/f and DPY-24-KpnI/r and the product inserted into pPD49_26/Plag-2 via the KpnI site.
Two unc-5 transcriptional gfp constructs were generated. The Punc-5(4.6 kb) DNA fragment was PCR-amplified from C. elegans genomic DNA using primers Punc-5_4.6 kb/f and Punc-5_4.6 kb/r and inserted into the vector pGEM-T Easy. The Punc-5(4.6 kb) fragment of the resulting construct was then inserted into the vector pPD95.77 (A. Fire) via SphI/SalI sites to generate Punc-5(4.6 kb)::gfp. A similar approach was used to generate Punc-5(1 kb)::gfp. The Punc-5(1 kb) fragment was PCR-amplified from genomic DNA using primers Punc-5_1 kb/f and Punc-5_1 kb/r and cloned into the vector pGEM-T Easy, then the Punc-5(1 kb) fragment was excised from the resulting plasmid and inserted into the vector pPD95.75 (A. Fire) via the XmaI site. To generate Punc-5(1 kb)::mCherry, the gfp fragment of the plasmid Punc-5(1 kb)::gfp was replaced by mCherry cDNA.
The Punc-5(1 kb) fragment was expected to contain the regulatory region for proper unc-5 function in guiding DTC migration, as the Punc-5(1 kb)::unc-5::gfp plasmid is sufficient to rescue the DTC migration defect of the unc-5 mutant (our unpublished data). The Punc-5(1 kb)::unc-5::gfp plasmid was generated by fusion PCR by fusing two PCR-amplified products corresponding to Punc-5(1 kb)::unc-5(cDNA) and gfp::unc-54 3′ UTR from plasmid pU5HA (J. Culotti) or pPD95.75, respectively.
The Pblmp-1::dgfp fragment was generated by fusion PCR by fusing two PCR products corresponding to Pblmp-1 and the region containing dgfp and the unc-54 3′ UTR. Pblmp-1 was PCR-amplified from genomic DNA using primers D24-5end5kb and d24Pgfp/r, and the region containing dgfp and unc-54 3′UTR was PCR-amplified from pPD95.75PEST (pYW807), which contains dgfp, using primers GFP/f and D. The two PCR products were then mixed and fused by fusion PCR using primers d24-5kbf-nest and D′. The Pblmp-1::blmp-1::gfp fragment was generated by fusion PCR by fusing two PCR products corresponding to blmp-1 genomic DNA and the region containing gfp and unc-54 3′ UTR from the pPD95.75 plasmid [50]. blmp-1 genomic DNA was PCR-amplified from wild-type genomic DNA using primers D24-5end5kb and d24gfp/r, and the region containing gfp and the unc-54 3′ UTR was PCR-amplified from the pPD95.75 plasmid using primers GFP/f and D, then the two PCR products were fused by fusion PCR using primers d24-5kbf-nest and D′.
The primers used in this work are listed in Table S6.
Transgenic worms
Transgenic worms were generated by microinjection of the indicated plasmid, as described previously [51]. For genetic rescue experiments, cosmids and plasmids were microinjected into the indicated strains and the indicated phenotype of the stably transmitting lines scored using DIC optics. Pblmp-1::blmp-1::gfp (20 ng/µl) was co-injected with the unc-119 rescuing plasmid (100 ng/µl) [52] into the unc-119(ed3) mutant to generate tpEx49 transgenic worms. For the unc-5 transcription assay, Punc-5(4.6 kb)::gfp, Punc-5(1 kb)::gfp, or Punc-5(1 kb)::mCherry (20 ng/µl) was co-injected with the marker Pmyo-2::gfp (2 ng/µl), which expresses gfp in the pharynx [26], [53], as described previously [51]. To determine how blmp-1 was down-regulated during larval development, the indicated plasmids (20 ng/µl) were co-injected with Pmyo-2::gfp (2 ng/µl) into wild-type worms to generate transgenic worms, and the resulting transgenes were crossed to the indicated heterochronic mutants. For the blmp-1 cell autonomy assay, Plag-2::blmp-1 or Pblmp-1::blmp-1 (20 ng/µl) was co-injected with Pmyo-2::gfp (2 ng/µl) into wild-type worms to generate worms carrying the respective transgenes, then the blmp-1(s71) mutation was introduced into these transgenic worms by crossing to generate the blmp-1(s71) mutant carrying either the Plag-2::blmp-1 or Pblmp-1::blmp-1 transgene.
The transgenes used in this work were listed in Table S5.
Antibodies and immunostaining
The blmp-1 cDNA fragment from the plasmid pYW687 was digested with EcoRI and cloned into the vectors pGEX5X-1 (GE Healthcare) and pRSET B (Invitrogen) at their EcoR I sites to generate the respective constructs pYW802 and pYW691. The GST-BLMP-1 and HIS-BLMP-1 fusion proteins were present in inclusion bodies and were purified using standard methods [54]. Polyclonal rabbit antibodies against GST-BLMP-1 were generated and affinity-purified using 6His-tagged BLMP-1 as described previously [55] and the purified antibodies recognized GST-BLMP-1, but not GST, on a Western blot, indicating their BLMP-1 specificity. The immunostaining method was adopted from a previously published protocol [56], with slight modification. The animals were fixed for 1 h at 4°C in 80 mM KCl, 20 mM NaCl, 10 mM EGTA, 5 mM spermidine, 0.03 mM PIPES, 25% methanol, and 2% formaldehyde, then incubated overnight at 4°C with a 1∶100 dilution of the purified anti-BLMP-1 antibodies in PBS, 0.2% BSA, 0.5% Triton X-100 (PBT), then for 2 h at room temperature with rhodamine red-X (RRX)-conjugated donkey-anti-rabbit IgG antibodies (Jackson ImmunoResearch Laboratories; 1∶100 in PBT). For immunostaining of the lin-29(RNAi);daf-12(rh61rh411) mutants, the worms were mounted on a gelatin-chromic potassium sulfate-subbed slide and immunostained with anti-BLMP-1 antibodies, as described previously [57]. Worms were freeze-cracked and fixed in 95% ethanol for 10 min, then in 2% paraformaldehyde for 10 min, then incubated sequentially overnight at room temperature with purified anti-BLMP-1 antibodies (1∶1000 in PBT), followed by RRX-conjugated donkey-anti-rabbit IgG antibodies (Jackson ImmunoResearch Laboratories; 1∶1000 in PBT). They were then mounted with 2 µl of DABCO anti-bleaching reagent (Fluka) and 1 µl of DAPI (0.5 µg/ml) and observed using a Zeiss Axioplan 2 microscope equipped with a digital camera (AxioCam; Carl Zeiss, Inc.).
RNA interference
To knock down blmp-1, skr-1, cul-1, rbx-1, dre-1, or lin-29, RNA interference (RNAi) was performed either by feeding worms with bacteria expressing the double stranded RNA for the indicated genes or by injecting the double stranded RNA for the indicated genes as described previously [58], [59]. To generate the lin-29 RNAi construct, the region corresponding to exons 3–9 of lin-29 was PCR-amplified from the yk1430g04 plasmid, which contains lin-29 cDNA (Y. Kohara, Japan), and cloned into the L4440 vector. The blmp-1, srk-1, cul-1, and rbx-1 RNAi constructs were obtained from the Ahringer RNAi library.
HEK293T cell culture and drug treatments
HEK293T cells were grown in Dulbecco's modified Eagle's medium (Thermo Inc) containing 10% fetal bovine serum, 100 U/ml of penicillin, and 100 µg/ml of streptomycin (all from Life technologies) and were transfected with the indicated plasmids using Lipofectamine 2000 (Invitrogen). MG132 (20 µM) was added to the medium 2 h before the cells were harvested to block protein degradation by the proteasome.
Co-immunoprecipitation and immunoblotting
Cells were harvested using pre-chilled PBS and lysed using 1% NP-40 lysis buffer with protease inhibitor (Roche). HA-tagged or Myc-tagged fusion proteins were immunoprecipitated by incubation overnight at 4°C with anti-HA or anti-Myc agarose beads (Sigma) and bound protein identified by Western blotting using antibodies against HA (Covance), Myc (BD Biosciences), or CUL1 (Zymed). Antibodies against tubulin (Sigma) or GAPDH (Abnova) were used on the loading controls.
Supporting Information
Zdroje
1. KilleenMT, SybingcoSS (2008) Netrin, Slit and Wnt receptors allow axons to choose the axis of migration. Dev Biol 323 : 143–151.
2. KimbleJ, HirshD (1979) The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Dev Biol 70 : 396–417.
3. HedgecockEM, CulottiJG, HallDH, SternBD (1987) Genetics of cell and axon migrations in Caenorhabditis elegans. Development 100 : 365–382.
4. FielenbachN, GuardavaccaroD, NeubertK, ChanT, LiD, et al. (2007) DRE-1: an evolutionarily conserved F box protein that regulates C. elegans developmental age. Dev Cell 12 : 443–455.
5. AntebiA, CulottiJG, HedgecockEM (1998) daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development 125 : 1191–1205.
6. RougvieAE, AmbrosV (1995) The heterochronic gene lin-29 encodes a zinc finger protein that controls a terminal differentiation event in Caenorhabditis elegans. Development 121 : 2491–2500.
7. ChanSS, ZhengH, SuMW, WilkR, KilleenMT, et al. (1996) UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87 : 187–195.
8. HedgecockEM, CulottiJG, HallDH (1990) The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4 : 61–85.
9. Leung-HagesteijnC, SpenceAM, SternBD, ZhouY, SuMW, et al. (1992) UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell 71 : 289–299.
10. IshiiN, WadsworthWG, SternBD, CulottiJG, HedgecockEM (1992) UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9 : 873–881.
11. WadsworthWG, BhattH, HedgecockEM (1996) Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16 : 35–46.
12. ColavitaA, KrishnaS, ZhengH, PadgettRW, CulottiJG (1998) Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science 281 : 706–709.
13. SuM, MerzDC, KilleenMT, ZhouY, ZhengH, et al. (2000) Regulation of the UNC-5 netrin receptor initiates the first reorientation of migrating distal tip cells in Caenorhabditis elegans. Development 127 : 585–594.
14. RoseAM, BaillieDL (1980) Genetic organization of the region around UNC-15 (I), a gene affecting paramyosin in Caenorhabditis elegans. Genetics 96 : 639–648.
15. WilliamsBD (1995) Genetic mapping with polymorphic sequence-tagged sites. Methods Cell Biol 48 : 81–96.
16. ChenN, HarrisTW, AntoshechkinI, BastianiC, BieriT, et al. (2005) WormBase: a comprehensive data resource for Caenorhabditis biology and genomics. Nucleic Acids Res 33: D383–9.
17. TurnerCA, MackDH, DavisMM (1994) Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 77 : 297–306.
18. NuttSL, FairfaxKA, KalliesA (2007) BLIMP1 guides the fate of effector B and T cells. Nat Rev Immunol 7 : 923–927.
19. LinY, WongK, CalameK (1997) Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science 276 : 596–599.
20. PiskurichJF, LinKI, LinY, WangY, TingJP, et al. (2000) BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat Immunol 1 : 526–532.
21. GhoshN, GyoryI, WrightG, WoodJ, WrightKL (2001) Positive regulatory domain I binding factor 1 silences class II transactivator expression in multiple myeloma cells. J Biol Chem 276 : 15264–15268.
22. TamaiKK, NishiwakiK (2007) bHLH transcription factors regulate organ morphogenesis via activation of an ADAMTS protease in C. elegans. Dev Biol 308 : 562–571.
23. SulstonJE, HorvitzHR (1977) Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56 : 110–156.
24. HendersonST, GaoD, LambieEJ, KimbleJ (1994) lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development 120 : 2913–2924.
25. EllisRE, KimbleJ (1995) The fog-3 gene and regulation of cell fate in the germ line of Caenorhabditis elegans. Genetics 139 : 561–577.
26. NiuW, LuZJ, ZhongM, SarovM, MurrayJI, et al. (2011) Diverse transcription factor binding features revealed by genome-wide ChIP-seq in C. elegans. Genome Res 21 : 245–254.
27. BettingerJC, LeeK, RougvieAE (1996) Stage-specific accumulation of the terminal differentiation factor LIN-29 during Caenorhabditis elegans development. Development 122 : 2517–2527.
28. LiX, ZhaoX, FangY, JiangX, DuongT, et al. (1998) Generation of Destabilized Green Fluorescent Protein as a Transcription Reporter. J Biol Chem 273 : 34970–34975.
29. ChiorazziM, RuiL, YangY, CeribelliM, TishbiN, et al. (2013) Related F-box proteins control cell death in Caenorhabditis elegans and human lymphoma. Proceedings of the National Academy of Sciences 110 : 3943–3948.
30. CardozoT, PaganoM (2004) The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol 5 : 739–751.
31. FerrellJE (2002) Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Current Opinion in Cell Biology 14 : 140–148.
32. MotolaDL, CumminsCL, RottiersV, SharmaKK, LiT, et al. (2006) Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 124 : 1209–1223.
33. TennessenJM, GardnerHF, VolkML, RougvieAE (2006) Novel heterochronic functions of the Caenorhabditis elegans period-related protein LIN-42. Dev Biol 289 : 30–43.
34. JeonM, GardnerHF, MillerEA, DeshlerJ, RougvieAE (1999) Similarity of the C. elegans developmental timing protein LIN-42 to circadian rhythm proteins. Science 286 : 1141–1146.
35. CassadaRC, RussellRL (1975) The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol 46 : 326–342.
36. RiddleDL, SwansonMM, AlbertPS (1981) Interacting genes in nematode dauer larva formation. Nature 290 : 668–671.
37. HochbaumD, ZhangY, StuckenholzC, LabhartP, AlexiadisV, et al. (2011) DAF-12 regulates a connected network of genes to ensure robust developmental decisions. PLoS Genet 7: e1002179.
38. TennessenJM, OppermanKJ, RougvieAE (2010) The C. elegans developmental timing protein LIN-42 regulates diapause in response to environmental cues. Development 137 : 3501–3511.
39. ZhangL, ZhouD, LiS, JinC (2012) BLMP-1 Contributes to Collagen-related Morphogenesis in C. elegans. Life Sci 9 : 1080–1088.
40. FerrierA, CharronA, SadozaiY, SwitajL, SzutenbachA, et al. (2011) Multiple phenotypes resulting from a mutagenesis screen for pharynx muscle mutations in Caenorhabditis elegans. PLoS One 6: e26594.
41. NelsonMD, ZhouE, KiontkeK, FradinH, MaldonadoG, et al. (2011) A Bow-Tie Genetic Architecture for Morphogenesis Suggested by a Genome-Wide RNAi Screen in Caenorhabditis elegans. PLoS Genet 7: e1002010.
42. DuanS, CermakL, PaganJK, RossiM, MartinengoC, et al. (2012) FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature 481 : 90–93.
43. CiW, PoloJM, MelnickA (2008) B-cell lymphoma 6 and the molecular pathogenesis of diffuse large B-cell lymphoma. Curr Opin Hematol 15 : 381–390.
44. StaudtLM, DaveS (2005) The biology of human lymphoid malignancies revealed by gene expression profiling. Adv Immunol 87 : 163–208.
45. CattorettiG, PasqualucciL, BallonG, TamW, NandulaSV, et al. (2005) Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell 7 : 445–455.
46. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
47. FayD, BenderA (2006) Genetic mapping and manipulation: Chapter 4-SNPs: Introduction and two-point mapping. WormBook
48. AbrahamMC, LuY, ShahamS (2007) A Morphologically Conserved Nonapoptotic Program Promotes Linker Cell Death in Caenorhabditis elegans. Developmental Cell 12 : 73–86.
49. HobertO (2002) PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32 : 728–730.
50. HobertO, MoermanDG, ClarkKA, BeckerleMC, RuvkunG (1999) A conserved LIM protein that affects muscular adherens junction integrity and mechanosensory function in Caenorhabditis elegans. J Cell Biol 144 : 45–57.
51. MelloCC, KramerJM, StinchcombD, AmbrosV (1991) Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10 : 3959–3970.
52. MaduroM, PilgrimD (1995) Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141 : 977–988.
53. OkkemaPG, FireA (1994) The Caenorhabditis elegans NK-2 class homeoprotein CEH-22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development 120 : 2175–2186.
54. Harlow E, Lane D (1988) Antibodies: a laboratory manual: Cold Spring Harbor Laboratory.
55. PerroneCA, YangP, O'TooleE, SaleWS, PorterME (1998) The Chlamydomonas IDA7 locus encodes a 140-kDa dynein intermediate chain required to assemble the I1 inner arm complex. Mol Biol Cell 9 : 3351–3365.
56. FinneyM, RuvkunG (1990) The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell 63 : 895–905.
57. Rowse-EagleD, WatsonHD, TignorGH (1981) Improved method for trypsin digestion of Paraplast sections before immunofluorescence staining. J Clin Microbiol 13 : 996–997.
58. FireA, XuS, MontgomeryMK, KostasSA, DriverSE, MelloCC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 : 806–811.
59. KamathRS, Martinez-CamposM, ZipperlenP, FraserAG, AhringerJ (2001) Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2: RESEARCH0002.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



