-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRecombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
One of the most challenging issues in evolutionary biology concerns the question of why most organisms exchange genetic material with each other, e.g. during sexual reproduction. Gene shuffling can create genetic diversity that facilitates adaptation to new environments, but theory shows that this effect is highly dependent on how different genes interact in determining the fitness of an organism. Using a large data set of fitness values based on HIV-1, we provide evidence that shuffling of genetic material indeed raises the level of genetic diversity, and as a result accelerates adaptation. Our results also propose genetic shuffling as a mechanism utilized by HIV to accelerate the evolution of multi-drug-resistant strains.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004439
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004439Summary
One of the most challenging issues in evolutionary biology concerns the question of why most organisms exchange genetic material with each other, e.g. during sexual reproduction. Gene shuffling can create genetic diversity that facilitates adaptation to new environments, but theory shows that this effect is highly dependent on how different genes interact in determining the fitness of an organism. Using a large data set of fitness values based on HIV-1, we provide evidence that shuffling of genetic material indeed raises the level of genetic diversity, and as a result accelerates adaptation. Our results also propose genetic shuffling as a mechanism utilized by HIV to accelerate the evolution of multi-drug-resistant strains.
Introduction
Recombination, here broadly defined as the shuffling of genetic material, is widespread in nature and occurs among a wide range of taxa, including most eukaryotes but also bacteria and viruses. It has long been believed that sex and recombination facilitate adaptation by increasing the genetic variance upon which natural selection can act [1]. However, recombination can also reduce variation if there is a preponderance of co-adapted allelic associations. This cost, referred to as recombination load, arises because recombination tends to unravel combinations of genes that are favored by selection, thus impeding adaptation [2]–[4]. Several hypotheses have been developed to account for the conditions under which recombination accelerates adaptation [4]–[8]. Epistasis-based hypotheses state that if beneficial mutations increase fitness less in combination than expected based on their individual effects (negative epistasis), recombination can accelerate adaptation by increasing genetic variance and thus enhancing the efficacy of selection [6], [7], [9]. In contrast, recombination is predicted to decelerate adaptation with positive as well as sign epistasis (i.e., when the direction of selection on an allele depends on the allelic status at other loci) [10]–[12]. According to another class of hypotheses, random genetic drift resulting from finite population size is believed to provide an advantage to recombination [4], [13]–[15]. This occurs because in finite populations, beneficial mutations are likely to occur on different backgrounds and compete with each other, thus reducing selection efficacy. Recombination alleviates this competition by bringing beneficial mutations together on the same background and consequently speeds up adaptation (the Fisher-Muller effect) [14]–[16].
Prior experimental studies have demonstrated that sex and recombination can facilitate adaptation [17]–[21]. Yet, our understanding of the costs and benefits of recombination during adaptation on empirical complex fitness landscapes is still limited. The structure of the underlying fitness landscape is a decisive factor for the effect of recombination on adaptation since it determines how natural selection creates non-random combinations of alleles. Although realistic fitness landscapes are believed to exhibit a complex structure characterized by intricate patterns of fitness interactions among genes, not much is known about the structure of large-scale fitness landscapes. This lack of knowledge hampered efforts to obtain insights into the effect of recombination on adaptation in realistic situations. To our knowledge, only one study explores the effect of recombination on an empirical fitness landscape [22], but this fitness landscape comprises only six loci, and thus, the results may not be generalizable to larger landscapes.
Recently, Hinkley et al. [23] have estimated a fitness landscape in HIV-1 for 1859 mutations, based on an in-vitro assay for viral replicative capacity. This empirical fitness landscape, by far the largest available empirical database characterizing epistatic interactions, allows us for the first time to scrutinize the impact of recombination on the evolutionary dynamics of an adapting population on a realistic fitness landscape. Through simulations on this fitness landscape, we examined the effect of recombination on adaptation under different conditions. We found benefits of recombination are sufficiently high to accelerate adaptation under a wide range of parameters. Our findings highlight the important evolutionary role of recombination in adaptation, and in particular, in HIV evolution.
Results
Recombination was found to produce a substantial increase in the rate of adaptation (Figure 1A). This effect was robust with respect to the initial composition of the population, as it is observed not only when starting from the reference sequence but also when initializing the population with a random sequence (Figure S1 and Section S1 in Text S1). The acceleration of adaptation by recombination can be attributed to increased genetic variance in fitness, which in turn enhances the efficacy of natural selection, as proposed by the fundamental theorem of natural selection [14]. Indeed, we see a markedly stronger increase in genetic variance in fitness over time in the recombining, compared to the non-recombining population (Figure 1B). These results on population fitness are also in agreement with patterns of genetic diversity within the evolving populations: the recombining populations accumulate within-population diversity faster than the non-recombining populations (Figure S2A and Section S2 in Text S1), and they diverge faster from the initial sequence (Figure S2B and Section S2 in Text S1).
Fig. 1. A) Population fitness and B) fitness variance in the recombining and non-recombining populations over time. 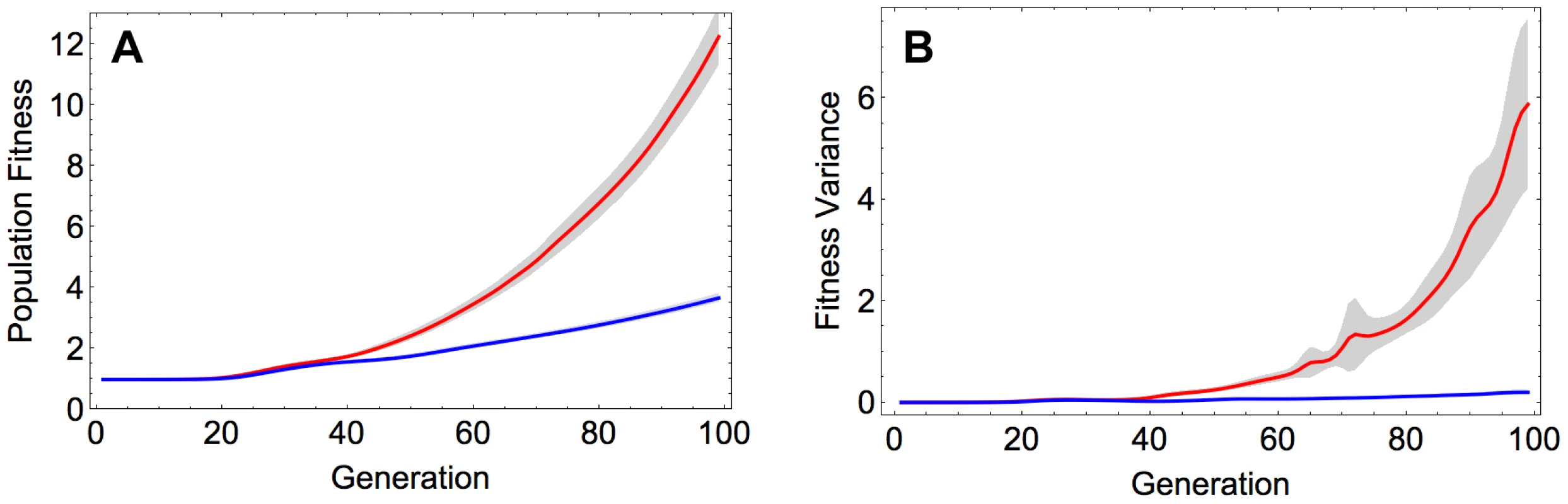
Red and blue curves correspond to the mean value across 100 simulations in the recombining and non-recombining settings, respectively. Fitness and fitness variance values are normalized with the fitness of the reference sequence. The shaded regions show 95% confidence intervals. Parameters take values: and . We also observed that with increasing recombination rate the fittest genotypes that emerged by the end of each simulation became more divergent from each other across replicate runs (Figure S3A and Section S3 in Text S1). However, it appears that this diversifying effect of recombination is primarily a consequence of the fact that recombination increases the rate at which the populations adapt and traverse the fitness landscape: when sequences are compared when the population reaches a certain threshold mean fitness, recombination has little effect on divergence (Figure S3B and Section S3 in Text S1). In other words, recombination accelerates adaptation but does not increase the number of evolutionary trajectories available to the populations in the course of adaptation.
We also considered how different drug treatments affect the impact of recombination on adaptation. To this end, we measured the effect of recombination using fitness landscapes obtained for 16 environments with different drug treatments. Our results indicate that the effect of recombination is markedly stronger in the presence of antiviral drugs compared to the drug-free environment (Figure S4 and Section S4 in Text S1), which appears to be due to the higher selection pressure in environments with drug treatments (results not shown).
We next explored how the population size and mutation rate affect the extent to which recombination accelerates adaptation. Figure 2A indicates that, for a given population size, adaptation is already accelerated with moderate recombination rates and this effect increases monotonically with increasing recombination rates. In contrast, the effect of recombination depends non-monotonically on mutation rate (Figure 2B): whereas modest mutation rates enhance the accelerating effect of recombination on adaptation, at very high mutation rates this effect is reduced (see Discussion). Finally, our results indicate that with increasing population size, the accelerating effect of recombination becomes stronger (Figure 2A and S5 and Section S5 in Text S1). This is because with increasing population size, more beneficial mutations co-segregate in the population on different backgrounds and as a result the Fisher-Muller effect is enhanced. We expect that in very large populations this acceleration would become weaker again (because then all combinations of beneficial alleles would be present in the population even without recombination), but in the range of population sizes that was computationally feasible this was not observed. We also found the effect of recombination for populations with the same population mutation rate (population size×mutation rate) to be dependent on the population size: the effect is maximized for intermediate values of the population mutation rate and this maximum occurs at higher values for larger populations (Figure S6 and Section S6 in Text S1). This is presumably because for larger populations a higher mutation rate is required for multiple beneficial mutations to occur on the same background and thereby mitigate the Fisher-Muller effect.
Fig. 2. Effect of model parameters on the impact of recombination on adaptation. 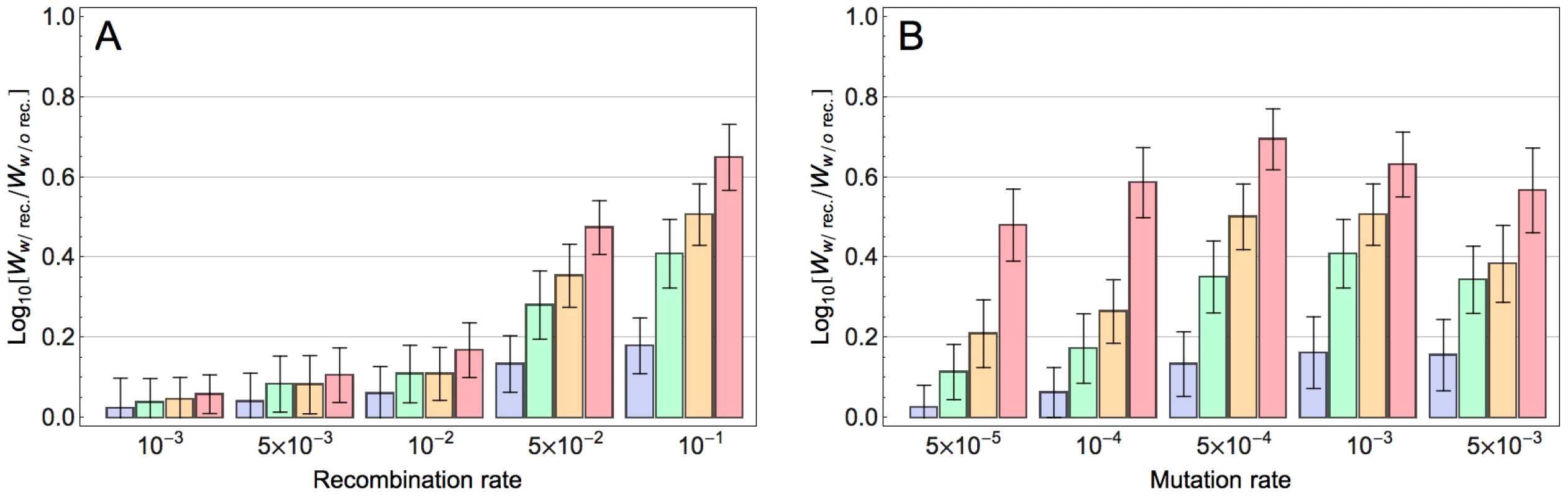
Each bar corresponds to the relative fitness of the recombining to the non-recombining population at generation 100, measured by taking the logarithm (base 10) of the ratio of mean fitness of the recombining population to that of the non-recombining population and averaged across 100 simulations. The blue, green, orange and red bars correspond to population sizes and , respectively. The error bars show the standard deviation of the difference between two log normal distributions (). A) The effect of recombination rate at different population sizes for mutation rate: . B) The effect of mutation rate at different population sizes for recombination rate: . (The effect of recombination is still significant for populations larger than 103 with this recombination rate; see Figure S5). Our fitness model incorporates both main and pairwise epistatic effects, both of which are known to influence the effect of recombination on the rate of adaptation. To assess the relative contribution of these effects, we simulated adaptation on fitness landscapes where, starting from the original MHL fitness matrix, we decreased the epistatic and main effects by varying amounts. Figure 3 indicates that both main effects and epistatic effects contribute to the acceleration of adaptation and that in combination these two effects appear to operate additively. Similar results were obtained for smaller recombination rates (Figure S7). We also found that both main effects and epistatic interactions increase the rate of adaptation and the effect of recombination becomes stronger with increasing adaptation rate (results not shown). These findings suggest that both main and epistatic effects can enhance selection. This seems to result in stronger interferences between arisen beneficial mutations and therefore a higher advantage of recombination (see Discussion).
Fig. 3. Contribution of the main and epistatic effects to the recombination effect on adaptation on the HIV-1 fitness landscape. 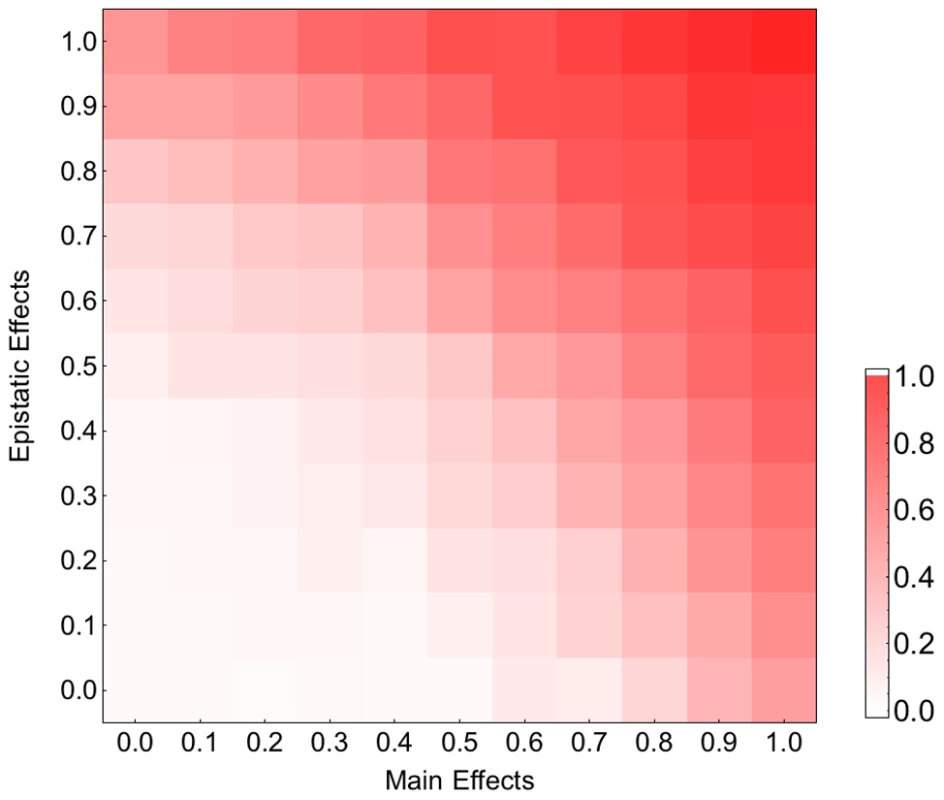
The x and y axes show the values of the constants by which the elements of the main and epistatic effects, respectively, are multiplied in the hierarchical fitness landscape. The plot shows the logarithm (base 10) of the ratio of mean fitness of the recombining to that of the non-recombining populations at generation 100, averaged across 100 simulations. Parameters take values: and . We next determined the predominant form of epistasis in the adapting populations. To infer epistasis, one can use the fitness values of a set of sequences that are chosen irrespective of the composition of the evolving population and that therefore may not represent the sequences formed during adaptation (‘physiological epistasis’). Alternatively, only fitness values of sequences that are present in the adapting population can be utilized to estimate epistasis. This form of epistasis, referred to as population epistasis, provides a real time estimate of the epistasis that is responsible for generating the standing linkage disequilibrium in the population, and is therefore more accurate than physiological epistasis (see Discussion). We calculated population epistasis by regressing log fitness against Hamming distance, i.e., the number of sites where the corresponding sites are different between two sequences. The regression was done for sequences that are present in the population at the end of simulation according to , where Hamming distance is measured relative to the reference sequence. The parameter , determining the curvature, is used as a measure of epistasis. Our results indicate that in the majority of simulations, population epistasis is significant (ANOVA test for comparison of a quadratic and a linear model, p≪0.001 for the simulations in Figure 4) and predominantly negative, indicating diminishing returns with each additional beneficial mutation in increasing fitness. Population epistasis in the recombining population becomes less negative on average than in the non-recombining population (Wilcoxon test for the significance of epistasis in Figure 4, p≪0.001 and for the significance of the difference between recombining and non-recombining simulations Figure 4B, p≪0.001). We obtained similar results for populations at other time points (results not shown).
Fig. 4. Population epistasis of the HIV-1 fitness landscape. 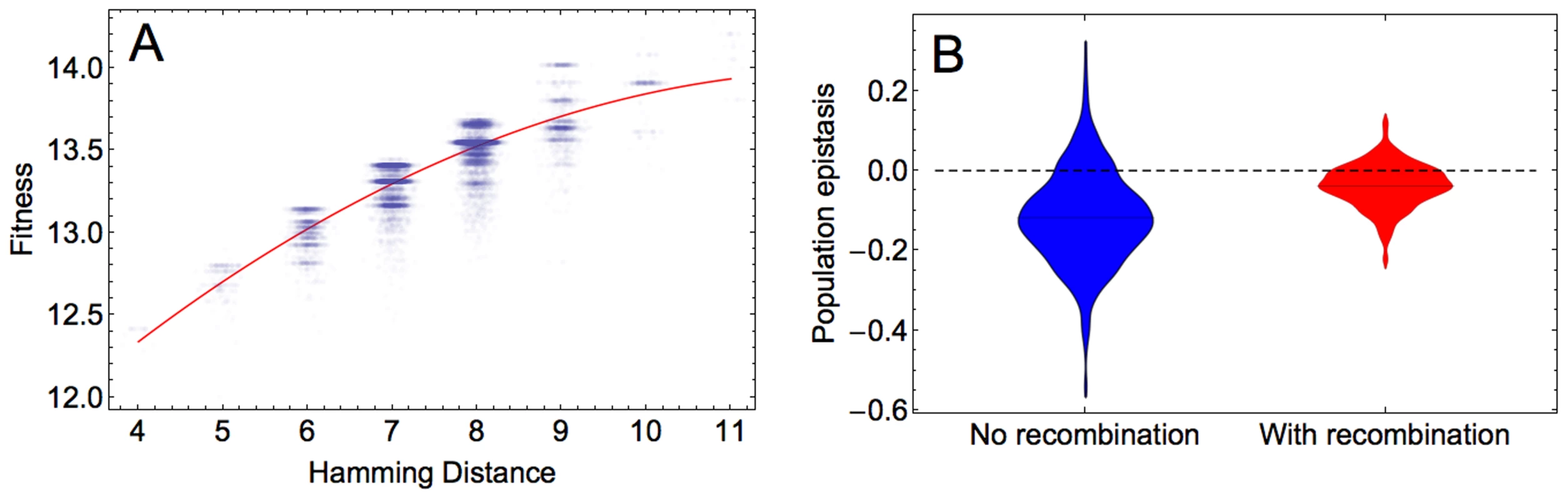
A) An example of the log fitness plot against the Hamming distance between the reference sequence and sequences at generation 100 of one simulation for 20000 data points. Each point corresponds to one fitness value. To improve the visualization of the data on x axis, random values taken from a normal distribution with mean 0 and standard deviation 0.1 are added to the Hamming distances. The curvature of the red curved fitted to the data points defines population epistasis, which in this example is −0.0405. B) Distribution of population epistasis values across 500 simulations in the absence (blue) and presence (red) of recombination measured at generation 100. Parameters take values: and . Thus far, we considered the effect of recombination on adaptation by comparing evolving populations characterized by different recombination rates (including the absence of recombination). To examine whether recombination is selectively favored within an adapting population, we performed additional simulations in which we competed a resident non-recombining with an invading recombining genotype during adaptation. Figure S8A shows that the frequency of the recombining genotype gradually increases over time if the recombination rate is high enough . In accordance with our previous findings, the results of the invasion analysis demonstrate that the benefit of recombination is most pronounced for intermediate mutation rates (Figure S8B and S8C and Section S7 in Text S1).
Discussion
Our results can be interpreted as support for the proposed accelerating role of recombination in the adaptive process through the Fisher-Muller effect. In our simulations, this effect seems to be sufficiently strong to outweigh potential costs of recombination. The Fisher-Muller effect is based on strong selective interference between beneficial mutations in an asexual (non-recombining) population. Previous mathematical models have provided important insights into how the strength of selection, mutation rate and population size affect selective interference and the Fisher-Muller effect, but these models have ignored epistatic interactions between mutations [24]–[30]. Our results demonstrate that both epistatic interactions and main fitness effects contribute to the accelerating effect of recombination. It is clear that this effect in the absence of epistatic interactions is due to the Fisher-Muller effect. Adding epistatic interactions to this model enhances the effect of recombination but the interpretation of why this occurs is challenging. On the one hand, this may be because the epistatic interactions increase the overall strength of selection and thereby produce stronger clonal interference, but on the other hand we cannot exclude explanations based purely on epistasis (see below).
One important determinant of the Fisher-Muller effect is the mutation rate. With a finite number of loci, an increasing mutation rate leads to a higher number of co-segregating beneficial mutations and this augments the Fisher-Muller effect. However, at very high mutation rates, it becomes increasingly likely that several beneficial mutations arise on the same genetic background so that recombination becomes less important (Figure 2B). A similar effect is expected for population size (with finite sites for beneficial mutations) but for the range of population sizes we examined here the effect of population size was monotonic.
In addition to the Fisher-Muller effect, recombination can also accelerate adaptation in the absence of random genetic drift when there is epistasis [9], [31]. Several studies have attempted to determine the prevailing form of epistasis in nature but have yielded inconsistent results: sometimes strong positive epistasis [32]–[36], negative epistasis [37], [38] or pervasive sign epistasis [39]–[42] was reported. The HIV-I fitness landscape is characterized by extensive epistatic interactions [23]. We demonstrated that population epistasis during adaptation is predominately negative. This result is in apparent contrast with the predominant positive epistasis in HIV-I sequences reported in Bonhoeffer et al. [33]. We think this discrepancy arises mainly because Bonhoeffer et al. estimated physiological epistasis, which is based only on the structure of fitness landscape itself and may be very different from population epistasis that we estimated here [43], [44]. The difference between the two estimates is that in determining population epistasis only the mutations that pass the sieve of natural selection are taken into account, whereas in measuring physiological epistasis all mutations are used indiscriminately. In addition to these different measures of epistasis, the two studies also differ in the way fitness was estimated. First, Bonhoeffer et al. [33] obtained fitness values from a much smaller data set than was used to estimate the fitness landscape in our study (9466 vs. 70,081 sequences). Second, in their study the main effects of a mutation and the epistatic effects for a given pair of mutations were calculated by averaging over the fitness effects of other mutations in the genetic background. By contrast, we obtained fitness using a predictive fitness model [23] that explicitly accounts for mutational effects in different genetic backgrounds during estimation of the fitness landscape, and therefore provides a more accurate estimate.
It is tempting to interpret the significant negative population epistasis that we observed as a support for the mechanism proposed by the mutational deterministic hypothesis, i.e. acceleration of adaptation through reduction of negative linkage disequilibria generated by negative epistasis. However, we would like to caution that it is very difficult to explain how population epistasis arises in our model and how it impacts the effect of recombination. For example, population epistasis on a complex fitness landscape can also be generated by variation in main fitness effects of mutations. The underlying causes of negative epistasis in our model and the extent to which it contributes to the accelerating effect of recombination (in isolation from the Fisher-Muller effect) is difficult to determine on a complex fitness landscape because deterministic models are not feasible. Therefore, we cannot exclude the possibility that stochastic benefits of recombination due to the Fisher-Muller effect may be sufficiently strong to override any direct effect of epistasis in our simulations, as reported by other studies [11], [45], [46].
Our study is based on an estimated fitness landscape and therefore the limitations of this approach should be taken into account while interpreting the results. First, in the fitness landscape that we used only main and pairwise epistatic effects were estimated but higher order fitness interactions (>2) were neglected. To accurately estimate higher orders epistasis, a much larger number of sequences with measured fitness values would be required. It is not clear what the strength of these higher-order interactions is and how they affect the impact of recombination. Second, although the accuracy of our fitness model (predicting 54.8% of the variance; see Methods) is acceptable as the only available fitness landscape, the predicted fitness landscape is yet to become more realistic by using a greater number of empirical fitness data. This is important as the estimated fitness values become increasingly unreliable for the regions in the sequence space far from the reference sequence due to the lack of data. To account for this problem, we focused on the population dynamics in the region of the fitness landscapes that is close to the reference sequence. Finally, the empirical fitness data used to predict the structure of the fitness landscape was obtained from an in vitro assay, and therefore might not completely correspond to in vivo fitness values.
It should also be noted that this study mainly examines the effect of recombination at the population level and does not address the evolution of recombination rate. One interesting extension of this work would be to incorporate variation in recombination rates in the model and study the spread of a recombination modifier gene in a non-recombining population. One problem with using the modifier approach in a realistic way with the current fitness model is that probably the best candidate for a recombination modifier gene in the HIV-1 genome is the reverse transcriptase gene [see 47], which is itself part of the fitness landscape and therefore changes during adaptation because of direct selection.
Our study relates to the debate over the advantage of recombination in retroviral, and in particular HIV, evolution. Recombination is believed by some to be beneficial because it generates genetic diversity to facilitate the development of multidrug resistance [48]–[50] or escape from host immune reaction [51]. Nonetheless, some studies have suggested that recombination has not evolved to facilitate adaptation but is a mere by-product of other mechanisms such as genomic organization [reviewed in 52], [ see also 45]. Unlike some prior studies [53]–[55], our model does not include any specific feature of HIV biology, such as viral dynamics during infection or specificities of recombination in HIV. Nonetheless, we believe that our findings are generic enough to highlight the potential role of recombination in accelerating HIV evolution.
This study utilizes data derived from a high-throughput fitness assay to address one of the long-standing questions in evolutionary biology. The advent of systems biology approaches made it possible to obtain a comprehensive picture of a large-scale fitness landscape. This serves as a framework for us to demonstrate that recombination has a substantial accelerating effect on adaptation on a realistic complex fitness landscape.
Methods
Fitness Model
Our model is based on a recently estimated fitness landscape of HIV-1 [23]. Briefly, to obtain this fitness landscape, the in vitro replicative capacity of 70,081 samples from HIV-1 subtype B infected individuals were measured and the corresponding amino acid sequences of the protease and partial sequences of the reverse transcriptase were obtained for all of these samples. This enabled estimation of the fitness effects of 1,857 single mutations and 257,536 pairs of mutations in these samples by fitting a fitness model to the data. This fitness model, as detailed in Hinkley et al. [23], invokes a generalized kernel ridge regression (GKRR) method to estimate the fitness effect of individual amino acid variants and the epistatic effects between variants.
Based on these results, we used the following fitness model to obtain fitness values for a given sequence:
Here, the amino acid sequence x is a binary vector indicating the presence or absence of amino acid variants. M is a triangular matrix where an entry Mii on the diagonal determines the main effects of the amino acid variant i and the off-diagonal entries Mij (with ) determine pairwise epistatic effects between variant i and j.
Higher order epistatic interactions were not considered. Note that the original model in Hinkley et al. [23] also includes an intercept term that gives the log fitness of a reference sequence (NL4-3) and that is added to Equation (1). However, since natural selection only depends on relative fitness in our model, this term was not considered in our simulation setting.
We used two different types of matrices M determining fitness. The first matrix type, MRL, describing the ‘reference fitness landscape’, was obtained by Hinkley et al. [23] by estimating both main and epistatic effects simultaneously. This estimation was done in 16 different environments: one drug-free environment and 15 environments each characterized by the presence of a different antiretroviral drug. On average, this matrix predicts 54.8% of the variance in fitness across different environments. Unless stated otherwise, we use the fitness landscape in the drug-free environment as the reference fitness landscape in our simulations.
To obtain the second matrix type, MHL, describing a ‘hierarchical fitness landscape’, two fitting steps were performed [56]. In the first step, MHL was estimated by assuming that there are only main effects (all off-diagonal elements set to zero), and in the second step, epistatic effects were estimated by fitting the residuals under the assumption that main effects are absent. This fitness landscape was obtained only for the drug free environment. Since the main and epistatic effects are estimated separately for these fitness landscapes, this approach allowed us to generate fitness landscapes where we could scale the magnitudes of main and epistatic effects and thus evaluate their relative contribution with respect to the effects of recombination. Hierarchical fitness landscapes with different magnitudes of epistatic effects were shown to provide accurate predictions of the reference fitness landscape [56].
For details about the estimation procedures, we refer to Hinkley et al. [23] and Kouyos et al. [56].
Simulation Framework
We consider a discrete-time model based on the classic the Wright-Fisher model to simulate adaptive evolution under mutation, recombination and natural selection on the HIV-1 fitness landscape. The population consists of a constant number of amino acid sequences, each of which contains the protease, as well as a partial reverse transcriptase, gene. Initially, this population is monomorphic, consisting only of copies of a reference sequence (NL4-3).
In each generation, the new population is formed from the previous one through three steps. First, reproduction and selection are implemented through random sampling of sequences, weighted according to the relative fitness value of each sequence. Second, to implement mutation events, sequences are randomly chosen from the population with replacement ( denotes the per genome mutation rate), thus allowing for several mutations per sequence. For <1, this number is treated as a random number with mean . For each of these sequences, the allele at a randomly selected site for which there exists more than one possible variant is substituted with one of the other possible allelic variant. Amino acid variants at a given site that are not present in the data set used to estimate fitness are neglected. In the final step, selected sequences undergo homologous recombination. We denote the recombination rate by . Here, pairs of sequences are chosen randomly (without replacement) and for each of these pairs, a single crossover site at which the two parental sequences exchange genetic material is determined at random. Note that recombination may result in daughter sequences that are identical to the parental sequences if identical pieces are exchanged.
The simulations were run for 100 generations. This period is long enough for the population to adapt but the adapting population still remains in the proximity of the reference sequence, where due to the availability of empirical fitness data, the estimation of the fitness by our model is reliable. To examine the effect of recombination on adaptation, we computed the ratio of the logarithm (base 10) of the population mean fitness of a population evolving with recombination to that of a population evolving without recombination. In this case, finding a proper definition of error bars is not straightforward since the data in question are the logarithms of ratios. However, this logarithm can be written as a difference (), so that we can use the standard deviation of the difference between two log normal distributions, , as error bars. This is justified because we found the log fitness values of sequences at generation 100 across 100 simulations to be normally distributed (for instance, non-significant Shapiro-Wilk and Anderson-Darling test to reject normal distribution for results in Figure 1, with p>0.05).
Supporting Information
Zdroje
1. Weismann A (1904) The evolution theory. London: Edward Arnold.
2. OttoSP, GersteinAC (2006) Why have sex? The population genetics of sex and recombination. Biochem Soc Trans 34 : 519–522.
3. BartonNH, CharlesworthB (1998) Why sex and recombination? Science 281 : 1986–1990.
4. FelsensteinJ (1974) The evolutionary advantage of recombination. Genetics 78 : 737–756.
5. Maynard SmithJM (1988) Selection for recombination in a polygenic model–the mechanism. Genet Res 51 : 59–63.
6. CharlesworthB (1993) Directional selection and the evolution of sex and recombination. Genet Res 61 : 205–224.
7. FelsensteinJ (1965) The effect of linkage on directional selection. Genetics 52 : 349–363.
8. BartonNH (1995) Linkage and the limits to natural selection. Genetics 140 : 821–841.
9. KondrashovAS (1988) Deleterious mutations and the evolution of sexual reproduction. Nature 336 : 435–440.
10. de VisserJAGM, ElenaSF (2007) The evolution of sex: empirical insights into the roles of epistasis and drift. Nature Reviews Genetics 8 : 139–149.
11. MoradigaravandD, EngelstädterJ (2012) The effect of bacterial recombination on adaptation on fitness landscapes with limited peak accessibility. PLoS Comput Biol 8: e1002735.
12. KondrashovFA, KondrashovAS (2001) Multidimensional epistasis and the disadvantage of sex. Proc Natl Acad Sci U S A 98 : 12089–12092.
13. HillWG, RobertsonA (1966) Effect of Linkage on Limits to Artificial Selection. Genetical Research 8 : 269–294.
14. Fisher RA (1930) The Genetical Theory Of Natural Selection USA: Oxford University Press.
15. MullerHJ (1932) Some genetic aspects of sex. American Naturalist 66 : 118–138.
16. KimY, OrrHA (2005) Adaptation in sexuals vs. asexuals: Clonal interference and the Fisher-Muller model. Genetics 171 : 1377–1386.
17. RiceWR, ChippindaleAK (2001) Sexual recombination and the power of natural selection. Science 294 : 555–559.
18. ColegraveN (2002) Sex releases the speed limit on evolution. Nature 420 : 664–666.
19. PoonA, ChaoL (2004) Drift increases the advantage of sex in RNA bacteriophage Phi6. Genetics 166 : 19–24.
20. GoddardMR, GodfrayHC, BurtA (2005) Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434 : 636–640.
21. BecksL, AgrawalAF (2012) The Evolution of Sex Is Favoured During Adaptation to New Environments. Plos Biology 10: e1001317.
22. de VisserJAGM, ParkSC, KrugJ (2009) Exploring the Effect of Sex on Empirical Fitness Landscapes. American Naturalist 174: S15–S30.
23. HinkleyT, MartinsJ, ChappeyC, HaddadM, StawiskiE, et al. (2011) A systems analysis of mutational effects in HIV-1 protease and reverse transcriptase. Nat Genet 43 : 487–489.
24. DesaiMM, FisherDS, MurrayAW (2007) The speed of evolution and maintenance of variation in asexual populations. Current Biology 17 : 385–394.
25. DesaiMM, FisherDS (2007) Beneficial mutation selection balance and the effect of linkage on positive selection. Genetics 176 : 1759–1798.
26. WeissmanDB, BartonNH (2012) Limits to the rate of adaptive substitution in sexual populations. Plos Genetics 8: e1002740.
27. SniegowskiPD, GerrishPJ (2010) Beneficial mutations and the dynamics of adaptation in asexual populations. Philosophical Transactions of the Royal Society B-Biological Sciences 365 : 1255–1263.
28. NeherRA (2013) Genetic Draft, Selective Interference, and Population Genetics of Rapid Adaptation. Annual Review of Ecology, Evolution, and Systematics, Vol 44 44 : 195–215.
29. ParkSC, KrugJ (2007) Clonal interference in large populations. Proceedings of the National Academy of Sciences of the United States of America 104 : 18135–18140.
30. ParkSC, KrugJ (2013) Rate of Adaptation in Sexuals and Asexuals: A Solvable Model of the Fisher-Muller Effect. Genetics 195 : 941–955.
31. BartonNH (1995) A General-Model for the Evolution of Recombination. Genetical Research 65 : 123–144.
32. TrindadeS, SousaA, XavierKB, DionisioF, FerreiraMG, et al. (2009) Positive epistasis drives the acquisition of multidrug resistance. Plos Genetics 5: e1000578.
33. BonhoefferS, ChappeyC, ParkinNT, WhitcombJM, PetropoulosCJ (2004) Evidence for positive epistasis in HIV-1. Science 306 : 1547–1550.
34. HeXL, QianWF, WangZ, LiY, ZhangJZ (2010) Prevalent positive epistasis in Escherichia coli and Saccharomyces cerevisiae metabolic networks. Nature Genetics 42 : 272–U120.
35. Maisnier-PatinS, RothJR, FredrikssonA, NystromT, BergOG, et al. (2005) Genomic buffering mitigates the effects of deleterious mutations in bacteria. Nature Genetics 37 : 1376–1379.
36. BurchCL, ChaoL (2004) Epistasis and its relationship to canalization in the RNA virus phi 6. Genetics 167 : 559–567.
37. KhanAI, DinhDM, SchneiderD, LenskiRE, CooperTF (2011) Negative epistasis between beneficial mutations in an evolving bacterial population. Science 332 : 1193–1196.
38. ChouHH, ChiuHC, DelaneyNF, SegreD, MarxCJ (2011) Diminishing Returns Epistasis Among Beneficial Mutations Decelerates Adaptation. Science 332 : 1190–1192.
39. KvitekDJ, SherlockG (2011) Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape. Plos Genetics 7: e1002056.
40. SilvaRF, MendoncaSCM, CarvalhoLM, ReisAM, GordoI, et al. (2011) Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations. Plos Genetics 7: e1002181.
41. WeinreichDM, DelaneyNF, DePristoMA, HartlDL (2006) Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312 : 111–114.
42. FrankeJ, KlozerA, de VisserJA, KrugJ (2011) Evolutionary accessibility of mutational pathways. PLoS Comput Biol 7: e1002134.
43. KouyosRD, SilanderOK, BonhoefferS (2007) Epistasis between deleterious mutations and the evolution of recombination. Trends Ecol Evol 22 : 308–315.
44. KouyosRD, OttoSP, BonhoefferS (2006) Effect of varying epistasis on the evolution of recombination. Genetics 173 : 589–597.
45. OttoSP, BartonNH (2001) Selection for recombination in small populations. Evolution 55 : 1921–1931.
46. KeightleyPD, OttoSP (2006) Interference among deleterious mutations favours sex and recombination in finite populations. Nature 443 : 89–92.
47. Onafuwa-NugaA, TelesnitskyA (2009) The remarkable frequency of human immunodeficiency virus type 1 genetic recombination. Microbiol Mol Biol Rev 73 : 451–480 Table of Contents
48. GuZ, GaoQ, FaustEA, WainbergMA (1995) Possible involvement of cell fusion and viral recombination in generation of human immunodeficiency virus variants that display dual resistance to AZT and 3TC. J Gen Virol 76(Pt 10): 2601–2605.
49. KellamP, LarderBA (1995) Retroviral Recombination Can Lead to Linkage of Reverse-Transcriptase Mutations That Confer Increased Zidovudine Resistance. Journal of Virology 69 : 669–674.
50. MoutouhL, CorbeilJ, RichmanDD (1996) Recombination leads to the rapid emergence of HIV-1 dually resistant mutants under selective drug pressure. Proc Natl Acad Sci U S A 93 : 6106–6111.
51. MalimMH, EmermanM (2001) HIV-1 sequence variation: Drift, shift, and attenuation. Cell 104 : 469–472.
52. Simon-LoriereE, HolmesEC (2011) Why do RNA viruses recombine? Nature Reviews Microbiology 9 : 617–626.
53. FraserC (2005) HIV recombination: what is the impact on antiretroviral therapy? Journal of the Royal Society Interface 2 : 489–503.
54. BretscherMT, AlthausCL, MullerV, BonhoefferS (2004) Recombination in HIV and the evolution of drug resistance: for better or for worse? Bioessays 26 : 180–188.
55. KouyosRD, FouchetD, BonhoefferS (2009) Recombination and drug resistance in HIV: Population dynamics and stochasticity. Epidemics 1 : 58–69.
56. KouyosRD, LeventhalGE, HinkleyT, HaddadM, WhitcombJM, et al. (2012) Exploring the complexity of the HIV-1 fitness landscape. Plos Genetics 8: e1002551.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




