-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
Cell communication is essential for eukaryotic development, but our knowledge of molecules and mechanisms required for intercellular communication is fragmentary. In particular, the connection between signal sensing and regulation of cell polarity is poorly understood. In the filamentous ascomycete Neurospora crassa, germinating spores mutually attract each other and subsequently fuse. During these tropic interactions, the two communicating cells rapidly alternate between two different physiological states, probably associated with signal delivery and response. The MAK2 MAP kinase cascade mediates cell–cell signaling. Here, we show that the conserved scaffolding protein HYM1/MO25 controls the cell shape-regulating NDR kinase module as well as the signal-receiving MAP kinase cascade. HYM1 functions as an integral part of the COT1 NDR kinase complex to regulate the interaction with its upstream kinase POD6 and thereby COT1 activity. In addition, HYM1 interacts with NRC1, MEK2, and MAK2, the three kinases of the MAK2 MAP kinase cascade, and co-localizes with MAK2 at the apex of growing cells. During cell fusion, the three kinases of the MAP kinase module as well as HYM1 are recruited to the point of cell–cell contact. hym-1 mutants phenocopy all defects observed for MAK2 pathway mutants by abolishing MAK2 activity. An NRC1-MEK2 fusion protein reconstitutes MAK2 signaling in hym-1, while constitutive activation of NRC1 and MEK2 does not. These data identify HYM1 as a novel regulator of the NRC1-MEK2-MAK2 pathway, which may coordinate NDR and MAP kinase signaling during cell polarity and intercellular communication.
Published in the journal: . PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1002950
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002950Summary
Cell communication is essential for eukaryotic development, but our knowledge of molecules and mechanisms required for intercellular communication is fragmentary. In particular, the connection between signal sensing and regulation of cell polarity is poorly understood. In the filamentous ascomycete Neurospora crassa, germinating spores mutually attract each other and subsequently fuse. During these tropic interactions, the two communicating cells rapidly alternate between two different physiological states, probably associated with signal delivery and response. The MAK2 MAP kinase cascade mediates cell–cell signaling. Here, we show that the conserved scaffolding protein HYM1/MO25 controls the cell shape-regulating NDR kinase module as well as the signal-receiving MAP kinase cascade. HYM1 functions as an integral part of the COT1 NDR kinase complex to regulate the interaction with its upstream kinase POD6 and thereby COT1 activity. In addition, HYM1 interacts with NRC1, MEK2, and MAK2, the three kinases of the MAK2 MAP kinase cascade, and co-localizes with MAK2 at the apex of growing cells. During cell fusion, the three kinases of the MAP kinase module as well as HYM1 are recruited to the point of cell–cell contact. hym-1 mutants phenocopy all defects observed for MAK2 pathway mutants by abolishing MAK2 activity. An NRC1-MEK2 fusion protein reconstitutes MAK2 signaling in hym-1, while constitutive activation of NRC1 and MEK2 does not. These data identify HYM1 as a novel regulator of the NRC1-MEK2-MAK2 pathway, which may coordinate NDR and MAP kinase signaling during cell polarity and intercellular communication.
Introduction
Appropriate cellular responses to external and internal stimuli depend on the highly orchestrated activity of interconnected signaling cascades. Within these networks, individual proteins can function in more than just one pathway. This raises the question of signal fidelity and the avoidance of undesired crosstalk in response to a specific signal. One crucial level of control arises from the formation of discrete complexes involving scaffold proteins that bind multiple components of a given pathway [1], [2]. By facilitating the transient spatio-temporal organization of the different signaling factors, scaffolds promote kinase-substrate interactions, or kinase activation by upstream components such as membrane bound receptors. Scaffold proteins may also actively participate in signal modulation, for example by recruiting opposing phosphatases to the complex [3]–[7].
The best-studied scaffold is the budding yeast protein Ste5p, which binds the three kinases Ste11p, Ste7p and Fus3p of the mitogen-activated protein kinase (MAP kinase) module of the pheromone response pathway [8], [9]. Upon binding of pheromone to its transmembrane receptor, dissociation of the receptor-associated heterotrimeric G protein triggers the recruitment of Ste5p to the plasma membrane through its interaction with the free Gβγ dimer. This membrane association facilitates the phosphorylation of the MAP kinase kinase kinase (MAPKKK) Ste11p by the membrane-associated p21-activated (PAK) kinase Ste20p and activates the mating pathway. The outcome of this activation includes cell cycle arrest, expression of mating specific genes, reorganization of the cytoskeleton and the control of cell fusion of the two mating partners. Although the mating MAP kinase cascade is highly conserved throughout evolution, the Ste5p scaffold is specific for budding yeast. Even close fungal relatives, such as Ashbya gossypii or Candida albicans lack obvious homologs.
Recent studies in Neurospora crassa indicate that a MAP kinase module homologous to the yeast pheromone response pathway is essential for the communication and subsequent fusion of vegetative cells. The three kinases involved are the MAPKKK NRC1, the MAPKK MEK2 and the MAP kinase MAK2 (homologous to budding yeast Ste11p, Ste7p, and Fus3p, respectively) [10]–[13]. Upstream activators of this pathway, including a postulated secreted signal and its cognate receptor are so far unknown. N. crassa and other filamentous fungi possess pheromone receptors, heterotrimeric G-proteins, and PAK kinases [14], [15]. However, these upstream components of the yeast pheromone response pathway are dispensable for vegetative cell communication [16], [17]. Cell-cell signaling and tropic growth of N. crassa germlings involves the unusual subcellular dynamics of the MAP kinase MAK2 and SOFT, a conserved, Pezizomycotina-specific protein of unknown molecular function. Both proteins are recruited to the plasma membrane of the tip region of communicating germlings in an oscillating and alternating manner [13]. These observations led to the hypothesis that the two fusion cells coordinately switch between two physiological stages, which are probably related to signal sending and receiving [13], [17], [18].
Genetic data indicate a functional connection between the MAK2 MAP kinase pathway and the nuclear Dbf2-related (NDR) kinase COT1 in N. crassa [12]. Mutations in MAK2 pathway components partially suppress cot-1 defects, such as defective tip elongation and hyperbranching. In addition, MAK2 pathway defects are partially overcome in a cot-1 background. These data suggest an intricate relationship between COT1 and MAK2 that coordinates polar growth, intercellular communication and cell fusion.
NDR kinases, such as COT1, are central elements for controlling cell polarity, proliferation and development [19], [20]. They associate with proteins of the Mps One binder (MOB) family and are activated by germinal center (GC) kinases of the STE20 super family. A given GC kinase can assemble with various NDR-MOB complexes into distinct signaling modules, which are determined by distinct scaffold proteins [21], [22]. The morphogenetic NDR kinase pathway MOR is defined by the conserved scaffold TAO3/SAX2/FRY in fungi and animals [23]–[26]. In addition, fungal NDR kinases also associate with HYM1, a conserved protein that functions as a second and non-redundant scaffold during MOR-dependent cell polarization [27]–[29]. The homologous animal protein MO25 regulates the activity of the tumor suppressor kinase LKB1, but seems not to be involved in NDR kinase signaling [30], [31]. Recently, it has been shown that MO25 interacts with and regulates the activity of multiple GC kinases, implicating MO25 as a master regulator of this subgroup of STE20-related kinases [32].
To date, N. crassa COT1, its co-activators MOB2A and MOB2B, and the activating GC kinase POD6 are among the best-characterized components that regulate polar tip extension and subapical branch formation [33]–[39]. Strains carrying mutations in these genes are viable, but display highly restricted colonial growth with growth-arrested needle-shaped tips and produce massive amounts of extension-arrested new tips along the entire cell. In the present study, we show that N. crassa HYM1 functions as scaffold of the COT1-MOB2-POD6 complex to regulate the interaction of the two kinases and thereby COT1 activity. Moreover, HYM1 is essential for activation of the MAK2 MAP kinase pathway. We suggest that this dual use of one scaffold in two kinase pathways may promote the coordination of chemotropic growth and cell-cell communication.
Results
HYM1 functions as scaffold protein for the COT1 NDR kinase pathway
HYM1 interacts with and regulates morphogenetic NDR kinases in unicellular yeasts, but its function during highly polar filamentous growth is unclear. We identified the uncharacterized ORF NCU03576 as the N. crassa homolog of HYM1. The N. crassa protein matched HymA of Aspergillus nidulans and MO25 of Schizosaccharomyces pombe with E-values of 2e−142 and 5e−67, respectively. To test a potential involvement of HYM1 in the NDR complex we analyzed its interaction with COT1 and POD6 in yeast two-hybrid assays. Both tests rendered positive results (Figure 1A). To corroborate these interactions in vivo by immunoprecipitation experiments, we generated a strain that expressed functionally tagged versions of the three proteins. We modified the endogenous loci of cot-1 and pod-6 to encode for HA - and myc-tagged kinase genes, respectively, and fused this strain with an isolate that ectopically expressed GFP-tagged HYM1 at the his-3 locus. The anti-GFP immunoprecipitation recovered both myc-COT1 and HA-POD6 from extracts of myc-cot-1;HA-pod-6 + hym-1-gfp::his-3, but not the myc-cot-1;HA-pod-6 control strain (Figure 1B). In order to test if HYM1 promotes COT1 function, the activity of the kinase was compared in wild type and Δhym-1. COT1 activity purified from Δhym-1 was reduced to 70% (SD +/−5; n = 5) of wild type level (Figure 1C). Expression of HYM1-GFP in Δhym-1 increased COT1 activity back to wild type level, confirming that the reduced kinase activity was due to the deletion of hym-1.
Fig. 1. HYM1 functions as scaffold protein for the COT1-POD6 complex. 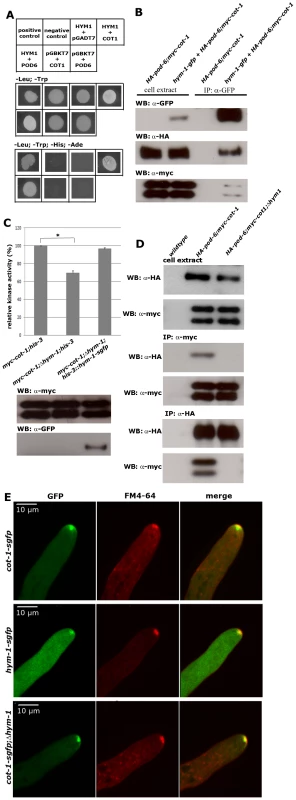
(A) Yeast two hybrid tests identify HYM1 as direct interaction partner of COT1 or POD6. Genes cloned into pGBKT7 and pGADT7 (mentioned as first or second label, respectively) were co-expressed as fusion proteins with the GAL4 DNA-binding domain and activation domains, respectively. Plasmids expressing the indicated proteins either as prey or as bait alone were used as negative controls and pGBKT7-53 (murine p53) and pGADT7-recT (SV40 large T antigen) fusion proteins as positive control. (B) Co-immunoprecipitation of GFP-tagged HYM1 recovered the small and large isoform of myc-COT1 and HA-POD6 from N. crassa cell extracts indicating the formation of a COT1-POD6-HYM1 complex in vivo. (C) Kinase activity assay of myc-COT1 purified from the indicated strains. Data represent means of five independent experiments (standard deviations are indicated as bars; the star denotes significantly different COT1 activity in the tested strains (p<0,01; unpaired Student's t-test). (D) Reciprocal anti-myc and anti-HA immunoprecipitation experiments from strains co-expressing myc-COT1 and HA-POD6 in a Δhym-1 background failed to recover HA-POD6 and myc-COT1, respectively. (E) COT1-GFP accumulated at the hyphal tip as bright spot at the distal side of the Spitzenkörper (co-stained with the marker dye FM4-64) and as apex-associated crescent. HYM1, in contrast, did not form this apex-associated crescent and its apical localization fully coincided with the Spitzenkörper. Note that both proteins co-localized at constricting septa (Videos S1, S2). COT1-GFP in a Δhym-1 background still accumulated as a bright spot at the distal side of the Spitzenkörper and as an apex-associated crescent at the hyphal tip. HYM1 might promote COT1 activation by connecting the NDR kinase with its activating kinase POD6. We predicted that in this case the interaction between COT1 and POD6 should be reduced or abolished in a Δhym-1 background. To test this hypothesis, we crossed myc-cot-1;HA-pod-6 with Δhym-1 to generate myc-cot-1;HA-pod-6;Δhym-1. Reciprocal co-precipitation experiments between myc-COT1 and HA-POD6 revealed that the weak interaction between the two kinases observed in wild type was abolished in a Δhym-1 background (Figure 1D). Together, these data indicate that HYM1 functions as a scaffold for COT1 and POD6, thereby promoting COT1 activity.
In order to determine the subcellular localization of COT1 and HYM1, we modified both gene loci to express C-terminally GFP-tagged proteins. The generated strains displayed wild type characteristics, indicating the functionality of the two fusion proteins under the control of their endogenous promoters. COT1-GFP formed an apical membrane-associated crescent in growing hyphal tips and accumulated as a bright, discrete dot that partly overlapped with the distal region of the Spitzenkörper (Figure 1E). The Spitzenkörper is an apex-associated cluster of vesicles, cytoskeletal elements and polarity factors that serves as vesicle supply center and guides polar tip growth [40]–[42]. HYM1-GFP, in contrast, did not form this apical cap and fully co-localized with the Spitzenkörper (Figure 1E). The intensity of the apical localization was higher in COT1-GFP compared to HYM1-GFP, a difference that was also reflected by a ca. 15-fold higher expression level of COT1 in comparison to HYM1 (Figure S1). Moreover, both proteins also labeled constricting septa and accumulated around the septal pore of mature septa (Videos S1, S2). Deletion of hym-1 did not alter the apical and septum localization of COT1-GFP (Figure 1E), indicating that the scaffold is dispensable for proper localization of the NDR kinase. In summary, these data establish HYM1 as a functional component of the COT1-POD6 kinase complex. However, in contrast to the situation in unicellular yeasts [27], [28], [43], [44], HYM1 is not essential for MOR signaling in N. crassa.
HYM1 is essential for MAK2 MAP kinase activity
Mutations in the cot-1, pod-6 or mob-2a/2b genes, which define the central components of the N. crassa MOR pathway, share characteristic defects, such as the inhibition of polar tip extension and the initiation of extension-arrested new tips along the entire cell [33]–[35], [37], [39]. In contrast, Δhym-1 did not display this barbed-wired appearance, but generated defects that were highly reminiscent of strains deficient in components of the mak-2 MAP kinase pathway [10], [12], [13], [45]. Both, Δhym-1 and Δmak-2 mutants were characterized by a reduced hyphal growth rate (ca. 35% of wild type) and highly knobby cell morphology, stunted aerial mycelium formation and de-repressed conidiation. In addition, both mutants were unable to develop female sexual structures, called protoperithecia (Figure 2A, 2B). Moreover, similar to MAK2 pathway mutants, germinating conidia of Δhym-1 or hyphal cells within an established Δhym-1 colony showed no mutual attraction and self-fusion (Figure 2C, 2D).
Fig. 2. Δhym-1 displays phenotypic characteristics of MAK2 MAP kinase pathway mutants. 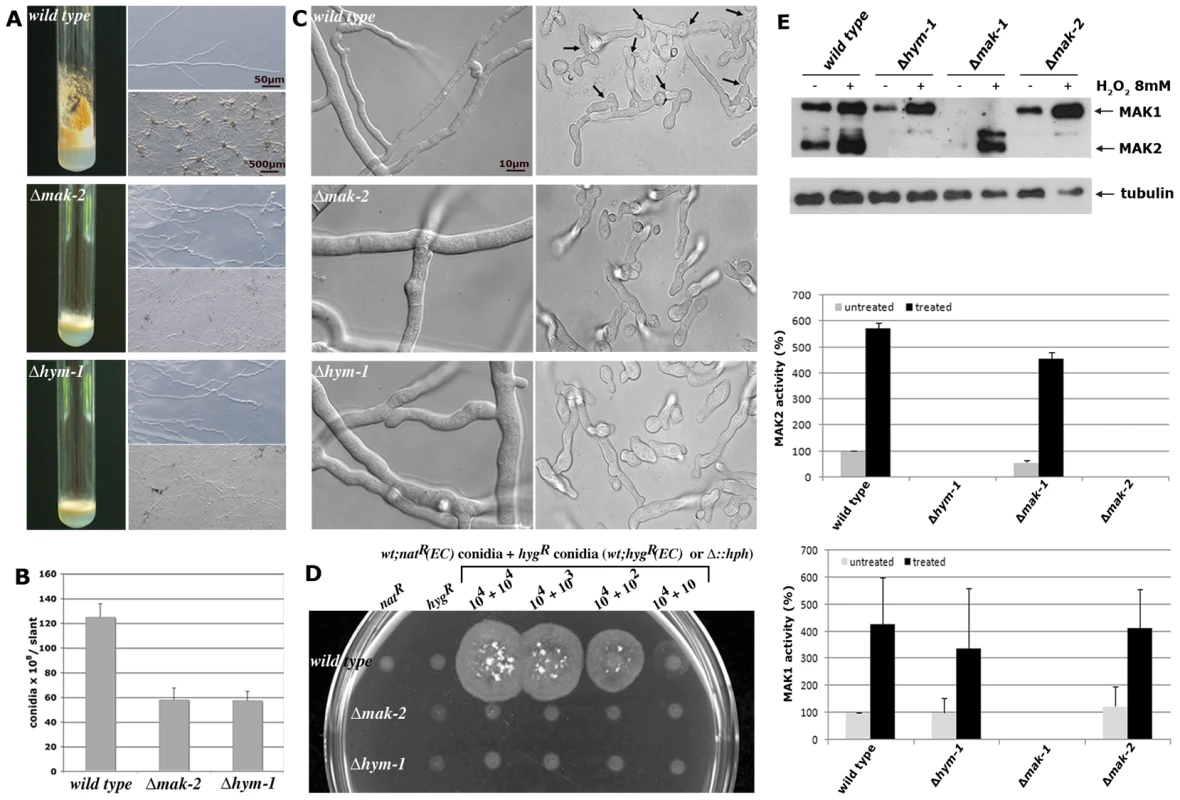
(A) Phenotypic characterization of Δhym-1, Δmak-2 and wild type regarding macroscopic morphology and conidiation pattern (left panel: growth in slants for 5 days on minimal medium), hyphal morphology (upper right panel; bar), and protoperithecia formation (lower right panel). (B) Production of conidiospores was quantified by counting conidia generated in slants grown at room temperature for 5 days (n = 5; standard deviations are indicated as bars). (C) HYM1 and MAK2 are required for vegetative cell fusion. Hyphal (left panel) and germling fusion events (right panel; fusion events are indicated by arrows) were assessed by light microscopy in wild type, Δhym-1 and Δmak-2 cultures. Cell fusion was not observed in Δhym-1 and Δmak-2. (D) Quantification of cell fusion competence and formation of forced heterokarya that grew on double-selective media supplemented with 150 µg/ml hygromycin and 20 µg/ml nourseothricin. 104 hygromycin-resistant conidiospores of the indicated strains (“wild type” carried an ectopically integrated (EC) hygromycin-resistance cassette, while the hygromycin-resistance cassette was used to replace the two gene deletions in Δhym-1 and Δmak-2) were plated alone (second column) or in decreasing concentrations together with a second “wild type” strain that carried an ectopically integrated nourseothricin-resistance cassette (column 3–6). Column 1 indicated lack of growth of the “wild type” natR control strain. 1% sorbose was added to Vogels minimal medium to restrict radial growth of the forming colonies generated by forced heterokaryons after incubation for 3 days at room temperature. (E) HYM1 is required for MAK2 activity. Total soluble protein was extracted from the indicated strains grown in the presence or absence of 8 mM H2O2. Western blot analysis with anti-phospho-p42/p44 antibody detected activated MAK1 and MAK2, respectively. Equal loading was confirmed by re-probing the membrane with anti-tubulin antibody. A typical Western blot is shown. MAK1 and MAK2 activities from 5 independent experiments were quantified for the diagrams. A similar cell fusion deficiency is described for strains defective in components of the MAK1 cell wall integrity MAP kinase pathway [12], [46]. However, the relationship between the MAK1 and MAK2 MAP kinase modules during hyphal fusion is unresolved. To test if HYM1 might influence the activity of these signaling modules, we compared the phosphorylation status of the two MAP kinases in wild type and Δhym-1 by using phospho-specific antibodies against the activated proteins. In wild type cells, MAK1 and MAK2 displayed basal activities that can be stimulated ca. 5 - to 10-fold under conditions of oxidative stress [12], [47]. MAK1 displayed wild type activities in non-stressed and H2O2-stimulated Δhym-1, but MAK2 activity was completely abolished in Δhym-1 (Figure 2E).
Driven by these results, we asked if HYM1 interacted with the MAK2 pathway in vivo by performing co-precipitation experiments. We detected weak, but consistent interactions between myc-HYM1 and flag - and HA-tagged versions of three kinases of the MAK2 module (Figure 3A). However, the interaction between HA-MAK2 and its upstream kinase flag-MEK2, which was used as positive control, was stronger and stable after several washes with 300 mM NaCl, while the interactions between HYM1 and the three kinases were only detected in immunoprecipitations lacking the high salt washing steps (Figure S2). Moreover, yeast two-hybrid tests between HYM1 and the three kinases of the MAK2 MAP kinase cascade were negative (Figure 3B). In control experiments, we observed interactions between NRC1 and MEK2 and between MEK2 and MAK2, confirming the functionality of the yeast two-hybrid constructs and the physical interaction of the NRC1-MEK2-MAK2 cascade. In summary, HYM1 is essential for MAK2 activity, but appears to interact with the three MAP kinases only in an indirect manner, probably as part of a larger protein complex. In line with this hypothesis, we found that the PAK kinase STE20/NCU03894 physically interacted with HYM1 and with NRC1 in yeast two-hybrid assays. The second PAK kinase present in N. crassa, CLA4/NCU00406, which we used as control, did not interact with HYM1 or NRC1 (Figure 3B).
Fig. 3. HYM1 interacts with components of the MAK2 MAP kinase pathway. 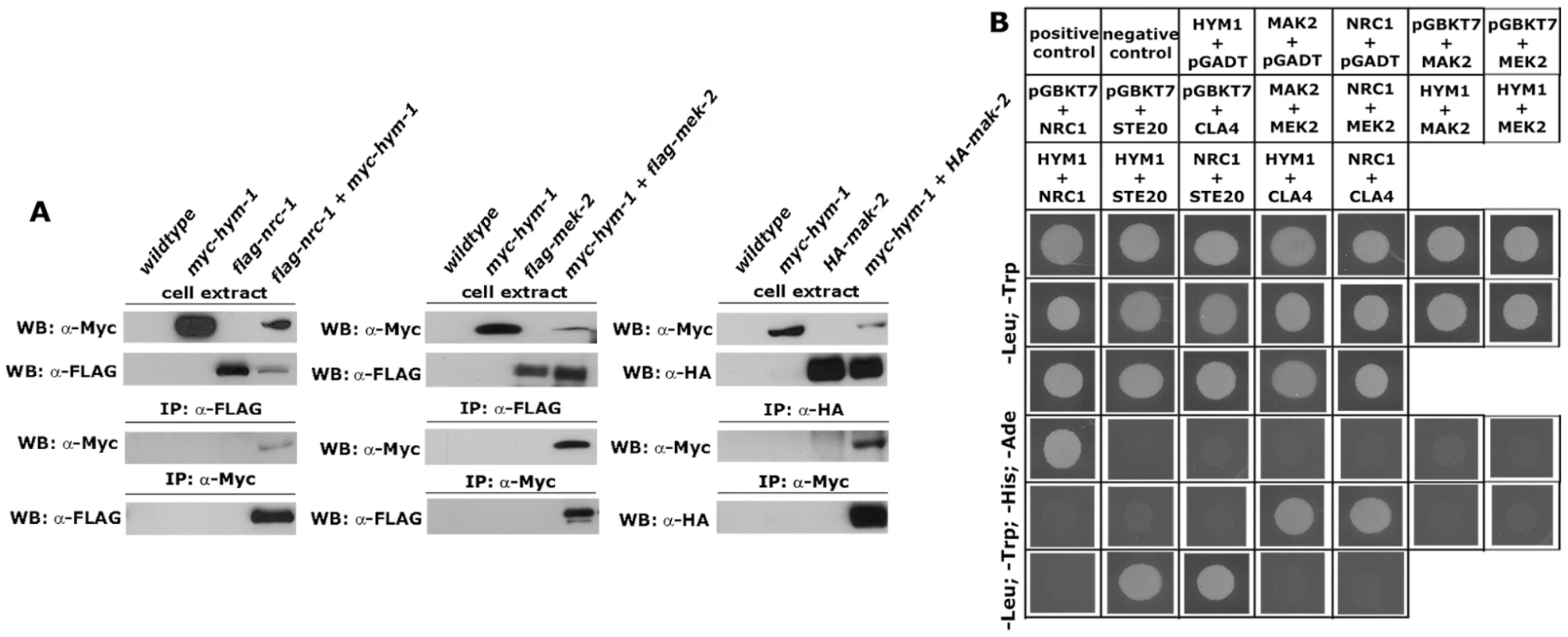
(A) Co-immunoprecipitation experiments of forced heterokaryons expressing myc-tagged HYM1 with flag-NRC1, flag-MEK2 or HA-MAK2. Interactions were observed between HYM1 and all three kinases of the MAK2 pathway. (B) Yeast two-hybrid analysis of HYM1 and the kinases NRC1, MEK2 and MAK2 revealed no physical interactions between the tested protein pairs. Interactions between NRC1 and MEK2 and between MEK2 and MAK2 were used as positive controls. Moreover, HYM1 and NRC1 interacted with the PAK kinase STE20, but not the homologous kinase CLA4. HYM1 is required for signal transduction through the NRC1-MEK2-MAK2 kinase cascade
Based on these results, HYM1 could function upstream of the MAK2 pathway or as a component of a scaffold complex that connects the three kinases of the MAP kinase cascade. To distinguish between these two possibilities we employed constitutive activated components of the MAK2 MAP kinase module. We hypothesized that this activation would complement Δhym-1 defects in the first case, while the activation should not be transferred to the downstream components in the latter one. First, we constructed a proline 448 to serine-substituted version of NRC1. Homologous mutations have previously been shown to confer constitutive activity of related fungal MAPKKKs [48], [49]. Ectopic expression of flag-NRC1(P448S) in the N. crassa wild type and Δnrc-1 control strains increased MAK2 activity ca. 12–fold (Figure 4A; Table 1). When we expressed this construct in Δmek-2 as control, hyperactivation of MAK2 was blocked, confirming that the constitutive NRC1 signal is transmitted through the MAPK cascade. No MAK2 activity was detected when we expressed flag-NRC1(P448S) in Δhym-1. Analogous results were obtained, when we expressed a constitutive active version of MEK2 in these strains. Expression of MEK2(S212D;T216D) resulted in MAK2 hyperactivation in wild type and Δmek-2, but not in Δhym-1. Thus, HYM1 is required for signal transmission through the entire NRC1-MEK2-MAK2 MAP kinase cascade (Table 1).
Fig. 4. HYM1 is required for signal transduction through the entire MAK2 kinase cascade. 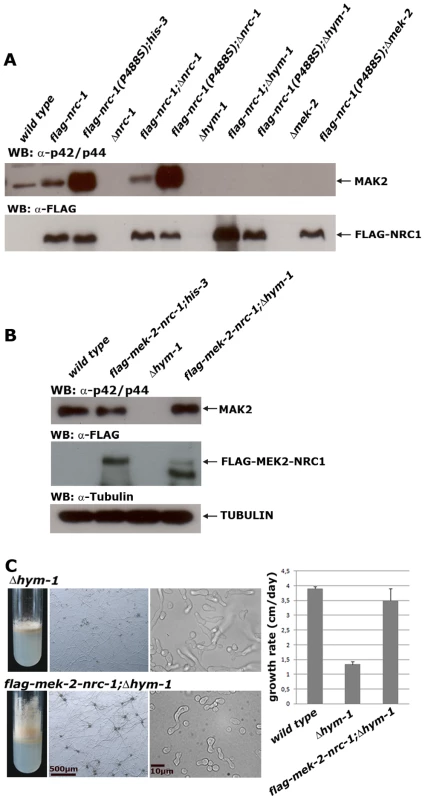
(A) Constitutive hyperactive versions of NRC1 (NRC1(P448S)) and MEK2 (MEK2(S212D;T216D) do not activate MAK2 in a Δhym-1 background. Total soluble protein was extracted from the indicated strains grown at 25°C and Western blot analyzed with anti-phospho-p42/p44 antibody to probe for MAK2 activity. Equal loading was confirmed by re-probing the membrane with anti-FLAG antibody. (B) Expression of a MEK2-NRC1 fusion protein in Δhym-1 resulted in wild type levels of active MAK2 (determined by Western blot experiments with anti-phospho-p42/p44 antibody). Note that the stability of the MEK2-NRC1 fusion protein seems reduced in Δhym-1, resulting in the consistent appearance of potential degradation products with smaller size (B). An anti-tubulin blot served as loading control to indicate equal protein levels. (C) Expression of a MEK2-NRC1 fusion protein complemented all Δhym-1 defects (panel 1: aerial mycelium formation in slants; panel 2: sexual development/protoperithecia formation; panel 3: germling fusion; panel 4: growth rate). Tab. 1. Phenotypic characteristics of constitutive hyperactive NRC1 and MEK2 variants in the indicated strains. 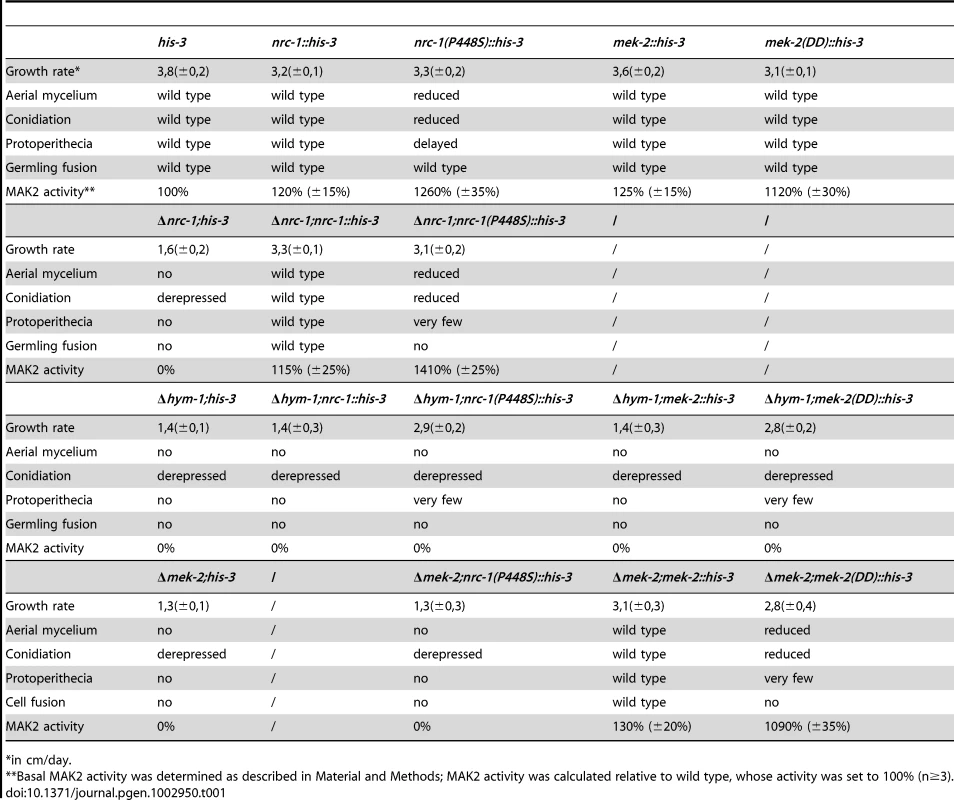
in cm/day. Consistent with the observed MAK2 activities in these strains, expression of flag-NRC1(P448S) rescued the vegetative hyphal growth defects of Δnrc-1, but not of Δmek-2, while flag-MEK2(S212D;T216D) complemented Δmek-2 (Table 1; Figure S3). Interestingly, the two constitutive hyperactive kinase variants also increased the growth rate of Δhym-1, although we were unable to detect MAK2 activity in this strain. Thus, a basal signal transduction rate below the detection level of the p42/44 anti-ERK antibodies seems sufficient for sustained vegetative growth in the absence of HYM1. In contrast, the cell fusion defects of Δnrc-1, Δmek-2 and Δhym-1 were not complemented by flag-NRC1(P448S) or MEK2(S212D;T216D), suggesting that cell fusion requires signal based, adjustable MAP kinase activation.
To further support the finding that HYM1 promotes signal transduction through the MAP kinase module, we tested if the artificial tethering of two of the kinases rescued the Δhym-1 defects. A flag-MEK2-NRC1 fusion protein was generated and expressed in the different mutants. This construct rescued all defects of Δmek-2 and Δnrc-1 and resulted in wild type levels of MAK2 activity in both strains, confirming the functionality of the two fused kinases. Moreover, this construct resulted in wild type MAK2 activity levels when expressed in Δhym-1 and fully complemented the vegetative and developmental defects of Δhym-1 (Figure 4B, 4C). These data indicate that HYM1 functions as part of an essential adaptor complex that connects the components of the NRC1-MEK2-MAK2 cascade to allow signaling through the cell fusion MAP kinase pathway.
The three kinases of the MAK2 MAP kinase module and HYM1 are recruited to the contact zone of germling fusion pairs
The interactions of the three MAK2 pathway kinases and of the kinases with HYM1 suggest a similar subcellular localization of all proteins. We have recently shown that MAK2 is recruited to the tips of two communicating germlings in an oscillatory manner [13]. Its localization in mature hyphae or the subcellular dynamics of the two upstream kinases were unknown. We determined that MAK2-GFP expressed under the control of the ccg-1 promoter localized in the cytoplasm, was not excluded from the nuclei and accumulated in the Spitzenkörper of growing hyphal tips (Figure 5A). Moreover, MAK2 labeled constricting septa and the septal pore of mature septa (Figure 5A; Video S3). Thus, MAK2 displayed a localization pattern highly similar to the localization described for HYM1. Co-localization experiments employing MAK2-mCherry and HYM1-GFP fusion proteins confirmed the co-localization of both factors in the Spitzenkörper of growing hyphal tips and at septa (Figure 5B). To exclude that the used promoter and thus the ca. two-fold higher expression level of MAK2 affected its localization, we compared two strains that expressed MAK2-GFP under the control of the native and the ccg-1 promoter, but no differences in MAK2 localization were observed (Figures S1, S4). Functional MEK2-GFP and NRC1-GFP fusion proteins, expressed under the control of their native and the ccg-1 promoters, did not display any apical accumulation, but localized in the cytosol and accumulated around mature septal pores (Figure 5A). Moreover, both proteins were associated with constricting septa (Videos S4, S5). In contrast to MAK2, MEK2 and NRC1 were excluded from nuclei (Figure 5A). When we addressed the localization of the three kinases in a Δhym-1 background, we found that MAK2-GFP, but not the other two kinases, strongly accumulated in the nucleus in addition to the described localization at the hyphal tip and the septum (Figure 5C). These data indicate differential functions of MAK2 and its upstream kinases NRC1 and MEK2 at the tips of vegetatively growing cells and suggest a function of HYM1 in regulating the nuclear versus cytosolic accumulation of MAK2.
Fig. 5. NRC1, MEK2, and MAK2 display different localization patterns in vegetative hyphae. 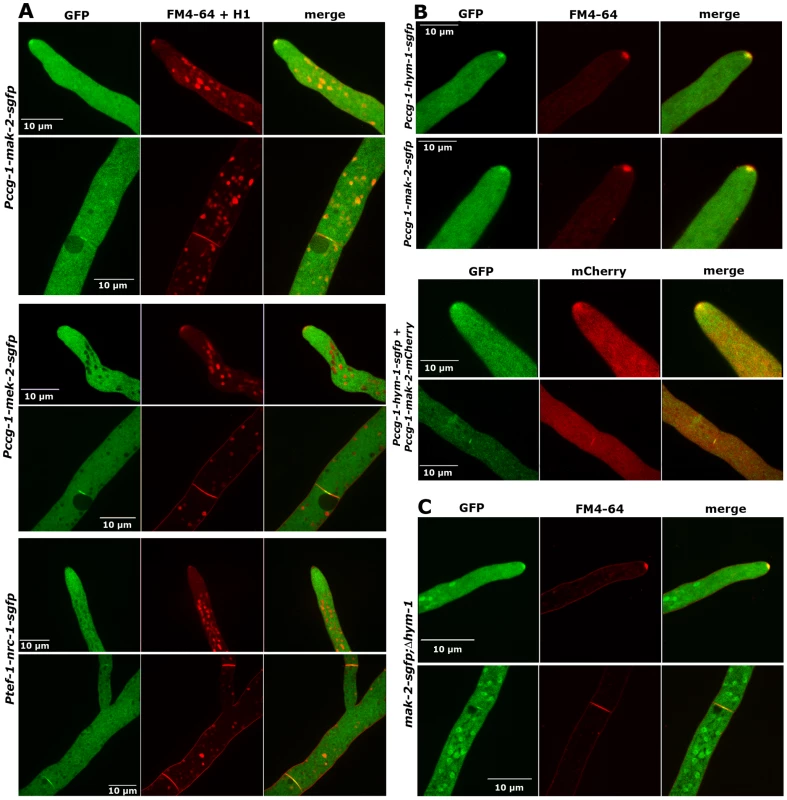
(A) MAK2-GFP accumulated at the hyphal tip and the forming septum, while MEK2 and NRC1 only labeled the septum, but not the cell apex. Note that MAK2 has a cytosolic and nuclear distribution, while MEK2 and NRC1 are exclusive cytosolic proteins. Nuclei, hyphal plasma membrane/septa were co-labeled with histone H1-RFP and FM4-64, respectively, and detected in the red channel. (B) HYM1 and MAK2 co-localized in the Spitzenkörper at the hyphal tip and at constricting septa. Upper panel: comparison of hyphal tips of cells expressing HYM1-GFP and MAK2-GFP; lower panel: co-localization of co-expressed HYM1-GFP and MAK2-mCherry. (C) MAK2-GFP accumulated in the nucleus in a Δhym-1 background. Since MEK2, NRC1 and HYM1 are essential for germling fusion, we tested if GFP fusion constructs of these components displayed a dynamic localization to communicating germling tips, as described for MAK2 [13]. In germlings of strains expressing either one the three constructs under their native promoters barely any fluorescence was detectable. Therefore, strains carrying functional gfp fusion constructs under the control of the stronger ccg-1 or tef-1 promoters were employed. MEK2-GFP showed subcellular dynamics comparable to MAK2 in communicating partner cells (Figure 6). Based on this observation and the physical interaction of the two kinases (Figure 3) we tested if the two proteins co-localized during the dynamic recruitment associated with tropic growth of fusing germlings, by employing GFP and mCherry tagged protein variants (Figure 7). While the majority of the protein aggregates that formed at the plasma membrane of communicating tips contained both kinases, we frequently observed spots containing only one of the two proteins. Together, these data indicate that MEK2 and MAK2 functionally interact at the plasma membrane of fusion tips, but that they appear to be independently recruited and/or released from these kinase complexes.
Fig. 6. Components of the MAP kinase cascade oscillate during germling fusion. 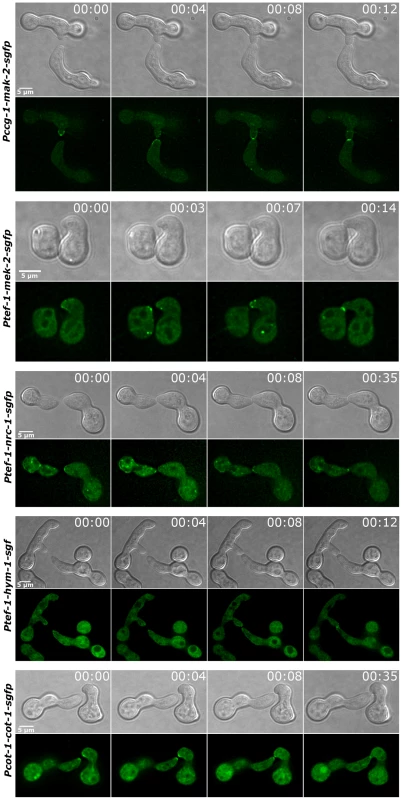
Dynamic localization of MAK2-GFP, MEK2-GFP and NRC1-GFP at opposing tips of interacting germlings. HYM1-GFP is recruited to the contact zone of germling fusion pairs. COT1-GFP associated with both tips during tropic growth of communicating germlings. Fig. 7. MAK2-GFP and MEK2-mCherry co-localize at the tips of interacting germlings. 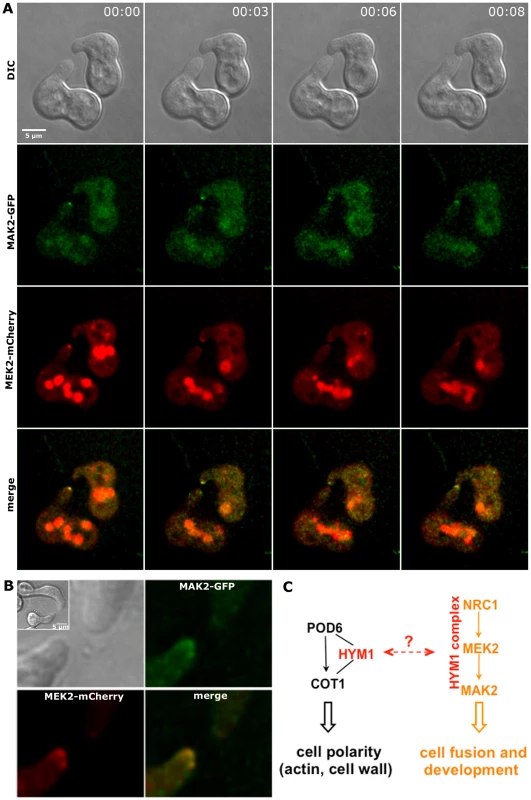
(A) MAK2-GFP and MEK2-mCherry recruitment to the germling tips oscillated synchronously during tropic growth. (B) MAK2-GFP and MEK2-mCherry co-localized in complexes at the tips of interacting germlings. Images were taken at one time point. DIC Inlay shows the entire germlings. (C) Model for the potential interactions of the COT1 and MAK2 signal pathways through the common scaffold HYM1; see text for details. NRC1-GFP and HYM1-GFP could barely be detected in germlings carrying constructs controlled by the native or the ccg-1 promoters. Expression of a functional nrc-1-gfp construct under control of the tef-1 promoter [50] resulted in a weak, but detectable GFP signal in young germlings (note that in germlings Pccg-1 is less active than Ptef-1). In rare cases, we observed oscillatory recruitment of the NRC1-GFP fusion protein to fusion tips, suggesting a similar subcellular dynamics of all three kinases of the MAK2 pathway (Figure 6, Figure S5). Expression of a ptef-1-hym1-gfp construct resulted in detectable GFP signals, but plasma membrane recruitment and dynamics of HYM1-GFP in tropic growing cell tips could not be unambiguously determined. However, after germlings had established physical contact, HYM1 and NRC1 both clearly accumulated at the future fusion site, similar to MAK2 and MEK2, indicating that the three kinases as well as HYM1 share their highly dynamic subcellular localization during the process of germling fusion. In contrast, the NDR kinase COT1 stably localized to both tips of interacting germ tubes, which likely represents its general function in hyphal polarity establishment and maintenance (Figure 6).
Discussion
Establishing cell polarity and maintaining cellular asymmetry are essential properties that govern morphogenesis and the development of uni - and multicellular organisms. These processes require the sensing of subtle intra - or extracellular signals and the transduction of this information to various cellular outputs via intricate signaling networks. An emerging theme is that signaling cascades form molecular assemblies within cells. Their spatial organization is ensured by scaffolding proteins, which allow the compartmentalization of signaling pathways.
The findings reported in this study allow three significant conclusions. First, we show that COT1, POD6 and HYM1 physically interact, and the interaction of both kinases is dependent on HYM1. Moreover, HYM1 regulates the activity of COT1. Thus, we propose that HYM1 functions as scaffold of the COT1 NDR kinase pathway in N. crassa to bridge the two kinases of the MOR pathway. Intriguingly, and in contrast to the situation in unicellular fungi, Δhym-1 mutants do not develop the polarity defects characteristic for MOR pathway mutants in N. crassa. One possible interpretation of these data may be that the presence of HYM1 in the COT1 pathway is an evolutionary relic and not physiologically relevant. However, we consider this hypothesis unlikely. We base this conclusion on the findings that the deletion of hym-1 resulted in COT1 activity that is reduced to a similar extent as described in Δpod-6 cells and that POD6-dependent Thr589 phosphorylation of COT1 is required for full activation of COT1 [39]. Moreover, Thr589 phosphorylation of COT1 is required for the proper localization of the NDR kinase [39]. The fact that the localization of COT1 in Δhym-1 is normal may explain a phenotype that does not fully resemble Δpod-6. Intriguingly, we found that also mutants in the uncharacterized N. crassa components SOG2/NCU03360 and TAO3/NCU09740 do not display the typical hyperbranching defects characteristic for the central MOR elements cot-1, pod-6 and mob-2a/b, but deletion strains have almost wild type characteristics (unpublished data). Thus, we speculate that overlapping functions between the two scaffolds HYM1 and TAO3 (and potentially with SOG2 - a fungal-specific protein with unknown function) may blur more drastic defects of deleting in hym-1 on COT1 signaling. This hypothesis is consistent with the fact that HYM1 is a functional component of the MOR pathway in all ascomycete and basidiomycete fungi analyzed to date, [26], [27], [43], [44], and it would be surprising, if N. crassa HYM1 would represent an exception.
Second, the co-precipitation of HYM1 with NRC1, MEK2 and MAK2, and the expression of constitutive-active NRC1 and MEK2 versions and of a MEK2-NRC1 fusion protein clearly demonstrate that HYM1 is critical for the organization of the MAK2 pathway. We also show that HYM1 co-localizes with MAK2 at the hyphal apex and with all three MAK2 pathway kinases at septa and the contact point of communicating fusion cells. However, our yeast two-hybrid data also suggest that HYM1 does not physically interact with any of the three kinases of the MAK2 cascade. Because animal MO25 functions as master regulator for multiple STE20-like kinases [32], we speculate that additional kinase(s) of this group may bridge HYM1 with the three kinases of the MAK2 module as part of a functional HYM1-kinase complex that organizes the MAK2 cascade. This hypothesis is supported by the physical interaction of HYM1 and NRC1 with the N. crassa PAK kinase STE20, but not the related PAK kinase CLA4 in our yeast two-hybrid experiments. Interestingly, Δste-20 is fusion competent and has only mild growth defects (data not shown), indicating that additional components must be part of the signal receiving machinery of the self-fusion pathway. Furthermore, our data indicate that self-signaling during germling fusion involves specific requirements for the regulation of the MAK2 module in addition to a general function of MAK2 in hyphal morphogenesis. Constitutive hyperactivation of NRC1 and MEK2 rescues the vegetative growth defects of MAK2 pathway mutants, but not their fusion defects. Together with our observation on the subcellular localization of MAK2 and its upstream kinases, this indicates that self-signaling, but not continuous hyphal growth requires a regulated on-off switch of the MAP kinase module.
Third, the individual components of the MAK2 kinase cascade display distinct localization behaviors. During self-communication and germling fusion, MEK2 and MAK2 show similar oscillating localization to the plasma membrane of interacting tips, but aggregates containing only one of the two kinases were frequently detected. The signal of the upstream kinase NRC1 was very weak and often close to the detection limit. Nevertheless, it appears to underlay a similar subcellular dynamics. This indicates that membrane-bound signaling complexes do not necessarily contain equimolar amounts of the three kinases required for self-communication, as also described for the homologous kinases of the yeast mating pathway [51]. Interestingly, MAK2 localizes in the cytosol and the nucleus, while MEK2 and NRC1 are exclusively cytosolic proteins. This suggests that only the MAP kinase shuttles between the two compartments. Moreover, MAK2 accumulates in the nucleus in Δhym-1 cells. This may indicate that HYM1 promotes cytosolic sequestration of MAK2. Alternatively, MAK2 in Δhym-1 cells is inactive and may therefore accumulate in the nucleus. During vegetative growth of mature hyphae MAK2 accumulates in the Spitzenkörper of the hyphal apex, while MEK2 and NRC1 are not enriched at the cell tips of hyphae. Nevertheless, all three kinases localize to the forming septum and around the mature septal pore. We have no explanation for the septum association of the MAK2 cascade, but an increasing array of signaling machinery is found to be associated with the septal pore in various filamentous fungi. Thus, this and other studies identify the septal pore as a potential signaling hub within the fungal cell (summarized in [42], [52]). The different localization behaviors of the three MAP kinase components are also reflected by different protein abundances of the three kinases. MAK2 is expressed at ca. 10-fold higher level than MEK2 and NRC1 when the three proteins are expressed from their native promoters. The expression level of HYM1 is comparable to those of NRC1 and MEK2. The relative protein abundance might thus reflect the hierarchical order within the kinase cascade.
In summary, we conclude that HYM1 functions as scaffold of the COT1 NDR kinase complex and as essential regulator of the MAK2 MAP kinase cascade. We have previously identified a complex and interdependent genetic relationships between cot-1 and mak-2 mutants [12]. Thus, we propose that this dual use of a common regulator in the two pathways may promote the coordination of intercellular communication, tropic tip growth and cell polarity. Further analysis is required to thoroughly test this intriguing hypothesis and to unravel this signaling network, which is essential for efficient fungal growth and adaptation.
Materials and Methods
Strains, media, and growth conditions
Strains used in this study are listed in Table S1 (see also [53]). General genetic procedures and media used in the handling of N. crassa are available through the Fungal Genetic Stock Center (www.fgsc.net).
Two-hybrid plasmids and methods
The Matchmaker Two-Hybrid system 3 (Clontech, USA) was used according to the manufacturer's instructions. cDNA of genes of interest for two hybrid tests was amplified with primers listed in Table S2 spanning the coding region from start to stop codon as annotated by the N. crassa database (http://www.broadinstitute.org/annotation/genome/neurospora/MultiHome.html) and cloned either into the pGADT7 vector containing the GAL4 activation domain or into pGBKT7 containing the DNA-binding domain. Fusion proteins were (co-) expressed in S. cerevisiae AH109 and potential interactions determined by growth tests on SD medium lacking the amino acids adenine, histidine, leucine and tryptophane.
Plasmid construction and fungal expression of tagged proteins
Modification of the endogenous cot-1 and hym-1 loci to allow expression of C-terminal GFP-tagged fusion proteins was achieved by PCR-amplification of the ORFs of both genes and 1 kb fragments of their 3′UTRs using genomic DNA with primers that incorporated XhoI and SacI/BamHI sites, respectively, at the ends. The fragments were cloned into the vector pGFP::hph::loxP [54] and transformed in Δmus-52::barR;his-3 to ensure homologous recombination with the endogenous cot-1 and hym-1 loci. Transformants were backcrossed with wild type to remove the Δmus-52::barR mutation and the correct genotype was confirmed by Southern analysis.
To obtain strains for the subcellular localization of MEK2 and NRC1, plasmids containing either gfp- or mCherry-fusion constructs were built based on the described plasmid pMF272 [55]. The ORFs of mek-2 and nrc-1 were amplified by PCR as annotated by the N. crassa database (http://www.broadinstitute.org/annotation/genome/neurospora/MultiHome.html) using primers listed in Table S2 and introduced via XbaI/PacI or BamHI/PacI, respectively, into pMF272. Constructs containing mek-2 and nrc-1 with the native promoters were obtained by using a forward primer binding 1 kb upstream of the ORF and the reverse primers described for the ccg-1 constructs, as listed in Table S2, and integrated into pMF272 using NotI and PacI, thereby replacing the ccg-1 promoter. Plasmids containing the tef-1 promoter were constructed by amplifying the 0.9 kb promoter region from the described plasmid pAB621 [50] using primers listed in Table S2 containing a T to A replacement at position 38, thereby destroying a present XbaI restriction site. The fragment was inserted into pMF272 using NotI and XbaI, replacing the ccg-1-promoter. mCherry constructs were obtained by replacing sgfp by mCherry, using the restriction sites PacI and EcoRI. The final plasmids were transformed into his-3 and his-3;Δmek-2 or his-3;Δnrc-1, respectively, and the transformants were selected on media lacking histidine. For co-localization experiments plasmids were additionally transformed into his-3;nic-3 and his-3;trp-1 and selected on media supplemented with nicotinic acid or tryptophane.
To allow expression of myc/flag/HA-tagged fusion proteins from the his-3 locus, the candidate ORFs were amplified with primers listed in Table S2 spanning the coding region from start to stop codon as annotated by the N. crassa database and cloned into the vectors pFLAGN1, pHAN1 or pCCG1-3×MYC [54], [56] to allow expression of N-terminally tagged flag-NRC1, flag-MEK2, HA-MAK2 and myc-HYM1. Constitutive-active versions of flag-NRC1 and flag-MEK2 were generated by site-directed mutagenesis according to the manufacturer's instructions (Stratagene, USA). For generation of the flag-MEK2-NRC1 fusion construct, the ORF of mek-2 was amplified by PCR and introduced via BamHI into vector pFLAGN1-NRC1. The final plasmids were transformed into his-3, nic-3;his-3 or trp-1;his-3, respectively, and were selected for complementation of the his-3 auxotrophy. Immunoprecipitation was performed with cell extracts from fused, heterokaryotic strains that were selected by their ability to grow on minimal media lacking supplements [37]. All fusion constructs were also expressed at the his-3 locus of the respective deletion mutant and tested for full complementation of the deletion mutant defects.
Microscopy
Low magnification documentation of fungal hyphae or colonies was performed as described [36] using an SZX16 stereomicroscope, equipped with a Colorview III camera and CellD imaging software (Olympus). Images were further processed using Photoshop CS2 (Adobe). Fluorescence microscopy was performed as described [57], [58]. An inverted Axio Observer. Z1 (Zeiss) microscope equipped with a QuantEM 512SC camera (Photometrics) and the slidebook 5.0 software (Intelligent Imaging Innovations), or an Zeiss Axiophot 2 equipped with a pco pixelfly camera and a modified version of 4D microscopy software [59], programmed by Christian Hennig and Ralf Schnabel, were used for image acquisition. Images were deconvolved using SVI Huygens Essential software and further processed using ImageJ.
Protein methods
Liquid N. crassa cultures were grown at room temperature, harvested gently by filtration using a Büchner funnel and ground in liquid nitrogen. Immunoprecipitation and COT1 kinase activity assays were performed as described [37], [39]. Monoclonal mouse α-HA (clone HA-7, Sigma Aldrich, Germany), monoclonal mouse α-FLAG M2 antibody (Sigma-Aldrich, Germany), monoclonal mouse α-myc (9E10, Santa Cruz, USA), monoclonal mouse α-GFP (Invitrogen GmbH, Germany) antibodies and GFP-Trap beads (ChromoTek, Germany) were used in this study.
Protein extraction for the analysis of the MAK2 phosphorylation status was performed as described in [47] with minor modifications. Briefly, the frozen mycelial powder was incubated in 95% ethanol at -20°C for ≥12 h, the supernatant removed after centrifugation and the pellet vacuum-dried in a SpeedVac concentrator (Thermo Fisher Scientific, USA). Extraction buffer (100 mM Tris pH7.0, 1% (w/v) SDS; supplemented with 5 mM NaF, 1 mM PMSF, 1 mM Na3VO4, 25 mM β-glycerophosphate, 2 mM benzamidine, 2 ng/µl pepstatin A, 10 ng/µl aprotinin, 10 ng/µl leupeptin) was added, the samples mixed and incubated at 80°C for 5 min and the supernatant collected after centrifugation. After a second round of extraction, the supernatants pooled, subjected to another centrifugation step, and the protein concentration determined using a Nanodrop spectrophotometer (ND-1000, Peqlab, Germany). Sample volumes corresponding to 75 µg total protein per lane were subjected to SDS polyacrylamide gel electrophoresis and subsequent Western blotting using polyclonal rabbit α-Phospho-p42/44 MAPK (Cell Signaling Technology, Inc., USA) and goat α-rabbit IgG-HRP (Santa Cruz, USA) as primary and secondary antibodies, respectively. For quantification of MAK1 and MAK2 phosphorylation levels, exposed films were scanned at a resolution of 600 dpi and densitometry was performed on the resulting tiff-files employing the AIDA Image Analyzer (version 4.22; raytest Isotopenmessgeräte, Germany) in transmission mode. Intensity values [arbitrary units] measured within a region of interest of fixed size containing the MAK1 or MAK2 protein bands were corrected by subtraction of local background, normalized to the protein amount loaded and used for further evaluation.
Supporting Information
Zdroje
1. DardN, PeterM (2006) Scaffold proteins in MAP kinase signaling: more than simple passive activating platforms. Bioessays 28 : 146–156.
2. SaitoH (2010) Regulation of cross-talk in yeast MAPK signaling pathways. Curr Opin Microbiol 13 : 677–683.
3. MartinH, FlandezM, NombelaC, MolinaM (2005) Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol Microbiol 58 : 6–16.
4. KolchW (2005) Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6 : 827–837.
5. BudayL, TompaP (2010) Functional classification of scaffold proteins and related molecules. FEBS J 277 : 4348–4355.
6. GoudreaultM, D'AmbrosioLM, KeanMJ, MullinMJ, LarsenBG, et al. (2009) A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol Cell Proteomics 8 : 157–171.
7. BloemendalS, BernhardsY, BarthoK, DettmannA, VoigtO, et al. (2012) A homologue of the human STRIPAK complex controls sexual development in fungi. Mol Microbiol 84 : 310–323.
8. BardwellL (2005) A walk-through of the yeast mating pheromone response pathway. Peptides 26 : 339–350.
9. DohlmanHG, SlessarevaJE (2006) Pheromone signaling pathways in yeast. Sci STKE 2006: cm6.
10. PandeyA, RocaMG, ReadND, GlassNL (2004) Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa. Eukaryot Cell 3 : 348–358.
11. LiD, BobrowiczP, WilkinsonHH, EbboleDJ (2005) A mitogen-activated protein kinase pathway essential for mating and contributing to vegetative growth in Neurospora crassa. Genetics 170 : 1091–1104.
12. MaerzS, ZivC, VogtN, HelmstaedtK, CohenN, et al. (2008) The nuclear Dbf2-related kinase COT1 and the mitogen-activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa. Genetics 179 : 1313–1325.
13. FleissnerA, LeederAC, RocaMG, ReadND, GlassNL (2009) Oscillatory recruitment of signaling proteins to cell tips promotes coordinated behavior during cell fusion. Proc Natl Acad Sci U S A 106 : 19387–19392.
14. LiL, WrightSJ, KrystofovaS, ParkG, BorkovichKA (2007) Heterotrimeric G protein signaling in filamentous fungi. Annu Rev Microbiol 61 : 423–452.
15. RispailN, SoanesDM, AntC, CzajkowskiR, GrunlerA, et al. (2009) Comparative genomics of MAP kinase and calcium-calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet Biol 46 : 287–298.
16. FleissnerA, SimoninAR, GlassNL (2008) Cell fusion in the filamentous fungus, Neurospora crassa. Methods Mol Biol 475 : 21–38.
17. ReadND, LichiusA, ShojiJY, GoryachevAB (2009) Self-signalling and self-fusion in filamentous fungi. Curr Opin Microbiol 12 : 608–615.
18. GoryachevAB, LichiusA, WrightGD, ReadND (2012) Excitable behavior can explain the “ping-pong” mode of communication between cells using the same chemoattractant. Bioessays 34 : 259–266.
19. HergovichA, CornilsH, HemmingsBA (2008) Mammalian NDR protein kinases: from regulation to a role in centrosome duplication. Biochim Biophys Acta 1784 : 3–15.
20. MaerzS, SeilerS (2010) Tales of RAM and MOR: NDR kinase signaling in fungal morphogenesis. Curr Opin Microbiol 13 : 663–671.
21. EmotoK, ParrishJZ, JanLY, JanYN (2006) The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature 443 : 210–213.
22. ChibaS, IkedaM, KatsunumaK, OhashiK, MizunoK (2009) MST2 - and Furry-mediated activation of NDR1 kinase is critical for precise alignment of mitotic chromosomes. Curr Biol 19 : 675–681.
23. ZallenJA, PeckolEL, TobinDM, BargmannCI (2000) Neuronal cell shape and neurite initiation are regulated by the Ndr kinase SAX-1, a member of the Orb6/COT-1/warts serine/threonine kinase family. Mol Biol Cell 11 : 3177–3190.
24. CongJ, GengW, HeB, LiuJ, CharltonJ, et al. (2001) The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development 128 : 2793–2802.
25. DuLL, NovickP (2002) Pag1p, a novel protein associated with protein kinase Cbk1p, is required for cell morphogenesis and proliferation in Saccharomyces cerevisiae. Mol Biol Cell 13 : 503–514.
26. HirataD, KishimotoN, SudaM, SogabeY, NakagawaS, et al. (2002) Fission yeast Mor2/Cps12, a protein similar to Drosophila Furry, is essential for cell morphogenesis and its mutation induces Wee1-dependent G(2) delay. EMBO J 21 : 4863–4874.
27. NelsonB, KurischkoC, HoreckaJ, ModyM, NairP, et al. (2003) RAM: a conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol Biol Cell 14 : 3782–3803.
28. KanaiM, KumeK, MiyaharaK, SakaiK, NakamuraK, et al. (2005) Fission yeast MO25 protein is localized at SPB and septum and is essential for cell morphogenesis. EMBO J 24 : 3012–3025.
29. RayS, KumeK, GuptaS, GeW, BalasubramanianM, et al. (2010) The mitosis-to-interphase transition is coordinated by cross talk between the SIN and MOR pathways in Schizosaccharomyces pombe. J Cell Biol 190 : 793–805.
30. AlessiDR, SakamotoK, BayascasJR (2006) LKB1-dependent signaling pathways. Annu Rev Biochem 75 : 137–163.
31. HeY, EmotoK, FangX, RenN, TianX, et al. (2005) Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol Biol Cell 16 : 4139–4152.
32. FilippiBM, de los HerosP, MehellouY, NavratilovaI, GourlayR, et al. (2011) MO25 is a master regulator of SPAK/OSR1 and MST3/MST4/YSK1 protein kinases. EMBO J 30 : 1730–1741.
33. YardenO, PlamannM, EbboleDJ, YanofskyC (1992) cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J 11 : 2159–2166.
34. SeilerS, PlamannM (2003) The genetic basis of cellular morphogenesis in the filamentous fungus Neurospora crassa. Mol Biol Cell 14 : 4352–4364.
35. SeilerS, VogtN, ZivC, GorovitsR, YardenO (2006) The STE20/germinal center kinase POD6 interacts with the NDR kinase COT1 and is involved in polar tip extension in Neurospora crassa. Mol Biol Cell 17 : 4080–4092.
36. VogtN, SeilerS (2008) The RHO1-specific GTPase-activating protein LRG1 regulates polar tip growth in parallel to Ndr kinase signaling in Neurospora. Mol Biol Cell 19 : 4554–4569.
37. MaerzS, DettmannA, ZivC, LiuY, ValeriusO, et al. (2009) Two NDR kinase-MOB complexes function as distinct modules during septum formation and tip extension in Neurospora crassa. Mol Microbiol 74 : 707–723.
38. ZivC, Kra-OzG, GorovitsR, MarzS, SeilerS, et al. (2009) Cell elongation and branching are regulated by differential phosphorylation states of the nuclear Dbf2-related kinase COT1 in Neurospora crassa. Mol Microbiol 74 : 974–989.
39. MaerzS, DettmannA, SeilerS (2012) Hydrophobic Motif Phosphorylation Coordinates Activity and Polar Localization of the Neurospora crassa Nuclear Dbf2-Related Kinase COT1. Mol Cell Biol 32 : 2083–2098.
40. HarrisSD, ReadND, RobersonRW, ShawB, SeilerS, et al. (2005) Polarisome meets spitzenkorper: microscopy, genetics, and genomics converge. Eukaryot Cell 4 : 225–229.
41. ViragA, HarrisSD (2006) The Spitzenkorper: a molecular perspective. Mycol Res 110 : 4–13.
42. RiquelmeM, YardenO, Bartnicki-GarciaS, BowmanB, Castro-LongoriaE, et al. (2011) Architecture and development of the Neurospora crassa hypha - a model cell for polarized growth. Fungal Biol 115 : 446–474.
43. SongY, CheonSA, LeeKE, LeeSY, LeeBK, et al. (2008) Role of the RAM network in cell polarity and hyphal morphogenesis in Candida albicans. Mol Biol Cell 19 : 5456–5477.
44. SartorelE, and, Perez-MartinJ (2012) The distinct wiring between cell cycle regulation and the widely conserved Morphogenesis-Related (MOR) pathway in the fungus Ustilago maydis determines the morphological outcome. J Cell Sci doi:10.1242/jcs.107862.
45. KotheGO, FreeSJ (1998) The isolation and characterization of nrc-1 and nrc-2, two genes encoding protein kinases that control growth and development in Neurospora crassa. Genetics 149 : 117–130.
46. MaddiA, DettmannA, FuC, SeilerS, FreeSJ (2012) WSC-1 and HAM-7 Are MAK-1 MAP Kinase Pathway Sensors Required for Cell Wall Integrity and Hyphal Fusion in Neurospora crassa.. PLoS ONE 7: e42374 doi:10.1371/journal.pone.0042374.
47. RichthammerC, EnseleitM, Sanchez-LeonE, HeiligY, RiquelmeM, et al. (2012) The Neurospora crassa RHO1 and RHO2 GTPase modules share partially overlapping functions in the regulation of cell wall integrity and hyphal polarity. Mol Microbiol 85 : 716–733.
48. StevensonBJ, RhodesN, ErredeB, SpragueGFJr (1992) Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of the G protein. Genes Dev 6 : 1293–1304.
49. MuellerP, WeinzierlG, BrachmannA, FeldbruggeM, KahmannR (2003) Mating and pathogenic development of the Smut fungus Ustilago maydis are regulated by one mitogen-activated protein kinase cascade. Eukaryot Cell 2 : 1187–1199.
50. BerepikiA, LichiusA, ShojiJY, TilsnerJ, ReadND (2010) F-actin dynamics in Neurospora crassa. Eukaryot Cell 9 : 547–557.
51. MaederCI, HinkMA, KinkhabwalaA, MayrR, BastiaensPI, et al. (2007) Spatial regulation of Fus3 MAP kinase activity through a reaction-diffusion mechanism in yeast pheromone signalling. Nat Cell Biol 9 : 1319–1326.
52. SeilerS, Justa-SchuchD (2010) Conserved components, but distinct mechanisms for the placement and assembly of the cell division machinery in unicellular and filamentous ascomycetes. Mol Microbiol 78 : 1058–1076.
53. McCluskeyK (2003) The Fungal Genetics Stock Center: from molds to molecules. Adv Appl Microbiol 52 : 245–262.
54. HondaS, SelkerEU (2009) Tools for fungal proteomics: multifunctional neurospora vectors for gene replacement, protein expression and protein purification. Genetics 182 : 11–23.
55. FreitagM, HickeyPC, RajuNB, SelkerEU, ReadND (2004) GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet Biol 41 : 897–910.
56. KawabataT, InoueH (2007) Detection of physical interactions by immunoprecipitation of FLAG - and HA-tagged proteins expressed at the his-3 locus in Neurospora crassa. Fungal Genetics Newsletter 54 : 5–8.
57. Justa-SchuchD, HeiligY, RichthammerC, SeilerS (2010) Septum formation is regulated by the RHO4-specific exchange factors BUD3 and RGF3 and by the landmark protein BUD4 in Neurospora crassa. Mol Microbiol 76 : 220–235.
58. Araujo-PalomaresCL, RichthammerC, SeilerS, Castro-LongoriaE (2011) Functional characterization and cellular dynamics of the CDC-42 - RAC - CDC-24 module in Neurospora crassa. PLoS ONE 6: e27148 doi: 10.1371/journal.pone.0027148.
59. SchnabelR, HutterH, MoermanD, SchnabelH (1997) Assessing normal embryogenesis in Caenorhabditis elegans using a 4D microscope: variability of development and regional specification. Dev Biol 184 : 234–265.
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 9
-
Všechny články tohoto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



