-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIs a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
Metastasis is a complex process utilizing both tumor-cell-autonomous properties and host-derived factors, including cellular immunity. We have previously shown that germline polymorphisms can modify tumor cell metastatic capabilities through cell-autonomous mechanisms. However, how metastasis susceptibility genes interact with the tumor stroma is incompletely understood. Here, we employ a complex genetic screen to identify Cadm1 as a novel modifier of metastasis. We demonstrate that Cadm1 can specifically suppress metastasis without affecting primary tumor growth. Unexpectedly, Cadm1 did not alter tumor-cell-autonomous properties such as proliferation or invasion, but required the host's adaptive immune system to affect metastasis. The metastasis-suppressing effect of Cadm1 was lost in mice lacking T cell–mediated immunity, which was partially phenocopied by depleting CD8+ T cells in immune-competent mice. Our data show a novel function for Cadm1 in suppressing metastasis by sensitizing tumor cells to immune surveillance mechanisms, and this is the first report of a heritable metastasis susceptibility gene engaging tumor non-autonomous factors.
Published in the journal: . PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1002926
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002926Summary
Metastasis is a complex process utilizing both tumor-cell-autonomous properties and host-derived factors, including cellular immunity. We have previously shown that germline polymorphisms can modify tumor cell metastatic capabilities through cell-autonomous mechanisms. However, how metastasis susceptibility genes interact with the tumor stroma is incompletely understood. Here, we employ a complex genetic screen to identify Cadm1 as a novel modifier of metastasis. We demonstrate that Cadm1 can specifically suppress metastasis without affecting primary tumor growth. Unexpectedly, Cadm1 did not alter tumor-cell-autonomous properties such as proliferation or invasion, but required the host's adaptive immune system to affect metastasis. The metastasis-suppressing effect of Cadm1 was lost in mice lacking T cell–mediated immunity, which was partially phenocopied by depleting CD8+ T cells in immune-competent mice. Our data show a novel function for Cadm1 in suppressing metastasis by sensitizing tumor cells to immune surveillance mechanisms, and this is the first report of a heritable metastasis susceptibility gene engaging tumor non-autonomous factors.
Introduction
Metastatic disease remains a major problem for the clinical treatment of many different malignancies. Metastases can appear years after treatment of the primary tumor and is frequently refractory to therapy [1]. It has been estimated that approximately 90% of cancer-related deaths are directly attributable to the development of metastatic disease, rather than the primary tumor [2]. In order for a tumor cell to form a clinically-relevant metastatic lesion, it must undergo a highly complex process termed the invasion-metastasis cascade, which includes escaping from the primary tumor, entering the circulation, evading the immune system, seeding the secondary organ, and adapting to growth in this foreign environment [3]. Evidence suggests that the invasion-metastasis cascade is driven by a complex interplay of tumor cell-autonomous properties and host derived factors [3]. There is also accumulating evidence that germline polymorphism modifies tumor cell metastatic capability, indicating that heritable genetic variability can predetermine a tumor cell's propensity to metastasize [4]–[6]. In this study, we employ a complex genetics screen that exploits the differential heritable metastatic susceptibility observed among strains of inbred mice to identify tumor-autonomous expression of Cadm1 as a germline modifier of metastatic susceptibility. We demonstrate that over-expression of Cadm1 by as little as 1.5-fold can specifically suppress metastasis without any resultant difference in primary tumor growth.
In addition to tumor-autonomous cellular phenotypes, metastatic efficiency is also impacted by tumor non-autonomous, host-derived factors including the immune system [3]. However, mechanisms by which tumor cells interact with the immune system remain poorly understood. Here, we show that the metastasis suppressive effects of Cadm1 are lost in mice lacking functional T cell–mediated immunity, an effect which is partially phenocopied by the depletion of CD8+ T cells in immune-competent mice, suggesting that Cadm1 sensitizes tumor cells to immune-surveillance mechanisms by CD8+ T cells. Since differences in expression of Cadm1 are inherited in mice, our data links the contribution of the genetic background in determining metastatic risk to the adaptive immune system, suggesting that individuals with higher levels of Cadm1 expression may be more resistant to metastasis.
Results
Complex genetic screen identifies Cadm1 as a candidate metastasis susceptibility gene
Previous work from our laboratory demonstrated that the progeny of FVB-MMTV-PyMT, a mouse model of metastatic breast cancer, and NZB/B1NJ or C58/J mice have significantly reduced pulmonary metastasis relative to the parental FVB-MMTV-PyMT [7]. Preliminary genetic mapping in an NZBxFVB backcross (Figure 1A) suggested the presence of a metastasis susceptibility gene on chromosome 9 [8] which was subsequently validated by the development of a chromosomal substitution strain [9]. Reproducible association of a metastasis susceptibility locus on proximal chromosome 9 was obtained by analysis of a 226 animal C58 x FVB backcross. Linkage analysis of the C58 backcross replicated the association with metastasis susceptibility for the proximal half of mouse chromosome 9 (Figure 1B and Figure S1). Despite the reduction of metastasis in the C58xPyMT or the NZBxPyMT F1 progeny, inheritance of either the C58 or NZB proximal chromosome 9 was associated with increased metastasis in the backcrosses. These data are consistent with the possibility that NZB and C58 harbor a common metastasis promoting allele whose presence was unmasked by segregation of unlinked compensatory metastasis-resistance genes in the backcross populations.
Fig. 1. A genetic screen in mice identifies Cadm1 as a candidate germline modifier of metastasis. 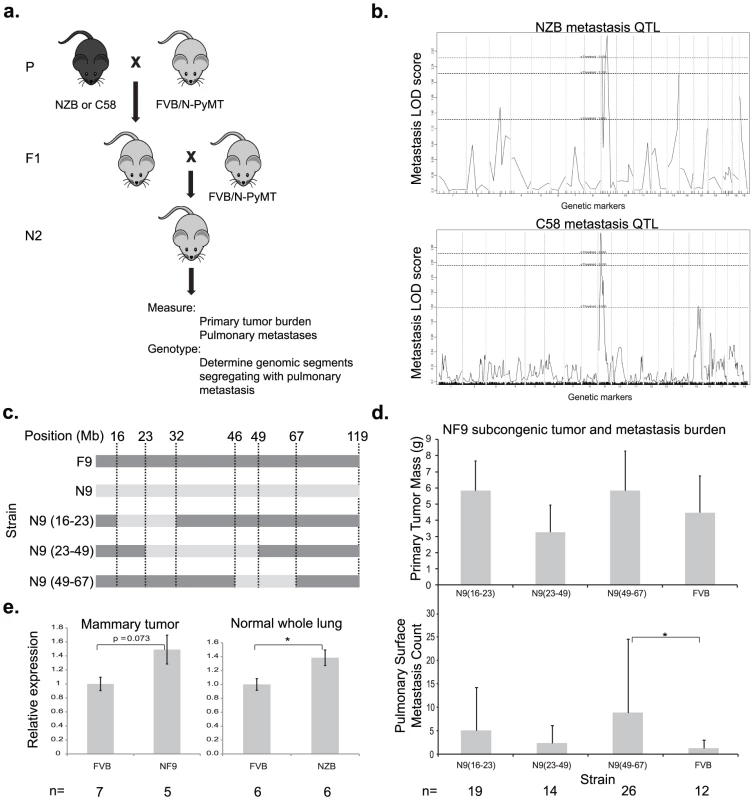
(a) NZB and C58 mice were crossed to FVB mice then re-crossed with MMTV-PyMT-transgenic FVB mice. (b) Primary tumor, pulmonary metastasis, and genotype data was obtained from transgene-positive mice developed tumors and was used to generate QTL maps for pulmonary metastasis. Subcongenic lines containing fragments of NZB chromosome 9 (c) were crossed to MMTV-PyMT transgenic FVB mice and tumor size and pulmonary surface metastases were measured in the progeny (d). No significant change in tumor mass was observed, however a significant increase in metastasis was observed in the N9(49–60) subcongenic line (p = 0.022), resolving the locus of susceptibility was resolved to 49–60 Mb of chromosome 9. (e) Real time quantitative PCR measurement of cDNA from mammary tumor and lung tissue demonstrated a 1.5 fold higher expression of Cadm1 in NZB and NF9 relative to FVB. To further resolve the segment of chromosome 9 carrying this susceptibility locus, three congenic strains were developed that contained overlapping segments of NZB chromosome 9, spanning from 16–67 megabases (Figure 1C). Each congenic was bred to the PyMT transgenic animal and the effect of the NZB chromosomal segments on metastatic progression was assessed. No significant difference was observed for primary tumor burden. Only the congenic strain containing NZB chromosome 9 from 46–67 megabases was found to significantly increase metastatic susceptibility relative to FVB (p = 0.022, Figure 1D), consistent with the original quantitative trait locus (QTL) analysis. These results indicate that the gene(s) of interest resided within 46 Mb and 67 Mb of chromosome 9 and therefore subsequent analysis focused on this region.
To filter the gene list in the chromosome 9 metastasis modifier interval for potential candidate genes, haplotype analysis was performed. Based on the hypothesis that NZB and C58 shared a common metastasis susceptibility gene, screening for genes with identical haplotypes in NZB and C58 that were distinct from the FVB haplotype identified 80 potential candidate genes. Since the majority of polymorphisms occur in non-coding sequence it is hypothesized that the majority of quantitative traits are due to subtle changes in gene expression rather than non-synonymous amino acid substitutions. Based on this assumption the candidate gene list was further filtered by RNA expression microarray analysis. Tumors from PyMT-FVB or NZB chromosome 9 substitution (NF9) strains were subjected to microarray analysis to identify differentially expressed transcripts. Three genes within the candidate interval, Cadm1, Zbtb16 and Pias1, had the appropriate haplotype structure and were differentially expressed between the two genotypes. Of the three Cadm1 had the greatest magnitude of differential expression on microarray. Since aberrations in Cadm1 expression have previously been associated with poor prognosis in numerous malignancies [10]–[14], Cadm1 was selected for further characterization. Zbtb16 and Pias1 are under investigation in separate studies.
Exon sequencing of the Cadm1 protein-coding exons was performed to confirm the predicted haplotype differences between NZB and FVB and to screen for undescribed polymorphisms. Sequence analysis confirmed that NZB and FVB have distinct haplotypes at the Cadm1 locus and validated one synonymous polymorphism in exon 2 (rs32721609, Figure S2). Since no non-synonymous polymorphisms were observed, subsequent efforts were focused on the possible effect of expression levels on the metastatic phenotype.
To validate microarray data that Cadm1 was differentially expressed in FVB and NZB, quantitative real time PCR (qRT-PCR) was conducted on reverse transcribed RNA extracted from PyMT-FVB and NF9 tumors. The results showed a trend toward increased Cadm1 expression in NF9 tumors relative to FVB that was of borderline significance (p = 0.073, Figure 1E). To rule out the possibility that differential expression might have resulted from somatic alterations, expression was assessed in normal lung tissue as a representative epithelial sample. qRT-PCR in normal lung tissue from high-metastatic FVB and low-metastatic (NZB x FVB)F1 transgene-negative females showed a statistically significant increase in Cadm1 expression in NZB F1 mice of about 1.5-fold (Figure 1E). These results, in combination with linkage and haplotype data, were consistent with the hypothesis that Cadm1 expression is likely to be a germline modifier of metastasis.
Cadm1 expression inversely impacts metastasis
Cadm1, also known as Tslc1, Necl2, Ra175, IgSF4a and SynCAM is an immunoglobulin superfamily adhesion molecule reported to be involved in homotypic and heterotypic cell-cell interactions [15]. It has also been identified as a tumor suppressor in lung adenocarcinoma [16]. To test whether variation in Cadm1 expression had an impact on tumor growth and metastasis, two independent mouse mammary tumor cell lines stably expressing Cadm1 were generated. Mvt-1 and 6DT1 cells [17] were transduced with Pol2-driven empty vector or Cadm1 expression vector lentivirus particles and selected with blasticidin. Stable expression of exogenous Cadm1 was confirmed by Western Blot and qRT-PCR (Figure 2A and 2D) and quantification revealed that 6DT1 cells over-expressed Cadm1 by approximately 1.5 fold, similar to the difference observed in NF9 tumors and lung relative to FVB (Figure 1E and Figure 2D). One hundred thousand cells stably expressing Cadm1 or empty vector were then injected into the fourth mammary fat pad of syngeneic mice. Thirty days post-injection mice were euthanized and primary tumors were resected and weighed to assess the effect of Cadm1 on primary tumor growth. A statistically significant reduction in primary tumor growth was observed for the Mvt-1 cell line (Figure 2B). In contrast, no significant difference in primary tumor growth was observed 6DT1 cells.
Fig. 2. The effect of Cadm1 over-expression on tumor growth and metastasis in vivo. 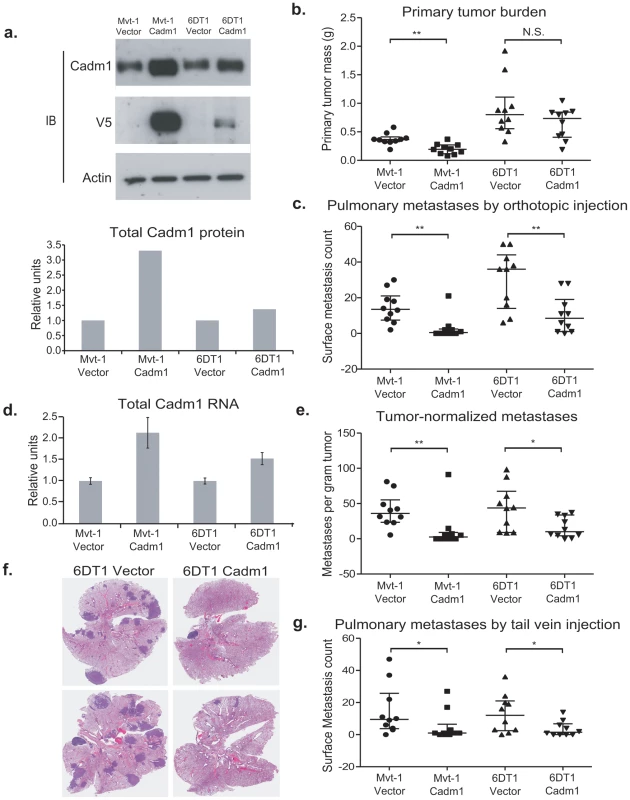
(a) Expression of endogenous and exogenous V5-epitope tagged Cadm1 protein (IB, immunoblot) and total protein by ECL densitometry on immunoblot. (d) Total RNA levels of Cadm1 measured by qRT-PCR (mean +/− standard error of the mean [SEM]). Mvt-1 and 6DT1 mouse mammary tumor cells stably expressing Cadm1 were implanted into mammary fat pads of syngeneic FVB mice. Data on primary tumor burden (Mvt-1: p = 0.0031; 6DT1: p = 0.2261) (b), pulmonary surface metastases (c) was collected on day 30 (Mvt-1: p = 0.0026; 6DT1: p = 0.0089; median +/− interquartile range; n = 10 mice per group). (e) Pulmonary surface metastases were normalized by primary tumor mass to determine if Cadm1 overexpression showed an effect on metastasis independent of its potential tumor suppressive activity (Mvt-1: p = 0.0048; 6DT1: p = 0.0185; median +/− interquartile range; n = 10 mice per group). Arrows indicate several but not all visible metastatic nodules present on lung surfaces. (f) Images of whole lungs of two mice implanted with 6DT1 control cells and two mice implanted with 6DT1 cells expressing Cadm1. (g) Results of tail vein injection study (Mvt-1: p = 0.0361; 6DT1: p = 0.0475; median +/− interquartile range; n = 10 mice per group). (N.S., not significant; * defined as p<0.05; ** defined as p<0.01.) The effect of ectopic Cadm1 expression on metastasis was then assessed by pulmonary surface metastasis counts. To account for the reduction of primary tumor growth for the Mvt-1/Cadm1 cell line, metastasis counts were also normalized by primary tumor burden to allow better comparisons between cell lines. As shown in Figure 2C, 2E, and 2F, Cadm1 expression significantly reduced the metastatic capability of both Mvt-1 and 6DT1 relative to controls, consistent with a metastasis susceptibility role for Cadm1. The metastatic nodule size was measured on lung sections of orthotopically injected mice and did not differ significantly between control and Cadm1 expressing cells (Figure S3). Significant reductions in pulmonary metastasis were also observed by intravenous injection of tumor cells into the tail-vein, a model that assays tumor cell colonization of the lung (Figure 2G), suggesting that the effect of Cadm1 on metastasis operates downstream of local invasion and intravasation.
Expression of Cadm1 in 6DT1 cells suppressed metastasis without significantly impacting primary tumor growth, suggesting that, Cadm1 might be functioning purely as a metastasis suppressor in this cell line. To test whether Cadm1 had metastasis-specific effect on metastatic progression, knockdown of the endogenous gene in 6DT1 cells was performed as Cadm1 expression in 6DT1 cells reproducibly has no effect on primary tumor growth. Due to the potentially confounding effect on the primary tumor the Mvt-1 cell line was excluded from this analysis. 6DT1 cells were transduced with two independent shRNA lentivirus constructs targeting Cadm1 and were selected with puromycin, achieving 44% and 81% reduction of total Cadm1 protein, respectively (Figure 3A and 3D). These cells were subsequently injected orthotopically into immune-competent syngeneic mice for in vivo analysis. Cells expressing shRNA-14 showed a statistically significant reduction in primary tumor mass while those expressing shRNA-15 showed no change in primary tumor mass (Figure 3B). The tumor suppression observed with shRNA-14 is likely to be an off-target effect since it was only observed with one of two shRNAs. Although only shRNA-14 significantly promoted metastasis (Figure 3C), both constructs showed significant increases in pulmonary metastasis after normalization for primary tumor burden (Figure 3E). Since increases in metastases were observed for both constructs Cadm1 is likely to have a metastasis-specific function in this model system.
Fig. 3. The effect of Cadm1 knockdown on tumor growth and metastasis in vivo. 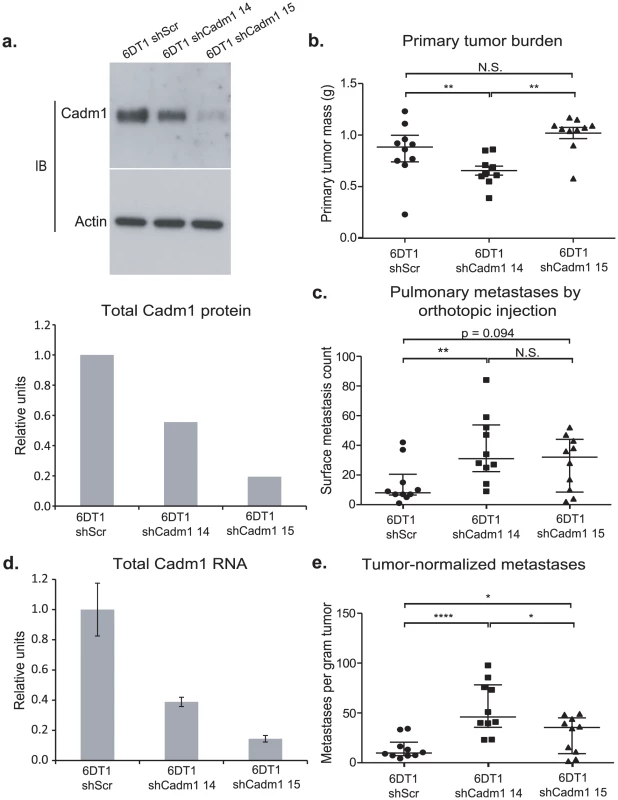
6DT1 cells expressing shRNA constructs targeting Cadm1 showed (a) protein level knockdown of Cadm1 by immunoblot and ECL densitometry quantification and (d) total RNA level knockdown (mean +/− SEM). Orthotopic implant data showing the effects of Cadm1 knockdown on primary tumor burden (shScr vs. sh14: p = 0.0090; shScr vs. sh15: p = 0.0943; sh14 vs. sh15: p = 0.0001) (b), pulmonary surface metastases (shScr vs. sh14: p = 0.0085; shScr vs. sh15: p = 0.0709; sh14 vs. sh15: p = 0.3485) (c), and tumor-normalized metastases (e) ((shScr vs. sh14: p = 0.00005; shScr vs. sh15: p = 0.0444; sh14 vs. sh15: p = 0.0152; median +/− interquartile range; n = 10 mice per group). Cadm1 transgene expression is attenuated in metastatic nodules
To assess if the reduction in metastasis observed in Cadm1 over-expressing Mvt-1 cells was due to a tumor suppressor or metastasis-specific effect, immunohistochemistry was performed. Matched tumor and lung specimens were sectioned and staining was conducted against the V5 epitope to determine if pulmonary metastases from Cadm1 positive tumors retained expression of V5-tagged Cadm1. As shown in Figure 4A–4F, the primary tumors (Figure 4A–4B) stained positive for the V5 epitope, however, pulmonary metastases (Figure 4C–4D) from matched tumors did not stain for V5, indicating that these metastases had lost expression of the Cadm1 transgene.
Fig. 4. Pulmonary metastases of Mvt-1/Cadm1 cells express attenuated levels of Cadm1. 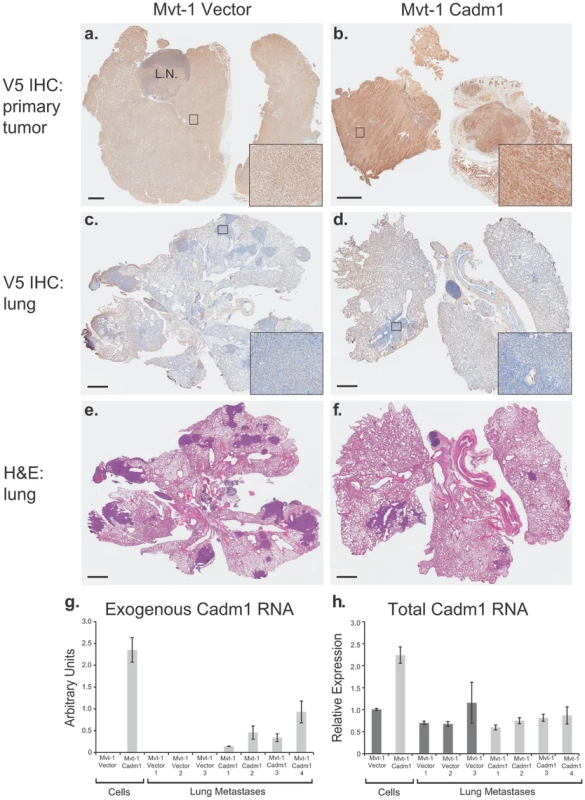
Matched primary tumor and lung sections were immunohistochemically (IHC) stained with antibody against the V5 epitope. Immunohistochemical staining of the primary tumors produced by control (a) and Cadm1-V5 (b) expressing cells. IHC stained lung sections of mice implanted with control (c) and Cadm1-V5 (d). Hematoxylin and eosin (H&E) stained lung sections of mice implanted with control (e) and Cadm1-V5 (f) expressing cells. Insets show 20× magnification of boxed area (L.N., lymph node; black bar indicates 1 um). Level of (g) exogenous and (h) total Cadm1 mRNA from grossly dissected pulmonary metastatic nodules of mice injected with Mvt-1/Vector versus Mvt-1/Cadm1 relative to the original cell lines prior to injection. Further, gross dissection with subsequent qRT-PCR of metastatic nodules from the lungs of mice injected intravenously with Mvt-1/Cadm1 cells revealed that exogenous Cadm1 mRNA expression was attenuated in all four mice relative to the levels expressed in those cells upon injection (Figure 4G) and total Cadm1 expression was not significantly different between Mvt-1/Vector and Mvt-1/Cadm1 bearing mice (Figure 4H). Since these cells were injected directly into the venous circulation and likely seeded the lungs immediately after injection, they had little time to lose Cadm1 expression suggesting that tumor cells with lower overall Cadm1 expression upon injection were selected for colonization. Together, this data suggests that low Cadm1 expression may be required for successful lung colonization by Mvt-1 cells, consistent with a specific metastasis-suppressing role in this cell line.
Cadm1 expression does not affect cell-autonomous properties in vitro
Previous studies demonstrated that expression of Cadm1 could affect in vitro phenotypes associated with metastatic potential [18]. To examine the mechanism by which Cadm1 influences metastasis in breast cancer, proliferation, migration and invasion assays were performed. Ectopic expression of Cadm1 had no significant impact on proliferation rates in either 2-D or 3-D culture, and no difference in trans-well migration or invasion (Figures S4, S5 and S6). Though Cadm1-expressing 6DT1 cells demonstrated a slight, marginally significant reduction in motility relative to control cells by scratch wound assay, Mvt-1 cells showed no difference in motility (Figure S7). These data would suggest that the effects of Cadm1 are likely distinct from events leading to the initial escape from the primary tumor, which is supported by the observed suppression of metastasis upon tail vein injection (Figure 2G).
Cadm1 interacts with the cell-mediated immunity
Cadm1 has been shown to engage in heterotypic interactions with class-I restricted T cell adhesion molecule (Crtam), a marker of activated CD8+ T cells and NK-cells [19]. This interaction has been shown to induce interferon-γ production by CD8+ T cells, enhance CD8+ T cell and NK-cell cytotoxicity in vitro, and tumor rejection in vivo [20], [21]. In order to test the role of cell-mediated immunity in Cadm1-mediated metastasis suppression, control and Cadm1-expressing cells were orthotopically implanted into athymic nude mice. As illustrated in Figure 5, the metastatic phenotype of Cadm1-expressing cells was rescued in immunocompromised mice. In addition, the tumor suppressive effect of Cadm1 in Mvt-1 cells was also lost. This suggests that T cell mediated immunity may be critical for the tumor and metastasis suppressive effects of Cadm1.
Fig. 5. The effect of Cadm1 expression on tumor and metastasis in athymic mice. 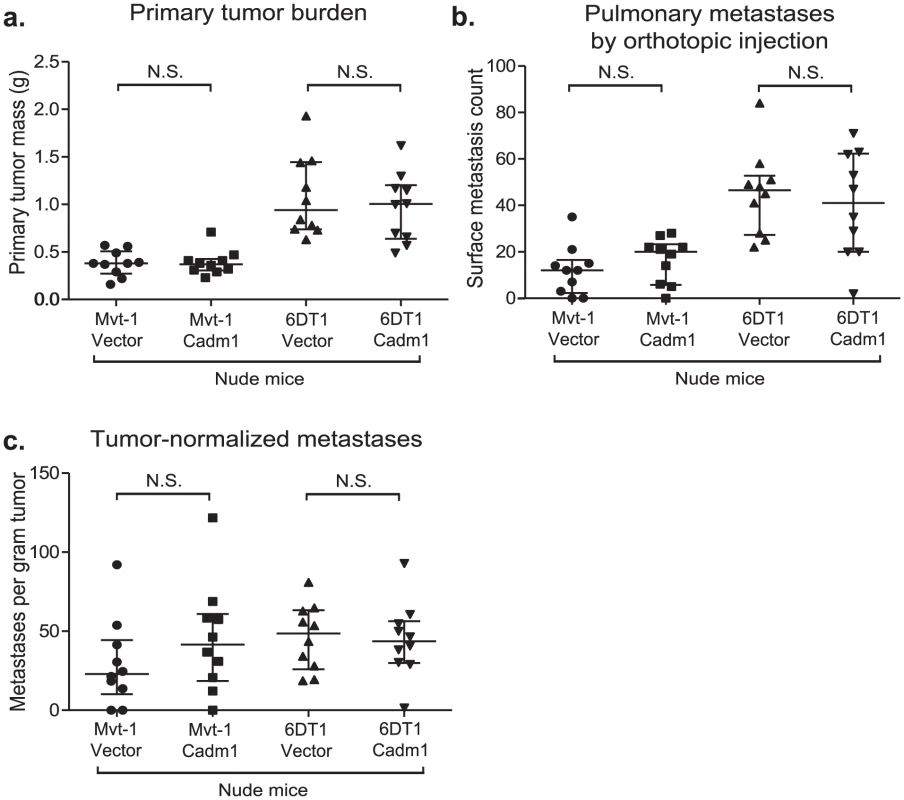
Orthotopic injections of control and Cadm1-expressing cells into athymic nude mice resulted in no significant difference in (a) primary tumor burden, (b) pulmonary metastasis, or (c) tumor-normalized metastasis. (median +/− interquartile range; n = 10 mice per group). It has been reported that Crtam is present on activated natural killer (NK) and CD8+ T cells. Since athymic mice possess functional NK cells, we hypothesized that the loss of Cadm1-mediated metastasis suppression might be mediated by CD8+ T cells. Thus we orthotopically injected Mvt-1 cells expressing Cadm1 in immune-competent mice in which CD8+ lymphocytes were depleted with anti-CD8 antibody (Figure 6A and Figure S8). Unexpectedly, Cadm1-expressing Mvt-1 did not suppress tumor growth in this experiment as previously observed (Figure 2C). However, subsequent protein-level expression analysis of the cells used in this experiment revealed that the level of Cadm1 over-expression was lower than in previous studies (Figure S9) and similar to the levels observed in the 6DT1 cell line, suggesting that higher Cadm1 expression is required to suppress primary tumor growth than to achieve metastasis suppression.
Fig. 6. The effect of Cadm1 expression on tumor and metastasis in CD8+ T cell depleted mice. 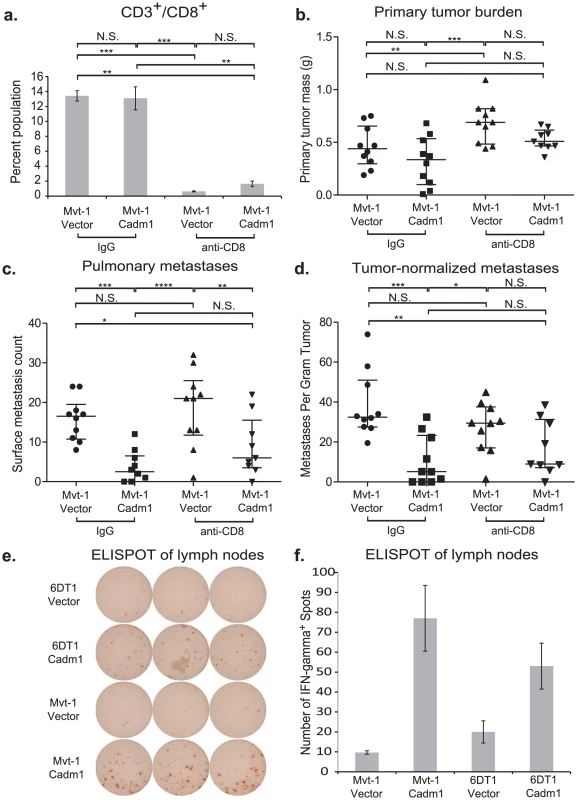
Orthotopic injections of control and Cadm1-expressing Mvt-1 cells into immune-competent FVB mice that were treated with IgG or anti-CD8 resulted in (a) >90% depletion of CD8+ T cells. (b) No significant differences were observed in tumor burden between control and Cadm1 expressing within the same antibody treatment group, though a significant increase was observed in control Mvt-1 cells upon anti-CD8 treatment. (c) Total pulmonary metastases showed significant suppression in Mvt-1/Cadm1 in both control and anti-CD8 treatment groups. (d) Metastases normalized by matched primary tumor mass showed highly significant Cadm1-mediated metastasis suppression in the control IgG treatment group but was not significant in the anti-CD8 group (median +/− interquartile range; n = 10 mice per group). ELISPOT plate (e) and quantitative representation (f) of interferon-γ secreting T cells (mean +/− SEM). (N.S., not significant; * defined as p<0.05; ** defined as p<0.01; *** defined as p<0.001, **** defined as p<0.0001). Although pulmonary metastases were significantly reduced in both control IgG treated and CD8+ lymphocyte-depleted mice, this effect was lost specifically in CD8+ lymphocyte-depleted mice when metastases were normalized by matched primary tumor mass (Figure 6B, 6C, and 6D). This suggests that CD8+ lymphocytes contribute to Cadm1-mediated metastasis and implicates the role of cell-mediated immunity in metastasis suppression by Cadm1.
The engagement of Cadm1 on tumor cells and Crtam on CD8+ T cells has been reported to induce interferon-γ production of CD8+ T cells [20], [21]. We hypothesized that enhanced interferon-γ may mediate an inflammatory response to induce tumor cytotoxicity. To test whether interferon-γ secretion was enhanced in mice bearing Cadm1-expressing tumors relative to those bearing control tumors, we performed an ELISPOT assay. Cells derived from the draining lymph nodes contralateral to the tumor were used because the tumors frequently enveloped the ipsilateral lymph nodes. We observed a marked increase in interferon-γ secretion in lymphocytes derived from the lymph nodes of Cadm1+ tumor bearing mice (Figure 6E and 6F), indicating that an interferon-γ-mediated immune response local to the primary tumor may be involved in Cadm1-mediated metastasis suppression.
Cadm1 expression predicts outcome in breast cancer patients
The down-regulation of Cadm1 in invasive breast cancer, primarily by promoter hypermethylation, has been reported in at least four studies [22]–[25]. This loss or reduction in Cadm1 expression is associated with increased tumor grade, stage, and local invasiveness [22]–[25]. To determine whether levels of Cadm1 expression in breast tumors correlated with survival in patients, we searched three publicly available datasets from Oncomine, two of which have associated publications [26], [27] and the Gene expression-Based Outcome for Breast cancer Online (GOBO, http://co.bmc.lu.se/gobo/), a web-based meta-analysis tool containing microarray-based tumor expression data on 1881 patients from 11 public datasets [28].
With the exception of the Boersma set, high Cadm1 expression consistently correlated with significantly improved survival in estrogen receptor (ER) positive tumor datasets, (Figure 7A, 7B, 7C, 7E, and Table S1). In contrast, high Cadm1 levels only significantly correlated with improved survival in ER negative patients in the Boersma dataset (Figure 7D and 7F), suggesting that any effect Cadm1 levels may have on tumor progression are primarily relevant to ER positive tumors. Since metastasis is the primary determinant of overall survival in cancer, this suggests that high tumor cell Cadm1 expression may also be protective against metastasis in human breast cancer.
Fig. 7. The effect of Cadm1 expression levels on survival in patient datasets. 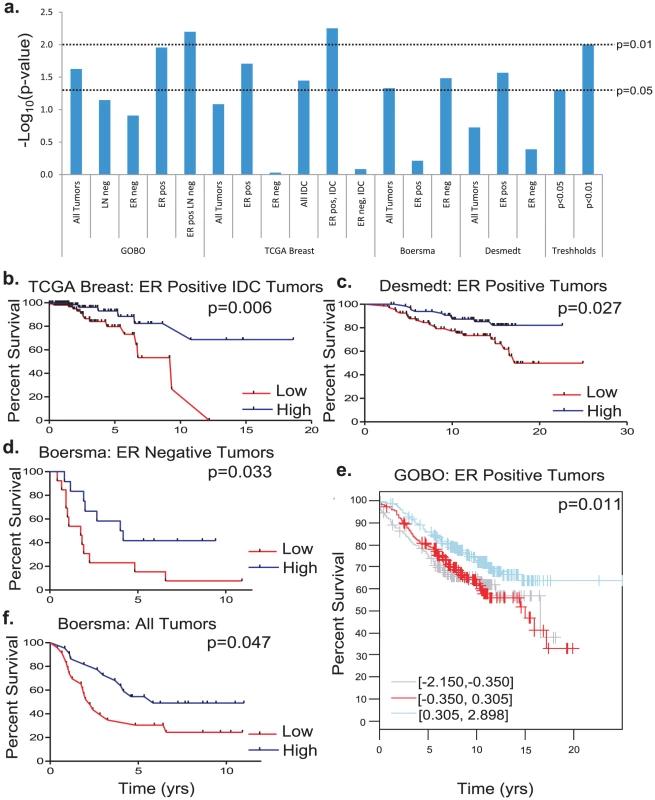
(a) Compiled results of -Log10 transformed p-values for all survival analyses. Kaplan-Meier plots of (b) estrogen receptor (ER) positive invasive ductal carcinoma (IDC) tumors from the Oncomine TCGA set, (c) ER positive tumors from the Desmedt set, (d) ER negative tumors from the Boersma set, (e) ER positive tumors from GOBO, (f) all tumors from the Boersma set. Discussion
Numerous studies have implicated down-regulation of Cadm1 with poor patient prognosis [10], [12]–[14], [29], [30]; however, to the best of our knowledge no studies have investigated the mechanism responsible. Here we demonstrate that expression of Cadm1 in two independent cell lines resulted in either specific metastasis suppression or tumor suppression accompanied by a reduction in metastasis. As metastasis is the primary cause of cancer-induced mortality, these findings imply a direct role for Cadm1 in metastatic progression in breast cancer and provide a link between diminished Cadm1 expression and reduced progression-free survival.
Two key observations suggest a direct role for Cadm1 in metastasis suppression. First, in the Mvt-1 cell line, Cadm1 ectopic expression was lost in all metastases, suggesting that down-regulation of Cadm1 may be a prerequisite for completion of the metastatic cascade. Second, manipulation of Cadm1 in 6DT1 cells specifically affected the ability to form pulmonary metastases, without any significant effect on primary tumor growth. Thus, in the 6DT1 cell line, Cadm1 appears to function as a metastasis susceptibility gene, based on variations in functional levels. Together, the results suggest that Cadm1 may have both tumor suppressive and metastasis suppressive activities which may depend on the level of Cadm1 expressed.
The identification of Cadm1 as an inherited metastasis suppressor gene was not predicted by the initial linkage analysis. Genetic analysis of both backcross populations and the congenic mouse strains indicated that inheritance of the C58 or NZB chromosome 9 candidate region was associated with an increase in pulmonary metastasis. In contrast, the up-regulation seen for both the C58 and NZB Cadm1 alleles was associated with a decrease in pulmonary metastasis in the orthotopic transplant studies.
A likely explanation for this discrepancy lies with the complexity of the genome. Every individual carries numerous susceptibility and resistance factors and the final phenotype is dictated by the balance of these genes. The pro-metastatic chromosome 9 interval that was investigated in this study contains more than one gene that fit the criteria for a metastasis susceptibility gene. In addition, the strategy followed here does not capture candidates based on non-synonymous amino acid substitutions. We speculate the paradox between the identification of Cadm1 as a metastasis-suppressing gene in a metastasis-promoting genomic region is explained by the presence of one or more metastasis-promoting genes within the interval. When inherited together the metastasis-promoting factors dominate, masking the effect of Cadm1.
Finally, our results potentially implicate Cadm1 in cancer immunoediting, a process by which the adaptive immune system protects the host from developing cancer and, in turn, alters tumor progression by driving the outgrowth of tumor cells with decreased sensitivity to immune attack [31], [32]. We have shown that Cadm1 may regulate metastasis by sensitizing tumor cells to surveillance by the immune system. Our findings show the metastatic potential of Cadm1-expressing cells was fully rescued in mice deficient of T cell mediated immunity, indicating that Cadm1-mediated metastasis suppression specifically involves the adaptive cell mediated immunity rather than the innate immunity, as the innate immune system is intact in athymic mice. However, we cannot rule out the possibility that thymus-dependent, Cadm1-sensitive immune cells are indirectly associated with metastasis suppression by recruiting innate immune cells, including NK-cells, to eliminate tumor cells. Since the effect of CD8+ T cells depletion did not result in a full rescue, we hypothesize that NKT cells, which also express the Cadm1 receptor, Crtam, [33] and are absent in athymic mice [34] may also contribute to tumor cell cytotoxicity and clearance.
Further, the partial rescue of the metastatic phenotype by depletion of CD8+ T cells suggests that Cadm1 may promote the detection of tumor cells by an immune surveillance mechanism which potentially results in the CD8+ T cell–mediated cytotoxicity and clearance of Cadm1 expressing cells from circulation and secondary sites. While we have not directly shown that Cadm1 expression resulted in tumor cell death by T cells, this notion is supported by previous studies [20], [21] showing Cadm1 expression on target cells enhances T cell cytotoxicity in an MHC-dependent manner. Thus loss or reduction in Cadm1 expression may contribute to the final step of cancer immunoediting: tumor cell “escape” from detection by the immune system [31]. Reports that the Crtam-Cadm1 interaction induces interferon-γ secretion [20], [21] combined with our observation that lymph nodes draining Cadm1+ tumors contain lymphocytes that secrete higher levels of interferon-γ than controls suggest that the interaction with the immune system may be mediated by engaging Crtam on cytotoxic lymphocytes. Further, the role of Cadm1 as a tumor-autonomous component of immune surveillance is consistent with our observations that 1) knockdown of endogenous Cadm1 potentiates metastatic capability, and 2) pulmonary metastases have lost expression of exogenous Cadm1. Together with the numerous [10]–[14] reports in the literature that Cadm1 expression is lost in more invasive tumors, our data implicates Cadm1 loss as an important step in cancer immunoediting that enhances the immune evasive and metastatic properties of tumor cells.
Materials and Methods
Mouse genetics (FVBxNZB backcross, generating and genotyping subcongenic mice, QTL analysis)
The NZB backcross has been described previously [8]. NZB chromosome 9 subcongenics were generated by breeding the NF9 chromosomal substitution line [9] to FVB/NJ, backcrossing the F1 progeny to FVB/NJ and screening the N2 backcross progeny for retention of NZB chromosomal segments. Chromosomal segments of interest were made homozygous by breeding to FVB/NJ followed by brother-sister mating of animals containing the segment of interest. Genotyping was performed by using a combination of microsatellite and SNP genotyping.
The C58 backcross was generated by crossing PyMT male animals with C58/J females to generate PyMT-positive F1 males, which were subsequently bred to FVB/NJ females to generate N2 backcross animals. Tumor phenotyping was performed as previously described [35]. Genotyping was performed using the Illumina Mouse Medium Density Linkage array at the Center for Inherited Disease Research (CIDR) using spleen DNA isolated by the Wizard DNA purification kit. Quantitative trait mapping (QTL) was performed using J/QTL [36].
Exon sequencing of Cadm1 from genomic DNA
Exons were amplified from genomic DNA then run on a 2% agarose gel. Amplified bands were purified by the Qiagen Gel Extraction Kit and cloned into TOPO-TA vectors (Invitrogen) and transformed into One-shot Top10 chemically competent E. Coli (Invitrogen) as per manufacture's protocol. Plasmid was purified using the Mini Prep kit (Qiagen). Sequencing reactions were conducted on ∼350 ng of purified plasmid using Big Dye Terminator reaction mix (Applied Biosystems) following the manufacturers protocol and sequenced by the NCI Sequencing Facility.
Cell lines and culture conditions
All experiments were conducted using FVB/NJ background mouse mammary tumor explants cell lines 6DT1 and Mvt-1 kindly provided by Dr. Lalage Wakefield [17]. Cell lines were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Gibco) supplemented with L-Glutamate (Gibco), 9% fetal bovine serum (FBS) (Gemini BioProducts), and 1% Penicillin and Streptomycin (P/S) (Gemini BioProducts).
Oligonucleotides and plasmids
pDonr253 is a Gateway Donor vector modified from pDonr201 (Invitrogen). pDonr253 replaces the kanamycin resistance gene with a gene encoding spectinomycin resistance, and contains several sequencing primer sites to aid in sequence verification of Entry clones. The following oligonucleotides (Eurofins MWG Operon) were used in this study: L10379 : 5′-GGGGACAACTTTGTACAAAAAAGTTGGCACCATGGCGAGTGCTGTGCTGCCGAGCGGATC
L10383 : 5′-GGGGACAACTTTGTACAAGAAAGTTGAGATGAAGTACTCTTTCTTTTCTTC
The sequences of short hairpin RNA pLKO.1 vectors for Cadm1 knockdown were
shRNA14: CCGGCGGACTGGTTTGTAAAGGAAACTCGAGTTTCCTTTACAAACCAGTCCGTTTTTG
shRNA15: CCGGCCTGTTCATCAATAACCTAAACTCGAGTTTAGGTTATTGATGAACAGGTTTTTG
Cloning of Cadm1
Murine Cadm1 was cloned into a Gateway Entry clone by PCR from a cDNA template (BC095986, RefSeq NM_018770.3). The Entry clone contains the complete ORF preceded by a Kozak translation initiation sequence and an ATG start codon, and lacking a stop codon at the 3′ end to allow C-terminal fusions to be generated. DNA was amplified using specific primers L10379 and 10383 containing the Gateway recombination sequences, and PCR was carried out using Pfusion polymerase (New England Biolabs). The final PCR product contains the gene of interest flanked on the 5′ side with a Gateway attB1 site and on the 3′ side with a Gateway attB2 site. The PCR product was cleaned using the QiaQuick PCR purification kit (Qiagen), and recombined into pDonr253 using the Gateway BP recombination reaction (Invitrogen) by the manufacturer's protocols. The subsequent Entry clone was sequence verified throughout the entire cloned region.
Subcloning of Cadm1 and control lentivectors
A lentiviral vector expressing a C-terminal V5 epitope-tagged fusion of Cadm1 was generated using Multisite Gateway recombination. An entry clone using the murine Pol2 promoter was recombined with the Cadm1 entry clone and a C-terminal entry clone encoding the V5 epitope tag (GKPIPNPLLGLDST) into a Gateway destination vector pDest-659. This vector is a modified version of the pFUGW lentiviral vector which contains the enhanced polypurine tract (PPT) and woodchuck regulatory element (WRE) to provide higher titer virus. In addition, it contains an antibiotic resistance gene for blasticidin resistance. Entry clones were subcloned by Gateway Multisite LR recombination using the manufacturer's protocols (Invitrogen). Expression clones were transformed into E. coli STBL3 cells to minimize unwanted LTR repeat recombination, and verified by agarose gel electrophoresis and restriction digest. Transfection-ready DNA for the final clones was prepared using the GenElute XP Maxiprep kit (Sigma). A control vector (8166-M24-658) was generated by standard Gateway LR recombination of a stuffer fragment made up of a non-coding DNA into the pLenti6-V5-DEST vector (Invitrogen). All lentivector constructs and lentivirus particles were generated by the Protein Expression Laboratory and the Viral Technology Group in NCI, Frederick.
Viral transduction
Two milliliter suspensions of 5×104 cells were incubated at 37°C in 5% CO2 overnight. Cells were then infected with 50 uL of concentrated lentivirus suspension, and selected 30 hours post-infection with 5 mg/mL blasticidin for over-expression (Invitrogen) constructs or 10 ug/mL (shRNA) puromycin for shRNA constructs.
Immunoblot and antibodies
Protein was extracted by cell lysis in 400 uL of Pierce lysis buffer, vigorous homogenization, and incubation on ice for one hour. Twenty micrograms of protein extract per sample in NuPage LDS Sample Buffer and NuPage Reducing Agent (Invitrogen) were used for western blotting. PVDF membrane (Millipore) containing transferred proteins was incubated overnight with the primary antibodies: mouse anti-V5 (Invitrogen), chicken IgY anti-SynCAM/TSLC1/Cadm1 (MBL International), or mouse anti-β-actin (Abcam). The membrane was then incubated with horse-radish peroxidase linked anti-mouse (GE Healthcare) or anti-chicken IgY secondary antibodies (Abcam). Immunoblot was visualized using Amersham ECL Prime Western Blotting Detection System and Amersham Hyperfilm ECL (GE Healthcare). Densitometry data were obtained and analyzed with a ChemiDoc-It Imaging System and VisionWorksLS software (UVP).
RNA isolation, reverse transcription, and real-time polymerase chain reaction (qRT-PCR)
RNA was isolated from tumors and cell lines using RNeasy kit (Qiagen) and reverse transcribed using iScript (Bio-Rad). Real-Time PCR was conducted using QuantiTect SYBR Green PCR kit (Qiagen). See Table S2 for primer sequences.
Scratch wound assay
One-milliliter suspensions of 6×105 cells were seeded in triplicate into each well of 24-well Essen ImageLock (Essen Bioscience) plates in selective media and incubated at 37°C and 5% CO2 overnight. The following day, the cells washed twice with PBS and incubated at 37°C and 5% CO2 for 3 hours in 10 ug/mL mitomycin C. Scratch wounds were made using Essen 4-channel scratch instrument loaded with Eppendorf 10 uL micropipette tips. Cells were subsequently washed three times with PBS, placed in selective media and placed in Incucyte (Essen Bioscience). Incucyte was programmed to image each well at 2-hour intervals. Data analysis was conducted using Incucyte 2011A software.
Proliferation assay
One-milliliter suspensions of 5×103 cells were seeded in six replicates into each well of 24-well cell culture plates (Corning, Inc.) in selective media and placed in Incucyte. Incucyte was programmed to image each well at 3-hour intervals. Data analysis was conducted using Incucyte 2011A software.
Transwell migration and invasion assays
Matrigel inserts were hydrated for 2 hours in 500 uL serum-free DMEM then suspensions of 7.5×104 cells were seeded in triplicate into each well of the 24-well plate format BD BioCoat Control and Matrigel Invasion Chambers (BD Biosciences) and incubated for 20 hours at 37°C in 5% CO2. Enriched media was used as chemoattractant. Membranes were subsequently fixed with methanol, stained with crystal violet, and cells present on the underside of the membrane were counted.
Three-dimensional culture
Cultrex 3-D culture matrix (Trevigen, Inc.) was injected into 8-chamber slides and allowed to solidify at 37°C for 30 minutes. Cells were trypsinized and 5,000 counted then resuspended in 400 uL DMEM, 9%FBS, 5 ug/mLBlasticidin, and 2% Cultrex and incubated at 37°C with 5% CO2 for 3 days prior to microscopy.
Mouse work: Orthotopic and tail vein injections
Female FVB/NJ mice from Jackson Laboratories were injected at 6–8 weeks of age. Two days prior to orthotopic injections, cells were placed in non-selective media. On the day of injection, 1×105 cells were injected orthotopically into the fourth mammary fat pad of age-matched virgin females. After 30 days the mice were euthanized by intraperitoneal injection of 1 mL Tribromoethanol with subsequent cervical dislocation. Primary tumors were resected, weighed, and snap frozen in liquid nitrogen. Lungs were resected, surface metastases were counted; lungs were inflated with 10% nitrate-buffered formalin and sent for sectioning and staining. For tail vein injection, 7×105 were injected into the lateral tail vein, mice were euthanized 22 days post-injection. For the CD8-antibody treatment study, orthotopic transplantation was conducted but mice were treated with 0.5 mg of either control rat IgG or rat monoclonal anti-CD8 IgG (Harlan Bioproducts, kindly provided by Dr. Lalage Wakefield) on days −4, −3, −2, +3, +10, +17, +24, and +30; cells were injected on day 0 and mice were euthanized on day 37. All procedures were performed under the Animal Safety Proposal (LCBG-004) and approved by the NCI-Bethesda Animal Care and Use Committee.
Immunohistochemistry
Immunohistochemical staining was performed on LeicaBiosystems' Bond Autostainer on paraffin embedded tissue sections with biotinylated rabbit anti-Goat IgG; primary antibody, anti-V5 rabbit polyclonal antibody (Abcam Catalog #: ab95038); LeicaBiosystems Intense R Detection Kit. All histological analysis including paraffin embedding, sectioning, hematoxylin and eosin staining and immunohistochemistry were conducted by the Pathology/Histotechnology Laboratory, Laboratory Animal Sciences Program, SAIC, Frederick.
IFN-γ ELISPOT
For IFN-γ production by T cells in either Cadm1-expressing or control tumor bearing mice: Single cell suspensions from the lymph nodes of tumor bearing mice were prepared. 1.6 million cells were loaded into each well of an IFN-γ ELISPOT plate, stimulated with anti-CD3 (0.5 ug/ml, eBioscience, San Diego, CA), and cultured overnight. The procedure was done according to recommendations from the manufacture (BD). Three to four mice for each experimental group, and triplicates for each sample were examined. The ELISPOT plate was scanned in ImmunoSpot (Cellular Technology Ltd. Shaker Heights, OH) and quantification was assessed using the CTL Scanning and CTL counting 4.0.
Statistical analysis
Statistical analysis comparing two samples were conducted using the Mann-Whitney test on Prism Version 5.03 (GraphPad Software, La Jolla, CA). Multiple-comparison data was analyzed by Kruskal-Wallis test with post-hoc Conover-Inman correction for multiple analyses by R-script. Survival data was conducted with the Mantel-Cox test on Prism.
Supporting Information
Zdroje
1. SteegPS, TheodorescuD (2008) Metastasis: a therapeutic target for cancer. Nat Clin Pract Oncol 5 : 206–219.
2. ChafferCL, WeinbergRA (2011) A perspective on cancer cell metastasis. Science 331 : 1559–1564.
3. ValastyanS, WeinbergRA (2011) Tumor metastasis: molecular insights and evolving paradigms. Cell 147 : 275–292.
4. ParkYG, ZhaoX, LesueurF, LowyDR, LancasterM, et al. (2005) Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat Genet 37 : 1055–1062.
5. CrawfordNP, AlsarrajJ, LukesL, WalkerRC, OfficewalaJS, et al. (2008) Bromodomain 4 activation predicts breast cancer survival. Proc Natl Acad Sci U S A 105 : 6380–6385.
6. CrawfordNP, QianX, ZiogasA, PapageorgeAG, BoersmaBJ, et al. (2007) Rrp1b, a new candidate susceptibility gene for breast cancer progression and metastasis. PLoS Genet 3: e214 doi:10.1371/journal.pgen.0030214.
7. HunterK (2004) Genetic, genomic, and bioinformatic tools for studying breast cancer progression. Breast Dis 19 : 83–91.
8. HunterKW, BromanKW, VoyerTL, LukesL, CozmaD, et al. (2001) Predisposition to efficient mammary tumor metastatic progression is linked to the breast cancer metastasis suppressor gene Brms1. Cancer Res 61 : 8866–8872.
9. LancasterM, RouseJ, HunterKW (2005) Modifiers of mammary tumor progression and metastasis on mouse chromosomes 7, 9, and 17. Mamm Genome 16 : 120–126.
10. ItoT, ShimadaY, HashimotoY, KaganoiJ, KanT, et al. (2003) Involvement of TSLC1 in progression of esophageal squamous cell carcinoma. Cancer Res 63 : 6320–6326.
11. KikuchiS, YamadaD, FukamiT, MaruyamaT, ItoA, et al. (2006) Hypermethylation of the TSLC1/IGSF4 promoter is associated with tobacco smoking and a poor prognosis in primary nonsmall cell lung carcinoma. Cancer 106 : 1751–1758.
12. YangG, HeW, CaiM, LuoF, KungH, et al. (2011) Loss/Down-regulation of tumor suppressor in lung cancer 1 expression is associated with tumor progression and is a biomarker of poor prognosis in ovarian carcinoma. Int J Gynecol Cancer 21 : 486–493.
13. YouY, MaL, YouM, LiX, WangS, et al. (2010) TSLC1 gene silencing in cutaneous melanoma. Melanoma Res 20 : 179–183.
14. SuraceEI, LusisE, MurakamiY, ScheithauerBW, PerryA, et al. (2004) Loss of tumor suppressor in lung cancer-1 (TSLC1) expression in meningioma correlates with increased malignancy grade and reduced patient survival. J Neuropathol Exp Neurol 63 : 1015–1027.
15. KuramochiM, FukuharaH, NobukuniT, KanbeT, MaruyamaT, et al. (2001) TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat Genet 27 : 427–430.
16. WatabeK, ItoA, KomaYI, KitamuraY (2003) IGSF4: a new intercellular adhesion molecule that is called by three names, TSLC1, SgIGSF and SynCAM, by virtue of its diverse function. Histol Histopathol 18 : 1321–1329.
17. PeiXF, NobleMS, DavoliMA, RosfjordE, TilliMT, et al. (2004) ExplanT cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In Vitro Cell Dev Biol Anim 40 : 14–21.
18. SussanTE, PletcherMT, MurakamiY, ReevesRH (2005) Tumor suppressor in lung cancer 1 (TSLC1) alters tumorigenic growth properties and gene expression. Mol Cancer 4 : 28.
19. GalibertL, DiemerGS, LiuZ, JohnsonRS, SmithJL, et al. (2005) Nectin-like protein 2 defines a subset of T cell zone dendritic cells and is a ligand for class-I-restricted T cell-associated molecule. J Biol Chem 280 : 21955–21964.
20. BolesKS, BarchetW, DiacovoT, CellaM, ColonnaM (2005) The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T cell responses through the cell-surface receptor CRTAM. Blood 106 : 779–786.
21. GiangrecoA, HosteE, TakaiY, RosewellI, WattFM (2012) Epidermal Cadm1 expression promotes autoimmune alopecia via enhanced T cell adhesion and cytotoxicity. J Immunol 188 : 1514–1522.
22. HellerG, GeradtsJ, ZieglerB, NewshamI, FilipitsM, et al. (2007) Downregulation of TSLC1 and DAL-1 expression occurs frequently in breast cancer. Breast Cancer Res Treat 103 : 283–291.
23. TakahashiY, IwaiM, KawaiT, ArakawaA, ItoT, et al. (2011) Aberrant expression of tumor suppressors CADM1 and 4.1B in invasive lesions of primary breast cancer. Breast Cancer
24. BertoloC, GuerreroD, VicenteF, CordobaA, EstellerM, et al. (2008) Differences and molecular immunohistochemical parameters in the subtypes of infiltrating ductal breast cancer. Am J Clin Pathol 130 : 414–424.
25. AllinenM, PeriL, KujalaS, Lahti-DomeniciJ, OutilaK, et al. (2002) Analysis of 11q21-24 loss of heterozygosity candidate target genes in breast cancer: indications of TSLC1 promoter hypermethylation. Genes Chromosomes Cancer 34 : 384–389.
26. BoersmaBJ, ReimersM, YiM, LudwigJA, LukeBT, et al. (2008) A stromal gene signature associated with inflammatory breast cancer. Int J Cancer 122 : 1324–1332.
27. DesmedtC, PietteF, LoiS, WangY, LallemandF, et al. (2007) Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res 13 : 3207–3214.
28. RingnerM, FredlundE, HakkinenJ, BorgA, StaafJ (2011) GOBO: gene expression-based outcome for breast cancer online. PLoS ONE 6: e17911 doi:10.1371/journal.pone.0017911.
29. OvermeerRM, HenkenFE, SnijdersPJ, Claassen-KramerD, BerkhofJ, et al. (2008) Association between dense CADM1 promoter methylation and reduced protein expression in high-grade CIN and cervical SCC. J Pathol 215 : 388–397.
30. LungHL, CheungAK, XieD, ChengY, KwongFM, et al. (2006) TSLC1 is a tumor suppressor gene associated with metastasis in nasopharyngeal carcinoma. Cancer Res 66 : 9385–9392.
31. SchreiberRD, OldLJ, SmythMJ (2011) Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331 : 1565–1570.
32. DuPageM, MazumdarC, SchmidtLM, CheungAF, JacksT (2012) Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482 : 405–409.
33. KennedyJ, VicariAP, SaylorV, ZurawskiSM, CopelandNG, et al. (2000) A molecular analysis of NKT cells: identification of a class-I restricted T cell-associated molecule (CRTAM). J Leukoc Biol 67 : 725–734.
34. ColesMC, RauletDH (2000) NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol 164 : 2412–2418.
35. LifstedT, Le VoyerT, WilliamsM, MullerW, Klein-SzantoA, et al. (1998) Identification of inbred mouse strains harboring genetic modifiers of mammary tumor age of onset and metastatic progression. Int J Cancer 77 : 640–644.
36. SmithR, SheppardK, DiPetrilloK, ChurchillG (2009) Quantitative trait locus analysis using J/qtl. Methods Mol Biol 573 : 175–188.
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 9
-
Všechny články tohoto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



