-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
Tinkering with pre-existing genes has long been known as a major way to create new genes. Recently, however, motherless protein-coding genes have been found to have emerged de novo from ancestral non-coding DNAs. How these genes originated is not well addressed to date. Here we identified 24 hominoid-specific de novo protein-coding genes with precise origination timing in vertebrate phylogeny. Strand-specific RNA–Seq analyses were performed in five rhesus macaque tissues (liver, prefrontal cortex, skeletal muscle, adipose, and testis), which were then integrated with public transcriptome data from human, chimpanzee, and rhesus macaque. On the basis of comparing the RNA expression profiles in the three species, we found that most of the hominoid-specific de novo protein-coding genes encoded polyadenylated non-coding RNAs in rhesus macaque or chimpanzee with a similar transcript structure and correlated tissue expression profile. According to the rule of parsimony, the majority of these hominoid-specific de novo protein-coding genes appear to have acquired a regulated transcript structure and expression profile before acquiring coding potential. Interestingly, although the expression profile was largely correlated, the coding genes in human often showed higher transcriptional abundance than their non-coding counterparts in rhesus macaque. The major findings we report in this manuscript are robust and insensitive to the parameters used in the identification and analysis of de novo genes. Our results suggest that at least a portion of long non-coding RNAs, especially those with active and regulated transcription, may serve as a birth pool for protein-coding genes, which are then further optimized at the transcriptional level.
Published in the journal: . PLoS Genet 8(9): e32767. doi:10.1371/journal.pgen.1002942
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002942Summary
Tinkering with pre-existing genes has long been known as a major way to create new genes. Recently, however, motherless protein-coding genes have been found to have emerged de novo from ancestral non-coding DNAs. How these genes originated is not well addressed to date. Here we identified 24 hominoid-specific de novo protein-coding genes with precise origination timing in vertebrate phylogeny. Strand-specific RNA–Seq analyses were performed in five rhesus macaque tissues (liver, prefrontal cortex, skeletal muscle, adipose, and testis), which were then integrated with public transcriptome data from human, chimpanzee, and rhesus macaque. On the basis of comparing the RNA expression profiles in the three species, we found that most of the hominoid-specific de novo protein-coding genes encoded polyadenylated non-coding RNAs in rhesus macaque or chimpanzee with a similar transcript structure and correlated tissue expression profile. According to the rule of parsimony, the majority of these hominoid-specific de novo protein-coding genes appear to have acquired a regulated transcript structure and expression profile before acquiring coding potential. Interestingly, although the expression profile was largely correlated, the coding genes in human often showed higher transcriptional abundance than their non-coding counterparts in rhesus macaque. The major findings we report in this manuscript are robust and insensitive to the parameters used in the identification and analysis of de novo genes. Our results suggest that at least a portion of long non-coding RNAs, especially those with active and regulated transcription, may serve as a birth pool for protein-coding genes, which are then further optimized at the transcriptional level.
Introduction
For decades, people believed that “mother gene”-based mechanisms such as gene duplication or its modified forms such as exon shuffling, gene fusion, gene fission, and retroposition are the major means of creating new genes [1]–[4]. All of these mechanisms modify pre-existing genes or mother genes as the building blocks for new genes. However, recently identified motherless genes or de novo genes in primates [5]–[8] and other species [9]–[12] challenged this idea, in that some protein-coding genes might have emerged de novo from ancestral non-coding DNAs, providing another explanation for the complex genetic background underlying species - or lineage-specific traits.
In spite of the discovery of dozens of de novo protein-coding genes, an issue of great complexity and general interest that remains poorly addressed is how they originated from ancestral non-coding DNAs [13]–[15]. The ancestral non-coding DNA must become transcribed and gain a translatable open reading frame (ORF) before becoming a protein-coding gene, but either order of these two steps seems possible [7]. The “transcription-first” hypothesis was initially raised in [12], [16]. Later on, functional genomics data seemed to favor this hypothesis, given the high transcriptional activity of the genome [13], [14], [17], [18]. A case-study in yeast further supported this model, showing that both coding and non-coding orthologs of BSC4 are transcribed across multiple species [10].
However, even if we assume that the ancestral non-coding locus is firstly transcribed, there are still two possibilities that need to be distinguished. First, such an event may represent some sort of transcriptional leakage or noise, and only after the locus acquires a functional ORF may the transcriptional profile become regulated and optimized. Second, alternatively, the ancestral locus may encode a functional non-coding RNA with a specific transcriptional profile and transcript structure, and after the acquisition of an ORF, the new protein-coding gene largely adopts the pre-existing transcriptional profile. If the latter possibility is true, we would expect to see that the transcriptional structure and profile of a de novo protein is correlated with that of an out-group species that has the corresponding DNA but does not encode the ORF. On the other hand, if the initial transcription is promiscuous before translation starts, we would not expect to find a strong correlation in terms of expression pattern and gene structure.
In this work, we identified 24 hominoid-specific de novo genes and performed a comparative transcriptome study in human, chimpanzee and rhesus macaque, on the basis of next-generation sequencing technology [19]. We found generally similar transcriptional structures and profiles between de novo proteins and corresponding non-coding loci in the chimpanzee or rhesus macaque, suggesting that most de novo proteins were born out of non-coding RNAs. More interestingly, although the transcription was largely correlated, the protein-coding version of de novo genes tended to show higher abundance than non-coding orthologs, hinting that the transcription of de novo genes continued to be optimized. Taken together, our work presents a “semi-product” model of origination and evolution of de novo genes.
Results
Identification and sequence features of hominoid-specific de novo protein-coding genes
We performed genome-wide identification of hominoid-specific de novo genes. On the basis of the locus age assignments, we assigned precise origination timing for the ORFs of these genes, inferred by summing up the information on the presence and absence of orthologs in vertebrate phylogeny with the rule of parsimony (Figure 1A, Materials and Methods). Sequence search in the human genome ensured that the new genes did not originate through other mechanisms such as gene duplication. Hominoid-specific de novo genes without coding potential in rhesus macaque were manually curated, using genome-wide expression filters to ensure the in-group transcriptional/translational expression, a newly-assembled transcriptome to validate the transcript structure in out-group species, and the identification of common ancestral disablers to ensure de novo origination rather than gene loss (Materials and Methods). In total, 24 hominoid-specific de novo protein-coding genes were identified: eleven encode proteins only in human (Class I) and thirteen encode proteins in both human and chimpanzee but not in rhesus macaque (Class II) (Figure 1A; Dataset S1; Tables S1, S2, S3).
Fig. 1. Genome-wide identification of hominoid-specific de novo protein-coding genes. 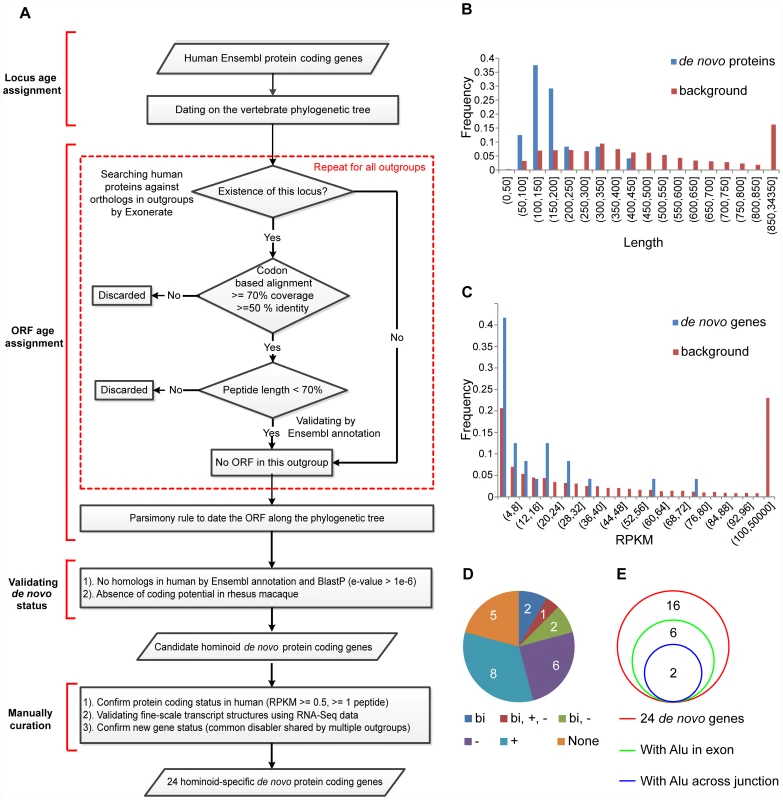
(A) On the basis of the gene locus and ORF age assignments, hominoid-specific de novo protein-coding genes were identified. Regions within dotted red lines indicate the repeating steps for each out-group species. We further filtered this list using stringent inclusion criteria and generated a smaller convincing list of 24 de novo genes. (B) Distribution of protein length for the 24 de novo genes, compared with the human genome as background. (C) Distribution of summed RPKM scores of the 24 de novo genes in seven human tissues, compared with the human genome as background. (D) Pie chart showing the distribution of the 24 de novo protein-coding genes in terms of the reuse of preexisting transcriptional context. Gene numbers in each category are marked. None: no evidence for the reuse of transcriptional context; bi: located downstream of bi-directional promoter; +: overlapping with preexisting genes on the same strand; −: overlapping with preexisting genes on the opposite strand. (E) Venn diagrams showing the contribution of Alu sequences to exons and splicing junctions in de novo protein-coding genes. We analyzed the characteristics of these de novo protein-coding genes. Consistent with previous reports [4], we found that the gene products were smaller, with a median length of 150.5 amino-acids, compared with 416 amino-acids in the human genome, suggesting the difficulty in de novo origination of long ORFs (Wilcoxon test, p-value = 4.06e-10, Figure 1B). The transcripts were generally expressed at lower levels compared with the human genome background (Wilcoxon test, p-value = 0.037, Figure 1C). Nineteen of the 24 de novo genes (79.2%) showed evidence to co-opt the transcriptional context, including seventeen (70.8%) that overlapped with another gene (nine anti-sense and nine at the same strand) and five (20.8%) located downstream of bi-directional promoters (Figure 1D, Table S2). Consistent with the case of FLJ33706 or C20orf203 [8], Alu elements contributed to eight exons in eight genes (33.3%), and to three splicing junction sites in two genes (Figure 1E, Tables S4, S5), further supporting the previous observation that repeat elements might be involved in the origination of some de novo genes [11].
Transcriptomes across human, chimpanzee, and rhesus macaque
To test whether the ancestral non-coding locus of a de novo protein-coding gene is already transcribed in a regulated way, we performed comparative transcriptome profiling of 24 de novo genes between human and two relatives, the chimpanzee, which did not encode about half of the corresponding human ORFs and the rhesus macaque, which did not encode any.
Because de novo genes tend to co-opt the transcriptional context by overlapping with neighboring and anti-sense genes, it is difficult to accurately define the gene structures, especially the gene borders, and determine the RNA expression using probe-based cDNA microarray technology or strand-non-specific RNA-Seq. We performed high-quality strand-specific RNA-Seq in five rhesus macaque tissues (liver, prefrontal cortex, skeletal muscle, adipose and testis) to identify polyadenylated RNAs (Table 1). A total of 477.7 million 90-bp paired-end reads were uniquely mapped to the rhesus macaque genome, covering over 18,000 polyadenylated RNAs with correct transcript strand information, clear transcript structure and accurate quantification of expression (Figure 2, Figure S1, Table 1). All data have been submitted to NCBI Gene Expression Omnibus (Accession Number: GSE34426).
Fig. 2. Strand-specific RNA–Seq in five rhesus tissues reveals clear transcript structure for de novo genes. 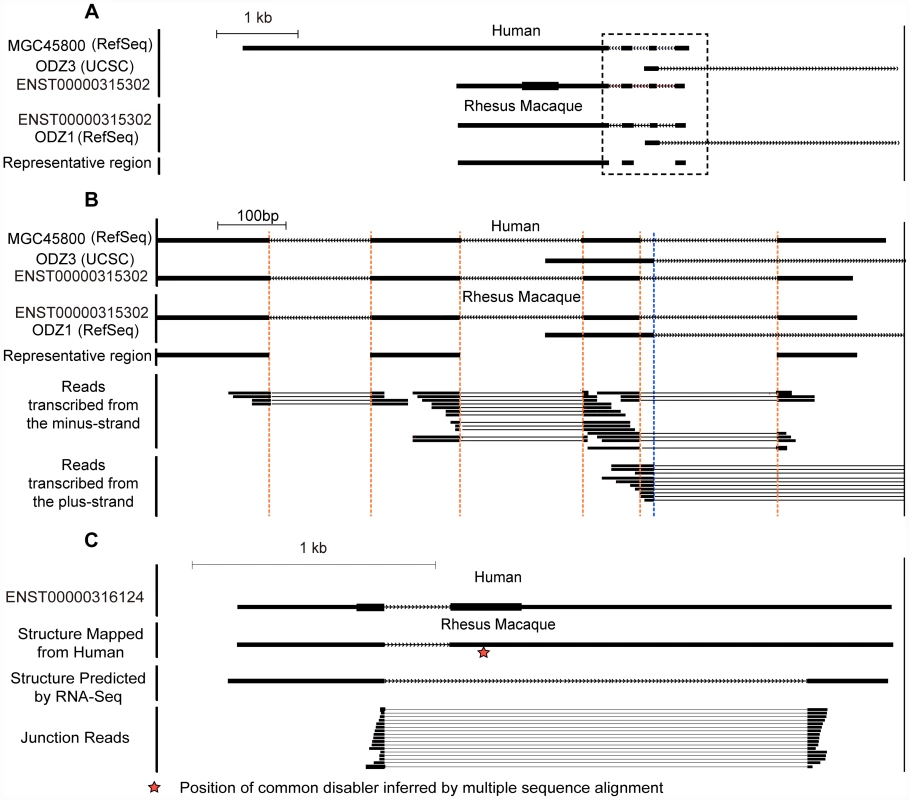
(A) An example of de novo gene ENST00000315302 partially overlapped with a pre-existing gene ODZ3, transcribed by the other strand of the DNA. The ortholog of ENST00000315302 in rhesus macaque was aligned according to genome-wide multiple alignments in UCSC. The junction reads generated by strand-specific RNA-Seq assays are highlighted by black bold lines, with fragments of junction reads crossing splicing junctions connected by thinner lines. The mapped reads well supported the transcription of the target de novo gene on the reverse strand, as most reads appeared in the track for ‘reads transcribed from the minus-strand’. Regions for all four splicing junctions are highlighted in dotted boxes and expanded in (B), including three in ENST00000315302 transcribed from the minus strand and one from the other strand. All of these splicing junctions were well supported by the RNA-Seq reads mapped on the corresponding strand of the DNA. Vertical dotted lines in brown or blue highlight the exon boundaries in transcripts on the minus or plus strands, respectively. (C) Demo case for a discarded de novo gene in the manual curation process, in which the RNA-Seq data in rhesus macaque were not consistent with the putative splicing pattern predicted on the basis of human gene models. The common disabler is marked with a red star, and this was actually spliced out in rhesus macaque as indicated by the junction reads. Scale bar shown as benchmark for gene size. Tab. 1. Statistics of RNA–Seq data. 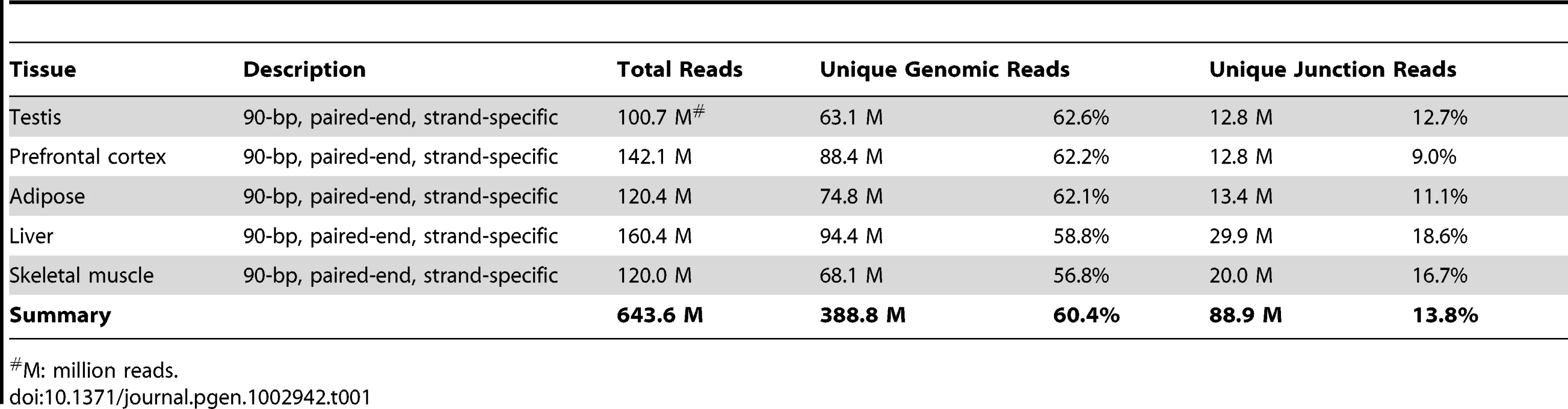
M: million reads. Based on this RNA-Seq dataset, we selected representative regions for the 24 de novo genes that did not overlap with other genes (Table S6, Materials and Methods). We then integrated all publicly available strand-non-specific RNA-Seq data including RNA-Seq data from seven human tissues [20], [21], two additional rhesus macaque tissues [20] and five chimpanzee tissues [20], [22] (Table S7, Materials and Methods). RNA expression levels were calculated in terms of RPKMs (Reads Per Kilobase of exon model per Million mapped reads) of representative regions in all three species. Splicing junctions were assembled. These efforts made it possible to study the transcript structure, transcription activity and tissue expression profile for these de novo loci in species with or without protein-coding potential.
High correlation of non-coding genes with their protein-coding orthologs with respect to gene structure, transcription activity, and tissue expression profile
We found that 21 out of 24 (87.5%) hominoid-specific de novo genes appeared to be transcribed in at least one chimpanzee tissue, and twenty (83.3%) in at least one rhesus tissue as non-coding RNAs (Figure S2, Materials and Methods). However, eighteen showed lower expression levels in rhesus macaque than their protein-coding orthologs in human (Figure 3A, Table S8). Such a difference suggests that the de novo origination of protein-coding genes probably happened in a step-by-step fashion, in which the ancestral non-coding DNA may already have been transcribed before it acquired coding potential, while the expression level was further optimized at the transcriptional level after the coding potential was acquired.
Fig. 3. Orthologs of human de novo protein-coding genes encode structure-matched non-coding RNAs in rhesus macaque or chimpanzee. 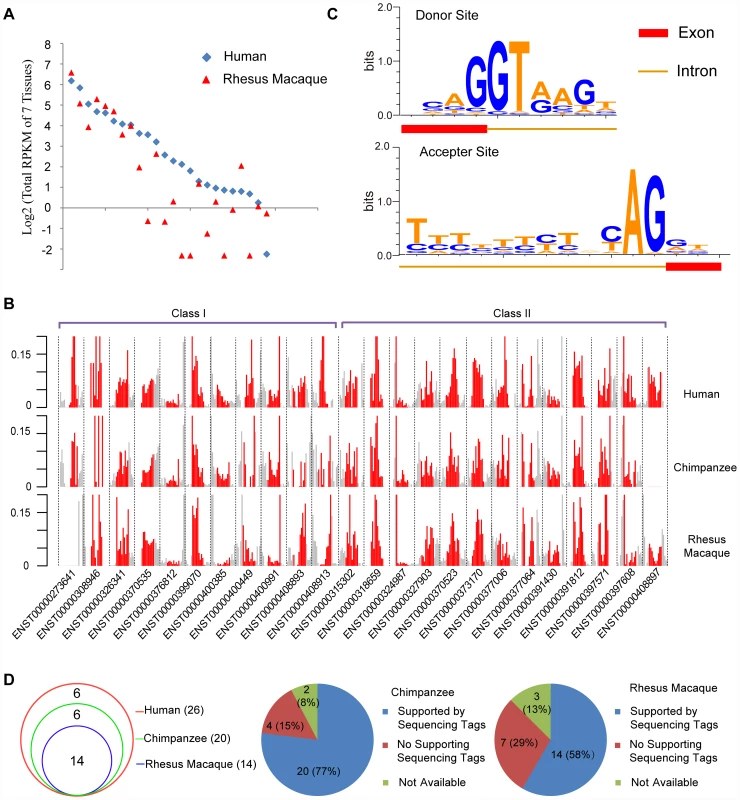
(A) Summed RPKM scores (log2 transformed) of de novo genes in seven tissues from human and rhesus macaque. The human genes were ordered by decreasing expression level as a reference, and the rhesus genes were aligned accordingly. (B) For each de novo gene in Classes I and II, the base-level densities of RNA-Seq reads across the transcript (red), as well as the upstream/downstream regions (grey, 50% of the length of the transcript), are shown. The raw density scores computed from RNA-Seq reads coverage were normalized with the total reads across the region. (C) Splicing junctions with the sequence motifs near both the donor site and acceptor site, summarized by all splicing junctions in human de novo genes. (D) Venn diagram showing the numbers of human splicing junctions detected also in chimpanzee or rhesus macaque. Pie charts further illustrate the detailed status of human splicing junctions in chimpanzee and rhesus macaque. We then compared the base-level density of RNA-Seq reads across the transcript with that in the upstream/downstream flanking regions. These de novo loci showed significantly higher expression than the corresponding flanking regions (Figure 3B). For both Class I and II genes, the distributions of expression densities were largely comparable among the three species, suggesting that the overall transcription structure of these de novo genes had been formed before these species diverged (Figure 3B). We further examined the exon/intron gene structure of these de novo transcripts. The 24 de novo genes formed 26 splicing junctions in human, each supported by at least one RNA-Seq read (Figure 3D, Table S9). Among these human splicing junctions, 24 in chimpanzee and 23 in rhesus macaque were located in regions with enough read coverage to infer the exon/intron structures (Figure 3D, Tables S10, S11). Among them twenty (20/24, 83.3%) in chimpanzee and fourteen in rhesus macaque (14/23, 60.9%) were supported by reads covering both donor and acceptor sites, forming exons separated by standard introns marked with GT-AG splicing junctions (Figure 3C and 3D, Figure S3). Thus the majority of human splicing junctions were detectable in chimpanzee and rhesus macaque, suggesting that the exon/intron structures of human de novo genes were largely shared by their non-coding orthologs.
Finally, we analyzed the expression profiles of these newly-originated de novo protein-coding genes. The de novo genes showed brain-enriched transcriptional expression (Figure 4A, Figure S4), consistent with the recent discovery of accelerated recruitment of new genes into the human brain [23] as well as other independent studies reporting the expression profiles of newly originated de novo genes in human [5], [7], [8]. On the basis of cross-species tissue expression profiles, we further investigated the transcriptional correlations of de novo genes across different tissues in human, chimpanzee and rhesus macaque. For the twenty de novo genes with convincing transcription in both human and rhesus macaque, sixteen in rhesus macaque (80.0%) showed tissue expression profiles similar to human (Figure 4A). Based on 10,000 Monte Carlo simulations neglecting ortholog relationships for the tissue expression profile, such an observation represents a statistically significant excess (p-value<10−4, Figure 4B, Materials and Methods). Transcriptome data from five chimpanzee tissues had similar patterns of correlation with human and rhesus macaque (Figure 4A). Considering these de novo genes as a whole, the hierarchical clustering further supported the between-species similarity of tissue expression profiles, in that the same tissues from different species were clustered together (Figure 4A). Spearman correlation coefficients were computed separately for each pair of tissues and the extent of tissue-specific differences in de novo gene expression were shown in Figure 4C. Besides the strongest correlations across the diagonal line representing self-comparisons, strong correlations were also detected for pairs of the same tissues in different species (shorter lines parallel to the diagonal line) (Figure 4C). No significant difference was detected for the expression profile correlation between the two categories of de novo genes (Figure S5).
Fig. 4. Non-coding orthologs of human de novo protein-coding genes in rhesus macaque and chimpanzee show tissue expression profiles similar to human. 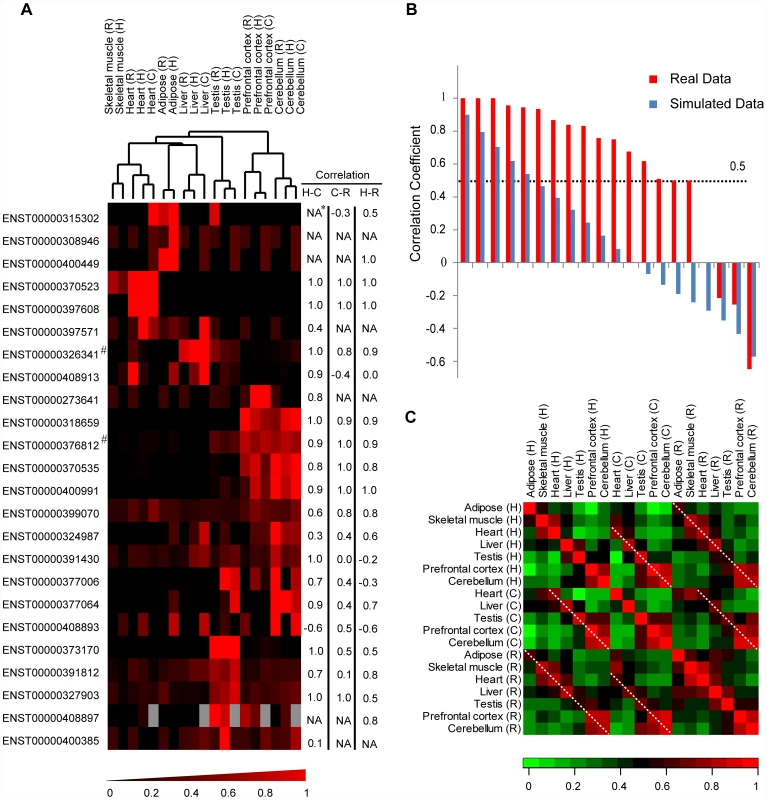
(A) Hierarchical clustering chart of tissue expression proportions. For each gene in one species, tissue expression proportions were calculated by normalizing RPKM scores with the total expression level of the gene in that species. The scores were then clustered according to similarity using complete linkage hierarchical clustering. For each gene, cross-tissue correlation coefficients between human and chimpanzee (H–C), chimpanzee and rhesus macaque (C–R) and human and rhesus macaque (H–R) are shown. (B) Correlation coefficient scores for tissue expression profiles between human and rhesus macaque. Correlation coefficients for de novo genes (brown histograms) are illustrated with background simulated by 10,000 Monte Carlo simulations neglecting ortholog relationship for the tissue expression profile (blue histograms, mean scores are shown). (C) For each pair of tissues, Spearman correlation coefficients were computed separately and the extent of tissue-specific differences in de novo gene expressions are shown. Dotted lines highlight comparisons between pairs of corresponding tissues in different species. Grey boxes: missing data. *Correlation coefficient not available due to low tissue expressions in one or both species. #Gene reported in previous study as human-specific de novo protein-coding gene. The pattern of correlated expression is insensitive to parameters in de novo gene identification
As reviewed recently, the identification of de novo genes faces tremendous challenges [14], [24]. We thus investigated whether the major findings we reported here, that de novo protein-coding genes appear to have acquired a regulated transcript structure and expression profile before acquiring coding potential, are robust and insensitive to these parameters.
Firstly, all patterns remained unchanged when stricter criteria were considered (Figure S6, S7, S8; Table S4): i) when using a more conservative list of fifteen de novo genes where the hominoid ORF should be 50% (instead of 30%) longer than all other out-group species, the conclusion remained the same (Figure S6, Table S4); ii) Although unlikely, it cannot be completely ruled out that a stop codon-containing exon might be spliced out, or an alternative splice site might be used to avoid a stop codon. Thus we repeated our analysis with eighteen de novo genes encoding only a single coding exon and found the conclusion unchanged (Figure S7, Table S4); and iii) the conclusion was unchanged when de novo genes partially contributed by Alu-elements were removed (Figure S8, Table S4) and when the sequence alignment parameters for identifying de novo genes were modified (Table S4). Secondly, comparative transcriptome analysis of both previously reported lists of human-specific de novo protein supported the main findings in our study. Specifically, among the three human-specific de novo genes identified previously by Knowles et al, two were supported by convincing RNA-Seq transcription evidence. Both were also transcribed in rhesus macaque with tissue expression profiles similar to human (Spearman correlation coefficient = 0.9 for both genes), although at slightly lower expression levels (Table S8). The recently-identified sixty human-specific de novo genes [5] showed a similar result: 22 of 34 genes showed lower expression levels in rhesus macaque compared with human orthologs (Figure S9). Most of these de novo genes had similar transcription structure and splicing junction structure in rhesus macaque (Figure S9), and significantly more genes showed correlated tissue expression profiles with human, based on Monte Carlo simulations neglecting orthologous relationships (p-value = 0.0009, Figure S9, Materials and Methods).
Our data supports that de novo origination rather than ORF expansion drove the origination of hominoid-specific ORFs. The ORF expansion model, which believes that a shorter ORF may predate the human-rhesus split, predicts that the longer the ORF in rhesus macaque relative to the ORF in human, the stronger the transcriptional correlation between them. The model also predicts that human de novo genes encoding multiple exons are more likely to be transcriptionally correlated between human and rhesus macaque, given that these genes may be generated by exon-level expansion of an ancestral protein-coding gene. However, our data showed that the ratio between the length of a human protein and that of the corresponding truncated protein in rhesus macaque did not have strong predictive power for the expressional correlation between these two species, measured by either relative expression intensity (Figure S10A, Spearman correlation coefficient: −0.16) or cross-tissue transcriptional correlation (Figure S10B, Spearman correlation coefficient: −0.38). In addition, rhesus orthologs of human de novo genes with multiple coding exons did not show higher transcription than orthologs of human de novo genes with only one exon (one-sided Fisher's exact test, p-value = 0.97). Both lines of evidence favor the de novo origination model instead of the ORF expansion model.
Discussion
Challenges to identify de novo protein-coding genes
As reviewed recently, the detection of de novo genes faces tremendous challenges [14], [24]. Using different bioinformatics strategies and annotation databases, the number of human-specific de novo proteins identified had ranged from three [7] to sixty [5]. Such a remarkable difference is largely due to different strategies used to confirm the in-group transcript structure and coding potential, verify the absence of proteins from out-group species, and confirm that the gene is in the process of de novo origination rather than gene loss or other gene origination mechanisms such as gene duplication [14], [24].
Here, to confirm the transcriptional and translational expression of these genes in human, we applied genome-wide filters using genome-wide RNA and protein expression data. We took advantage of the newly-assembled genomes and transcriptomes to validate the fine transcript structure of these genes. Specifically, half of the splicing junctions in these genes (twenty out of forty) were supported by at least two uniquely mapped RNA-Seq reads across the junction in our own RNA-seq data (Figure S1), and three were supported by one RNA-Seq read. When considering more public RNA-Seq datasets, additional evidence was found to support three other splicing junctions (Table S9). Considering that these newly-originated transcripts were generally expressed at low levels (Figure 1C), the likelihood that two high-quality RNA-seq reads spanned the same splicing junction by chance was low. In addition, we also required that at least one mRNA or EST sequence supported the transcript structure (Table S9). Combining evidence from spliced ESTs, mRNAs and RNA-Seq junction reads, all of the forty splicing junctions were well supported. The de novo genes we used in the subsequent comparative transcriptome analyses thus had a clear transcript structure with in-group coding potential.
Because it is technically and economically prohibitive to fully profile the proteomes across species, individuals, tissues and developmental stages, conceptual translation of ORFs remains the main strategy in the field to verify the absence of proteins from out-group species. However, it is difficult to conclusively say which parameter is better. The ORF length cutoff in out-group species ranged from 50% to 80% in previous studies [5], [7]. In our study, we chose 70% as the cutoff based on data from previous studies [5], [7]. In fact, 62.5% (fifteen out of 24) of the de novo genes conceptually encode a peptide at least 50% shorter in the out-group species than in human, based on phylogenetic distribution of “disabling” mutations.
We introduced three additional filters in our identification pipeline. First, we inferred the locus and ORF ages in the phylogenetic tree and removed genes without a convincing gene locus or ORF age assignment. We used as many high-quality out-group species as possible. For example, a human-specific de novo gene was defined as a gene without intact orthologous ORF in multiple out-group species including chimpanzee, orangutan, rhesus macaque, mouse, dog and so on. Second, since unambiguous sequence information in multiple out-group species was needed to trace the detailed origination process, we only retained reliable codon-based alignments with high sequence coverage (≥70%) and DNA sequence identity (≥50%). In this way, rapidly-evolving loci at the DNA level were excluded. Finally, as a stop codon-containing exon could be spliced out, or an alternative splice site could be used to avoid the stop codon, for the de novo genes with at least two coding exons, we manually curated the transcript structure based on RNA-Seq data and removed genes with ambiguous transcript structures in out-group species (a demo case is shown in Figure 2C). Furthermore, if the proteins were indeed absent from out-group species, we would expect that their ratio of non-synonymous substitution rate to synonymous substitution rate (dN/dS) should be close to 1 [25]. In order to test this hypothesis, we aligned the truncated forms of the human de novo proteins in non-human primates and calculated the merged dN/dS ratio using DnaSP [26]. Indeed, the value did not deviate significantly from 1 (dN/dS = 0.904), suggesting that these truncated forms of ORFs in non-human primates may largely not encode bona fide proteins.
The last question was to distinguish de novo gene origination from gene loss and other gene-origination mechanisms such as gene duplication. In our study, to verify that these were newly created genes in hominoid instead of dying old genes in out-group species, we manually checked the corresponding ORF regions in multiple primate out-group species. For each de novo gene, the phylogenetic distribution of ORF-disabling mutations in multiple primate out-group species as well as the detailed multiple sequence alignments are shown in Dataset S1 and Table S1. The existence of common ancestral disablers shared by multiple out-groups suggested that gene origination rather than gene loss was more likely for these genes (Dataset S1, Table S1). To confirm that they originated through de novo evolution instead of by other mechanisms such as gene duplication, we searched these human proteins against all Ensembl-annotated proteins by BLASTP, and a de novo gene had to have no significant paralog (BLASTP E-value≤10−6) in the human genome. BLASTP E-value cutoffs ranging from 10−4 to 10−10 had been used in previous identification of de novo genes [5], 7,12,27. In our study, we used 10−6 as the cutoff in the initial screening followed by two additional checks: first, in order to account for the fact that short proteins generally have a large E-value in the alignment, we also required that a de novo gene should not align well (coverage ≥70% and identity ≥50%) with any other human genes; and second, a human de novo protein should not have any paralog annotated by the Ensembl pipeline, which has been shown to be quite sensitive [28].
After removing the many possibilities that could introduce false-positives, our strategy may cause some false-negatives. However, our gene list does appear convincing as it did not miss any of the three human-specific genes reported by Knowles et al in the genome-wide identification of human de novo genes (Figure 1A). Also, the de novo gene dataset verified several previously proposed features of de novo protein-coding genes such as shorter ORFs and co-option of the neighboring transcriptional context. The main finding we proposed in this study was insensitive to the parameters used in defining de novo genes, as discussed in detail in the previous sections (Figures S6, S7, S8).
Brain and testis may be favorable locations for newly created de novo genes
The out-of-testis hypothesis states that the testis is a hotbed of new gene origination, given its permissive chromatin state allowing widespread transcription [13], [29]. We analyzed the expression profiles of the de novo protein-coding genes we identified and tested the out-of-testis pattern by following the idea described in [29]. First, we investigated the relative abundance of each de novo gene across testis, skeletal muscle, liver, heart, adipose, prefrontal cortex and cerebellum in terms of RNA-Seq read counts. As shown in Figure 4A, the de novo genes were most often transcribed in cerebellum. Testis ranked number two or three, depending on the species. Second, we tested whether class I (human-specific) de novo genes are more often biased to testis compared to class II (human/chimpanzee shared) de novo genes. As discussed in [29], Class I should be more often expressed in testis if the out-of-testis hypothesis stands. As shown in Figure S4, different from this prediction, class II de novo genes had a much higher proportion of reads expressed in testis. Thus, de novo genes may not generally conform to the out-of-testis hypothesis. Testis-biased expression of de novo genes had been reported so far in the fruit fly [9], [12]. By contrast, none of the previously reported human-specific de novo genes appeared to have such a pattern: i) the three de novo genes identified in [7] are broadly transcribed; ii) higher expression levels of FLJ33706 [8] were observed in several brain regions such as cerebellum or cerebral cortex compared to testis (relative expression levels, 2.4–4.9 versus 1.5); and iii) sixty de novo genes identified in [5] are most often transcribed in cerebral cortex with testis following as the second top tissue. In summary, testis may still be the location of some de novo genes, but brain appears to be a favorable location for the origination of many human de novo genes.
Long non-coding RNAs may serve as a birth pool of protein-coding genes
Our work demonstrated how origination of de novo genes occurred by Darwinian step-by-step evolution as speculated in a previous study [14]. Specifically, our comparative transcriptome analysis suggests that transcription tends to occur first, since the de novo locus with coding potential is transcriptionally correlated with its non-coding counterparts in rhesus macaque or chimpanzee with respect to transcript structure and tissue expression profile. Such a result also suggests that the ancestral form was a functional non-coding RNA, given its regulated rather than promiscuous transcription. More interestingly, after acquiring an ORF, the transcript is subject to further transcriptional optimization as supported by higher abundance expression compared to their non-coding orthologs. Thus, a protein-coding gene is not built in a single step but by the gradual acquisition of various elements. Our work sheds new light on the challenging problem of finding the potential functions of long non-coding RNAs [30]. At least a portion of these polyadenylated long non-coding RNAs may represent a “birth pool” of protein-coding genes, especially those with active and regulated transcription. It would be interesting to delineate the functional differences between non-coding RNAs and their orthologous de novo protein-coding genes, especially since the origination of protein-coding potential and further optimized transcription might render the protein-coding version more functional.
Materials and Methods
Ethics statement
Rhesus macaque tissue samples were obtained from the internationally-accredited (Association for Assessment and Accreditation of Laboratory Animal Care, AAALAC) animal facility of the Institute of Molecular Medicine in Peking University. The present study was approved by the Institutional Animal Care and Use Committee of Peking University. All animals were handled in strict accordance with good animal practice as defined by the relevant national and local animal welfare bodies.
Dating protein-coding genes in the phylogenetic tree
Genome-alignment-based pipelines were developed to infer the origination time of a given genomic region by modifying a previous gene-alignment-based method [31], [32]. Briefly, UCSC netted chained files [33] were analyzed to verify whether a given locus had a reciprocal syntenic alignment in the out-group genomes. Multiple out-group species were scanned to control the false discovery rate raised by occasional sequencing gaps. A specific branch was then assigned to this locus by following the rule of parsimony. As we investigated whether a best-to-best match could be found between a locus and out-group loci regardless of chromosomal linkage information, orthologous genes could be identified independent of gene annotation of out-groups and robust with different chromosomal locations due to gene fusions or translocations [32].
Genome-wide identification of hominoid-specific de novo protein-coding genes
We developed a sophisticated pipeline to identify hominoid-specific de novo protein-coding genes [7], [8]. First, for each locus, the existence of the ORF in multiple out-group species (chimpanzee, orangutan, rhesus macaque, mouse, guinea pig, dog, hedgehog and armadillo) was inferred separately. Specifically, on the basis of the gene locus age assignment described in the previous section, if the locus was absent from one out-group, we directly classified this case as the ORF not existing in this out-group neither. Otherwise, the fine-scale gene structure in this out-group was inferred by Exonerate [34], using human Ensembl proteins [35] as reference. For the codon-based pairwise alignment generated by Exonerate, we retained those that were reliable and had ≥70% coverage and ≥50% identity. If the alignment generated by Exonerate failed to pass these criteria, we classified such a scenario as ambiguous, namely, we did not know whether the orthologous ORF existed or not. Next, if the sequence in this out-group encoded at least one ORF disabler, such as frame-disrupting indels or premature stop codons, and the subsequent maximum continuous peptide was shorter than 70% of the human ORF length, the ORF was inferred to be non-existent in this out-group. To ensure the absence of coding potential in this out-group, we also required that the Ensembl automatic annotation [35] did not identify any homolog in this out-group to avoid potential misalignments by Exonerate [34]. The exhaustive search functionality of Exonerate was enabled to conservatively identify species - or lineage-specific de novo genes (Figure 1A).
We then inferred the origination timing of ORFs for these de novo genes by summing up the presence or absence information in multiple out-group species, along the phylogenetic tree with the rule of parsimony. As the genomic assembly of non-human species is generally error-prone, the existence information for orthologous ORFs in multiple out-group species was used to avoid the identification of false-positive frame disruption potentially caused by genome sequencing errors, e.g. one human-specific ORF was defined as not existing in chimpanzee, orangutan, rhesus macaque, mouse, guinea pig, dog, hedgehog and armadillo. We discarded those cases with contradictory information in several out-group species.
We further searched these human proteins against all Ensembl-annotated proteins by BLASTP to verify that they originated through de novo evolution, instead of other gene origination mechanism such as gene duplication. A de novo gene was defined only if there were no other hits passing the BLAST E-value cutoff of 10−6 in human Ensembl proteins and no annotated paralogs by Ensembl [35]. Finally, only hominoid-specific de novo genes without coding potential in rhesus macaque were kept for further manual curation (Figure 1A). The initial gene list covered all four human-specific de novo genes reported in previous studies [7], [8], as well as five of the human-specific de novo genes reported in a more recent study [5] using a relatively lenient criterion [24]. Ensembl [35] release 51 was downloaded as the basic gene dataset for this analysis. We used MySQL to organize the data, BioPerl [36] and BioEnsembl [37] to fold the pipeline, and R (v2.13.1) to perform all statistical analyses.
Inclusion criteria for hominoid-specific de novo protein-coding genes
We manually curated the initial gene list to identify convincing hominoid-specific de novo protein-coding genes for subsequent statistical analyses.
First, we introduced genome-wide filters such as genome-wide RNA and protein expression data to make sure these genes had convincing evidence for transcriptional and translational expression in human. Public RNA-Seq data from nine human tissues were analyzed to estimate the gene expression level of each de novo gene, following the protocols in the original report [21]. Peptide evidence from large-scale mass spectrometry studies was further extracted from the proteomics identifications database (PRIDE) [38] and the PeptideAtlas project [39]. Briefly, all human peptide sequence data were downloaded and all-against-all BLAST similarity searches were performed. A peptide was considered to show convincing peptide evidence for a de novo gene only if its whole sequence completely and identically matched the CDS region, with the second-best hit in the genome (if existing) including at least two mismatches (Ensembl release 51 annotation). Only genes with i) RNA-Seq RPKM >0.5 in at least one of the nine human tissues, and ii) at least one convincing item of peptide evidence in support, were retained (Figure 1A).
Second, besides the EST and mRNA evidence used in the initial genome-wide pipeline, in the manual curation, we also took advantage of newly-assembled genomes and transcriptomes to validate the fine-scale transcript structure of these genes in human and out-group species (Figure 1A). Genes with the stop codon-containing exon spliced out, or a totally different splicing structure in out-group species were discarded (Figure 2C).
Third, to verify that these genes were newly created instead of old dying genes, we manually checked the corresponding ORF regions in multiple primate out-groups in the context of the revised transcript structure, to ensure the existence of common ancestral disablers shared by multiple out-group species. Genes without common ancestral disablers were not included in the subsequent statistical analyses (Figure 1A). For each de novo gene, the phylogenetic distribution of ORF-disabling mutations in multiple primate out-groups, as well as the detailed multiple sequence alignments are shown (Dataset S1, Table S1). Newly-assembled genomes and transcriptomes from the UCSC genome browser annotations [33] were also considered in the manual process to ensure the gene structure, locus age assignment and ORF integrity.
Overall, 24 hominoid-specific de novo genes were identified, with unambiguous gene structures and confirmed locus and ORF age assignment (Figure 1A). As no arbitrary criteria were included in the manual curation process, the de novo gene dataset is not biased with respect to age dating and transcription profiles.
Characteristics of de novo protein-coding genes
The basic characteristics of the de novo genes and background genes were downloaded from UCSC [33], Ensembl [35] and Swiss-Prot [40]. R scripts (v2.13.1) were implemented to perform all statistical analyses and calculate the distributions of these characteristics for de novo genes, using the human genome as the background. For each human-specific de novo gene, we aligned the truncated form of the human ORF between chimpanzee and orangutan, and calculated the merged dN/dS score with DnaSP [26].
Library preparation and strand-specific poly (A)–positive RNA–Seq
Total RNA was extracted from five rhesus macaque tissues using the Trizol method and analyzed by an Agilent 2100 bio-analyzer (Agilent Technologies). Poly (A)-positive RNA was purified with the Dynabeads mRNA purification kit (Invitrogen) from 5 µg of total RNA with high quality (RNA integrity number ≥7.5), following the manufacturer's instructions. A strand-specific RNA-Seq library preparation on the basis of the incorporation of deoxy-UTP was performed as reported previously [41]. Briefly, after the first-strand cDNA synthesis, non-incorporated nucleotides were removed and dTTP was substituted by dUTP during the synthesis of the second strand. Then after ligation with a Y-shaped adaptor, the deoxyuridine-containing strand was selectively removed with UNG, leaving only the first cDNA strand [41]. Amplified material was loaded onto a flow-cell and sequencing was carried out on the Illumina HiSeq2000 platform by running 90 cycles (paired-end design) according to the manufacturer's instructions.
Computational processing of RNA–Seq data
In-house paired-end mRNA sequence tags were mapped to the rhesus macaque genome (rheMac2) by TopHat (v1.2.0) [42]. Multiple alignment reads were discarded. The RPKM scores for each gene were calculated as previous described [43]. FastQC (v0.10.0) and a series of Perl (v5.12.2) and R (v2.13.1) scripts were implemented to evaluate the quality of the RNA-Seq data, including i) the average quality of reads, ii) the evenness of short-read distributions on transcripts, iii) the contrasts of read distributions in exon, intron and intergenic regions, iv) the efficiency of strand-specific strategy, v) mutation rates across the reads, and vi) correlations of RPKM scores with real-time PCR results, by calculating the Spearman correlation coefficient across 877 transcripts as measured by Taqman Gene Expression Assays and by strand-specific RNA-Seq read counts [21]. Representative regions for each de novo gene-encoded transcript were selected, which met the criteria of i) being located in the exonic regions of de novo genes, ii) having no overlap with other known genes, and iii) accurate identification of syntenic regions in rhesus macaque and chimpanzee genomes based on UCSC genomic alignment. The RPKM scores for each de novo gene were calculated for the representative regions. Public RNA-Seq datasets for human [20], [21], rhesus macaque [20] and chimpanzee tissues [20], [22] were downloaded from SRA and mapped to the human (hg18), chimpanzee (panTro2) or rhesus genome (rheMac2) respectively with similar pipelines. Public strand non-specific RNA-Seq data meeting the inclusion criteria were further integrated, in which i) the RNA-Seq study was performed in tissues derived from male individuals; ii) the RNA-Seq library was generated using high-quality RNA samples (RIN >7.0); and iii) the RNA-Seq reads were mapped to the genome with high mapping rates (uniquely mapped reads >40%). Similarly, for the orthologous loci of each de novo gene, RPKM scores were calculated for the representative regions as noted above and an RPKM cutoff of 0.2 was set for convincing transcription, a value significantly higher than that in intergenic region (Monte Carlo p-value<0.05, Figure S2).
Uniquely mapped RNA-Seq reads covering splicing junctions were extracted by Samtools (v 0.1.16) [44]. The correlation coefficient between the RPKM scores in human tissues and the parallel rhesus macaque tissues were calculated using R scripts (v2.13.1) and a correlation coefficient of 0.5 was used as the cutoff for positive correlations between tissue expression profiles. 10,000 Monte Carlo simulations were performed to generate the distribution of correlation coefficient scores, under the assumption of neglecting ortholog relationships between the tissue expression profiles in human and rhesus macaque, while keeping all other parameters identical to those used for the real data. Statistical analyses were further performed using this distribution as background and Monte Carlo p-values<0.05 were considered significant.
Our RNA-Seq data was of high quality after extensive evaluation. Specifically, the mean PHRED quality score was >30 across the whole reads (one possible sequencing error per 1000 bases, Figure S1A). The even distribution of short reads on transcripts further revealed well-controlled randomized fragmentation of the transcripts in the RNA-Seq experiments (Figure S1B). The strand-specific strategy worked well since the reads with correct strand information were over 100-fold more than strand-mislabeled reads (Figure S1C). As expected, RNA-Seq read counts showed very sharp peaks at exonic regions, with a read density >100-fold higher than that in introns or intergenic regions, revealing that most reads derived from mature polyadenylated RNAs (Figure S1D). The percentage of mis-mapped reads across the genome was <0.1%, as estimated using a method previously reported [21]. The average mutation rate was as low as 1.51 errors per read, with mutations evenly distributed across the reads (Figure S1E). Two standard human RNA samples, from Brain Reference and Universal Human Reference (UHR) [21], were then quantified using the identical experimental and computational pipelines, and obtained highly consistent results with Taqman-based real-time PCR quantification (Spearman correlation coefficient = 0.94) (Figure S1F). The data also made it possible to accurately assemble the gene structures of de novo genes with complex transcriptional contexts and infer the representative regions as described. In summary, high-quality RNA-Seq studies were performed in five rhesus tissues, with accurate strand information and transcript expression quantification (Figure 2, Figure S1).
Supporting Information
Zdroje
1. Susumu O (1970) Evolution by gene duplication. Springer-Verlag ISBN 0-04-575015-7.
2. JacobF (1977) Evolution and tinkering. Science 196 : 1161–1166.
3. LongM, BetranE, ThorntonK, WangW (2003) The origin of new genes: glimpses from the young and old. Nat Rev Genet 4 : 865–875.
4. SiepelA (2009) Darwinian alchemy: Human genes from noncoding DNA. Genome Res 19 : 1693–1695.
5. WuD-D, IrwinDM, ZhangY-P (2011) De Novo Origin of Human Protein-Coding Genes. PLoS Genet 7: e1002379 doi:10.1371/journal.pgen.1002379.
6. Toll-RieraM, BoschN, BelloraN, CasteloR, ArmengolL, et al. (2008) Origin of primate orphan genes: a comparative genomics approach. Mol Biol Evol
7. KnowlesDG, McLysaghtA (2009) Recent de novo origin of human protein-coding genes. Genome Res 19 : 1752–1759.
8. LiCY, ZhangY, WangZ, ZhangY, CaoC, et al. (2010) A human-specific de novo protein-coding gene associated with human brain functions. PLoS Comput Biol 6: e1000734 doi:10.1371/journal.pcbi.1000734.
9. BegunDJ, LindforsHA, KernAD, JonesCD (2007) Evidence for de novo evolution of testis-expressed genes in the Drosophila yakuba/Drosophila erecta clade. Genetics 176 : 1131–1137.
10. CaiJ, ZhaoR, JiangH, WangW (2008) De novo origination of a new protein-coding gene in Saccharomyces cerevisiae. Genetics 179 : 487–496.
11. ChenST, ChengHC, BarbashDA, YangHP (2007) Evolution of hydra, a recently evolved testis-expressed gene with nine alternative first exons in Drosophila melanogaster. PLoS Genet 3: e107 doi:10.1371/journal.pgen.0030107.
12. LevineMT, JonesCD, KernAD, LindforsHA, BegunDJ (2006) Novel genes derived from noncoding DNA in Drosophila melanogaster are frequently X-linked and exhibit testis-biased expression. Proc Natl Acad Sci U S A 103 : 9935–9939.
13. KaessmannH (2010) Origins, evolution, and phenotypic impact of new genes. Genome Res 20 : 1313–1326.
14. TautzD, Domazet-LosoT (2011) The evolutionary origin of orphan genes. Nat Rev Genet 12 : 692–702.
15. KhalturinK, HemmrichG, FrauneS, AugustinR, BoschTC (2009) More than just orphans: are taxonomically-restricted genes important in evolution? Trends Genet 25 : 404–413.
16. BegunDJ, LindforsHA, ThompsonME, HollowayAK (2006) Recently evolved genes identified from Drosophila yakuba and D. erecta accessory gland expressed sequence tags. Genetics 172 : 1675–1681.
17. BirneyE, StamatoyannopoulosJA, DuttaA, GuigoR, GingerasTR, et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 : 799–816.
18. Bornberg-BauerE, HuylmansAK, SikosekT (2010) How do new proteins arise? Curr Opin Struct Biol 20 : 390–396.
19. SchusterSC (2008) Next-generation sequencing transforms today's biology. Nat Methods 5 : 16–18.
20. BrawandD, SoumillonM, NecsuleaA, JulienP, CsardiG, et al. (2011) The evolution of gene expression levels in mammalian organs. Nature 478 : 343–348.
21. WangET, SandbergR, LuoS, KhrebtukovaI, ZhangL, et al. (2008) Alternative isoform regulation in human tissue transcriptomes. Nature 456 : 470–476.
22. BlekhmanR, MarioniJC, ZumboP, StephensM, GiladY (2010) Sex-specific and lineage-specific alternative splicing in primates. Genome Res 20 : 180–189.
23. ZhangYE, LandbackP, VibranovskiMD, LongM (2011) Accelerated recruitment of new brain development genes into the human genome. PLoS Biol 9: e1001179 doi:10.1371/journal.pbio.1001179.
24. GuerzoniD, McLysaghtA (2011) De Novo Origins of Human Genes. PLoS Genet 7: e1002381 doi:10.1371/journal.pgen.1002381.
25. KimuraM (1977) Preponderance of synonymous changes as evidence for the neutral theory of molecular evolution. Nature 267 : 275–276.
26. LibradoP, RozasJ (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25 : 1451–1452.
27. ZhouQ, WangW (2008) On the origin and evolution of new genes–a genomic and experimental perspective. J Genet Genomics 35 : 639–648.
28. VilellaAJ, SeverinJ, Ureta-VidalA, HengL, DurbinR, et al. (2009) EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Research 19 : 327–335.
29. VinckenboschN, DupanloupI, KaessmannH (2006) Evolutionary fate of retroposed gene copies in the human genome. Proc Natl Acad Sci U S A 103 : 3220–3225.
30. MercerTR, DingerME, MattickJS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10 : 155–159.
31. ZhouQ, ZhangG, ZhangY, XuS, ZhaoR, et al. (2008) On the origin of new genes in Drosophila. Genome Res 18 : 1446–1455.
32. ZhangYE, VibranovskiMD, LandbackP, MaraisGA, LongM (2010) Chromosomal redistribution of male-biased genes in mammalian evolution with two bursts of gene gain on the X chromosome. PLoS Biol 8 doi:10.1371/journal.pbio.1000494.
33. FujitaPA, RheadB, ZweigAS, HinrichsAS, KarolchikD, et al. (2011) The UCSC Genome Browser database: update 2011. Nucleic Acids Res 39: D876–882.
34. SlaterGS, BirneyE (2005) Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 6 : 31.
35. FlicekP, AmodeMR, BarrellD, BealK, BrentS, et al. (2011) Ensembl 2011. Nucleic Acids Res 39: D800–806.
36. StajichJE, BlockD, BoulezK, BrennerSE, ChervitzSA, et al. (2002) The Bioperl toolkit: Perl modules for the life sciences. Genome Res 12 : 1611–1618.
37. StabenauA, McVickerG, MelsoppC, ProctorG, ClampM, et al. (2004) The Ensembl core software libraries. Genome Res 14 : 929–933.
38. VizcainoJA, CoteR, ReisingerF, BarsnesH, FosterJM, et al. (2010) The Proteomics Identifications database: 2010 update. Nucleic Acids Res 38: D736–742.
39. DeutschEW (2010) The PeptideAtlas Project. Methods Mol Biol 604 : 285–296.
40. The-UniProt-Consortium (2011) Ongoing and future developments at the Universal Protein Resource. Nucleic Acids Res 39: D214–219.
41. ParkhomchukD, BorodinaT, AmstislavskiyV, BanaruM, HallenL, et al. (2009) Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res 37: e123.
42. TrapnellC, PachterL, SalzbergSL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25 : 1105–1111.
43. MortazaviA, WilliamsBA, McCueK, SchaefferL, WoldB (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5 : 621–628.
44. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
Štítky
Genetika Reprodukční medicína
Článek Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese SubjectsČlánek Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in RiceČlánek Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System inČlánek An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural CrestČlánek A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of BacteriaČlánek A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 9
-
Všechny články tohoto čísla
- Heterozygous Mutations in DNA Repair Genes and Hereditary Breast Cancer: A Question of Power
- GWAS of Diabetic Nephropathy: Is the GENIE out of the Bottle?
- The Conflict within and the Escalating War between the Sex Chromosomes
- Proteome-Wide Analysis of Disease-Associated SNPs That Show Allele-Specific Transcription Factor Binding
- Exome Sequencing Identifies Rare Deleterious Mutations in DNA Repair Genes and as Potential Breast Cancer Susceptibility Alleles
- A Gene Family Derived from Transposable Elements during Early Angiosperm Evolution Has Reproductive Fitness Benefits in
- Genome-Wide Association Study for Serum Complement C3 and C4 Levels in Healthy Chinese Subjects
- Role of Transposon-Derived Small RNAs in the Interplay between Genomes and Parasitic DNA in Rice
- Co-Evolution of Mitochondrial tRNA Import and Codon Usage Determines Translational Efficiency in the Green Alga
- SIRT6/7 Homolog SIR-2.4 Promotes DAF-16 Relocalization and Function during Stress
- CNV Formation in Mouse Embryonic Stem Cells Occurs in the Absence of Xrcc4-Dependent Nonhomologous End Joining
- Tetraspanin Is Required for Generation of Reactive Oxygen Species by the Dual Oxidase System in
- Citrullination of Histone H3 Interferes with HP1-Mediated Transcriptional Repression
- Variation in Genes Related to Cochlear Biology Is Strongly Associated with Adult-Onset Deafness in Border Collies
- The Long Non-Coding RNA Affects Chromatin Conformation and Expression of , but Does Not Regulate Its Imprinting in the Developing Heart
- Rif2 Promotes a Telomere Fold-Back Structure through Rpd3L Recruitment in Budding Yeast
- Is a Metastasis Susceptibility Gene That Suppresses Metastasis by Modifying Tumor Interaction with the Cell-Mediated Immunity
- The p38/MK2-Driven Exchange between Tristetraprolin and HuR Regulates AU–Rich Element–Dependent Translation
- Rare Copy Number Variants Contribute to Congenital Left-Sided Heart Disease
- A Genetic Basis for a Postmeiotic X Versus Y Chromosome Intragenomic Conflict in the Mouse
- An Essential Role of Variant Histone H3.3 for Ectomesenchyme Potential of the Cranial Neural Crest
- Characterization of Inducible Models of Tay-Sachs and Related Disease
- Hominoid-Specific Protein-Coding Genes Originating from Long Non-Coding RNAs
- Transcriptional Repression of Hox Genes by HP1/HPL and H1/HIS-24
- Integrative Genomic Analysis Identifies Isoleucine and CodY as Regulators of Virulence
- Convergence of the Transcriptional Responses to Heat Shock and Singlet Oxygen Stresses
- Genomics of Adaptation during Experimental Evolution of the Opportunistic Pathogen
- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Vsx2 Controls Eye Organogenesis and Retinal Progenitor Identity Via Homeodomain and Non-Homeodomain Residues Required for High Affinity DNA Binding
- The Long Path from QTL to Gene
- TCF7L2 Modulates Glucose Homeostasis by Regulating CREB- and FoxO1-Dependent Transcriptional Pathway in the Liver
- The Non-Flagellar Type III Secretion System Evolved from the Bacterial Flagellum and Diversified into Host-Cell Adapted Systems
- Complex Chromosomal Rearrangements Mediated by Break-Induced Replication Involve Structure-Selective Endonucleases
- Factors That Promote H3 Chromatin Integrity during Transcription Prevent Promiscuous Deposition of CENP-A in Fission Yeast
- A Mimicking-of-DNA-Methylation-Patterns Pipeline for Overcoming the Restriction Barrier of Bacteria
- Determinants of Human Adipose Tissue Gene Expression: Impact of Diet, Sex, Metabolic Status, and Genetic Regulation
- Genome-Wide Association Studies Identify Heavy Metal ATPase3 as the Primary Determinant of Natural Variation in Leaf Cadmium in
- Tethering of the Conserved piggyBac Transposase Fusion Protein CSB-PGBD3 to Chromosomal AP-1 Proteins Regulates Expression of Nearby Genes in Humans
- A Genome-Wide Association Study Identifies Five Loci Influencing Facial Morphology in Europeans
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- H4K20me1 Contributes to Downregulation of X-Linked Genes for Dosage Compensation
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Coevolution within and between Regulatory Loci Can Preserve Promoter Function Despite Evolutionary Rate Acceleration
- New Susceptibility Loci Associated with Kidney Disease in Type 1 Diabetes
- SWI/SNF-Like Chromatin Remodeling Factor Fun30 Supports Point Centromere Function in
- A Response Regulator Interfaces between the Frz Chemosensory System and the MglA/MglB GTPase/GAP Module to Regulate Polarity in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
- Two Distinct Repressive Mechanisms for Histone 3 Lysine 4 Methylation through Promoting 3′-End Antisense Transcription
- Genetic Modifiers of Chromatin Acetylation Antagonize the Reprogramming of Epi-Polymorphisms
- UTX and UTY Demonstrate Histone Demethylase-Independent Function in Mouse Embryonic Development
- A Comparison of Brain Gene Expression Levels in Domesticated and Wild Animals
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Enrichment of HP1a on Drosophila Chromosome 4 Genes Creates an Alternate Chromatin Structure Critical for Regulation in this Heterochromatic Domain
- Normal DNA Methylation Dynamics in DICER1-Deficient Mouse Embryonic Stem Cells
- The NDR Kinase Scaffold HYM1/MO25 Is Essential for MAK2 MAP Kinase Signaling in
- Functional Variants in and Involved in Activation of the NF-κB Pathway Are Associated with Rheumatoid Arthritis in Japanese
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



