-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMicroevolution of during Prolonged Infection of Single Hosts and within Families
Our understanding of basic evolutionary processes in bacteria is still very limited. For example, multiple recent dating estimates are based on a universal inter-species molecular clock rate, but that rate was calibrated using estimates of geological dates that are no longer accepted. We therefore estimated the short-term rates of mutation and recombination in Helicobacter pylori by sequencing an average of 39,300 bp in 78 gene fragments from 97 isolates. These isolates included 34 pairs of sequential samples, which were sampled at intervals of 0.25 to 10.2 years. They also included single isolates from 29 individuals (average age: 45 years) from 10 families. The accumulation of sequence diversity increased with time of separation in a clock-like manner in the sequential isolates. We used Approximate Bayesian Computation to estimate the rates of mutation, recombination, mean length of recombination tracts, and average diversity in those tracts. The estimates indicate that the short-term mutation rate is 1.4×10−6 (serial isolates) to 4.5×10−6 (family isolates) per nucleotide per year and that three times as many substitutions are introduced by recombination as by mutation. The long-term mutation rate over millennia is 5–17-fold lower, partly due to the removal of non-synonymous mutations due to purifying selection. Comparisons with the recent literature show that short-term mutation rates vary dramatically in different bacterial species and can span a range of several orders of magnitude.
Published in the journal: . PLoS Genet 6(7): e32767. doi:10.1371/journal.pgen.1001036
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001036Summary
Our understanding of basic evolutionary processes in bacteria is still very limited. For example, multiple recent dating estimates are based on a universal inter-species molecular clock rate, but that rate was calibrated using estimates of geological dates that are no longer accepted. We therefore estimated the short-term rates of mutation and recombination in Helicobacter pylori by sequencing an average of 39,300 bp in 78 gene fragments from 97 isolates. These isolates included 34 pairs of sequential samples, which were sampled at intervals of 0.25 to 10.2 years. They also included single isolates from 29 individuals (average age: 45 years) from 10 families. The accumulation of sequence diversity increased with time of separation in a clock-like manner in the sequential isolates. We used Approximate Bayesian Computation to estimate the rates of mutation, recombination, mean length of recombination tracts, and average diversity in those tracts. The estimates indicate that the short-term mutation rate is 1.4×10−6 (serial isolates) to 4.5×10−6 (family isolates) per nucleotide per year and that three times as many substitutions are introduced by recombination as by mutation. The long-term mutation rate over millennia is 5–17-fold lower, partly due to the removal of non-synonymous mutations due to purifying selection. Comparisons with the recent literature show that short-term mutation rates vary dramatically in different bacterial species and can span a range of several orders of magnitude.
Introduction
When did modern pathogenic bacteria evolve? Current wisdom teaches that 10,000–50,000 years have elapsed since a variety of genetically highly monomorphic bacterial pathogens evolved from their last common ancestors [1]–[6] and the ages of pathogenic bacteria with greater levels of genetic diversity have been estimated as reflecting millions of years of evolution [7], [8]. Age estimates for bacteria are higher than those of viruses, many of which appeared a few hundred years ago [9], primarily because many bacterial estimates are based on a supposedly universal molecular clock rate, μS, for synonymous polymorphisms in genes that encode proteins. In 1987, Ochman and Wilson calibrated this clock rate as 3.4×10−9 per nucleotide per year by dating the split between Escherichia coli and Salmonella enterica within the framework of a universal clock rate for bacterial rRNA sequences [10]. The divergence between E. coli and S. enterica was equated with the age of mammals, estimated as ∼160 Myr. However, the validity of this molecular clock rate for dating bacterial evolution is highly questionable.
Some of the geological dates used to calibrate the rRNA clock rate have since been revised (Table 1). These revisions are so drastic that the original linear regression of diversity with time [10] is no longer valid [11] (Figure 1). Furthermore, the estimate of ∼160 Mya for the age of the split between E. coli and S. enterica depends on the assumption that E. coli is specific for mammalian hosts, unlike S. enterica which infects reptiles as well as mammals. But E. coli can be readily isolated from reptiles or birds [12], which invalidates this argument. An independent recent study also dates the split between E. coli and S. enterica at 57–176 Mya on the basis of long-term phylogenies of protein-encoding sequences [13]. However, both this recent estimate and the original estimate of Ochman and Wilson share the problem that geological events that occurred billions of years ago are extrapolated to speciation events that supposedly occurred ∼100 Mya, which implicitly assumes that molecular clock rates are linear over large time scales for diverse microorganisms. This is unlikely to be the case (see below). The use of such long-term clock rates is even more problematical for age estimates of divergence within genetically monomorphic or recently emerged pathogens [1]–[6], which require extrapolations over a further three to four orders of magnitude.
Fig. 1. Percentage diversity in rRNA versus age (million years). 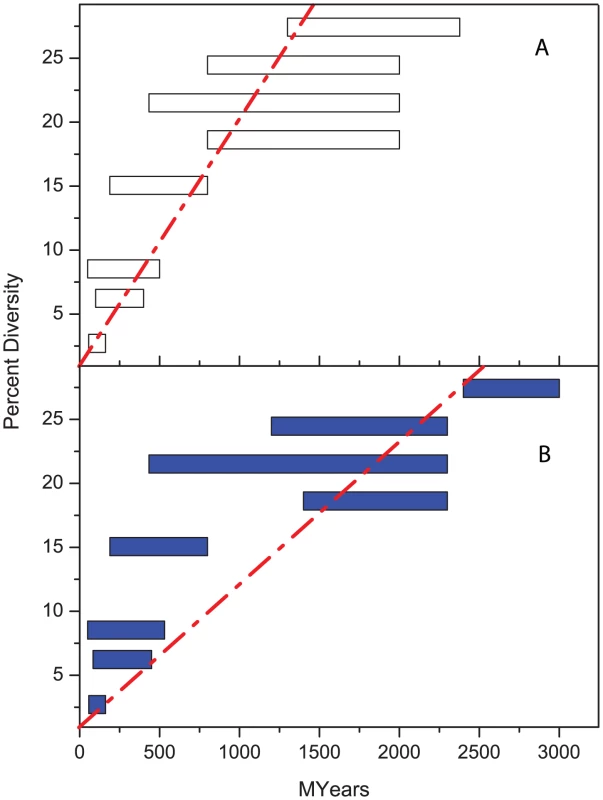
(A) original correlation by Ochman and Wilson [10]. (B) lack of good correlation according to modern estimates of age ranges (Table 1). Tab. 1. Comparisons of dating used by Ochman and Wilson, 1987 <em class="ref">[10]</em> with current estimates. ![Comparisons of dating used by Ochman and Wilson, 1987 <em class="ref">[10]</em> with current estimates.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/1f783a4a70a15f6ed459671730a7e481.png)
Long-term clock rates are now thought to accelerate by one to two orders of magnitude for recent events [14], [15]. Furthermore, clock rates for genetic diversity between species should not be used for dating within a species. Diversity between species represents fixation events whereas diversity within a species reflects the accumulation of polymorphisms [16], [17]. Finally, molecular clock rates probably vary between different bacterial species, which can differ by up to two orders of magnitude in their relative ratios of divergence of rRNA to protein-encoding genes [18]. As a result of these considerations, almost all age estimates for recently evolved bacterial pathogens need to be reconsidered [19] and should be based on species-specific short-term molecular clock rates.
Age estimates for viruses depend on the use of archival samples that were stored over several years or decades. Only very few attempts, summarized in Table 2, have been made to estimate ages in bacteria with this approach, in part because their clock rates were thought to be too slow. In the case of Yersinia pestis which was introduced to Madagascar in the early 20th century, the clock rate was similar to that of Ochman and Wilson (Table 2). However, a clock rate dated by migration of Buchnera, an aphid endosymbiote, to North America in the late 19th century is two orders of magnitude higher (Table 2).
Tab. 2. Published ages and clock rates for microevolution in selected bacteria. 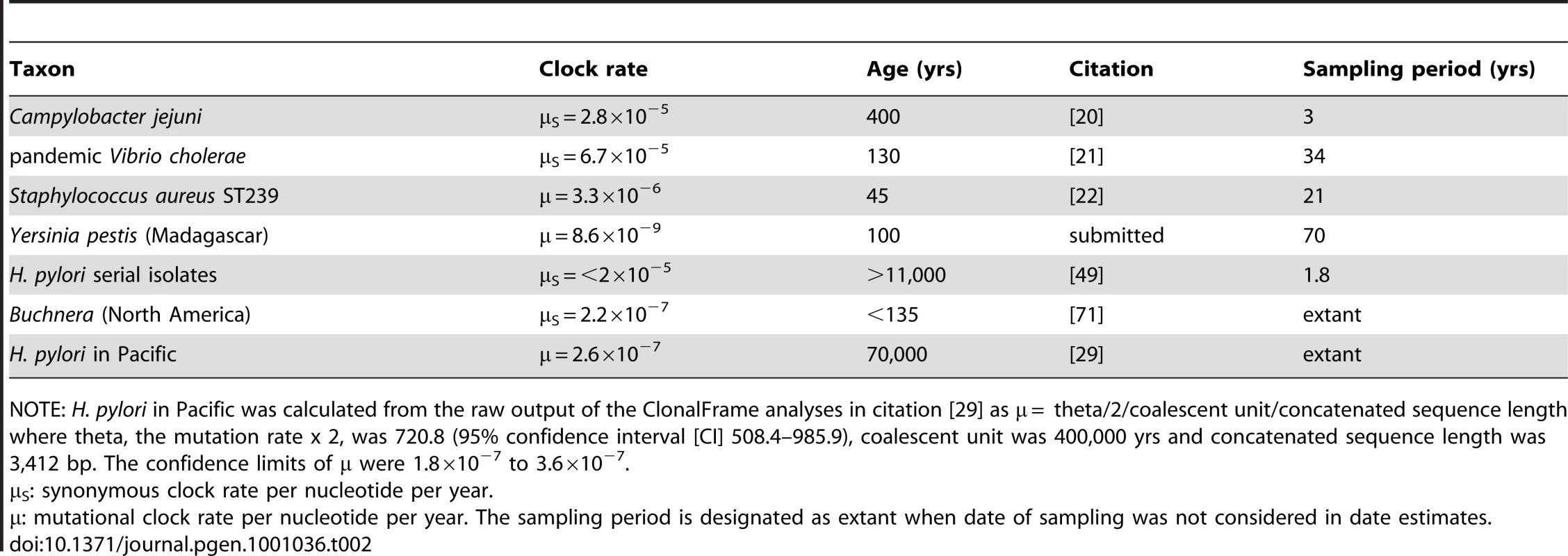
NOTE: H. pylori in Pacific was calculated from the raw output of the ClonalFrame analyses in citation [29] as μ = theta/2/coalescent unit/concatenated sequence length where theta, the mutation rate x 2, was 720.8 (95% confidence interval [CI] 508.4–985.9), coalescent unit was 400,000 yrs and concatenated sequence length was 3,412 bp. The confidence limits of μ were 1.8×10−7 to 3.6×10−7. Two recent studies of Campylobacter jejuni and Vibrio cholerae have found synonymous clock rates of >10−6 per site per year, several orders of magnitude higher than the clock rate of Wilson and Ochman. However, we are sceptical about the validity of these two estimates due to problems with their sampling schemes. The C. jejuni isolates were obtained over a three year period from infected humans within a sampling area of only 968 km2 in Lancashire, England [20], and might reflect admixture due to the import of novel polymorphisms from outside the catchment area. Similarly, the V. cholerae estimates were based on a comparison of only three genomes whose epidemiological patterns suggested that they had evolved soon before the dates of sampling [21]. A third recent study found a clock rate of 3×10−6 for ST239 of Staphylococcus aureus, which would mean that ST239 evolved in the mid-1960's [22]. However, the ST239 genealogy consists of multiple, early radiations, which suggests adaptation due to selective pressures.
Clock rates are distorted when based on polymorphisms that are under positive selection because adaptation can increase the fixation rate for mutations by orders of magnitude [2]. As an extreme example, serial isolates from human infections that are repeatedly treated with antibiotics acquire mutations that are associated with antibiotic resistance and can result in hyper-mutation [23]. 68 mutations in the 6.5 Mbp genome were observed over eight years of lung infection by Pseudomonas aeruginosa in a patient with cystic fibrosis [24] and 35 mutations in the 2.9 Mbp genome during 12 weeks of endocarditis caused by S. aureus [25]. Similarly, patho-adaptive, transient mutations in an E. coli adhesin are selected during infection of the urinary tract but rapidly disappear due to source-sink dynamics [26]. Short-term positive selection may be common because an appreciable fraction of E. coli genes show traces of such selection [27].
These various analyses show that mutation rates may be sufficiently high in some bacteria that microevolution can be observed within serial bacterial isolates from individual humans. Here we analyze such microevolution within Helicobacter pylori. H. pylori is commonly acquired in childhood, after which, in the absence of antibiotic therapy, it can continue to infect the stomachs of humans over their entire lifespan [28]. H. pylori has infected humans for at least 60,000 years because it accompanied anatomically modern humans out of Africa [29]–[33]. H. pylori also exhibits an atypically high genetic diversity: every third nucleotide in housekeeping gene fragments is polymorphic in global analyses [30], [34], and the pair-wise synonymous diversity of individual genes ranges from 0.1–0.3 [34]. High genetic diversity can reflect a long evolutionary history but can also result from a high mutation rate. Indeed, the frequency of mutants per cell among natural isolates is approximately 10–100 fold higher in laboratory experiments than for E. coli [35], [36], with some variation between individual isolates. That high mutation frequency may reflect the lack of genes encoding the MutHLS1 mismatch repair system [28], [37]. A high mutation rate in the laboratory suggests that the mutational clock rate may also be high during natural infection, possibly facilitating the adaptation of these bacteria to individual human hosts [38]. However, as for most bacteria, robust estimates of the microevolutionary mutation rate are lacking.
In addition to a high mutation rate, recombination is also particularly frequent in H. pylori. This conclusion was originally reached on the basis of homoplasy analysis [39]. Although this methodology has been recently criticized [40], recombination is clearly frequent in nature because mosaic imports have been observed, a direct signal for homologous recombination. In laboratory experiments, DNA transformation followed by homologous recombination introduces mosaic stretches of 1.3–3.9 Kbp into the recipient, occasionally interrupted by interspersed segments of recipient DNA sequences that have not been replaced [41], [42]. In nature, mixed infection of individual humans with multiple distinct strains [43]–[48] occurs sufficiently frequently that unambiguous mosaics were detected in serial isolates [49] or isolates from members of a family [47]. Recombination is also indicated by analyses using Structure [33] and the three gamete test [29] on random isolates from diverse global sources. In the analyses of serial isolates [49], the sequences of 10 gene fragments were compared between pairs of strains that were isolated from 26 individuals in Louisiana and Colombia at intervals of 3–36 months (mean 1.8 years). No sequence differences were found in 14 pairs, three pairs of isolates differed by a single nucleotide, and six pairs of isolates differed by eight mosaic stretches. (Four other pairs were excluded from analysis because they either reflected mixed infections with genetically unrelated strains or an infection with a cloud of related isolates whose genetic diversity had arisen prior to infection.) For the 6 pairs of isolates with mosaic stretches, homologous recombination had introduced imports of an average size of 417 bp (CI [95% confidence interval] 259–732) at a rate per nucleotide per year of 6.9×10−5 (CI 3.5×10−5 to 1.2×10−4). The three pairs that differed by a single polymorphism were used to calculate a maximal mutation rate per nucleotide per year of 4.1×10−5, but these polymorphisms could not be definitively ascribed to mutation because they might have represented atypically short imports [49].
Here we have reanalyzed the same pairs of isolates plus others that spanned longer time periods. We examined the sequence diversity in 78 gene fragments in order to provide robust short-term clock rates for mutation and recombination. These clock rates were compared to long-term clock rates that were calibrated by the dates of human migrations.
Results
Novel nucleotide sequences
We sequenced 78 gene fragments from 97 isolates (Table 3, Table S1, Table S2). Two of these fragments are parts of genes that encode outer membrane proteins and all others are within housekeeping genes. We first sequenced an average of 398 bp from each gene fragment; for fragments with polymorphisms we also sequenced ∼500 bp from each of the flanking regions. This resulted in an average total of 39,301 bp that was sequenced per isolate, almost ten times more than in our previous study [49]. The 97 isolates included 34 pairs of serial isolates from continuously infected individuals, of which 22 had been the subject of our previous analysis [49]. Twelve other pairs were from chronically infected patients in the Netherlands [50] with an average sampling interval of 8.4 years (Table 3). The remaining 29 isolates were from 10 families consisting of siblings plus their parents with an average age of 44.5 years from Colombia (4 families), Korea (3), the UK (2) and the USA (1) [47]. The strains within each pair or group of isolates must have diverged very recently because each pair/group shared identical sequences within at least four of the seven MLST housekeeping fragments. In contrast, in previous population genetic studies based on these seven gene fragments [30], [32], [33], [47], random pairs of isolates were usually distinct at all or most of the seven gene fragments. Despite the limited differences found here between pairs of isolates, the frequency of polymorphic sites across the entire data set was high (0.18±0.04), almost as high as in a comparison of the same 78 gene fragments from seven genomic sequences (0.27±0.07; Table S4).
Tab. 3. Sequence comparisons and sources of isolates. 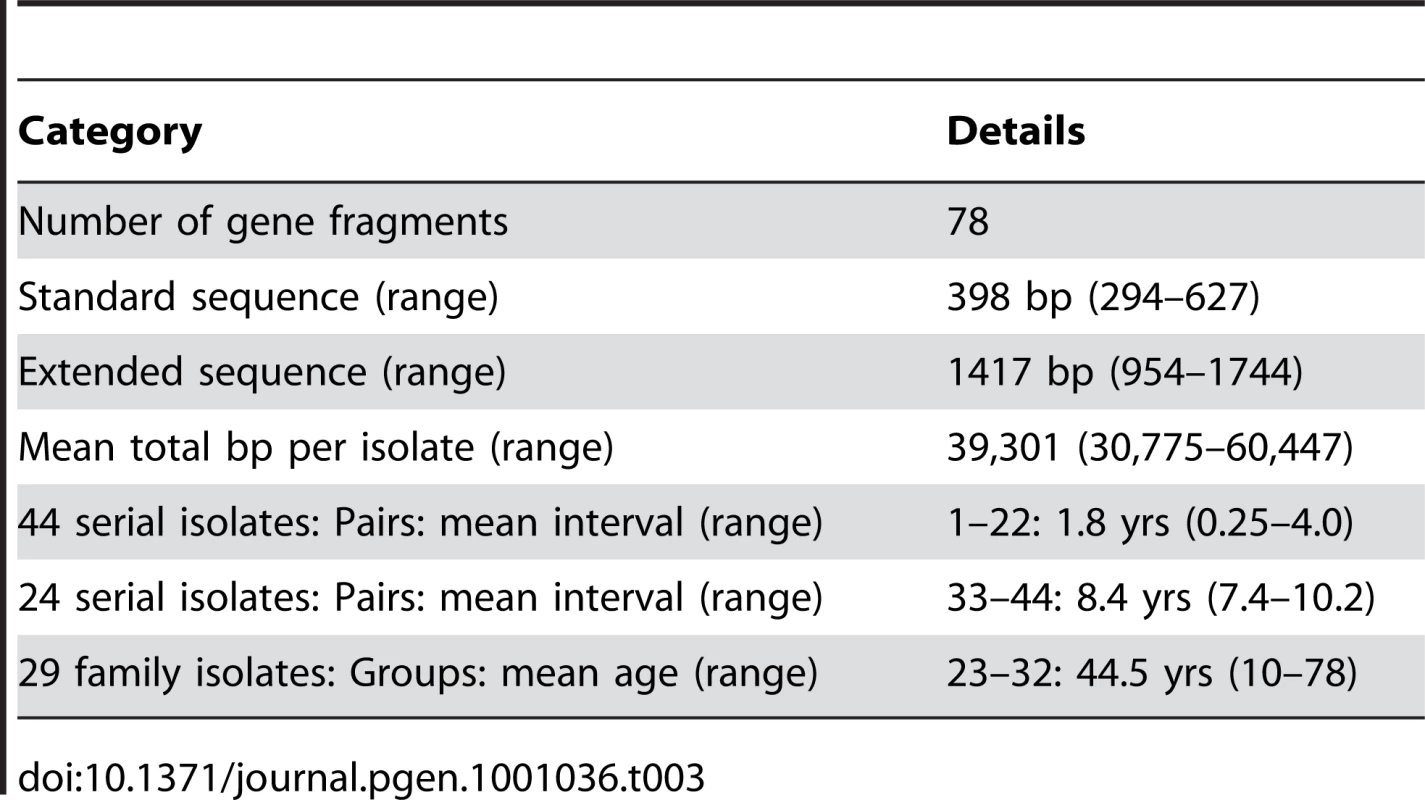
Sequence comparisons
Figure 2 shows a comparison of the paired sequences from the serial isolates. Out of a total of 2650 pair-wise sequence comparisons of gene fragments, 62 contained 1 polymorphic site, 12 showed two polymorphisms and 50 showed more than two polymorphisms. The total number of fragments with sequence differences correlates significantly with the time difference between the serial samples (R = 0.4, p = 0.02; Figure 3A), referred to as the minimal age below. Thus, sequence diversity introduced by mutation plus recombination seems to accumulate in a clock-like manner in infected individuals. We note that minimal age represents only a lower bound for the time of divergence between those isolates because the variant might have arisen earlier and persisted together with the parent in the form of a mixed infection. The maximal age is the extreme opposite scenario to the minimal age, namely that the variants evolved soon after birth. We approximated the maximal time of divergence within each individual as the sum of the ages at sampling. There is apparently no correlation between this maximal age and the number of polymorphic fragments (R = 0.07, p = 0.7, Figure 3B).
Fig. 2. Sequence differences for 78 gene fragments (X axis) that were tested from 34 pairs of sequential isolates (Y axis). 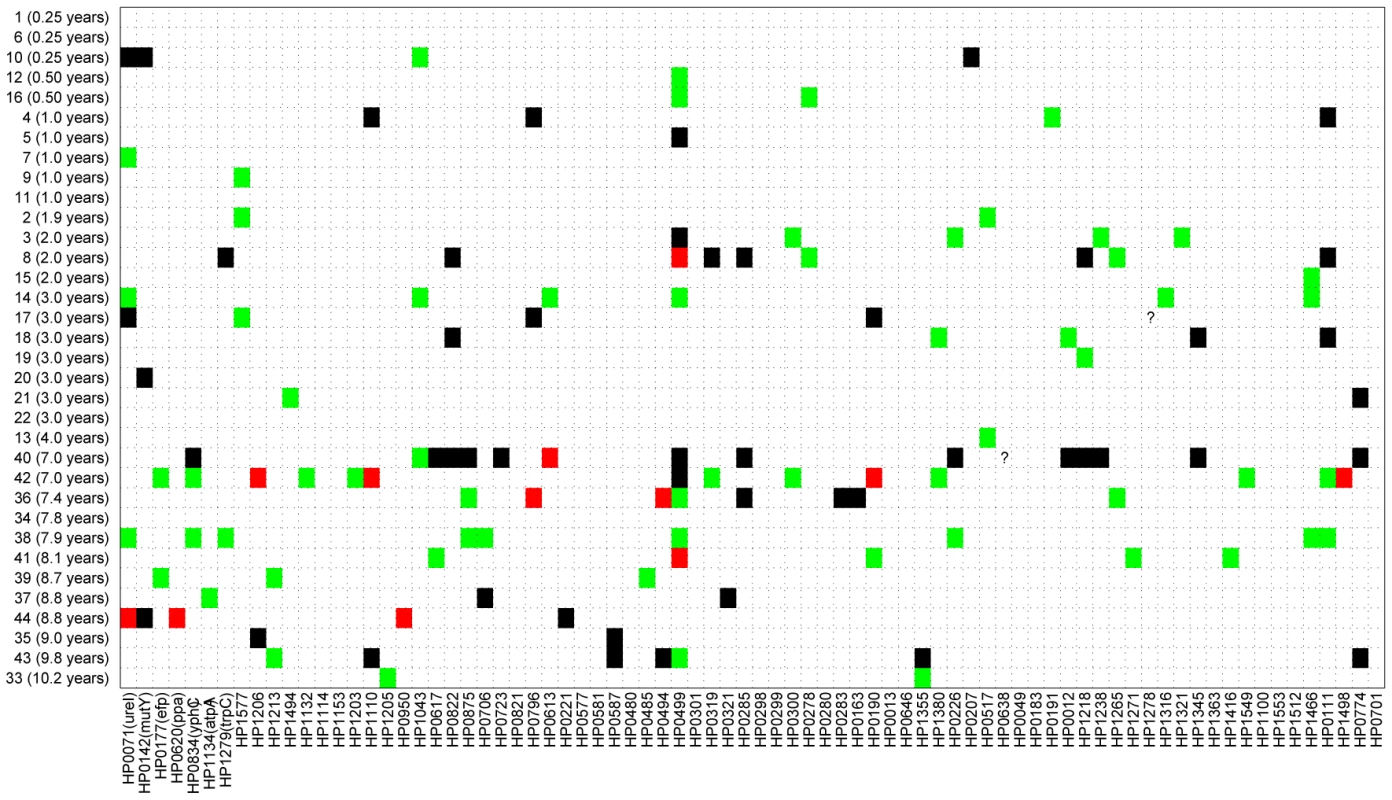
Of 2,650 pairs of sequenced gene fragments, 2,526 were identical (white), 62 differ by one polymorphism (green), 12 had two polymorphisms (red), and 50 had at least three (black). Two question marks indicate missing data that were not used for comparisons. Gene fragments are designated by their designations in the genome of 26695 (HPxxxx) [63], except that the first seven gene fragments that are used for MLST of H. pylori [34] also include the gene designations. Fig. 3. Age versus number of different gene fragments in pairwise comparisons. 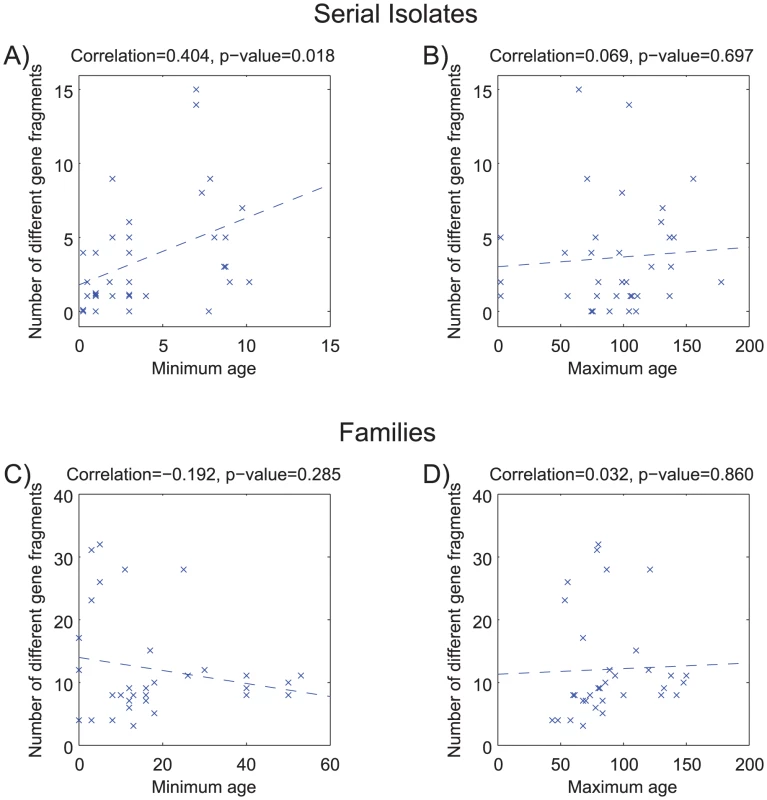
(A,B) serial isolates. (C, D) isolates within each family. (A) Minimum age was the time separation between pairs of isolates. (B) Maximum age was the sum of the ages of the infected person upon isolation of the serial isolates. (C) Minimum age was the minimum age of the two subjects—20. (D) Maximum age was the sum of the ages of the two family members. Each plot contains a linear regression of the data, whose correlation (R) and probability (p) are indicated above the plot. Pair-wise comparisons of sequences from the family isolates revealed even greater diversity (Figure 4), as expected because the time of separation of these pairs is greater. Out of 2568 pair-wise gene fragment comparisons, 183 showed one nucleotide difference, 30 had two and 186 had at least three. However, although the longer time span for divergence of the family isolates was expected to show even stronger correlations with time, this was not the case. Instead, we could not find a significant correlation between the numbers of non-identical gene fragments and any function of the age of the family members that was tested. For example, if infection were transmitted to siblings or children when they reached 20 years of age, a significant correlation should have been observed between the numbers of distinct gene fragment sequences and the minimum age of the two family members – 20 (minimal age), but this was not the case (R = −0.19; p = 0.28) (Figure 3C). Similarly, if each of the family members were infected at birth, a significant correlation would have been expected against the sum of the ages of the two family members (maximal age), but again this was not the case (R = 0.03; p = 0.86; Figure 3D). Visual examination of the data indicated that this lack of correlation with age largely reflected two families, numbers 23 and 26, which had unusually high levels of polymorphism. After removal of data from these two families, the number of differences was significantly correlated with maximal age (R = 0.4; p = 0.045; Figure S1D).
Fig. 4. Pair-wise comparison of sequences from 29 isolates acquired from members of 10 families. 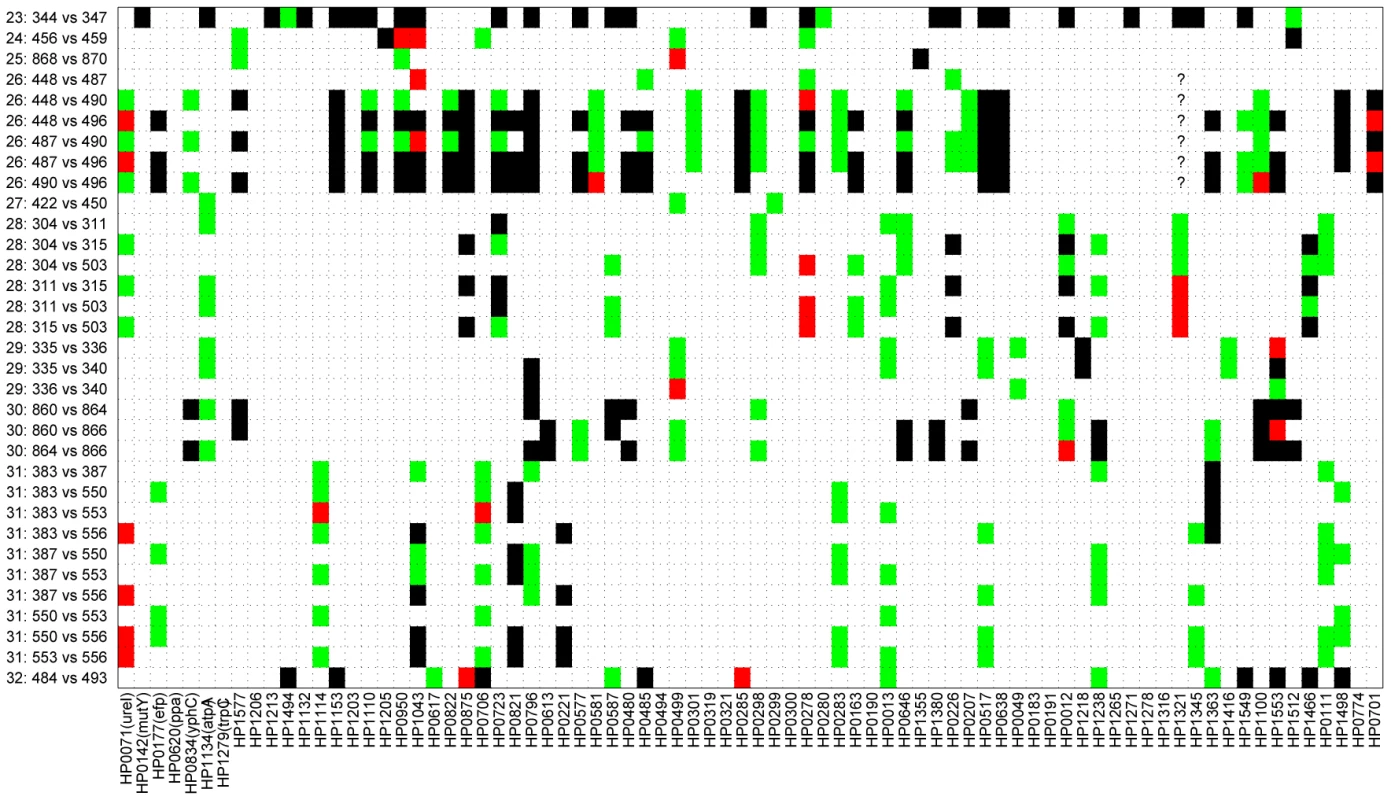
Of 2,568 sequenced gene fragments, 2,169 were identical (white), 183 had one difference (green), 30 had two differences (red), and 186 had at least four differences (black). Six question marks indicate missing data that were not used for comparisons. Model-based analysis
We designed a statistical model of the microevolutionary process in order to analyze our data. Our model assumes that each sequenced fragment evolved independently for an unknown number of years. During that time, mutation events happen according to a molecular clock with a constant rate m per site and per year, and independent recombination events occur in and around the fragment at a constant rate r per initiation site and per year. We follow Falush et al. [49] in assuming that when a recombination event happens, it affects a stretch of DNA with a geometrically distributed length of mean λ from the initiation point. In the affected region, each site has a probability of being substituted which is drawn from a normal distribution with mean equal to ν. Our recombination model is therefore similar to that of ClonalFrame [51], except that the rate of substitution introduced by each recombination event is drawn from a distribution rather than being constant. The use of such a distribution is advantageous because it reflects the diversity of the level of relatedness between donor and recipient for all recombination events.
We applied this microevolutionary model to our data using Approximate Bayesian Computation (ABC). ABC is a Monte-Carlo method to perform statistical inference on the parameters of a model using summary statistics [52], and is well suited to deal with the complex models that arise in population genetics [53]–[55]. We therefore performed ABC inference under the model described above, using the algorithm described by Marjoram et al. [56]. This algorithm uses a Monte-Carlo Markov Chain, but instead of guiding the random walk on the parameter space according to the likelihood, as is usually done, it is guided according to the ability of the parameters to produce a dataset with similar summary statistics (see Materials and Methods).
Our model can be directly applied to the serial isolate data since it describes the evolution between a pair of isolates, resulting in the parameter estimates that are summarized in the first column of Table 4. However, we also wanted to perform the same statistical analysis with the family isolate data as for the serial isolate data. To do so, we first attempted to deduce the genealogical relationships between the isolates within each family using ClonalFrame [51], but the statistical uncertainty found in these reconstructions was too high to make this approach practical, i.e. it is unclear who infected whom. Therefore, we made no assumptions about phylogeny but rather performed pair-wise comparisons of each pair of isolates within a family. This technique has the disadvantage that it might count some microevolutionary events several times in the pair-wise comparisons, but it is the only approach available in the absence of a robust estimate of phylogenies. The parameter estimates for the family data are also reported in Table 4.
Tab. 4. Estimated average [95% credibility intervals] of parameters from ABC analysis. ![Estimated average [95% credibility intervals] of parameters from ABC analysis.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/8a409d3af57009618ae2e8cb147657db.png)
Model validation
We assessed the validity of our model by comparing the observed distributions for two summary statistics that were not used in the ABC inference with their posterior predictive distributions [57], i.e. the distribution obtained by simulations using parameters from the posterior sample (Figure 5). This method of model criticism has been applied previously in multiple ABC studies [58], [59]. The distribution of the number of polymorphisms per gene fragment was quite similar between the data and the posterior simulations from the serial isolates: most gene fragments contained only one polymorphism, several contained two or three polymorphisms, and the frequencies of larger numbers of polymorphisms were spread fairly uniformly over the entire data set (Figure 5A). The length of the polymorphic stretches was less uniform (Figure 5B). The data contained multiple fragments with polymorphisms in stretches of less than 50 bp whereas larger polymorphic stretches were distributed fairly evenly up to the maximum length of just under 1,600 bp. In contrast, the posterior predictive distribution of lengths of polymorphic stretches was fairly uniform, except that stretches of 500–900 bp and of 1,300–1,500 bp were somewhat more frequent. However, these differences between observed data and simulations were relatively minor, again providing support for the validity of our model and inference methodology. Similarly, only minor differences were found when comparing the family data in the same way (Figure S2).
Fig. 5. Comparisons of observed and simulated data for sequences from serial isolates. 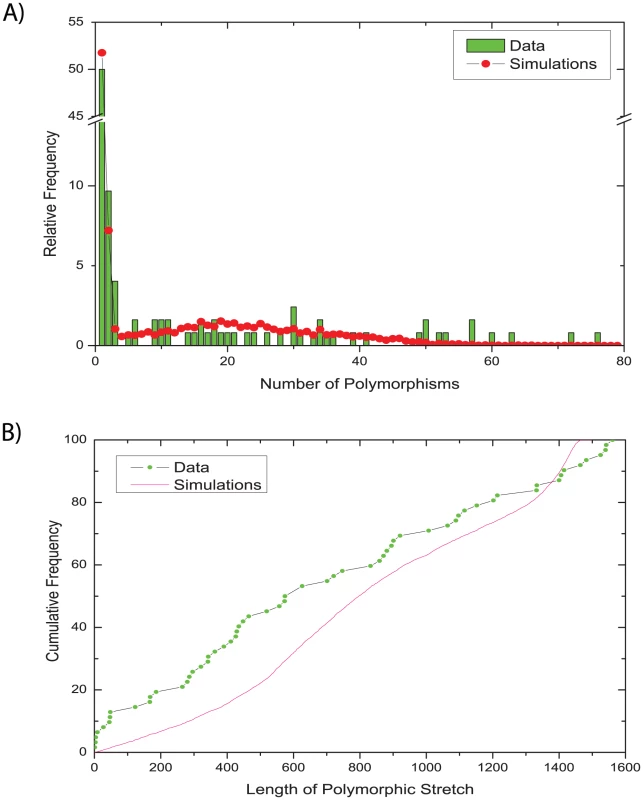
(A) relative frequency of numbers of polymorphisms. (B) cumulative frequency of the lengths of polymorphic stretches. Comparable data for the family isolates are presented in Figure S2. Discussion
Parameter estimates for the sequential isolate data
The average rate of polymorphism introduced by recombination events (ν) was 0.02 (Table 4), which is somewhat lower than the average genetic distance between unrelated members of H. pylori from Ladakh in northern India (0.03) [32] or Europe (0.04) [33]. In turn, this lower rate indicates that donors and recipients were somewhat more closely related than are random, unrelated isolates, and may reflect increased opportunities for recombination within members of the same subpopulations due to geographical structure [47]. Local geographic structure arises due to isolation by distance [30] and isolates within families may have had more opportunities for prior recombination events that would reduce diversity than do geographically separated isolates.
The mean length of imports (λ) was 1247 bp, which is in good agreement with recent estimates from experimental work [41], [42], but considerably greater than the value of 417 bp found previously among serial isolates by Falush et al. [49]. We ascribe this discrepancy to the limited number (eight) of recombination events examined by Falush et al. rather than to differences in methodology. The combination of these two estimates (λ ⋅ ν) indicates that on average 18.6 nucleotide substitutions were introduced by each recombination event, although this number ranged greatly between individual recombination events (Figure 5A).
The average rate of mutation m (per nucleotide site, per year) was estimated as 1.4×10−6 and the average rate of recombination r (per initiation site, per year) was 2.4×10−7. These estimates are sensitive to our choice of prior for the evolutionary time of split between isolates, on which there is much uncertainty. However, Figure 3A provides support for clock-like microevolution versus the time of isolation of the paired isolates (minimal age) and the ABC analyses were performed using very uninformative priors for their time of separation, consisting of the range since birth to the time of isolation of the bacterial strains. Furthermore, data and simulations based on the estimated parameters correspond well in regard to the frequencies of numbers of polymorphisms and reasonably well for the lengths of polymorphic stretches (Figure 5). We therefore conclude that these estimates are reasonably accurate as measures of mutation and recombination rates over very short time periods of up to 10 years.
The ratio r/m should be a robust measure of the relative frequencies at which mutation and recombination are initiated at a given site because both r and m are equally affected by any under - or over-estimation of the split times. The mean estimate for r/m is 0.19. Thus mutations are on average 5 times more frequent than recombination events over the genome of H. pylori. However, even though it happens less often than does mutation, the effect of recombination is much more dramatic than that of mutation, as indicated in Table 4 by the estimate of 3.4 for r ⋅ λ ⋅ ν/m, which represents the ratio of rates at which a site is substituted through recombination and mutation. According to this estimate, a site is >3 times as likely to be substituted by recombination than by mutation.
Parameter estimates for the family isolate data
The average estimates for m and r were about 3 times higher within the families than in the paired isolates (Table 4). We considered the possibility that the different estimates of r and m between serial and family data might reflect the fact that families 23 and 26 exhibited elevated numbers of polymorphisms. However, after excluding these two families, the resulting parameter estimates did not differ dramatically from the estimates summarized in Table 4. We note, however, that in the absence of specific evidence from the data, Bayesian analysis with a broad uniform prior will tend to settle on values within the range of the prior rather than at the extremes. Genetic diversity within families correlated with maximal age (after excluding families 23 and 26; Figure S1D) whereas diversity between serial isolates correlated with minimal age (Figure 3A). Thus, this tendency to use internal values within a broad prior range would shift our parameter estimates for the serial and family isolates in opposite directions away from the extreme age that best correlated with diversity, and could well account for the threefold difference between the two sets of parameter estimates. Finally, we also note that we tested 10 family isolates to see whether the elevated numbers of polymorphisms in families 23 and 26 were accompanied by extreme in vitro frequencies of mutation and DNA transformation (from strain J99). However, although a broad range was measured for the frequencies of both mutation (sevenfold) and transformation (200 fold) (each with one outlier), there was no clear correlation between the two exceptional families and the extremes of the laboratory rates (data not shown).
In contrast to r and m themselves, the ratio r/m is independent of time and should be robust. This ratio has a mean value of 0.18, very similar to the estimate of 0.19 for the serial isolates (Table 4). Similarly, the tract length λ and the frequency ν at which polymorphisms were introduced are also independent of time, and were only slightly higher in the family data than in the serial isolate data (Table 4). ν remains lower than the average pair-wise distance between two random strains of H. pylori and λ is consistent with recent estimates of tract lengths introduced by recombination in the laboratory [41], [42]. Finally, the relative effect of recombination and mutation, r ⋅ λ ⋅ ν/m, should also be relatively robust in regard to uncertainties about time of separation. The mean value of 5.5 was 50% higher than for the serial isolates (3.4), possibly reflecting more opportunities for recombination over the longer time period of infection in the families than within the serial isolates.
Mutation rates in H. pylori versus other bacteria
The estimated short-term mutation rates in the serial and family isolates were 1.4×10−6 and 4.5×10−6, respectively. This range is a robust estimate of the mutation rate over years to decades. It is also possible to calculate a longer term mutation rate for genetic diversity between H. pylori from different global sources, because isolation by distance over the last 60,000 years has resulted in parallel trends in changes in genetic diversity between these bacteria and their human hosts [30]. As a result, diversity between H. pylori from different global sources has accumulated in a clock-like manner that correlates with, and can be dated by, the times of separation of their human hosts [29]. We estimated the long-term mutation rate on the basis of the ClonalFrame analyses described by Moodley et al. [29], yielding a long-term estimate for m of 2.6×10−7 (Table 2). This value is 5–17 fold lower than the short-term rates calculated here, which is probably a general phenomenon among bacteria according to theoretical considerations [14], [15]. One reason for such discrepancies is that even neutral polymorphisms are usually lost over time through genetic drift. A second reason is that non-synonymous mutations will be selected against with time because many of them are slightly deleterious, which should result in a lower dN/dS ratio, the relative rates of non-synonymous to synonymous mutations. A loss of non-synonymous mutations will reduce the apparent mutation rate because approximately 75% of all mutations in coding genes are non-synonymous.
We estimated what proportion of the 5–17 fold reduction in the long-term mutation rate could be accounted for by the loss of non-synonymous mutations. Based on our simulations with the serial isolates, approximately 99% of paired fragments with only one polymorphism resulted from mutation rather than recombination. Thus we could equate the polymorphisms within fragments containing only one SNP to mutations, allowing the calculation of dN/dS even when other fragments had undergone recombination. The resulting dN/dS ratio was 0.5, which indicates that only little purifying selection had taken place over the time period considered here, as is also the case in other examples of recent microevolution [2], [22]. Over longer time periods, purifying selection of deleterious non-synonymous mutations does take place in H. pylori, resulting in an average dN/dS ratio of 0.07 (sevenfold lower) in housekeeping genes among unrelated isolates [34], which is in good agreement with the 5–17 fold difference in mutation rates.
Finally, we return to the general question of the short-term clock rate within bacteria. The results presented here demonstrate that the short-term clock rate in H. pylori is approximately the same (0.4–1.4 fold) as the short-term clock rate in S. aureus ST239, 6.2–20.5 times the rate in Buchnera and 158–524 times the rate in Y. pestis (Table 2). These comparisons show that the short-term clock rate varies dramatically among different bacteria, and in some cases overlaps with those of RNA viruses [60]. However, in all cases considered here, it is higher than the long-term (synonymous) clock rate of 3.4×10−9 that has often been used until now to calculate the ages of genetically monomorphic bacteria.
Materials and Methods
Bacterial isolates
We studied two types of bacterial isolates of H. pylori: serial isolates which were collected from individual persons after a specified time interval, and family isolates which were collected concurrently from two or more members of the same family (Table S2). The 68 serial isolates were collected from 34 patients at intervals ranging from 3 months to 10.2 years. The 29 family isolates were collected from 2 to 5 members of 10 families.
Nucleotide sequencing
Fragments of 78 genes were sequenced (Table S1). Additional extended flanking regions were also sequenced when sequence polymorphisms were detected in the standard fragments. PCR products were amplified and sequences were performed by standard Sanger sequencing on an ABI 3730 XL as described [47] using the oligonucleotide primers listed in Table S3, except that PCR products were cleaned by using shrimp alkaline phosphatase plus exonuclease I. All sequence data has been deposited in the Helicobacter pylori Multi Locus Sequence Typing website (http://pubmlst.org/helicobacter/projects/microevolution/alldata.zip) developed by Keith Jolley and sited at the University of Oxford [61].
Microevolutionary model
We designed a microevolutionary model which describes the evolution of the genome of a strain over a certain period of time T. During this time, each nucleotide of the genome is mutated with probability T×m and is the initiation site of a recombination with probability T×r. When a recombination occurs, it affects a segment of the genome starting from the initiation site and stretching to the right over a length which is geometrically distributed with mean λ. Each site of the affected segment has a probability to be substituted which is normally distributed with mean ν.
The parameters of this microevolutionary model are the time T separating each compared pair of isolates, the mutation rate m per site per year, the recombination rate r per initiation site per year, the average tract length of recombination λ and the average rate of polymorphism introduced by recombination ν. The prior for the time of divergence between the paired isolates is described below. Priors for the four other parameters were uniform from 0 to infinity (improper prior).
Prior on the evolutionary time separating pairs of isolates
Because the evolutionary time separating pairs of isolates is unknown, we had to assume a prior for this quantity in order to perform Bayesian inference. For the serial isolates, we know that the time spent between successive isolations represents a lower bound. If we further assume that the two isolates originated from the same infection, and since this infection must have happened after the birth of the patient, we get an upper bound equal to twice the age of the infected person. We thus assumed a uniform prior for the evolutionary time separating serial isolates between these lower and upper bounds.
For the evolutionary time separating a pair of family isolates, we took a lower bound equal to the minimum of the ages of the two family members minus 20, based on the idea that H. pylori infection usually occurs before the age of 20. We took an upper bound equal to the sum of the ages of the two family members. We assumed a uniform prior for the evolutionary time separating pairs of family isolates between these lower and upper bounds.
Approximate Bayesian Computation analysis
We performed inference under the model above using the Approximate Bayesian Computation (ABC) algorithm described by Marjoram et al. [56]. This algorithm was run independently for the serial isolates and the family isolates. The length of each run was set at 100,000 iterations, which took approximately 5 hours on a Desktop computer. Several independent runs were performed and compared manually in order to ensure that good convergence and mixing properties were achieved.
One essential step in ABC analysis is the choice of the summary statistics used, which determines how exact the inference is [52]. If the whole data were used as a summary, the algorithm would be exact but unfeasibly slow. If no summary statistic were used at all, the Markov chain would explore the prior on the parameters. It is thus important to find a handful of statistics that summarize the information contained in the data about the parameters as well as possible. Here we found that the data was well summarized by the numbers of gene fragments with zero, one, two or at least three substitutions, and the average spread of substitutions for the fragments with at least 3 substitutions. The rationale behind this choice is that fragments with one substitution are likely to be caused by mutation whereas fragments with at least 3 substitutions are likely to be caused by recombination. Therefore, even though our model makes no assumption about the cause of observed polymorphisms, the number of fragments with one substitution is informative about the mutation rate m and the number of fragments with at least 3 substitutions is informative about the recombination rate r. Furthermore, the average spread of substitutions for the fragments with at least 3 substitutions is informative about the average tract length of recombination λ.
We note that this model determines mutation and recombination by a phylogenetic approach, which implicitly assumes that each mutation is fixed rather than resulting in a polymorphism. This approach allows comparisons with the other mutation rates in Table 2, which were also calculated by a phylogenetic approach, except C. jejuni. However, as pointed out by one of the reviewers, Joshua B. Plotkin, the sequence differences we have analyzed correspond to segregating polymorphisms, which might have implications for our estimated mutation rates [16], [17], [62].
Supporting Information
Zdroje
1. AchtmanM
MorelliG
ZhuP
WirthT
DiehlI
2004 Microevolution and history of the plague bacillus, Yersinia pestis. Proc Natl Acad Sci (USA) 101 17837 17842
2. RoumagnacP
WeillF-X
DolecekC
BakerS
BrisseS
2006 Evolutionary history of Salmonella Typhi. Science 314 1301 1304
3. NübelU
RoumagnacP
FeldkampM
SongJH
KoKS
2008 Frequent emergence and limited geographic dispersal of methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci (USA) 105 14130 14135
4. SreevatsanS
PanX
StockbauerK
ConnellND
KreiswirthBN
1997 Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci (U S A) 94 9869 9874
5. PupoGM
LanR
ReevesPR
2000 Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc Natl Acad Sci (U S A) 97 10567 10572
6. LeopoldSR
MagriniV
HoltNJ
ShaikhN
MardisER
2009 A precise reconstruction of the emergence and constrained radiations of Escherichia coli O157 portrayed by backbone concatenomic analysis. Proc Natl Acad Sci (USA) 106 8713 8718
7. ParkhillJ
SebaihiaM
PrestonA
MurphyLD
ThomsonN
2003 Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nature Genet 35 32 40
8. WirthT
MorelliG
KusecekB
Van BelkumA
van der ScheeC
2007 The rise and spread of a new pathogen: seroresistant Moraxella catarrhalis. Genome Research 17 1647 1656
9. HolmesEC
2008 Evolutionary history and phylogeography of human viruses. Annu Rev Microbiol 62 307 328
10. OchmanH
WilsonAC
1987 Evolution in bacteria: Evidence for a universal substitution rate in cellular genomes. J Mol Evol 26 74 86
11. SheridanPP
FreemanKH
BrenchleyJE
2003 Estimated minimal divergence times of the major bacterial and archaeal phyla. Geomicrobiology Journal 20 1 14
12. WirthT
FalushD
LanR
CollesF
MensaP
2006 Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60 1136 1151
13. BattistuzziFU
FeijaoA
HedgesSB
2004 A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land. BMC Evol Biol 4 44
14. HoSY
LarsonG
2006 Molecular clocks: when times are a-changin'. Trends Genet 22 79 83
15. HoSY
ShapiroB
PhillipsMJ
CooperA
DrummondAJ
2007 Evidence for time dependency of molecular rate estimates. Syst Biol 56 515 522
16. RochaEP
SmithJM
HurstLD
HoldenMT
CooperJE
2006 Comparisons of dN/dS are time dependent for closely related bacterial genomes. J theor Biol 239 226 235
17. KryazhimskiyS
PlotkinJB
2008 The population genetics of dN/dS. PLoS Genet 4 e1000304 doi:10.1371/journal.pgen.1000304
18. OchmanH
ElwynS
MoranNA
1999 Calibrating bacterial evolution. Proc Natl Acad Sci (U S A) 96 12638 12643
19. AchtmanM
2008 Evolution, population structure and phylogeography of genetically monomorphic bacterial pathogens. Annu Rev Microbiol 62 53 70
20. WilsonDJ
GabrielE
LeatherbarrowAJ
CheesbroughJ
GeeS
2009 Rapid evolution and the importance of recombination to the gastroenteric pathogen Campylobacter jejuni. Mol Biol Evol 26 385 397
21. FengL
ReevesPR
LanR
RenY
GaoC
2008 A recalibrated molecular clock and independent origins for the cholera pandemic clones. PLoS ONE 3 e4053 doi:10.1371/journal.pone.0004053
22. HarrisSR
FeilEJ
HoldenMT
QuailMA
NickersonEK
2010 Evolution of MRSA during hospital transmission and intercontinental spread. Science 327 469 474
23. MenaA
SmithEE
BurnsJL
SpeertDP
MoskowitzSM
2008 Genetic adaptation of Pseudomonas aeruginosa to the airways of cystic fibrosis patients is catalyzed by hypermutation. J Bacteriol 190 7910 7917
24. SmithEE
BuckleyDG
WuZ
SaenphimmachakC
HoffmanLR
2006 Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci (USA) 103 8487 8492
25. MwangiMM
WuSW
ZhouY
SieradzkiK
De LencastreH
2007 Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci (USA) 104 9451 9456
26. WeissmanSJ
BeskhlebnayaV
ChesnokovaV
ChattopadhyayS
StammWE
2007 Differential stability and trade-off effects of pathoadaptive mutations in the Escherichia coli FimH adhesin. Infect Immun 75 3548 3555
27. ChattopadhyayS
WeissmanSJ
MininVN
RussoTA
DykhuizenDE
2009 High frequency of hotspot mutations in core genes of Escherichia coli due to short-term positive selection. Proc Natl Acad Sci (USA) 106 12412 12417
28. SuerbaumS
JosenhansC
2007 Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol 5 441 452
29. MoodleyY
LinzB
YamaokaY
WindsorHM
BreurecS
2009 The peopling of the Pacific from a bacterial perspective. Science 323 527 530
30. LinzB
BallouxF
MoodleyY
ManicaA
LiuH
2007 An African origin for the intimate association between humans and Helicobacter pylori. Nature 445 915 918
31. EppingerM
BaarC
LinzB
RaddatzG
LanzC
2006 Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLoS Genet 2 e120 doi:10.1371/journal.pgen.0020120
32. WirthT
WangX
LinzB
NovickRP
LumJK
2004 Distinguishing human ethnic groups by means of sequences from Helicobacter pylori: lessons from Ladakh. Proc Natl Acad Sci (USA) 101 4746 4751
33. FalushD
WirthT
LinzB
PritchardJK
StephensM
2003 Traces of human migrations in Helicobacter pylori populations. Science 299 1582 1585
34. AchtmanM
AzumaT
BergDE
ItoY
MorelliG
1999 Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol Microbiol 32 459 470
35. BjorkholmB
SjolundM
FalkPG
BergOG
EngstrandL
2001 Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc Natl Acad Sci (USA) 98 14607 14612
36. KangJM
IovineNM
BlaserMJ
2006 A paradigm for direct stress-induced mutation in prokaryotes. FASEB J 20 2476 2485
37. LinZ
NeiM
MaH
2007 The origins and early evolution of DNA mismatch repair genes–multiple horizontal gene transfers and co-evolution. Nucleic Acids Res 35 7591 7603
38. KangJ
BlaserMJ
2006 Bacterial populations as perfect gases: genomic integrity and diversification tensions in Helicobacter pylori. Nat Rev Microbiol 4 826 836
39. SuerbaumS
Maynard SmithJ
BapumiaK
MorelliG
SmithNH
1998 Free recombination within Helicobacter pylori. Proc Natl Acad Sci (U S A) 95 12619 12624
40. MeinersmannRJ
Romero-GalloJ
BlaserMJ
2008 Rate heterogeneity in the evolution of Helicobacter pylori and the behavior of homoplastic sites. Infect Genet Evol 8 593 602
41. KulickS
MocciaC
DidelotX
FalushD
KraftC
2008 Mosaic DNA imports with interspersions of recipient sequence after natural transformation of Helicobacter pylori. PLoS ONE 3 e3797 doi:10.1371/journal.pone.0003797
42. LinEA
ZhangXS
LevineSM
GillSR
FalushD
2009 Natural transformation of Helicobacter pylori involves the integration of short DNA fragments interrupted by gaps of variable size. PLoS Pathog 5 e1000337 doi:10.1371/journal.ppat.1000337
43. TaylorNS
FoxJG
AkopyantsNS
BergDE
ThompsonN
1995 Long-term colonization with single and multiple strains of Helicobacter pylori assessed by DNA fingerprinting. J Clin Microbiol 33 918 923
44. BergDE
GilmanRH
Lelwala-GurugeJ
SrivastavaK
ValdezY
1997 Helicobacter pylori populations in Peruvian patients. Clin Infect Dis 25 996 1002
45. KersulyteD
ChalkauskasH
BergDE
1999 Emergence of recombinant strains of Helicobacter pylori during human infection. Mol Microbiol 31 31 43
46. RaymondJ
ThibergJM
ChevalierC
KalachN
BergeretM
2004 Genetic and transmission analysis of Helicobacter pylori strains within a family. Emerg Infect Dis 10 1816 1821
47. SchwarzS
MorelliG
KusecekB
ManicaA
BallouxF
2008 Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog 4 e1000180 doi:10.1371/journal.ppat.1000180
48. TalaricoS
GoldBD
FeroJ
ThompsonDT
GuarnerJ
2009 Pediatric Helicobacter pylori isolates display distinct gene coding capacities and virulence gene marker profiles. J Clin Microbiol 47 1680 1688
49. FalushD
KraftC
TaylorNS
CorreaP
FoxJG
2001 Recombination and mutation during long-term gastric colonization by Helicobacter pylori: Estimates of clock rates, recombination size and minimal age. Proc Natl Acad Sci (U S A) 98 15056 15061
50. KuipersEJ
IsraelDA
KustersJG
GerritsMM
WeelJ
2000 Quasispecies development of Helicobacter pylori observed in paired isolates obtained years apart from the same host. J Infect Dis 181 273 282
51. DidelotX
FalushD
2007 Inference of bacterial microevolution using multilocus sequence data. Genetics 175 1251 1266
52. BeaumontMA
ZhangWY
BaldingDJ
2002 Approximate Bayesian computation in population genetics. Genetics 162 2025 2035
53. PritchardJK
SeielstadMT
Perez-LezaunA
FeldmanMW
1999 Population growth of human Y chromosomes: a study of Y chromosome microsatellites. Mol Biol Evol 16 1791 1798
54. TavareS
MarshallCR
WillO
SoligoC
MartinRD
2002 Using the fossil record to estimate the age of the last common ancestor of extant primates. Nature 416 726 729
55. VoightBF
AdamsAM
FrisseLA
QianY
HudsonRR
2005 Interrogating multiple aspects of variation in a full resequencing data set to infer human population size changes. Proc Natl Acad Sci (USA) 102 18508 18513
56. MarjoramP
MolitorJ
PlagnolV
TavareS
2003 Markov chain Monte Carlo without likelihoods. Proc Natl Acad Sci (USA) 100 15324 15328
57. GelmanA
MengXL
SternH
1996 Posterior predictive assessment of model fitness via realized discrepancies. Statistica Sinica 6 733 760
58. IngvarssonPK
2008 Multilocus patterns of nucleotide polymorphism and the demographic history of Populus tremula. Genetics 180 329 340
59. ThorntonK
AndolfattoP
2006 Approximate Bayesian inference reveals evidence for a recent, severe bottleneck in a Netherlands population of Drosophila melanogaster. Genetics 172 1607 1619
60. HolmesEC
2010 The comparative genomics of viral emergence. Proc Natl Acad Sci (USA) 107 Supplement 1 1742 1746
61. JolleyKA
ChanMS
MaidenMC
2004 mlstdbNet - distributed multi-locus sequence typing (MLST) databases. BMC Bioinformatics 5 86
62. PetersonGI
MaselJ
2009 Quantitative prediction of molecular clock and Ka/Ks at short timescales. Mol Biol Evol 26 2595 2603
63. TombJF
WhiteO
KerlavageAR
ClaytonRA
SuttonGG
1997 The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388 539 547
64. TomitaniA
KnollAH
CavanaughCM
OhnoT
2006 The evolutionary diversification of cyanobacteria: molecular-phylogenetic and paleontological perspectives. Proc Natl Acad Sci (USA) 103 5442 5447
65. ButterfieldNJ
2000 Bangiomorpha pubescens n. gen., n. sp.: implications for the evolution of sex, multicellularity, and the Mesoproterozoic/Neoproterozoic radiation of eukaryotes. Paleobiology 26 386 404
66. HollandHD
2002 Volcanic gases, black smokers, and the Great Oxidation Event. Geochimica et Cosmochimica Acta 66 3811 3826
67. JavauxEJ
KnollAH
WalterMR
2001 Morphological and ecological complexity in early eukaryotic ecosystems. Nature 412 66 69
68. Date-a-Clade service for the molecular tree of life 2009 Age of crown Bilateria. http://www.fossilrecord.net/dateaclade/clade_crown_Bilateria.html
69. Date-a-Clade service for the molecular tree of life 2009 Age of mammals. http://www.fossilrecord.net/dateaclade/clade-platypus-elephant.html
70. Tree of Life web project 2009 Age of legumes. http://tolweb.org/Fabaceae/21093
71. MoranNA
McLaughlinHJ
SorekR
2009 The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria. Science 323 379 382
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 7
-
Všechny články tohoto čísla
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
- Tinkering Evolution of Post-Transcriptional RNA Regulons: Puf3p in Fungi as an Example
- The Importance of Imprinting in the Human Placenta
- Regulator of G Protein Signaling 3 Modulates Wnt5b Calcium Dynamics and Somite Patterning
- Lysosomal Dysfunction Promotes Cleavage and Neurotoxicity of Tau
- Combinatorial Binding Leads to Diverse Regulatory Responses: Lmd Is a Tissue-Specific Modulator of Mef2 Activity
- Variation, Sex, and Social Cooperation: Molecular Population Genetics of the Social Amoeba
- Comparative Analysis of DNA Replication Timing Reveals Conserved Large-Scale Chromosomal Architecture
- The Fitness Landscapes of -Acting Binding Sites in Different Promoter and Environmental Contexts
- Cohesin Is Limiting for the Suppression of DNA Damage–Induced Recombination between Homologous Chromosomes
- Genome-Wide Analysis Reveals Novel Genes Essential for Heme Homeostasis in
- Genome-Wide Meta-Analysis for Serum Calcium Identifies Significantly Associated SNPs near the Calcium-Sensing Receptor () Gene
- Rad3 Decorates Critical Chromosomal Domains with γH2A to Protect Genome Integrity during S-Phase in Fission Yeast
- Quantitative and Molecular Genetic Analyses of Mutations Increasing Life Span
- Association of Variants at with Chronic Kidney Disease and Kidney Stones—Role of Age and Comorbid Diseases
- Breast Cancer DNA Methylation Profiles Are Associated with Tumor Size and Alcohol and Folate Intake
- Calpain 8/nCL-2 and Calpain 9/nCL-4 Constitute an Active Protease Complex, G-Calpain, Involved in Gastric Mucosal Defense
- A Collection of Target Mimics for Comprehensive Analysis of MicroRNA Function in
- A Genome-Wide Analysis Reveals No Nuclear Dobzhansky-Muller Pairs of Determinants of Speciation between and , but Suggests More Complex Incompatibilities
- Microevolution of during Prolonged Infection of Single Hosts and within Families
- Id4, a New Candidate Gene for Senile Osteoporosis, Acts as a Molecular Switch Promoting Osteoblast Differentiation
- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Chromatin Remodeling in Development and Disease: Focus on CHD7
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Requirement of Male-Specific Dosage Compensation in Females—Implications of Early X Chromosome Gene Expression
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



