-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation of Variants at with Chronic Kidney Disease and Kidney Stones—Role of Age and Comorbid Diseases
Chronic kidney disease (CKD) is a worldwide public health problem that is associated with substantial morbidity and mortality. To search for sequence variants that associate with CKD, we conducted a genome-wide association study (GWAS) that included a total of 3,203 Icelandic cases and 38,782 controls. We observed an association between CKD and a variant with 80% population frequency, rs4293393-T, positioned next to the UMOD gene (GeneID: 7369) on chromosome 16p12 (OR = 1.25, P = 4.1×10−10). This gene encodes uromodulin (Tamm-Horsfall protein), the most abundant protein in mammalian urine. The variant also associates significantly with serum creatinine concentration (SCr) in Icelandic subjects (N = 24,635, P = 1.3×10−23) but not in a smaller set of healthy Dutch controls (N = 1,819, P = 0.39). Our findings validate the association between the UMOD variant and both CKD and SCr recently discovered in a large GWAS. In the Icelandic dataset, we demonstrate that the effect on SCr increases substantially with both age (P = 3.0×10−17) and number of comorbid diseases (P = 0.008). The association with CKD is also stronger in the older age groups. These results suggest that the UMOD variant may influence the adaptation of the kidney to age-related risk factors of kidney disease such as hypertension and diabetes. The variant also associates with serum urea (P = 1.0×10−6), uric acid (P = 0.0064), and suggestively with gout. In contrast to CKD, the UMOD variant confers protection against kidney stones when studied in 3,617 Icelandic and Dutch kidney stone cases and 43,201 controls (OR = 0.88, P = 5.7×10−5).
Published in the journal: . PLoS Genet 6(7): e32767. doi:10.1371/journal.pgen.1001039
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001039Summary
Chronic kidney disease (CKD) is a worldwide public health problem that is associated with substantial morbidity and mortality. To search for sequence variants that associate with CKD, we conducted a genome-wide association study (GWAS) that included a total of 3,203 Icelandic cases and 38,782 controls. We observed an association between CKD and a variant with 80% population frequency, rs4293393-T, positioned next to the UMOD gene (GeneID: 7369) on chromosome 16p12 (OR = 1.25, P = 4.1×10−10). This gene encodes uromodulin (Tamm-Horsfall protein), the most abundant protein in mammalian urine. The variant also associates significantly with serum creatinine concentration (SCr) in Icelandic subjects (N = 24,635, P = 1.3×10−23) but not in a smaller set of healthy Dutch controls (N = 1,819, P = 0.39). Our findings validate the association between the UMOD variant and both CKD and SCr recently discovered in a large GWAS. In the Icelandic dataset, we demonstrate that the effect on SCr increases substantially with both age (P = 3.0×10−17) and number of comorbid diseases (P = 0.008). The association with CKD is also stronger in the older age groups. These results suggest that the UMOD variant may influence the adaptation of the kidney to age-related risk factors of kidney disease such as hypertension and diabetes. The variant also associates with serum urea (P = 1.0×10−6), uric acid (P = 0.0064), and suggestively with gout. In contrast to CKD, the UMOD variant confers protection against kidney stones when studied in 3,617 Icelandic and Dutch kidney stone cases and 43,201 controls (OR = 0.88, P = 5.7×10−5).
Introduction
Chronic kidney disease (CKD) is a common disorder that can progress to kidney failure and is associated with an increased risk of cardiovascular disease and mortality [1], [2]. The cause of CKD is not always known and frequently appears multifactorial with hypertension (HTN) and diabetes mellitus (DM) being the most important causes [3]–[6]. Other causes include intrinsic kidney disorders, atherosclerosis and nephrotoxic drugs [7], [8]. Studies also indicate a dramatic increase in the prevalence of CKD with advancing age [9], [10]. With greater lifespan, the burden of CKD is thus steadily rising in the Western world [11], resulting in a substantial impact on the health care system [12].
Previous studies have suggested a genetic contribution to the risk of kidney disease. Heritability estimates of serum creatinine (SCr) and estimated glomerular filtration rate based on SCr (eGFRcrea), both common measures of kidney function, have been reported as 29% and 33%, respectively [13]. Recently, Köttgen et al. [14] published the first genome-wide association study (GWAS) on eGFRcrea, eGFR based on cystatin C (eGFRcys), another measure of kidney function, and CKD, reporting significant association with eGFRcrea at three loci (UMOD, SHROOM3 (GeneID: 57619) and GATM-SPATA5L1 (GeneIDs: 2628 and 79029)), with eGFRcys at two loci (CST3-CST9 (GeneIDs: 1471 and 128822) and STC1 (GeneID: 6781)) and with CKD at one locus (UMOD) [14].
With the aim of discovering sequence variants that associate with kidney function, we conducted a GWAS in a total of 3,203 Icelanders with CKD and 38,782 controls and in 24,635 Icelandic and 1,819 Dutch subjects with SCr information. We found a sequence variant at the UMOD locus that associates with both CKD and SCr at a genome wide-significant (GWS) level, providing an independent replication of the result by Köttgen et al [14]. We also show that this variant interacts with age-related increase in SCr levels with little or no effect on SCr before the age of 50 years, followed by a rapidly growing effect with increasing age. We demonstrate that this variant associates significantly with serum urea, uric acid and suggestively with gout. In contrast to the deleterious effect on kidney function, the variant confers protection against kidney stone disease.
Results/Discussion
Genome-wide association of variants at the UMOD locus with CKD and SCr
A GWAS of 2.5 million SNPs, either directly genotyped (Illumina HumanHap300 or HumanHapCNV370 bead chips) or imputed based on the HapMap CEU samples [15], was performed on a sample set of 2,903 Icelanders with CKD (see Materials and Methods for sample set description) and 35,818 controls and also on 22,256 Icelandic subjects with SCr information (See QQ-plots in Figure S1 and Figure S2). The Icelandic SCr measurements came from two laboratories; the Laboratory in Mjodd, a private outpatient laboratory, and the Clinical Biochemistry Laboratory of Landspitali University Hospital (LUH), serving both inpatients and outpatients. These subjects had 5.2 SCr measurements on average (geometric mean) and we used the median SCr value for each individual in the subsequent analysis. The SCr values from the two Icelandic laboratories showed similar dependence on age and sex but there was clearly a trend towards higher SCr in the hospital laboratory compared with the outpatient laboratory (Figure S3).
The GWAS on CKD and SCr both yielded several SNPs in high linkage disequilbrium (LD) on chromosome 16p12 with GWS (P<5×10−8) association to increased risk of CKD and elevated SCr. For both phenotypes, this signal is tagged by rs4293393-T (Table 1 and Table 2). For CKD, the odds ratio (OR) conferred by rs4293393-T was 1.25 (95% CI = 1.16–1.34) with a corresponding P value of 6.2×10−9. In an attempt to replicate this finding, rs4293393 was typed in additional 300 Icelandic subjects with CKD and 2,964 controls. The association was nominally significant in the replication sample set and the effect size consistent with the initial observation (Table 1). The combined OR for rs4293393-T in the two Icelandic CKD sample sets was 1.25 (95% CI = 1.17–1.35) and P = 4.1×10−10. The association between SCr and rs4293393-T on 16p12 was very strong with an effect of 1.86 µmol/L per allele carried and P = 6.7×10−20 (Table 2). To follow up on these results, we genotyped rs4293393 in 2,379 additional Icelanders with SCr information, significantly replicating the initially observed effect (P = 1.4×10−5, Table 2). Analysis of the combined Icelandic datasets showed a strong GWS association between rs4293393-T and elevated SCr (effect = 1.93 µmol/L per allele, 95% CI = 1.55–2.31 µmol/L; P = 1.3×10−23). Our findings provide an independent replication of the recently reported results by Köttgen et al [14] of an association of this 16p12 locus with CKD and eGFRcrea. The strongest SNP associations outside the UMOD region on chromosome 16p12 are shown in Table S1 (for CKD) and Table S2 (for SCr), respectively.
Tab. 1. Association of rs4293393-T with chronic kidney disease (CKD). 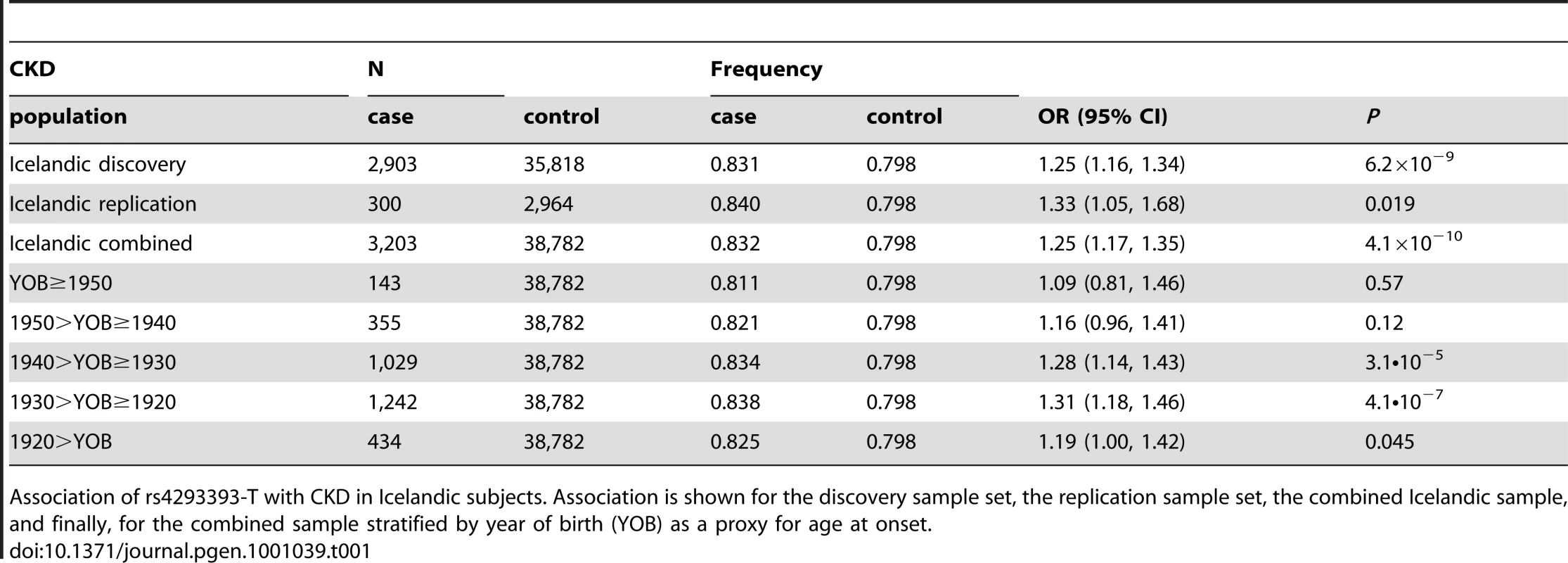
Association of rs4293393-T with CKD in Icelandic subjects. Association is shown for the discovery sample set, the replication sample set, the combined Icelandic sample, and finally, for the combined sample stratified by year of birth (YOB) as a proxy for age at onset. Tab. 2. Association of rs4293393-T with serum creatinine concentration (SCr). 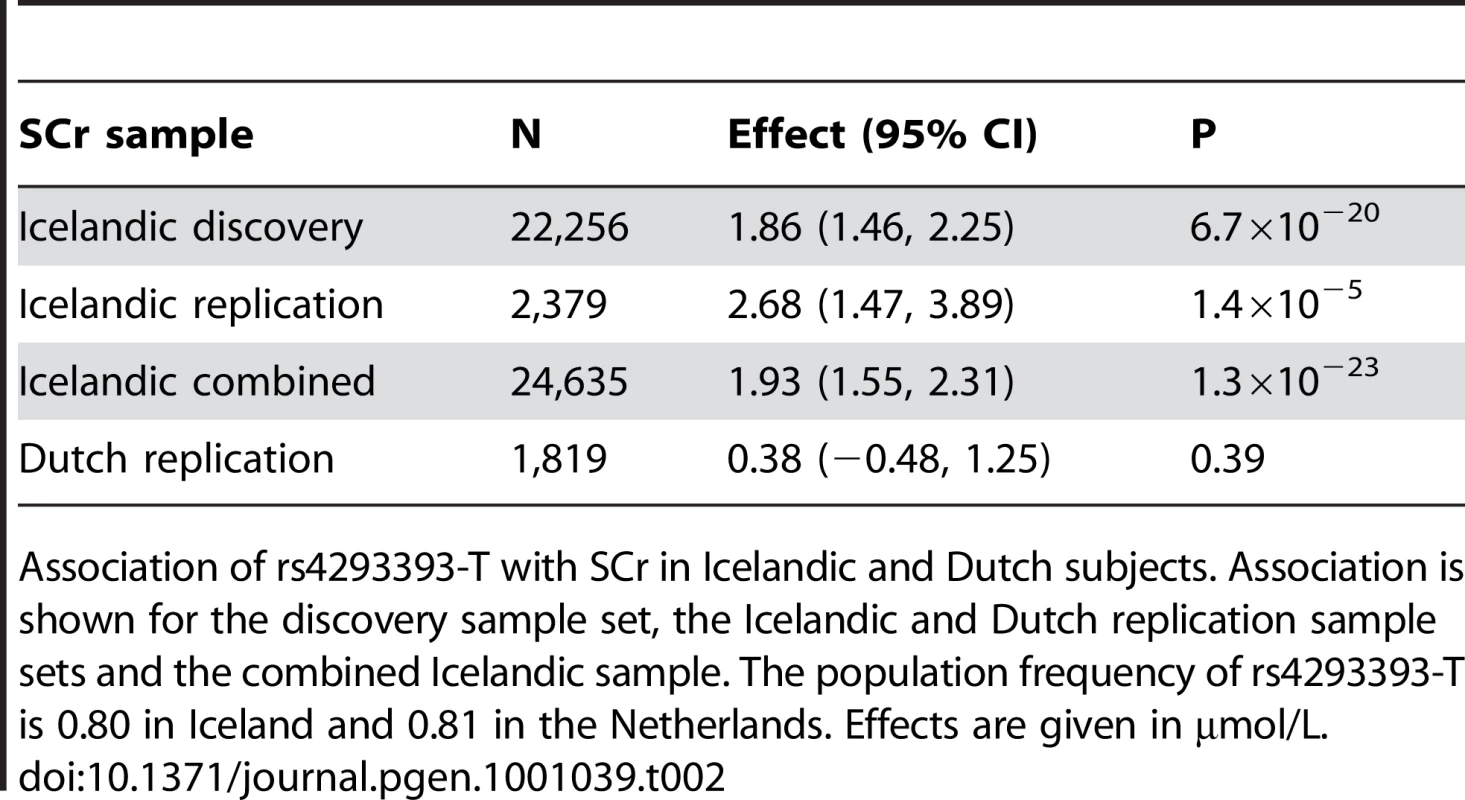
Association of rs4293393-T with SCr in Icelandic and Dutch subjects. Association is shown for the discovery sample set, the Icelandic and Dutch replication sample sets and the combined Icelandic sample. The population frequency of rs4293393-T is 0.80 in Iceland and 0.81 in the Netherlands. Effects are given in µmol/L. For further assessment, we tested rs4293393 in 1,819 Dutch subjects with SCr information. These were healthy population-based controls (see Materials and Methods for sample set description) with SCr values substantially different from the Icelandic measurements, generally showing lower values and much less variability (Figure S3). Interestingly, no association was observed in the 1,819 healthy Dutch subjects (effect = 0.38 µmol/L, 95%CI = −0.48–1.25 µmol/L; P = 0.39) (Table 2). Significant heterogeneity was observed between the SCr association results for the Icelandic and Dutch populations (I2 = 90.4%, P = 0.0013).
The SNP rs4293393 is located 550 basepairs upstream of UMOD, encoding uromodulin, also known as the Tamm-Horsfall protein (Figure 1). The protein is a glycosylphosphatidylinositol (GPI)-anchored glycoprotein, exclusively expressed in the thick ascending loop of Henle [16] and distal convoluted tubule [17] of the mammalian kidney. It is the most abundant protein in urine of healthy individuals, where it is present in a highly aggregated state [18], [19]. While the exact physiological function of uromodulin remains to be elucidated, it has the capacity to bind to a variety of ligands. It has been reported to prevent bacteria from adhering to human kidney cells [20] and to inhibit calcium oxalate crystallization [21]. It may also have a role in ion transport and immunological processes [22], [23]. UMOD knockout mice have been shown to have decreased creatinine clearance [24] and predilection for both urinary tract infections [25] and calcium oxalate stone formation [26].
Fig. 1. An overview of the region around rs4293393. 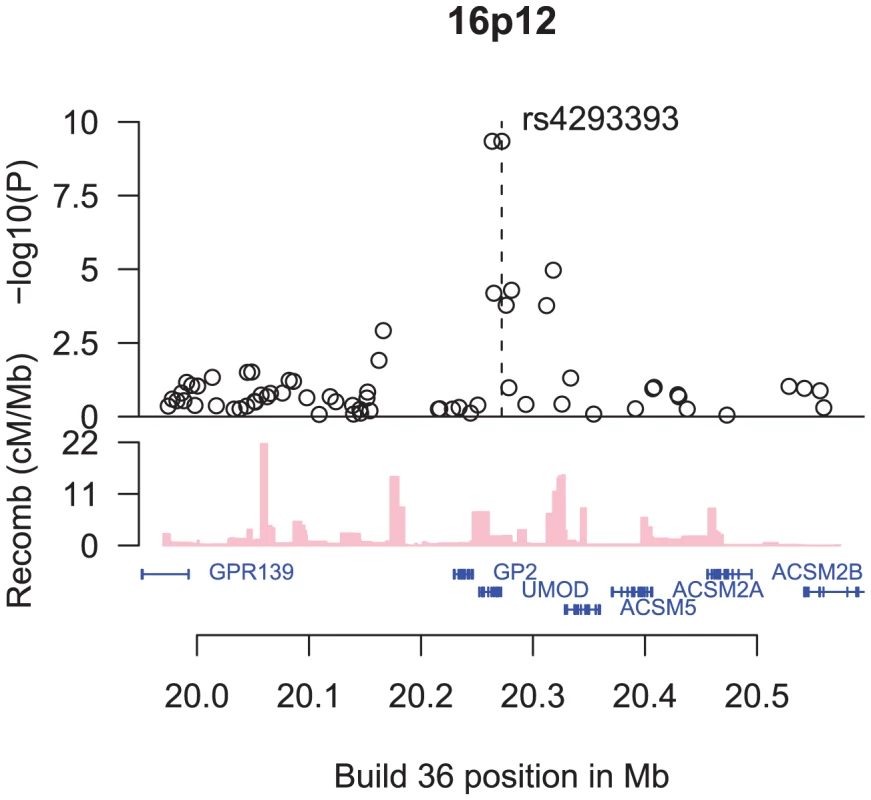
Shown are the −log10 association P values of SNPs in the region with CKD (black circles), the SNPs' build 36 coordinates, the genes in the region and their exons (in blue), and recombination rates in centimorgans (cM) per megabase (Mb) (pink histogram). The rs4293393 variant is in perfect LD in the HapMap CEU samples [15] with a synonymous SNP in UMOD, rs13335818, coding for V264V (D′ = 1.0, r2 = 1.0). The same perfect correlation between rs4293393 and rs13335818 was observed in a set of 3,364 Icelanders (D′ = 1.0, r2 = 1.0). Both rs4293393 and rs13335818 are also in perfect correlation with rs12917707 (D′ = 1.0, r2 = 1.0 for both markers in the HapMap CEU samples [15]) near the UMOD, the variant reported by Köttgen et al [14] to associate with both CKD and eGFRcrea with similar effect, indicating that these SNPs are tagging the same signal. As rs4293393 is on the Illumina 300/370K chips we used for direct genotyping, we refer to rs4293393 in the remainder of this article.
The effect of the UMOD variant on SCr is age-dependent
Given that SCr varies substantially with both age and sex, we tested for an interaction between the effect of rs4293393-T and effects of age and sex on SCr. No interaction was found between the UMOD variant and sex (P = 0.41). In contrast, a strong interaction was observed between the UMOD variant and age in the Icelandic sample set (P = 3.0×1017). On average, SCr increased by an additional 0.09 µmol/L per year per allele of rs4293393-T (95% CI = 0.07–0.11). In order to visualize this interaction, we stratified our Icelandic samples based on age and sex and tested for association within each stratum (Figure 2A). Interestingly, rs4293393-T has little or no effect on SCr before the age of 50 years, but thereafter the effect increases rapidly with advancing age, especially around 70 years. Thus, the variant does not affect SCr in young individuals but rather how SCr increases with age. We note that due to the relatively short time span in which the SCr data were collected there is an inherent confounding between age and generation in our study, which will require further investigation to resolve. Similar interaction between the UMOD variant, age and CKD was also observed when the association analysis for CKD was stratified by year of birth used here as a proxy for age of onset (Table 1).
Fig. 2. An overview of the effect of age and the number of comorbid conditions on SCr levels, directly and through the rs4293393-T allele count. 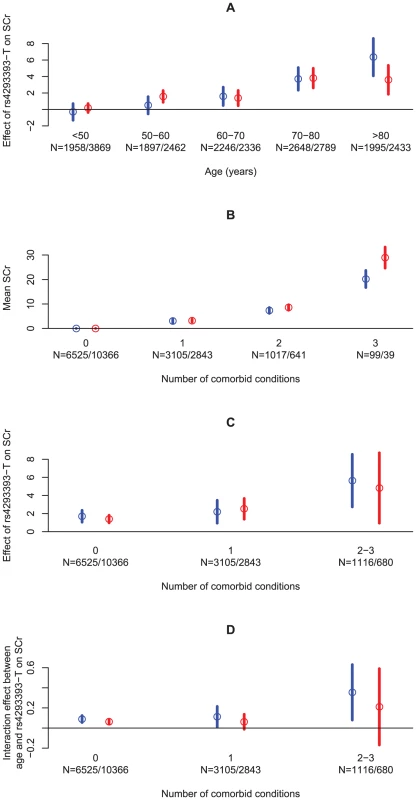
(A) The effect of rs4293393-T on SCr stratified on age and sex. (B) The mean SCr stratified by the number of comorbid conditions and sex, compared to the mean SCr in those without any comorbid conditions. (C) The effect of rs4293393-T on SCr stratified by the number of comorbid conditions and sex. (D) The interaction effect between age and rs4293393-T allele count on SCr stratified by the number of comorbid conditions and sex. The circles give the point estimates and the vertical lines show their 95% confidence intervals. Estimates and confidence intervals are given in blue for males and red for females. Sample sizes (N) are given for each strata for males and females, respectively. Effects are given in µmol/L in (A–C) and µmol/L/year in (D). Although it is well known that kidney function declines with age, GFR has been shown to decrease more slowly with senescence in healthy individuals than previously thought [3]–[5], [11]. Comorbid conditions that increase in frequency with aging, including HTN, DM, atherosclerosis and heart failure are, however, increasingly recognized as important contributors of age-related decline in kidney function [3], [4], [6]–[8]. We thus proceeded to investigate whether the age effect observed in carriers of the UMOD variant is influenced by interaction with age-related risk factors for decline in kidney function.
UMOD-associated increase in SCr with age is affected by the number of comorbid conditions present
As HTN, type 2 DM and atherosclerosis are all well recognized age-dependent risk factors for CKD [3], [4], [6]–[8], the association analysis was repeated after stratifying the SCr data based on these conditions. Incomplete information regarding history of HTN (5,705 cases), type 2 DM (1,422 cases) and myocardial infarction (MI, 2,551 cases) was available for the Icelandic SCr sample set. In parallel with previous studies, the rate of increase in SCr with age was significantly higher in individuals with HTN than in individuals without this diagnosis (effect = 0.23 µmol/L/year, 95% CI = 0.19–0.26 µmol/L/year; P = 2.9×10−35). Similar results were obtained for type 2 DM (effect = 0.26 µmol/L/year, 95% CI = 0.19–0.34 µmol/L/year; P = 1.1×10−11) and MI (effect = 0.36 µmol/L/year, 95% CI = 0.30–0.42 µmol/L/year; P = 1.4×10−32) as well as the number of comorbid conditions (Figure 2B). We also found that the effect of rs4293393-T on SCr increases with the number of comorbid conditions present (Figure 2C).
To further assess the relationship between genotype, age and risk factors for reduced kidney function, we investigated the effect of the rs4293393-T allele count on the increase in SCr with age stratifying on HTN and type 2 DM. A trend was observed for a higher rate of increase in SCr with age and rs4293393-T allele count in individuals with HTN compared to those without a diagnosis of HTN (P = 0.077) as well as in those with type 2 DM compared to those without (P = 0.063). In other words, the age-related increase in SCr levels appears to be greater in rs4293393-T carriers that have either HTN or type 2 DM than in carriers who do not have these risk factors. However, an age effect was still observed after accounting for these age-related risk factors. Furthermore, we also observed a significantly higher rate of SCr increase with age and rs4293393-T allele count stratifying on the number of comorbid conditions present (P = 0.0080) (Figure 2D).
To determine whether rs4293393-T influenced kidney function by directly affecting known risk factors, we tested the association of rs4293393-T in well powered Icelandic case-control sets of HTN, MI, stroke, and type 2 DM (Table S3). A weak nominally significant association of rs4293393-T with increased risk of HTN was observed (OR = 1.07, 95% CI = 1.01–1.12; P = 0.014), but not with the other diseases tested. These data demonstrate that the effect of rs4293393-T on kidney function is not mediated through increased risk of these comorbid conditions, but rather suggest that the variant may affect the vulnerability of the kidney to these risk factors.
These findings, demonstrating not only the effect of age on UMOD-associated increase in SCr but also the effect of age-related comorbid conditions, may explain why no association was observed between rs4293393-T and kidney function in the Dutch sample set of healthy population-based subjects with much lower SCr values and of much less variability than observed in the Icelandic samples (Figure S3).
Association of the UMOD variant with serum urea
Urea is another small solute that is commonly used to assess renal function together with SCr. The correlation between SCr and serum urea in our data was 58%. We tested for association between rs4293393-T and serum urea in an Icelandic sample set that had urea measurements performed at the Laboratory in Mjodd (N = 4,084) and found significant association with increased serum urea concentration (effect = 0.36 mg/dL, 95% CI = 0.23–0.50 mg/dL; P = 1.0×10−6).
Association of the UMOD variant with uric acid and gout
In humans, rare mutations in the UMOD gene that cause accumulation of abnormal uromodulin in the nephron and reduced urinary excretion of the normal protein [27] have been associated with two autosomal dominant kidney diseases with overlapping clinical features, medullary cystic kidney disease and familial juvenile hyperuricemic nephropathy [28]. These disorders are characterized by hyperuricemia, gout and progressive renal failure due to tubulointerstitial nephropathy. Given the link between UMOD and hyperuricemia, we tested rs4293393-T in Icelandic subjects with serum uric acid values from the Laboratory in Mjodd (N = 6,583) and observed significant association (effect = 6.1, 95% CI = 1.7–10.4; P = 0.0064). We then tested for association with gout in a set of 377 Icelandic cases and 39,916 controls (see Materials and Methods for sample set description) and found a suggestive association (OR = 1.17, 95% CI = 0.97–1.41; P = 0.097). These data contrast the work of Köttgen et al [14] that neither detected association with serum uric acid nor gout.
Reduced risk of kidney stone formation in carriers of the UMOD CKD risk variant
As uromodulin is thought to prevent the formation of calcium-containing kidney stones [21], we tested rs4293393 in a sample set of 1,689 Icelandic patients with radiopaque kidney stones and 37,076 Icelandic population controls. We observed a significant association between rs4293393-T and reduced risk of kidney stones (OR = 0.88, 95% CI = 0.81–0.96; P = 0.0053). In an attempt to replicate this observation, we genotyped rs4293393 in two additional sample sets of European ancestry, one from Iceland (1,271 cases and 3,177 controls) and the other from the Netherlands (701 cases and 2,948 controls) (Table 3). The effect size in both replication samples is consistent with the initial observation and the association is significant in the combined replication samples (OR = 0.89, 95% CI = 0.81–0.97; P = 0.0089). There was no correlation between the effect size and year of birth of the kidney stone patients (Table S4).
Tab. 3. Association of rs4293393-T with kidney stones. 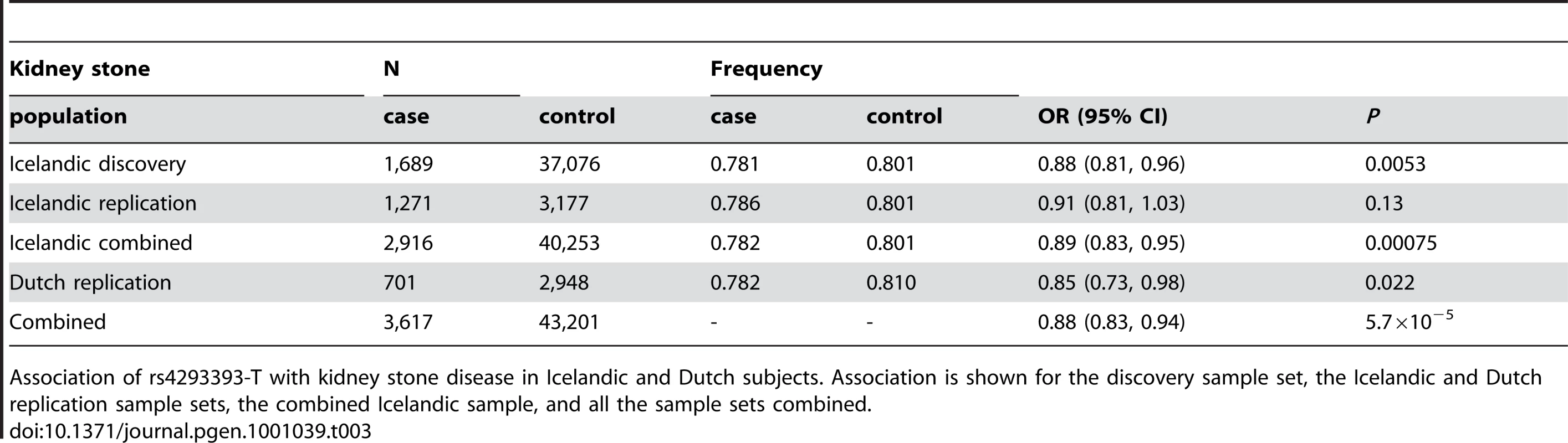
Association of rs4293393-T with kidney stone disease in Icelandic and Dutch subjects. Association is shown for the discovery sample set, the Icelandic and Dutch replication sample sets, the combined Icelandic sample, and all the sample sets combined. Replication of the SHROOM3 and GATM-SPATA5L1 eGFRcrea loci and the STC1 and CST3-CST9 eGFRcys loci
Köttgen et al [14] reported on variants at additional loci with GWS association to eGFRcrea (SHROOM3 and GATM-SPATA5L1) or eGFRcys (STC1 and CST3-CST9). We tested these variants in our Icelandic datasets, including a small sample set with cystatin C measurements (Table 4). The association with SCr replicated for both the SHROOM3 and GATM-SPATA5L1 SNPs (P = 0.00057 and P = 0.0067, respectively) and the association with cystatin C replicated for the CST3-CST9 SNP but not the STC1 SNP (P = 0.00037 and P = 0.73, respectively). It should be noted, however, that the Icelandic cystatin C sample set is very small (N = 284) and possibly underpowered to replicate the reported association with the STC1 SNP. The STC1 SNP did show association with SCr in our dataset (P = 1.6·10−6) as was observed in the analysis by Köttgen et al [14]. The SHROOM3 and GATM-SPATA5L1 SNPs showed suggestive association with CKD in the analysis by Köttgen et al [14] and our data support this association but do not constitute a conclusive replication. In contrast to the UMOD variant, we did not observe an interaction between the variants at these other loci and age. Furthermore, none of the SNPs did associate with kidney stones in Iceland (data not shown). Finally, Köttgen et al [14] reported suggestive association between eGFRcrea and variants at JAG1 (GeneID: 182); we did not replicate this finding in our SCr scan (for rs6040055-T: effect = −0.27, 95% CI = −0.60−0.05), P = 0.17).
Tab. 4. Replication of loci recently found to associate with eGFRcrea and eGFRcys. 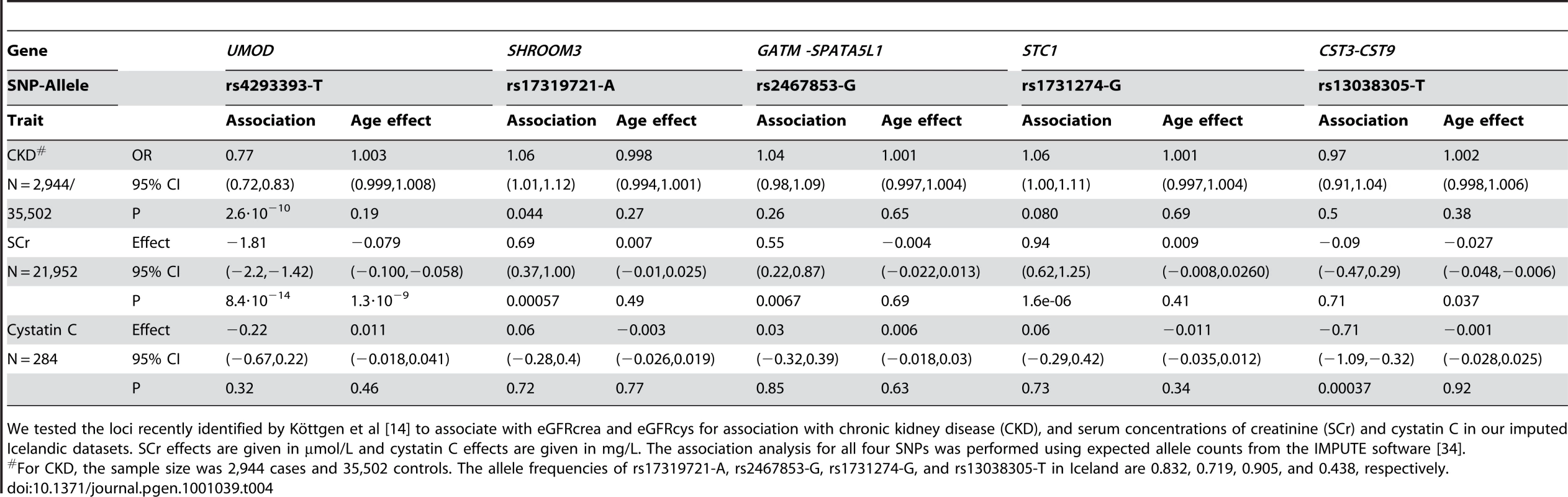
We tested the loci recently identified by Köttgen et al [14] to associate with eGFRcrea and eGFRcys for association with chronic kidney disease (CKD), and serum concentrations of creatinine (SCr) and cystatin C in our imputed Icelandic datasets. SCr effects are given in µmol/L and cystatin C effects are given in mg/L. The association analysis for all four SNPs was performed using expected allele counts from the IMPUTE software [34]. In summary, we describe sequence variants next to and in UMOD that associate with increased risk of CKD and elevated SCr but confer protection against kidney stones. We also demonstrate an interaction between these variants and both age and comorbid conditions that are related to decline in kidney function. Our observations indicate that UMOD is important for maintaining kidney function with advancing age and exposure to risk factors that are associated with aging, such as HTN, type 2 DM and cardiovascular disease.
Materials and Methods
Study subjects from Iceland
Landspitali University Hospital (LUH) is a regional hospital for the greater Reykjavík area and a tertiary referral center for the entire Icelandic nation. The population of Iceland is comprised of 330,000 inhabitants of whom approximately 200,000 reside in the greater Reykjavik area. The nation's only nephrology clinic is located at LUH and all laboratory tests for the primary care clinics of the greater Reykjavik area are performed in the hospital's laboratories. We obtained results of all SCr measurements performed during the period 2003 to 2008 from the computerized database of the Clinical Laboratories at LUH and used the SCr values to identify those with chronic kidney disease (CKD) based on calculation of the estimated glomerular filtration rate (eGFR) by the original 4-variable Modification of Diet in Renal Disease (MDRD) study equation. We classified those with eGFR<60 ml/min/1.73 m2 as having CKD. All individuals with acute kidney injury and those who had eGFR<60 ml/min/1.73 m2 of less than 3 months duration were excluded from the CKD sample set. The study included CKD patients that had donated blood through various genetic programs at deCODE genetics.
Biochemical measurements including SCr, serum urea, serum uric acid and serum cystatin C were available from two laboratories, the Laboratory in Mjodd, Reykjavik, Iceland, a private outpatient laboratory, and the Clinical Biochemistry Laboratory of LUH, serving both inpatients and outpatients. The main referral center for the Laboratory in Mjodd is a multispecialty medical clinic in Reykjavik (Laeknasetrid). The laboratory tests were done in the years 1997–2008 at the Laboratory in Mjodd and in the years 2003–2008 at LUH. The Icelandic SCr measurements came from both laboratories, the Laboratory in Mjodd (N = 10,260) and LUH (N = 22,898, of whom 8,523 also had measurements from the Laboratory in Mjodd). At the LUH, the same enzymatic method was used for measurement of SCr during the study period (Vitros 950 Autoanalyzer, Ortho Clinical Diagnostics, Rochester, MN, USA), whereas at the Laboratory in Mjodd, SCr measurements were performed by modified kinetic Jaffe rection assays until May 2007 when an enzymatic method was introduced.
The Icelandic kidney stone cases consisted of patients with confirmed radiopaque kidney stones from the Icelandic Kidney Stone Disease Registry at LUH. The study included kidney stone patients that had donated blood through various genetic programs at deCODE genetics.
The coronary artery disease [29], stroke [30] and type 2 DM [31], [32] patient groups from Iceland have been described previously. The HTN sample set includes individuals who have been recruited into various genetic programs at deCODE and have: (1) self-reported HTN; (2) received the diagnosis of HTN at discharge from the LUH; or (3) have attended the Hypertension Clinic at LUH. The gout sample set includes subjects who were recruited into various genetic programs at deCODE and reported the use of either one of two specific anti-gout medications, allopurinol or colchicine.
The Icelandic controls used in the case-control genome-wide association studies (GWAS) and replication studies were selected among individuals who had participated in the various genetic programs at deCODE genetics; tremor, preeclampsia, endometriosis, psoriasis, type 2 DM, Alzheimer's disease, osteoarthritis, schizophrenia, peripheral artery disease, abdominal aortic aneurysm, chronic obstructive pulmonary disease, stroke, osteoporosis, coronary artery disease, HTN, asthma, Parkinson's disease, sleep apnea, age-related macular degeneration, polycystic ovary syndrome, rheumatoid arthritis, lung cancer, longevity, benign prostatic hyperplasia, enuresis, migraine, glaucoma, attention deficit hyperactivity disorder, prostate cancer, infectious diseases, anxiety, expression studies, autism, dyslexia, melanoma, colorectal cancer, deep vein thrombosis, restless leg syndrome, studies on addiction and population controls. Individuals who reported a history of the trait being tested (e.g. CKD) were excluded from the control set. Some of the controls participated in more than one genetic program in which case their genotypes are only included once.
The study was approved by the Icelandic Data Protection Authority and the National Bioethics Committee. All patients signed informed consent and donated blood samples. Personal identities of the patients and biological samples were encrypted by a third party system provided by the Icelandic Data Protection Authority.
Study subjects from The Netherlands
All samples with SCr measurements came from the Nijmegen Biomedical Study. The details of this study have been reported previously [33]. Briefly, this is a population-based survey conducted by the Department of Epidemiology and Biostatistics and the Department of Clinical Chemistry of the Radboud University Nijmegen Medical Center (RUNMC), in which 9,371 individuals participated from a total of 22,500 age - and sex-stratified, randomly selected inhabitants of Nijmegen. The subjects were invited to participate in a study on gene-environment interactions in complex diseases. All participants filled out a questionnaire on lifestyle and medical history at baseline and 6500 of them donated blood samples for DNA isolation and biochemical studies. A fraction of the study participants were previously genotyped with the Illumina HumanHap300 or HumanHapCNV370 bead chips; these were selected to serve as controls in GWAS on prostate and breast cancer and were selected primarily based on age. A total of 1,819 individuals had both serum creatinine measurements and genome-wide SNP data available for analysis in this study.
The Dutch patients with kidney stones were recruited from two sources: The outpatient clinics of the RUNMC and The Nijmegen Biomedical Study. All patients who present to the outpatient clinics of the RUNMC are invited to participate in a study on the effects of genes and lifestyle on the development of urological diseases. In case of consent, the patients fill out a questionnaire on lifestyle and donate a blood sample for DNA isolation. The controls for the analysis of kidney stone disease were also taken from the biobank of the Nijmegen Biomedical Study. All patients and controls were of self-reported European descent and were fully informed about the goals and the procedures of these studies. The study protocols for the recruitment of patients from outpatient clinics and the recruitment of participants to the Nijmegen Biomedical Study were approved by the RUNMC Institutional Review Board. All study subjects gave written informed consent.
Illumina genome-wide genotyping
All Icelandic case and control samples were assayed with the Illumina HumanHap300 or HumanHapCNV370 bead chips (Illumina, SanDiego, CA, USA), containing 317,503 and 370,404 haplotype tagging SNPs derived from phase I of the International HapMap project, respectively. Only SNPs present on both chips were included in the analysis and SNPs were excluded if they had: (a) yield lower than 95% in cases or controls; (b) minor allele frequency less than 1% in the population; or (c) showed significant deviation from Hardy-Weinberg equilibrium in the controls (P<0.001). Any samples with a call rate below 98% were excluded from the analysis. The final analysis included 302,379 SNPs.
Imputation of SNP genotypes
The genome-wide association scan was based on expected allele counts obtained with the IMPUTE software [34], using the HapMap CEU samples as a training set [15]. The test for association was then performed using the expected allele counts as covariates. The imputation information was estimated by the ratio of the observed likelihood of allele counts and the likelihood of allele counts assuming perfect information under the assumption of Hardy-Weinberg equilibrium. The estimated information for the four SNPs imputed in Table 4 was high in all instances (>0.96).
Single SNP genotyping
Single SNP genotyping of all samples was carried out at deCODE genetics in Reykjavik, Iceland, applying the same platform to all populations studied. All single SNP genotyping was carried out using the Centaurus (Nanogen) platform [35]. The quality of each Centaurus SNP assay was evaluated by genotyping each assay on the CEU samples and comparing the results with the HapMap data. All assays had a mismatch rate <0.5%. Additionally, all markers were re-genotyped on more than 10% of samples typed with the Illumina platform resulting in an observed mismatch in less than <0.5% of samples.
Association analysis
For case-control association analysis, e.g. for CKD and kidney stones, we utilized a standard likelihood ratio statistic, implemented in the NEMO software [32] to calculate two-sided P values and odds ratios (ORs) for each individual allele, assuming a multiplicative model for risk, i.e. that the risk of the two alleles carried by a person multiplies [36]. Allelic frequencies, rather than carrier frequencies, are presented for the markers and P values are given after adjustment for the relatedness of the subjects. When estimating genotype specific OR, genotype frequencies in the population were estimated assuming Hardy-Weinberg equilibrium.
Results from multiple case-control groups were combined using a Mantel-Haenszel model [37] in which the groups were allowed to have different population frequencies for alleles, haplotypes and genotypes but were assumed to have common relative risks.
For the quantitative trait association analysis, e.g. for SCr and cystatin C, we utilized a robust linear regression based on an M estimator [38] as implemented in the rlm function of the R software package [39]. An additive model for SNP effects was assumed in all instances. All associations with quantitative traits were performed adjusting for age and sex.
Estimation and testing of interaction effects
Interaction effects were tested by assuming all main effects and lower order interaction effects were present under the null model but not the interaction effect, resulting in a one degree of freedom model. For example, when testing for an interaction effect on SCr between age and the rs4293393-T allele count, the null model included as covariates age, sex and the rs4293393-T allele count. The alternative model included all these covariates as well as the product of the interaction of age and the rs4293393-T allele counts. Similarly, when testing for the interaction between age, the number of comorbid conditions and the rs4293393-T allele count, the null model included as covariates age, sex, rs4293393-T allele count, the product of interaction of age and rs4293393-T allele count, the product of interaction of the number of comorbid conditions and rs4293393-T allele count, and the product of interaction of age and the number of comorbid conditions and the alternative model added the product of interaction of age, the rs4293393-T allele count and the number of comorbid conditions. In the instances when an interaction effect was estimated, the main effect estimates and P values shown were obtained from fitting the appropriate model without an interaction effect.
Correction for relatedness of the subjects and genomic control
Some of the individuals in the Icelandic patient and control groups are related to each other, causing the chi-square test statistic to have a mean >1 and median >0.675. We estimated the inflation factor for the genome-wide association by calculating the average of the 302,379 chi-square statistics, which was a method of genomic control [40] to adjust for both relatedness and potential population stratification. The inflation factor was estimated as 1.15 for CKD and 1.22 for SCr and all the results presented from association with these traits were adjusted based on these inflation factors.
Supporting Information
Zdroje
1. National Kidney Foundation. 2002 K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39 S1 266
2. SarnakMJ
LeveyAS
SchoolwerthAC
CoreshJ
CulletonB
2003 Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108 2154 2169
3. LindemanRD
TobinJD
ShockNW
1984 Association between blood pressure and the rate of decline in renal function with age. Kidney Int 26 861 868
4. FliserD
FranekE
JoestM
BlockS
MutschlerE
1997 Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int 51 1196 1204
5. FliserD
RitzE
1998 Relationship between hypertension and renal function and its therapeutic implications in the elderly. Gerontology 44 123 131
6. RibsteinJ
Du CailarG
MimranA
2001 Glucose tolerance and age-associated decline in renal function of hypertensive patients. J Hypertens 19 2257 2264
7. KasiskeBL
1987 Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int 31 1153 1159
8. BleyerAJ
ShemanskiLR
BurkeGL
HansenKJ
AppelRG
2000 Tobacco, hypertension, and vascular disease: risk factors for renal functional decline in an older population. Kidney Int 57 2072 2079
9. ViktorsdottirO
PalssonR
AndresdottirMB
AspelundT
GudnasonV
2005 Prevalence of chronic kidney disease based on estimated glomerular filtration rate and proteinuria in Icelandic adults. Nephrol Dial Transplant 20 1799 1807
10. CoreshJ
SelvinE
StevensLA
ManziJ
KusekJW
2007 Prevalence of chronic kidney disease in the United States. JAMA 298 2038 2047
11. LindemanRD
TobinJ
ShockNW
1985 Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33 278 285
12. SmithDH
GullionCM
NicholsG
KeithDS
BrownJB
2004 Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. J Am Soc Nephrol 15 1300 1306
13. FoxCS
YangQ
CupplesLA
GuoCY
LarsonMG
2004 Genomewide linkage analysis to serum creatinine, GFR, and creatinine clearance in a community-based population: the Framingham Heart Study. J Am Soc Nephrol 15 2457 2461
14. KöttgenA
GlazerNL
DehghanA
HwangSJ
KatzR
2009 Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41 712 717
15. The International HapMap Consortium 2005 A haplotype map of the human genome. Nature 437 1299 1320
16. BachmannS
Koeppen-HagemannI
KrizW
1985 Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83 531 538
17. PeachRJ
DayWA
EllingsenPJ
McGivenAR
1988 Ultrastructural localization of Tamm-Horsfall protein in human kidney using immunogold electron microscopy. Histochem J 20 156 164
18. PookMA
JeremiahS
ScheinmanSJ
PoveyS
ThakkerRV
1993 Localization of the Tamm-Horsfall glycoprotein (uromodulin) gene to chromosome 16p12.3–16p13.11. Ann Hum Genet 57 285 290
19. DevuystO
DahanK
PirsonY
2005 Tamm-Horsfall protein or uromodulin: new ideas about an old molecule. Nephrol Dial Transplant 20 1290 1294
20. LeekerA
KreftB
SandmannJ
BatesJ
WasenauerG
1997 Tamm-Horsfall protein inhibits binding of S - and P-fimbriated Escherichia coli to human renal tubular epithelial cells. Exp Nephrol 5 38 46
21. HessB
NakagawaY
CoeFL
1989 Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol 257 F99 106
22. SaemannMD
WeichhartT
ZeydaM
StafflerG
SchunnM
2005 Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest 115 468 475
23. YuCL
TsaiCY
LinWM
LiaoTS
ChenHL
1993 Tamm-Horsfall urinary glycoprotein enhances monokine release and augments lymphocyte proliferation. Immunopharmacology 26 249 258
24. BachmannS
MutigK
BatesJ
WelkerP
GeistB
2005 Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. Am J Physiol Renal Physiol 288 F559 567
25. BatesJM
RaffiHM
PrasadanK
MascarenhasR
LaszikZ
2004 Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int 65 791 797
26. MoL
HuangHY
ZhuXH
ShapiroE
HastyDL
2004 Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int 66 1159 1166
27. RampoldiL
CaridiG
SantonD
BoarettoF
BernasconeI
2003 Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics. Hum Mol Genet 12 3369 3384
28. HartTC
GorryMC
HartPS
WoodardAS
ShihabiZ
2002 Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 39 882 892
29. HelgadottirA
ThorleifssonG
ManolescuA
GretarsdottirS
BlondalT
2007 A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316 1491 1493
30. GretarsdottirS
ThorleifssonG
ManolescuA
StyrkarsdottirU
HelgadottirA
2008 Risk variants for atrial fibrillation on chromosome 4q25 associate with ischemic stroke. Ann Neurol 64 402 409
31. SteinthorsdottirV
ThorleifssonG
ReynisdottirI
BenediktssonR
JonsdottirT
2007 A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39 770 775
32. GretarsdottirS
ThorleifssonG
ReynisdottirST
ManolescuA
JonsdottirS
2003 The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet 35 131 138
33. WetzelsJF
KiemeneyLA
SwinkelsDW
WillemsHL
den HeijerM
2007 Age - and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 72 632 637
34. MarchiniJ
HowieB
MyersS
McVeanG
DonnellyP
2007 A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 906 913
35. KutyavinIV
MilesiD
BelousovY
PodyminoginM
VorobievA
2006 A novel endonuclease IV post-PCR genotyping system. Nucleic Acids Res 34 e128
36. RiceJA
1995 Mathematical Statistics and Data Analysis Belmont, CA Wadsworth Inc
37. MantelN
HaenszelW
1959 Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22 719 748
38. HuberPJ
1981 Robust statistics New York Wiley 308
39. Team RDC 2009 R: A Language for Statistical Computing Vienna, Austria R Foundation for Statistical Computing
40. DevlinB
RoederK
1999 Genomic control for association studies. Biometrics 55 997 1004
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 7
-
Všechny články tohoto čísla
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
- Tinkering Evolution of Post-Transcriptional RNA Regulons: Puf3p in Fungi as an Example
- The Importance of Imprinting in the Human Placenta
- Regulator of G Protein Signaling 3 Modulates Wnt5b Calcium Dynamics and Somite Patterning
- Lysosomal Dysfunction Promotes Cleavage and Neurotoxicity of Tau
- Combinatorial Binding Leads to Diverse Regulatory Responses: Lmd Is a Tissue-Specific Modulator of Mef2 Activity
- Variation, Sex, and Social Cooperation: Molecular Population Genetics of the Social Amoeba
- Comparative Analysis of DNA Replication Timing Reveals Conserved Large-Scale Chromosomal Architecture
- The Fitness Landscapes of -Acting Binding Sites in Different Promoter and Environmental Contexts
- Cohesin Is Limiting for the Suppression of DNA Damage–Induced Recombination between Homologous Chromosomes
- Genome-Wide Analysis Reveals Novel Genes Essential for Heme Homeostasis in
- Genome-Wide Meta-Analysis for Serum Calcium Identifies Significantly Associated SNPs near the Calcium-Sensing Receptor () Gene
- Rad3 Decorates Critical Chromosomal Domains with γH2A to Protect Genome Integrity during S-Phase in Fission Yeast
- Quantitative and Molecular Genetic Analyses of Mutations Increasing Life Span
- Association of Variants at with Chronic Kidney Disease and Kidney Stones—Role of Age and Comorbid Diseases
- Breast Cancer DNA Methylation Profiles Are Associated with Tumor Size and Alcohol and Folate Intake
- Calpain 8/nCL-2 and Calpain 9/nCL-4 Constitute an Active Protease Complex, G-Calpain, Involved in Gastric Mucosal Defense
- A Collection of Target Mimics for Comprehensive Analysis of MicroRNA Function in
- A Genome-Wide Analysis Reveals No Nuclear Dobzhansky-Muller Pairs of Determinants of Speciation between and , but Suggests More Complex Incompatibilities
- Microevolution of during Prolonged Infection of Single Hosts and within Families
- Id4, a New Candidate Gene for Senile Osteoporosis, Acts as a Molecular Switch Promoting Osteoblast Differentiation
- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Chromatin Remodeling in Development and Disease: Focus on CHD7
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Requirement of Male-Specific Dosage Compensation in Females—Implications of Early X Chromosome Gene Expression
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



