-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide Meta-Analysis for Serum Calcium Identifies Significantly Associated SNPs near the Calcium-Sensing Receptor () Gene
Calcium has a pivotal role in biological functions, and serum calcium levels have been associated with numerous disorders of bone and mineral metabolism, as well as with cardiovascular mortality. Here we report results from a genome-wide association study of serum calcium, integrating data from four independent cohorts including a total of 12,865 individuals of European and Indian Asian descent. Our meta-analysis shows that serum calcium is associated with SNPs in or near the calcium-sensing receptor (CASR) gene on 3q13. The top hit with a p-value of 6.3×10-37 is rs1801725, a missense variant, explaining 1.26% of the variance in serum calcium. This SNP had the strongest association in individuals of European descent, while for individuals of Indian Asian descent the top hit was rs17251221 (p = 1.1×10-21), a SNP in strong linkage disequilibrium with rs1801725. The strongest locus in CASR was shown to replicate in an independent Icelandic cohort of 4,126 individuals (p = 1.02×10-4). This genome-wide meta-analysis shows that common CASR variants modulate serum calcium levels in the adult general population, which confirms previous results in some candidate gene studies of the CASR locus. This study highlights the key role of CASR in calcium regulation.
Published in the journal: . PLoS Genet 6(7): e32767. doi:10.1371/journal.pgen.1001035
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001035Summary
Calcium has a pivotal role in biological functions, and serum calcium levels have been associated with numerous disorders of bone and mineral metabolism, as well as with cardiovascular mortality. Here we report results from a genome-wide association study of serum calcium, integrating data from four independent cohorts including a total of 12,865 individuals of European and Indian Asian descent. Our meta-analysis shows that serum calcium is associated with SNPs in or near the calcium-sensing receptor (CASR) gene on 3q13. The top hit with a p-value of 6.3×10-37 is rs1801725, a missense variant, explaining 1.26% of the variance in serum calcium. This SNP had the strongest association in individuals of European descent, while for individuals of Indian Asian descent the top hit was rs17251221 (p = 1.1×10-21), a SNP in strong linkage disequilibrium with rs1801725. The strongest locus in CASR was shown to replicate in an independent Icelandic cohort of 4,126 individuals (p = 1.02×10-4). This genome-wide meta-analysis shows that common CASR variants modulate serum calcium levels in the adult general population, which confirms previous results in some candidate gene studies of the CASR locus. This study highlights the key role of CASR in calcium regulation.
Introduction
Calcium is the most abundant mineral in the human body contributing approximately one kilogram to the average adult human body mass. Whereas 99% of calcium is stored in the skeleton and teeth, the remaining 1% circulates in the bloodstream and is involved in many physiological processes including its function as a universal cellular signaling molecule [1]–[2]. Calcium plays a key role in membrane potential, which is important for muscle contraction, heart rate regulation and generation of nerve impulses. Calcium also influences bone metabolism, ion transport and many other cellular processes [3]. Approximately 2/5 of calcium in the extracellular fluid is found in blood serum. The level of serum calcium is under tight hormonal control with a normal concentration of 2.15–2.55 mmol/L. Serum calcium is under strong genetic control, with twin studies showing that the variance in total calcium due to genetic effects is between 50% and 78% [4]–[5].
While skeletal calcium is important in numerous clinical disorders, in particular bone and mineral disorders, the clinical role of serum calcium is less clear. Several [6]–[7] (but not all [8]) studies indicated that elevated serum calcium levels are associated with an increased risk of cardiovascular disease. Patients with hyperparathyroidism, who suffer from chronic hypercalcemia, have a high prevalence of hypertension and increased cardiovascular mortality [9]–[11]. However, the mechanisms underlying the putative association of serum calcium with increased cardiovascular morbidity and mortality remain unclear.
Rare monogenic forms of hypo - or hypercalcemia have been described, including disorders involving the calcium-sensing receptor (CASR, locus 3q13) gene. Heterozygous and homozygous CASR mutations that inactivate CASR are responsible, respectively, for familial hypocalciuric hypercalcemia, type 1 (also known as familial benign hypercalcemia) (OMIM #145980) [12]–[13] and neonatal severe hyperparathyroidism (OMIM #239200) [13]. On the other hand, mutations that result in CASR activation lead to autosomal dominant hypocalcemia (OMIM #146200) [14]. Mutations in many other genes have also been found to lead to disturbances of serum calcium levels (Table 1).
Tab. 1. Serum calcium candidate genes. 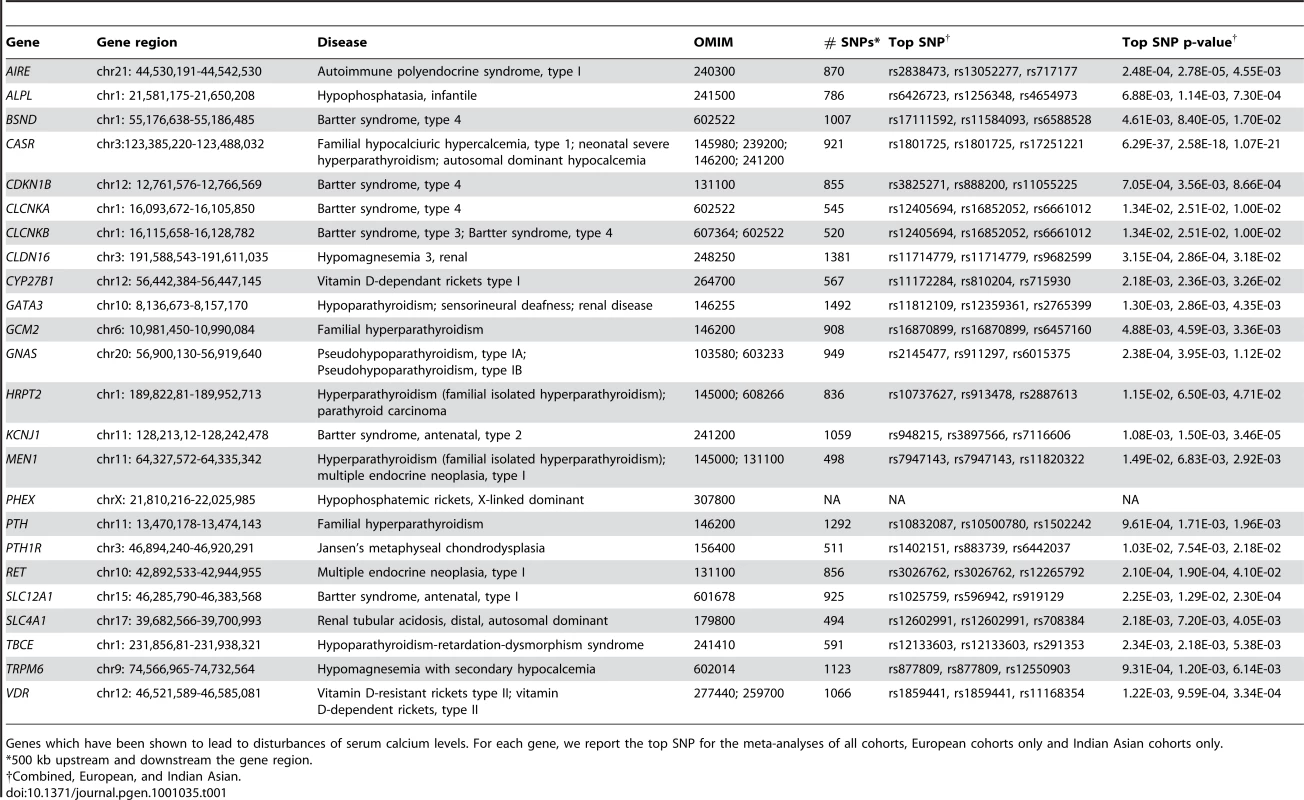
Genes which have been shown to lead to disturbances of serum calcium levels. For each gene, we report the top SNP for the meta-analyses of all cohorts, European cohorts only and Indian Asian cohorts only. In the present study, we report results obtained from meta-analysis of genome-wide associations of serum calcium levels from four cohorts with a total of 12,865 participants. We first describe the design of the study and its main finding, that variants in CASR give rise to the strongest signals associated with serum calcium levels in both European and Indian Asian populations. Our results confirm previous studies showing that mutations in CASR are associated with serum calcium levels in young healthy women [15]–[16] and extend this observation to men and women across a large spectrum of age. We show that CASR is a key player in the genetic regulation of serum calcium in men and women from the general adult population.
Results
We performed a meta-analysis for genome-wide associations of serum calcium, determined by subtracting the estimated amount of calcium bound to albumin from the total serum calcium, to infer the amount of ionized calcium (see Materials and Methods). Our study included four cohorts: (i) 5404 European individuals from the Cohorte Lausanne (CoLaus) [17]–[18], (ii) 5548 European and Indian Asian individuals from the London Life Sciences Population (LOLIPOP) Study from West London UK [19]–[20], (iii) 1196 European individuals from the InCHIANTI Study (Tuscany, Italy) [21], and (iv) 717 individuals of European descent from the Baltimore Longitudinal Study of Aging (BLSA) study based in the Baltimore-Washington DC area [22], totaling 12,865 participants (see Table 2 for more detailed characteristics of each cohort).
Tab. 2. Characteristics of participants, by study. 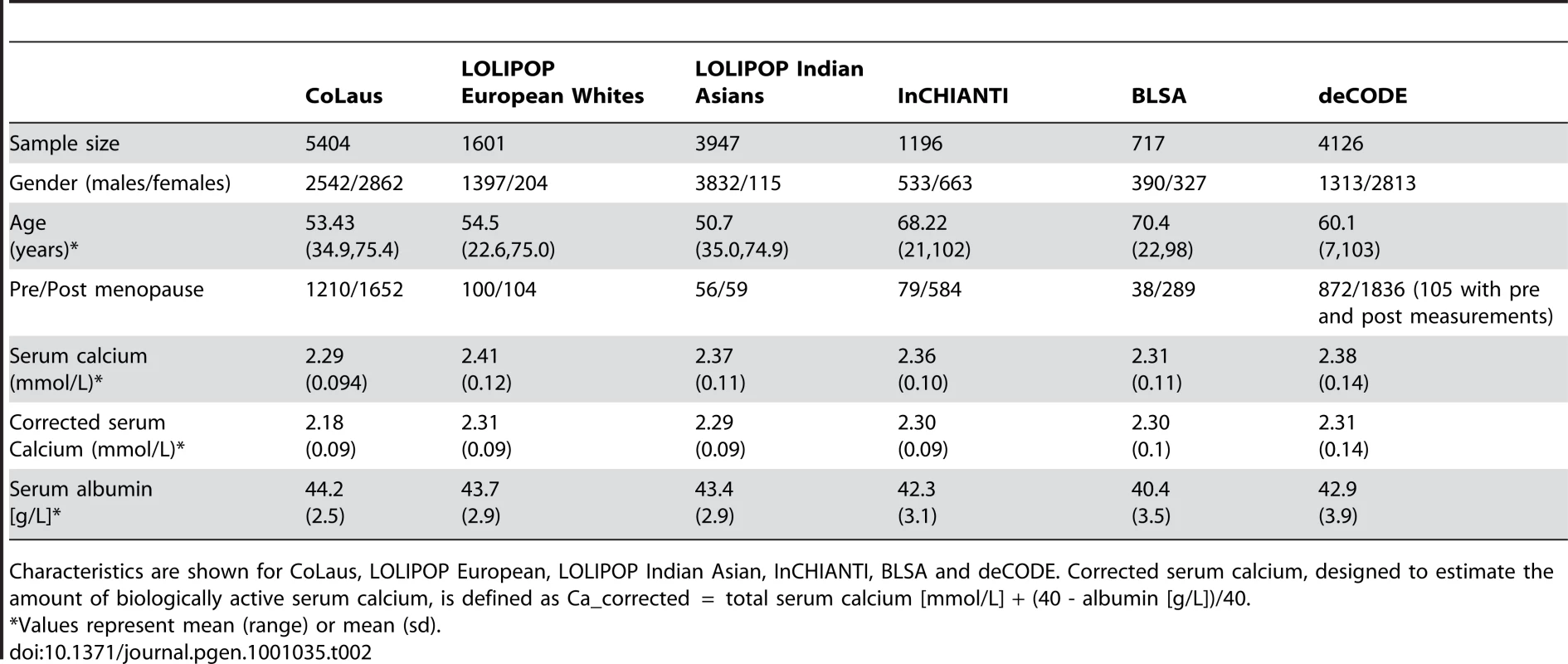
Characteristics are shown for CoLaus, LOLIPOP European, LOLIPOP Indian Asian, InCHIANTI, BLSA and deCODE. Corrected serum calcium, designed to estimate the amount of biologically active serum calcium, is defined as Ca_corrected = total serum calcium [mmol/L] + (40 - albumin [g/L])/40. Genome-wide association scans were performed first independently for each cohort using linear regression and then the effect sizes from each cohort were meta-analyzed (see Materials and Methods). Due to the possibility of population substructure obscuring effects of genetic variants, meta-analysis was performed separately for (i) combined European and Indian Asian cohorts (N = 12,865) and restricted to cohorts of (ii) European (N = 8,919), and (iii) Indian Asian descent (N = 3,947). The meta-analyses yielded 100 SNPs from the combined cohorts, 70 SNPs when restricting to European cohorts and 22 SNPs restricting to Indian Asian cohorts that exceeded the genome-wide significance threshold of 5×10-8 (Figure 1A–1C) (the full list is provided in Table S2A, S2B, S2C). All SNPs reaching statistical significance clustered around the CASR locus at 3q13. The most significant SNP in the (i) combined and (ii) European meta-analyses was rs1801725 (p = 6.29×10-37, p = 2.58×10-18, respectively) and in the (iii) Indian Asian meta-analysis was rs17251221 (p = 1.07×10-21). These two SNPs are less than 11 kb apart and are in high linkage disequilibrium with each other (r2 = 0.946, 0.494, 1.0, 1.0 in HapMap CEU, CHB, JPT, YRI, respectively), and therefore most likely derive from the same association signal. We find that rs1801725 explains 1.26% of the variance in serum calcium, with the effect sizes and standard errors of the serum calcium increasing T allele in individual cohorts shown in Figure 2 and Table S3. According to our additive model, each rs1801725 T allele increases log10 serum calcium (in units mmol/L) by 3.61×10-3, equivalent to a multiplicative effect of 1.008 on serum calcium (see also Table S2). At an average serum calcium level of 2.25 mmol/L, each rs1801725 T allele yields an increase of 0.01874 mmol/L, or 21% of one standard deviation of serum calcium levels in a normal population. The regional pattern of association of SNPs around the CASR locus, and their linkage disequilibrium with rs1801725, are shown in Figure 3. Of note, rs1042636, which has been associated with decreased serum calcium [23], also achieved genome-wide significance with the G minor allele associated with decreased serum calcuim (p = 4.96×10-9). However, conditional on the rs1801725 locus, located 12 bps upstream, the rs1042636 p-value became 3.32×10−4, indicating that the two loci share contributions to serum calcium levels.
Fig. 1. Genome-wide association results. 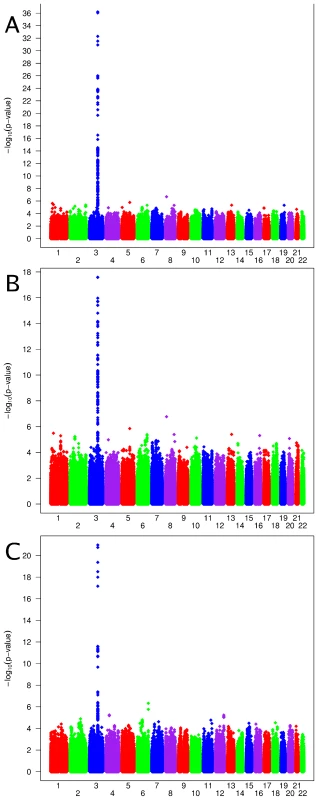
Manhattan plots showing significance of association of all SNPs in the meta-analysis for (A) combined European and Indian Asian cohorts, (B) European cohorts only and (C) Indian Asian cohorts only. SNPs are plotted on the x-axis according to their position on each chromosome against association with serum calcium concentrations on the y-axis (shown as −log10 p-values). Fig. 2. Comparison of rs1801725 significance across cohorts. 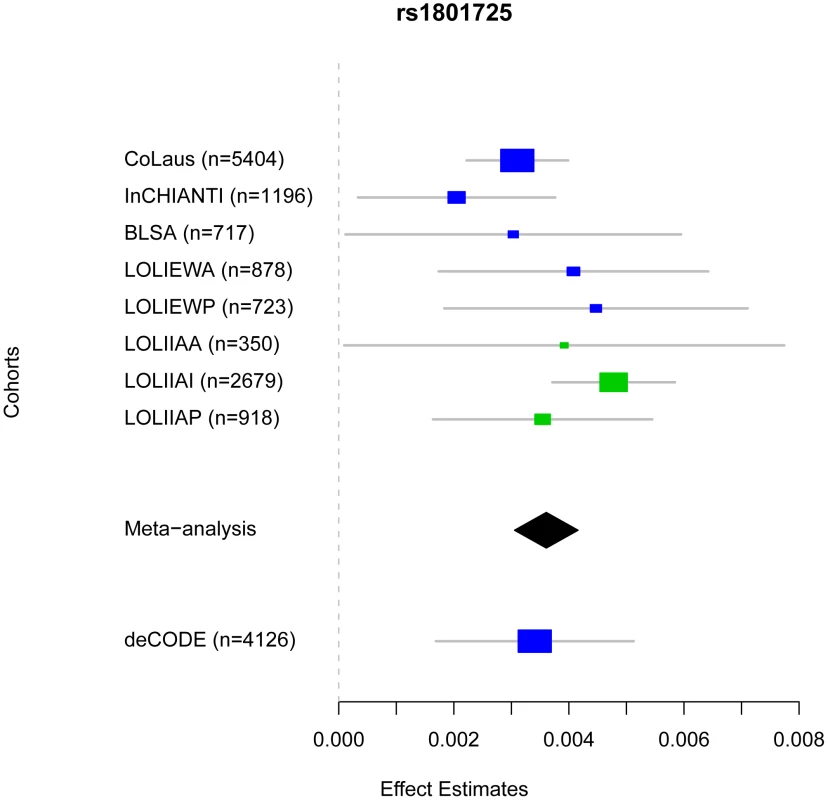
The effect size and 95% confidence intervals of the serum calcium increasing T allele of SNP rs1801725 are shown separately for each cohort (CoLaus, LOLIPOP_EWA, LOLIPOP_EWP, LOLIPOP_IAA, LOLIPOP_IAI, LOLIPOP_IAP, BLSA, InCHIANTI) and for the replication cohort deCODE. European cohorts are shown in blue and Indian Asian cohorts are drawn in green. The size of the box is proportional to the precision 1/se2 and the meta-analysis estimate and 95% confidence interval across all cohorts is given by a diamond. Fig. 3. Regional association plot of the CASR locus. 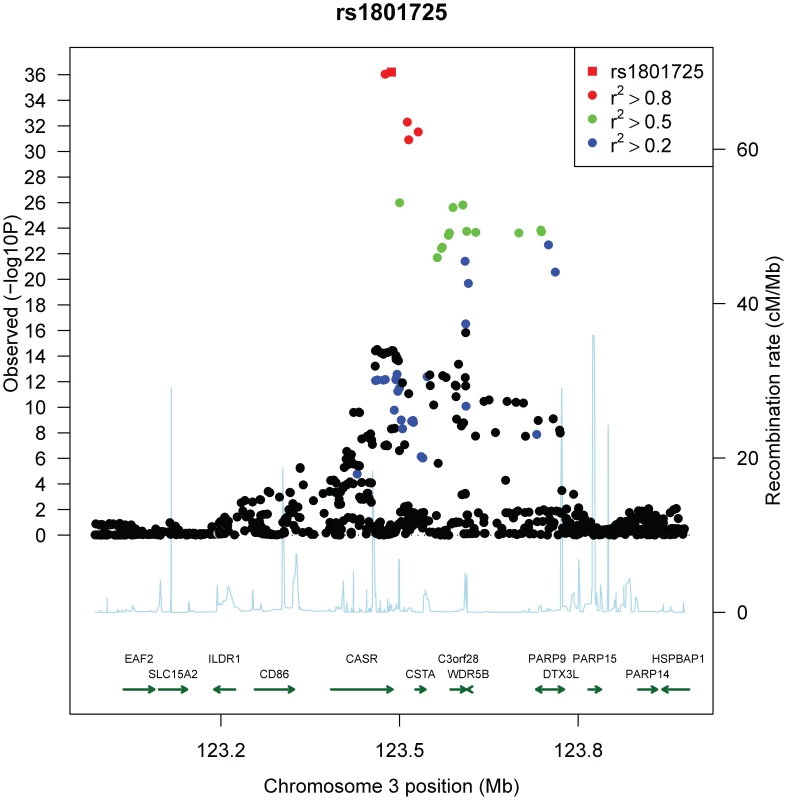
Plots show genomic position on the x-axis and −log10 p-values on the y-axis for SNPs in the CASR locus on chromosome 3. The sentinel hit is shown as a red square. Patterns of linkage disequilibrium between the sentinel SNP and all other SNPs are color-coded. Red circles indicate high correlation (r2>0.8), green circles indicate moderate correlation (r2>0.5), blue circles indicate low correlation (r2>0.2) and black circles indicate no correlation (r2<0.2). The fine-scale recombination rates from [72]–[73] are plotted in light blue. To confirm the rs1801725 signal, we analyzed the association pattern with serum calcium in a separate cohort. We used a subset of 4,126 Icelandic individuals from the deCODE study [24]–[25] with serum calcium measurements. We found the rs1801725 T allele to be strongly associated with increased serum calcium (p = 1.02×10-4), replicating the key meta-analysis result.
While only the CASR locus reached nominal genome-wide significance for association with serum calcium, the top regions with p<10-5 are shown in Table 3. These SNPs cover 12 regions, the significance of which is displayed across cohorts in Figure S3. There were no SNPs in other candidate genes (which have previously been shown to be involved in disorders associated with disturbed serum calcium levels) that were associated with serum calcium at genome-wide significance. The most significant SNPs within 500 kb of the gene transcripts are shown in Table 1. Considering the set of 18,611 distinct SNPs mapping to the set of serum calcium candidate genes excluding CASR, we find no significant association (at significance level 0.05 and applying the Bonferroni correction for multiple testing, giving a cut-off p-value of 2.69×10-6, see also Figure S4). Indeed, fixing the sample size and genome-wide significance threshold our study is well-powered (≥0.80) to detect SNPs explaining at least 0.31% of the variance. Therefore the common SNPs within the candidate genes (excluding CASR) likely play at best a small role in serum calcium regulation.
Tab. 3. Significance of top SNPs. 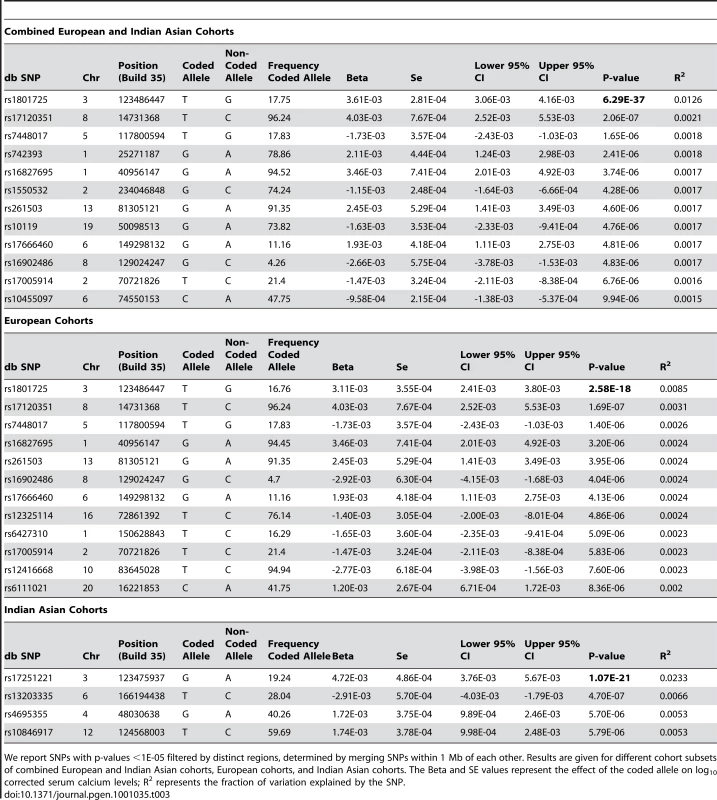
We report SNPs with p-values <1E-05 filtered by distinct regions, determined by merging SNPs within 1 Mb of each other. Results are given for different cohort subsets of combined European and Indian Asian cohorts, European cohorts, and Indian Asian cohorts. The Beta and SE values represent the effect of the coded allele on log10 corrected serum calcium levels; R2 represents the fraction of variation explained by the SNP. We analyzed the association of the top SNP with several calcium-related outcomes (coronary heart disease, myocardial infarction, hypertension, stroke, osteoarthritis, osteoporosis and kidney stones). The number of cases and controls for each outcome and each cohort is given in Table S4. Logistic regression including age and pseudosex (see Materials and Methods) as covariates did not find any significant association between rs1801725 and the calcium-related outcomes, after correcting for multiple testing (effect sizes and standard errors for the T allele are listed in Table S5). Power calculations show that given the sample sizes for the clinical traits above, our study has good power (≥0.80) to detect odds ratios of 1.20, 1.13, 1.77, 1.27, 1.27, 1.24 and 1.75, respectively. As the smallest p-values from calcium-related traits were for osteoarthritis and osteoporosis (bonferroni-corrected p = 0.21, 0.44, respectively), we further investigated bone density traits. None of deCODE hip bone mineral density or spine bone mineral density (N = 6657 and 6838, respectively) nor InCHIANTI total bone density, trabecular bone density, cortical bone density, cortical bone thickness or cortical bone area (N = 1196) bonferroni-adjusted p-values for eight traits were significant.
Discussion
This genome-wide scan of 12,865 individuals revealed CASR as the most significant (and only genome-wide significant) locus influencing serum calcium levels. Specifically, we found evidence for a strong association of SNPs located in the CASR locus with serum calcium levels in both Europeans and Indian Asians. The strongest locus in CASR was further shown to replicate in an independent Icelandic cohort of 4,126 individuals.
The top signal (rs1801725, 2956G>T) explains 1.26% of the variance in serum calcium. Indeed, this is similar to results from other GWAS of human height [26]–[29], body mass index [30]–[31], serum urate [32]–[34] and serum lipid concentrations [34]–[36], for which the genome-wide significant loci uncovered thus far collectively explain only a small fraction of the phenotypic variance (usually at least one order of magnitude less than the total additive genetic variance estimated from heritability studies [37]–[38]). The rs1801725 T allele (A986S) was associated with higher serum calcium, consistent with previous findings (see Table S6). The rs1801725 polymorphism (with T allele frequencies of 16.76%, 19.98% in European and Indian Asian cohorts, respectively) affects serum calcium levels of a substantial proportion of the population.
The rs1801725 polymorphism encodes a missense variant in exon 7 of the CASR gene, which leads to a non-conservative amino-acid change (serine substitution for alanine-986, A986S corresponding to nucleotides 2956G>T) in the cytoplasmic tail of CASR. In vitro studies showed that mutations within the C-terminal tail may influence several aspects of CASR function, such as signal transduction, intracellular trafficking and cell surface expression [39]–[41]. However, PolyPhen predicts rs1801725 to be a benign substitution. It is presently unclear whether this substitution gives rise to functional variants, as functional studies have yielded conflicting results [42]–[43]. Deep sequencing of this region may help identifying the causal variants. While it is still not possible to infer a direct causal role, it is of interest to note that the SNP gives rise to an amino acid change in the C-terminal tail of CASR, a domain which plays a key role in the receptor function and may potentially influence intracellular trafficking following CASR activation by extracellular calcium.
Several studies have reported associations of A986S and nearby CASR mutations with various phenotypes. The A986S CASR polymorphism has been associated with variations in circulating calcium levels in healthy adults in some studies [15], [23], [44]–[45], but not in others [46]–[47]. The fact that the latter studies were underpowered (sample size ranging from 148 to 1252) to detect a small effect size likely explains these inconsistent results. The rs1042636 (R990G) polymorphism has been associated with the magnitude of parathyroid hormone (PTH) secretion in patients with primary hyperparathyroidism [48], and preliminary results suggest that it could influence response to cinacalcet, a calcimimetic used to treat secondary hyperparathyroidism in patients with end-stage renal disease [49]. In a meta-analysis, 986S was associated with a 49% increased risk (P = 0.002) of primary hyperparathyroidism [47], [50]–[51]. Among patients with primary hyperparathyroidism, the AGQ haplotype (i.e. 986A, 990G, 1011Q, which is associated with lower serum calcium levels and hypercalciuria [52]) was associated with increased risk, and the SRQ haplotype with decreased risk, of kidney stones [50].
CASR has been previously considered as a candidate gene for osteoporosis [53] and coronary heart disease as well as increased total and cardiovascular mortality [54]. In our meta-analysis, we found no significant association of rs1801725 with these calcium-related phenotypes. A recent meta-analysis focusing on effects of candidate genes on osteoporosis also reports negative results for CASR [55]. Furthermore, results on the association of elevated serum calcium with increased cardiovascular risk in the general population are controversial [6]–[8]. It is therefore not clear to what extent serum calcium might predict cardiovascular risk. The SNPs identified in this meta-analysis could serve as genetic instruments in future studies, such as Mendelian randomization analysis in longitudinal cohorts, to further investigate the causal effect of serum calcium on osteoporosis and on cardiovascular disease risk (see Table S5 for rs1801725 effects and standard errors).
Our meta-analysis suffers from some limitations. First, we used corrected serum calcium and not directly measured ionized serum calcium. The correlation between corrected serum calcium and ionized serum calcium varies between 0.66 and 0.87 [56]–[58]. We can hypothesize that the association of ionized serum calcium with CASR variants would be stronger than the one with corrected serum calcium because ionized calcium is the form physiologically active on CASR. Second, data on serum phosphate, PTH or vitamin D are not available, so that we cannot explore further these relationships. Third, sample sizes for calcium-related clinical traits were limited, many clinical traits in CoLaus were self-reported instead of clinically diagnosed, and we incur a multiple testing penalty due to the number of clinical traits posited to be associated with serum calcium. However, the major strengths of the study are the hypothesis-free nature of GWAS studies, the large sample meta-analysis and the inclusion of multiple populations.
Materials and Methods
Cohorts
CoLaus is a population-based sample from Lausanne, Switzerland, consisting of 5435 individuals between 35 and 75 years old (after QC) of which a subset of 5404 had available serum calcium measurements. The study design and protocols have been described previously [17]–[18]. The CoLaus study was approved by the Institutional Ethic's Committee of the University of Lausanne. The London Life Sciences Prospective Population Study (LOLIPOP) is an ongoing population-based cohort study of ∼30,000 Indian Asian and European white men and women, aged 35–75 years living in West London, United Kingdom [59]. All study participants gave written consent including for genetic studies. The LOLIPOP study is approved by the local Research Ethics Committee. The participants included in the present study are a subset of 3947 Indian Asians and 1601 Europeans from the LOLIPOP cohort study. LOLIPOP individuals are separated by origin as well as the genotyping platform, with IAA, IAI or IAP denoting Indian Asians genotyped on Affymetrix, Illumina or Perlegen platforms, respectively, and EWA and EWI denoting Europeans genotyped on Affymetrix or Illumina platforms, respectively (see Table S1). The InCHIANTI study is a population-based epidemiological study aimed at evaluating the factors that influence mobility in the older population living in the Chianti region in Tuscany, Italy. The details of the study have been previously reported [60]. Overnight fasted blood samples were taken for genomic DNA extraction, and measurement of serum calcium. For this study, 1196 subjects with serum calcium and GWAS data were analyzed. The study protocol was approved by the Italian National Institute of Research and Care of Aging Institutional Review and Medstar Research Institute (Baltimore, MD). The Baltimore longitudinal study on Aging (BLSA) study is a population-based study aimed to evaluate contributors of healthy aging in the older population residing predominantly in the Baltimore-Washington DC area [61]. Starting in 1958, participants are examined every one to four years depending on their age. Blood samples were collected for DNA extraction. This analysis focused on a subset of the participants (N = 717) of European ancestry. The BLSA has continuing approval from the Institutional Review Board (IRB) of Medstar Research Institute. Approval was obtained from local ethic committees for all studies and all participants signed informed written consent. The deCODE study consists of individuals who visited a private outpatient laboratory, the Laboratory in Mjodd, Reykjavik, Iceland between 1997 and 2008. The main referral center for this laboratory is a multispecialty medical clinic in Reykjavik (Laeknasetrid). For the serum calcium analysis we used information on 4,126 individuals with both genome-wide SNP data and measured serum calcium and serum albumin. The samples for bone density analysis have previously been described in detail [24]–[25]. For this study 6,657 individuals with total hip bone mineral density (BMD) and 6,838 individuals with lumbar spine BMD and SNP data were available for analysis. All participants gave informed consent and the study was approved by the Data Protection Commission of Iceland (DPC) and the National Bioethics Committee of Iceland.
Clinical data
For each CoLaus participant a venous blood sample was collected under fasting conditions. Measurements were conducted using a Modular P apparatus (Roche Diagnostics, Switzerland). Total serum calcium was measured by O-cresolphtalein (2.1% – 1.5% maximum inter and intra-batch CVs); albumin was measured by bromocresol green (2.5% – 0.4%). To further characterize the identified genetic variants, we analyzed the association with several outcomes postulated to be correlated with serum calcium. Within the CoLaus study, we have questionnaire responses to queries about personal histories of osteoporosis, osteoarthritis, myocardial infarction and stroke in addition to clinical data determining hypertension status, defined as previously described [17]. The assessment of LOLIPOP study participants was carried out by a trained research nurse, during a 45 minute appointment according to a standardized protocol and with regular QC audits. An interviewer-administered questionnaire was used to collect data on medical history, family history, current prescribed medication, and cardiovascular risk factors. Physical assessment included anthropometric measurements (height, weight, waist, hip) and blood pressure. Blood was collected after an 8 hour fast for biochemical analysis, including glucose, insulin, total and HDL cholesterol and triglycerides, and whole blood was taken for DNA extraction [59]. InCHIANTI serum albumin concentrations were determined as percentage of total protein using agarose electrophoretic technique (Hydragel Protein (E) 15/30, Sebia, Issy-les-Moulineaux, France). Serum calcium was measured using calorimetric assay (Roch Diagnostic, GmbH, Mannheim, Germany) by a Roche-Hitachi autoanalyzer (The intra-assay CV and 0.9% and the inter-assay CV was 1.5%). Measures of bone density, bone dimensions and osteoporosis diagnosis were assessed by peripheral quantitative computed tomography (pQCT) using the XCT 2000 device (Stratec Medizintechnik, Pforzheim, Germany) [62]. BLSA albumin concentrations were measured by a calorimetric assay using bromocresol green (Ortho-Clinical Diagnostics). Calcium concentrations were measured by a calorimetric assay (Vitros 5,1,FS).
Genome-wide genotyping and imputation
CoLaus participants were genotyped using Affymetrix Human Mapping 500 K Array. For the genome-wide association stage, genotyping in LOLIPOP participants was carried out using the Illumina 317 K mapping array, Affymetrix Human Mapping 500 K array, and Perlegen, 284 K platforms (Table S1). Participants of the InCHIANTI and BLSA studies were genotyped using Illumina Infinium HumanHap 550 K SNP arrays were used for genotyping [21]. Imputation of allele dosage of SNPs was performed using either MACH [63] or IMPUTE [64] with parameters and quality control filters as described in . All European cohorts imputed SNPs typed in the HapMap CEU population; LOLIPOP Indian Asian cohort imputed SNPs using mixed HapMap populations, given that this showed greater concordance with real genotypes compared with use of any one HapMap population. SNPs were excluded if cohort-specific imputation quality as assessed by r2.hat (MACH) or .info (IMPUTE) metrics were <0.30. In total, 2,557,252 genotyped or imputed SNPs had data from one or more cohorts and were analyzed. Genotypes in deCODE were measured using either humanHap300, humanHap300-duo or humanCNV370.
Statistical analysis
Individual genome-wide association analysis
Biologically active serum calcium is estimated by the correction, Ca_corrected = total serum calcium [mmol/L] + (40 - albumin [g/L])/40. Individuals with values <1.9 or >3 were removed as these were extreme outliers. Linear-regression analyses were carried out using an additive genetic model on log10-transformed corrected calcium levels adjusted for age and pseudosex (a factor variable with three values: males, pre-menopausal females and post-menopausal females). BLSA also included the first two and LOLIPOP included the first four ancestry principal components in the regression, respectively. Regression analyses were performed with QUICKTEST [65] (CoLaus), MACH2qtl (LOLIPOP) [63] or MERLIN [66] (InCHIANTI, BLSA).
Meta-analysis
The results from all cohorts were combined into a fixed-effects meta-analysis using inverse variance weighting. Tests for heterogeneity were assessed using Cochran's Q statistic and the log of the related H statistic [67] after grouping LOLIPOP subsets into European and Indian subsets. For rs1801725 and rs1042636 the p-values were (0.07657, 0.1432) and (0.3450, 0.8876), respectively, indicating limited between-study variability. The analysis was implemented in R and run on a quad-core Linux machine. SNPs were reported provided they had effect size estimates in at least 2 of the 5 European cohorts, in at least 2 of the 3 Indian Asian cohorts, or in at least 3 of the 8 total cohorts. For the overall meta-analysis, residual inflation of the test statistic was corrected using genomic control [68]. The inflation factor was 1.0207 for the all combined cohorts, 1.0068 for European cohorts and 1.0286 for Indian Asian cohorts. Where reported, individual study p-values are corrected for inflation using genomic control methods for genotyped and imputed SNPs combined (inflation factors for individual studies were 1.0139 (CoLaus), 0.9891 (LOLIPOP EWA), 0.9994 (LOLIPOP EWP), 0.9967 (LOLIPOP IAA), 1.0131 (LOLIPOP IAI), 0.9985 (LOLIPOP IAP), 0.9842 (InCHIANTI), 1.0019 (BLSA)). The regional association plot (Figure 3) was created modifying a publically available R script [69]. The map of fine-scale recombination rates was downloaded from the HapMap website http://www.hapmap.org/downloads/recombination/ using Phase II HapMap data (release 21). Quantile-quantile plots of the association results are shown in Figure S1A, S1B, S1C, study-specific quantile-quantile plots are shown in Figure S2. Associations below p = 5×10−8 were considered genome-wide significant, which corresponds to a Bonferroni correction for the estimated one million independent common variant tests in the human genome of European individuals [70]. The analysis of osteoporosis status in CoLaus and InCHIANTI was performed using logistic regression including age and pseudosex as covariates in QUICKTEST [65]. Linkage disequilibrium was estimated from HapMap CEU (2007-01, build 35 non-redundant) genotypes. LD r2 statistics were estimated for SNPs within 500 kb using Haploview [71].
Association of rs1801725 with calcium-related outcomes
For each related trait (coronary heart disease, hypertension, kidney stones, myocardial infarction, osteoarthritis, osteoporosis and stroke) we performed a fixed-effects meta-analysis of the logistic regression coefficients. We applied the bonferroni correction to adjust for multiple testing. We performed Wald-based power calculations using a type I error of 0.05/7 and meta-analysis coefficient estimates and standard errors to estimate the sample size for each trait giving power 0.80.
Supporting Information
Zdroje
1. CarafoliE
2004 Calcium-mediated cellular signals: a story of failures. Trends Biochem Sci 29 371 379
2. CarafoliE
2005 Calcium–a universal carrier of biological signals. Delivered on 3 July 2003 at the Special FEBS Meeting in Brussels. FEBS J 272 1073 1089
3. CarafoliE
2004 The ambivalent nature of the calcium signal. J Endocrinol Invest 27 134 136
4. WhitfieldJB
MartinNG
1984 The effects of inheritance on constituents of plasma: a twin study on some biochemical variables. Ann Clin Biochem 21 (Pt 3) 176 183
5. WilliamsPD
PuddeyIB
MartinNG
BeilinLJ
1992 Platelet cytosolic free calcium concentration, total plasma calcium concentration and blood pressure in human twins: a genetic analysis. Clin Sci (Lond) 82 493 504
6. LeifssonBG
AhrenB
1996 Serum calcium and survival in a large health screening program. J Clin Endocrinol Metab 81 2149 2153
7. LindL
SkarforsE
BerglundL
LithellH
LjunghallS
1997 Serum calcium: a new, independent, prospective risk factor for myocardial infarction in middle-aged men followed for 18 years. J Clin Epidemiol 50 967 973
8. DhingraR
SullivanLM
FoxCS
WangTJ
D'AgostinoRBSr
2007 Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167 879 885
9. PalmerM
AdamiHO
BergstromR
JakobssonS
AkerstromG
1987 Survival and renal function in untreated hypercalcaemia. Population-based cohort study with 14 years of follow-up. Lancet 1 59 62
10. LundgrenE
LindL
PalmerM
JakobssonS
LjunghallS
2001 Increased cardiovascular mortality and normalized serum calcium in patients with mild hypercalcemia followed up for 25 years. Surgery 130 978 985
11. WermersRA
KhoslaS
AtkinsonEJ
GrantCS
HodgsonSF
1998 Survival after the diagnosis of hyperparathyroidism: a population-based study. Am J Med 104 115 122
12. HeathHIII
OdelbergS
JacksonCE
TehBT
HaywardN
1996 Clustered inactivating mutations and benign polymorphisms of the calcium receptor gene in familial benign hypocalciuric hypercalcemia suggest receptor functional domains. J Clin Endocrinol Metab 81 1312 1317
13. PollakMR
BrownEM
ChouYH
HebertSC
MarxSJ
1993 Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell 75 1297 1303
14. PollakMR
BrownEM
EstepHL
McLainePN
KiforO
1994 Autosomal dominant hypocalcaemia caused by a Ca(2+)-sensing receptor gene mutation. Nat Genet 8 303 307
15. ColeDE
PeltekovaVD
RubinLA
HawkerGA
ViethR
1999 A986S polymorphism of the calcium-sensing receptor and circulating calcium concentrations. Lancet 353 112 115
16. ColeDE
ViethR
TrangHM
WongBY
HendyGN
2001 Association between total serum calcium and the A986S polymorphism of the calcium-sensing receptor gene. Mol Genet Metab 72 168 174
17. FirmannM
MayorV
Marques-VidalPM
BochudM
PecoudA
2008 The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BMC Cardiovasc Disord
18. RodondiN
CornuzJ
Marques-VidalP
ButlerJ
HayozD
2008 Aspirin use for the primary prevention of coronary heart disease: A population-based study in Switzerland. Prev Med 46 137 144
19. ChambersJC
ElliottP
ZabanehD
ZhangW
LiY
2008 Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet 40 716 718
20. KoonerJS
ChambersJC
Aguilar-SalinasCA
HindsDA
HydeCL
2008 Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat Genet 40 149 151
21. MelzerD
PerryJR
HernandezD
CorsiAM
StevensK
2008 A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet 4 e1000072 doi:10.1371/journal.pgen.1000072
22. SchockNW
GreulichRC
AndresR
ArenbergD
CostaPT
1984 Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington D.C
23. ScillitaniA
GuarnieriV
DeGS
MuscarellaLA
BattistaC
2004 Blood ionized calcium is associated with clustered polymorphisms in the carboxyl-terminal tail of the calcium-sensing receptor. J Clin Endocrinol Metab 89 5634 5638
24. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2009 New sequence variants associated with bone mineral density. Nat Genet 41 15 17
25. StyrkarsdottirU
HalldorssonBV
GretarsdottirS
GudbjartssonDF
WaltersGB
2008 Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358 2355 2365
26. GudbjartssonDF
WaltersGB
ThorleifssonG
StefanssonH
HalldorssonBV
2008 Many sequence variants affecting diversity of adult human height. Nat Genet 40 609 615
27. LettreG
JacksonAU
GiegerC
SchumacherFR
BerndtSI
2008 Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet 40 584 591
28. WeedonMN
LettreG
FreathyRM
LindgrenCM
VoightBF
2007 A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat Genet 39 1245 1250
29. WeedonMN
LangoH
LindgrenCM
WallaceC
EvansDM
2008 Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet 40 575 583
30. FraylingTM
TimpsonNJ
WeedonMN
ZegginiE
FreathyRM
2007 A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316 889 894
31. LoosRJ
LindgrenCM
LiS
WheelerE
ZhaoJH
2008 Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40 768 775
32. DoringA
GiegerC
MehtaD
GohlkeH
ProkischH
2008 SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet 40 430 436
33. LiS
SannaS
MaschioA
BusoneroF
UsalaG
2007 The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet 3 e194 doi:10.1371/journal.pgen.0030194
34. WallaceC
NewhouseSJ
BraundP
ZhangF
TobinM
2008 Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 82 139 149
35. SandhuMS
WaterworthDM
DebenhamSL
WheelerE
PapadakisK
2008 LDL-cholesterol concentrations: a genome-wide association study. Lancet 371 483 491
36. WillerCJ
SannaS
JacksonAU
ScuteriA
BonnycastleLL
2008 Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40 161 169
37. GoldsteinDB
2009 Common genetic variation and human traits. N Engl J Med 360 1696 1698
38. MaherB
2008 Personal genomes: The case of the missing heritability. Nature 456 18 21
39. BaiM
TrivediS
BrownEM
1998 Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem 273 23605 23610
40. BaiM
TrivediS
LaneCR
YangY
QuinnSJ
1998 Protein kinase C phosphorylation of threonine at position 888 in Ca2+o-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem 273 21267 21275
41. GamaL
BreitwieserGE
1998 A carboxyl-terminal domain controls the cooperativity for extracellular Ca2+ activation of the human calcium sensing receptor. A study with receptor-green fluorescent protein fusions. J Biol Chem 273 29712 29718
42. VezzoliG
TerranegraA
ArcidiaconoT
BiasionR
CovielloD
2007 R990G polymorphism of calcium-sensing receptor does produce a gain-of-function and predispose to primary hypercalciuria. Kidney Int 71 1155 1162
43. HardingB
CurleyAJ
HannanFM
ChristiePT
BowlMR
2006 Functional characterization of calcium sensing receptor polymorphisms and absence of association with indices of calcium homeostasis and bone mineral density. Clin Endocrinol (Oxf) 65 598 605
44. KellyC
GunnIR
GaffneyD
DevgunMS
2006 Serum calcium, urine calcium and polymorphisms of the calcium sensing receptor gene. Ann Clin Biochem 43 503 506
45. LaaksonenMM
OutilaTA
KarkkainenMU
KemiVE
RitaHJ
2009 Associations of vitamin D receptor, calcium-sensing receptor and parathyroid hormone gene polymorphisms with calcium homeostasis and peripheral bone density in adult Finns. J NutrigenetNutrigenomics 2 55 63
46. BollerslevJ
WilsonSG
DickIM
DevineA
DhaliwalSS
2004 Calcium-sensing receptor gene polymorphism A986S does not predict serum calcium level, bone mineral density, calcaneal ultrasound indices, or fracture rate in a large cohort of elderly women. Calcif Tissue Int 74 12 17
47. CetaniF
BorsariS
VignaliE
PardiE
PiconeA
2002 Calcium-sensing receptor gene polymorphisms in primary hyperparathyroidism. J Endocrinol Invest 25 614 619
48. YamauchiM
SugimotoT
YamaguchiT
YanoS
KanzawaM
2001 Association of polymorphic alleles of the calcium-sensing receptor gene with the clinical severity of primary hyperparathyroidism. Clin Endocrinol (Oxf) 55 373 379
49. RotheHM
ShapiroWB
SunWY
ChouSY
2005 Calcium-sensing receptor gene polymorphism Arg990Gly and its possible effect on response to cinacalcet HCl. Pharmacogenet Genomics 15 29 34
50. ScillitaniA
GuarnieriV
BattistaC
DeGS
MuscarellaLA
2007 Primary hyperparathyroidism and the presence of kidney stones are associated with different haplotypes of the calcium-sensing receptor. J Clin Endocrinol Metab 92 277 283
51. MiedlichS
LameschP
MuellerA
PaschkeR
2001 Frequency of the calcium-sensing receptor variant A986S in patients with primary hyperparathyroidism. Eur J Endocrinol 145 421 427
52. VezzoliG
TaniniA
FerrucciL
SoldatiL
BianchinC
2002 Influence of calcium-sensing receptor gene on urinary calcium excretion in stone-forming patients. J Am Soc Nephrol 13 2517 2523
53. KimKS
KimGS
HwangJY
LeeHJ
ParkMH
2007 Single nucleotide polymorphisms in bone turnover-related genes in Koreans: ethnic differences in linkage disequilibrium and haplotype. BMC Med Genet 8 70
54. MarzW
SeelhorstU
WellnitzB
TiranB
Obermayer-PietschB
2007 Alanine to serine polymorphism at position 986 of the calcium-sensing receptor associated with coronary heart disease, myocardial infarction, all-cause, and cardiovascular mortality. J Clin Endocrinol Metab 92 2363 2369
55. RichardsJB
KavvouraFK
RivadeneiraF
StyrkarsdottirU
EstradaK
2009 Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151 528 537
56. BjörkmanMP
SorvaAJ
TilvisRS
1979 Calculated serum calcium is an insufficient surrogate for measured ionized calcium. Archives of Gerontology and Geriatrics 49 348 350
57. RobertsonWG
MarshallRW
1979 Calcium measurements in serum and plasma–total and ionized. CRC Crit Rev Clin Lab Sci 11 271 304
58. LadensonJH
LewisJW
BoydJC
1978 Failure of total calcium corrected for protein, albumin, and pH to correctly assess free calcium status. J Clin Endocrinol Metab 46 986 993
59. ChambersJC
ZhangW
LiY
SehmiJ
WassMN
2009 Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nature Genetics
60. FerrucciL
BandinelliS
BenvenutiE
Di IorioA
MacchiC
2000 Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 48 1618 1625
61. ShockNW
GreulichRC
ArenbergD
CostaPT
LakattaEG
1984 Normal Human Aging: The Baltimore Longitudinal Study of Aging. Washington, D.C. National Institutes of Health
62. RussoCR
LauretaniF
BandinelliS
BartaliB
Di IorioA
2003 Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int 14 531 538
63. LiY
WillerC
SannaS
AbecasisG
2009 Genotype imputation. Annu Rev Genomics Hum Genet 10 387 406
64. MarchiniJ
HowieB
MyersS
McVeanG
DonnellyP
2007 A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 906 913
65. JohnsonT
KutalikZ
2008 QUICKTEST
66. AbecasisGR
ChernySS
CooksonWO
CardonLR
2002 Merlin–rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30 97 101
67. HigginsJP
ThompsonSG
2002 Quantifying heterogeneity in a meta-analysis. Stat Med 21 1539 1558
68. BacanuSA
DevlinB
RoederK
2000 The power of genomic control. Am J Hum Genet 66 1933 1944
69. SaxenaR
VoightBF
LyssenkoV
BurttNP
de BakkerPI
2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 1331 1336
70. DudbridgeF
GusnantoA
2008 Estimation of significance thresholds for genomewide association scans. Genet Epidemiol 32 227 234
71. BarrettJC
FryB
MallerJ
DalyMJ
2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 263 265
72. McVeanGA
MyersSR
HuntS
DeloukasP
BentleyDR
2004 The fine-scale structure of recombination rate variation in the human genome. Science 304 581 584
73. WincklerW
MyersSR
RichterDJ
OnofrioRC
McDonaldGJ
2005 Comparison of fine-scale recombination rates in humans and chimpanzees. Science 308 107 111
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 7
-
Všechny články tohoto čísla
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
- Tinkering Evolution of Post-Transcriptional RNA Regulons: Puf3p in Fungi as an Example
- The Importance of Imprinting in the Human Placenta
- Regulator of G Protein Signaling 3 Modulates Wnt5b Calcium Dynamics and Somite Patterning
- Lysosomal Dysfunction Promotes Cleavage and Neurotoxicity of Tau
- Combinatorial Binding Leads to Diverse Regulatory Responses: Lmd Is a Tissue-Specific Modulator of Mef2 Activity
- Variation, Sex, and Social Cooperation: Molecular Population Genetics of the Social Amoeba
- Comparative Analysis of DNA Replication Timing Reveals Conserved Large-Scale Chromosomal Architecture
- The Fitness Landscapes of -Acting Binding Sites in Different Promoter and Environmental Contexts
- Cohesin Is Limiting for the Suppression of DNA Damage–Induced Recombination between Homologous Chromosomes
- Genome-Wide Analysis Reveals Novel Genes Essential for Heme Homeostasis in
- Genome-Wide Meta-Analysis for Serum Calcium Identifies Significantly Associated SNPs near the Calcium-Sensing Receptor () Gene
- Rad3 Decorates Critical Chromosomal Domains with γH2A to Protect Genome Integrity during S-Phase in Fission Yeast
- Quantitative and Molecular Genetic Analyses of Mutations Increasing Life Span
- Association of Variants at with Chronic Kidney Disease and Kidney Stones—Role of Age and Comorbid Diseases
- Breast Cancer DNA Methylation Profiles Are Associated with Tumor Size and Alcohol and Folate Intake
- Calpain 8/nCL-2 and Calpain 9/nCL-4 Constitute an Active Protease Complex, G-Calpain, Involved in Gastric Mucosal Defense
- A Collection of Target Mimics for Comprehensive Analysis of MicroRNA Function in
- A Genome-Wide Analysis Reveals No Nuclear Dobzhansky-Muller Pairs of Determinants of Speciation between and , but Suggests More Complex Incompatibilities
- Microevolution of during Prolonged Infection of Single Hosts and within Families
- Id4, a New Candidate Gene for Senile Osteoporosis, Acts as a Molecular Switch Promoting Osteoblast Differentiation
- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Chromatin Remodeling in Development and Disease: Focus on CHD7
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Requirement of Male-Specific Dosage Compensation in Females—Implications of Early X Chromosome Gene Expression
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CHD7 Targets Active Gene Enhancer Elements to Modulate ES Cell-Specific Gene Expression
- Extensive DNA End Processing by Exo1 and Sgs1 Inhibits Break-Induced Replication
- Question and Answer: An Anniversary Interview with Jane Gitschier
- Multi-Variant Pathway Association Analysis Reveals the Importance of Genetic Determinants of Estrogen Metabolism in Breast and Endometrial Cancer Susceptibility
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



