-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Single Nucleotide Polymorphism within the Acetyl-Coenzyme A Carboxylase Beta Gene Is Associated with Proteinuria in Patients with Type 2 Diabetes
It has been suggested that genetic susceptibility plays an important role in the pathogenesis of diabetic nephropathy. A large-scale genotyping analysis of gene-based single nucleotide polymorphisms (SNPs) in Japanese patients with type 2 diabetes identified the gene encoding acetyl-coenzyme A carboxylase beta (ACACB) as a candidate for a susceptibility to diabetic nephropathy; the landmark SNP was found in the intron 18 of ACACB (rs2268388: intron 18 +4139 C > T, p = 1.4×10−6, odds ratio = 1.61, 95% confidence interval [CI]: 1.33–1.96). The association of this SNP with diabetic nephropathy was examined in 9 independent studies (4 from Japan including the original study, one Singaporean, one Korean, and two European) with type 2 diabetes. One case-control study involving European patients with type 1 diabetes was included. The frequency of the T allele for SNP rs2268388 was consistently higher among patients with type 2 diabetes and proteinuria. A meta-analysis revealed that rs2268388 was significantly associated with proteinuria in Japanese patients with type 2 diabetes (p = 5.35×10−8, odds ratio = 1.61, 95% Cl: 1.35–1.91). Rs2268388 was also associated with type 2 diabetes–associated end-stage renal disease (ESRD) in European Americans (p = 6×10−4, odds ratio = 1.61, 95% Cl: 1.22–2.13). Significant association was not detected between this SNP and nephropathy in those with type 1 diabetes. A subsequent in vitro functional analysis revealed that a 29-bp DNA fragment, including rs2268388, had significant enhancer activity in cultured human renal proximal tubular epithelial cells. Fragments corresponding to the disease susceptibility allele (T) had higher enhancer activity than those of the major allele. These results suggest that ACACB is a strong candidate for conferring susceptibility for proteinuria in patients with type 2 diabetes.
Published in the journal: . PLoS Genet 6(2): e32767. doi:10.1371/journal.pgen.1000842
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000842Summary
It has been suggested that genetic susceptibility plays an important role in the pathogenesis of diabetic nephropathy. A large-scale genotyping analysis of gene-based single nucleotide polymorphisms (SNPs) in Japanese patients with type 2 diabetes identified the gene encoding acetyl-coenzyme A carboxylase beta (ACACB) as a candidate for a susceptibility to diabetic nephropathy; the landmark SNP was found in the intron 18 of ACACB (rs2268388: intron 18 +4139 C > T, p = 1.4×10−6, odds ratio = 1.61, 95% confidence interval [CI]: 1.33–1.96). The association of this SNP with diabetic nephropathy was examined in 9 independent studies (4 from Japan including the original study, one Singaporean, one Korean, and two European) with type 2 diabetes. One case-control study involving European patients with type 1 diabetes was included. The frequency of the T allele for SNP rs2268388 was consistently higher among patients with type 2 diabetes and proteinuria. A meta-analysis revealed that rs2268388 was significantly associated with proteinuria in Japanese patients with type 2 diabetes (p = 5.35×10−8, odds ratio = 1.61, 95% Cl: 1.35–1.91). Rs2268388 was also associated with type 2 diabetes–associated end-stage renal disease (ESRD) in European Americans (p = 6×10−4, odds ratio = 1.61, 95% Cl: 1.22–2.13). Significant association was not detected between this SNP and nephropathy in those with type 1 diabetes. A subsequent in vitro functional analysis revealed that a 29-bp DNA fragment, including rs2268388, had significant enhancer activity in cultured human renal proximal tubular epithelial cells. Fragments corresponding to the disease susceptibility allele (T) had higher enhancer activity than those of the major allele. These results suggest that ACACB is a strong candidate for conferring susceptibility for proteinuria in patients with type 2 diabetes.
Introduction
Diabetic nephropathy is a leading cause of end-stage renal disease (ESRD) in Western countries [1] and in Japan [2]. The rising incidence of diabetic nephropathy, especially among patients with type 2 diabetes, is a serious worldwide concern in terms of both poor prognosis and medical costs. The pathogenesis of diabetic nephropathy has not been fully elucidated. However, susceptibility to diabetic nephropathy appears to be determined by multiple genetic and environmental risk factors, and genetic susceptibility plays an important role in its development and progression [3],[4].
Both candidate gene approaches and genome-wide linkage analyses have suggested several candidate genes with potential impact on diabetic nephropathy. However, these findings have not been robustly replicated [5],[6], and many susceptibility genes for diabetic nephropathy remain to be identified. The recent development of single nucleotide polymorphism (SNP) typing technology and insights into patterns of linkage disequilibrium (LD) in the human genome have facilitated genome-wide association studies (GWASs) for investigating genes associated with disease susceptibility across the entire human genome. GWASs conducted by several independent research groups in Europe, United States [7],[8] and Japan [9],[10] have identified multiple loci associated with susceptibility to common complex traits, including type 2 diabetes. Recently conducted GWAS in a population of European descent identified 4 distinct loci associated with diabetic nephropathy in type 1 diabetes. Two of these loci were replicated in a population of the Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) cohorts [11].
With the aim of identifying loci involved in susceptibility to common diseases, we initiated a large-scale association study using SNPs from a Japanese SNP database (JSNP: http://snp.ims.u-tokyo.ac.jp/) [12],[13], that was established before creation of the HapMap database. Through this project, we have previously identified genes encoding solute carrier family 12 (sodium/chloride) member 3 (SLC12A3: MIM 600968, Online Mendelian Inheritance in Man: http://www.ncbi.nlm.nih.gov/omim)[14], engulfment and cell motility 1 (ELMO1: MIM 606420)[15], and neurocalcin δ (NCALD: MIM 606722)[16] as being associated with susceptibility to diabetic nephropathy. The ELMO1 association has been replicated in African Americans [17] and European Americans [18].
In the present study, we extended a previous large-scale association study for diabetic nephropathy, and provide evidence that a SNP within the acetyl-coenzyme A (CoA) carboxylase beta gene (ACACB; MIM: 601557) contributes to an increased prevalence of proteinuria in patients with type 2 diabetes across different ethnic populations.
Results
We extended our prior analysis to SNPs with p values between 0.01 and 0.05, and examined the association of these SNPs with diabetic nephropathy in a larger study sample. In this analysis, a SNP within ACACB showed the strongest association with diabetic nephropathy in Japanese patients with type 2 diabetes (rs2268388: intron 18 +4139 C > T, p = 1.4×10−6, odds ratio [OR] = 1.61, 95% confidence interval [Cl]: 1.33–1.96, Table 1).
Tab. 1. Top 4 SNPs associated with diabetic nephropathy in a genome-wide screening. 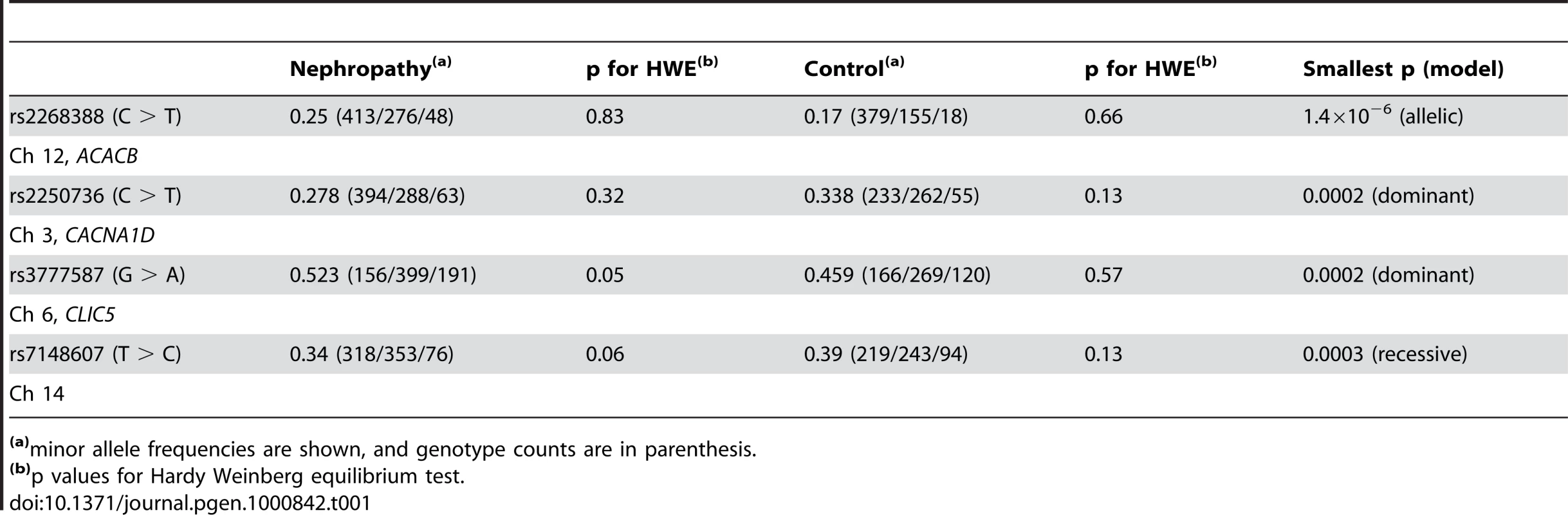
(a)minor allele frequencies are shown, and genotype counts are in parenthesis. Subsequent LD mapping around this region with data for 264 SNPs with allele frequencies ≥0.1 from HapMap database (HapMap: http://hapmap.ncbi.nlm.nih.gov/) for the Japanese, identified a 20-kb LD block that included an original marker SNP (rs2268388), which corresponded to a part of the ACACB gene (Figure 1A and 1B). Therefore, we concluded that ACACB was likely a candidate for conferring susceptibility to diabetic nephropathy. We next analyzed 51 SNPs, including 31 tagging SNPs, within ACACB in our Japanese population (Japanese1). Several SNPs within the same LD block as rs2268388 were nominally associated with diabetic nephropathy (Figure 1C, Table S1). No single SNP or haplotype showed stronger association with diabetic nephropathy than the original marker SNP (Figure S1).
Fig. 1. Schematic view of the association of SNPs in the ACACB region with diabetic nephropathy. 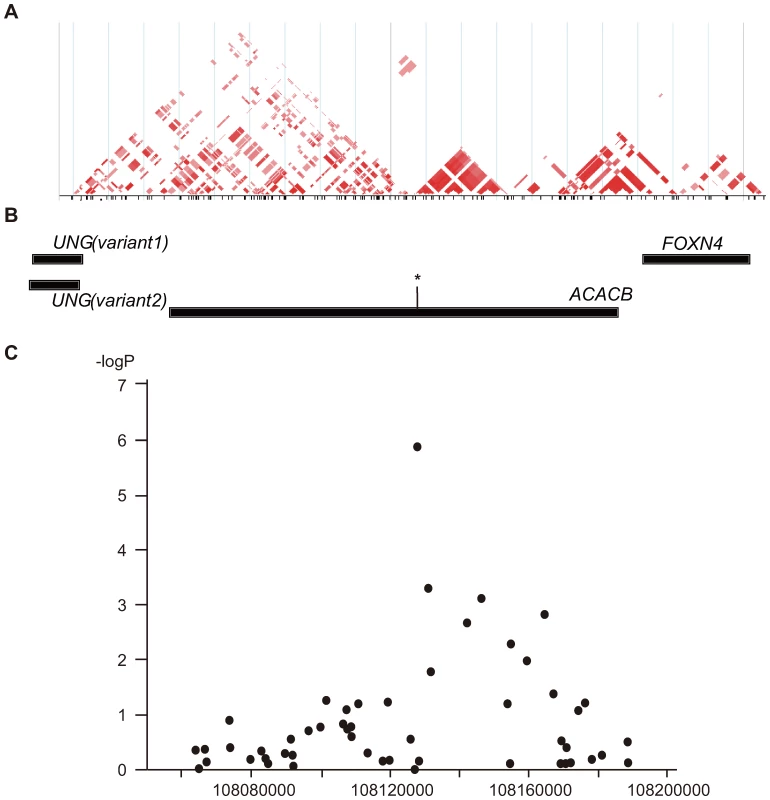
(A) Pairwise correlation structure at a 200-kb interval around SNP rs2268338 analyzed by Haploview (Haploview: http://www.broadinstitute.org/haploview/haploview). The plot includes pairwise r2 values from the HapMap release 24 for the JPT population. (B) Genes located at this locus. The asterisk indicates the SNP rs2268388 at intron 18 of the ACACB. (C) Results of a case-control association study for diabetic nephropathy in 754 Japanese individuals with type 2 diabetes having overt proteinuria and 558 control individuals with type 2 diabetes and normoalbuminuria. The log10-transformed p values for an additive model are plotted on the Y-axis. The X-axis indicates chromosomal position at this locus. To validate the association of this SNP with diabetic nephropathy, we examined the effects of the SNP on susceptibility to the disease in several independent populations from different ethnic groups (Table 2). The results indicated that the frequency of the T allele of rs2268388 was consistently higher among patients with type 2 diabetes with proteinuria (combined meta-analysis gave a p value of 5.35×10−8 in the Japanese, 2.34×10−7 for all populations). Significant association with ESRD was detected in the relatively large European 2 samples (481 cases and 427 controls). The SNP was also modestly associated with ESRD in East Asian type 2 diabetes, but the direction of association differed. Overall, the distribution of the genotype for rs2268388 did not differ significantly between patients with ESRD and control patients having type 2 diabetes (p = 0.47). No significant association was detected in patients with type 1 diabetes having proteinuria.
Tab. 2. Association of the SNP in the ACACB (rs2268388) with diabetic nephropathy in several independent cohorts. 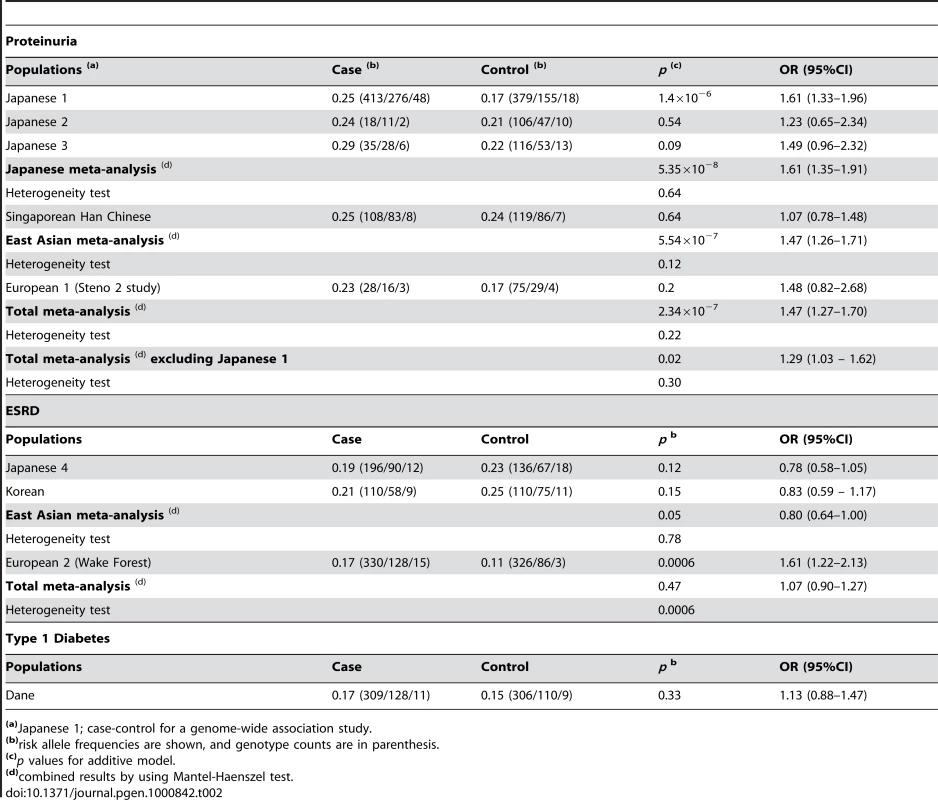
(a)Japanese 1; case-control for a genome-wide association study. We next examined the expression profile of ACACB in various human tissues. Expression of ACACB was observed in adipose tissue, heart and skeletal muscle, and, to a lesser extent, in the kidney (Figure 2A). The results of in situ hybridization with normal mouse kidney revealed that Acacb was localized to glomerular epithelial cells and tubular epithelial cells (Figure 2B). We also observed the expression of ACACB in cultured human renal proximal tubular epithelial cells (hRPTECs).
Fig. 2. Expression profiles of ACACB. 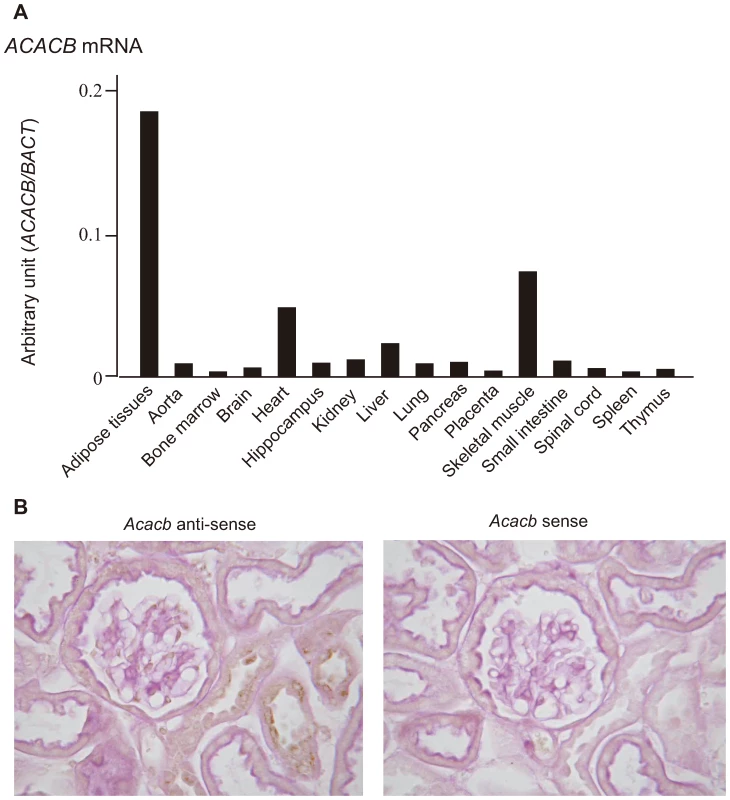
(A) Expression profiles of ACACB in various human tissues evaluated by real-time PCR. (B) Results of in situ hybridization for 20-week-old normal mouse kidneys using mouse Acacb anti-sense (left) and sense (right) probes. To investigate the functional role of this SNP region, we examined the effects of a 29-bp DNA fragment containing the associated SNP (rs2268388) on transcriptional activity in cultured hRPTECs. As shown in the Figure 3, the 29-bp DNA fragments had significant enhancer activity (promoter alone [P]: 39.4±13.1; susceptibility allele [T]: 384.3±104.1; major allele [C]: 238.5±81.9; relative luciferase activity, p = 0.0005 for P vs. T, p = 0.016 for P vs. C). Fragments corresponding to the disease susceptibility allele had stronger enhancer activity than those for the major allele ([T] 10.5±3.4 vs. [C] 5.9±1.3, fold increase over promoter alone, p = 0.045, Figure 3B).
Fig. 3. Effect of a 29-bp DNA fragment containing the associated SNP (rs2268388) on transcriptional activity in cultured hRPTECs. 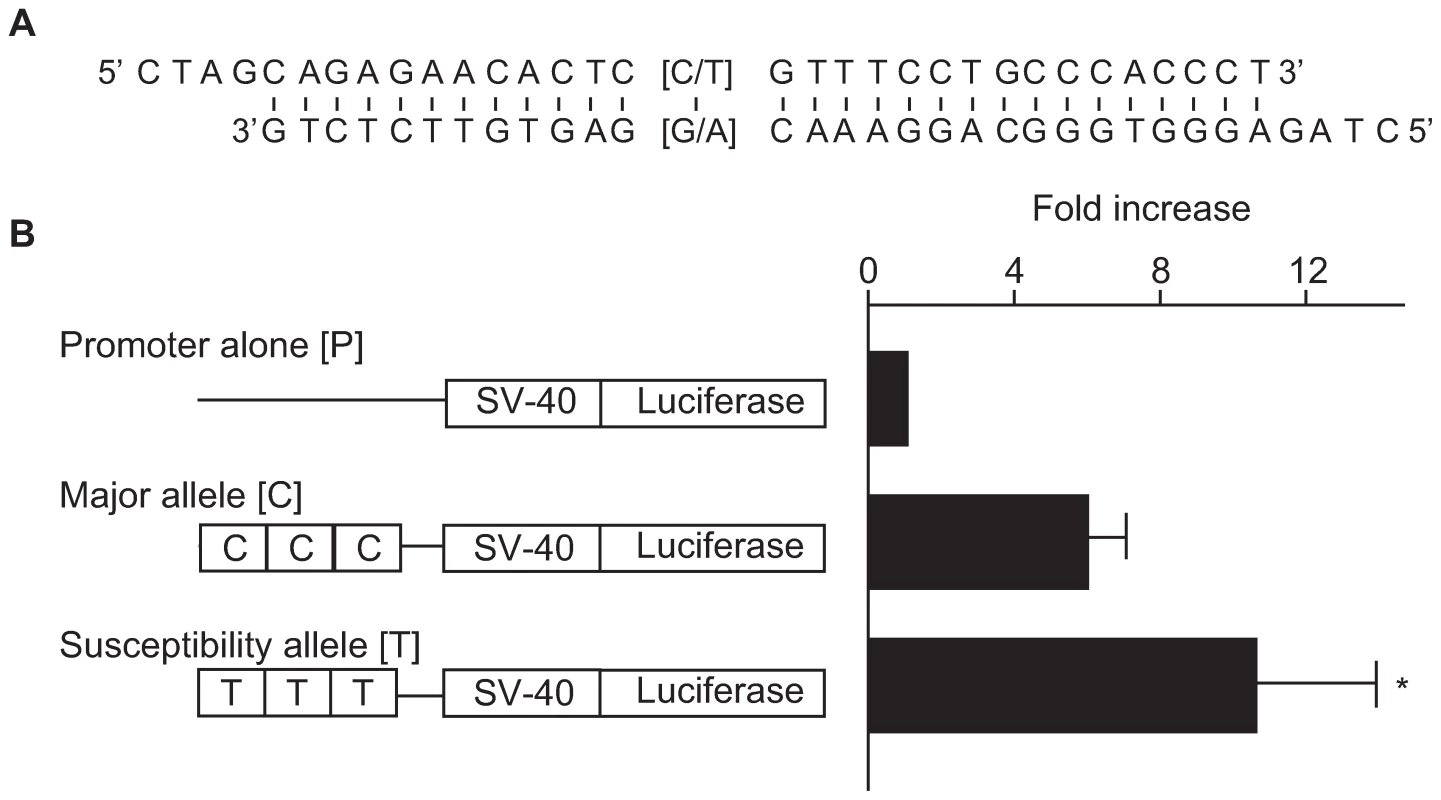
(A) A 29-bp sequence including SNP rs2268338. Overhung sequences are compatible ends for concatenation. (B) Enhancer activities of the DNA fragments corresponding to each allele in human RPTECs. Results are shown as mean ± SD of the ratio of activity to that of the promoter alone obtained from 4 independent experiments. * p = 0.045 vs. major allele. Discussion
In the present study, we showed ACACB located at chromosome 12q24.1 to be a strong susceptibility gene for diabetic nephropathy in patients with type 2 diabetes. Our findings suggest that a SNP within ACACB (rs2268388, intron 18 + 4139 C > T) contributes to the development of proteinuria in patients with type 2 diabetes.
ACACB encodes acetyl-coenzyme A (CoA) carboxylase beta, which catalyzes the carboxylation of acetyl-CoA to malonyl-CoA, and controls fatty acid oxidation by means of the ability of malonyl-CoA to inhibit carnitine palmitoyl transferase I (CPT1A; MIM 600528), the rate-limiting step in fatty acid uptake and oxidation by mitochondria in non-lipogenic tissues. Mice lacking Acacb have a normal life span, a higher rate of fatty acid oxidation, lower amounts of fat, and increased insulin sensitivity [19]–[21]; therefore, ACACB might affect insulin sensitivity via modulation of fatty acid metabolism. However evidence suggesting a role for the ACACB in the pathogenesis of diabetic nephropathy was previously lacking. In this study, expression of ACACB was detected in heart, skeletal muscle and adipose tissues by real-time quantitative polymerase chain reaction (PCR) as previously reported [22]. We also showed that ACACB was expressed in human kidney, and in situ hybridization revealed that Acacb expression was localized to glomerular epithelial cells and tubular epithelial cells in normal mouse kidneys. Abnormalities in lipid metabolism [23],[24], including fatty acid metabolism have been shown to contribute to the development and/or progression of chronic kidney diseases, including diabetic nephropathy. Hence, genotype-based differences in expression and/or activity of this enzyme in the kidney might contribute to conferring susceptibility to diabetic nephropathy.
Elucidating these functional differences will help us to understand how variation in this gene contributes to susceptibility to diabetic nephropathy. In this study, the 29-bp fragment that included the landmark SNP (rs2268388) was shown to have significant enhancer activity. We also demonstrated that the DNA corresponding to the disease susceptibility allele had significantly higher enhancer activity than that for the major allele in cultured human RPTECs. Therefore, the intronic variation in the gene seems to be causal. We hypothesize that higher expression of ACACB in the kidneys of subjects having the disease susceptibility allele (T) may increase the susceptibility to diabetic nephropathy in type 2 diabetes.
Interestingly, the association of the T allele of rs2268388 with type 2 diabetes-associated nephropathy was consistently observed in cases with proteinuria, whereas there was less consistent association between type 2 diabetic patients under chronic renal replacement therapy (ESRD). Discrepancies in genomic loci underlying susceptibility to proteinuria versus ESRD were previously noted in a genome-wide linkage scan for diabetic nephropathy in type 2 diabetes [25]. Because most of the patients with ESRD were considered to have had proteinuria, and there is significant heterogeneity in the association with diabetic ESRD among the East Asian and European American populations (heterogeneity p = 0.0006, Table 2), some selection bias, such as a survival effect, or ethnic differences might exist when patients with ESRD were used as cases. Since the presence of proteinuria is also recognized as a predictor of cardio-vascular diseases, the association of ACACB with proteinuria might reflect an association between the gene and cardio-vascular diseases or metabolic syndrome. However elucidation of a precise mechanism will require further investigation.
Association of the ACACB with diabetic nephropathy could not be replicated in patients with type 1 diabetes, although these nephropathy cases had proteinuria. The clinical features or histological characteristics of diabetic nephropathy are similar in both type1 and type 2 diabetes mellitus; however, there are some differences in the background circumstances between both types of the disease. For example, patients with type 2 diabetes are generally older, and more obese than those with type 1 diabetes. Therefore, it is possible that genetic factors for nephropathy are different for type 1 and type 2 diabetes, although some overlap may exist. Because the statistical power of this study for patients with type 1 diabetes was probably not sufficient, the association of variations in ACACB with diabetic nephropathy in patients with type 1 diabetes should be re-evaluated in future studies.
Recently, GWASs have been performed to identify susceptibility genes for common diseases. Convincing susceptibility genes for many diseases including type 2 diabetes have been successfully identified by GWASs. A GWAS for diabetic nephropathy was conducted in the Genetics of Kidneys in Diabetes (GoKinD) collection, and several novel loci were identified for diabetic nephropathy in type 1 diabetes [11]. Compared to our study, recent GWASs conducted in European and American groups had greater power to detect true associations. The power of the first and second test in the present study is estimated >40% and >90% respectively, for SNPs with minor allele frequency of 0.2 as in our sample, if we set a cut-off value at the p = 0.05 level, a genotypic relative risk (γ) of 1.5, and the prevalence of diabetic nephropathy is assumed to be 10% (CaTS power calculator, CaTS: http://www.sph.umich.edu/csg/abecasis/CaTS/). Therefore, other important loci contributing to diabetic nephropathy in Japanese populations may not have been detected and should be searched for using a larger scale of GWAS. Limitations exist in increasing the numbers of subjects with diabetic nephropathy and diabetes lacking nephropathy in single center studies. Therefore, we examined several independently collected study populations, allowing for the presence of different selection criteria and the small sample size in some replication cohorts. Future analyses should attempt to avoid these limitations so as not to produce spurious results.
In summary, based upon extensive genome-wide gene-centric SNP analyses, we identified ACACB as a candidate gene for conferring susceptibility to diabetic nephropathy. Subsequent replication studies in several different ethnic groups and a functional study suggest that the T-allele of a common intronic SNP (rs2268338) within ACACB is a risk factor for the development and progression of proteinuria in patients with type 2 diabetes.
Materials and Methods
Subjects, DNA preparation, and genotyping
Japanese 1 (genome-wide screening); DNA samples were obtained from the peripheral blood of patients with type 2 diabetes who regularly attended outpatient clinics at Shiga University of Medical Science, Tokyo Women's Medical University, Juntendo University, Kawasaki Medical School, Iwate Medical University, Toride Kyodo Hospital, Kawai Clinic, Osaka City General Hospital, Chiba Tokusyukai Hospital or Osaka Rosai Hospital. All subjects provided informed consent before enrolling in this study. DNA extraction was performed by a standard phenol-chloroform method. Diabetic patients were divided into 2 groups according to the following diagnostic criteria: 1) nephropathy cases, i.e., patients with diabetic retinopathy and overt nephropathy, indicated by a urinary albumin excretion rate (AER) ≥200 µg/min or a urinary albumin/creatinine ratio (ACR) ≥300 mg/g creatinine (Cr), and 2) control patients who have diabetic retinopathy but no evidence of renal dysfunction (i.e. AER less than 20 µg/min or ACR less than 30 mg/g Cr). Measurements of AER or ACR were performed at least twice for each patient. The SNPs for genotyping were randomly selected from our gene-based Japanese SNP (JSNP) database. The genotype of each SNP locus was analyzed with multiplex PCR-invader assays, as described previously [14]–[16].
Our first screening involved genotyping 94 nephropathy cases and 94 control patients for more than 100,000 SNP loci. In total, 76,767 SNP loci, which distributed to 13,707 gene-centric regions and covered approximately 35% of common SNPs (MAF >0.15) in these regions, were successfully genotyped by the invader assay (success rates >0.95). We estimated identity by descent (IBD) sharing to assess relatedness among our initial GWAS populations. As shown in Figure S2, the result indicates that there were no close relative pairs in this population. We also performed principal component analysis (PCA) using our initial GWAS populations to evaluate population structures. Since all subjects were in a single cluster in the PCA analysis (Figure S3), no evidence for population stratification between case and control groups appeared to exist in the present GWAS.
After the first round of analysis that evaluated SNPs with p values less than 0.01 in the first screening (previously published [14]–[16]), we extended our analysis to include SNPs with p values between 0.01 and 0.05 and examined them in a larger number of patients (754 nephropathy cases vs. 558 control patients). The study protocol was approved by the ethics committees of RIKEN Yokohama Institute and each participating institution.
Subjects in replication studies
Japanese replication studies
We obtained DNA samples from 2 longitudinal studies (Japanese 2, Japanese 3) and one case-control study (Japanese 4).
Japanese 2; Patients with type 2 diabetes were recruited from among the participants in the Shiga Prospective Observational Follow-Up Study for Diabetic Complications [26]. On the basis of at least 2 measurements of AER in 24-h urine collections, those classified as having microalbuminuria (200 µg/min > AER ≥20 µg/min) were followed for up to 6 years. The progressors (cases) were defined as those had progressed to overt proteinuria (AER ≥200 µg/min, n = 32), and the remaining patients were defined as non-progressors (controls, n = 168). The ACACB genotype was analyzed with multiplex PCR-invader assays. The study protocol and informed consent procedure were approved by the ethics committee of Shiga University of Medical Science.
Japanese 3; Patients with type 2 diabetes and normoalbuminuria or microalbuminuria, as determined by at least two measurements of ACR or AER (normoalbuminuria; ACR <30 mg/g Cr or AER <20 µg/min, microalbuminuria; 30≤ ACR <300 mg/g Cr or 20≤ AER <200 µg/min) who could be followed for 10 years were recruited from among diabetic outpatients at Juntendo University Hospital or Saiseikai Central Hospital [27]. Progressors (cases, n = 71) were defined as patients who progressed from a given stage to a more advanced stage of diabetic nephropathy; the remaining patients were defined as non-progressors (controls, n = 193). The ACACB genotype was analyzed with multiplex PCR-invader assays. All patients gave informed consent and the protocol was approved by the ethics committee of Juntendo University or that of Saiseikai Central Hospital.
Japanese 4; Patients with type 2 diabetes regularly visiting Tokai University Hospital or its affiliated hospitals were enrolled. All nephropathy cases were receiving chronic hemodialysis therapy (n = 300), and control patients were those with normoalbuminuria determined by at least 2 measurements of the urinary ACR, and diabetes for more than 10 years (n = 218). The ACACB genotype was analyzed with multiplex PCR-invader assays. Patients gave informed consent and the protocol was approved by the ethics committee of Tokai University School of Medicine.
Korean replication study
Korean patients with type 2 diabetes comprising two groups according to the following criteria were examined [28]: 1) the control group (n = 196): patients with diabetic retinopathy and who had diabetes for more than 15 years but no renal involvement (i.e., ACR<30 mg/g Cr and creatinine clearance [using the Cockroft equation] of >60 ml/min per 1.73 m2); 2) the ESRD group (n = 177): patients with diabetic retinopathy and ESRD due to type 2 diabetes, as indicated by a creatinine clearance rate of <15 ml/min per 1.73 m2 or receiving renal replacement therapy. The TaqMan method for genotyping was applied in the Korean replication study. The institutional review board of the Clinical Research Institute at Seoul National University Hospital approved the study protocol, and informed consent for genetic analysis was obtained from each patient.
Singaporean replication study
Cases and controls were selected from Chinese patients with type 2 diabetes who had been enrolled into the Singapore Diabetes Cohort Study (SDCS) as previously reported [29]. Patients with ACR >300 mg/g Cr or dipstick positive were considered nephropathy cases (n = 199). Controls were patients who were normalbuminuric with ACR <30 mg/g Cr and had diabetes for more than 7 years (n = 212). The ACACB genotype was analyzed using a Taqman genotyping assay available from Applied Biosystems (Foster city, CA, U.S.A.). The research protocol for SDCS was approved by both the National University of Singapore Institutional Review Board (NUS-012) and the National Healthcare Group Domain-Specific Review Board (C/05/118).
European replication studies
European 1 (Steno 2): Patients were recruited from the Steno Diabetes Center between 1992 and 1993. Microalbuminuria was defined as an AER of 30–300 mg per 24 h in 4 of 6 samples of sterile urine. These patients were enrolled and followed up for an average of 7.8 years [30]. Patients who progressed to nephropathy (AER >300 mg per 24 h, n = 47) were used as cases, and the remaining patients were defined as controls (n = 110). The ACACB genotype was analyzed with multiplex PCR-invader assays. Informed consent was obtained from all participants. The protocol was in accordance with the Declaration of Helsinki and was approved by the ethics committee of Copenhagen County.
European 2 (Wake Forest): Patients with European ancestry who were born in North Carolina, South Carolina, Georgia, Tennessee, or Virginia were enrolled. Cases all had type 2 diabetes mellitus for 5 or more years before the development of ESRD with overt proteinuria and/or diabetic retinopathy (n = 481). Control patients had type 2 diabetes for more than 5 years with ACR <30 mg/g Cr and serum creatinine <1.5 mg/dl (n = 427). The ACACB SNP was genotyped using the MassARRAY genotyping system (Sequenom, San Diego, CA, U.S.A.). PCR primers were designed using the MassARRAY Design 3.4 Software (Sequenom). This study was conducted under Institutional Review Board approval from Wake Forest University School of Medicine, and adhered to the tenets of the Declaration of Helsinki.
Type 1 diabetes: Adults with type 1 diabetes attending the outpatient clinic at the Steno Diabetes Center were invited to participate in a study of genetic risk factors for the development of diabetic micro - and macrovascular complications [31]. Patients were considered to have type 1 diabetes if the age at onset of diabetes was ≤35 years and if the time to definitive insulin therapy was ≤1 year. Established diabetic nephropathy (cases, n = 458) was defined as persistent albuminuria (≥300 mg/24 h) in 2 out of 3 consecutive measurements on sterile urine in the presence of retinopathy. The absence of diabetic nephropathy (controls, n = 442) was defined as persistent normoalbuminuria (urinary albumin excretion rate: <30 mg/24 h) after at least 15 years of diabetes duration in patients not treated with angiotensin converting enzyme inhibitors or angiotensin II receptor blockers. The ACACB genotype was analyzed with multiplex PCR-invader assays. The study was performed in accordance with the Declaration of Helsinki. The local ethics committee approved the study and all patients gave their informed consent.
The clinical characteristics of patients in all studies are shown in Table S2.
Real-time quantitative RT–PCR
We obtained human cDNAs from multiple tissues from CLONTECH Inc. (Palo Alto, CA, U.S.A.). The cDNAs were amplified by PCR with the following primers: human ACACB, sense 5′-CGG ATG CGT AAC TTC GAT CTG-3′, antisense 5′-CTA TGG TCC GTC ACT TCC ACA C-3′; BACT, sense 5′ - TCA CCC ACA CTG TGC CCA TCT ACG A -3′, antisense 5′ - CAG CGG AAC CGC TCA TTG CCA ATG G -3′. Amplification was performed in a 22 µl reaction volume that contained 1× EX Taq Buffer, 200 nM dNTP, 1/20,000 SYBR Green, 0.2 µM Rox, 800 nM gene-specific primer, 0.05 U/µl EX Taq Hot Start Version (Takara, Otsu, Japan), and 5 ng of template DNA. The thermal profile was 50°C for 2 min, at 95°C for 10 min, followed by 40 cycles at 95°C for 30 s and at 60°C for 60 s in thermal cycler (Mx3000P Multiplex Quantitative PCR system; Stratagene, La Jolla, CA, U.S.A.). The results were normalized with human BACT.
In situ hybridization
Under pentobarbital anesthesia, 20-week-old mice were flushed with PBS through the abdominal aorta followed by perfusion with 4% paraformaldehyde buffered with PBS (pH 7.4). The kidneys were quickly removed and cut into small pieces. The renal cortex tissue was immediately dissected and immersed into a fresh portion of the same fixative at 4°C overnight. All steps were carefully carried out to avoid contamination with RNase. Diethylpyrocarbonate-treated water was used at 0.1% to prepare each buffer. The fixed samples were thoroughly rinsed with PBS (pH 7.4) and subsequently dehydrated by passage through an alcohol series and cleared in xylene. In situ hybridization was performed on paraffin-embedded sections using a previously described method [15]. Antisense and sense single-strand cRNAs were synthesized from cDNA fragments encoding Acacb using reverse-transcription PCR. The Acacb cDNA fragment was consisted of a 500 bp mouse sequence (nucleotides 181–680, GenBank accession number NM_133904, GenBank: http://www.ncbi.nlm.nih.gov/Genbank/).
Plasmid construction and transfection experiments
Three copies of the 29-bp DNA fragments including rs2268388 in ACACB were subcloned into a pGL3-promoter vector (Promega, Madison, WI, U.S.A.) at its multi-cloning site upstream of the SV-40 promoter. We introduced constructs corresponding to each allele into the human renal proximal tubular epithelial cells (hRPTEC, Lonza, Basel, Switzerland) along with a sea-pansy luciferase control vector, pRL-TK (Promega), using the liposome transfection procedure (Lipofectoamine 2000, Life Technology Inc, Carlsbad, CA, U.S.A.). Twenty-four hours after transfection, luciferase activitiy was determined by means of the Dual Luciferase Reporter Assay System (Promega). The luminescence of firefly luciferase was corrected by use of the sea-pansy luciferase, which reflected transfection efficiency.
Statistical analyses
We tested the genotype and allele frequencies for Hardy-Weinberg equilibrium (HWE) proportions by use of the χ2 test [32]. We calculated the LD index, D' and r2, as described elsewhere [33]. We analyzed the differences between the case and control groups with regard to the genotype distribution and allele frequency in the genome-wide screen by Fisher's exact test with dominant, recessive and allelic models with autosomal SNPs. The association of the ACACB locus with diabetic nephropathy in the replication study was evaluated with the Armitage test for trends using an additive model, as described previously [34]. Combined meta-analysis was performed by using the Mantel-Haenszel procedure with a fixed effect model after testing for heterogeneity. The data from the transfection experiments were analyzed by one-way analysis of variance, followed by Scheffe's test to evaluate statistical differences among 3 groups or by an un-paired t test to evaluate differences between 2 groups.
Supporting Information
Zdroje
1. U S Renal Data System 2008 USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD
2. NakaiS
MasakaneI
AkibaT
ShigematsuT
YamagataK
2008 Overview of Regular Dialysis Treatment in Japan as of 31 December 2006. Ther Apher Dial 12 428 456
3. SeaquistER
GoetzFC
RichS
BarbosaJ
1989 Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 320 1161 1165
4. QuinnM
AngelicoMC
WarramJH
KrolewskiAS
1996 Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 39 940 945
5. FreedmanBI
BostromM
DaeihaghP
BowdenDW
2007 Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol 2 1306 1316
6. MaedaS
2008 Genetics of diabetic nephropathy Ther Adv Cardiovasc Dis 2 363 371
7. FraylingTM
2007 Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet 8 657 662
8. ProkopenkoI
McCarthyMI
LindgrenCM
2008 Type 2 diabetes: new genes, new understanding. Trends Genet 24 613 621
9. UnokiH
TakahashiA
KawaguchiT
HaraK
HorikoshiM
2008 SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 40 1098 1102
10. YasudaK
MiyakeK
HorikawaY
HaraK
OsawaH
2008 Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 40 1092 1097
11. PezzolesiMG
PoznikGD
MychaleckyjJC
PatersonAD
BaratiMT
2009 Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 58 1403 1410
12. HagaH
YamadaR
OhnishiY
NakamuraY
TanakaT
2002 Gene-based SNP discovery as part of the Japanese Millennium Genome Project: identification of 190,562 genetic variations in the human genome. Single-nucleotide polymorphism. J Hum Genet 47 605 610
13. HirakawaM
TanakaT
HashimotoY
KurodaM
TakagiT
2002 JSNP: a database of common gene variations in the Japanese population. Nucleic Acids Res 30 158 162
14. TanakaN
BabazonoT
SaitoS
SekineA
TsunodaT
2003 Association of solute carrier family 12 (sodium/chloride) member 3 with diabetic nephropathy, identified by genome-wide analyses of single nucleotide polymorphisms. Diabetes 52 2848 2853
15. ShimazakiA
KawamuraY
KanazawaA
SekineA
SaitoS
2005 Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes 54 1171 1178
16. KamiyamaM
KobayashiM
ArakiS
IidaA
TsunodaT
2007 Polymorphisms in the 3′ UTR in the neurocalcin δ gene affect mRNA stability, and confer susceptibility to diabetic nephropathy. Hum Genet 122 397 407
17. LeakTS
PerlegasPS
SmithSG
KeeneKL
HicksPJ
2009 Variants in intron 13 of the ELMO1 gene are associated with diabetic nephropathy in African Americans. Ann Hum Genet 73 152 159
18. PezzolesiMG
KatavetinP
KureM
PoznikGD
SkupienJ
2009 Confirmation of Genetic Associations at ELMO1 in the GoKinD Collection Support its Role as a Susceptibility Gene in Diabetic Nephropathy. Diabetes 58 2698 2702
19. Abu-ElheigaL
MatzukMM
Abo-HashemaKA
WakilSJ
2001 Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science 291 2613 2616
20. Abu-ElheigaL
OhW
KordariP
WakilSJ
2003 Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A 100 10207 10212
21. OhW
Abu-ElheigaL
KordariP
GuZ
ShaikenovT
2005 Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc Natl Acad Sci U S A 102 1384 1389
22. Abu-ElheigaL
Almarza-OrtegaDB
BaldiniA
WakilSJ
1997 Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J Biol Chem 272 10669 10677
23. KumeS
UzuT
IsshikiK
KoyaD
2008 Peroxisome proliferator-activated receptors in diabetic nephropathy. PPAR Res 2008 879523
24. WahbaIM
MakRH
2007 Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2 550 562
25. KrolewskiAS
PoznikGD
PlachaG
CananiL
DunnJ
2006 A genome-wide linkage scan for genes controlling variation in urinary albumin excretion in type II diabetes. Kidney Int 69 129 136
26. ArakiS
HanedaM
SugimotoT
IsonoM
IsshikiK
2005 Factors associated with frequent remission of microalbuminuria in patients with type 2 diabetes. Diabetes 54 2983 2987
27. NishiyamaK
TanakaY
NakajimaK
MokuboA
AtsumiY
2005 Polymorphism of the solute carrier family 12 (sodium/chloride transporters) member 3, SLC12A3, gene at exon 23 (+78G/A: Arg913Gln) is associated with elevation of urinary albumin excretion in Japanese patients with type 2 diabetes: a 10-year longitudinal study. Diabetologia 48 1335 1338
28. KimJH
ShinHD
ParkBL
MoonMK
ChoYM
2006 SLC12A3 (solute carrier family 12 member [sodium/chloride] 3) polymorphisms are associated with end-stage renal disease in diabetic nephropathy. Diabetes 55 843 848
29. NgDPK
FukushimaM
TaiBC
KohD
LeongH
2008 Reduced glomerular filtration rate and albuminuria in type 2 diabetes mellitus are both independently associated with activation of the TNF-alpha system. Diabetologia 51 2318 2324
30. GaedeP
VedelP
LarsenN
JensenGV
ParvingHH
2003 Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348 383 393
31. JorsalA
TarnowL
FrystykJ
LajerM
FlyvbjergA
2008 Serum adiponectin predicts all-cause mortality and end stage renal disease in patients with type I diabetes and diabetic nephropathy. Kidney Int 74 649 654
32. NielsenDM
EhmMG
WeirBS
1998 Detecting marker-disease association by testing for Hardy-Weinberg disequilibrium at a marker locus. Am J Hum Genet 63 1531 1540
33. DevlinB
RischN
1995 A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics 29 311 322
34. SladekR
RocheleauG
RungJ
DinaC
ShenL
2007 A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445 881 885
Štítky
Genetika Reprodukční medicína
Článek Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the GenomeČlánek Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in MiceČlánek Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' DiseaseČlánek Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD TagsČlánek Wing Patterns in the Mist
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 2
-
Všechny články tohoto čísla
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Scale of Population Structure in
- Allelic Exchange of Pheromones and Their Receptors Reprograms Sexual Identity in
- Genetic and Functional Dissection of and in Age-Related Macular Degeneration
- A Single Nucleotide Polymorphism within the Acetyl-Coenzyme A Carboxylase Beta Gene Is Associated with Proteinuria in Patients with Type 2 Diabetes
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Cdk2 Is Required for p53-Independent G/M Checkpoint Control
- Uncoupling of Satellite DNA and Centromeric Function in the Genus
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in the Clade
- Use of DNA–Damaging Agents and RNA Pooling to Assess Expression Profiles Associated with and Mutation Status in Familial Breast Cancer Patients
- Cheating by Exploitation of Developmental Prestalk Patterning in
- Replication and Active Demethylation Represent Partially Overlapping Mechanisms for Erasure of H3K4me3 in Budding Yeast
- Cdk1 Targets Srs2 to Complete Synthesis-Dependent Strand Annealing and to Promote Recombinational Repair
- A Genome-Wide Association Study Identifies Susceptibility Variants for Type 2 Diabetes in Han Chinese
- Genome-Wide Identification of Susceptibility Alleles for Viral Infections through a Population Genetics Approach
- Transcriptional Rewiring of the Sex Determining Gene Duplicate by Transposable Elements
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in
- Proteasome Nuclear Activity Affects Chromosome Stability by Controlling the Turnover of Mms22, a Protein Important for DNA Repair
- Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in Mice
- Structure, Function, and Evolution of the spp. Genome
- Human and Non-Human Primate Genomes Share Hotspots of Positive Selection
- A Kinase-Independent Role for the Rad3-Rad26 Complex in Recruitment of Tel1 to Telomeres in Fission Yeast
- Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' Disease
- Molecular Evolution and Functional Characterization of Insulin-Like Peptides
- Molecular Poltergeists: Mitochondrial DNA Copies () in Sequenced Nuclear Genomes
- Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD Tags
- Wing Patterns in the Mist
- DNA Binding of Centromere Protein C (CENPC) Is Stabilized by Single-Stranded RNA
- Genome-Wide Association Study Reveals Multiple Loci Associated with Primary Tooth Development during Infancy
- Mutations in , Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease
- Genome-Wide Identification of Binding Sites Defines Distinct Functions for PHA-4/FOXA in Development and Environmental Response
- Ku Regulates the Non-Homologous End Joining Pathway Choice of DNA Double-Strand Break Repair in Human Somatic Cells
- Nucleoporins and Transcription: New Connections, New Questions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Nucleoporins and Transcription: New Connections, New Questions
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



