-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Genome-Wide Association Study Identifies Susceptibility Variants for Type 2 Diabetes in Han Chinese
To investigate the underlying mechanisms of T2D pathogenesis, we looked for diabetes susceptibility genes that increase the risk of type 2 diabetes (T2D) in a Han Chinese population. A two-stage genome-wide association (GWA) study was conducted, in which 995 patients and 894 controls were genotyped using the Illumina HumanHap550-Duo BeadChip for the first genome scan stage. This was further replicated in 1,803 patients and 1,473 controls in stage 2. We found two loci not previously associated with diabetes susceptibility in and around the genes protein tyrosine phosphatase receptor type D (PTPRD) (P = 8.54×10−10; odds ratio [OR] = 1.57; 95% confidence interval [CI] = 1.36–1.82), and serine racemase (SRR) (P = 3.06×10−9; OR = 1.28; 95% CI = 1.18–1.39). We also confirmed that variants in KCNQ1 were associated with T2D risk, with the strongest signal at rs2237895 (P = 9.65×10−10; OR = 1.29, 95% CI = 1.19–1.40). By identifying two novel genetic susceptibility loci in a Han Chinese population and confirming the involvement of KCNQ1, which was previously reported to be associated with T2D in Japanese and European descent populations, our results may lead to a better understanding of differences in the molecular pathogenesis of T2D among various populations.
Published in the journal: . PLoS Genet 6(2): e32767. doi:10.1371/journal.pgen.1000847
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000847Summary
To investigate the underlying mechanisms of T2D pathogenesis, we looked for diabetes susceptibility genes that increase the risk of type 2 diabetes (T2D) in a Han Chinese population. A two-stage genome-wide association (GWA) study was conducted, in which 995 patients and 894 controls were genotyped using the Illumina HumanHap550-Duo BeadChip for the first genome scan stage. This was further replicated in 1,803 patients and 1,473 controls in stage 2. We found two loci not previously associated with diabetes susceptibility in and around the genes protein tyrosine phosphatase receptor type D (PTPRD) (P = 8.54×10−10; odds ratio [OR] = 1.57; 95% confidence interval [CI] = 1.36–1.82), and serine racemase (SRR) (P = 3.06×10−9; OR = 1.28; 95% CI = 1.18–1.39). We also confirmed that variants in KCNQ1 were associated with T2D risk, with the strongest signal at rs2237895 (P = 9.65×10−10; OR = 1.29, 95% CI = 1.19–1.40). By identifying two novel genetic susceptibility loci in a Han Chinese population and confirming the involvement of KCNQ1, which was previously reported to be associated with T2D in Japanese and European descent populations, our results may lead to a better understanding of differences in the molecular pathogenesis of T2D among various populations.
Introduction
Type 2 diabetes (T2D) affects at least 6% of the world's population; the worldwide prevalence is expected to double by 2025 [1]. T2D is a complex disorder that is characterized by hyperglycemia, which results from impaired pancreatic β cell function, decreased insulin action at target tissues, and increased glucose output by the liver [2]. Both genetic and environmental factors contribute to the pathogenesis of T2D. The disease is considered to be a polygenic disorder in which each genetic variant confers a partial and additive effect. Only 5%–10% of T2D cases are due to single gene defects; these include maturity-onset diabetes of the young (MODY), insulin resistance syndromes, mitochondrial diabetes, and neonatal diabetes [3]–[5]. Inherited variations have been identified from studies of monogenic diabetes, and have provided insights into β cell physiology, insulin release, and the action of insulin on target cells [6].
Much effort has been devoted to finding common T2D genes, including genome-wide linkage, candidate-gene, and genome-wide association studies (GWAS). Whole-genome linkage scans have identified chromosomal regions linked to T2D; however, with the exception of regions 1q [7]–[13] and 20q, which have been repeatedly mapped, linkage results vary from study to study [14]–[19]. Candidate-gene studies have provided strong evidence that common variants in the peroxisome proliferator-activated receptor-r (PPARG) [20], potassium inwardly-rectifying channel J11 (KCNJ11) [21]–[23], transcription factor 2 isoform b (TCF2) [24],[25], and Wolfram syndrome 1 (WFS1) [26] genes are associated with T2D. These genes all have strong biological links to diabetes, and rare, severe mutations cause monogenic diabetes. GWAS have accelerated the identification of T2D susceptibility genes, expanding the list from three in 2006 to over 20 genes in 2009. There are now at least 19 loci containing genes that increase risk of T2D, including PPARG [27], KCNJ11 [27], KCNQ1 [28],[29], CDKAL1 [27],[29]–[33], CDKN2A-2B [27],[32],[33], CDC123-CAMK1D [34], MTNR1B [35]–[37], TCF7L2 [31],[38],[39], TCF2 (HNF1B), HHEX-KIF11-IDE [27],[32],[33],[38], JAZF1 [34], IGF2BP2 [27],[29],[32], SLC30A8 [27],[32],[33],[38], THADA [34], ADAMTS9 [34], WFS1 [26], FTO [27],[31], NOTCH2 [34], and TSPAN8 [34]. Variants in these genes have been identified almost exclusively in populations of European descent, except for KCNQ1; individually, these variants confer a modest risk (odds ratio [OR] = 1.1–1.25) of developing T2D. KCNQ1 was identified as a T2D susceptibility gene in three GWA scans in Japanese individuals, highlighting the need to extend large-scale association efforts to different populations, such as Asian populations [28],[29],[40]. The association of other previously reported loci (CDKAL1, CDKN2A-2B, IGF2BP2, TCF7L2, SLC30A8, HHEX, and KCNJ11) with T2D were also replicated in the Japanese population [29],[40],[41].
To date, a GWA scan for T2D has not been conducted in the Han Chinese population, although the association of some known loci have been confirmed, including KCNQ1 and CDKAL1, CDKN2A-2B, MTNR1B, TCF7L2, HNF1β, and KCNJ11 [42]–[47]. Therefore, we conducted a two-stage GWA scan for T2D in a Han Chinese population residing in Taiwan. There were a total of 2,798 cases and 2,367 normal controls (995 cases and 894 controls in stage 1, 1,803 cases and 1,473 controls in stage 2). Our accomplished objective was to identify new diabetes susceptibility loci that were associated with increased risk of T2D in a Han Chinese population.
Results
Association analysis
We conducted a two-stage GWAS to identify genetic variants for T2D in the Han-Chinese residing in Taiwan. In the first stage, an exploratory genome-wide scan, we genotyped 995 T2D cases and 894 population controls using the Illumina Hap550duov3 chip (Figure 1 and Table S1). For each sample genotyped in this study, the average call rate was 99.92±0.12%. After applying stringent quality control criteria, high-quality genotypes for 516,737 SNPs (92.24%) were obtained, with an average call rate of 99.92±0.24% (Table S2). The results of principal component analysis in stage 1 revealed no evidence for population stratification between T2D cases and controls (P = 0.111, Fst statistics between populations <0.001) (Text S1; Figure S1). Multidimensional scaling analysis using PLINK [48] produced similar results (Text S1; Figure S2). Furthermore, genomic control (GC) with a variance inflation factor λ = 1.078 (trend test) did not substantially change the results of this GWAS (Table S3).
Fig. 1. Graphical summary of T2D GWAS in a Han Chinese population. 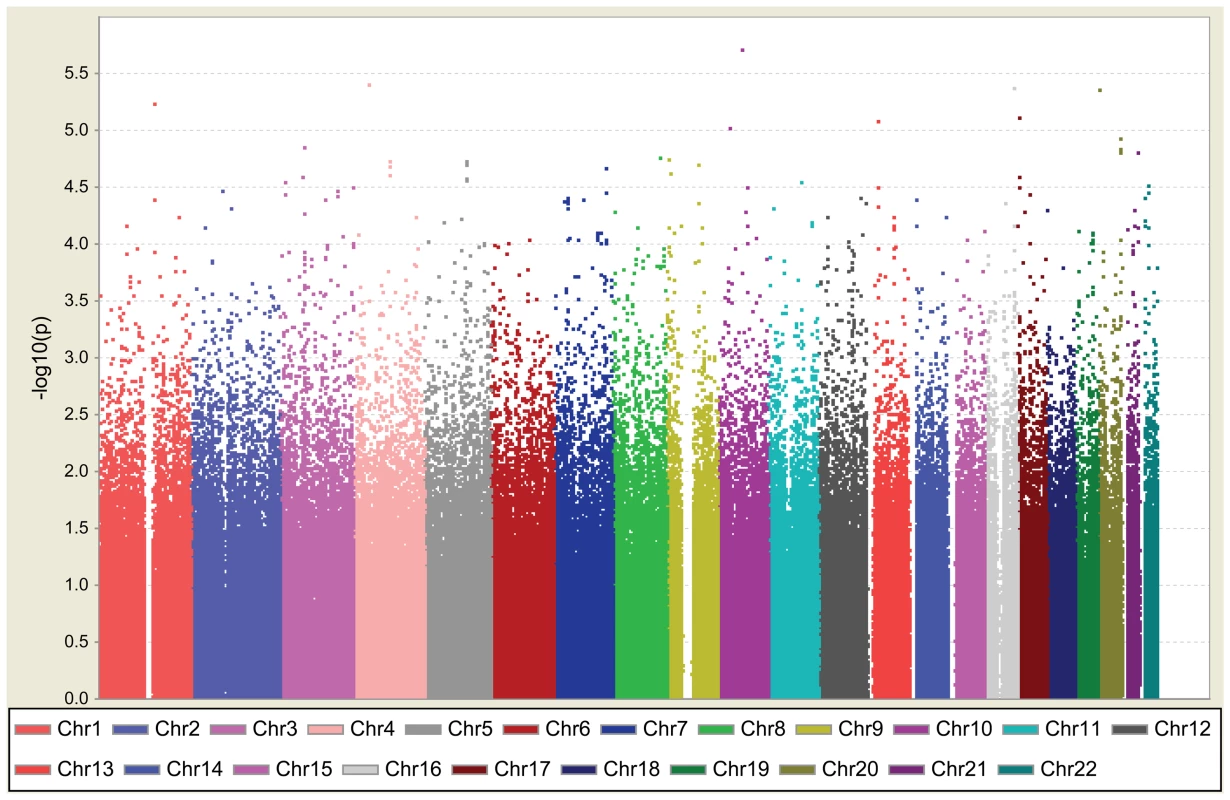
T2D association was determined for SNPs on the Illumina HumanHap550K-Duo chip. The y-axis represents the −log10 P value and the x-axis represents each of the 516,212 SNPs used in the primary scan of 995 T2D cases and 894 controls. We selected eight SNPs in seven regions: rs9985652 and rs2044844 on 4p13, rs7192960 on 16q23.1, rs7361808 on 20p13, rs1751960 on 10q11.23, rs4845624 on 1q21.3, rs391300 on 17p13.3, and rs648538 on 13q12.3. These SNPs had association P values of <10−5 at stage 1 with any of the genotype, allele, trend, dominant, and recessive models for subsequent cross-platform validation using Sequenom (Table 1; Table S3). For SNPs with weaker associations (P value between 10−4 and 10−5), we searched for novel susceptibility candidates for T2D as implicated by (1) gene function identified by a bioinformatics approach and (2) an animal model showing defects in glucose homeostasis caused by genes within the same subfamily. Therefore, we selected SNP rs17584499 (P = 2.4×10−5 under best model) for further investigation. rs17584499 lies within protein tyrosine phosphatase receptor type D (PTPRD). We hypothesized that PTPRD might play a role in the regulation of insulin signaling, because its subfamily members leukocyte common antigen-related (LAR) and protein tyrosine phosphatase sigma (PTPRS) exhibit defects in glucose homeostasis and insulin sensitivity in knockout and/or transgenic mice [49]–[51].
Tab. 1. Association results for Type 2 diabetes in Han Chinese. 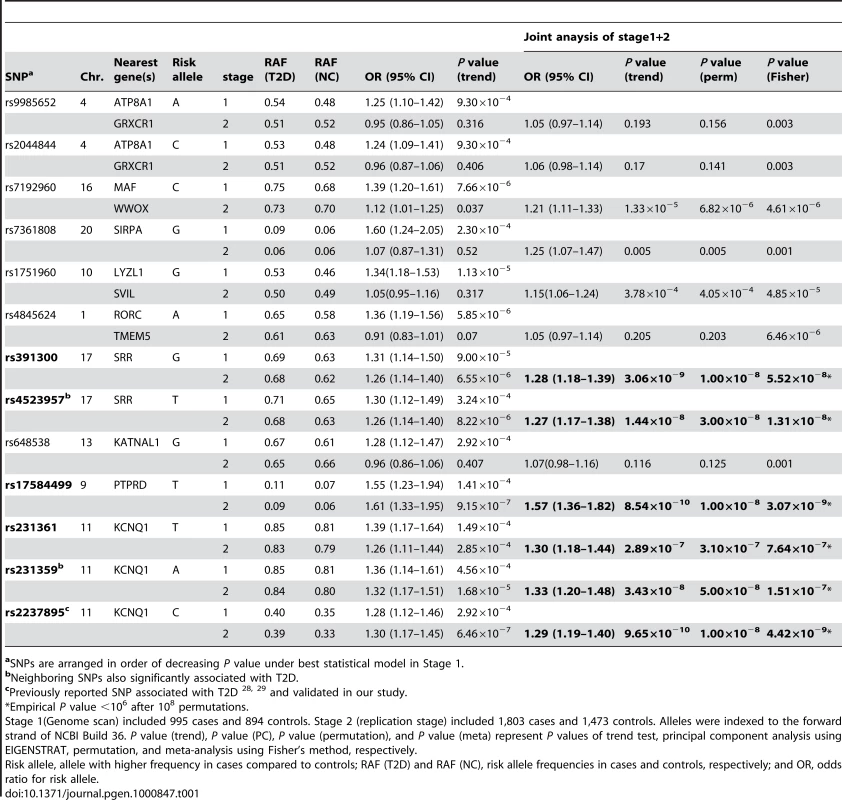
aSNPs are arranged in order of decreasing P value under best statistical model in Stage 1. We also evaluated the most significant SNP (rs231361) within KCNQ1, which was previously reported to be a diabetes susceptibility gene in a Japanese population, as well as in populations of Korean, Chinese, and European ancestry [28],[29]. Together, these ten SNPs—the 8 SNPs with association p<10−5, rs17584499, and rs231361—were cross-platform validated and yielded consistent results using both Illumina and Sequenom. The concordance rate for stage 1 samples typed on the Illumina and Sequenom platforms was 99.1%±0.84% (Table S4).
We took these ten SNPs and an additional 29 neighboring SNPs within the linkage disequilibrium (LD) block forward to replicate in 3,803 additional samples (stage 2; 1,803 cases and 1,473 controls). The average call rate for each sample was 96.13%±4.66%. After applying stringent quality control criteria, high-quality genotypes for 35 SNPs (89.7%) were obtained, with an average call rate of 98.96%±0.24% (Table S2). Of the ten SNPs selected in stage 1, only three SNPs still showed a strong association in the stage 2 analysis: rs17584499 in PTPRD at 9p24.1-p23, rs231359 in KCNQ1 at 11p15.5, and rs391300 in serine racemase (SRR) at 17p13.3 (Table 1). We were unable to replicate the association between T2D and the remaining seven SNPs in ATP8A1/GRXCR1, MAF/WWOX, SIRPA, LYZL1/SVIL, RORC/TMEM5, and KATNAL1 in the stage 2 analysis (Table 1). Joint analysis of stage 1 and stage 2 data revealed consistent results with stage 2. The most significant associations were found for rs391300, rs17584499, and rs231359 (Table 1; Figure 2). These associations remained significant after calculating P values using 108 permutations of the disease state labels. Joint association analysis was performed with all of the 2,798 T2D cases and 2,367 controls; this could achieve a power of 0.85 to detect a disease allele with a frequency of 0.15 and an OR of 1.5, assuming a disease prevalence of 0.06, at a significant level of 0.05 (Table S5).
Fig. 2. Regional plots of three significant associations. 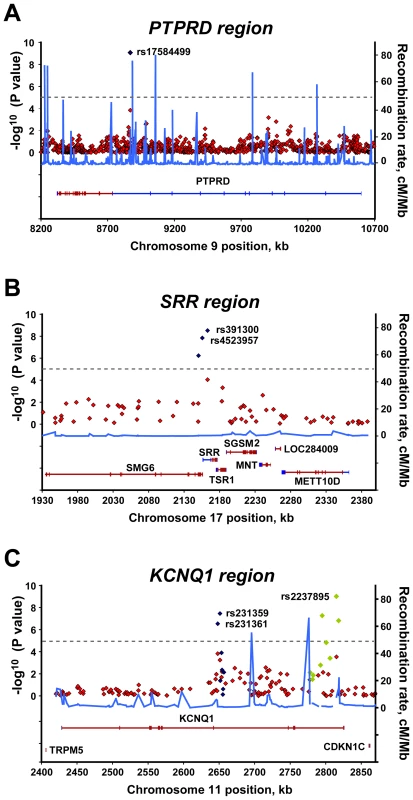
For each of the (A) PTPRD, (B) SRR, and (C) KCNQ1 regions, the −log10 P values for the trend test from the primary scan were plotted as a function of genomic position (NCBI Build 36). The SNPs with the strongest signal and neighboring genotyped SNPs in the joint analysis are denoted by blue diamonds. Green diamonds in the KCNQ1 region (C) represent reported T2D–associated SNPs genotyped in all samples of joint analysis. Estimated recombination rates (right y-axis) based on the Chinese HapMap population was plotted to reflect the local LD structure around the significant SNPs. Gene annotations were taken from NCBI. Identification of two novel T2D loci and confirmation of KCNQ1 association
Two previously unknown loci were detected in our joint analysis of GWAS data. The strongest new association signal was found for rs17584499 in intron 10 of PTPRD (P = 8.54×10−10 [trend test]; allelic OR = 1.57, 95% confidence interval [CI] = 1.36–1.82) (Table 1; Figure 2). The second strongest signal was found with rs391300 (P = 3.06×10−9 [trend test]; OR = 1.28, 95% CI = 1.18–1.39). The nearby SNP rs4523957 also demonstrated a significant association (P = 1.44×10−8; OR = 1.27, 95% CI = 1.17–1.38). SNPs rs391300 and rs4523957 were in tight LD with one another (r2 = 0.942 in HapMap HCB), and were located within the serine racemase gene (SRR).
SNP rs231361, located in intron 11 of KCNQ1, had a less significant association with T2D, and was selected in stage 1 (P = 1.49×10−4 [trend test]; OR = 1.39, 95% CI = 1.17–1.64) (Table 1). We further genotyped eight additional SNPs within the same LD block from the HapMap Asian group data: rs231359 yielded a P value of 4.56×10−4 with a trend test (OR = 1.36, 95% CI = 1.14–1.61) (Figure 2). rs231361 and rs231359 were in strong LD with one another (r2 = 1 in HapMap HCB), and were located approximately 164 kb upstream of SNP rs2237897, which was previously reported to be significantly associated with T2D in a Japanese population [28],[29]. We took rs231361, rs231359, and neighboring SNPs within the LD block forward to replicate in stage 2. Joint analysis of stage 1 and stage 2 data revealed that rs231359 had an even stronger association with T2D than did rs231361 (rs231359: P = 3.43×10−8, OR = 1.33, 95% CI = 1.2–1.48; rs231361: P = 2.89×10−7, OR = 1.3, 95% CI = 1.18–1.44).
Additional SNPs that were reported to be significantly associated with T2D in a Japanese population were further genotyped [28],[29]. The average call rate for each sample was 99.12%±7.21%. After applying stringent quality control criteria, we obtained high-quality genotypes with an average call rate of 99.16%±0.18% (Table S2). SNP rs2237895 showed the strongest association with T2D of all the genotyped SNPs in KCNQ1 (P = 9.65×10−10; OR = 1.29, 95% CI = 1.19–1.40) (Figure 2 and Figure S3; Table S6). Conditioning on the rs2237895, the statistical significance of rs231361 (or rs231359) disappeared. It seems the same underlying biological effect between the 2 SNPs (Table S7).
Subsequently, we sequenced all of the exons, intron–exon boundaries, and up to 1.2 kb of the promoter region of the KCNQ1 gene in 50 individuals with T2D, and identified 42 polymorphic variations, including one nonsynonymous P448R polymorphism and two novel SNPs with minor allele frequency >0.03. We then genotyped the two novel SNPs and one nonsynonymous polymorphism; however, none of these SNPs showed an association with T2D (Table S6).
Discussion
Our GWAS for T2D in a Han Chinese population found two previously unreported susceptibility genes. All of the significant variants detected in our study showed modest effects, with an OR between 1.21 and 1.57. Two loci with less-significant associations in our primary scan (stage 1), PTPRD and KCNQ1, were selected for further replication; both showed compelling evidence of association in joint analysis. The susceptibility loci we identified in this study need to be further replicated in additional populations. Of the 18 loci previously reported to be associated with T2D (with the exception of KCNQ1), none of the P values for any of the SNPs within or near the genes reached 10−5 using allele, genotype, trend, dominant, or recessive models (Table S8; Figure S4). Three SNPs within CDKAL1, JAZF1, and HNF1B had the lowest P values, ranging from 5×10−4 to 10−5, among the 18 known loci (Table S8). No significant associations were found within these regions in our Han Chinese population.
The strongest new signal was observed for rs17584499 in PTPRD. The overall Fst among 11 HapMap groups for rs17584499 was estimated to be 0.068 [52], which indicated a significant difference in allele frequencies among the populations (P<0.0001, chi-square test ) (Table S9). PTPRD is widely expressed in tissues, including skeletal muscle and pancreas, and is expressed highest in the brain. PTPRD-deficient mice exhibit impaired learning and memory, early growth retardation, neonatal mortality, and posture and motor defects [53]. Multiple mRNA isoforms are expressed by alternative splicing and/or alternative transcription start sites in a developmental and tissue-specific manner [54],[55]. PTPRD belongs to the receptor type IIA (R2A) subfamily of protein tyrosine phosphatases (PTPs). The R2A PTP subfamily comprises LAR, PTPRS, and PTPRD. The R2A family has been implicated in neural development, cancer, and diabetes [56]. Although the complex phenotype including neurological defects seen in knockout mice could obscure the roles of these genes in glucose homeostasis, LAR - and PTPRS-deficient mice were demonstrated to have altered glucose homeostasis and insulin sensitivity [49]–[51]. Transgenic mice overexpressing LAR in skeletal muscle show whole-body insulin resistance [57]. Because R2A subfamily members are structurally very similar [54], PTPRD could play a role in T2D pathogenesis and should be further characterized.
The second new association locus was found for rs391300 and rs4523957 in the biologically plausible candidate gene SRR. SRR encodes a serine racemase that synthesizes D-serine from L-serine [58],[59]. D-serine is a physiological co-agonist of the N-methyl D-aspartate (NMDA) class of glutamate receptors, the major excitatory neurotransmitter receptors mediating synaptic neurotransmission in the brain [60],[61]. NMDA receptor activation requires binding of glutamate and D-serine, which plays a neuromodulatory role in NMDA receptor transmission, synaptic plasticity, cell migration, and neurotoxicity [62]. D-serine and SRR are also present in the pancreas [63]. Glutamate signaling functions in peripheral tissues, including the pancreas, and positively modulates secretion of both glucagon and insulin in pancreatic islets [64]–[66]. The nearby SNP rs216193 also showed significant association (P = 2.49×10−6); this SNP resides 3.8 kb upstream from SRR, within Smg-6 homolog, nonsense mediated mRNA decay factor (C. elegans) (SMG6). rs216193 was in tight LD with rs391300 (r2 = 0.942 in HapMap HCB). Based on their biological functions and the association results, neither SMG6 nor any of the nearby genes TSR1, SGSM2, MNT, and METT10D were compelling candidates for association withT2D. However, SRR was significantly associated with T2D; thus, we suggest that dysregulation of D-serine could alter glutamate signaling and affect insulin or glucagon secretion in T2D pathogenesis.
rs7192960 also had a suggestive association with T2D (P = 1.32×10−5; OR = 1.21, 95% CI = 1.11–1.33). This SNP which lies approximately 211 kb downstream of v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian) (MAF) and 170 kb downstream of WW domain containing oxidoreductase (WWOX). WWOX is a tumor suppressor gene that spans the second most common human fragile site FRA16D [67],[68], and is disrupted in many tumors, including pancreatic carcinoma [67], [69]–[73]. MAF encodes the transcription factor c-Maf, a member of the Maf family of basic-Zip (bZip) transcription factors. c-Maf is involved in development and differentiation of the lens [74],[75], kidney [76], immune system [77], adipose tissue [78], and pancreas [79]. It is expressed in α cells of the pancreatic islets [80], and is a strong transactivator of the glucagon promoter that regulates glucagon gene expression [80],[81]. c-Maf is also associated with early-onset and morbid adult obesity [82].
Our GWAS revealed that KCNQ1, which was previously reported to be associated with T2D in several populations, was also associated with T2D in a Han Chinese population residing in Taiwan. KCNQ1 encodes the pore-forming α subunit of a voltage-gated K+ channel (KvLQT1), which is involved in repolarization of the action potential in cardiac muscle [83],[84]. Mutations in KCNQ1 cause long QT syndrome [85],[86] and familial atrial fibrillation [87]. KCNQ1 is widely expressed, including in the heart, brain, kidney, liver, intestine, and pancreas [88]–[90]. It is also expressed in pancreatic islets, and blockade of the KvLQT1 channel stimulates insulin secretion in insulin-secreting INS-1 cells [91]. KCNQ1 knockout mice have cardiac dysfunctions [88],[92] and enhanced systemic insulin sensitivity [93]. In our study, variants in the coding region did not show an association with T2D. The functional variant(s) could be located in the regulatory element of KCNQ1, rather than in the coding region. We did not find an association between either CDKAL1 or IGF2BP2 and T2D, in contrast with the results described in a previous study [29], nor did we find T2D associated with various other genes identified in populations of European descent.
In conclusion, we identified two previously unknown loci that are associated with T2D in a Han Chinese population, and confirmed the reported association of KCNQ1 with T2D. The novel T2D risk loci may involve genes that are implicated in insulin sensitivity and control of glucagon and insulin secretion: PTPRD may participate in the regulation of insulin action on its target cells, while SRR variants may alter glutamate signaling in the pancreas, thus regulating insulin and/or glucagon secretion. Our study suggests that in different patient populations, different genes may confer risks for diabetes, which may lead to a better understanding of the molecular pathogenesis of T2D.
Materials and Methods
Ethical statement
The study was approved by the institutional review board and the ethics committee of each institution. Written informed consent was obtained from each participant in accordance with institutional requirements and the Declaration of Helsinki Principles.
Subject participants
A total of 2,798 unrelated individuals with T2D, age >20 years, were recruited from China Medical University Hospital (CMUH), Taichung, Taiwan; Chia-Yi Christian Hospital (CYCH), Chia-Yi, Taiwan; and National Taiwan University Hospital (NTU), Taipei, Taiwan. All of the T2D cases were diagnosed according to medical records and fasting plasma glucose levels using American Diabetic Association Criteria. Subjects with type 1 diabetes, gestational diabetes, and maturity-onset diabetes of the young (MODY) were excluded from this study. For the two-stage GWAS, we genotyped 995 T2D cases and 894 controls in the first exploratory genome-wide scan (stage 1). In the replication stage (stage 2), we genotyped selected SNPs in additional samples from 1,803 T2D cases and 1,473 controls. The controls were randomly selected from the Taiwan Han Chinese Cell and Genome Bank [94]. The criteria for controls in the association study were (1) no past diagnostic history of T2D, (2) HbA1C ranging from 3.4 to 6, and (3) BMI<32. The two control groups were comparable with respect to BMI, gender, age at study, and level of HbA1C. All of the participating T2D cases and controls were of Han Chinese origin, which is the origin of 98% of the Taiwan population. Details of demographic data are shown in Table S10.
Genotyping
Genomic DNA was extracted from peripheral blood using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN, USA). In stage 1, whole genome genotyping using the Illumina HumanHap550-Duo BeadChip was performed by deCODE Genetics (Reykjavík, Iceland). Genotype calling was performed using the standard procedure implemented in BeadStudio (Illumina, Inc., San Diego, CA, USA), with the default parameters suggested by the platform manufacturer. Quality control of genotype data was performed by examining several summary statistics. First, the ratio of loci with heterozygous calls on the X chromosome was calculated to double-check the subject's gender. Total successful call rate and the minor allele frequency of cases and controls were also calculated for each SNP. SNPs were excluded if they: (1) were nonpolymorphic in both cases and controls, (2) had a total call rate <95% in the cases and controls combined, (3) had a minor allele frequency <5% and a total call rate <99% in the cases and controls combined, and (4) had significant distortion from Hardy–Weinberg equilibrium in the controls (P<10−7). Genotyping validation was performed using the Sequenom iPLEX assay (Sequenom MassARRAY system; Sequenom, San Diego, CA, USA). In the replication stage (stage 2), SNPs showing significant or suggestive associations with T2D and their neighboring SNPs within the same LD block were genotyped using the Sequenom iPLEX assay. The neighboring SNPs in the same LD were selected from the HapMap Asian (CHB + JPT) group data for fine mapping the significant signal.
Statistical analysis
T2D association analysis was carried out to compare allele frequency and genotype distribution between cases and controls using five single-point methods for each SNP: genotype, allele, trend (Cochran–Armitage test), dominant, and recessive models. The most significant test statistic obtained from the five models was chosen. SNPs with P values less than a = 2×10−8, a cut-off for the multiple comparison adjusted by Bonferroni correction, were considered to be significantly associated with the traits. The joint analysis was conducted by combining the data from the stage 1 and 2 samples. We also applied Fisher's method to combine P values for joint analysis. The permutation test was carried out genome-wide for 106 permutations, in which the phenotypes of subjects were randomly rearranged. For better estimation of empirical P values, the top SNPs were reexamined using 108 permutations. Each permutation proceeded as follows: (1) the case and control labels were shuffled and redistributed to subjects, and (2) the test statistics of the corresponding association test was calculated based on the shuffled labels. The empirical P value was defined as the number of permutations that were at least as extreme as the original divided by the total number of permutations. Detection of possible population stratification that might influence association analysis was carried out using principle component analysis, multidimensional scaling analysis, and genomic control (Text S1). Quantile–quantile (Q–Q) plots were then used to examine P value distributions (Figure 3 and Figure S5).
Fig. 3. Q–Q plot for the trend test. 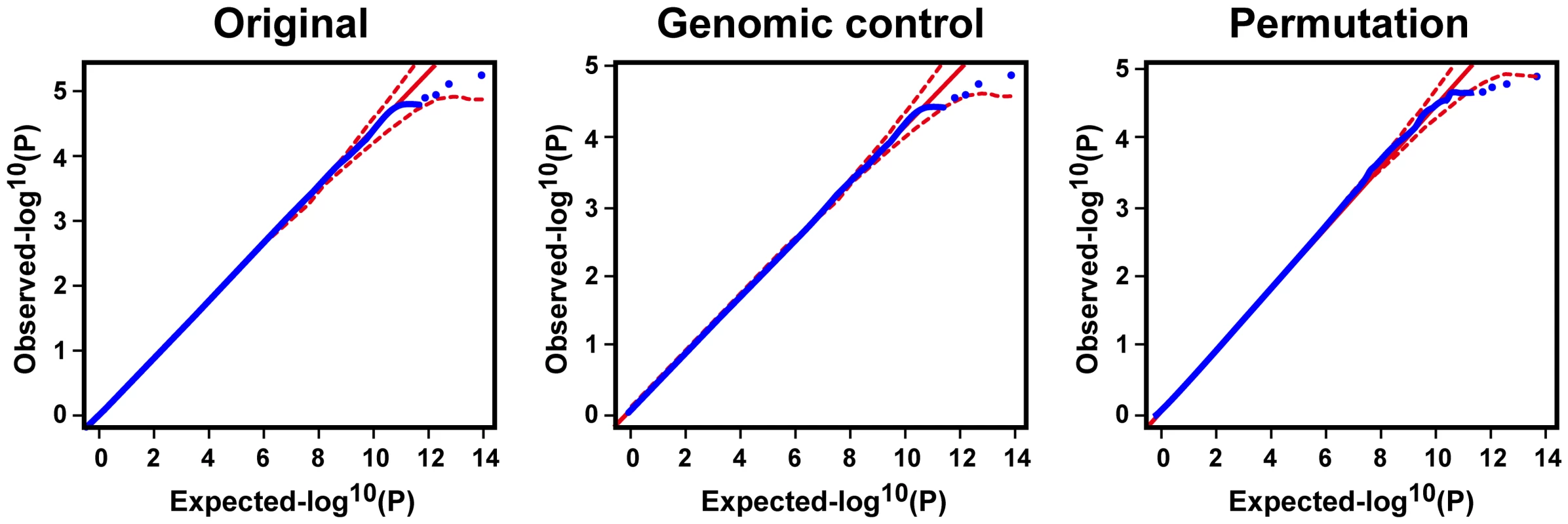
Q–Q plots are shown for the trend test based on the 516,212 quality SNPs of the initial analysis of 995 cases and 894 controls. The red lines represent the upper and lower boundaries of the 95% confidence bands. Supporting Information
Zdroje
1. ZimmetP
AlbertiKG
ShawJ
2001 Global and societal implications of the diabetes epidemic. Nature 414 782 787
2. StumvollM
GoldsteinBJ
van HaeftenTW
2005 Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365 1333 1346
3. LedermannHM
1995 Maturity-onset diabetes of the young (MODY) at least ten times more common in Europe than previously assumed? Diabetologia 38 1482
4. MaassenJA
LMTH
Van EssenE
HeineRJ
NijpelsG
2004 Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes 53 Suppl 1 S103 109
5. FajansSS
BellGI
PolonskyKS
2001 Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 345 971 980
6. MooreAF
FlorezJC
2008 Genetic susceptibility to type 2 diabetes and implications for antidiabetic therapy. Annu Rev Med 59 95 111
7. LangefeldCD
WagenknechtLE
RotterJI
WilliamsAH
HokansonJE
2004 Linkage of the metabolic syndrome to 1q23-q31 in Hispanic families: the Insulin Resistance Atherosclerosis Study Family Study. Diabetes 53 1170 1174
8. HansonRL
EhmMG
PettittDJ
ProchazkaM
ThompsonDB
1998 An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet 63 1130 1138
9. ElbeinSC
HoffmanMD
TengK
LeppertMF
HasstedtSJ
1999 A genome-wide search for type 2 diabetes susceptibility genes in Utah Caucasians. Diabetes 48 1175 1182
10. VionnetN
HaniEH
DupontS
GallinaS
FranckeS
2000 Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet 67 1470 1480
11. AnP
HongY
WeisnagelSJ
RiceT
RankinenT
2003 Genomic scan of glucose and insulin metabolism phenotypes: the HERITAGE Family Study. Metabolism 52 246 253
12. WiltshireS
HattersleyAT
HitmanGA
WalkerM
LevyJC
2001 A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (the Diabetes UK Warren 2 Repository): analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am J Hum Genet 69 553 569
13. HsuehWC
St JeanPL
MitchellBD
PollinTI
KnowlerWC
2003 Genome-wide and fine-mapping linkage studies of type 2 diabetes and glucose traits in the Old Order Amish: evidence for a new diabetes locus on chromosome 14q11 and confirmation of a locus on chromosome 1q21-q24. Diabetes 52 550 557
14. BowdenDW
SaleM
HowardTD
QadriA
SprayBJ
1997 Linkage of genetic markers on human chromosomes 20 and 12 to NIDDM in Caucasian sib pairs with a history of diabetic nephropathy. Diabetes 46 882 886
15. XiangK
WangY
ZhengT
JiaW
LiJ
2004 Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the Chinese: significant linkage to chromosome 6q21-q23 and chromosome 1q21-q24. Diabetes 53 228 234
16. JiL
MaleckiM
WarramJH
YangY
RichSS
1997 New susceptibility locus for NIDDM is localized to human chromosome 20q. Diabetes 46 876 881
17. ZoualiH
HaniEH
PhilippiA
VionnetN
BeckmannJS
1997 A susceptibility locus for early-onset non-insulin dependent (type 2) diabetes mellitus maps to chromosome 20q, proximal to the phosphoenolpyruvate carboxykinase gene. Hum Mol Genet 6 1401 1408
18. GhoshS
WatanabeRM
HauserER
ValleT
MagnusonVL
1999 Type 2 diabetes: evidence for linkage on chromosome 20 in 716 Finnish affected sib pairs. Proc Natl Acad Sci U S A 96 2198 2203
19. KlupaT
MaleckiMT
PezzolesiM
JiL
CurtisS
2000 Further evidence for a susceptibility locus for type 2 diabetes on chromosome 20q13.1-q13.2. Diabetes 49 2212 2216
20. AltshulerD
HirschhornJN
KlannemarkM
LindgrenCM
VohlMC
2000 The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat Genet 26 76 80
21. FlorezJC
BurttN
de BakkerPI
AlmgrenP
TuomiT
2004 Haplotype structure and genotype-phenotype correlations of the sulfonylurea receptor and the islet ATP-sensitive potassium channel gene region. Diabetes 53 1360 1368
22. GloynAL
WeedonMN
OwenKR
TurnerMJ
KnightBA
2003 Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 52 568 572
23. NielsenEM
HansenL
CarstensenB
EchwaldSM
DrivsholmT
2003 The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes 52 573 577
24. GudmundssonJ
SulemP
SteinthorsdottirV
BergthorssonJT
ThorleifssonG
2007 Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39 977 983
25. WincklerW
WeedonMN
GrahamRR
McCarrollSA
PurcellS
2007 Evaluation of common variants in the six known maturity-onset diabetes of the young (MODY) genes for association with type 2 diabetes. Diabetes 56 685 693
26. SandhuMS
WeedonMN
FawcettKA
WassonJ
DebenhamSL
2007 Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet 39 951 953
27. ScottLJ
MohlkeKL
BonnycastleLL
WillerCJ
LiY
2007 A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316 1341 1345
28. YasudaK
MiyakeK
HorikawaY
HaraK
OsawaH
2008 Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet 40 1092 1097
29. UnokiH
TakahashiA
KawaguchiT
HaraK
HorikoshiM
2008 SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet 40 1098 1102
30. SteinthorsdottirV
ThorleifssonG
ReynisdottirI
BenediktssonR
JonsdottirT
2007 A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39 770 775
31. 2007 Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 661 678
32. SaxenaR
VoightBF
LyssenkoV
BurttNP
de BakkerPI
2007 Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 1331 1336
33. ZegginiE
WeedonMN
LindgrenCM
FraylingTM
ElliottKS
2007 Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316 1336 1341
34. ZegginiE
ScottLJ
SaxenaR
VoightBF
MarchiniJL
2008 Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet 40 638 645
35. Bouatia-NajiN
BonnefondA
Cavalcanti-ProencaC
SparsoT
HolmkvistJ
2009 A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 41 89 94
36. LyssenkoV
NagornyCL
ErdosMR
WierupN
JonssonA
2009 Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 41 82 88
37. ProkopenkoI
LangenbergC
FlorezJC
SaxenaR
SoranzoN
2009 Variants in MTNR1B influence fasting glucose levels. Nat Genet 41 77 81
38. SladekR
RocheleauG
RungJ
DinaC
ShenL
2007 A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445 881 885
39. GrantSF
ThorleifssonG
ReynisdottirI
BenediktssonR
ManolescuA
2006 Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38 320 323
40. TakeuchiF
SerizawaM
YamamotoK
FujisawaT
NakashimaE
2009 Confirmation of multiple risk Loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes 58 1690 1699
41. OmoriS
TanakaY
TakahashiA
HiroseH
KashiwagiA
2008 Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes 57 791 795
42. HuC
WangC
ZhangR
MaX
WangJ
2009 Variations in KCNQ1 are associated with type 2 diabetes and beta cell function in a Chinese population. Diabetologia 52 1322 1325
43. WuY
LiH
LoosRJ
YuZ
YeX
2008 Common variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8, and HHEX/IDE genes are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Diabetes 57 2834 2842
44. ChangYC
ChangTJ
JiangYD
KuoSS
LeeKC
2007 Association study of the genetic polymorphisms of the transcription factor 7-like 2 (TCF7L2) gene and type 2 diabetes in the Chinese population. Diabetes 56 2631 2637
45. RonnT
WenJ
YangZ
LuB
DuY
2009 A common variant in MTNR1B, encoding melatonin receptor 1B, is associated with type 2 diabetes and fasting plasma glucose in Han Chinese individuals. Diabetologia 52 830 833
46. WangC
HuC
ZhangR
BaoY
MaX
2009 Common variants of hepatocyte nuclear factor 1beta are associated with type 2 diabetes in a Chinese population. Diabetes 58 1023 1027
47. ZhouD
ZhangD
LiuY
ZhaoT
ChenZ
2009 The E23K variation in the KCNJ11 gene is associated with type 2 diabetes in Chinese and East Asian population. J Hum Genet 54 433 435
48. PurcellS
NealeB
Todd-BrownK
ThomasL
FerreiraMA
2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
49. RenJM
LiPM
ZhangWR
SweetLJ
ClineG
1998 Transgenic mice deficient in the LAR protein-tyrosine phosphatase exhibit profound defects in glucose homeostasis. Diabetes 47 493 497
50. ChagnonMJ
ElcheblyM
UetaniN
DombrowskiL
ChengA
2006 Altered glucose homeostasis in mice lacking the receptor protein tyrosine phosphatase sigma. Can J Physiol Pharmacol 84 755 763
51. BattJ
AsaS
FladdC
RotinD
2002 Pituitary, pancreatic and gut neuroendocrine defects in protein tyrosine phosphatase-sigma-deficient mice. Mol Endocrinol 16 155 169
52. WrightS
1951 The genetical structure of populations. Annals of Eugenics 15 323 354
53. UetaniN
KatoK
OguraH
MizunoK
KawanoK
2000 Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. Embo J 19 2775 2785
54. PulidoR
Serra-PagesC
TangM
StreuliM
1995 The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc Natl Acad Sci U S A 92 11686 11690
55. SatoM
TakahashiK
NagayamaK
AraiY
ItoN
2005 Identification of chromosome arm 9p as the most frequent target of homozygous deletions in lung cancer. Genes Chromosomes Cancer 44 405 414
56. ChagnonMJ
UetaniN
TremblayML
2004 Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochem Cell Biol 82 664 675
57. ZabolotnyJM
KimYB
PeroniOD
KimJK
PaniMA
2001 Overexpression of the LAR (leukocyte antigen-related) protein-tyrosine phosphatase in muscle causes insulin resistance. Proc Natl Acad Sci U S A 98 5187 5192
58. WoloskerH
BlackshawS
SnyderSH
1999 Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A 96 13409 13414
59. WoloskerH
ShethKN
TakahashiM
MothetJP
BradyROJr
1999 Purification of serine racemase: biosynthesis of the neuromodulator D-serine. Proc Natl Acad Sci U S A 96 721 725
60. De MirandaJ
PanizzuttiR
FoltynVN
WoloskerH
2002 Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-D-aspartate (NMDA) receptor coagonist D-serine. Proc Natl Acad Sci U S A 99 14542 14547
61. MothetJP
ParentAT
WoloskerH
BradyROJr
LindenDJ
2000 D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A 97 4926 4931
62. WoloskerH
DuminE
BalanL
FoltynVN
2008 D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. Febs J 275 3514 3526
63. ImaiK
FukushimaT
SantaT
HommaH
HuangY
1998 Whole body autoradiographic study on the distribution of 14C-D-serine administered intravenously to rats. Amino Acids 15 351 361
64. GonoiT
MizunoN
InagakiN
KuromiH
SeinoY
1994 Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem 269 16989 16992
65. InagakiN
KuromiH
GonoiT
OkamotoY
IshidaH
1995 Expression and role of ionotropic glutamate receptors in pancreatic islet cells. Faseb J 9 686 691
66. BertrandG
GrossR
PuechR
Loubatieres-MarianiMM
BockaertJ
1993 Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur J Pharmacol 237 45 50
67. BednarekAK
LaflinKJ
DanielRL
LiaoQ
HawkinsKA
2000 WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res 60 2140 2145
68. RiedK
FinnisM
HobsonL
MangelsdorfM
DayanS
2000 Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet 9 1651 1663
69. IliopoulosD
GulerG
HanSY
JohnstonD
DruckT
2005 Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene 24 1625 1633
70. KurokiT
YendamuriS
TrapassoF
MatsuyamaA
AqeilanRI
2004 The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin Cancer Res 10 2459 2465
71. PaigeAJ
TaylorKJ
TaylorC
HillierSG
FarringtonS
2001 WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci U S A 98 11417 11422
72. KurokiT
TrapassoF
ShiraishiT
AlderH
MimoriK
2002 Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res 62 2258 2260
73. YendamuriS
KurokiT
TrapassoF
HenryAC
DumonKR
2003 WW domain containing oxidoreductase gene expression is altered in non-small cell lung cancer. Cancer Res 63 878 881
74. SakaiM
ImakiJ
YoshidaK
OgataA
Matsushima-HibayaY
1997 Rat maf related genes: specific expression in chondrocytes, lens and spinal cord. Oncogene 14 745 750
75. KimJI
LiT
HoIC
GrusbyMJ
GlimcherLH
1999 Requirement for the c-Maf transcription factor in crystallin gene regulation and lens development. Proc Natl Acad Sci U S A 96 3781 3785
76. ImakiJ
TsuchiyaK
MishimaT
OnoderaH
KimJI
2004 Developmental contribution of c-maf in the kidney: distribution and developmental study of c-maf mRNA in normal mice kidney and histological study of c-maf knockout mice kidney and liver. Biochem Biophys Res Commun 320 1323 1327
77. AgnelloD
LankfordCS
BreamJ
MorinobuA
GadinaM
2003 Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol 23 147 161
78. SerriaMS
IkedaH
OmoteyamaK
HirokawaJ
NishiS
2003 Regulation and differential expression of the c-maf gene in differentiating cultured cells. Biochem Biophys Res Commun 310 318 326
79. TsuchiyaM
TaniguchiS
YasudaK
NittaK
MaedaA
2006 Potential roles of large mafs in cell lineages and developing pancreas. Pancreas 32 408 416
80. KataokaK
ShiodaS
AndoK
SakagamiK
HandaH
2004 Differentially expressed Maf family transcription factors, c-Maf and MafA, activate glucagon and insulin gene expression in pancreatic islet alpha - and beta-cells. J Mol Endocrinol 32 9 20
81. GosmainY
AvrilI
MaminA
PhilippeJ
2007 Pax-6 and c-Maf functionally interact with the alpha-cell-specific DNA element G1 in vivo to promote glucagon gene expression. J Biol Chem 282 35024 35034
82. MeyreD
DelplanqueJ
ChevreJC
LecoeurC
LobbensS
2009 Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 41 157 159
83. SanguinettiMC
CurranME
ZouA
ShenJ
SpectorPS
1996 Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384 80 83
84. BarhaninJ
LesageF
GuillemareE
FinkM
LazdunskiM
1996 K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384 78 80
85. NeyroudN
TessonF
DenjoyI
LeiboviciM
DongerC
1997 A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet 15 186 189
86. WangQ
CurranME
SplawskiI
BurnTC
MillhollandJM
1996 Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet 12 17 23
87. ChenYH
XuSJ
BendahhouS
WangXL
WangY
2003 KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science 299 251 254
88. LeeMP
RavenelJD
HuRJ
LustigLR
TomaselliG
2000 Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest 106 1447 1455
89. DemolombeS
FrancoD
de BoerP
KuperschmidtS
RodenD
2001 Differential expression of KvLQT1 and its regulator IsK in mouse epithelia. Am J Physiol Cell Physiol 280 C359 372
90. ChouabeC
NeyroudN
GuicheneyP
LazdunskiM
RomeyG
1997 Properties of KvLQT1 K+ channel mutations in Romano-Ward and Jervell and Lange-Nielsen inherited cardiac arrhythmias. Embo J 16 5472 5479
91. UllrichS
SuJ
RantaF
WittekindtOH
RisF
2005 Effects of I(Ks) channel inhibitors in insulin-secreting INS-1 cells. Pflugers Arch 451 428 436
92. CasimiroMC
KnollmannBC
EbertSN
VaryJCJr
GreeneAE
2001 Targeted disruption of the Kcnq1 gene produces a mouse model of Jervell and Lange-Nielsen Syndrome. Proc Natl Acad Sci U S A 98 2526 2531
93. BoiniKM
GrafD
HennigeAM
KokaS
KempeDS
2009 Enhanced insulin sensitivity of gene-targeted mice lacking functional KCNQ1. Am J Physiol Regul Integr Comp Physiol 296 R1695 1701
94. PanWH
FannCS
WuJY
HungYT
HoMS
2006 Han Chinese cell and genome bank in Taiwan: purpose, design and ethical considerations. HumHered 61 27 30
Štítky
Genetika Reprodukční medicína
Článek Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the GenomeČlánek Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in MiceČlánek Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' DiseaseČlánek Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD TagsČlánek Wing Patterns in the Mist
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 2
-
Všechny články tohoto čísla
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Scale of Population Structure in
- Allelic Exchange of Pheromones and Their Receptors Reprograms Sexual Identity in
- Genetic and Functional Dissection of and in Age-Related Macular Degeneration
- A Single Nucleotide Polymorphism within the Acetyl-Coenzyme A Carboxylase Beta Gene Is Associated with Proteinuria in Patients with Type 2 Diabetes
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Cdk2 Is Required for p53-Independent G/M Checkpoint Control
- Uncoupling of Satellite DNA and Centromeric Function in the Genus
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in the Clade
- Use of DNA–Damaging Agents and RNA Pooling to Assess Expression Profiles Associated with and Mutation Status in Familial Breast Cancer Patients
- Cheating by Exploitation of Developmental Prestalk Patterning in
- Replication and Active Demethylation Represent Partially Overlapping Mechanisms for Erasure of H3K4me3 in Budding Yeast
- Cdk1 Targets Srs2 to Complete Synthesis-Dependent Strand Annealing and to Promote Recombinational Repair
- A Genome-Wide Association Study Identifies Susceptibility Variants for Type 2 Diabetes in Han Chinese
- Genome-Wide Identification of Susceptibility Alleles for Viral Infections through a Population Genetics Approach
- Transcriptional Rewiring of the Sex Determining Gene Duplicate by Transposable Elements
- Genomic Hotspots for Adaptation: The Population Genetics of Müllerian Mimicry in
- Proteasome Nuclear Activity Affects Chromosome Stability by Controlling the Turnover of Mms22, a Protein Important for DNA Repair
- Deletion of the Huntingtin Polyglutamine Stretch Enhances Neuronal Autophagy and Longevity in Mice
- Structure, Function, and Evolution of the spp. Genome
- Human and Non-Human Primate Genomes Share Hotspots of Positive Selection
- A Kinase-Independent Role for the Rad3-Rad26 Complex in Recruitment of Tel1 to Telomeres in Fission Yeast
- Analysis of the Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires' Disease
- Molecular Evolution and Functional Characterization of Insulin-Like Peptides
- Molecular Poltergeists: Mitochondrial DNA Copies () in Sequenced Nuclear Genomes
- Population Genomics of Parallel Adaptation in Threespine Stickleback using Sequenced RAD Tags
- Wing Patterns in the Mist
- DNA Binding of Centromere Protein C (CENPC) Is Stabilized by Single-Stranded RNA
- Genome-Wide Association Study Reveals Multiple Loci Associated with Primary Tooth Development during Infancy
- Mutations in , Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease
- Genome-Wide Identification of Binding Sites Defines Distinct Functions for PHA-4/FOXA in Development and Environmental Response
- Ku Regulates the Non-Homologous End Joining Pathway Choice of DNA Double-Strand Break Repair in Human Somatic Cells
- Nucleoporins and Transcription: New Connections, New Questions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study in Asian Populations Identifies Variants in and Associated with Systemic Lupus Erythematosus
- Nucleoporins and Transcription: New Connections, New Questions
- Nuclear Pore Proteins Nup153 and Megator Define Transcriptionally Active Regions in the Genome
- The Genetic Interpretation of Area under the ROC Curve in Genomic Profiling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



