-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTimed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
Annual epidemics of influenza result in 3 to 5 million cases of severe illness and approximately 300,000 deaths around the world. Although most patients infected with normal circulating influenza A viruses recover from the illness, complications arise during infections with highly pathogenic strains of the virus, resulting in increased mortality associated with severe immunopathology and acute respiratory distress. Previous studies suggested a major contribution of the vigorous immune response to lung damage. How the immune system constrains the negative impact of inflammation might therefore be of significant importance for future therapies. Our study in a mouse model of influenza shows that the cytokine IL-27 plays a crucial role in survival by protecting against lung damage. Its actions include regulation of innate (neutrophil influx) and adaptive (inflammatory cytokine production of T cells) arms of immunity during the acute respiratory infection. The data also suggest a therapeutic potential of IL-27, as mice treated with recombinant cytokine at later stages of infection exhibited decreased immunopathology and showed improved survival. The findings uncover an important role of IL-27 in limiting the collateral damages of anti-viral immunity and provide initial evidence that these mechanisms might be exploited for the management of severe immunopathology after infection.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004110
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004110Summary
Annual epidemics of influenza result in 3 to 5 million cases of severe illness and approximately 300,000 deaths around the world. Although most patients infected with normal circulating influenza A viruses recover from the illness, complications arise during infections with highly pathogenic strains of the virus, resulting in increased mortality associated with severe immunopathology and acute respiratory distress. Previous studies suggested a major contribution of the vigorous immune response to lung damage. How the immune system constrains the negative impact of inflammation might therefore be of significant importance for future therapies. Our study in a mouse model of influenza shows that the cytokine IL-27 plays a crucial role in survival by protecting against lung damage. Its actions include regulation of innate (neutrophil influx) and adaptive (inflammatory cytokine production of T cells) arms of immunity during the acute respiratory infection. The data also suggest a therapeutic potential of IL-27, as mice treated with recombinant cytokine at later stages of infection exhibited decreased immunopathology and showed improved survival. The findings uncover an important role of IL-27 in limiting the collateral damages of anti-viral immunity and provide initial evidence that these mechanisms might be exploited for the management of severe immunopathology after infection.
Introduction
Infection with highly pathogenic strains of influenza viruses, such as the pandemic 1918 Spanish flu, which resulted in 30–50 million deaths, is still a major threat to health [1], [2]. Pathological findings suggest that the vigorous mobilization of innate and adaptive arms of host immunity upon infection leads to uncontrolled inflammation and potentially fatal lung injury [3]. Rapid leukocyte infiltration of the lung and a subsequent cytokine storm involving the excessive production of inflammatory cytokines and chemokines have been strongly implicated in mediating lung immunopathology [3]–[5]. A better understanding of the factors that regulate the balance between viral clearance, tissue damage and resolution of inflammation is therefore necessary [6].
Interleukin 27 (IL-27) might be one important player in this context. The heterodimeric IL-27 belongs to the IL-12 superfamily and is composed of the Epstein-Barr virus inducible gene-3 (EBI3) and the IL-27p28 subunit [7]. The IL-27 receptor complex consists of IL-27Rα (WSX-1, TCCR) and the gp130 subunit, and is expressed by a wide range of cell types including T cells, monocytes, and neutrophils [8]. Initially, IL-27 was thought to promote TH1 responses because of its ability to induce T-bet expression, thereby triggering the upregulation of IL-12β2 receptor and IFN-γ under some conditions [9]–[11]. However, a series of subsequent studies using in vivo models of infection or autoimmune diseases provided evidence that its dominant function is rather to limit immune-mediated pathology [12].
Mice deficient in IL-27 receptor displayed increased immunopathology associated with overwhelming TH1 responses following infection with a number of parasites and intracellular bacteria [13]–[18]. Moreover, the lack of IL-27 receptor signaling resulted in augmented IL-17 production by CD4+ T cells in several animal models, including experimental autoimmune encephalomyelitis (EAE) [15], [19], [20]. Most studies have characterized the ability of IL-27 to suppress CD4+ T cell responses, but accumulating evidence suggests that the regulatory function of IL-27 also extends to cells of the innate immune system [8]. Consistent with its regulatory function, IL-27-mediated activation of STAT1, STAT3, STAT4 or BLIMP-1 promotes IL-10 and suppresses IL-17 production by CD4+ T cells [15], [21]–[23]. Additionally, IL-27 induced the expression of SOCS3 in CD4+ T cells, resulting in reduced IL-2 secretion in these cells [24].
The role of IL-27 in influenza has not been comprehensively studied. Liu et al. reported that influenza virus infection of epithelial cells or leukocytes induced IL-27, which correlates with increased serum levels of IL-27 in influenza patients [25]. Additionally, they showed a STAT1-dependent antiviral action of IL-27 in vitro [25]. Mayer et al. reported that IL-27 induces IFN-γ in transgenic CD8+ T cells [26]. In contrast, Sun et al. found no effect on T cell derived IFN-γ but a reduced IL-10 production by CD8+ T cells and increased leukocyte infiltration in infected Ebi-3−/− or conditional Prdm1−/− mice [23], [27].
We therefore investigated the impact of IL-27 and its receptor IL-27Rα on immunopathology using the highly mouse pathogenic [28] strain A/PR/8 (H1N1) and subsequently explored the therapeutic potential of recombinant IL-27 (rIL-27) to treat inflammatory lung disease in influenza. We found that, Il-27ra−/− mice exhibited increased mortality after influenza virus infection due to exaggerated immunopathology, in conjunction with augmented numbers of IFN-γ or IL-17-producing CD4+ and CD8+ T cells, and a strongly increased neutrophil infiltration. These effects were only partially attributed to diminished IL-27-induced IL-10. Thus, IL-27 plays an important role in limiting destructive inflammation, notably in the resolving phase of infection. Well-timed treatment with rIL-27 improved lung injury and accelerated recovery without affecting viral clearance. Our findings suggest that therapeutic application of rIL-27 predominantly suppresses innate cell recruitment but hardly affects the T cell response in the local tissue. These data demonstrate that IL-27 has a unique role in controlling immunopathology without impacting on host defense, and might therefore represent a promising candidate for immunomodulatory therapy of viral pneumonia.
Results
Impaired IL-27Rα signaling leads to increased mortality following influenza virus challenge
To determine the role of IL-27 in shaping the immune response against influenza virus, we first examined the kinetics of Il-27p28 and Ebi3 mRNA expression in the lungs of sublethally infected C57BL/6 mice (Fig. 1A). While Ebi3 was constitutively expressed and not significantly upregulated in the lungs and other organs (Fig. 1B), Il-27p28 expression displayed a pronounced peak on day 7 post-infection (d.p.i.), two days after the peak of the viral load (Fig. 1C). Coinciding with the peak of Il-27p28 expression was the maximal expression of Il-10 mRNA, which is consistent with the assumption that IL-27 is an important inducer of IL-10 [15]. These mRNA data were confirmed at the protein level where IL-27 and IL-10 peaked at 7 d.p.i (Fig. 1D). In contrast, the inflammatory cytokines IL-12 and IL-23 were maximal already at 3 d.p.i (Fig. 1D). Thus, the expression kinetic of IL-27 in the infected lungs follows, with some delay, the kinetic of the virus load, being highest when virus is already declining and coming down when immunopathology has resolved. This is compatible with its role for dampening uncontrolled inflammation in a late phase while initially allowing for a rapid start of immune defense.
Fig. 1. Absence of IL-27Rα leads to increased mortality and immunopathology during influenza. 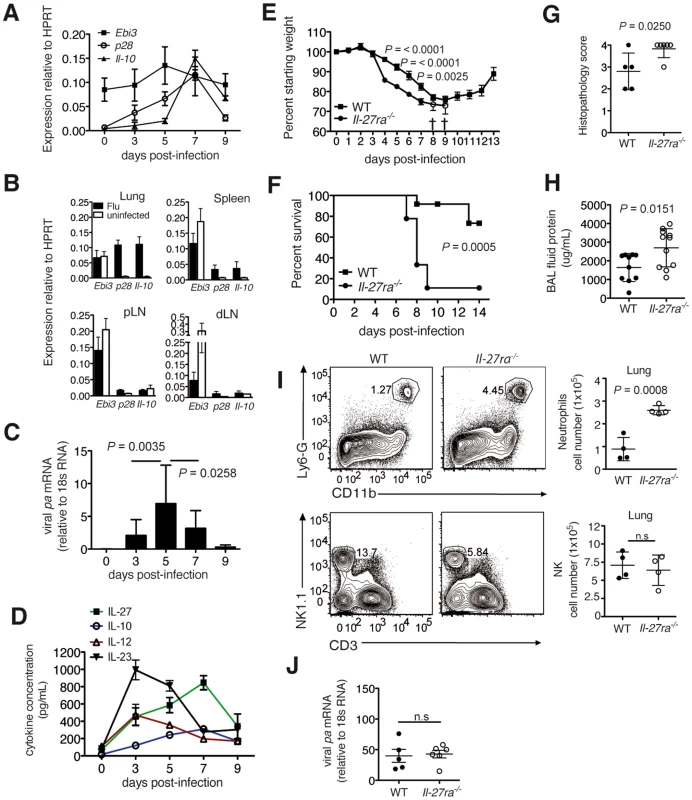
C57BL/6 mice were infected with a sublethal dose influenza virus. Ebi3, Il-27p28 and Il-10 mRNA in the (A) lung at indicated d.p.i or (B) spleen, peripheral (pLN) and lung-draining (dLN) lymph nodes of infected or uninfected C57BL/6 mice at 7 d.p.i. (C) Influenza virus polymerase (pa) mRNA expression or (D) cytokine concentration in the lung homogenate of infected C57BL/6 mice were analyzed at indicated d.p.i. (E) Weight loss or (F) survival of infected Il-27ra−/− (n = 9) or wild-type (WT) C57BL/6 (n = 12) mice after challenge with 3000 EID influenza virus. Open circles in E represent remaining live Il-27ra−/− mice (n = 2). (G) Pathological scores of H&E-stained lungs of Il-27ra−/− mice after 7 d.p.i. with 2500 EID influenza virus (H). Protein content in the BAL fluid of Il-27ra−/− mice at 9 d.p.i. was quantified by BCA. (I) Representative FACS plots and numbers of lung-infiltrating neutrophils or NK cells of Il-27ra−/− at 8 d.p.i. (J) Viral pa mRNA in lungs of Il-27ra−/− mice at 7 d.p.i was analyzed by qRT-PCR. Lung homogenates are a 20-fold dilution of homogenized whole lung tissue. All data sets were pooled from at least two independent experiments. Values represent means ± s.d. except for E, s.e.m. P values for F were determined by log-rank survival test. P values for C, E, G, H, I and J were determined by unpaired two-tailed Student's t test. ns, not significant. To assess the impact of IL-27 on survival during influenza, we challenged wild-type (WT) C57BL/6 or IL-27 receptor-deficient (Il-27ra−/−) mice with 3000 egg infectious dose (EID) influenza virus. Il-27ra−/− mice displayed accelerated weight loss and increased mortality following infection (Fig. 1E, F). Accordingly, Il-27ra−/− mice displayed a more severe lung pathology compared to control mice at 7 d.p.i (using a slightly lower virus dose, 2500 EID, to allow survival of all mice) (Fig. 1G). Furthermore, Il-27ra−/− mice had increased capillary leakage in the respiratory tract, leading to increased protein content in the bronchoalveolar lavage (BAL) fluid of these mice (Fig. 1H). A higher neutrophil, but not NK cell infiltration was observed in the lungs of Il-27ra−/− mice at 8 d.p.i (Fig. 1I). Remarkably, the increase in immunopathology and mortality in Il-27ra−/− mice was not due to a compromised viral elimination, as virus load was not significantly different between Il-27ra−/− and control mice (Fig. 1J). These findings demonstrate that IL-27 plays a critical role in limiting immunopathology during the later stages of infection.
Uncontrolled IFN-γ and IL-17 T cell production in influenza virus infected Il-27ra−/− mice
Consistent with the ability of IL-27 to suppress TH1 and TH17 responses [15], [29], influenza virus infected Il-27ra−/− mice exhibited significantly increased IFN-γ levels in BAL fluid, lung homogenate (Fig. 2A), and supernatants of enriched lymphocytes from Il-27ra−/− mice after polyclonal stimulation using PMA/ionomycin (Fig. 2B). Accordingly, we detected increased numbers of CD4+ and CD8+ T cells able to produce IFN-γ upon re-stimulation in the BAL and lungs of the Il-27ra−/− mice (Fig. 2C). In contrast, IFNα levels in the BAL of infected Il-27ra−/− mice were not different from WT animals (Fig. S1A). The results suggest that IL-27 dampens IFN-γ-production by T cells during influenza.
Fig. 2. Absence of IL-27Rα leads to increased numbers of IFN-γ or IL-17-producing T cells in the respiratory tract. 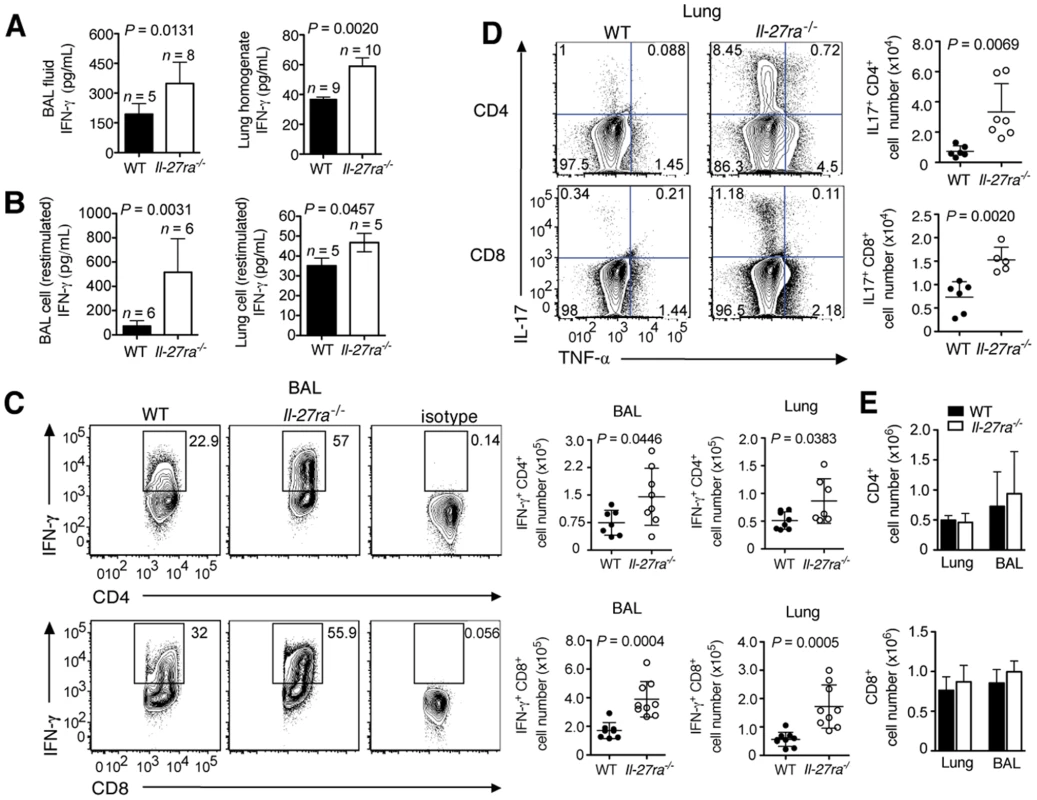
Il-27ra−/− or C57BL/6 mice were infected with 2500 EID influenza virus. At 9 d.p.i. IFN-γ concentrations in the (A) BAL fluid and lung homogenate of Il-27ra−/− mice or (B) supernatants of enriched Il-27ra−/− lymphocytes after PMA/ionomycin restimulation was quantified by ELISA. Numbers of (C) IFN-γ+ T cells in the BAL or lungs and (D) IL-17+ T cells from the lung were analyzed by FACS after PMA/ionomycin restimulation. (E) Total numbers of CD4+ or CD8+ T cells in the lungs and BAL of infected Il-27ra−/− and WT mice. Lung homogenates are a 20-fold dilution of homogenized whole lung tissue. All data sets were pooled from two independent experiments with similar results. P values were determined by unpaired two-tailed Student's t test. Values are means ± s.d. Similar to the increased numbers of IFN-γ+ T cells, we observed augmented numbers of IL-17+CD4+, IL-17+CD8+ (Fig. 2D) and TNF-α+CD4+ T cells (Fig. S1B) in the lungs of infected Il-27ra−/− mice. A slight but not significant increase was also found for IL-4+CD4+ T cells (Fig. S1C). Total numbers of CD4+ and CD8+ T cells in the lungs at 9 d.p.i were not different between Il-27ra−/− and WT mice (Fig. 2E). Taken together, these data demonstrate that endogenous IL-27 limits the magnitude of effector cytokine production by T cells during influenza.
Production of IL-10 by CD4+ T cells is induced by IL-27
Consistent with the role of IL-27 in inducing IL-10 production by CD4+ T cells in other models [15], [30]–[32], infected Il-27ra−/− mice had decreased levels of IL-10 in the BAL fluid (Fig. 3A) and in the supernatant of PMA/ionomycin-restimulated lymphocytes (Fig. 3B). This decrease correlated with the impaired ability of Il-27ra−/− CD4+ T cells from infected mice to produce IL-10 after in vitro restimulation with PMA/ionomycin (Fig. 3C). Moreover, Il-27ra−/− mice had reduced numbers of IL-10+IFN-γ+ double-positive cells, while total IFN-γ+CD4+ T cells were increased, resulting in a significantly reduced IL-10:IFN-γ ratio in CD4+ T cells of Il-27ra−/− mice compared to WT animals (Fig. S2). Although reduced, IL-10-producing CD4+ T cells were not completely lacking in Il-27ra−/− mice, suggesting that other factors besides IL-27 contribute to the induction of IL-10 [33].
Fig. 3. IL-27 directly modulates IFN-γ production by T cells and indirectly regulates IL-17 response via IL-10. 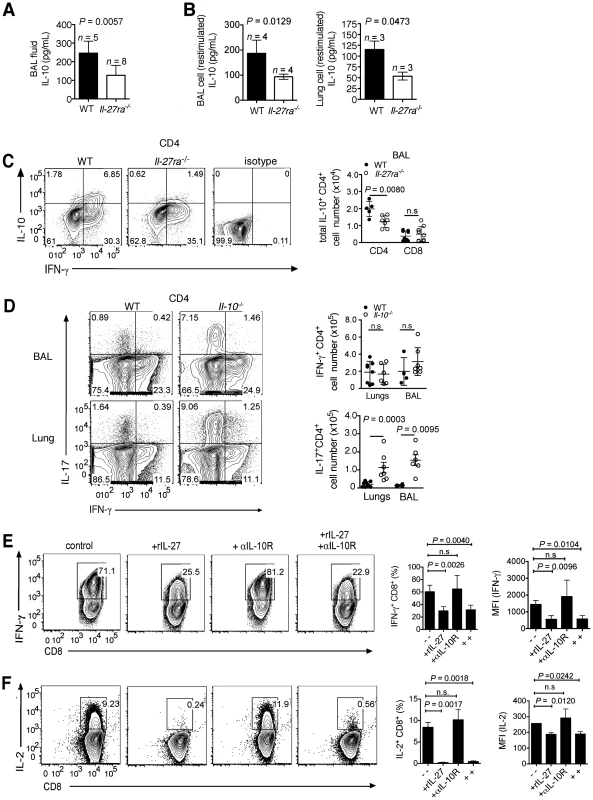
Il-27ra−/− and C57BL/6 mice were infected with 2500 EID influenza virus. At 9 d.p.i, IL-10 levels in the (A) BAL fluid or (B) supernatants of enriched lymphocytes after PMA/ionomycin restimulation were determined by ELISA. (C) Total numbers of IL-10+ T cells in the BAL were analyzed by FACS after PMA/ionomycin restimulation. Representative FACS plots shown are gated CD4+ T cells from BAL. (D) Il-10−/− or C57BL/6 (WT) mice were infected with a sublethal dose influenza virus. At 7 d.p.i., influenza virus peptide-specific IFN-γ or IL-17-producing CD4+ T cells in the respiratory tract were assessed by FACS. (E and F) Naive CD8+ T cells were activated with plate-bound anti-CD3 and anti-CD28 in Tc1 polarizing conditions in the presence or absence of rIL-27 and/or anti-IL-10 receptor blocking antibody (αIL-10R). After 3 days (d), cells were transferred to plates, then media was replenished with rIL-2, rIL-27 and/or αIL-10R antibody for additional 2 d, for a total of 5 d in culture. IFN-γ or IL-2 production by Tc1 cells were analyzed by FACS. A to D were pooled from two independent experiments with similar results. E and F represent data from three independent experiments with similar results. P values were determined by unpaired two-tailed Student's t test. Values are means ± s.d. ns, not significant. Numerous studies have established that IL-27 is signaling via STAT factors such as STAT1, STAT3 and STAT4. Among these, STAT4 has been shown to be involved in the induction of IL-10 production by CD4+ T cells [22], [34]. STAT4 is, however, also the main intermediate of IL-12 signaling. Sublethal influenza virus infection of Stat4−/− mice resulted in significantly fewer lung-infiltrating IL-10+IFN-γ+CD4+ T cells; yet this was not the case in IL-12p40−/− mice. Thus, IL-27 but not IL-12 is responsible for STAT4 mediated induction of IL-10 (Fig. S3A, B). Viral loads in the lungs of infected Stat4−/− mice at 9 d.p.i were not significantly different to WT animals (Fig. S3C).
Thus, IL-10 becomes induced in IFN-γ+CD4+ T cells during influenza by IL-27, in part mediated via STAT4.
IL-27 regulates IL-17 via IL-10 and directly modulates IFN-γ production in T cells
We next determined whether IL-10 mediates the anti-inflammatory effects of IL-27. To this end, we infected Il-10−/− mice with influenza virus and analyzed influenza peptide-specific IL-17 or IFN-γ-producing T cells. Indeed, Il-10−/− mice had elevated numbers of IL-17+CD4+ (Fig. 3D) and a slightly increased numbers of IL-17+CD8+ T cells in the lungs (Fig. S4), similar to that of infected Il-27ra−/− mice. These results indicate IL-17 suppression is largely mediated via IL-10.
In contrast, the increased numbers of IFN-γ-producing CD4+ or CD8+ T cells in the respiratory tract of infected Il-27ra−/− mice were not observed in Il-10−/− mice (Fig. 3D and Fig. S4). These findings were verified by blocking IL-10 signaling in vivo by administration of an anti-IL-10 receptor antibody (αIL-10R; Fig. S5A, B). Viral titers in the lungs of Il-10−/− mice were not different to that of WT mice (Fig. S5C).
The ability of IL-27 to directly modulate IFN-γ production in CD8+ T cells was confirmed in vitro, where addition of rIL-27 to the cultures strongly suppressed IFN-γ and IL-2 production by activated IFN-γ+CD8+ T cells (Tc1 cells), even when IL-10R signaling was blocked (Fig. 3E, F and Fig. S6). An IL-27-dependent suppression of IFN-γ and IL-2 in CD4+ T cells had already been described previously [21].
These results demonstrate that IL-10-dependent effects only partially account for IL-27 mediated suppression. Notably the IL-10-independent effects on IFNγ-production, but also on distinct recruitment events (see below) might explain why deficiency in IL-27 signaling has a strong impact on the disease course in influenza while IL-10 deficiency has not (Fig. S7; for the latter see also [35]).
Treatment with recombinant IL-27 alleviates immunopathology when administered in a late phase of the infection
Having demonstrated the pronounced role of IL-27 in regulating immunopathology, we wondered whether this property could be exploited for therapeutic purposes. In accordance with the delayed endogenous production of IL-27, we administered exogenous rIL-27 from 5–10 d.p.i. Indeed, this treatment regimen resulted in decreased weight loss and accelerated recovery (Fig. 4A), a striking improvement in lung immunopathology (Fig. 4B), and in reduced capillary leakage as indicated by a lower BAL fluid protein content (Fig. 4C). Notably, rIL-27 therapy did not impair viral clearance (Fig. 4D). In line with this, the numbers of CD8+ (Fig. 4E) or CD4+ (Fig. S8) T cells from the infected respiratory tract producing either TNF, IL-17 or IFN-γ upon antigen-specific stimulation were not changed. Only a slight decrease of secreted IL-17 (Fig. 4F) and increase of IL-10 levels (Fig. 4G) was found in the BAL fluid. The protective effect of treatment with rIL-27 was also found when mice were infected with a lethal dose of influenza virus (Fig. 4H). We did not observe significant effects of IL-27 treatment on the activity of virus-specific CTLs as measured by the CD107 mobilization assay or the fraction of IFN-γ-producing CD8+ cells (Fig. S8).
Fig. 4. Late-phase treatment with rIL-27 alleviates lung immunopathology. 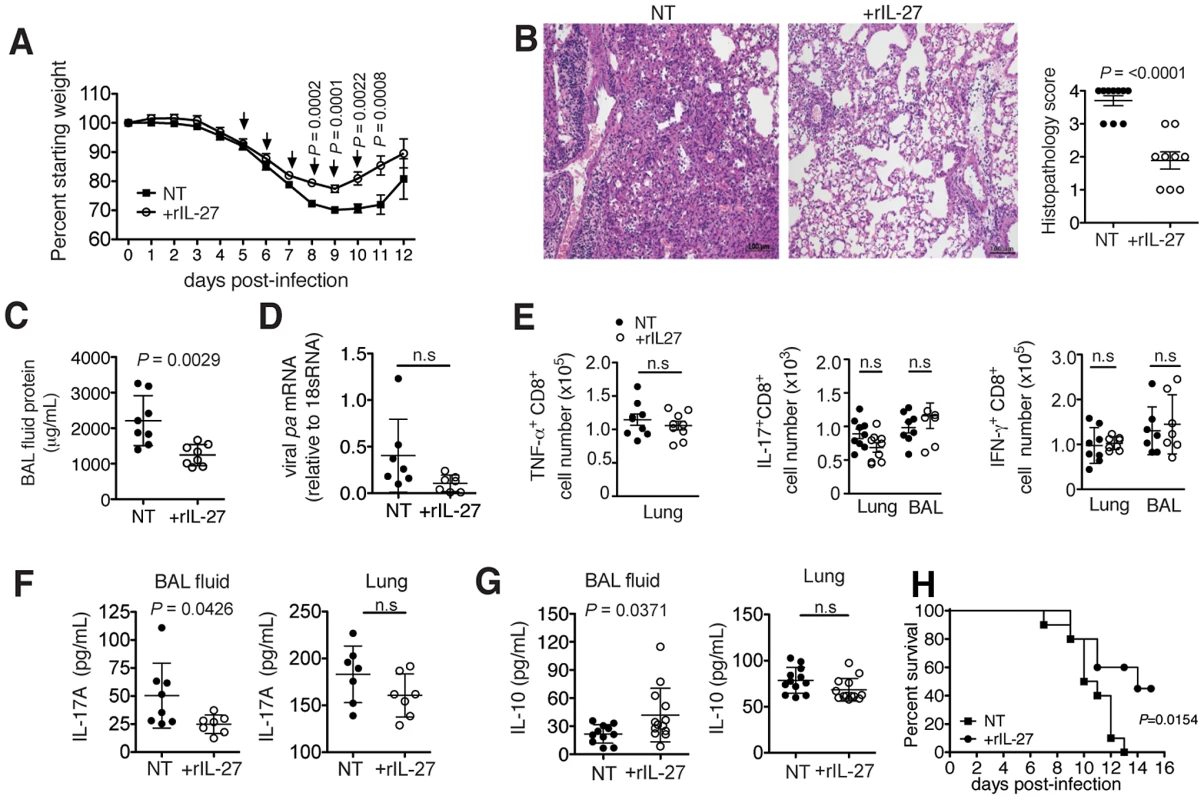
C57BL/6 mice were challenged with a sublethal (A to G) or lethal (H) dose of influenza virus and treated daily with rIL-27 from 5–10 (A, H) or 5–9 (B to G) d.p.i. Non-treated control mice (NT) were injected with PBS. (A) Weight loss of rIL-27-treated or NT mice. Arrows (↓) indicate points of treatment. At 9 d.p.i., (B) histological comparison of H&E-stained lungs was performed, (C) protein content in the BAL fluid was measured by BCA, (D) viral pa mRNA expression in the lungs was measured by qRT-PCR and (E) influenza virus peptide-specific cytokine production by CD8+ T cells was determined by FACS. Levels of (F) IL-17 and (G) IL-10 in the BAL fluid or lung homogenates of late-treated or NT mice at 9 d.p.i. were measured by ELISA. Lung homogenates are a 20-fold dilution of homogenized whole lung tissue. (H) Survival of mice treated with rIL27 or PBS from day 5–10 after challenge with a lethal dose of influenza virus. Values for weight loss curves are data pooled from at least two independent experiments, representing the means ± s.d. of the following numbers of mice per group: day 1–9, n = 14; day 10–11, n = 7; day 12, n = 6. Data from B to H are pooled from at least two independent experiments with similar results. P values were determined by unpaired two-tailed Student's t test. Values are means ± s.d. except for A, s.e.m.; ns, not significant. P values for H were determined by log-rank survival test (n = 10). To test whether the delayed kinetics of endogenous IL-27 is relevant for an unhindered initial response to infection, we applied exogenous rIL-27 from 1–7 d.p.i. (early phase). Mice treated under this regimen exhibited stronger weight loss (Fig. 5A) and reached the limits for euthanasia at 7 d.p.i. To assess the impact of treatment on immunopathology and other parameters, all animals were sacrificed at this time point. Although a diminished lung histopathology was observed (Fig. 5B), a significantly higher viral load was found (Fig. 5C). Impaired viral clearance was not due to a suppressed T cell cytokine response as treated mice had unchanged numbers of influenza peptide specific IL-17+ or IFN-γ+ T cells in the respiratory tract (Fig. 5D). However, mice treated in the early phase with rIL-27 had significantly reduced frequencies of neutrophils (Fig. 5E) and monocytes, but not NK cells (Fig. 5F). In our model, NK cells played a minimal role in viral clearance, as NK1.1 cell-depletion did not influence viral loads (Fig. S9).
Fig. 5. rIL-27 treatment at an early stage of infection aggravates disease severity and impairs viral clearance. 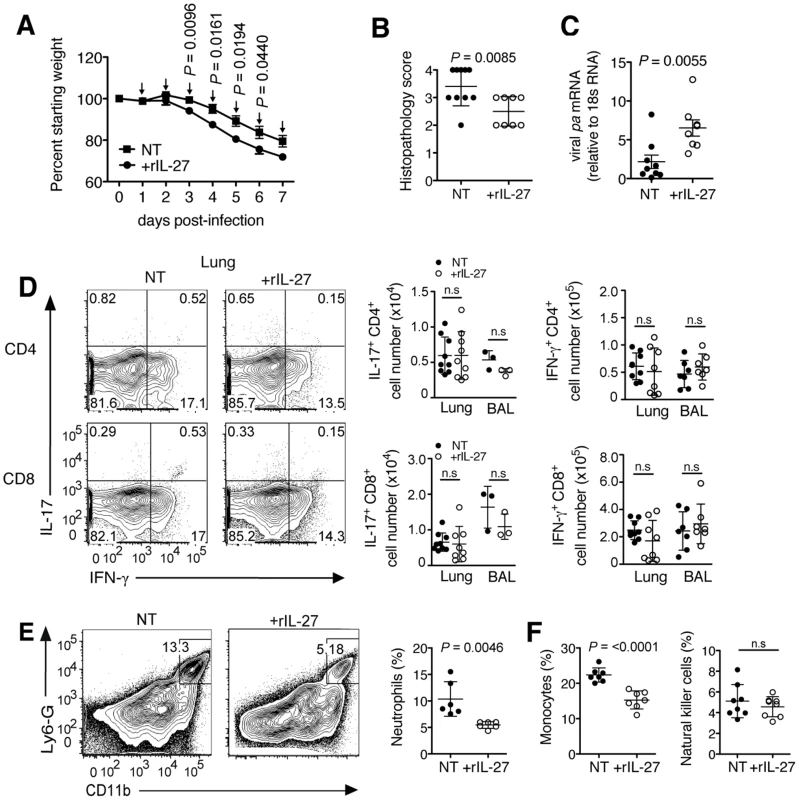
C57BL/6 mice were challenged with a sublethal dose influenza virus then treated daily with rIL-27 from 1–7 d.p.i. Non-treated control mice (NT) were injected with PBS. (A) Weight loss of rIL-27 treated (n = 5) or NT (n = 5) mice. Arrows (↓) indicate time of treatment. At 7 d.p.i., (B) histological scores of H&E-stained lungs from treated or NT groups and (C) viral pa mRNA expression were determined. (D) Numbers of influenza virus peptide-specific IL-17 or IFN-γ-producing T cells, (E) influx of neutrophils, (F) monocytes and NK cells in the lungs at 9 d.p.i. were determined by FACS. Gated cells in FACS plots in e indicate the percentage of neutrophils from total lung cells. Data from A are representative of two independent treatment experiments. Data from B to F are pooled from at least two independent experiments. P values were determined by unpaired two-tailed Student's t test. Values are means ± s.d. except for A, s.e.m.; ns, not significant. These findings suggest that systemic rIL-27 treatment during the early stages of influenza has little impact on the local antigen-specific T cell response, but suppresses neutrophil and monocyte influx that are crucial for the control of infection at this stage [28], [36]. In contrast, treatment at a later time-point, starting at the peak of viral load, did not impair viral clearance but immunopathology and disease course were markedly improved.
IL-27 treatment works by suppressing leukocyte recruitment into the infected lungs
Therapeutic application of rIL-27 from 5–9 d.p.i. had surprisingly little impact on the T cell compartment, but strongly suppressed the accumulation of neutrophils (Fig. 6A), monocytes and partially NK cells in the lung (Fig. 6B). We therefore conclude that IL-27 can regulate innate cell trafficking independently of any effect on T cell responses. Reduction of neutrophils, but not of NK cells was mediated via IL-10, as is the reduction of some chemokines in the BAL (Fig. S12)
Fig. 6. Late IL-27 treatment suppresses innate cell migration and chemokine production by CD11b+ and CD11c+ cells. 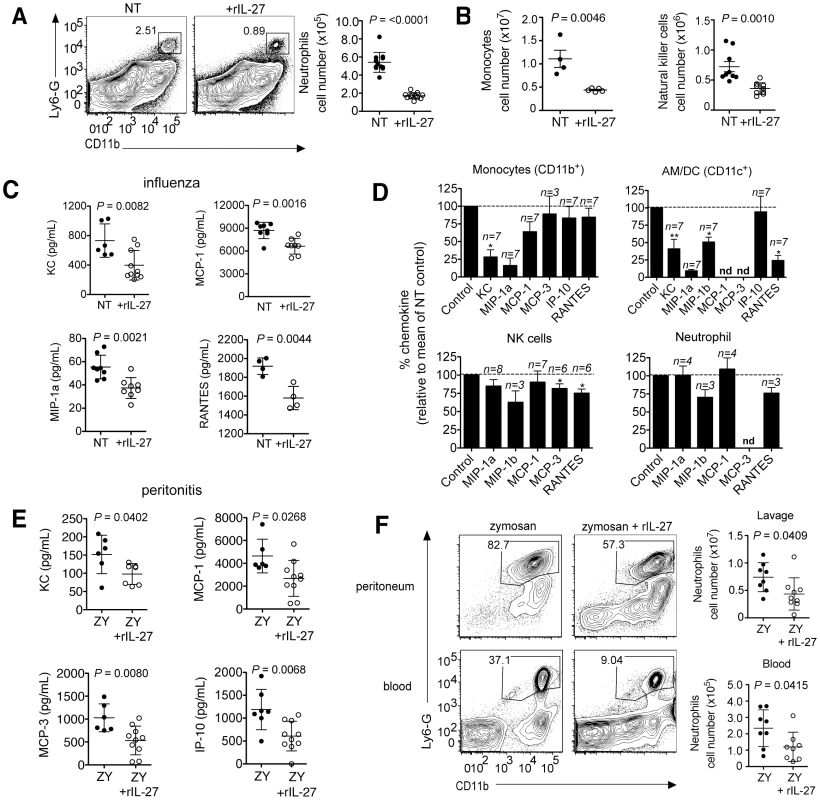
C57BL/6 mice were challenged with a sublethal dose of influenza virus then treated daily with rIL-27 from 5–9 d.p.i. At 9 d.p.i., (A) numbers of neutrophils, (B) monocytes or NK cells in the lungs were analyzed by FACS. Chemokine concentration in the (C) BAL fluid of late IL-27-treated influenza virus infected mice. (D) Chemokine levels in cultures of CD11b+, CD11c+, NK (NK1.1+CD3−) cells or neutrophils (Ly6G+CD11b+) isolated from influenza virus-infected lungs after a 24 h treatment with rIL-27. (E) C57BL/6 mice were co-injected i.p. with zymosan (ZY) and rIL-27. Injection of zymosan only was used as control. After 24 hours, chemokine levels in the peritoneal fluid were analyzed and (F) the numbers of neutrophils in the peritoneum and blood were determined by FACS and. All data sets are pooled from at least two independent experiments with similar results. P values were determined by unpaired two-tailed Student's t test. Values in A, B, C, E and F are means ± s.d.; D indicate means ± s.e.m. *P<0.05; **P<0.01; nd, not detected. Leukocyte accumulation in sites of inflammation is regulated by chemokines and adhesion molecules governing both the entry and exit from tissue. Indeed, rIL-27-treatment during influenza reduced the levels of multiple chemokines in the BAL fluid (Fig. 6C). To identify the cellular targets of IL-27-dependent chemokine suppression, different leukocyte subsets from the lungs of influenza virus infected mice were isolated and cultured overnight in the absence or presence of rIL-27. IL-27 suppressed chemokine production by CD11b+ or CD11c+ cells but had little impact on NK cells or neutrophils (Fig. 6D). Especially the chemokines KC (CXCL1), MIP-1β (CCL4) and RANTES (CCL5), which are prototypic attractors of neutrophils, monocytic, and lymphocytic cells, were suppressed. A similar suppression by IL-27 was found for IL-1β or IL-6-induced chemokine production by endothelial cells isolated from the lungs (Fig. S10).
To confirm the ability of IL-27 to suppress leukocyte recruitment in the absence of a significant contribution from T cells, we determined the impact of IL-27 treatment in the T cell-independent zymosan-induced peritonitis model. Similar to influenza, rIL-27 significantly reduced the levels of chemokines in the peritoneal lavage as well as the numbers of neutrophils in peritoneum and blood (Fig. 6E, F). As only minimal levels of IL-17 are present in the peritoneal lavage (Fig. S11), this effect does not rely on IL-17 suppression by IL-27. These data unravel a novel mode of action of IL-27 that is based on suppression of innate cell recruitment into sites of inflammation.
Discussion
The tight regulation of both the induction and subsequent down-regulation of inflammatory responses during influenza is imperative in minimizing severe immunopathology. Infection with highly pathogenic strains of influenza viruses results in increased leukocytic pulmonary infiltrates and leads to the exaggerated production of inflammatory cytokines (“cytokine storm”) that causes massive inflammation with increased mortality [4], [5], [37]. Therefore, understanding the regulatory pathways during infection not only sheds light on the mechanisms controlling the delicate balance of efficient viral clearance and disastrous immunopathology, but also reveals potential therapeutic approaches to target resolution of inflammation [6]. Few studies have evaluated the therapeutic potential of anti-inflammatory agents in influenza; while broad-acting immunosuppressants such as corticosteroids were found to worsen the disease, a combination of antiviral therapy and anti-inflammatory non-steroidals inhibiting cyclooxygenases (COX) improved survival in mice [38]. Similarly, targeting inhibitory pathways such as macrophage CD200, PAR2 and endothelial S1P1 receptors have been found to reduce immunopathology in influenza infection models [39]–[41]
Our findings suggest that IL-27 is a potential candidate for the treatment of immunopathology, as endogenous IL-27 was found to play a major role in dampening of exaggerated inflammation in influenza while having little impact on virus elimination. The absence of IL-27Rα signaling during acute virus infection worsened immunopathology and disease course; this ultimately resulted in increased mortality, despite controlled viral loads. Additionally, increased neutrophil accumulation and augmented IFN-γ or IL-17 production by T cells were observed in the infected Il-27ra−/− mice while local IFNα levels appeared not to be affected. These data are in agreement with a number of in vivo models of bacterial or parasitic infection that underline a crucial role of IL-27 in dampening inflammation [14], [16]–[18], [42].
That IL-27 acts in vivo predominantly as an anti-inflammatory cytokine was not foreseen in the beginning, as several studies demonstrated activating effects of IL-27, e.g. on the production of IFN-γ in vitro [9], [10], [43]. In an influenza model, Mayer et al. reported that WT CD8+ T cells displayed higher IFN-γ production than IL-27Rα-deficient cells [26]. In this chimera model, non-hematopoietic and half of the hematopoietic cells responded to IL-27 so that only T cell - intrinsic effects of deficiency were effective. In contrast, under the conditions of global absence of IL-27Rα as used here, we observed increased IFN-γ levels and two-fold higher numbers of IFN-γ+ T cells in the infected respiratory tract of Il-27ra−/− mice, in line with the findings of the above-mentioned parasite infection models. We assume, that the global effect of IL-27 in vivo involves a complex network of cell types including myeloid cells or even non-hematopoietic cells. In addition, timing and conditions might be crucial for the quality of IL-27 effects, as we also found a direct, IL-10-independent suppression of IFN-γ and IL-2 in activated Tc1 cells by IL-27 in vitro. Thus, the environmental context plays a significant role for the action of IL-27 in vivo, and its impact on the innate response might dominate over effects restricted to the T cell compartment. Indeed, the strong increase in the number of lung-infiltrating neutrophils in the absence of IL-27 signaling was one of the most impressive findings and appears to be crucial for the worsened immunopathology.
Major effects of compromised IL-27 signaling were also found on the number of IL-17 producing T cells. Both IFN-γ and IL-17 have been reported to play a significant role for lung injury during influenza [4], [44], [45]. IL-17 has been described as a major factor boosting expansion, recruitment and activation of neutrophils by inducing hematopoietic growth factors, chemokines and other activating signals [46]–[48]. IL-27-dependent regulation of TH17 responses was reported to occur through a number of mechanisms [15], [20], [49]–[51]. Here we provide evidence that suppression of IL-17 is largely dependent on IL-10 acting as an intermediate, since infected Il-10−/− mice displayed augmented numbers of IL-17+ T cells, similar to that observed in Il-27ra−/− mice. This is in agreement with a previous study in which blocked IL-10R signaling during high dose influenza virus infection resulted in elevated numbers of IL-17+CD4+ T cells [35]. In contrast, IFN-γ-producing T cells were not affected by absence of IL-10.
Based on these data demonstrating the important role of IL-27 in controlling inflammation, we reasoned that application of rIL-27 might be of value in situations in which exaggerated immunopathology, rather than virus elimination, becomes a critical issue for host survival as it is often the case in severe influenza. Indeed, systemic application of daily doses of rIL-27 at 5–9 d.p.i accelerated recovery and alleviated immunopathology by suppressing the influx of neutrophils, monocytes and, to a lesser degree, NK cells into the infected lungs of mice. Reduced infiltration appears to be the major cause of the improved overall status of treated mice, as large numbers of these cells can contribute to lethal lung damage by producing inflammatory cytokines, chemokines and reactive oxygen species, which results in the amplification of inflammatory signals [4], [5], [37]. Again, the reduction in infiltrating neutrophils upon IL-27 therapy was largely dependent on IL-10 (Fig. S12). In contrast, the reduced infiltration of NK cells was not dependent on IL-10, underlining that not all effects of IL-27 are mediated by induced IL-10 and that IL-27 has a broader suppressive effect than its downstream-mediator IL-10. This latter conclusion is supported by the finding that infected Il-27ra−/−, but not Il-10−/− mice exhibited a more severe disease course compared to WT animals.
Surprisingly, the number and cytokine profile of influenza virus-specific T cells in the lung was not significantly affected by treatment with rIL-27. Moreover, virus elimination was not impaired, if not even improved, upon treatment in the late phase. Whether this is due to the reported induction of antiviral activity by IL-27 that activates an interferon-induced antiviral protein kinase-R (PKR) via STAT1 in human lung epithelial cells [25], or whether destructive inflammation counteracts an efficient antiviral defense, remains to be shown.
Although some reduction in the level of IL-17 and increase in IL-10 was found in the BAL fluid upon treatment, these findings suggest that rIL-27 applied systemically predominantly regulates innate cell accumulation in the lungs rather than limiting the activity of the adaptive arm of the immune system such as IFN-γ or IL-17 producing T cells within the inflamed tissue. An explanation could be that local levels of IL-27 calculated for the lung tissue of infected WT animals are two orders of magnitude higher than plasma levels after systemic application of rIL-27 and are therefore hardly increased upon treatment (Fig. S13). We therefore propose that systemically applied rIL-27 predominantly acts on cells exposed directly to blood or plasma exudate and/or on innate cells before or during their journey to the inflamed lung.
To test the hypothesis that IL-27 treatment is able to suppress the accumulation of neutrophils independent of T cells, we applied rIL-27 in an acute model of TLR-induced sterile inflammation, the zymosan-induced peritonitis model. In this model, T cells are virtually absent in the inflammatory site, and IL-17 is hardly detectable. Indeed, rIL-27 inhibited the accumulation of neutrophils also under these conditions.
As leukocyte trafficking is controlled by adhesion molecules and chemokines presented on endothelial cells, we tested whether IL-27 affects key molecules involved in the recruitment or retention of innate leukocytes in influenza. Consistent with a role for IL-27 in modulating the trafficking of neutrophils and monocytes, treatment with rIL-27 reduced the production of neutrophil and monocyte chemoattractants KC (CXCL1), MIP-1α (CCL3), and RANTES (CCL5) produced in vitro by pulmonary monocytes (CD11b+), alveolar macrophages/dendritic cells (CD11c+) or NK cells isolated from infected lungs and resulted in reduced chemokine levels in the BAL fluid of infected mice. Similar effects were found with lung endothelial cells. These data complement recent findings that IL-27 suppresses the response of macrophages to TNF-α and IL-1 [52].
While the altered levels of chemokines in the BAL might affect the retention of leukocytes in the alveolar space, the deposition of chemokines on the endothelial surface by macrophages lining the blood vessels would directly affect the adhesion and transmigration of circulating leukocytes. Indeed, a major fraction of monocytic cells in the lung is not situated in the parenchyma but sitting within the vessel wall (“marginal pool”), rendering these cells sensitive to the cytokines in the blood, including exogenously administered cytokines [53], [54]. In addition we found that the chemokine production of endothelial cells upon stimulation with IL-1β or IL-6 was suppressed by IL-27. Moreover, IL-27 has been reported to directly affect adhesion and activation of neutrophils [55].
The expression of the IL-27p28 subunit in the influenza virus-infected respiratory tract peaks at the later phase of infection when viral titers are at a decline, which is consistent with the suggested role of IL-27 in limiting the immune response. Interferons can elicit IL-27 production as the Il-27p28 gene promoter contains an IFN-stimulated response element region (ISRE), which becomes activated through IRF-1 [56]–[58]. In contrast to the inflammatory cytokines IL-12 or IL-23, which are rapidly produced by myeloid cells, e.g. upon triggering TLR receptors, and accordingly found in early time points in the influenza infection, the expression of IL-27 is turned on in a delayed fashion by the inflammatory microenvironment and serves as a negative feedback mechanism, thereby dampening the immune response in the later phase when adaptive immunity is established and the risk of severe immunopathology comes to the fore.
In line with this concept, we observed protective effects when rIL-27 was administered in a later phase of infection, starting at the peak of viral load when also the endogenous IL-27 production is near its highest level. To test whether timing is crucial, we additionally applied rIL-27 in the early phase of infection, starting 1 day after infection. Indeed, under these conditions IL-27 treatment also reduced leukocyte infiltration and immunopathology, but simultaneously impaired virus elimination, resulting in a worsened disease course. This suggests that interference with leukocyte recruitment in the early phase of influenza aggravates the infection, and the low level of endogenously produced IL-27 in this early phase is appropriate to allow their unhindered rapid activity in virus defense.
Indeed, previous studies have demonstrated that neutrophils are essential for early host protection in influenza infection, as neutrophil depletion before infection led to increased viral titers and accelerated mortality [28], [36]. The same was found for alveolar macrophages [36]. While their mode of action is still unknown, these studies suggest that neutrophils and macrophages contribute to protection in the early phase. In the late phase, depletion of neutrophils or macrophages was not affecting the disease course, while even infection with a low pathogenic strain was fatal in RAG-2γc−/− mice lacking NK, T and B cells [28]. On the other side, recruited leukocytes have a major role in immunopathology, e.g. by inducing apoptosis in epithelial cells [59]. These findings are compatible with the paradigm that the innate system contributes to early protection in viral infection, while at later time points, antigen-specific T cells take over and eliminate the virus.
In conclusion, our study shows that endogenous IL-27 has a crucial role in preventing a fatal disease course in influenza where it acts to limit and resolve the inflammatory process while allowing an unimpaired antiviral response (Fig. S14). Based on its physiological role as a master factor regulating IL-10-dependent as well as -independent anti-inflammatory mechanisms, we here demonstrate that well-timed therapeutic application of recombinant IL-27 can successfully counteract detrimental immunopathology while keeping the antiviral response intact. Combination of IL-27 treatment with anti-viral or anti-microbial treatment might further expand the applicability of this concept, especially when the role of IL-27 in secondary bacterial infection [60] is appropriately taken into account.
These data suggest that strategies to target natural multifunctional pathways involved in the resolution of inflammation might be a valuable alternative for the treatment of inflammation-caused immunopathology and complement current therapeutic approaches focused on the inhibition of isolated effector mechanisms.
Materials and Methods
Mice and infection
Wild-type (WT) C57BL/6, BALB/c, Il-10−/−, Il-12p40−/− and Stat4−/− mice were purchased from Taconic Farms, Charles River, or The Jackson Laboratory. Il-27ra−/− mice were backcrossed more than nine generations to C57BL/6 mice [11] and housed in the Department of Pathobiology at The University of Pennsylvania, Philadelphia, USA. Mice were infected with influenza A/PR/8/34 virus intranasally (i.n) while under dexdomitor (0.5 mg/mL, Pfizer) and anti-mepazole (5 mg/mL, Pfizer) anesthesia, or with light isofluorane. For sublethal infection with influenza virus, BALB/c and Stat4−/− mice were infected with 12.5 plaque forming units (PFU), while C57BL/6, Il-10−/− and Il-27ra−/− mice were infected with 25 PFU or 2500 egg infectious dose (EID). Survival assays with Il-27ra−/− mice was performed with 3000 EID. For experiments addressing the therapeutic potential of rIL-27, 45 PFU of the virus were used as sublethal and 55 PFU as lethal dose. Infected mice were weighted daily and assessed for clinical symptoms of infection. When reaching a weight loss of >30%, mice were euthanatized. Animal care and experiments were performed in accordance with the institutional guidelines of German Federal Law and local authorities of Berlin (LAGESO), or Animal Care and Use Committee of the University of Pennsylvania.
Ethics statement
Animal procedures were performed in accordance with the German “Tierschutzgesetz in der Fassung vom 18. Mai 2006 (BGBI.IS.1207)” and the guideline 2010/63/EU from the European Union and the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. Animal protocols were approved by the ethics committee and the Berlin state authorities (LAGeSo Registration # G03310/08). Experiments performed at the University of Pennsylvania were carried out in accordance with the guidelines in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol (# 802004) was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania, Philadelphia PA (Federal assurance # FWA00004028; Office of Laboratory Animal Welfare assurance # A3079-01).
Influenza virus
Influenza A/PR/8/34 virus was grown in the allantoic cavaties of 11-day old embryonated chicken eggs or was purchased from Charles River.
Bronchoalveolar lavage fluid and tissue preparation
Bronchoalveolar lavage (BAL) was obtained by flushing the airways 3× with 1 mL sterile PBS. Lungs (approximately 200 mg of tissues) were mashed through a 70 µm cell strainer and suspended in 10 mL of PBS/BSA. Lung lymphocytes were enriched using a percoll gradient 40∶70 (vol/vol). Erythrocytes were lysed with an erylysis buffer (Sigma). BAL fluid and lung homogenates were collected and stored at −80°C for ELISA and BCA.
Leukocyte preparation for chemokine determination
CD11b+, CD11c+, NK1.1+CD3− (NK cells) and Ly6G+CD11b+ (neutrophils) cells were isolated from infected lungs of C57BL/6 mice at 5 d.p.i. and sorted by FACS. Cells (1×105/50 µL cRPMI) were incubated in the presence or absence of rIL-27 (50 ng/mL) for 24 hours (h) without further stimulation and chemokine concentrations in supernatants analyzed.
Assessment of lung injury, ELISA and multiple chemokine detection
Total BAL fluid protein was measured using a bicinchoninic protein assay (BCA) kit (Pierce Chemical). Cytokine or chemokine concentration in BAL fluid, lung homogenates or cell cultures were measured by enzyme-linked immunosorbent assay (BD Biosciences or eBiosciences) or FlowCytomix multiple detection kit (eBioscience).
Zymosan-induced peritonitis
C57BL/6 mice were injected with zymosan (1 mg in 1 mL PBS) intraperitoneally (i.p) with or without co-injection of rIL-27 (200 ng, R&D Systems). After 24 h, blood or 5 mL peritoneal lavage (PBS) was obtained.
Lymphocyte culture
Naive CD8+ T cells (CD8+CD62L+) were isolated from pooled splenocyte and lymph nodes using magnetic bead separation (Miltenyi). For activation, purified CD8+ T cells (1×106 cells per mL) were cultured with plate-bound anti-CD3 (3 µg/mL; clone 145-2C11) and anti-CD28 (3 µg/mL; clone 37.51). CD8+ T cells were supplemented with murine rIL-12 (5 ng/mL; eBioscience), rIFN-γ (20 ng/mL; eBioscience), and rIL-2 (10 ng/mL; R&D Systems) plus anti-mouse IL-4 antibody (5 µg/mL; clone 11B11) in complete RPMI media (RPMI 1640 (Gibco®) plus 10% FCS (vol/vol) and antibiotics) for 3 days (d). After 3 d in culture, Tc1 cells were transferred to a plate without anti-CD3 and anti-CD28 and supplemented with fresh medium plus rIL-2 and cultured for 2 d for a total of 5 d in culture. In cases where rIL-27 (rIL-27) was added or IL-10 receptor was blocked, the media were supplemented with rIL-27 (50 ng/mL; eBioscience) and/or anti-IL-10 receptor blocking antibody (αIL-10R) (40 µg/mL; 1B1-2). In some experiments, cells were labeled with CFSE (Sigma) and cultured under the same conditions as mentioned above.
T cell re-stimulation and intracellular cytokine staining
Intracellular cytokine staining (ICS) was performed as previously described (Hamada et al., 2009). Briefly, enriched lymphocyte samples were restimulated either with phorbol 12-myristate 13-acetate (PMA; 100 ng/mL) plus ionomycin for 4 h, or a combination of immunodominant influenza virus peptides (Anaspec; Table S1) for 6 h in the presence of Brefeldin A (10 µg/mL, Sigma). For analysis of CD107 expression, anti-CD107a antibody (10 µg/mL) and anti-FcRγ (10 µg/mL) was added to the culture during 6 h peptide restimulation. Cells were incubated with anti-FcRγ receptor antibody prior to staining for surface markers. Surface-labeled cells were fixed for 20 minutes using 2% paraformaldehyde. Cytokines were stained using fluorochrome-labeled anti-mouse monoclonal antibodies (mAbs) in 0.1% saponin buffer for 20 minutes. Cells were washed and resuspended in PBS/BSA then analyzed on a FACS Canto II (BD Biosciences). Data were analyzed using Flowjo analysis software (TreeStar). For FACS antibodies used, see supplemental methods.
Quantitative reverse-transcription PCR
RNA was extracted using RNeasy Mini Kit with oncolumn DNase digestion (Qiagen). cDNA was synthesized using SuperscriptII Reverse Transcriptase (Invitrogen) with random hexamers and oligo(dT) primers (Qiagen). Quantitative reverse-transcription PCR (qRT-PCR) was performed using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) on a Stratagene MX3000 thermo cycler. For qRT-PCR primers used, see Table S2.
In vivo treatments
Blockade of IL-10 signaling in vivo was achieved by administration of an anti-IL-10 receptor-specific mAb (αIL-10R; clone 1B1-2) after 3 d (i.p., 1 mg in 200 µL PBS), 4 d (i.n., 0.15 mg in 30 µL PBS) and 6 d.p.i (i.p., 1 mg in 200 µL PBS) [27]. rIL-27 (200 ng in 100 µL PBS; eBioscience) was injected i.p. from 1–7 d.p.i. (early) or 5–9/10 d.p.i. (late treatment). In parallel, mice were injected solely with PBS (NT) as control. NK cell depletion was performed by i.p injection of anti-NK1.1 depleting antibody (500 µg in 500 µL; clone PK136) at −1, 1 and 5 d.p.i.
Histolopathological analysis
Lung samples were fixed with 4% formaldehyde, embedded in paraffin and stained with hematoxylin and eosin (H&E). Images were acquired using an AxioImager Z1 microscope equipped with a charge-coupled device (CCD) camera (AxioCam MRm) and processed with AxioVision software (all purchased from Carl Zeiss MicroImaging, Inc.). H&E stained lung sections were scored in a blinded manner as follows: (0) normal, (1) minor perivascular inflammation around large blood vessels, (2) moderate perivascular and peribronchial inflammation, (3) increased perivascular and peribronchial inflammation, (4) severe formation of perivascular, peribronchial, and interstitial inflammation.
Antibodies and flow cytometry analysis
The following murine monoclonal antibodies (mAbs) were purchased from BD Biosciences or eBioscience: CD4 (RM4-5), Ly6-G (RB6-8C5), CD11c (N418), CD49b (pan-NK), CD11b (M1-70), CD8a+ (53-6.7), CD62L (16A/MEL-14), CD3 (145-2C11), IFN-γ (AN18.17.24), IL-10 (JES5-A6E3), IL-17A (TC11-18H10), TNF-α (MP6-XT22), CD107 (1D4B). CD31 (MEC13.3), ICAM-1 (KAT1) and MHCII (M5/114) were obtained in house. In certain cases, PI was added at 40 µg/ml to the cells immediately prior to cell acquisition.
Statistical analysis
Data are means ± s.d. or s.e.m. Statistical tests used include Kaplan-Meier log-rank survival test, and unpaired two-tailed Students t test. All P values>0.05 are considered not to be significant.
Supporting Information
Zdroje
1. BarryJM (2004) The site of origin of the 1918 influenza pandemic and its public health implications. J Transl Med 2 : 3.
2. WebbyRJ, WebsterRG (2003) Are we ready for pandemic influenza? Science 302 : 1519–1522.
3. PeirisJS, HuiKP, YenHL (2010) Host response to influenza virus: protection versus immunopathology. Current opinion in immunology 22 : 475–481.
4. La GrutaNL, KedzierskaK, StambasJ, DohertyPC (2007) A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol 85 : 85–92.
5. TisoncikJR, KorthMJ, SimmonsCP, FarrarJ, MartinTR, et al. (2012) Into the eye of the cytokine storm. Microbiol Mol Biol Rev 76 : 16–32.
6. BuckleyCD, GilroyDW, SerhanCN, StockingerB, TakPP (2013) The resolution of inflammation. Nat Rev Immunol 13 : 59–66.
7. PflanzS, TimansJC, CheungJ, RosalesR, KanzlerH, et al. (2002) IL-27, a Heterodimeric Cytokine Composed of EBI3 and p28 Protein, Induces Proliferation of Naive CD4+ T Cells. Immunity 16 : 779–790.
8. HunterCA (2005) New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol 5 : 521–531.
9. HibbertL, PflanzS, De Waal MalefytR, KasteleinRA (2003) IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res 23 : 513–522.
10. ChenQ, GhilardiN, WangH, BakerT, XieMH, et al. (2000) Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature 407 : 916–920.
11. YoshidaH, HamanoS, SenaldiG, CoveyT, FaggioniR, et al. (2001) WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity 15 : 569–578.
12. StumhoferJS, HunterCA (2008) Advances in understanding the anti-inflammatory properties of IL-27. Immunology letters 117 : 123–130.
13. FindlayEG, GreigR, StumhoferJS, HafallaJC, de SouzaJB, et al. (2010) Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. Journal of immunology 185 : 2482–2492.
14. RosasLE, SatoskarAA, RothKM, KeiserTL, BarbiJ, et al. (2006) Interleukin-27R (WSX-1/T-cell cytokine receptor) gene-deficient mice display enhanced resistance to leishmania donovani infection but develop severe liver immunopathology. Am J Pathol 168 : 158–169.
15. StumhoferJS, LaurenceA, WilsonEH, HuangE, TatoCM, et al. (2006) Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature Immunology 7 : 937–945.
16. HolscherC, HolscherA, RuckerlD, YoshimotoT, YoshidaH, et al. (2005) The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol 174 : 3534–3544.
17. HamanoS, HimenoK, MiyazakiY, IshiiK, YamanakaA, et al. (2003) WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19 : 657–667.
18. VillarinoA, HibbertL, LiebermanL, WilsonE, MakT, et al. (2003) The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity 19 : 645–655.
19. Amadi-ObiA, YuCR, LiuX, MahdiRM, ClarkeGL, et al. (2007) TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med 13 : 711–718.
20. BattenM, LiJ, YiS, KljavinNM, DanilenkoDM, et al. (2006) Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 7 : 929–936.
21. YoshimuraT, TakedaA, HamanoS, MiyazakiY, KinjyoI, et al. (2006) Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol 177 : 5377–5385.
22. RutzS, JankeM, KassnerN, HohnsteinT, KruegerM, et al. (2008) Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci U S A 105 : 3497–3502.
23. SunJ, DoddH, MoserEK, SharmaR, BracialeTJ (2011) CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nature Immunology 12 : 327–334.
24. OwakiT, AsakawaM, MorishimaN, HataK, FukaiF, et al. (2005) A role for IL-27 in early regulation of Th1 differentiation. J Immunol 175 : 2191–2200.
25. LiuL, CaoZ, ChenJ, LiR, CaoY, et al. (2012) Influenza A virus induces interleukin-27 through cyclooxygenase-2 and protein kinase A signaling. The Journal of biological chemistry 287 : 11899–11910.
26. MayerKD, MohrsK, ReileyW, WittmerS, KohlmeierJE, et al. (2008) Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol 180 : 693–697.
27. SunJ, MadanR, KarpCL, BracialeTJ (2009) Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 15 : 277–284.
28. TateMD, IoannidisLJ, CrokerB, BrownLE, BrooksAG, et al. (2011) The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One 6: e17618.
29. FitzgeraldDC, ZhangGX, El-BehiM, Fonseca-KellyZ, LiH, et al. (2007) Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol 8 : 1372–1379.
30. AwasthiA, CarrierY, PeronJP, BettelliE, KamanakaM, et al. (2007) A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol 8 : 1380–1389.
31. FitzgeraldDC, CiricB, TouilT, HarleH, GrammatikopolouJ, et al. (2007) Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. Journal of immunology 179 : 3268–3275.
32. AndersonCF, StumhoferJS, HunterCA, SacksD (2009) IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. Journal of immunology 183 : 4619–4627.
33. JankovicD, KuglerDG, SherA (2010) IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol 3 : 239–246.
34. SaraivaM, ChristensenJR, VeldhoenM, MurphyTL, MurphyKM, et al. (2009) Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity 31 : 209–219.
35. McKinstryKK, StruttTM, BuckA, CurtisJD, DibbleJP, et al. (2009) IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol 182 : 7353–7363.
36. TumpeyTM, Garcia-SastreA, TaubenbergerJK, PaleseP, SwayneDE, et al. (2005) Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol 79 : 14933–14944.
37. KuikenT, RiteauB, FouchierRA, RimmelzwaanGF (2012) Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol 2 : 276–286.
38. ZhengBJ, ChanKW, LinYP, ZhaoGY, ChanC, et al. (2008) Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci U S A 105 : 8091–8096.
39. SnelgroveRJ, GouldingJ, DidierlaurentAM, LyongaD, VekariaS, et al. (2008) A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol 9 : 1074–1083.
40. KhoufacheK, LeBouderF, MorelloE, LaurentF, RiffaultS, et al. (2009) Protective role for protease-activated receptor-2 against influenza virus pathogenesis via an IFN-gamma-dependent pathway. J Immunol 182 : 7795–7802.
41. TeijaroJR, WalshKB, CahalanS, FremgenDM, RobertsE, et al. (2011) Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146 : 980–991.
42. YoshidaH, MiyazakiY (2008) Regulation of immune responses by interleukin-27. Immunological reviews 226 : 234–247.
43. MorishimaN, MizoguchiI, OkumuraM, ChibaY, XuM, et al. (2010) A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol 2010 : 605483.
44. CroweCR, ChenK, PociaskDA, AlcornJF, KrivichC, et al. (2009) Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 183 : 5301–5310.
45. HsiehSM, ChangSC (2006) Insufficient perforin expression in CD8+ T cells in response to hemagglutinin from avian influenza (H5N1) virus. J Immunol 176 : 4530–4533.
46. SchwarzenbergerP, HuangW, YeP, OliverP, ManuelM, et al. (2000) Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol 164 : 4783–4789.
47. SchwarzenbergerP, La RussaV, MillerA, YeP, HuangW, et al. (1998) IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J Immunol 161 : 6383–6389.
48. NembriniC, MarslandBJ, KopfM (2009) IL-17-producing T cells in lung immunity and inflammation. The Journal of allergy and clinical immunology 123 : 986–994; quiz 995–986.
49. RajaiahR, PuttabyatappaM, PolumuriSK, MoudgilKD (2010) Interleukin-27 and interferon-gamma are involved in regulation of autoimmune arthritis. J Biol Chem 286 : 2817–2825.
50. StumhoferJS, SilverJ, HunterCA (2007) Negative regulation of Th17 responses. Seminars in immunology 19 : 394–399.
51. MurugaiyanG, MittalA, WeinerHL (2010) Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A 107 : 11495–11500.
52. KallioliasGD, GordonRA, IvashkivLB (2010) Suppression of TNF-alpha and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. Journal of immunology 185 : 7047–7056.
53. BarlettaKE, CagninaRE, WallaceKL, RamosSI, MehradB, LindenJ (2012) Leukocyte compartments in the mouse lung: distinguishing between marginated, interstitial, and alveolar cells in response to injury. J Immunol Methods 375 : 100–110.
54. TschernigT, PabstR (2009) What is the clinical relevance of different lung compartments? BMC Pulm Med 9 : 39.
55. LiJP, WuH, XingW, YangSG, LuSH, et al. (2010) Interleukin-27 as a negative regulator of human neutrophil function. Scandinavian Journal of Immunology 72 : 284–292.
56. LiuJ, GuanX, MaX (2007) Regulation of IL-27 p28 gene expression in macrophages through MyD88 - and interferon-{gamma}-mediated pathways. J Exp Med 204 : 141–152.
57. PirhonenJ, SirenJ, JulkunenI, MatikainenS (2007) IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol 82 : 1185–1192.
58. RemoliME, GafaV, GiacominiE, SeveraM, LandeR, et al. (2007) IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol 37 : 3499–3508.
59. HeroldS, SteinmuellerM, von WulffenW, CakarovaL, PintoR, et al. (2008) Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med 205 : 3065–3077.
60. CaoJ, WangD, XuF, GongY, WangH, et al. (2014) Activation of IL-27 signalling promotes development of postinfluenza pneumococcal pneumonia. EMBO Mol Med 6 : 120–140..
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



