-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEpigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
Schistosoma mansoni is a parasitic worm that causes schistosomiasis, a debilitating disease in Africa and South America. Female worms mated with males produce hundreds of eggs that can reach the environment to propagate the biological cycle, or become trapped in host tissues, triggering inflammation and pathology. Because eggshell formation is a key step in egg development and viability, we have studied the molecular mechanisms of S. mansoni eggshell development, focusing on a major eggshell gene, Smp14. Using a variety of technical and biological approaches, we obtained strong evidence that eggshell formation depends on nuclear receptors and coactivators with chromatin modifying activities, mainly histone acetylation. Inhibition or partial deletion of S. mansoni histone acetyltransferases impaired the expression of Smp14, culminating in a severe negative effect on eggshell formation. Our findings will contribute not only to a better understanding of sex and tissue-specific gene regulation in S. mansoni but also provide an alternative strategy for interfering with the egg production, which might be targeted in novel therapeutics directed against this parasite.
Published in the journal: . PLoS Pathog 10(5): e32767. doi:10.1371/journal.ppat.1004116
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004116Summary
Schistosoma mansoni is a parasitic worm that causes schistosomiasis, a debilitating disease in Africa and South America. Female worms mated with males produce hundreds of eggs that can reach the environment to propagate the biological cycle, or become trapped in host tissues, triggering inflammation and pathology. Because eggshell formation is a key step in egg development and viability, we have studied the molecular mechanisms of S. mansoni eggshell development, focusing on a major eggshell gene, Smp14. Using a variety of technical and biological approaches, we obtained strong evidence that eggshell formation depends on nuclear receptors and coactivators with chromatin modifying activities, mainly histone acetylation. Inhibition or partial deletion of S. mansoni histone acetyltransferases impaired the expression of Smp14, culminating in a severe negative effect on eggshell formation. Our findings will contribute not only to a better understanding of sex and tissue-specific gene regulation in S. mansoni but also provide an alternative strategy for interfering with the egg production, which might be targeted in novel therapeutics directed against this parasite.
Introduction
Schistosomes are large metazoan pathogens that parasitize over 200 million people worldwide, resulting in up to 300,000 deaths per year [1]. Praziquantel is the only drug available, and despite its efficacy, it does not prevent re-infection, it is not effective against juvenile schistosomes, its mechanism of action has not been elucidated, and most importantly, resistance to this drug is already a concern [2]. The search for new drug targets is critical for developing novel strategies to combat this major pathogen. Sexually mature adult female S. mansoni lay a large number of eggs daily, which are responsible for both the transmission and pathogenesis of schistosomiasis [3]; therefore, understanding the mechanisms involved in schistosome reproductive biology is of particular interest. Because eggshell formation is a key step for determining the quality and quantity of eggs laid [4], we focused our studies on the molecular mechanisms of S. mansoni eggshell development. The major S. mansoni eggshell protein is Smp14 [5]–[7]. The Smp14 gene is exclusively expressed in mature vitelline cells in the vitelline duct and within the egg, enclosed by the eggshell, inside the ootype [4]. Smp14 is the most abundant mRNA transcript in sexually mature females and accounts for 10% of the mRNA of the entire organism [8]. Our previous research suggested that Smp14 transcription is regulated by the nuclear receptor heterodimer SmRXR1/SmNR1, which recruits coactivators with histone modifying (acetylation and methylation) activities [9]–[14]. We previously demonstrated the in vitro assembly of the SmRXR1/SmNR1 heterodimer and the two major histone acetyltransferases (HATs) in S. mansoni, SmGCN5 and SmCBP1, on a DNA response element within the Smp14 promoter region [14]. In addition, the physical interactions between SmRXR1, SmNR1, SmGCN5 and SmCBP1 have been mapped [13]. Importantly, we have demonstrated that SmGCN5 is localized on the transcriptionally active chromatin within the mature vitelline cells of female parasites [12]. In this study, we extended our original findings by characterizing, in vivo, the role of SmGCN5 and SmCBP1 in the transcriptional regulation of Smp14. We demonstrated that histone acetylation and chromatin remodeling by both schistosome HATs are essential for the activation of Smp14. More significantly, the inhibition of the histone acetyltransferase activity of SmCBP1 or SmGCN5 by a HAT inhibitor or RNA interference decreased Smp14 transcription and was associated with a severe negative effect on eggshell formation. Taken together, our data strongly suggest that histone acetyltransferase activity can represent a possible target for limiting parasite transmission, i.e., by decreasing the number of viable eggs deposited in the environment and reducing the production of the soluble egg and eggshell antigens involved in the immunopathogenesis of schistosomiasis.
Results
Smp14 transcriptional activation is dependent on histone acetylation
First, we investigated whether the SmRXR1/SmNR1 heterodimer activated the transcription of a reporter gene under the control of the Smp14 promoter. Because schistosome cell lines are not available, we used HEK293 cells as a surrogate system to investigate Smp14 transcriptional regulation by schistosome nuclear receptors. We observed a SmRXR1/SmNR1-dependent transcriptional activation of the Smp14 promoter (Fig. 1A). Because transcriptional activation is generally correlated with histone acetylation, we used classical histone deacetylase (HDAC) inhibitors (NaB or TSA) to demonstrate that histone acetylation indeed plays a key role in the Smp14 transcriptional activation by SmRXR1/SmNR1 (Fig. 1B). To further define the role of histone acetylation in the transcriptional activation of the Smp14 promoter by the nuclear receptor-coactivator complex, expression vectors for the S. mansoni HATs SmGCN5 and SmCBP1 were co-transfected into HEK293. As shown in Fig. 1C and D, SmGCN5 and SmCBP1 significantly enhanced the transcriptional activation of the Smp14 promoter in a dose-dependent manner. To determine, in vivo, the assembly of this transcriptional complex on the Smp14 promoter, we performed ChIP assays with the chromatin extracted from adult worm pairs and antibodies directed against SmRXR1, SmNR1, SmGCN5, SmCBP1 or acetylated H3 (a marker of open chromatin). As seen in Fig. S1, the Smp14 promoter was immunoprecipitated along with the nuclear receptors and HAT coactivators. These data strongly suggest that in vivo, SmGCN5 and SmCBP1 are recruited to the Smp14 promoter through the stable interaction with SmRXR1/SmNR1, and acetylate histone H3.
Fig. 1. Smp14 promoter-dependent activation by the SmRXR1/SmNR1 heterodimer and the HATs SmGCN5 and SmCBP1. 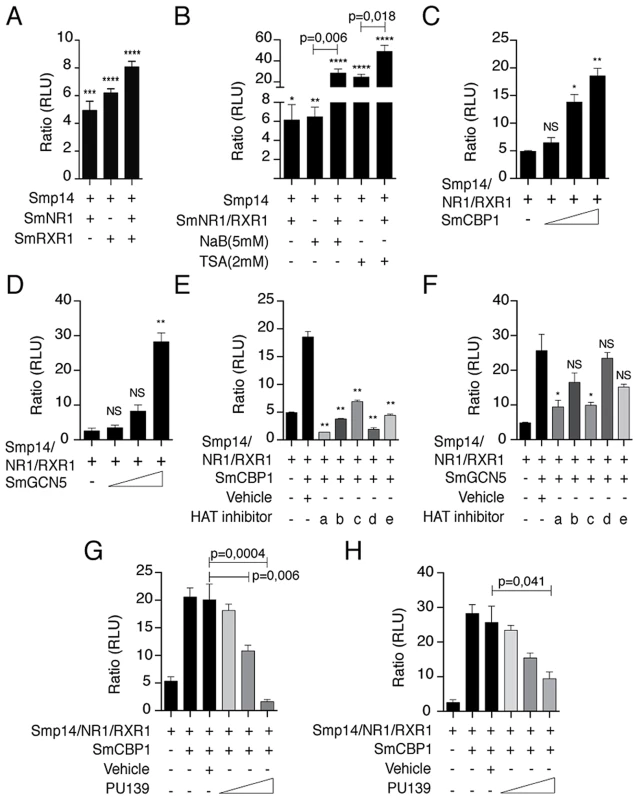
The luciferase gene reporter assays were performed in HEK293 cells. The Smp14 promoter-target sequence was cloned upstream of the luciferase reporter gene in the vector pGL4.23 (Smp14/3X-pGL4.23). This vector was co-transfected with the SmRXR1/SmNR1 heterodimer and/or co-transfected with SmCBP1 or SmGCN5 (A–H). The HDAC inhibitors NaB (5 mM) and TSA (2 µM) were tested (B). The dose-dependent transcriptional activation of the Smp14 promoter by SmCBP1 or SmGCN5 is shown (C and D). Small synthetic HAT inhibitors (2 µM) were tested in the transfected cells (E and F): PU139 (a), PU141 (b), SF7 (c), SF18 (d) and SF19 (e). Dose-dependent inhibition (0.5 nM, 1 µM and 2 µM) of SmCBP1 and SmGCN5 by the most potent inhibitor, PU139 (G and H). Results were plotted in relation to the firefly luciferase activity obtained from cells transfected with the Smp14/3X-pGL4.23 vector alone. Graphs are pooled from three independent experiments. Student's t-test was applied, with *p<0.05, **p<0,01, ***p<0.001 and ***p<0.001. Western blot data are representative of three independent experiments. HAT inhibition by small synthetic pyridoisothiazolones has been previously investigated [15]. In this study, these potential HAT inhibitors were evaluated for the inhibition of the human HAT subtypes GCN5, p300 and CBP in vitro using an immobilized histone peptide substrate and a heterogeneous assay format. The quantification of acetylation was measured using anti-acetyl lysine antibodies and a europium-labeled secondary antibody. Time-resolved fluorescence was used to determine the europium concentration, which is an indication of the degree of histone acetylation. Whereas the N-aryl derivatives PU139 and SF7 acted as broad-spectrum HAT inhibitors, the N-benzyl congener PU141 was selective for the closely related HAT subtypes CBP and p300 (Table S1). SF18 and SF19 demonstrated moderate selectivity for PCAF (Table S1). Because the targets of these inhibitors are the HAT catalytic domains and these enzyme domains are largely conserved across species, we performed additional receptor-reporter assays to assess the effects of five potential synthetic HAT inhibitors on the SmRXR1/SmNR1-, SmGCN5 - or SmCBP1-mediated transcriptional activity of the Smp14 promoter. All five compounds significantly inhibited the activity of SmCBP1; compound “a” (PU139) demonstrated the greatest inhibitory activity (Fig. 1E). When the SmGCN5-mediated transcriptional activity was investigated, only compounds “a” (PU139) and “c” (SF7) were effective, and the PAN-HAT inhibitor PU139 demonstrated the most effective inhibition of HAT (Fig. 1F). A clear dose-dependent inhibition of the transcriptional activity of the Smp14 promoter by PU139 was observed in HEK293 cells when SmCBP1 and SmGCN5 were co-transfected (Fig. 1G and H).
HAT inhibition halts Smp14 transcription and translation in S. mansoni
To confirm that histone acetylation is a prerequisite for Smp14 transcriptional activation in S. mansoni, adult worm pairs were cultivated for two days in the presence of increasing concentrations of PU139 (Fig. 2A). At concentrations that ranged from 1 to 20 µM, PU139 significantly blocked both the transcription (Fig. 2A) and translation (Fig. 2B) of Smp14. Furthermore, when the worms were treated for a longer period (3–4 days), the mRNA levels were decreased by the same amount as observed following the two-day incubation (Fig. 2C). Incubation with PU139 for a longer period practically abolished the Smp14 protein levels in the worms (Fig. 2D; compare the two-day incubation period (panel B) with the four-day incubation period (panel D). Although the 20 µM (0.1% DMSO) PU139 treatment was not overtly toxic to the worms (they remained paired and exhibited normal motility, data not shown), increasing the PU139 exposure concentration to 50 µM (0.5% DMSO) was harmful to the parasites (indicated by the separation of the male and female worms, data not shown), and the expected reduction in Smp14 expression was observed [16] (Fig. 2A and B). We believe that the negative effects of PU139 on the Smp14 transcriptional activity can be attributed to the inhibition of histone acetyltransferase activities and not to the alteration in coactivator or transcription factor expression levels, transcription factor binding to the DNA or coactivator recruitment. Therefore, we performed qPCR analysis of the SmRXR1 and SmNR1 transcription factors and of the SmGCN5 and SmCBP1 coactivators. As shown in Fig. S2, the PU139 treatment did not affect the mRNA levels of these transcription factor or coactivator targets. From the data presented in Fig. 2, we concluded that the deregulation of chromatin acetylation directly affects Smp14 production.
Fig. 2. HAT inhibition impairs Smp14 transcription and translation in S. mansoni adult worms. 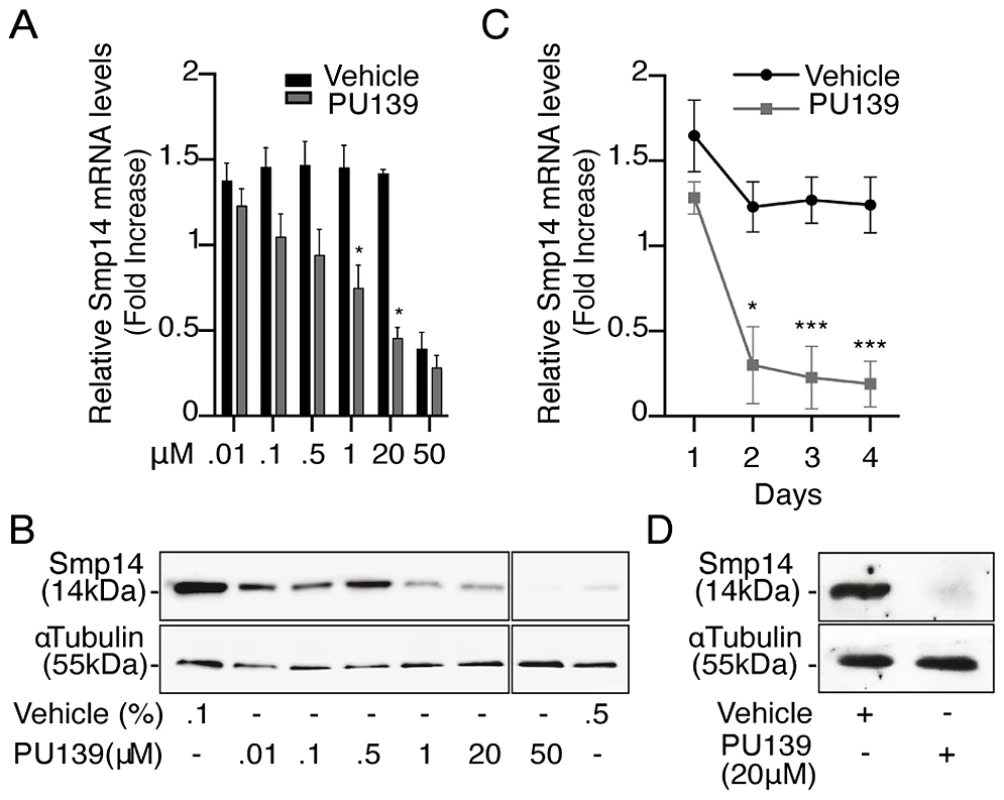
Ten adult worm pairs were cultivated with increasing concentrations of the HAT inhibitor PU139 or DMSO as vehicle. PU139 concentrations between 0.01 and 20 µM were solubilized in 0.1% DMSO, and 50 µM PU139 was only soluble in 0.5% DMSO. (A) qRT-PCR analysis of Smp14 mRNA levels after a two-day incubation time. (B) Western blot analysis of total protein extract from a two-day worm pair culture using polyclonal antibodies against Smp14 (upper panels) and monoclonal antibodies against α-tubulin (lower panels) as loading control. (C) qRT-PCR of Smp14 after a longer period (four days) of PU139 (20 µM) treatment. (D) Smp14 protein levels at the fourth day of treatment with 20 µM PU139. Graphs are pooled from three independent experiments. Student's t-test was applied, with *p<0.05 and ***p<0.001. Western blot data are representative of three independent experiments. The HAT inhibitor PU139 prevents chromatin decondensation at the Smp14 promoter
Based on the data presented above, we analyzed the acetylation status of the chromatin at the Smp14 promoter site. First, we evaluated the overall acetylation levels of histones H3 and H4, which are substrates of SmGCN5 and SmCBP1 [9], [11], in the worm pairs cultivated for two days with or without the HAT inhibitor PU139. The Western blot analysis demonstrated that histones H3 and H4 were acetylated in the worms treated with the vehicle, and H3 was highly acetylated (Fig. 3A). Importantly, the worms treated with PU139 demonstrated a significant reduction in H3 and H4 acetylation (Fig. 3A). Chromatin loci undergoing transcriptional activation are known to exhibit an enhanced activator-dependent recruitment of the HAT machinery [17], [18]. We therefore performed ChIP assays (Fig. 3B–E) to determine the histone acetylation status at the Smp14 promoter site. ChIP was performed using the chromatin prepared from the worm pairs cultivated for up to 48 h in the presence of vehicle or PU139. The results (Fig. 3B and C) suggested that schistosome HATs occupied the Smp14 promoter site and maintained the acetylation of H3 and H4 in vivo (black bars). The Smp14 promoter was co-immunoprecipitated by the anti-RNA polymerase II antibody (Fig. 3D, black bars), indicating that the Smp14 gene was transcribed. Most importantly, when we analyzed the immunoprecipitated chromatin from PU139-treated worms, we observed a significant decrease in the levels of acetylated H3 or H4 (Fig. 3B and C, gray bars). These data suggested that the histone hypo-acetylation at the Smp14 promoter site resulted in an inactive, repressed chromatin state. Indeed, the lack of RNA polymerase II recruitment to the Smp14 promoter loci (Fig. 3D, gray bars) suggested that the Smp14 gene was inactivated. We next confirmed that the Smp14 promoter assumed a transcriptionally inactive configuration through the immunoprecipitation of the chromatin using the anti-H3K27me3 antibody (Fig. 3E, 48 h), a known biomarker of repressed chromatin. The data (Fig. 3) also suggested that although the PU139 treatment altered the levels of schistosome chromatin acetylation as early as 30 min after the initial exposure for H3 (Fig. 3B) and 6 h for H4 (Fig. 3C), the inactivation of the Smp14 promoter was achieved after 48 h of histone acetylation inhibition. These data are in agreement with the data shown in Fig. 2.
Fig. 3. PU139 can reverse histone acetylation, keeping the Smp14 promoter in a repressed state. 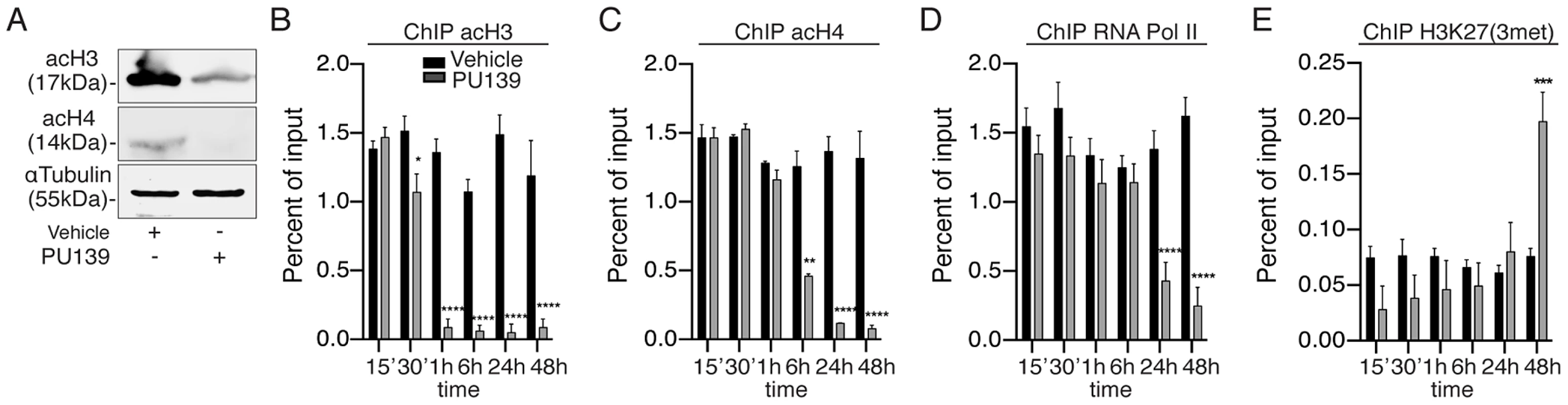
Ten adult worm pairs were cultivated with 20 µM PU139 or vehicle for 48 h. (A) Western blot analysis of total worm extract. The levels of S. mansoni acetylated histone H3 and H4 are shown. (B–E) ChIP analysis of S. mansoni histone modifications at the Smp14 promoter. Fifty worm pairs were treated with 20 µM PU139 or vehicle at different time points and submitted to chromatin extraction. Chromatin was immunoprecipitated with antibodies directed against acetylated H3 and H4, RNA pol II (markers of transcriptionally active chromatin) and H3K27me3 (a marker of repressed chromatin). ChIP DNA (Smp14 promoter) was quantified by real-time PCR and normalized as a percentage of input DNA. Results are pooled from four independent experiments. Student's t-test was applied, with *p<0.05, ***p<0.001 and ****p<0.0001. HAT inhibition impairs the production of normal S. mansoni eggs
The data presented in Figs. 1, 2 and 3 demonstrated clearly that histone acetylation plays a key role in Smp14 expression. Because Smp14 is a major eggshell component, we reasoned that the inhibition of histone acetylation would affect both egg production and structure. The worm pairs cultivated in the presence of 20 µM PU139 for two days laid significantly fewer eggs compared with the controls (Fig. 4A). A more detailed analysis revealed that the eggs were much smaller (Fig. 4B) and exhibited morphological defects, including invaginations of the egg surface and abnormal lateral spines (Fig. 4C, lower panel). Confocal laser scanning microscopy (Fig. 4D) demonstrated that the eggs inside the ootype (ot) of the paired female worms treated with vehicle were typical, normal eggs (Fig. 4D, panel a, ne) with a fully developed lateral spine (s) and several vitelline cells (vc). Control worms also showed a healthy egg inside the uterus, ready to be laid (Fig. 4D, panel c). In contrast, when the paired females incubated with PU139 were analyzed, we observed an undeveloped egg within the ootype (Fig. 4D, panel b, ae). That the PU139 treatment indeed affected egg development could be confirmed by the observation that the egg inside the uterus is malformed, and with a compromised eggshell (Fig. 4D, panel d, arrowhead). The normal physiology of the paired male worms was evaluated by the integrity of the tegumental tubercles (tt). Notably, the culture conditions used, including the presence of vehicle or PU139, did not change the worm behavior, i.e., they remained active and paired (Fig. 4D), or alter their morphology (data not shown). To confirm that the negative effects on the eggs (Fig. 4) were caused by the disruption of the eggshell integrity, we used scanning electron microscopy to examine the eggshell. As expected, the eggshells from the vehicle treatment group exhibited the typical smooth coat (Fig. 5A, panel a) and surface microspines (Fig. 5A, panel b refers to the boxed area in panel “a” at a higher magnification). Strikingly, the eggs from the PU139-treated parasites were much smaller and exhibited severe eggshell defects (panels c, d, e and f), including holes (panels e and f, arrows) and a large fissure (panel f, line), which ultimately led to the leakage of egg contents (panel f, arrowheads) [19], [20]. To analyze these eggshells in more detail, we made use of transmission electron microscopy (Fig. 5B). Normal eggs (panels a, d and e) show thick and continuous eggshells (Es). The characteristic microspines (ms) of S. mansoni eggs are indicated by arrows. When we analyzed the eggs laid by PU139-treated females (Fig. 5B, panels b, d and f), remarkable alterations in their eggshells were observed, which were notably much thinner and discontinuous (arrows). The holes in the eggshells could also be visualized in detail (arrows in panels d and f).
Fig. 4. HAT inhibition affects the normal development of S. mansoni eggs. 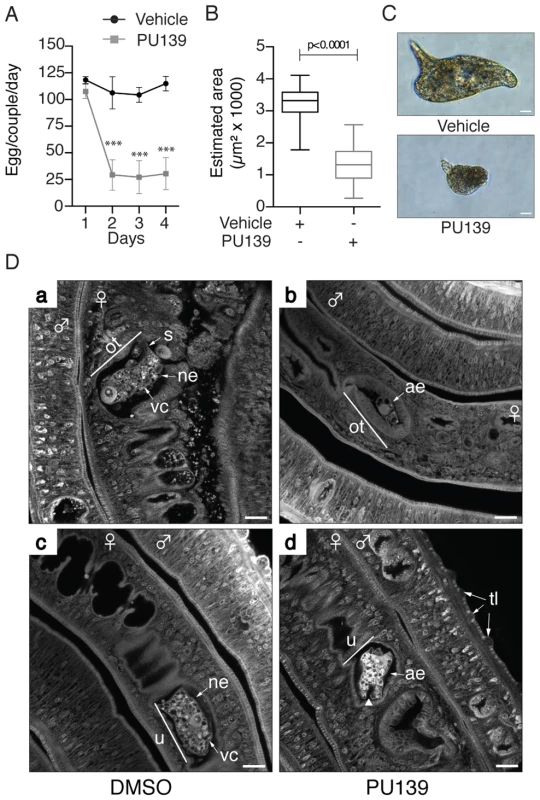
Ten adult worm pairs were cultivated with 20 µM PU139 or vehicle up to four days, and the number of eggs was counted on a daily basis (A). The estimated areas (length and width) of these same eggs were measured (B). Student's t-test was applied, with ***p<0.001. The morphology of the eggs was analyzed under optical microscopy. Scale bars: 10 µm (C). Adult worm pairs under the same treatment were fixed and stained with hydrochloric carmine for confocal laser scanning microscopy (CLSM) analysis. ne: normal egg; ae: abnormal egg; ot: ootype; vc: vitelline cells; u: uterus. The arrowhead in “ae” points to a fissure. tt: tegument tubercles. Scale bars: 20 µm (D). Fig. 5. HAT inhibition compromises eggshell integrity. 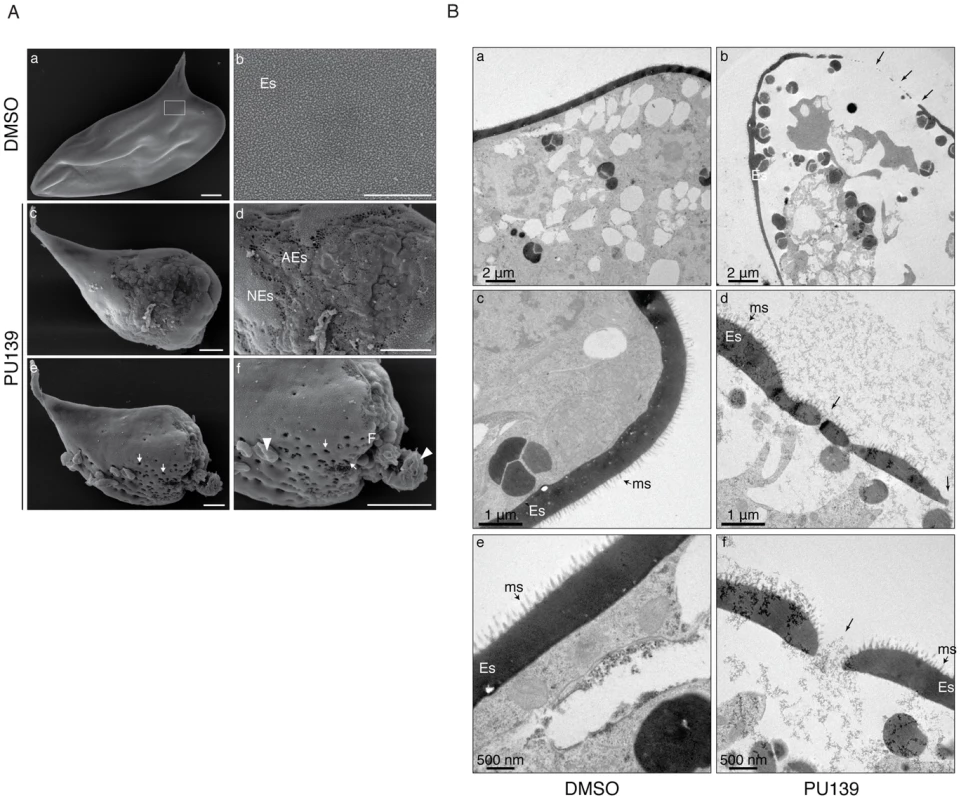
(A) Scanning electron microscopy of eggs laid by worm pairs cultivated for two days with vehicle (panels a and b) or 20 µM PU139 (panels c, d, e and f). A normal S. mansoni egg is shown (panel a) with the typical smooth coat of microspines on the eggshell (Es, panel b) seen at higher magnification (boxed area in panel a). PU139 treatment severely affected eggshell formation and integrity (panel c). Gross structural defects on the eggshell can be visualized at higher magnification (panel d; NEs: normal eggshell, AEs: abnormal eggshell). A remarkable phenotypic defect observed in eggs laid by PU139-treated parasites was the presence of holes in the eggshell (arrows in panel e). At a higher magnification, it can be clearly observed that, besides the holes (panel e and f, arrows), a large fissure in the eggshell was formed (panel f, F), leading to leakage of egg contents (panel f, arrowheads). Scale bars 10 µm. (B). Transmission electron microscopy of eggs laid by worm pairs cultivated for two days with vehicle (panels a, c and e) or 20 µM PU139 (panels b, d, and f). Normal eggs (panels a, d and e) show a thick and continuous eggshell (Es). The characteristic microspines (ms) of S. mansoni eggs are indicated by the arrows. The eggshells of the eggs laid by PU139-treated females (panels b, d and f) revealed remarkable differences in their structure, when compared with the control eggs, showing much thinner and discontinuous (arrows) eggshells. The holes in the eggshells were confirmed by TEM. HAT inhibition affects the development of the female reproductive system
In the course of our microscopy analysis for the effect of PU139 treatment on egg development, we noticed that the HAT inhibition had a broader negative phenotype on females. When we analysed the ovaries of DMSO-treated females (Fig. 6, panel a), we observed the typical morphology of a healthy ovary, with a large number of immature (io) and mature (mo) oocytes. In contrast, PU139-treated females (Fig. 6, panel b) revealed a smaller ovary, with fewer mature oocytes (compare with panel a).
Fig. 6. HAT inhibition compromises the reproductive system of female worms. 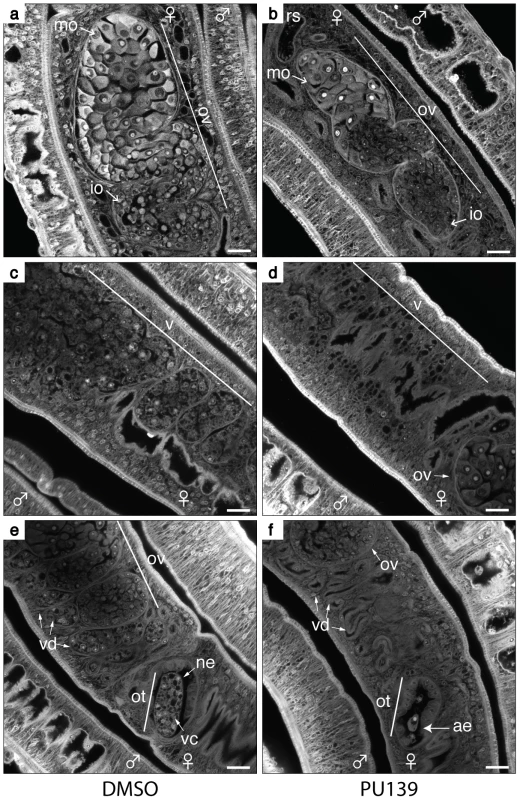
Ten adult worm pairs were cultivated with 20 µM PU139 (panels b, d and f) or vehicle (panels a, c and e) for two days. The worms were fixed and stained with Certistain (Merck) for confocal laser scanning microscopy (CLSM) analysis. ne: normal egg; ae: abnormal egg; ot: ootype; ov: ovary; mo: mature oocytes; io: immature oocytes; vd: vitelline ducts; vc: vitelline cells; rs: receptaculum seminis. The PU139 treatment provoked several negative phenotypes: 1 - the morphology of the ovaries and oocyte maturation (panel b); 2 - the vitellaria size and the number of vitelline cells (panel d); 3 – The vitelline duct and ootype depleted of vitelline cells (panel f). Scale bars: 20 µm. The importance of the vitellaria in the production of eggshell precursors and nutrients to develop the egg is well known [16]. In the DMSO-treated females, we observed a large and fully developed vitellaria (Fig. 6, panel c), as well as visible vitelline cells within the vitelline ducts (panel e). In contrast, in females treated with PU139, the vitellaria revealed a poriferous structure, with very low numbers of vitelline cells (panel d). In addition, many fewer vitelline cells were observed in the vitelline ducts (panel f).
SmCBP1 and SmGCN5 are required for Smp14 expression and egg development
To directly correlate the HAT activities of SmGCN5 and SmCBP1 with the regulation of Smp14 expression and the consequent effects on eggshell formation and egg development, we performed functional dsRNAi assays in adult worm pairs. The mRNA levels of SmCBP1 and SmGCN5 were knocked down 38.4% and 80.5%, respectively (Fig. 7A, SmCBP1; 7B, SmGCN5). Next, we demonstrated that the dsRNAi significantly affected the expression of Smp14, which is mediated by SmCBP1 and SmGCN5 (Fig. 7C and D, see protein levels; top panels). Most significantly, the knockdown of both of the schistosome HATs led to an approximate 50% reduction in the transcription of Smp14 (Fig. 7A and B, Smp14) and an effective decrease in the Smp14 protein level (Fig. 7C and D, middle panels). The cultivated worms that received the SmCBP1 or SmGCN5 dsRNAi demonstrated a significant reduction in the number of eggs (Fig. 7E and F) after the first four days in culture. In addition, these eggs exhibited an abnormal morphology, similar to that observed following the PU139 treatment (Fig. 4A). The dsRNAi experiments provided additional confirmation that both SmCBP1 and SmGCN5 play major roles in egg development.
Fig. 7. SmCBP1 and SmGCN5 play a key role in Smp14 expression and female sexual reproductive development. 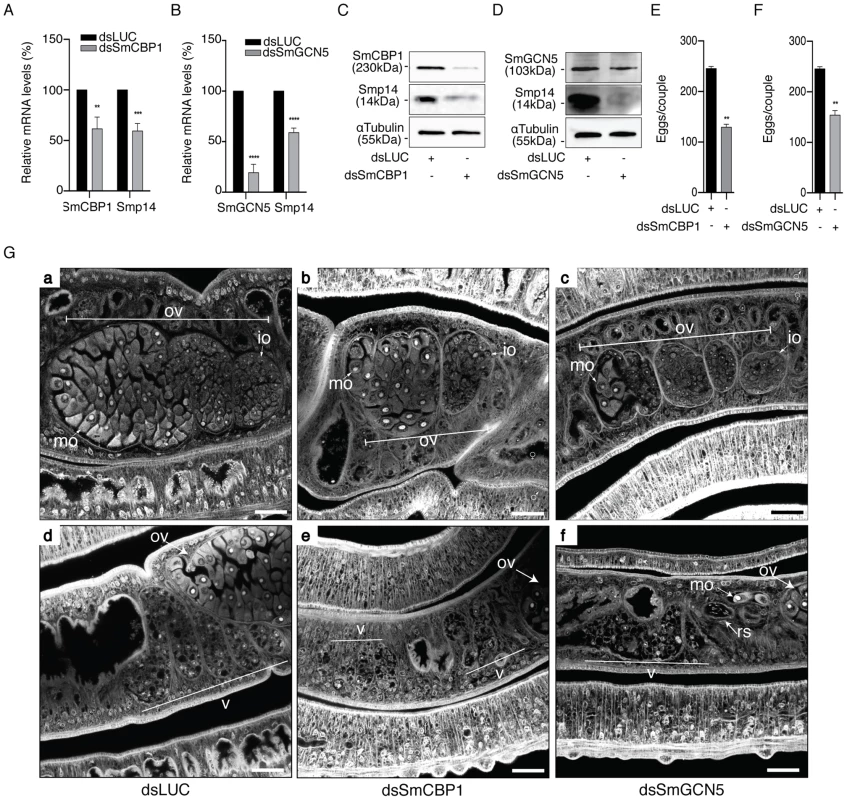
A dsRNAi experiment was carried out on sixteen adult worm pairs cultivated for seven days. Worms were electroporated and soaked with dsRNAi from SmCBP1 or SmGCN5 or with the non-specific dsRNAi LUC. On the seventh day of culture, the mRNA levels of SmCBP1, SmGCN5 or Smp14 (graphs A and B) were determined by qRT-PCR, normalized by the α-tubulin transcript levels. The results are depicted in relation to the non-specific dsRNAi (dsLUC) mRNA levels. The effects of the dsRNAi on targeted-protein synthesis were assessed by Western blot (panels C and D). The total number of eggs laid by the same parasites that received the dsRNAi was counted (graphs E and F). Adult worms from the dsRNAi experiments were analyzed by confocal laser scanning microscopy (G). Worms received either the control dsRNAi LUC (panels a and d) or the dsRNAi for SmCBP1 (panels b and e) or SmGCN5 (panels c and f). Panels a–c, and d–f, reveal the effect of the dsRNAi in the ovary, and vitellaria, respectively. OV: ovary; mo: mature oocytes; io: immature oocytes; v: vitellaria; rs: receptaculum seminis. Results are pooled from three independent experiments. Western blots were repeated three times. The confocal microscopy images are representative of several parasites analyzed. Scale bars 10 µm. Student's t-test was applied, with *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001. Not surprisingly, the knockdown of SmCBP1 and SmGCN5 also affected the reproductive system of the female worms (Fig. 7G), illustrated by the decrease in ovary size and the number and developmental stage of the oocytes produced (panel b for SmCBP1, and panel c, for SmGCN5). As expected, partial depletion of SmCBP1 (panel e) or SmGCN5 (panel f), also affected the vitellaria of females, visible by their reduced size, with fewer vitelline cells, when compared to the control RNAi (panel d). However, upon inspection of the male worm reproductive system, no apparent negative phenotype was observed; the male worms had fully developed testicular lobes containing plenty of germinal cells (Fig. S3B). Nevertheless, it is important to point out that we do not exclude the possibility of a negative effect of HAT inhibitors in males. We believe that mature females are readily affected due to their high metabolic rate required for sexual maturation and egg production. Thus, it is likely that males that were exposed to longer incubation periods in the presence of PU139 would also be affected. It is expected then, that female and male worms would undoubtedly experience a general transcriptional deregulation upon inhibition of their two main HAT enzymes, which would result in various physiological disorders.
Discussion
Schistosomiasis, which is caused by the blood fluke S. mansoni, has afflicted humans for thousands of years, and represents a major and still largely unacknowledged burden [1], particularly in sub-Saharan Africa where about 90% of infected individuals live [21]. In the absence of an effective prophylactic vaccine, a viable approach to controlling the disease has been the preventative mass-treatment of human populations in endemic areas using Praziquantel, the only effective drug available [2]. However, Praziquantel, although safe, effective and cheap is far from a perfect drug. First, it is not active against the juvenile parasites; second, the mechanism of action is not known, which limits the development of improved derivatives; and third, most concerning, Praziquantel-resistant parasites may eventually prevent the continued use of the drug [2] particularly in view of its massive use in mass chemotherapy campaigns [22]. Therefore, there is an urgent need to identify new parasite targets for the development of novel therapeutics.
Epigenetic targets, including enzymes that introduce histone modifications affecting gene transcription, are particularly attractive because these enzymes are already targeted in a variety of pathologies, particularly cancer [23] and a large number of drug candidates have been developed that can serve as the basis for developing selective inhibitors of schistosome enzymes.
Sexually mature female schistosomes produce 200–300 eggs daily, and the eggs constitute key components of the transmission and immunopathology of schistosomiasis. We have long been interested in understanding the molecular mechanisms involved in egg development, with the objective of finding the means to block egg production. Therefore, we have focused our studies on the mechanisms that regulate the expression of the major S. mansoni eggshell gene, Smp14. Our previous research suggested that Smp14 transcription is regulated by nuclear hormone receptors and coactivators that contain histone-modifying activities [9]–[14], such as acetylation. The two main S. mansoni HATs, SmCBP1 and SmGCN5, have been suggested to act as transcription activators [11]–[13]. Importantly, SmGCN5 has been directly linked to Smp14 activation in vivo via the localization in the nuclei of mature vitelline cells [12] where the Smp14 protein is produced [24]. SmGCN5 contains two unique sequences immediately flanking its catalytic domain [9] that are not found in any other GCN5 orthologues. This distinction might be targeted in novel therapeutics directed against this parasite.
Cellular homeostasis in eukaryotes is regulated by a fine balance between histone acetylation and deacetylation. Any loss of HAT and HDAC function via mutation or inhibition generally results in a disease state. As an example, the imbalance in histone acetylation activity mediated by HAT inhibitors compromises the development of protozoan parasites [25], [26]. In the specific case of S. mansoni, recent studies [27]–[29] demonstrated the feasibility of targeting histone deacetylation activity as a promising intervention strategy.
To demonstrate the relationship between histone acetylation and egg development, we investigated the effect of inhibitors of human HATs (Table S1) on the activities of SmCBP1 and SmGCN5. The inhibition of the acetyltransferase activities of SmCBP1 and SmGCN5, along with the concomitant inactivation of Smp14 expression, would be proof of concept for the inhibition of histone acetylation in female schistosomes as a feasible strategy for controlling eggshell formation.
The data presented in this study clearly demonstrate that the in vivo transcription of Smp14 requires nuclear receptor signaling, including the recruitment of coactivators with HAT activities (Fig. 8). Despite the fact that S. mansoni expresses six families of proteins with HAT activities [30], we hypothesized that SmCBP1 and SmGCN5 are the most important for Smp14 transcriptional activation. HAT1B and Tip60, members of the HAT family in S. mansoni, did not interact with SmRXR1 or SmNR1 in vitro, although both exhibited HAT activities (data not shown). Surprisingly, the S. mansoni genome does not present genes encoding members of the p160 HAT coactivators [31]. In other species, the p160 HAT coactivators are the first to recognize the nuclear receptors, followed by the recruitment of other HATs and the RNA Pol II complex [31]. Because of this distinction, we believe that SmGCN5 and/or SmCBP1 act as the main platform for the assembly of the transcriptional initiation complex on the Smp14 promoter.
Fig. 8. Proposed model of the acetylation-dependent activation of Smp14 transcription and eggshell formation. 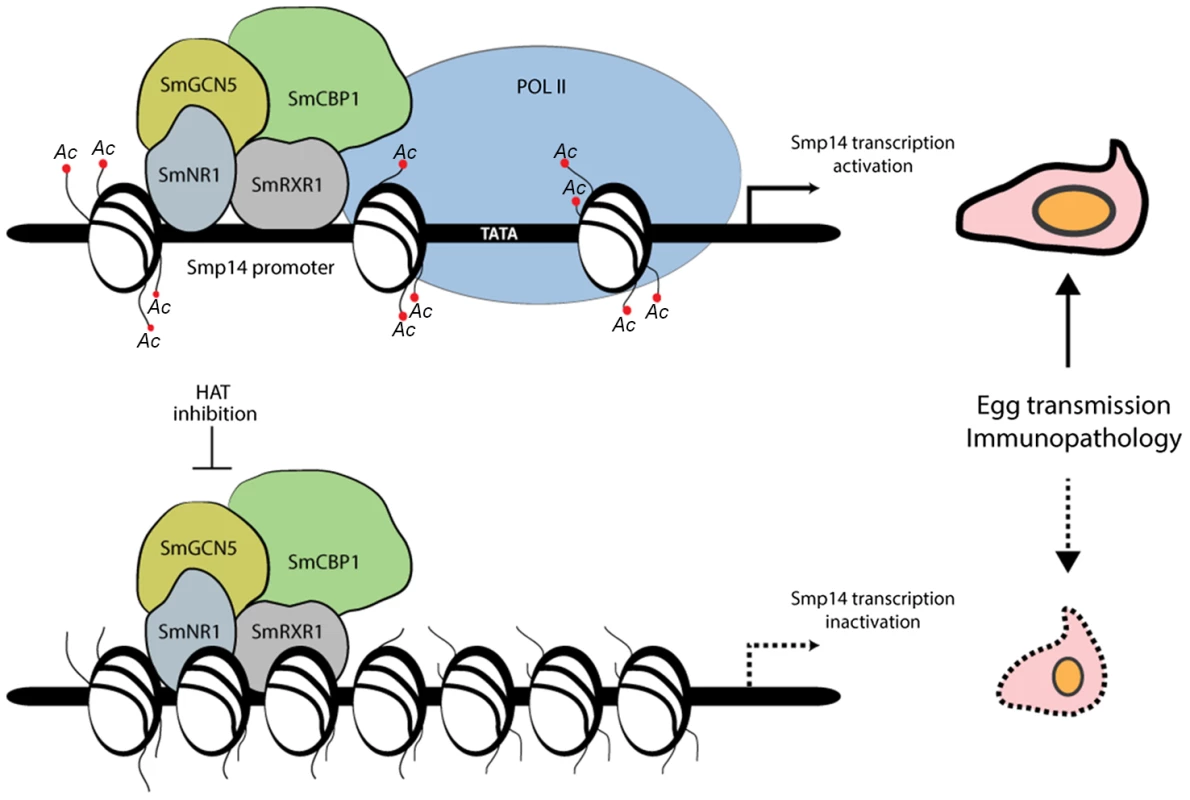
The nuclear receptor heterodimer SmRXR1/SmNR1 binds to a specific DNA response element present in the Smp14 promoter [14] and recruits the two histone acetyltransferases SmGCN5 and SmCBP1. Chromatin is then remodeled, RNA Pol II is attracted to the promoter and transcription occurs. Synthesis of Smp14 proteins will lead to eggshell formation, which is a prerequisite for egg dissemination and granuloma formation. Interfering with the histone acetyltransferase activities of either SmCBP1 or SmGCN5 reverses chromatin decondensation, with no access for RNA Poll II. Transcription of the Smp14 gene is halted, compromising eggshell integrity, which can ultimately lead to a reduction in the transmission and immunopathology of schistosomiasis. If SmCBP1 and SmGCN5 are the key coactivators of Smp14 expression, then the inhibition of these enzymes should have a direct effect on egg development in vivo. Indeed, this study demonstrated that the targeted inhibition of the catalytic domains of the schistosome HATs by PU139 treatment or the disruption of HAT transcription/translation by dsRNAi significantly decreased the Smp14 mRNA and protein levels, culminating in gross morphological eggshell defects and hindering egg development (Fig. 8). Interestingly, a similar phenotype in the S. mansoni eggshell was observed previously when two tyrosinases were inhibited by kojic acid [19]. These schistosome tyrosinases catalyze the cross-linking of tyrosine-rich Smp14 proteins, which is the final step in eggshell hardening [32]. Notably, other studies have contributed to the current understanding of S. mansoni egg development and have suggested potential targets for schistosome management [16], [33]–[35]. For example, Herbimycin A, an inhibitor of protein tyrosine kinases, blocked mitotic activity and egg production in paired females [33]. Recently, the same authors suggested that mitosis and eggshell formation were controlled via the cooperation of the Src-kinase and TGFβ receptor pathways [35]. Previous studies demonstrated that Herbimycin A decreased the mRNA levels of a variety of different genes, including genes that encode eggshell proteins. Although the molecular mechanism(s) involved in the regulation of eggshell genes by Src-kinase and the TGFβ receptor has not been elucidated, it is possible that tyrosine phosphorylation plays a role in the activation of the nuclear receptor heterodimer SmRXR1/SmNR1. SmRXR1/SmNR1 ligands have not been identified. However, the ligand-independent activation of nuclear receptors has been attributed to the phosphorylation of the AF-1 activation domain [36], [37]. Moreover, it is known that neither SmRXR1 nor SmNR1 binds retinoic acid or 9-cis-retinoic acid in vitro [13].
Modulators of enzyme activity are attractive agents for deciphering enzyme function [22]. Although silencing studies are used extensively to understand protein function, the lack of a particular protein in a specific cell type might lead to aberrant signaling and spurious conclusions. The modulator-based approach affects only the functional activity, enabling more precise conclusions regarding protein function, distinct from protein expression. In this study, we applied both approaches and concluded that histone acetylation by SmCBP1 and SmGCN5 creates the required epigenetic state for Smp14 transcriptional activation and eggshell formation. Our findings will contribute not only to a better understanding of sex - and tissue-specific gene regulation in S. mansoni but also provide an alternative strategy for interfering with egg production.
Materials and Methods
Ethics statement
The experiments involving hamsters as the final hosts to maintain the schistosome life cycle were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS No. 123; revised Appendix A) and were approved by the committee for ethics in animal experimentation of the Nord-Pas de Calais region (Authorization No. AF/2009) and the Pasteur Institute of Lille (Agreement No. A59-35009).
Parasite stock
A Puerto-Rican isolate of S. mansoni was maintained in Biomphalaria glabrata as the intermediate host and in Syrian hamsters (Mesocricetus auratus) as the definitive host [38] The adult worms were obtained by hepatoportal perfusion at 42–49 days post-infection.
Plasmids and gene reporter assay
The full-length cDNA of SmGCN5 [9] was cloned into pcDNA 3.1 A(+) (Invitrogen). The target sequence of the Smp14 promoter [5] (position −400 to −369 bp from the transcription start site, repeated three times) was cloned into pGL4.23 (Promega) upstream of the luciferase reporter gene, and the construct was named Smp14/3X-pGL4.23. The full-length SmCBP1-pcDNA 3.1 A(+) has been described previously [11]. The SmNR1-pcDNA 3.1 A(+) and SmRXR1-pcDNA 3.1 A(+) were a kind gift from Pr. Philip T. LoVerde (University of Texas, San Antonio). The oligonucleotides used in this study are described in Table S2. The HEK293 cells were maintained in DMEM supplemented with 10% fetal bovine serum at 37°C with 5% CO2. The cells were plated one day before transfection in 24-well plates at a density of 80,000 cells per well. The transfections were performed using 150 ng of each plasmid (Smp14/3X-pGL4.23, pGL4.74, SmCBP1-pcDNA, SmGCN5-pcDNA, SmNR1-pcDNA, SmRXR1-pcDNA and/or empty pcDNA3.1A). The cells were lysed 24 h after transfection, and the analysis of luminescence as a measure of luciferase activity was performed (dual-luciferase reporter assay system, Promega). The sodium butyrate (NaB), trichostatin A (TSA) or HAT inhibitors were added to the plated cells at 6 hours after transfection. MTT and LDH assays were used to measure the cell viability (data not shown). Statistical significance was determined using Student's t-test.
Schistosome in vitro culture, inhibitor treatment and microscopy analysis
The adult worms were obtained by whole-body perfusion of hamsters following a 6-week infection, and the worms were cultivated as previously reported [38]. For each treatment condition, 10 worm pairs were maintained in 60-mm diameter culture dishes in 2 mL of culture medium, supplemented with 10 nM to 50 µM PU139. The medium and the HAT inhibitor PU139 [15] were refreshed every 24 h during the treatment period (1–4 days). After treatment, the total RNA was extracted, and qRT-PCR was performed for the target genes (the oligonucleotides are listed in Table S2). The Western blots were performed using antibodies directed against acetylated histones H3 and H4 (Upstate), Smp14 (polyclonal serum, a kind gift from Pr. Philip T. LoVerde) and human α-tubulin (Abcam). For the microscopic analysis, the adult worms were fixed and stained as previously described [39]. Confocal scanning laser microscopy was performed on a Zeiss LSM 710 microscope equipped with a 488-nm HE/Ne laser and a 470-nm long-pass filter but without the reflection mode.
The eggs were counted and photographed every 24 h, and the worm motility and morphology were monitored daily. Approximately 300 eggs from three independent experiments were measured (length and width) as previously reported [20]. Approximately 200 eggs pooled from three independent experiments were fixed in 2.5% glutaraldehyde; 4% freshly prepared formaldehyde in 0.1 M cacodylate buffer, pH 7.2, for 1 h at room temperature and maintained at 4°C. The samples were then subsequently washed in 0.1 M cacodylate buffer, pH 7.2 and only the eggs were adhered to the coverslips coated with 1% gelatin. Samples were post-fixed in 1% OsO4 and 0.8% K3Fe (CN)6; washed in 0.1 M cacodylate buffer, pH 7.2; dehydrated in a graded ethanol series (20°–100° GL) for one hour each step; critical-point dried in CO2; mounted on metallic stubs and coated with gold (20–25 nm deposited). The samples were examined under FEI Quanta 250 scanning electron microscope, operating at 15–20 kV and a pressure of 2–8×10−4 Pa. Transmission electron microscopy was performed on a Tecnai G2 microscope (FEI Company, Eindhoven). Eggs (chemical fixed) were washed in 0.1 M cacodylate buffer, pH 7.2; post-fixed in 1% OsO4 and 0.8% K3Fe (CN)6; washed in 0.1 M cacodylate buffer, pH 7.2; dehydrated in a graded acetone series (20°–100° GL) for one hour each step and embedded in Polybed 812 epoxide resin. Thin-sections (60 nm) were collected on copper grids, stained for 30 minutes in 5% aqueous uranyl acetate, for 5 minutes in lead citrate.
Chromatin immunoprecipitation (ChIP)
ChIP analysis was performed following an adapted protocol for schistosomes, as previously reported [40]. Briefly, control and PU139-treated parasites were fixed in 1% formaldehyde and sonicated on a Bioruptor UCD-300 (Diagenode) to fragment the chromatin. The immunoprecipitation was performed using rabbit antibodies raised against acetylated histones H3 or H4 (ChIP grade, Upstate), RNA Pol II (ChIP grade, Abcam) or tri-methylated lysine 27 of histone H3 (H3K27-3met, ChIP grade, Abcam), SmNR1, SmRXR1, SmCBP1 and SmGCN5 (Rhea Biotechnology). Non-immunized rabbit serum (Sigma) was used as the control. The precipitated DNA was purified and quantified by qPCR or conventional PCR, using the oligonucleotides described in Table S2. The data were expressed as the percentage of the input DNA not subjected to immunoprecipitation.
Double-stranded RNAi
In preparation for the double-stranded RNA (dsRNA) synthesis, 500-bp fragments of the firefly luciferase (dsLUC, negative control), SmCBP1 or SmGCN5 cDNAs were amplified by PCR using the gene-specific primers listed in Table S2. The dsRNAs were synthesized in accordance with the manufacturer's instructions (MEGAscript RNA transcription kit, Ambion). Annealing and the integrity of the dsRNAs were confirmed using agarose gel electrophoresis. After electroporation, as previously reported [39], the worms were cultivated for seven days, and the first medium change was performed after the second day. Thereafter, the medium was refreshed every 24 h. The eggs were counted and the worm motility and morphology were monitored daily. For the Western blot analysis, an additional protein extraction step was performed using the RiboPure kit (Ambion) protocol. The SmCBP1 and SmGCN5 polyclonal antibodies were obtained from Rhea Biotech (Brazil). Both the control worms and worms that received dsRNAi were analyzed by confocal scanning laser microscopy.
Supporting Information
Zdroje
1. KingCH, Dangerfield-ChaM (2008) The unacknowledged impact of chronic schistosomiasis. Chronic Illn 4 : 65–79.
2. CioliD, BassoA, ValleC, Pica-MattocciaL (2012) Decades down the line: the viability of praziquantel for future schistosomiasis treatment. Expert Rev Anti Infect Ther 10 : 835–837.
3. PearceEJ, MacDonaldAS (2002) The immunobiology of schistosomiasis. Nat Rev Immunol 2 : 499–511.
4. deWalickS, TielensAG, van HellemondJJ (2012) Schistosoma mansoni: the egg, biosynthesis of the shell and interaction with the host. Exp Parasitol 132 : 7–13.
5. BobekL, RekoshDM, van KeulenH, LoVerdePT (1986) Characterization of a female-specific cDNA derived from a developmentally regulated mRNA in the human blood fluke Schistosoma mansoni. Proc Natl Acad Sci U S A 83 : 5544–5548.
6. KosterB, DargatzH, SchroderJ, HirzmannJ, HaarmannC, et al. (1988) Identification and localisation of the products of a putative eggshell precursor gene in the vitellarium of Schistosoma mansoni. Mol Biochem Parasitol 31 : 183–198.
7. KunzW, OpatzK, FinkenM, SymmonsP (1987) Sequences of two genomic fragments containing an identical coding region for a putative egg-shell precursor protein of Schistosoma mansoni. Nucleic Acids Res 15 : 5894.
8. ChenLL, RekoshDM, LoVerdePT (1992) Schistosoma mansoni p48 eggshell protein gene: characterization, developmentally regulated expression and comparison to the p14 eggshell protein gene. Mol Biochem Parasitol 52 : 39–52.
9. de Moraes MacielR, de Silva DutraDL, RumjanekFD, JulianoL, JulianoMA, et al. (2004) Schistosoma mansoni histone acetyltransferase GCN5: linking histone acetylation to gene activation. Mol Biochem Parasitol 133 : 131–135.
10. MansureJJ, FurtadoDR, de OliveiraFM, RumjanekFD, FrancoGR, et al. (2005) Cloning of a protein arginine methyltransferase PRMT1 homologue from Schistosoma mansoni: evidence for roles in nuclear receptor signaling and RNA metabolism. Biochem Biophys Res Commun 335 : 1163–1172.
11. BertinB, OgerF, CornetteJ, CabyS, NoelC, et al. (2006) Schistosoma mansoni CBP/p300 has a conserved domain structure and interacts functionally with the nuclear receptor SmFtz-F1. Mol Biochem Parasitol 146 : 180–191.
12. de Moraes MacielR, da CostaRF, de OliveiraFM, RumjanekFD, FantappieMR (2008) Protein acetylation sites mediated by Schistosoma mansoni GCN5. Biochem Biophys Res Commun 370 : 53–56.
13. FantappieMR, Bastos de OliveiraFM, de Moraes MacielR, RumjanekFD, WuW, et al. (2008) Cloning of SmNCoA-62, a novel nuclear receptor co-activator from Schistosoma mansoni: assembly of a complex with a SmRXR1/SmNR1 heterodimer, SmGCN5 and SmCBP1. Int J Parasitol 38 : 1133–1147.
14. FantappieMR, FurtadoDR, RumjanekFD, LoverdePT (2008) A unique nuclear receptor direct repeat 17 (DR17) is present within the upstream region of Schistosoma mansoni female-specific p14 gene. Biochem Biophys Res Commun 371 : 689–693.
15. FurdasSD, ShekfehS, BissingerEM, WagnerJM, SchlimmeS, et al. (2011) Synthesis and biological testing of novel pyridoisothiazolones as histone acetyltransferase inhibitors. Bioorg Med Chem 19 : 3678–3689.
16. GalantiSE, HuangSC, PearceEJ (2012) Cell death and reproductive regression in female Schistosoma mansoni. PLoS Negl Trop Dis 6: e1509.
17. PerissiV, RosenfeldMG (2005) Controlling nuclear receptors: the circular logic of cofactor cycles. Nat Rev Mol Cell Biol 6 : 542–554.
18. KatoS, YokoyamaA, FujikiR (2011) Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem Sci 36 : 272–281.
19. FitzpatrickJM, HiraiY, HiraiH, HoffmannKF (2007) Schistosome egg production is dependent upon the activities of two developmentally regulated tyrosinases. FASEB J 21 : 823–835.
20. NevesRH, de Lamare BiolchiniC, Machado-SilvaJR, CarvalhoJJ, BranquinhoTB, et al. (2005) A new description of the reproductive system of Schistosoma mansoni (Trematoda: Schistosomatidae) analyzed by confocal laser scanning microscopy. Parasitol Res 95 : 43–49.
21. HotezPJ, KamathA (2009) Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 3: e412.
22. FenwickA, WebsterJP, Bosque-OlivaE, BlairL, FlemingFM, et al. (2009) The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology 136 : 1719–1730.
23. ArrowsmithCH, BountraC, FishPV, LeeK, SchapiraM (2012) Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Discov 11 : 384–400.
24. EbersbergerI, KnoblochJ, KunzW (2005) Cracks in the shell–zooming in on eggshell formation in the human parasite Schistosoma mansoni. Dev Genes Evol 215 : 261–267.
25. CuiL, MiaoJ, FuruyaT, FanQ, LiX, et al. (2008) Histone acetyltransferase inhibitor anacardic acid causes changes in global gene expression during in vitro Plasmodium falciparum development. Eukaryot Cell 7 : 1200–1210.
26. VanagasL, JeffersV, BogadoSS, DalmassoMC, SullivanWJJr, et al. (2012) Toxoplasma histone acetylation remodelers as novel drug targets. Expert Rev Anti Infect Ther 10 : 1189–1201.
27. DuboisF, CabyS, OgerF, CosseauC, CapronM, et al. (2009) Histone deacetylase inhibitors induce apoptosis, histone hyperacetylation and up-regulation of gene transcription in Schistosoma mansoni. Mol Biochem Parasitol 168 : 7–15.
28. LancelotJ, CabyS, Dubois-AbdesselemF, VanderstraeteM, TroletJ, et al. (2013) Schistosoma mansoni Sirtuins: characterization and potential as chemotherapeutic targets. PLoS Negl Trop Dis 7: e2428.
29. MarekM, KannanS, HauserAT, Moraes MouraoM, CabyS, et al. (2013) Structural basis for the inhibition of histone deacetylase 8 (HDAC8), a key epigenetic player in the blood fluke Schistosoma mansoni. PLoS Pathog 9: e1003645.
30. PierceRJ, Dubois-AbdesselemF, LancelotJ, AndradeL, OliveiraG (2012) Targeting schistosome histone modifying enzymes for drug development. Curr Pharm Des 18 : 3567–3578.
31. YorkB, O'MalleyBW (2010) Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem 285 : 38743–38750.
32. SmythJD, CleggJA (1959) Egg-shell formation in trematodes and cestodes. Exp Parasitol 8 : 286–323.
33. KnoblochJ, KunzW, GreveldingCG (2006) Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni. Int J Parasitol 36 : 1261–1272.
34. HuangSC, FreitasTC, AmielE, EvertsB, PearceEL, et al. (2012) Fatty acid oxidation is essential for egg production by the parasitic flatworm Schistosoma mansoni. PLoS Pathog 8: e1002996.
35. BuroC, OliveiraKC, LuZ, LeutnerS, BeckmannS, et al. (2013) Transcriptome analyses of inhibitor-treated schistosome females provide evidence for cooperating Src-kinase and TGFbeta receptor pathways controlling mitosis and eggshell formation. PLoS Pathog 9: e1003448.
36. DebloisG, GiguereV (2003) Ligand-independent coactivation of ERalpha AF-1 by steroid receptor RNA activator (SRA) via MAPK activation. J Steroid Biochem Mol Biol 85 : 123–131.
37. WilliamsCC, BasuA, El-GharbawyA, CarrierLM, SmithCL, et al. (2009) Identification of four novel phosphorylation sites in estrogen receptor alpha: impact on receptor-dependent gene expression and phosphorylation by protein kinase CK2. BMC Biochem 10 : 36.
38. SmithersSR, TerryRJ (1965) The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 55 : 695–700.
39. BeckmannS, QuackT, DissousC, CailliauK, LangG, et al. (2012) Discovery of platyhelminth-specific alpha/beta-integrin families and evidence for their role in reproduction in Schistosoma mansoni. PLoS One 7: e52519.
40. CabyS, PierceRJ (2009) Quantitative chromatin immunoprecipitation (Q-ChIP) applied to Schistosoma mansoni. Mol Biochem Parasitol 166 : 77–80.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge inČlánek Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Surveillance for Emerging Biodiversity Diseases of Wildlife
- The Emerging Role of Urease as a General Microbial Virulence Factor
- PARV4: An Emerging Tetraparvovirus
- Epigenetic Changes Modulate Schistosome Egg Formation and Are a Novel Target for Reducing Transmission of Schistosomiasis
- The Human Adenovirus E4-ORF1 Protein Subverts Discs Large 1 to Mediate Membrane Recruitment and Dysregulation of Phosphatidylinositol 3-Kinase
- A Multifactorial Role for Malaria in Endemic Burkitt's Lymphoma Pathogenesis
- Structural Basis for the Ubiquitin-Linkage Specificity and deISGylating Activity of SARS-CoV Papain-Like Protease
- Cathepsin-L Can Resist Lysis by Human Serum in
- Epstein-Barr Virus Down-Regulates Tumor Suppressor Expression
- BCA2/Rabring7 Targets HIV-1 Gag for Lysosomal Degradation in a Tetherin-Independent Manner
- The Evolutionarily Conserved Mediator Subunit MDT-15/MED15 Links Protective Innate Immune Responses and Xenobiotic Detoxification
- Suppressor of Cytokine Signaling 4 (SOCS4) Protects against Severe Cytokine Storm and Enhances Viral Clearance during Influenza Infection
- T Cell Inactivation by Poxviral B22 Family Proteins Increases Viral Virulence
- Dynamics of HIV Latency and Reactivation in a Primary CD4+ T Cell Model
- HIV and HCV Activate the Inflammasome in Monocytes and Macrophages via Endosomal Toll-Like Receptors without Induction of Type 1 Interferon
- Virus and Autoantigen-Specific CD4+ T Cells Are Key Effectors in a SCID Mouse Model of EBV-Associated Post-Transplant Lymphoproliferative Disorders
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- Squalene Synthase As a Target for Chagas Disease Therapeutics
- The Contribution of Viral Genotype to Plasma Viral Set-Point in HIV Infection
- Combined Systems Approaches Reveal Highly Plastic Responses to Antimicrobial Peptide Challenge in
- Anthrax Lethal Factor as an Immune Target in Humans and Transgenic Mice and the Impact of HLA Polymorphism on CD4 T Cell Immunity
- Ly49C-Dependent Control of MCMV Infection by NK Cells Is -Regulated by MHC Class I Molecules
- Two Novel Human Cytomegalovirus NK Cell Evasion Functions Target MICA for Lysosomal Degradation
- A Large Family of Antivirulence Regulators Modulates the Effects of Transcriptional Activators in Gram-negative Pathogenic Bacteria
- Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response
- Malaria Parasite Infection Compromises Control of Concurrent Systemic Non-typhoidal Infection via IL-10-Mediated Alteration of Myeloid Cell Function
- A Role for in Higher Order Structure and Complement Binding of the Capsule
- Hip1 Modulates Macrophage Responses through Proteolysis of GroEL2
- CD8 T Cells from a Novel T Cell Receptor Transgenic Mouse Induce Liver-Stage Immunity That Can Be Boosted by Blood-Stage Infection in Rodent Malaria
- Phosphorylation of KasB Regulates Virulence and Acid-Fastness in
- HIV-Infected Individuals with Low CD4/CD8 Ratio despite Effective Antiretroviral Therapy Exhibit Altered T Cell Subsets, Heightened CD8+ T Cell Activation, and Increased Risk of Non-AIDS Morbidity and Mortality
- A Novel Mechanism Inducing Genome Instability in Kaposi's Sarcoma-Associated Herpesvirus Infected Cells
- Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin
- Hepatitis C Virus Cell-Cell Transmission and Resistance to Direct-Acting Antiviral Agents
- Different Modes of Retrovirus Restriction by Human APOBEC3A and APOBEC3G
- TNFα and IFNγ but Not Perforin Are Critical for CD8 T Cell-Mediated Protection against Pulmonary Infection
- Large Scale RNAi Reveals the Requirement of Nuclear Envelope Breakdown for Nuclear Import of Human Papillomaviruses
- The Cytoplasmic Domain of Varicella-Zoster Virus Glycoprotein H Regulates Syncytia Formation and Skin Pathogenesis
- A New Class of Multimerization Selective Inhibitors of HIV-1 Integrase
- Are We There Yet? The Smallpox Research Agenda Using Variola Virus
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
- Dynamic Functional Modulation of CD4 T Cell Recall Responses Is Dependent on the Inflammatory Environment of the Secondary Stimulus
- Bacterial Superantigens Promote Acute Nasopharyngeal Infection by in a Human MHC Class II-Dependent Manner
- Follicular Helper T Cells Promote Liver Pathology in Mice during Infection
- A Nasal Epithelial Receptor for WTA Governs Adhesion to Epithelial Cells and Modulates Nasal Colonization
- Unexpected Role for IL-17 in Protective Immunity against Hypervirulent HN878 Infection
- Human Cytomegalovirus Fcγ Binding Proteins gp34 and gp68 Antagonize Fcγ Receptors I, II and III
- Expansion of Murine Gammaherpesvirus Latently Infected B Cells Requires T Follicular Help
- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Molecular Signatures of Hemagglutinin Stem-Directed Heterosubtypic Human Neutralizing Antibodies against Influenza A Viruses
- The Downregulation of GFI1 by the EZH2-NDY1/KDM2B-JARID2 Axis and by Human Cytomegalovirus (HCMV) Associated Factors Allows the Activation of the HCMV Major IE Promoter and the Transition to Productive Infection
- Inactivation of Fructose-1,6-Bisphosphate Aldolase Prevents Optimal Co-catabolism of Glycolytic and Gluconeogenic Carbon Substrates in
- New Insights into Rotavirus Entry Machinery: Stabilization of Rotavirus Spike Conformation Is Independent of Trypsin Cleavage
- Prophenoloxidase Activation Is Required for Survival to Microbial Infections in
- SslE Elicits Functional Antibodies That Impair Mucinase Activity and Colonization by Both Intestinal and Extraintestinal Strains
- Timed Action of IL-27 Protects from Immunopathology while Preserving Defense in Influenza
- HIV-1 Envelope gp41 Broadly Neutralizing Antibodies: Hurdles for Vaccine Development
- The PhoP-Dependent ncRNA Mcr7 Modulates the TAT Secretion System in
- Cellular Superspreaders: An Epidemiological Perspective on HIV Infection inside the Body
- The Inflammasome Pyrin Contributes to Pertussis Toxin-Induced IL-1β Synthesis, Neutrophil Intravascular Crawling and Autoimmune Encephalomyelitis
- Papillomavirus Genomes Associate with BRD4 to Replicate at Fragile Sites in the Host Genome
- Integrative Functional Genomics of Hepatitis C Virus Infection Identifies Host Dependencies in Complete Viral Replication Cycle
- Co-assembly of Viral Envelope Glycoproteins Regulates Their Polarized Sorting in Neurons
- Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Venus Kinase Receptors Control Reproduction in the Platyhelminth Parasite
- Dual-Site Phosphorylation of the Control of Virulence Regulator Impacts Group A Streptococcal Global Gene Expression and Pathogenesis
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis
- High-Efficiency Targeted Editing of Large Viral Genomes by RNA-Guided Nucleases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



