-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFunctionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
Phytophthora species are among the most devastating crop pathogens worldwide. P. infestans is a pathogen of tomato and potato plants. The genome of P. infestans has been sequenced, revealing the presence of a large number of host-targeting RXLR effector proteins that are thought to manipulate cellular activities to the benefit of the pathogen. One step toward disease management comprises understanding the molecular basis of host susceptibility. In this paper, we used a protoplast-based system to analyze a subset of P. infestans RXLR (PiRXLR) effectors that interfere with plant immunity initiated by the recognition of microbial patterns (MAMP-triggered immunity - MTI). We identified PiRXLR effectors that suppress different stages early in the signaling cascade leading to MTI in tomato. By conducting a comparative functional analysis, we found that some of these effectors attenuate early MTI signaling in Arabidopsis, a plant that is not colonized by P. infestans. The PiRXLR effectors localize to different sub-cellular compartments, consistent with their ability to suppress different steps of the MTI signaling pathway. We conclude that the effector complement of P. infestans contains functional redundancy in the context of suppressing early signal transduction and gene activation associated with plant immunity.
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1004057
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004057Summary
Phytophthora species are among the most devastating crop pathogens worldwide. P. infestans is a pathogen of tomato and potato plants. The genome of P. infestans has been sequenced, revealing the presence of a large number of host-targeting RXLR effector proteins that are thought to manipulate cellular activities to the benefit of the pathogen. One step toward disease management comprises understanding the molecular basis of host susceptibility. In this paper, we used a protoplast-based system to analyze a subset of P. infestans RXLR (PiRXLR) effectors that interfere with plant immunity initiated by the recognition of microbial patterns (MAMP-triggered immunity - MTI). We identified PiRXLR effectors that suppress different stages early in the signaling cascade leading to MTI in tomato. By conducting a comparative functional analysis, we found that some of these effectors attenuate early MTI signaling in Arabidopsis, a plant that is not colonized by P. infestans. The PiRXLR effectors localize to different sub-cellular compartments, consistent with their ability to suppress different steps of the MTI signaling pathway. We conclude that the effector complement of P. infestans contains functional redundancy in the context of suppressing early signal transduction and gene activation associated with plant immunity.
Introduction
Plants possess innate defense mechanisms to resist microbial infection [1], [2]. Efficient plant disease resistance is based on two evolutionarily linked layers of innate immunity. One layer involves cell surface transmembrane receptors that recognize invariant microbial structures termed pathogen - or microbe-associated molecular patterns (PAMPs/MAMPs), hereafter referred to as MAMPs [3]–[5]. MAMPs are not only shared by particular pathogen races, but are broad signatures of a given class of microorganisms. They constitute evolutionarily conserved structures that are unique to microorganisms and have important roles in microbial physiology. Typical MAMPs include lipopolysaccharides (LPS) of Gram-negative bacteria, bacterial flagellin and fungal cell wall-derived carbohydrates or proteins, some of which were shown to trigger plant defense in a non-cultivar-specific manner [3], [6]. The best-studied MAMP receptor in plants is FLAGELLIN-SENSITIVE 2 (FLS2) from Arabidopsis, a receptor-like kinase (RLK) with extracellular leucine-rich repeat domains [7]. The 22 amino acid peptide (flg22) corresponding to the highly conserved amino-terminus of flagellin is sufficient to trigger immune responses in Arabidopsis, tomato, tobacco and barley but not in rice [8]–[12]. Although different MAMPs are perceived by different receptors, convergent early-signaling events, including MAP kinase activation and specific defense-gene induction, have been observed in Arabidopsis plants and protoplasts [13]–[15].
Suppression of flg22-induced defenses by bacterial virulence effectors suggests that manipulation of MAMP-triggered immunity (MTI) in plants is a key strategy for successful pathogens to grow and multiply (reviewed in [16]–[19]). A major target of bacterial effectors is the plant MAP kinase cascade, probably because of the central role of MAP kinase signaling in MTI. The Pseudomonas syringae effector HopAI1 displays phosphothreonine lyase activity and inactivates MPK3, MPK6, and MPK4 in Arabidopsis by dephosphorylating them [20]. P. syringae effector HopF2 blocks MAMP-induced signaling by targeting MKK5, a MAP kinase activating MPK3/MPK6, through a different mechanism of action i.e. ADP-ribosylation [21]. Bacterial effectors can also suppress MAP kinase signaling by targeting the pattern recognition receptor complex as illustrated by the P. syringae effectors AvrPto and AvrPtoB that block FLS2-mediated signal transduction in Arabidopsis and tomato [22]–[24]. Other effectors appear to act downstream of the activation of the MAPK cascade by blocking the expression of defense-associated genes in the nucleus. Such an effector is XopD from Xanthomonas campestris that inhibits the activity of the transcription factor MYB30, resulting in suppression of basal immune responses and promotion of pathogen growth [25], [26].
Unlike bacterial effectors, little is known about the molecular functions of effectors from eukaryotic plant pathogens. It remains to be demonstrated whether these pathogens have evolved effectors that subvert early-induced MTI signaling above, at, or immediately downstream of MAP kinase cascades. Oomycetes, including downy mildews and Phytophthora species, establish intimate association with host plant cells through structures such as appressoria, infection vesicles and haustoria, which are believed to facilitate the delivery of effectors into the host cytoplasm [27]. The genome sequences of Phytophthora sojae, P. ramorum, P. infestans and Hyaloperonospora arabidopsidis are published [28]–[30]. Each genome encodes several hundred putative RXLR effectors. Most oomycete Avirulence (Avr) proteins characterized so far carry a signal peptide followed by a conserved motif centered on the consensus RXLR-(EER) sequence, where X is any amino acid [31]. It has been shown that the RXLR peptide motif acts as a host-targeting signal for translocation into plant cells [31], [32].
Amongst the best-characterized oomycete RXLR effectors are AVR3a, AVRblb2 and PITG_03192 from P. infestans, AVR1b and AVR3b from the soybean pathogen P. sojae and ATR1 and ATR13 from H. arabidopsidis [33]–[47]. P. infestans Avr3a alleles encode secreted proteins of 147 amino acids that differ in two residues which determine recognition; only the isoform AVR3aKI is recognized by the potato resistance protein R3a, whereas AVR3aEM evades detection by R3a. When expressed in Nicotiana benthamiana cells, AVR3a suppresses host cell death induced by the elicitin INF1, a typical MAMP [35], [37]. It has since been shown to suppress cell death elicited by perception of a range of pathogen molecules by direct interaction with, and stabilization of, the plant E3 ligase CMPG1 [36], [42]. The Avrblb2 gene family is highly polymorphic and different forms/alleles are present in different P. infestans isolates. Sequence alignment of the deduced amino acid sequences of the Avrblb2 family members showed that the C-terminal effector domain undergoes positive selection, which is strong evidence for co-evolution with host resistance and/or target proteins [44]. The amino acid residue at position 69 was shown to be crucial for recognition by the cognate resistance protein Rpi-blb2 [44]. AVRblb2 was shown to block the secretion of a C14 cysteine protease that is involved in plant resistance against P. infestans [38]. Recently, the RXLR effector PITG_03192 has been shown to enhance P. infestans colonization of N. benthamiana by its interaction with NAC DNA binding proteins at the host endoplasmic reticulum, preventing their re-localization into the nucleus following pathogen perception [43]. Suppression of MTI has also been reported for ATR1 and ATR13 in Arabidopsis [47]. Nevertheless, for the majority of RXLR effectors, their biological functions and potential host targets are unknown.
Transient expression in protoplasts has proven fast and reliable for studying the function of bacterial type III effectors that suppress early MAMP signaling [48], [49]. Moreover, the assay allows the measurement of synchronized responses and it does not require the use of bacteria for protein or DNA transfer into the host cell. In addition, the protoplast system offers the possibility to test large sets of effectors in a medium-high throughput manner. In this study, we have used tomato mesophyll protoplasts to screen a library of 33 P. infestans RXLR effector candidates (PiRXLRs) for their ability to suppress flg22-triggered defense signaling. Our additional aim was to test whether PiRXLRs that suppress early MTI signaling in the host plant tomato retain that ability in the distantly-related non-host plant Arabidopsis. For the experimental read-out we measured the abilities of these effectors to suppress: i) flg22-induced promoterFLG22-INDUCED RECEPTOR-LIKE KINASE 1 - LUCIFERASE (pFRK1-Luc) reporter gene activity; ii) flg22-induced post-translational MAP kinase activation; and iii) flg22-induced gene expression. In addition, we performed sub-cellular localization studies of fluorescent protein-tagged PiRXLR effectors by confocal microscopy. Finally, we tested the potential of the PiRXLR effectors suppressing early MTI signaling to enhance N. benthamiana susceptibility to P. infestans. Unraveling the mode-of-action of PiRXLR effectors within plant cells will help to gain insight into the specific mechanisms that coordinate different signaling and metabolic pathways to ensure proper plant development and response to environmental changes or stresses.
Results
A subset of RXLR effectors from P. infestans suppresses flg22-inducible reporter gene activation in both tomato and Arabidopsis
A prerequisite to performing a screen that would allow us to identify PiRXLR effector candidates suppressing early events of MAMP signaling pathways in both a host (tomato) and a non-host (Arabidopsis) of P. infestans was to develop comparative bio-assays. Several components of the flg22-triggered signaling pathway are conserved in Arabidopsis and tomato. SlFLS2, the ortholog of AtFLS2, binds flg22 [50]. The MAP kinase orthologs of AtMPK3 and 6 in tomato are SlMPK3 and 1, respectively [51].
We adapted most of the techniques and materials that were generated for the identification and functional characterization of the P. syringae type III effector AvrPto, a well-studied suppressor of early MAMP signaling in both Arabidopsis [48], [49] and tomato [52]. Figure S1 shows that we could reproduce the AvrPto-mediated suppression of early MTI signaling observed in Arabidopsis protoplasts [48]. Moreover, we were able to extend this assay to tomato, and the induction of luciferase activity under control of the flg22-responsive promoter of FRK1 (pFRK1-Luc) was strongly impaired in Arabidopsis and tomato protoplasts expressing AvrPto with a C-terminal Green Fluorescent Protein (GFP) fusion (Figure S1A, B). An inactive AvrPto in which the Gly residue in position 2 is replaced by an Ala (AvrPto G2A-GFP), preventing the myristoylation and membrane localization of the effector protein [53], could not suppress pFRK1-Luc activation by flg22 (Figure S1A, B). Furthermore, we confirmed that AvrPto-GFP but not the AvrPto G2A-GFP mutant blocks post-translational activation of flg22-responsive MAP kinases in both protoplast systems (Figure S1 C, D).
We searched for PiRXLR effectors interfering with flg22-induced early immune responses in protoplasts of tomato, a host for P. infestans. Thirty-three PiRXLR effector genes, most of which were selected on the basis of their up-regulation during the biotrophic phase of infection [28], [32], [44], were cloned without the native secretion signal peptide into pDONR Gateway vectors (Table S1). We sub-cloned these sequences into Gateway destination vectors of the p2GW7 series to allow transient expression with/without an N-terminally fused GFP tag.
For the initial read-out, we measured pFRK1-Luc activity upon flg22 treatment. Of the 33 PiRXLR effectors screened, 8 (PITG_04097, PITG_04145, PITG_06087, PITG_09585, PITG_13628, PITG_13959, PITG_18215 and PITG_20303) reduced consistently and reproducibly flg22-induced pFRK1-Luc activation in tomato protoplasts, when compared to control protoplasts expressing only GFP (p-value<0.05 - Figure 1: S.lycopersicum). We named these effectors Suppressor of early Flg22-induced Immune response (SFI) 1 to 8, respectively. Protoplast staining with vital dyes, 24 h after plasmid transformation, showed that the percentage of dead cells is, with the exception of a higher (but non-significant) value for SFI6, similar for each of the tested PiRXLR effectors and the GFP control (Figure S2). Therefore, the suppression of reporter gene activity is not the consequence of a toxic or a programmed cell death process in transformed protoplasts.
Fig. 1. Inhibition of MAMP-inducible reporter gene activation by PiRXLR effectors. 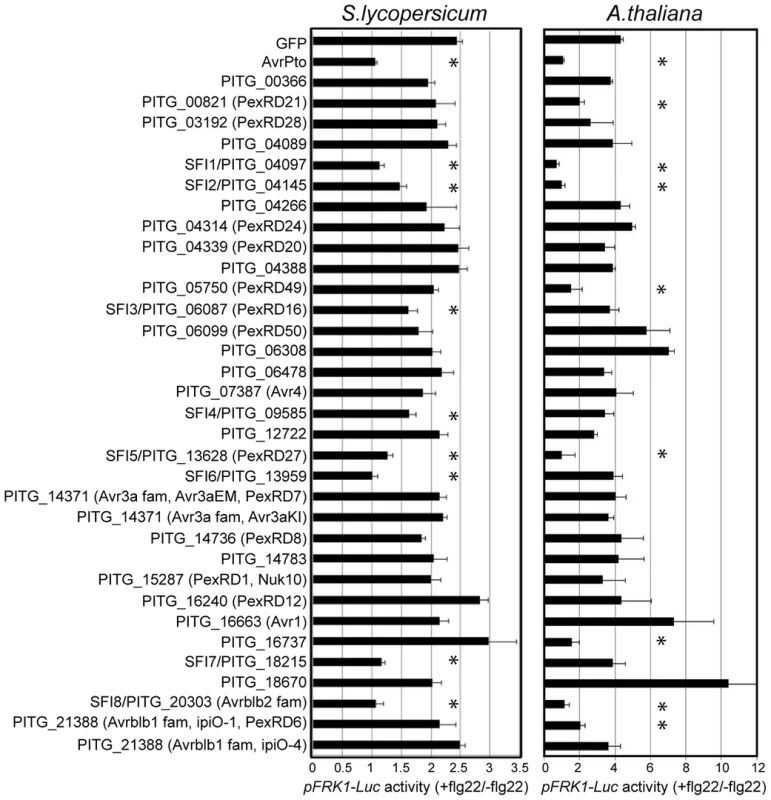
Luciferase reporter gene activity in flg22-challenged S. lycopersicum and A. thaliana protoplasts expressing PiRXLR effector genes. Mesophyll protoplasts were co-transfected with a p35S-effector construct (or a p35S-GFP control vector) and the two reporter gene constructs pFRK1-Luc and pUBQ10-GUS. Reporter gene activity was assessed 3 or 6 h later for S. lycopersicum and A. thaliana, respectively. For each data set, flg22-induced luciferase activity was calculated relative to the untreated sample and was normalized by the corresponding GUS activities in flg22 and untreated sample (pFRK1-Luc activity (+flg22/−flg22)). AvrPto was used as a positive control for pFRK1-Luc activity suppression. Four independent biological experiments were carried out per effector. Within each experiment three technical replicates were performed. Pooled data are presented as mean ± SEM. Differences in luciferase/GUS activity between control and effector gene-expressing protoplasts were determined using one-way ANOVA followed by Dunnett's multiple comparison test. An asterisk marks data sets with a p-value<0.05. Five PiRXLR effectors (SFI1 and SFI5-8) reduced pFRK1-Luc activation by flg22 with an efficiency comparable to the bacterial effector AvrPto (+flg22/−flg22≅1). Among PiRXLR effectors with a reported avirulence function in potato, only AVRblb2 (SFI8) [44] was able to suppress flg22-induced pFRK1-Luc activity. SFI8 is a representative member of a large family of AVRblb2-related proteins but it bears a Phe residue at position 69 in its sequence and, therefore, is predicted not to be recognized by Rpi-blb2 [44]. Thus, we extended our analysis to three more AVRblb2 family members with either an Ala (PITG_20300 and PITG_04090) or Ile (PITG_04085) at position 69 and crucial for Rpi-blb2-mediated HR (Table S2). Both predicted Rpi-blb2-recognized and -unrecognized isoforms of AVRblb2 equally suppressed reporter gene activation (Figure S3A). Other PiRXLR effectors identified as avirulence proteins such as AVR1 [28], AVR3a [34], AVR4 [54] and AVRblb1/IPI-O1 or IPI-O4 [55], [56] did not interfere with early flg22-induced responses in our assay (Figure 1: S. lycopersicum). In the case of AVR3a, both R3a-recognized AVR3aKI and R3a-unrecognized AVR3aEM had no effect on flg22-induced pFRK1-Luc activity (Figure 1: S.lycopersicum).
Using quantitative real-time PCR (qRT-PCR) we monitored the expression levels of the eight PiRXLR effector genes that suppressed pFRK1-Luc activation in tomato protoplasts at different stages of potato infection, relative to their expression in sporangia. Previous expression analyses of P. infestans RXLR effector genes showed that, when detected by either qRT-PCR [24] or in microarray experiments [28], [57], they are up-regulated in the first 48–72 hours of infection, i.e. during biotrophy. Transcripts of SFI1-8 accumulated during the first 48 hours post-inoculation (Figure S4), consistent with a potential role in effector-triggered susceptibility.
We extended our analyses to determine whether PiRXLR effectors that suppress pFRK1-Luc activity in the host tomato are able to also suppress such responses in the non-host plant Arabidopsis.
The pFRK1-Luc reporter gene assay, which turned out to be more sensitive in Arabidopsis than in tomato, showed that four effectors (SFI1, SFI2, SFI5 and SFI8/AVRblb2) were also able to attenuate activation in Arabidopsis (p-value<0.05 - Figure 1: A. thaliana). As observed in tomato, each tested AVRblb2 isoform suppressed reporter gene activation by flg22 in Arabidopsis protoplasts (Figure S3B), whereas AVR3a had no effect (Figure 1: A. thaliana). We found a further four effectors (PITG_00821, PITG_05750, PITG_16737 and AVRblb1/PITG_21388) that attenuated the flg22-dependent pFRK1-Luc activation only in Arabidopsis (p-value<0.05 - Figure 1: A. thaliana). Like in tomato, transient expression of PiRXLR effectors in Arabidopsis protoplasts did not cause significant cell death (Figure S5). One effector, PITG_18670, significantly induced a stronger flg22-dependent pFRK1-Luc activity than did the GFP control (p-value<0.05 – Figure 1: A. thaliana), but did not do so in the host plant tomato (Figure 1: S. lycopersicum). This effector was not pursued further in this work.
The observation that 4 PiRXLR effectors suppress flg22-mediated pFRK1-Luc induction in the non-host plant Arabidopsis, but not in the host plant tomato, was unexpected. This prompted us to test whether all 8 PiRXLR effectors that suppress pFRK1-Luc induction in Arabidopsis also inhibit the endogenous expression of early MAMP-regulated genes. First, we measured the level of endogenous FRK1 in Arabidopsis following flg22 treatment. This experiment confirmed the data obtained in the reporter gene assay with 3 PiRXLR effectors (SFI1, SFI2 and SFI8/AVRblb2) attenuating the up-regulation of FRK1 expression by flg22 (Figure 2A). In contrast, SFI5, as well as PITG_00821, PITG_05750, PITG_16737 and AVRblb1/PITG_21388, failed to suppress flg22-induced FRK1 expression (Figure 2A). We extended our analysis to an additional MAMP-induced gene, WRKY DNA-BINDING PROTEIN 17 (WRKY17), and observed that its up-regulation was also notably diminished by SFI1, SFI2 and SFI8/AVRblb2 (Figure 2B), whereas SFI5, PITG_00821, PITG_05750, PITG_16737 and AVRblb1/PITG_21388 again had no effect. The expression of the housekeeping gene ELONGATION FACTOR 1A (EF1α) was generally not altered. Only with SFI2 did we observe a 2–3 fold decrease of the EF1α transcript level, possibly as a consequence of reduced cellular fitness due to effector expression (Figure 2C). Indeed, the expression of all genes tested was barely detectable in the presence of this effector.
Fig. 2. Transcriptional profiling of MAMP-inducible genes in A. thaliana protoplasts transfected with SFI effector constructs. 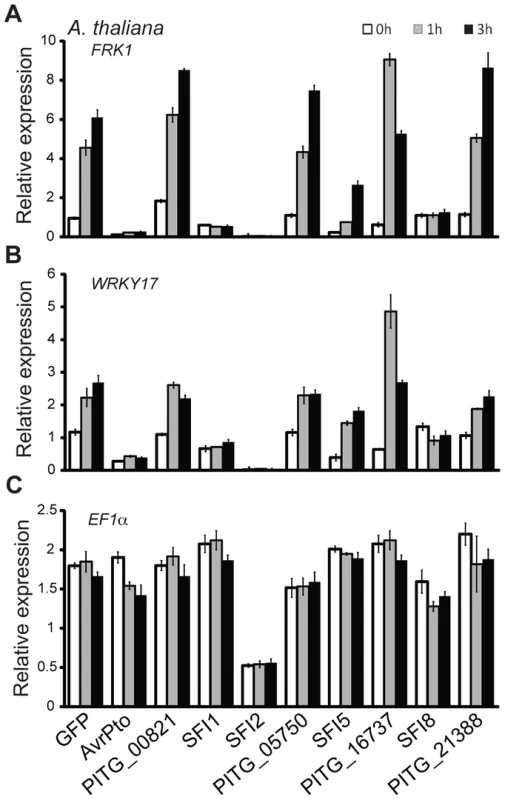
(A–C) Relative gene expression for the flg22-inducible genes FRK1 and WRKY17 (A, B) and the housekeeping gene EF1α (C) was assessed by quantitative real-time polymerase chain reaction (qRT-PCR) 0, 1 and 3 h after protoplasts were exposed to flg22. Transcript levels of the analyzed genes were normalized to the levels of the Actin transcript. GFP was used as a negative and AvrPto as a positive control for suppression of gene expression. One representative independent experiment out of four is plotted. Data is presented as mean ± SEM from three technical replicates. Together, our initial results revealed a set of 8 PiRXLR effectors that are candidate suppressors of early flg22-mediated MTI signaling in tomato, and assigned a novel function to the previously described AVRblb2 effector family. Moreover, our data predict that 3 of these PiRXLR effectors target processes contributing to MTI that are conserved in Arabidopsis and tomato. We proceeded to study all 8 effectors that suppress flg22-inducible reporter gene activation in tomato in more detail.
PiRXLR effectors suppressing flg22-inducible reporter gene activation display similar sub-cellular localizations in tomato and Arabidopsis protoplasts and in N. benthamiana leaves
From the initial screen for MTI signaling suppression we hypothesized that the function of 3 PiRXLR effectors (SFI1, SFI2 and SFI8/AVRblb2) may be conserved in both tomato and Arabidopsis while 5 effectors (SFI3, SFI4, SFI5, SFI6 and SFI7) may function specifically in tomato. We expected that the sub-cellular distribution of PiRXLR effectors might provide additional important information about their function in the cell. Therefore, these PiRXLR effectors, N-terminally fused to GFP, were transiently expressed in tomato (all SFI effectors) and Arabidopsis (only SFI1, SFI2 and SFI8/AVRblb2) protoplasts, and in N. benthamiana leaves for comparison, and visualized by confocal microscopy (Figure 3). We performed immunoblot analysis to confirm protein expression and stability of intact GFP-fusion proteins (Figure S6), and verified that GFP-tagged PiRXLR effectors were still functional and effectively suppressed pFRK1-Luc activity in protoplasts (Figure S7). Most of the GFP-tagged PiRXLR effectors were as active as the un-tagged proteins. Notably, GFP-SFI8/AVRblb2 functioned only weakly or not at all in Arabidopsis, but retained its function in tomato (Figure S7). SFI8/AVRblb2, C-terminally fused to GFP (SFI8-GFP) was also unable to suppress pFRK1-Luc activity in Arabidopsis protoplasts (Figure S8).
Fig. 3. Sub-cellular localization of N-terminally GFP-tagged SFI effectors in S. lycopersicum and A. thaliana protoplasts and in N. benthamiana leaves. 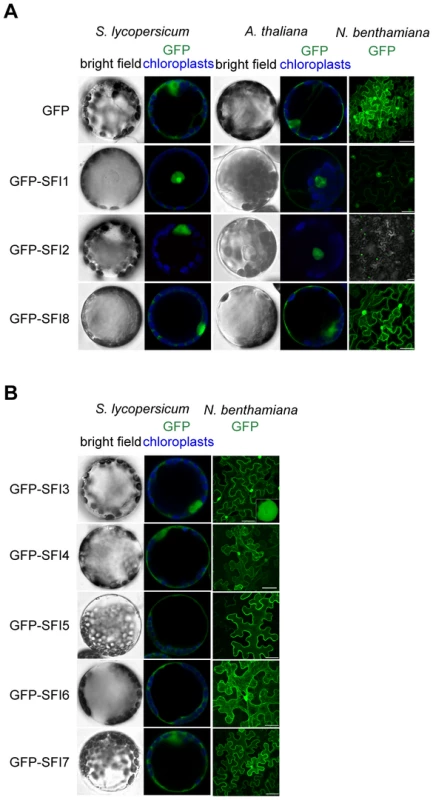
(A, B) Mesophyll protoplasts and N. benthamiana leaves were monitored using confocal microscopy 12 h and 48 h after transfection with a p35S-GFP-effector construct, respectively. Representative optical sections of bright field and merged GFP (in green) and chloroplast (in blue) fluorescence images are shown for protoplasts as indicated. The sub-cellular localizations of the 3 PiRXLR effectors (SFI1, SFI2 and SFI8/AVRblb2) affecting pFRK1-Luc/MAMP gene activation in both tomato and Arabidopsis are similar in each plant species (Figure 3A). GFP-SFI8/AVRblb2 showed nuclear-cytoplasmic localization whereas GFP-SFI1 and GFP-SFI2 localized predominantly in the nucleus, and were also apparent in the nucleolus (Figure 3A). In the case of GFP-SFI1, additional fluorescence signal was observed in the cytoplasm (and possibly at the plasma membrane [PM]) (Figure 3A). The 5 PiRXLR effectors (GFP-SFI3, -SFI4, -SFI5, -SFI6 and -SFI7) with a tomato-specific effect showed different subcellular localizations. GFP-SFI3 was enriched in the nucleus/nucleolus, GFP-SFI4 showed nuclear-cytoplasmic localization, and GFP-SFI5, -SFI6 and -SFI7 showed differing degrees of cytoplasmic localization and association with the PM (Figure 3B), with GFP-SFI5 almost exclusively localized to the PM.
Additional sub-cellular localization studies performed upon Agrobacterium-mediated expression in N. benthamiana leaves confirmed the results obtained in protoplasts, suggesting that protoplasts are accurate in reflecting sub-cellular localizations of these effectors in planta. Confocal microscopy revealed distinct sub-nuclear localization patterns for the 3 PiRXLR effectors (GFP-SFI1-3) that were predominant in this compartment. GFP-SFI1 appears to localize in the nucleolus, GFP-SFI3 forms a ring around the nucleolus, whereas GFP-SFI2 showed a range of sub-nuclear localizations (Figure S9). The obvious differences in sub-cellular localization between effectors imply that different steps and/or pathways may be targeted by individual effectors that have in common the suppression of flg22-triggered pFRK1-Luc activity.
SFI5, SFI6 and SFI7 suppress flg22-induced post-translational MAP kinase activation in tomato but not in Arabidopsis
We performed an epistatic analysis to find out which step of the flg22-triggered signaling pathway in tomato or Arabidopsis is affected by the PiRXLR effectors that suppressed pFRK1-Luc/MAMP responsive gene activation. We conducted immunoblot assays using the p44/42 antibody, raised against phosphorylated MAP kinases, to assess the impact of our effectors on the activation by flg22 of endogenous SlMPK1/3 and AtMPK3/6 in tomato and Arabidopsis protoplasts, respectively. AvrPto was used as a positive control, as it is known to block MTI signaling upstream of the MAP kinase cascade at the FLS2/BAK1 receptor complex [23], [24], [48].
In tomato, 3 effectors (SFI5-SFI7) consistently suppressed flg22-dependent post-translational MAP kinase activation (Figure 4A). We confirmed this result by performing transient expression of HA-tagged SlMPK1 and SlMPK3 in protoplasts followed by immunoprecipitation and in vitro MAP kinase assay (Figure 4B). In contrast, none of the 8 SFI effectors attenuated flg22-dependent post-translational MAP kinase activation in Arabidopsis (Figure 4C). This suggests that the effectors (SFI1, SFI2 and SFI8/AVRblb2) that were shown to attenuate flg22-induced gene activation in both tomato and Arabidopsis are most likely doing so downstream of MAP kinase activation. In the case of SFI5, the demonstration that it attenuates MAP kinase activation only in tomato (Figure 4A, 4C) is consistent with the observation that, although this effector suppressed pFRK1-Luc activation in Arabidopsis, it failed to suppress flg22-mediated up-regulation of endogenous FRK1 in that plant.
Fig. 4. MAP kinase activation upon flg22 treatment in protoplasts expressing SFI effector genes. 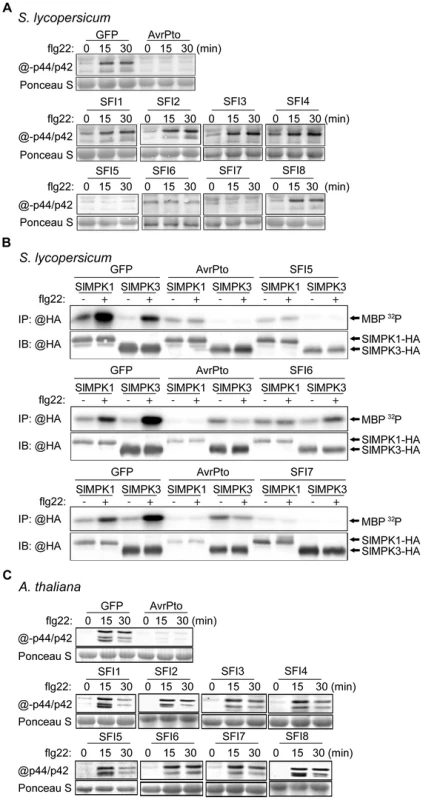
(A, C) Immunoblotting of phosphorylated MAP kinase in p35S-effector-transfected S. lycopersicum (A) and A. thaliana (C) protoplast samples collected 0, 15 and 30 min after flg22 treatment. Antibody raised against activated MAP kinase p44/p42 was used for detection. The experiments are representative of at least two repeats. Ponceau S staining served as a loading control. (B) MAP kinase in vitro kinase assay in S. lycopersicum protoplasts. GFP, AvrPto, SFI5, SFI6 or SFI7 were co-expressed with hemagglutinin (HA)-tagged tomato MAP kinase SlMPK1 or SlMPK3. HA-tagged MAP kinase were immunoprecipitated with anti-HA antibody for an in vitro kinase assay with [γ-32P] ATP and myelin basic protein (MBP) as phosphorylation substrate. The lower panel presents an immunoblot with anti-HA antibody showing the expression of HA-tagged proteins. The upper panel shows an autoradiography visualizing MBP phosphorylation (MBP 32P) in the presence of immunoprecipitated MAP kinase. The experiment was repeated twice with similar results. SFI5 and SFI6 specifically suppress flg22-induced MAP kinase signaling, whereas SFI7 also partially attenuates INF1-triggered cell death
To further elucidate the molecular mechanism(s) underlying the mode of action of SFI5-SFI7 in suppressing flg22-induced post-translational MAP kinase activation in tomato, we performed gain-of-function experiments using components that activate the MAP kinases SlMPK1 and SlMPK3 in the absence of flg22 signal. The ectopic expression of known key players of MAMP-signaling pathways, such as MAPK kinases and MAPKK kinases [48], [58] have helped to elucidate the steps at which bacterial effectors such as AvrPto interfere with MTI in Arabidopsis [48], [59].
In tomato and other solanaceous plants, MAP kinase signaling cascades are best studied in the context of programmed cell death (PCD) associated with effector-triggered immunity [51], [60], [61]. In N. benthamiana, PCD triggered by perception of the P. infestans MAMP INF1 requires NbMKK1 and its interaction with SIPK (salicylic acid-induced protein kinase; an ortholog of SlMPK1) [62]. The role of MAPKK kinases in tomato immunity is only documented for SlMAP3Kα and SlMAP3Kε [60], [61] and the best characterized MAPK kinases are SlMEK1 and SlMEK2 [60]. Whether these kinases contribute to flg22-triggered signaling in tomato is unknown. As shown in Figure S10, transient expression in tomato protoplasts of a constitutively active SlMEK2 (SlMEK2-DD), or the kinase domain of SlMAP3Kα (SlMAP3Kα-KD), led to post-translational activation of SlMPK1 and SlMPK3 in the absence of flg22. The constitutively active SlMEK1 (SlMEK1-DD) and kinase domain of SlMAP3Kε (SlMAP3Kε-KD) did not activate SlMPK1 and SlMPK3. The expression of the constitutively active SlMEK2 (SlMEK2-DD) and the kinase domain of SlMAP3Kα (SlMAP3Kα-KD) overrode the suppression of flg22-dependent activation of SlMPK1 and SlMPK3 by SFI5-SFI7 (Figure 5A, 5B). These results indicate that the three effectors suppress the signaling cascade very early; either upstream of MAPKK kinase activation, or specifically at the MAPK - and/or MAPKK kinase(s) involved in flg22 signaling. This is consistent with association of these effectors with the plant plasma membrane, where they may interfere with the earliest components of MAMP perception or signal transduction.
Fig. 5. Epistatic analysis of MAP kinase activation upon flg22 treatment in S. lycopersicum protoplasts expressing SFI effector genes. 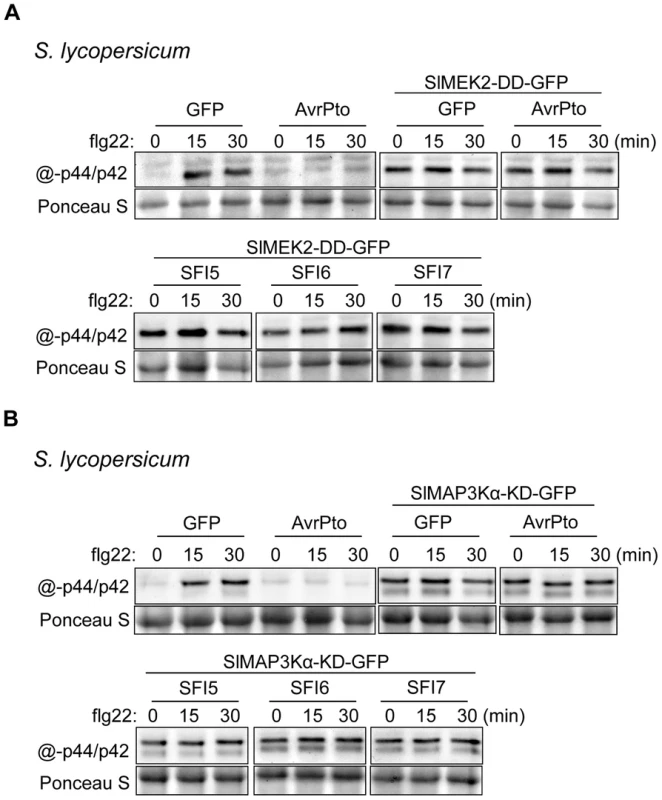
Immunoblotting of phosphorylated MAP kinase in p35S-effector- and p35S-SlMEK2-DD-GFP- (A) and in p35S-effector- and p35S-SlMAP3Kα-KD-GFP- (B) co-transfected S. lycopersicum protoplast samples collected 0, 15 and 30 min after flg22 treatment. Antibody raised against activated MAP kinase p44/p42 was used for detection. The experiments are representative of at least two repeats. Ponceau S staining served as a loading control. Since in N. benthamiana PCD triggered by perception of the MAMP INF1 [62], or perception of Cladosporium fulvum effectors Avr4/9 by tomato Cf-4/9 receptors [60], [61], involves MAP kinase cascades, we tested whether SFI5-SFI7 were able to suppress either PCD event. In contrast to AVR3a, which is known to suppress PCD triggered by INF1 or by co-expression of Cf-4/Avr4 ([42]); Figure 6a, 6b – p-value<0.01), GFP-SFI5 and GFP-SFI6 did not attenuate PCD triggered by either recognition event (Figure 6A, 6B). However, whereas GFP-SFI7 also failed to suppress Cf-4/Avr4-mediated PCD (Figure 6B), this effector significantly attenuated INF1-mediated PCD, albeit less efficiently than AVR3a (Figure 6B – p-value<0.01). Our results indicate that SFI5 and SFI6 display functional specificity by targeting the flg22/FLS2 MAP kinase cascade, but not suppressing MAP kinase cascades leading to Cf-4 - or INF1-mediated PCD, whereas SFI7 has a broader suppressive effect which includes INF1 - but not Cf-4-mediated PCD.
Fig. 6. Effect of GFP-SFI5, GFP-SFI6 and GFP-SFI7 on PCD triggered by INF1 or by co-expression of Cf-4 with Cladosporium fulvum Avr4. 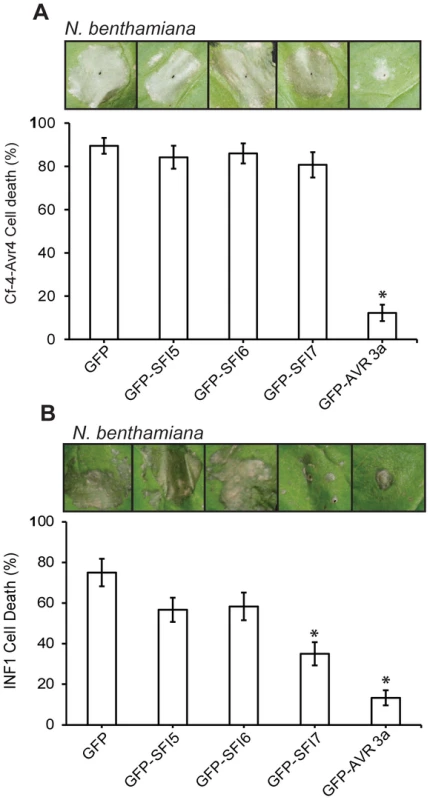
(A) Percentage of inoculation sites showing confluent cell death at 7 days post-infiltration of Agrobacterium strains expressing each GFP-effector fusion protein with a strain expressing Cf-4 and Avr4. (B) Percentage of inoculation sites showing confluent cell death at 7 days post-infiltration of Agrobacterium strains expressing each GFP-effector fusion protein with a strain expressing INF1. Results in (A) and (B) represent five biological replicates, each involving 18 inoculation sites. Error bars represent SEM. * represents statistical significance (p<0.01) using one-way ANOVA. PiRXLR effectors suppressing early MTI signaling contribute to P. infestans virulence
The 8 PiRXLR effectors suppressing early MTI signaling in tomato are assumed to contribute significantly to the virulence of P. infestans. N. benthamiana was further used to explore the role of the 8 selected PiRXLR effectors in host colonization. Agrobacterium tumefaciens strains containing the PiRXLR effector construct were infiltrated into leaves of 2–3 week-old N. benthamiana plants. Leaves were challenged with P. infestans 1 day after agro-infiltration and lesion size (Figure 7A), as well as disease symptoms (Figure 7B), were recorded after an additional 7 days. Except SFI2, whose overexpression in N. benthamiana leaves caused cell death that interfered with the pathogen assay, we found that the remaining 7 PiRXLR effectors enhanced colonization of N. benthamiana by P. infestans (Figure 7A, 7B). Compared to the empty vector control, the expression of the PiRXLR effectors caused a two - to five-fold increase of the lesion size (p<0.001) due to enhanced P. infestans colonisation. The strongest effect was observed when expressing GFP-SFI1. Interestingly, this is one of the effectors that localizes predominantly to the nucleus/nucleolus and suppresses flg22-mediated induction of MTI response genes in both Arabidopsis and tomato, but does not suppress MAP kinase activation, suggesting that it may act downstream of this step. We were thus prompted to look further at the significance of the nuclear/nucleolar localization of SFI1 on its virulence function.
Fig. 7. Effect of transient expression of SFI effectors enhances P. infestans colonization of N. benthamiana. 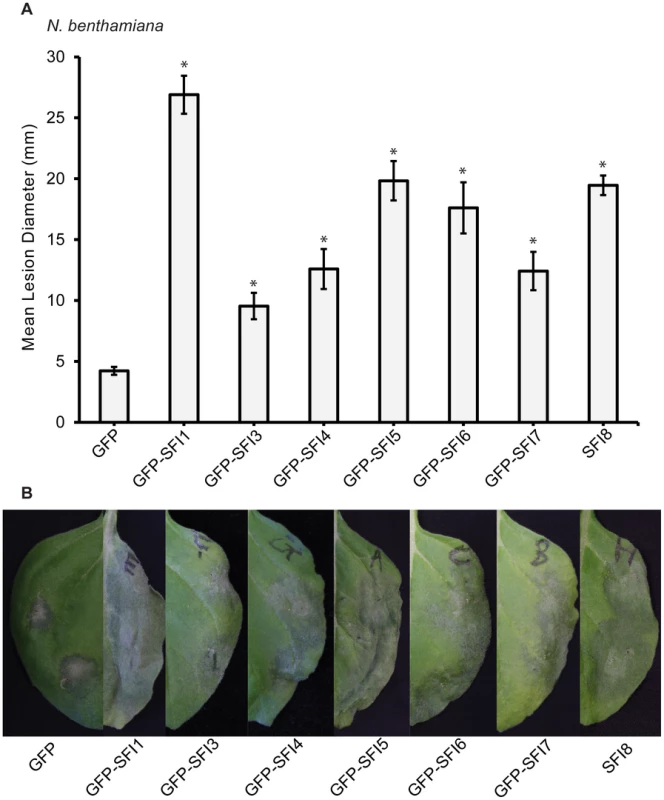
Mean lesion diameter (A) and typical disease development symptoms (B) are shown for P. infestans 7 days post-inoculation over sites on leaves where an effector construct or empty vector was agro-infiltrated 1 day earlier. Each effector was expressed as an N-terminal GFP fusion protein as indicated, except for SFI8. Error bars represent SEM, and significant difference (* = p<0.001) in lesion size compared to empty vector control was determined by one-way ANOVA. Three biological replicates were performed, each using 24 inoculation sites per construct. The nuclear localization of SFI1 is required for suppression of MTI signaling in Arabidopsis, and for enhancing P. infestans colonization of N. benthamiana
We attempted to address the importance of the nuclear localization for the function of SFI1 and hypothesized that mis-targeting of the effector away from the nucleus could impact its virulence function. We generated a construct introducing a myristoylation site at the N-terminus of GFP-SFI1. Transient expression of myr-GFP-SFI1 in planta showed that the myristoylation site was functional in targeting SFI1 to the plasma membrane in Arabidopsis protoplasts (Figure 8A) and N. benthamiana leaves (Figure 8B). Both GFP-SFI1 and myr-GFP-SFI1 fusion proteins were stable and intact in planta (Figure 8F). Strikingly, whereas the flg22-dependent induction of pFRK1-Luc activity was suppressed by GFP-SFI1 in Arabidopsis protoplasts, no such suppression was observed in the presence of myr-GFP-SFI1 (p-value<0.05 - Figure 8C). Notably, myr-GFP-SFI1 lost its ability to enhance P. infestans colonization of N. benthamiana (Figure 8D, 8E), providing strong evidence that suppression of MAMP-induced immune responses by this effector in both host and non-host plants requires its localization to the nucleus/nucleolus.
Fig. 8. Importance of the nuclear localization of SFI1 for suppression of flg22-triggered pFRK1-Luc expression in A. thaliana and for P. infestans colonization in N. benthamiana. 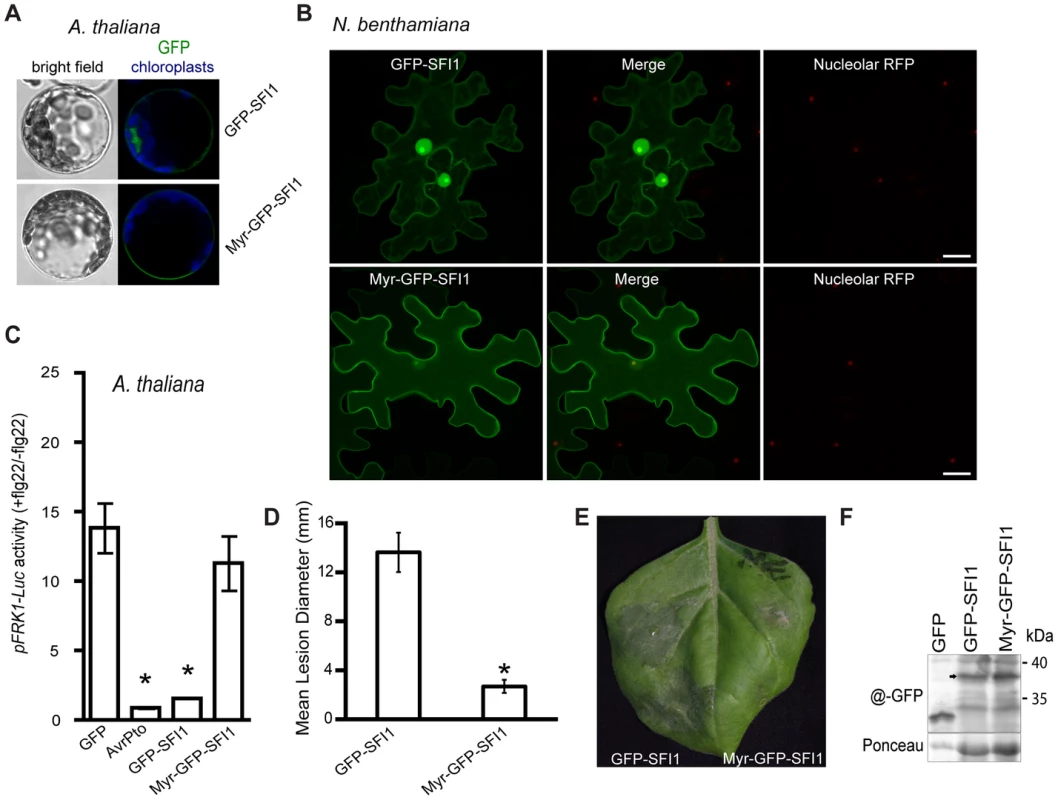
(A) Confocal microscopy of A. thaliana protoplasts expressing GFP-SFI1 or myr-GFP-SFI1 12 h after transfection. Representative optical sections of bright field and merged GFP (in green) and chloroplast (in blue) fluorescence images are shown. (B) Representative confocal microscope images of N. benthamiana cells expressing GFP-SFI1 and myr-GFP-SFI1 (left panels, in green) with the nucleolar marker RFP-fibrillarin (right panels, in red); the merged images are shown in the central panels. (C) Measurement of pFRK1-Luc reporter activity in A. thaliana protoplasts 6 h after flg22 treatment in the presence of GFP (control), AvrPto, GFP-SFI1 or myr-GFP-SFI1. Pooled data from four experiments are presented as mean ± SEM. Significant differences (p<0.05) in luciferase activity (denoted *) were determined using one-way ANOVA followed by Dunnett's multiple comparison test. (D, E) Effect of transient expression of GFP-SFI1 and myr-GFP-SFI1 on P. infestans colonization of N. benthamiana. Mean lesion diameter (D) and typical disease symptoms (E) are shown for P. infestans 7 days post-inoculation over sites on leaves where an effector construct was agro-infiltrated 1 day earlier. * represents statistical significance (p<0.001) using one-way ANOVA. (F) Immunoblot using anti-GFP antibody showing that both GFP-SFI1 and myr-GFP-SFI1 are stable and intact fusion proteins (arrowed) in planta. Discussion
In this study, we used a protoplast-based system to assess the potential for RXLR effectors from P. infestans (PiRXLRs) to manipulate MAMP-triggered early signaling in both a host and non-host plant species. Of 33 PiRXLR effector candidates, selected on the basis of up-regulation during the biotrophic phase of late blight infection, 8 (SFI1-SFI8) were able to suppress flg22-mediated induction of pFRK1-Luc activity in protoplasts of the host plant tomato (summarized in Table 1). Of these, three (SFI5-SFI7) were shown to suppress flg22-dependent MAP kinase activation at - or upstream of - the step of MAPK - and/or MAPKK kinase activation, indicating that they target the earliest stages of MTI signal transduction in tomato (Table 1). As P. infestans does not possess flagellin, the ability of these effectors to attenuate flg22-mediated MAP kinase activation and early defense gene expression indicates that these events are likely stimulated following perception of as yet undefined oomycete MAMPs. We confirmed that 7 of the 8 PiXRLR effectors that suppress early MTI signaling in tomato also enhance colonization by P. infestans in the host plant N. benthamiana (Table 1).
Tab. 1. Summary of PiRXLR effectors with suppressing activity on MTI. 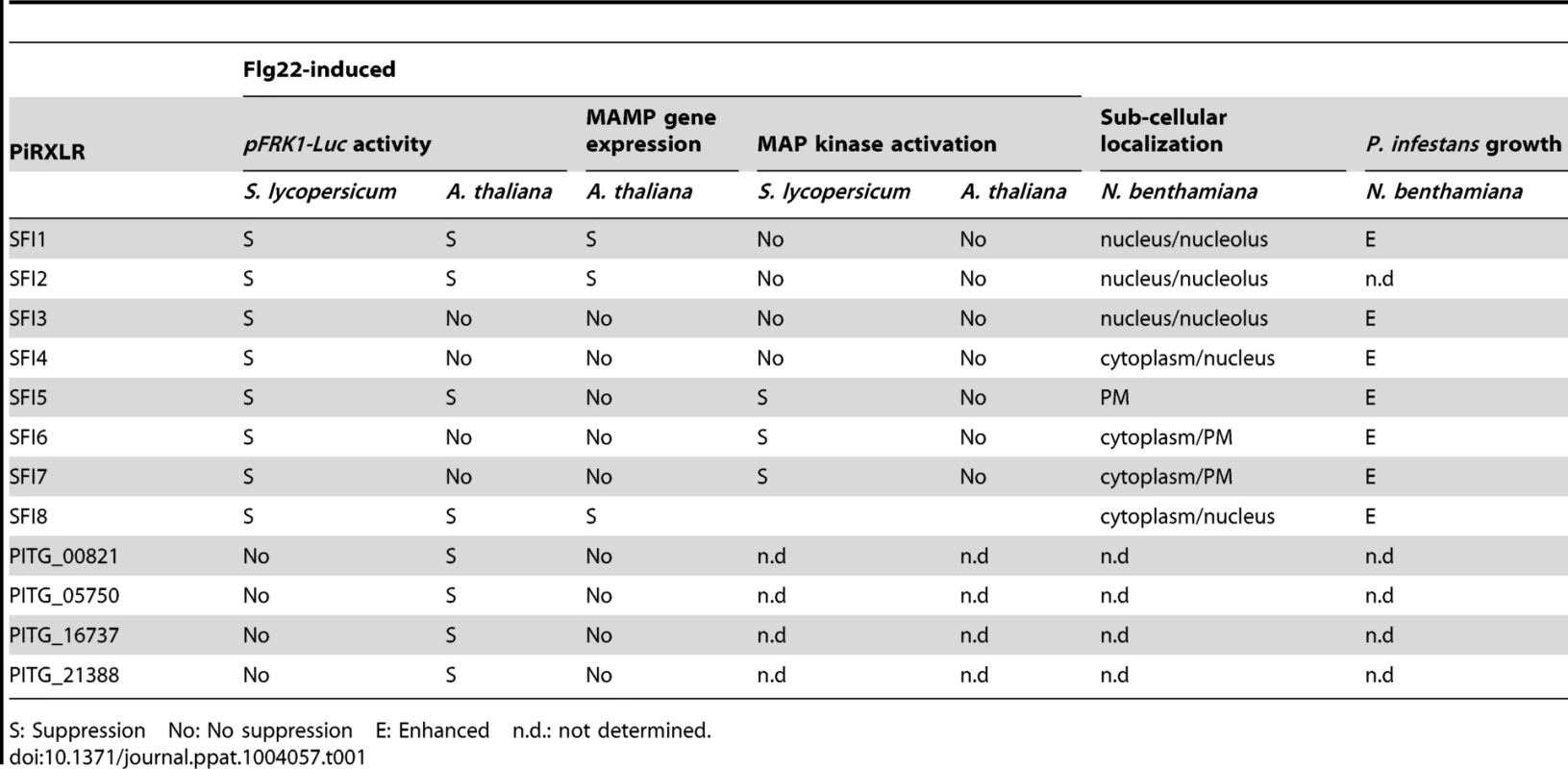
S: Suppression No: No suppression E: Enhanced n.d.: not determined. We found that 3 PiRXLR effectors (SFI1, SFI2 and SFI8/AVRblb2) suppress flg22-mediated induction of pFRK1-Luc activity in protoplasts of both the host plant tomato and the non-host plant Arabidopsis. We confirmed that suppression by all 3 effectors attenuates transcriptional activation of endogenous MAMP-induced marker genes in Arabidopsis (Table 1), indicating that some effectors may function efficiently across diverse (host and non-host) plant species. Interestingly, we found another set of 4 PiRXLR effectors that suppressed pFRK1-Luc activation only in the non-host Arabidopsis. This was a surprise, albeit the assay is potentially less sensitive in the host plant tomato. However, none of these effectors were able to prevent the activation of endogenous (Arabidopsis) MAMP-induced marker genes (Table 1). Therefore, additional experiments are necessary to determine to what extent suppression of flg22-induced post-transcriptional or translational processes may account for the activity of these effectors on the pFRK1-Luc reporter system in this plant.
While 3 PiRXLR effectors (SFI5-SFI7) suppressed MAMP-dependent MAP kinase activation in tomato, no PiRXLR effector had a similar effect in Arabidopsis (Table 1). This is an important finding, consistent with the hypothesis of Schulze-Lefert and Panstruga [63] that non-host resistance in plants (in this case Arabidopsis), which are distantly related to the host of P. infestans, is likely to include failures in effector-triggered susceptibility, due to effectors that are not sufficiently adapted to adequately manipulate plant immunity. Each of these observations will be discussed below.
The large number of RXLR effector gene candidates in Phytophthora genomes complicates their functional analysis by reverse and forward genetics. Thus, the development of a medium/high - throughput system to explore their function in plants is strongly desired. Other large-scale effector functional screens have been conducted recently. A study by Fabro et al. [64] identified 39 out of 64 RXLR effectors from Hyaloperonospora arabidopsidis that enhance P. syringae growth in Arabidopsis Col-0 when delivered via the type III secretion system (T3SS). A majority of these effectors was additionally able to suppress callose deposition in response to bacterial MAMP perception. Thirteen of the H. arabidopsidis RXLR effectors promoted bacterial growth in turnip (Brassica rapa), a member of the Brassicaceae that is a non-host of H. arabidopsidis, indicating that they likely retain their virulence function in this closely related plant. Although the authors did not provide molecular evidence of the influence of these RXLR effectors on MTI in turnip, their results are in line with our conclusions, in that the activity of some RXLR effectors is not restricted to the pathogen's host(s). Nevertheless, a number of H. arabidopsidis RXLR effectors that promoted P. syringae growth in Arabidopsis either failed to do so (44 effectors) in turnip, suggesting that they fail to function in the non-host plant, or reduced P. syringae growth (7 effectors), suggesting that they had activated ETI. Whereas we have identified a set of PiRXLRs that suppress early MTI signaling in tomato but not in Arabidopsis protoplasts, none of the tested PiRXLRs in our study significantly promoted cell death in Arabidopsis protoplasts. In apparent contradiction to the molecular evolutionary concept of non-host resistance [63] we have also identified three PiRXLR effectors that potentially attenuate early flg22-mediated MTI signaling events in Arabidopsis. In order to demonstrate whether failure to suppress MTI has the potential to contribute to non-host resistance to P. infestans in Arabidopsis, it would be necessary to extend the analysis to all PiRXLR effectors and provide an in-depth study of their precise function in both host and non-host plant.
Our primary goal was to identify and ascribe functions to PiRXLR effector proteins that interfere with early plant defense responses. Interestingly, AVRblb2 family members (such as SFI8), but not AVR3a, were among effectors suppressing flg22-induced pFRK1-Luc activity. This apparently contrasts with the results obtained from the screen for suppression of cell death mediated by the MAMP INF1 in N. benthamiana, in which AVR3a but not AVRblb2 family members acted as a suppressor [35], [37], [44]. Similarly, PITG_14736/PexRD8 also suppressed INF1-mediated PCD [44] whilst failing to attenuate flg22-mediated responses in this study, and SFI5/PexRD27 suppressed flg22-mediated MAP kinase activation here, whilst failing to suppress INF1-mediated PCD ([44]; Figure 6). Possible explanations would be that AVR3a and PexRD8 disable components located downstream of the MAMP signal transduction and early transcriptional changes studied here, or that these effectors act specifically on alternative signal transduction events related to INF1-mediated cell death, but not the FLS2/flg22 pathway. The opposite may be true for SFI8/AVRblb2 and SFI5. Moreover, SFI7 suppresses flg22/FLS2-mediated signal transduction and attenuates INF1-mediated PCD, but not Cf-4-mediated PCD, whereas AVR3a attenuates both INF1-mediated and Cf-4-mediated PCD. Evidence is thus emerging of effectors with overlapping functions, at the phenotypic level, that are likely mediated by distinct modes of action at the mechanistic level.
The epistatic analysis of the MAP kinase signaling cascade showed that SFI5-SFI7 presumably act upstream of the activation of the SlMPK1/SIPK and SlMPK3/WIPK MAP kinases in tomato protoplasts following flg22 perception. These effectors potentially function at the FLS2 receptor complex, or upon MAPKKK or MAPKK activity, or upon alternative regulators associated with this signal transduction pathway. As P. infestans does not possess flg22, and is thus unlikely to activate FLS2, the activity of any effectors upon the receptor complex must involve targets that are associated with bacterial and oomycete MAMP detection. Nevertheless, the absence of any suppressive activity of these effectors against CF4-mediated cell death and the modest suppression of INF1-mediated PCD only by SFI7 – two defense pathways that utilize alternative MAPKK kinases - imply specificity in the signal transduction pathways targeted by these effectors. It is important to note that all three effectors, to differing degree, associate with the plasma membrane, consistent with a potential action at the level of signal perception. Mukhtar et al., [65], postulated that an overlapping subset of host proteins, so-called hubs, are targeted by oomycete (H. arabidopsidis) and bacterial (P. syringae) effectors that have arisen independently through convergent evolution. Therefore, future work will focus on identification of host proteins with which SFI5-SFI7 interact to better elucidate the molecular mechanisms underlying the action of these effectors.
An effect of AVRblb2 on early MAMP signaling in solanaceous plant species has not been reported before, but it is has been shown that AVRblb2 affects plant immunity by inhibiting the secretion of C14, an apoplastic papain-like cysteine protease [38]. It is worth noting that in that study, AVRblb2 was exclusively localized at the plasma membrane, whereas in our experiments SFI8/AVRblb2 appeared mainly in the nucleus and cytosol. Yet, as AVRblb2 forms a large family and it is not clear which AVRblb2 isoform was exactly tested for the inhibition of C14 secretion [38], any apparent discrepancies in our results raise the possibility that different members of the AVRblb2 family have distinct or multiple cellular activities. Nevertheless, in our study all tested members of the AVRblb2 family were able to significantly suppress flg22-mediated induction of pFRK1-Luc activity in protoplasts of the host plant tomato.
As the effectors SFI1, SFI2 and SFI8/AVRblb2 interfere with transcriptional up-regulation of MAMP-responsive genes in both host and non-host plants, we presume that they target conserved processes upstream of the earliest transcriptional responses. None of these effectors prevented MAP kinase activation, suggesting that they act downstream of such signal transduction. The nuclear localization of SFI1 and SFI2 in Arabidopsis, tomato and N. benthamiana may indicate that they directly manipulate regulatory processes leading to transcriptional up-regulation. For SFI1 we showed that its mislocalization to the plasma membrane, via addition of a myristoylation signal, prevented both its ability to suppress flg22-mediated MTI gene activation in Arabidopsis and its ability to enhance P. infestans colonization of the host plant N. benthamiana. This strongly implicates the nucleus as the site of effector activity for SFI1. It also indicates the importance of determining subcellular localization of effectors, as mis-targeting them provides a strategy for investigating their virulence function. The fact that SFI1 activity is apparently conserved in the non-host plant Arabidopsis indicates that we may draw on the wealth of genetic resources available in the model plant to further dissect the functions of this effector. Future work will employ additional mis-targeting approaches, for example nuclear export (NES) and nuclear localization (NLS) signals, to better elucidate the potential contributions of SFI1-SFI8 activities, either within or outside the nucleus, to suppress early MTI signaling.
Three of the PiRXLR effectors, SFI5-SFI7, suppressed flg22-mediated post-translational MAP kinase activation in tomato but not in the non-host Arabidopsis. A further two effectors, SFI3 and SFI4, were shown to suppress specifically pFRK1-Luc activation in tomato, although we need to confirm their inhibitory effect on the expression of endogenous MAMP-responsive genes. Nevertheless, each enhanced P. infestans colonization when transiently expressed in N. benthamiana, consistent with a role in MTI suppression. Functional characterization of all these effectors is thus better pursued in host plants within the Solanaceae. The availability of genome sequences for potato [66], tomato [67] and N. benthamiana [68], the genetic tractability of the diploid tomato [67], and the range of functional analyses that can be performed in N. benthamiana [52], considerably broaden opportunities to do this. Moreover, the adaptation of the Arabidopsis protoplast-based system [48], [49], [58] to investigate the earliest stages of MTI in tomato, presented here, further enhances capabilities to study the functions of effectors from pathogens that infect solanaceous hosts. Future work will employ transgenic host and nonhost plants expressing the effectors revealed here, and additional RXLR effectors from P. infestans, to more specifically investigate their precise mechanistic action. Such studies will also reveal those effectors which may act downstream of the earliest signaling events in order to suppress MAMP-triggered immunity.
Ectopic expression in N. benthamiana of 7 of the 8 SFI effectors selected through the protoplast-based screen enhanced plant susceptibility toward P. infestans infection. This result suggests that the suppression of early signaling events triggering basal immunity is an important step toward successful host colonization by this pathogen. P. infestans itself offers the possibility to further study functional aspects of PiRXLR effectors, and gain - and loss-of function experiments may confirm the importance of our candidate effectors for virulence. However, it should be noted that the functional redundancy of the PiRXLR effectors studied here in suppressing early FLS2/flg22 MTI signaling suggests that silencing of these effector genes in P. infestans may not lead to clear virulence phenotypes, as has been shown by deletion studies with type III effectors in P. syringae [69]. Nevertheless, silencing of single PiRXLR effector genes Avr3a [36], or PITG_03192 [43] compromised P. infestans pathogenicity, indicating that (at least some of) the functions of these effectors are not redundant.
In conclusion, the tomato protoplast system provides a new medium/high-throughput tool to identify effectors that modulate the earliest stages of MTI signal transduction. We have identified 8 PiRXLR effectors that suppress early flg22-mediated MTI in tomato. Three of these reveal association with the plant plasma membrane and act at, or upstream of, MAPKK activation specifically related to flg22-mediated MTI signal transduction. Two of these effectors, SFI5 and SFI6, apparently do not act on other MAP kinase-mediated signal transduction events studied in this investigation. In addition, five of the effectors act downstream of the MAP kinase cascade, 3 of which also clearly suppress early flg22-mediated gene induction in Arabidopsis. This demonstrates that the effector complement of P. infestans contains functional redundancy in the context of suppressing early MTI signal transduction and gene activation. It remains to be established why such functional redundancy is necessary, or is selected for, and it is consistent with studies of bacteria such as P. syringae [69] that plant pathogens evolve multiple means of confounding the host immune system.
Materials and Methods
Plant growth conditions
Solanum lycopersicum cv. Moneymaker was kept in a greenhouse under controlled growth conditions: 16 h light at 24°C/8 h dark at 22°C, 40%–45% humidity, ∼200 µE m−2 s−1 light intensity. They were grown on soil containing a 4.6∶4.6∶1 mixture of type P soil, type T soil (Patzer, Germany) and sand. Leaves from 3 to 4 week-old plants were used for experiments.
Arabidopsis thaliana plants of the Col-0 ecotype were cultivated in a phytochamber under stable climate conditions: 8 h light at 22–24°C/16 h dark at 20°C, 40%–60% humidity, ∼120 µE m−2 s−1 light intensity. They were grown on soil composed of a 3.5∶1 mixture of GS/90 (Patzer, Germany) and vermiculite. Leaves from 4 to 5 week-old plants were used for protoplast preparation.
Nicotiana benthamiana was grown as described previously [36].
Phytophthora infestans RXLR effector cloning
Phytophthora infestans putative RXLR effector genes (PiRXLR) were amplified minus the signal peptide from gDNA of the sequenced isolate T30-4 in a two-step nested PCR reaction in order to add flanking attB sites to the RXLR coding sequence. The cloning primers are shown in Table S1 and Table S2. The PCR products were recombined into pDONR201 or pDONR221 vectors (Invitrogen) to generate entry clones, which were further recombined into the vectors p2GW7, p2FGW7 (N-terminal GFP fusion), pB7WGF2 (N-terminal GFP fusion), p2GWF7 (C-terminal GFP fusion) or p2HAGW7 (N-terminal hemagglutinin-tag; derived from p2GW7) (VIB, Ghent University, Belgium) using the Gateway recombination cloning technology (Invitrogen). The myristoylation signal sequence MGCSVSK was added to the amino-termini of the GFP-PiRXLR fusions using PCR with modifying primers and restriction cloning into pENTR1a (Invitrogen) before recombination into p2GW7 or pB2GW7 (VIB, Ghent University, Belgium). The Gateway destination vectors used are designed for transient 35S promoter-driven gene expression in protoplasts or, in the case of pB7WGF2 and pB2GW7, in N. benthamiana plants.
S. lycopersicum MAPK kinase and MAPKK kinase cloning
To generate the constructs used for epistasic analysis of the MAP kinase signaling cascade, four primer combinations: SlMEK1-attB1/SlMEK1-attB2, SlMEK2-attB1/SlMEK2-attB2, SlMAP3Kα-attB1/SlMAP3Kα-attB2 and SlMAP3Kε-attB1/SlMAP3Kε-attB2 (listed in Table S3) were used to amplify by PCR SlMEK1-DD, SlMEK2-DD, SlMAP3Kα-KD and SlMAP3Kε-KD from pER8 plasmid constructs, respectively. Subsequently, Gateway attB linkers were added via PCR using primers attB1-adapter and attB2-adapter. The obtained PCR products were introduced into pDONR201 to generate entry clones using the Gateway recombination cloning technology (Invitrogen). The genes were further recombined into the vector p2GWF7 (C-terminal GFP fusion – VIB, Ghent University, Belgium). The resulting plasmid constructs, p35S-SlMEK1-DD-GFP, p35S-SlMEK2-DD-GFP, p35S-SlMAP3Kα-KD-GFP and p35S-SlMAP3Kε-KD-GFP were used for protoplast transfection as described below.
Plasmid DNA preparation
Plasmid DNA was isolated from E. coli DH5α liquid cultures by column purification using the PureYield Plasmid Midi-prep system (Promega) following the manufacturer's instructions. For selected candidate gene, control genes and reporter gene constructs, higher amount of the corresponding plasmids were purified on a cesium chloride density gradient.
Protoplast preparation and transfection
S. lycopersicum mesophyll protoplasts were prepared as described by Nguyen et al., [52] with slight modifications. The lower epidermis of fully expended leaflets was gently rubbed with grated quartz, rinsed with sterile water and leaf strips were floated on enzyme solution containing 2% cellulose ‘Onozuka’ R10 (Yakult Pharmaceutical Industry), 0.4% pectinase (Sigma) and 0.4 M sucrose in K3 medium. After 30 min vacuum-infiltration and 3 h incubation at 30°C in the dark, the enzyme-protoplast mixture was filtered through a 45–100 µm nylon mesh. Viable protoplasts were collected by sucrose gradient centrifugation and washed in W5 buffer. After recovery on ice for 2 h, protoplasts were harvested by centrifugation and suspended to a density of 6*105 cells/ml in MMG buffer prior polyethylene glycol-mediated transfection. 100 µg plasmid DNA/ml protoplast suspension was used during transfection. Protoplasts samples were then incubated in W1 buffer at 20°C in the dark for 12 to 16 h allowing plasmid gene expression.
The preparation of A. thaliana mesophyll protoplasts was performed according to the protocol from Yoo et al., [70] with minor changes. Briefly, thin leaf stripes were dipped into 1.5% cellulose ‘Onozuka’ R10 – 0.4% macerozyme R10 solution (Yakult Pharmaceutical Industry), vacuum-infiltrated for 30 min and digested for 3 h at 20°C in the dark. After two subsequent washing steps with W5 buffer Arabidopsis protoplasts were suspended in MMG buffer to a concentration of 2*105 cells/ml. Arabidopsis protoplast transfection was performed as for tomato.
Luciferase and β-glucuronidase (GUS) reporter gene assays
Luciferase and GUS reporter gene assays were conducted to screen for immunity-suppressing effector genes. For this, A. thaliana or S. lycopersicum protoplasts were co-transfected with pFRK1-Luc, pUBQ10-GUS and an effector gene construct (or empty p2FGW7 serving as GFP control). For the luciferase assay, luciferin was added to 600 µl transfected protoplast solution to a final concentration of 200 µM. Protoplasts were transferred to an opaque 96-well plate (100 µl per well). For each sample, flg22 was added to 3 wells to a final concentration of 500 nM (+flg22). The remaining 3 replicates were left untreated (−flg22). The luminescence reflecting the luciferase activity was measured at different time-points using a Berthold Mithras LB 940 luminometer. For the GUS assay, 50 µl transfected protoplast solution of each sample was treated with 500 nM flg22 (+flg22) and 50 µl were left untreated (−flg22). Protoplast pellets were collected 3 or 6 h after flg22 elicitation. The cells were lysed in 100 µl CCLR solution (cell culture lysis reagent, Promega). For each sample, 3 technical replicates of 10 µl were incubated with 90 µl MUG substrate (1 mM 4-methyl-umbelliferyl-β-D-glucuronide, 100 mM Tris-HCl pH 8.0, 2 mM MgCl2) for 30 min at 37°C. The reaction was stopped with 900 µl 0.2 M Na2CO3. The fluorescence was monitored in an opaque plate using a MWG 96-well plate reader with λex = 360 nm and λem = 460 nm.
Statistical analysis of reporter gene assay data
Raw data of Luciferase and GUS assays were processed using Microsoft Excel. First the mean value of the +flg22 and the −flg22 triplicates was calculated for each sample in both assays of an experiment. Next, the +flg22/−flg22 ratio was calculated using the values from the 3 or 6 h time-point of the Luciferase assay and divided by the corresponding +flg22/−flg22 ratio of the GUS assay for normalization. Statistical analysis was performed using one-way ANOVA followed by Dunnett's multiple comparison test.
RNA isolation, cDNA synthesis and quantitative real-time PCR (qRT-PCR)
Total RNA from 400 µl A. thaliana protoplasts was extracted with TRI reagent (Ambion) and treated with DNase I (Machery-Nagel) following the suppliers' protocols. Poly A-tailed RNA (1 µg) was converted to cDNA using the RevertAid reverse transcriptase (Fermentas) and oligo-dT primers. qRT-PCR reactions were performed in triplicates with Maxtra SYBR Green Master Mix (Fermentas) and were run on a Biorad iCycler according to the manufacturers' instructions. Relative gene expression was determined with a serial cDNA dilution standard curve. The Actin transcript was used as an internal control in all experiments. Data was processed with the iQ software (Biorad).
qRT-PCR to measure PiRXLR gene expression was carried out on a time-course of potato leaves (cv Desiree) infected with P. infestans isolate 88069. Total RNA from infected leaf discs was extracted with RNeasy Plant mini kit (Qiagen) and treated with DNase I (Qiagen) following the suppliers' protocols. Poly A-tailed RNA (1 µg) was converted to cDNA using the Superscript II reverse transcriptase (Invitrogen) and oligo-dT primers. qRT-PCR reactions were performed in triplicate with Power SYBR Green Master Mix (ABgene) and run on a Biorad Chromo4 cycler according to the manufacturer's instructions. Relative gene expression was determined using the ΔΔCT method, and P. infestans ActA gene was used as an internal control in all experiments, as described in Whisson et al [32]. Data was processed with Opticon monitor software (Biorad). Primers used in qRT-PCR reactions are listed in Table S3.
Detection of phosphorylated MAP kinase and GFP-fusion proteins by immunoblotting
To monitor the activation of MAP kinase, transfected protoplasts were challenged with 500 nM flg22. Pellets from 100 µl protoplast solution were collected 0, 15 and 30 min after treatment and denatured in protein loading buffer. Proteins were loaded onto a 13.5% SDS-polyacrylamid gel and separated by electrophoresis (SDS-PAGE) using the Biorad MiniProtean equipment according to the manufacturer's instructions. PageRuler Prestained protein ladder (Fermentas) was used as a molecular weight marker. Proteins were blotted onto nitrocellulose membranes (Hybond–ECL, Amersham) and stained with 0.1% Ponceau S to visualize equal sample loading. The membranes were blocked for 1 h at room temperature in 5% skimmed milk in TBS-T buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% Tween 20), incubated overnight at 4°C in primary antibody solution (anti-phospho-p44/42 MAPK antibody, dilution 1/1000 in 5% BSA in TBS-T, Cell Signaling Technology) and finally incubated for 1 h at room temperature in secondary antibody solution (alkaline phosphatase-coupled anti-rabbit IgG antibody, dilution 1/3000 in TBS-T, Sigma). The immunoblot was revealed in NBT/BCIP detection solution.
The expression of GFP-tagged PiRXLR effectors was assessed by immunoblotting using polyclonal anti-GFP antibody produced in rabbit or in goat (Acris Antibodies) at a 1/3000 dilution in 5% BSA in TBS-T. For this, protoplast samples were collected 12 (for S. lycopersicum) or 24 h (for A. thaliana) after transfection and SDS-PAGE and immunoblotting were carried out as described above.
Immunoprecipitation and in vitro kinase assay
The MAP kinase in vitro kinase assay was carried out as described by He et al., [48]. Briefly, 1 ml transfected protoplasts were lysed in 1 ml of immunoprecipitation (IP) buffer (150 mM NaCl, 50 mM HEPES pH 7.4, 1 mM EDTA, 1 mM DTT, 0,1% Triton X-100, 1× phosphatase inhibitor cocktail [PhosphoSTOP, Roche Applied Science] and 1× protease inhibitor cocktail [Complete EDTA-free, Roche Applied Science]). HA-tagged SlMPK1 and SlMPK3 kinases [52] were immunoprecipitated from lysates after adding 20 µl anti-H antibody-coupled beads (Roche Applied Science) and incubated for 3 h at 4°C with gentle shaking. After centrifugation at 500 g for 1 min, the immunoprecipitated material was washed with IP buffer followed by a wash with kinase buffer (20 mM Tris-HCl pH 7.5, 20 mM MgCl2, 5 mM EDTA and 1 mM DTT). The kinase reaction was performed by adding 25 µl of kinase buffer (0.25 mg/ml MBP, 100 µM ATP and 5 µCi [γ-32P] ATP) for 30 min at RT. The reaction was stopped with 4× SDS-PAGE loading buffer. The 32P-labeled MBP was separated by SDS/PAGE (15%) gel and visualized by autoradiography.
Cell death and sub-cellular localization studies
To determine the cell death rate after transfection (percentage of dead cells/total number of cells), 100 µl protoplast samples were incubated for 24 h and subsequently stained with 1 µg propidium iodide. Stained protoplasts were counted using a Nikon Eclipse 80i epifluorescence microscope with the following filter: TRITC EX 540/40, DM 565, BA 605/55. For sub-cellular localization studies protoplasts were monitored 12 h post-transfection and N. benthamiana leaves at 2 days post-infiltration. Imaging was performed using Leica TCS SP2 AOBS confocal microscopes with HCX PL APO lbd.BL 63×1.20 W, L 40×0.8 and L 20×0.5 water immersion objectives. Samples were excited by an argon laser and fluorescence emission was detected at 496–552 nm for GFP and 620–726 nm for chloroplasts. The pinhole was set to 1.5 airy units for protoplasts and 1 airy unit for leaf cells. Single optical section images were acquired from protoplasts and z-stacks were collected from leaf cells and projected and processed using the Leica LCS software and Adobe Photoshop CS3.
Agrobacterium-mediated effector expression
A. tumefaciens transformed with pB7WG2 or pB7WGF2 vector constructs were grown overnight, pelleted, re-suspended in infiltration buffer (10 mM MES pH 5.6, 10 mM MgCl2 and 200 µM acetosyringone) and adjusted to the required OD600 before infiltration into N. benthamiana leaves.
Cell death suppression
A. tumefaciens cultures were grown as above and subsequently mixed together to a final optical density at 600 nm (OD600) of 0.3 for each construct except Cf4, which was used at 0.6, N. benthamiana plants were infiltrated using a 1 ml needleless syringe through the lower leaf surface. Three leaves on six plants were used for each biological replicate. Cell death was scored at 7 d post-infiltration (dpi). An individual inoculation was counted as positive if >50% of the inoculated area developed clear cell death. The mean percentage of total inoculations per plant developing cell death of combined data from at least two biological replicates was calculated. One-way ANOVA was performed to identify statistically significant differences.
P. infestans infection assay
A. tumefaciens Transient Assays (ATTA) in combination with P. infestans infection were carried out as described [36]. Briefly, Agrobacterium cultures were re-suspended in infiltration buffer at a final concentration of OD600 = 0.1 and infiltrated in N. benthamiana with the bacteria harboring the vector control on one side of the leaf midrib and the bacteria harboring the PiRXLR effector constructs to be tested on the other. P. infestans strain 88069 cultured on Rye Agar at 19°C for 2 weeks was used for plant infection. Plates were flooded with 5 ml cold H2O and scraped with a glass rod to release sporangia. The resulting solution was collected and sporangia numbers were counted using a haemocytometer and adjusted to 30,000 sporangia/ml. After 1 day, each agro-infiltration site was inoculated with 10 µl droplets of sporangia. Three leaves per plant for 4–6 intact plants were used for each biological replicate. Lesions were measured and photographed at 7 days post-infection and data of at least two biological replicates were combined. Statistically significant differences in lesion size were identified by one-way ANOVA with pairwise comparisons performed using the Holm-Sidak method.
Supporting Information
Zdroje
1. AkiraS, UematsuS, TakeuchiO (2006) Pathogen recognition and innate immunity. Cell 124 : 783–801.
2. ChisholmST, CoakerG, DayB, StaskawiczBJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124 : 803–814.
3. BollerT, FelixG (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60 : 379–406.
4. JonesJD, DanglJL (2006) The plant immune system. Nature 444 : 323–329.
5. NurnbergerT, BrunnerF, KemmerlingB, PiaterL (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198 : 249–266.
6. ZipfelC (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20 : 10–16.
7. Gomez-GomezL, BollerT (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5 : 1003–1011.
8. ChinchillaD, BauerZ, RegenassM, BollerT, FelixG (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 18 : 465–476.
9. FelixG, DuranJD, VolkoS, BollerT (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 : 265–276.
10. HannDR, RathjenJP (2007) Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. Plant J 49 : 607–618.
11. PeckSC, NuhseTS, HessD, IglesiasA, MeinsF, et al. (2001) Directed proteomics identifies a plant-specific protein rapidly phosphorylated in response to bacterial and fungal elicitors. Plant Cell 13 : 1467–1475.
12. TaguchiF, ShimizuR, InagakiY, ToyodaK, ShiraishiT, et al. (2003) Post-translational modification of flagellin determines the specificity of HR induction. Plant Cell Physiol 44 : 342–349.
13. GustAA, BiswasR, LenzHD, RauhutT, RanfS, et al. (2007) Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J Biol Chem 282 : 32338–32348.
14. ZhangWG, FraitureM, KolbD, LoffelhardtB, DesakiY, et al. (2013) Arabidopsis RECEPTOR-LIKE PROTEIN30 and Receptor-Like Kinase SUPPRESSOR OF BIR1-1/EVERSHED Mediate Innate Immunity to Necrotrophic Fungi. Plant Cell 25 : 4227–4241.
15. ZipfelC, KunzeG, ChinchillaD, CaniardA, JonesJD, et al. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125 : 749–760.
16. BollerT, HeSY (2009) Innate Immunity in Plants: An Arms Race Between Pattern Recognition Receptors in Plants and Effectors in Microbial Pathogens. Science 324 : 742–744.
17. DeslandesL, RivasS (2012) Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci 17 : 644–655.
18. FengF, ZhouJM (2012) Plant-bacterial pathogen interactions mediated by type III effectors. Curr Opin Plant Biol 15 : 469–476.
19. GohreV, RobatzekS (2008) Breaking the barriers: microbial effector molecules subvert plant immunity. Annu Rev Phytopathol 46 : 189–215.
20. ZhangJ, ShaoF, CuiH, ChenLJ, LiHT, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-Induced immunity in plants. Cell Host Microbe 1 : 175–185.
21. WangYJ, LiJF, HouSG, WangXW, LiYA, et al. (2010) A Pseudomonas syringae ADP-Ribosyltransferase Inhibits Arabidopsis Mitogen-Activated Protein Kinase Kinases. Plant Cell 22 : 2033–2044.
22. GohreV, SpallekT, HawekerH, MersmannS, MentzelT, et al. (2008) Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol 18 : 1824–1832.
23. ShanL, HeP, LiJ, HeeseA, PeckSC, et al. (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4 : 17–27.
24. XiangT, ZongN, ZouY, WuY, ZhangJ, et al. (2008) Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr Biol 18 : 74–80.
25. CanonneJ, MarinoD, JauneauA, PouzetC, BriereC, et al. (2011) The Xanthomonas Type III Effector XopD Targets the Arabidopsis Transcription Factor MYB30 to Suppress Plant Defense. Plant Cell 23 : 3498–3511.
26. KimJG, TaylorKW, HotsonA, KeeganM, SchmelzEA, et al. (2008) XopD SUMO protease affects host transcription, promotes pathogen growth, and delays symptom development in Xanthomonas-infected tomato leaves. Plant Cell 20 : 1915–1929.
27. JudelsonHS (2007) Genomics of the plant pathogenic oomycete Phytophthora: insights into biology and evolution. Adv Genet 57 : 97–141.
28. HaasBJ, KamounS, ZodyMC, JiangRHY, HandsakerRE, et al. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461 : 393–398.
29. McDowellJM, BaxterL, TripathyS, IshaqueN, BootN, et al. (2010) Signatures of Adaptation to Obligate Biotrophy in the Hyaloperonospora arabidopsidis Genome. Science 330 : 1549–1551.
30. TylerBM, TripathyS, ZhangX, DehalP, JiangRH, et al. (2006) Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science 313 : 1261–1266.
31. HeinI, GilroyEM, ArmstrongMR, BirchPRJ (2009) The zig-zag-zig in oomycete-plant interactions. Mol Plant Pathol 10 : 547–562.
32. WhissonSC, BoevinkPC, MolelekiL, AvrovaAO, MoralesJG, et al. (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450 : 115–118.
33. AllenRL, Bittner-EddyPD, Grenville-BriggsLJ, MeitzJC, RehmanyAP, et al. (2004) Host-parasite coevolutionary conflict between Arabidopsis and downy mildew. Science 306 : 1957–1960.
34. ArmstrongMR, WhissonSC, PritchardL, BosJI, VenterE, et al. (2005) An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc Natl Acad Sci U S A 102 : 7766–7771.
35. BosJI, KannegantiTD, YoungC, CakirC, HuitemaE, et al. (2006) The C-terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a-mediated hypersensitivity and suppress INF1-induced cell death in Nicotiana benthamiana. Plant J 48 : 165–176.
36. BosJIB, ArmstrongMR, GilroyEM, BoevinkPC, HeinI, et al. (2010) Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc Natl Acad Sci U S A 107 : 9909–9914.
37. BosJIB, Chaparro-GarciaA, Quesada-OcampoLM, GardenerBBM, KamounS (2009) Distinct Amino Acids of the Phytophthora infestans Effector AVR3a Condition Activation of R3a Hypersensitivity and Suppression of Cell Death. Mol Plant-Microbe Interact 22 : 269–281.
38. BozkurtTO, SchornackS, WinJ, ShindoT, IlyasM, et al. (2011) Phytophthora infestans effector AVRblb2 prevents secretion of a plant immune protease at the haustorial interface. Proc Natl Acad Sci U S A 108 : 20832–20837.
39. DongSM, YinWX, KongGH, YangXY, QutobD, et al. (2011) Phytophthora sojae Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity. PLoS Pathog 7: e1002353.
40. DouD, KaleSD, WangX, ChenY, WangQ, et al. (2008) Conserved C-terminal motifs required for avirulence and suppression of cell death by Phytophthora sojae effector Avr1b. Plant Cell 20 : 1118–1133.
41. DouD, KaleSD, WangX, JiangRH, BruceNA, et al. (2008) RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery. Plant Cell 20 : 1930–1947.
42. GilroyEM, TaylorRM, HeinI, BoevinkP, SadanandomA, et al. (2011) CMPG1-dependent cell death follows perception of diverse pathogen elicitors at the host plasma membrane and is suppressed by Phytophthora infestans RXLR effector AVR3a. New Phytol 190 : 653–666.
43. McLellanH, BoevinkPC, ArmstrongMR, PritchardL, GomezS, et al. (2013) An RxLR effector from Phytophthora infestans prevents re-localisation of two plant NAC transcription factors from the endoplasmic reticulum to the nucleus. PLoS Pathog 9: e1003670.
44. OhSK, YoungC, LeeM, OlivaR, BozkurtTO, et al. (2009) In Planta Expression Screens of Phytophthora infestans RXLR Effectors Reveal Diverse Phenotypes, Including Activation of the Solanum bulbocastanum Disease Resistance Protein Rpi-blb2. Plant Cell 21 : 2928–2947.
45. RehmanyAP, GordonA, RoseLE, AllenRL, ArmstrongMR, et al. (2005) Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell 17 : 1839–1850.
46. ShanW, CaoM, LeungD, TylerBM (2004) The Avr1b locus of Phytophthora sojae encodes an elicitor and a regulator required for avirulence on soybean plants carrying resistance gene Rps1b. Mol Plant Microbe Interact 17 : 394–403.
47. SohnKH, LeiR, NemriA, JonesJD (2007) The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell 19 : 4077–4090.
48. HeP, ShanL, LinNC, MartinGB, KemmerlingB, et al. (2006) Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 125 : 563–575.
49. LiXY, LinHQ, ZhangWG, ZouY, ZhangJ, et al. (2005) Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc Natl Acad Sci U S A 102 : 12990–12995.
50. RobatzekS, BittelP, ChinchillaD, KochnerP, FelixG, et al. (2007) Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Mol Biol 64 : 539–547.
51. PedleyKF, MartinGB (2004) Identification of MAPKs and their possible MAPK kinase activators involved in the Pto-mediated defense response of tomato. J Biol Chem 279 : 49229–49235.
52. NguyenHP, ChakravarthyS, VelasquezAC, McLaneHL, ZengLR, et al. (2010) Methods to Study PAMP-Triggered Immunity Using Tomato and Nicotiana benthamiana. Mol Plant Microbe Interact 23 : 991–999.
53. ShanLB, TharaVK, MartinGB, ZhouJM, TangXY (2000) The pseudomonas AvrPto protein is differentially recognized by tomato and tobacco and is localized to the plant plasma membrane. Plant Cell 12 : 2323–2337.
54. van PoppelPM, GuoJ, van de VondervoortPJ, JungMW, BirchPR, et al. (2008) The Phytophthora infestans avirulence gene Avr4 encodes an RXLR-dEER effector. Mol Plant Microbe Interact 21 : 1460–1470.
55. ChampouretN, BouwmeesterK, RietmanH, van der LeeT, MaliepaardC, et al. (2009) Phytophthora infestans Isolates Lacking Class I ipiO Variants Are Virulent on Rpi-blb1 Potato. Mol Plant Microbe Interact 22 : 1535–1545.
56. VleeshouwersVG, RietmanH, KrenekP, ChampouretN, YoungC, et al. (2008) Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes. PLoS one 3: e2875.
57. CookeDEL, CanoLM, RaffaeleS, BainRA, CookeLR, et al. (2012) Genome Analyses of an Aggressive and Invasive Lineage of the Irish Potato Famine Pathogen. PLoS Pathog 8: e1002940.
58. AsaiT, TenaG, PlotnikovaJ, WillmannMR, ChiuWL, et al. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 : 977–983.
59. PitzschkeA, SchikoraA, HirtH (2009) MAPK cascade signalling networks in plant defence. Curr Opin Plant Biol 12 : 421–426.
60. del PozoO, PedleyKF, MartinGB (2004) MAPKKK alpha is a positive regulator of cell death associated with both plant immunity and disease. Embo J 23 : 3072–3082.
61. Melech-BonfilS, SessaG (2010) Tomato MAPKKK epsilon is a positive regulator of cell-death signaling networks associated with plant immunity. Plant J 64 : 379–391.
62. TakahashiY, NasirKH, ItoA, KanzakiH, MatsumuraH, et al. (2007) A high-throughput screen of cell-death-inducing factors in Nicotiana benthamiana identifies a novel MAPKK that mediates INF1-induced cell death signaling and non-host resistance to Pseudomonas cichorii. Plant J 49 : 1030–1040.
63. Schulze-LefertP, PanstrugaR (2011) A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci 16 : 117–125.
64. FabroG, SteinbrennerJ, CoatesM, IshaqueN, BaxterL, et al. (2011) Multiple Candidate Effectors from the Oomycete Pathogen Hyaloperonospora arabidopsidis Suppress Host Plant Immunity. PLoS Pathog 7: e1002348.
65. MukhtarMS, CarvunisAR, DrezeM, EppleP, SteinbrennerJ, et al. (2011) Independently Evolved Virulence Effectors Converge onto Hubs in a Plant Immune System Network. Science 333 : 596–601.
66. XuX, PanSK, ChengSF, ZhangB, MuDS, et al. (2011) Genome sequence and analysis of the tuber crop potato. Nature 475 : 189–194.
67. SatoS, TabataS, HirakawaH, AsamizuE, ShirasawaK, et al. (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature 485 : 635–641.
68. BombarelyA, RosliHG, VrebalovJ, MoffettP, MuellerL, et al. (2012) A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol Plant Microbe Interact 25 : 1523–1530.
69. KvitkoBH, ParkDH, VelasquezAC, WeiCF, RussellAB, et al. (2009) Deletions in the Repertoire of Pseudomonas syringae pv. tomato DC3000 Type III Secretion Effector Genes Reveal Functional Overlap among Effectors. PLoS Pathog 5: e1000388.
70. YooSD, ChoYH, SheenJ (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2 : 1565–1572.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial PeptidesČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 ActivationČlánek Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



