-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGranzyme A Produced by γδ T Cells Induces Human Macrophages to Inhibit Growth of an Intracellular Pathogen
Human γ9δ2 T cells potently inhibit pathogenic microbes, including intracellular mycobacteria, but the key inhibitory mechanism(s) involved have not been identified. We report a novel mechanism involving the inhibition of intracellular mycobacteria by soluble granzyme A. γ9δ2 T cells produced soluble factors that could pass through 0.45 µm membranes and inhibit intracellular mycobacteria in human monocytes cultured below transwell inserts. Neutralization of TNF-α in co-cultures of infected monocytes and γ9δ2 T cells prevented inhibition, suggesting that TNF-α was the critical inhibitory factor produced by γ9δ2 T cells. However, only siRNA - mediated knockdown of TNF-α in infected monocytes, but not in γ9δ2 T cells, prevented mycobacterial growth inhibition. Investigations of other soluble factors produced by γ9δ2 T cells identified a highly significant correlation between the levels of granzyme A produced and intracellular mycobacterial growth inhibition. Furthermore, purified granzyme A alone induced inhibition of intracellular mycobacteria, while knockdown of granzyme A in γ9δ2 T cell clones blocked their inhibitory effects. The inhibitory mechanism was independent of autophagy, apoptosis, nitric oxide production, type I interferons, Fas/FasL and perforin. These results demonstrate a novel microbial defense mechanism involving granzyme A-mediated triggering of TNF-α production by monocytes leading to intracellular mycobacterial growth suppression. This pathway may provide a protective mechanism relevant for the development of new vaccines and/or immunotherapies for macrophage-resident chronic microbial infections.
Published in the journal: . PLoS Pathog 9(1): e32767. doi:10.1371/journal.ppat.1003119
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003119Summary
Human γ9δ2 T cells potently inhibit pathogenic microbes, including intracellular mycobacteria, but the key inhibitory mechanism(s) involved have not been identified. We report a novel mechanism involving the inhibition of intracellular mycobacteria by soluble granzyme A. γ9δ2 T cells produced soluble factors that could pass through 0.45 µm membranes and inhibit intracellular mycobacteria in human monocytes cultured below transwell inserts. Neutralization of TNF-α in co-cultures of infected monocytes and γ9δ2 T cells prevented inhibition, suggesting that TNF-α was the critical inhibitory factor produced by γ9δ2 T cells. However, only siRNA - mediated knockdown of TNF-α in infected monocytes, but not in γ9δ2 T cells, prevented mycobacterial growth inhibition. Investigations of other soluble factors produced by γ9δ2 T cells identified a highly significant correlation between the levels of granzyme A produced and intracellular mycobacterial growth inhibition. Furthermore, purified granzyme A alone induced inhibition of intracellular mycobacteria, while knockdown of granzyme A in γ9δ2 T cell clones blocked their inhibitory effects. The inhibitory mechanism was independent of autophagy, apoptosis, nitric oxide production, type I interferons, Fas/FasL and perforin. These results demonstrate a novel microbial defense mechanism involving granzyme A-mediated triggering of TNF-α production by monocytes leading to intracellular mycobacterial growth suppression. This pathway may provide a protective mechanism relevant for the development of new vaccines and/or immunotherapies for macrophage-resident chronic microbial infections.
Introduction
While γ9δ2 T cells comprise only 3% to 5% of total human blood lymphocytes they can expand to high frequencies in vivo and develop effector functions protective against multiple major human pathogens [1], [2]. In addition, γ9δ2 T cells can be stimulated by non-peptidic phosphoantigens, potentially broadening the host capacity for immune recognition of invading pathogens [3]. Furthermore, γ9δ2 T cells can develop memory responses critical for long-term vaccine-induced protective immunity [4]–[6]. Although phosphoantigens (e.g.-isopentenyl pyrophosphate) can induce polyclonal γ9δ2 T cell activation and anti-tumor immunity, we have shown that these same activation conditions do not generate γ9δ2 T cells capable of inhibiting intracellular mycobacterial growth [7]. Thus, the relevant TB antigens inducing protective γ9δ2 T cells so far remain undefined. However, despite the failure of simple phosphoantigens to induce mycobacteria-inhibitory γ9δ2 T cells, effector memory γ9δ2 T cells expanded with live BCG or mycobacterial lysates can display potent effector functions relevant for protective TB immunity including production of Th1 cytokines and cytolytic activity after co-culture with mycobacteria-infected human monocytes [7]–[10]. The specific effector mechanisms most important for γ9δ2 T cell-mediated suppression of intracellular mycobacterial growth in vivo are not known.
In the present work, we have identified a novel granzyme A-dependent, but perforin-independent mechanism utilized by mycobacteria-specific γ9δ2 T cells to inhibit the growth of intracellular mycobacteria. Co-culture of infected macrophages with either mycobacteria-specific γ9δ2 T cells or purified granzyme A resulted in the production of TNF-α by target macrophages and inhibition of intracellular mycobacterial growth without inducing apoptosis. These findings are consistent with recently published data demonstrating that soluble granzyme A can trigger the production of inflammatory cytokines, including TNF-α, by macrophages without inducing apoptosis [11]. However, our results are novel by showing for the first time that the inflammatory responses induced by granzyme A can lead to intracellular anti-microbial activity. Overall, these combined results indicate that granzyme A produced by γ9δ2 T cells can induce infected macrophages to produce TNF-α which by itself and/or in combination with other factors leads to intracellular control of mycobacterial replication. These results may provide an important new target for future vaccine development and/or lead to novel therapeutic strategies for combating chronic macrophage-resident microbial infections.
Materials and Methods
Human samples
PPD positive, HIV negative healthy volunteers were recruited following a Saint Louis University IRB approved protocol and consent process conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was provided by all study participants. Ficoll-Paque (GE Healthcare, Piscataway, NJ) was used to obtain PBMC from leukapheresed samples. Adherent monocytes were used for the generation of mature DC as previously described [12]. γ9δ2T cell lines were generated from primary BCG-expanded γ9δ2 T cells [7]. Antigen expanded γ9δ2 T cells were purified on day 7 with immunomagnetic beads and restimulated with BCG-infected macrophages (1∶1.2 ratio) then expanded by replenishing medium with 5 U/mL rhIL-2 every 3–4 days as needed. This stimulation/expansion protocol was repeated every 2 weeks for continued maintenance of the γ9δ2 T cell lines.
Reagents
IL-2 (Hoffmann-LaRoche Inc., Basel, Switzerland) was used to expand γ9δ2 T cell lines. Strontium chloride (Sigma Aldrich, St. Louis, MO) pre-treatment was used to induce degranulation [13]. Wortmannin and 3-Methyladenine (Sigma) were used to block autophagy [14], [15]. zVAD.fmk (BD Biosciences) was used to inhibit apoptosis [16]. The following neutralizing antibodies were used to block T cell effector functions: anti-FasL (clone NOK-1) and anti-TNF-α (clone MAb1) from BD Bioscience; anti-IFN-γ (clone 25718) from R&D Systems; and anti-Perforin (clone δG9) from Axxora, San Diego, CA. All antibodies used for flow cytometry were from BD Bioscience except anti-Granulysin from eBioscience (San Diego, CA). CFSE was from Molecular Probes (Eugene, OR). Phorbol myristate acetate (PMA, Sigma-Aldrich), ionomycin (Sigma-Aldrich) and Cytofix/Cytoperm kit (BD Biosciences) were used in the intracellular staining protocol. Soluble 4-hydroxy-but-2-enyl pyrophosphate (HMB-PP, Echelon Bioscience, Salt Lake City, UT) was used for stimulation of γ9δ2 T cells in some transwell conditions. Granzyme A was quantified with the Human Granzyme A CBA Flex Set (BD Biosciences), collected on a LSR II flow cytometer and analyzed with FCAP Array software (BD Biosciences). Native human granzyme A was isolated from NK92 cells, quantified by silver stain, shown not to be contaminated with granzyme B or M, and endotoxin depleted as previously described [17]. Briefly, the postnuclear fraction of centrifuged disrupted NK92 cells was spun at 14,500×g for 15 min to obtain the granule pellet. This was extracted 3 times by 0.5% Triton X-100 and each fraction subjected to cation-exchange chromatography. All gradient fractions were individually assayed for protein concentration and esterolytic activity. No contaminating proteins were observed by silver staining of the final native granzyme A preparation. In addition, the final native granzyme A preparation was shown to be free of granzyme B and M by Western blot. Any endotoxin contamination of purified granzyme A was removed using the EndoTrap-Blue kit (Cambrex Bioscience Walkersville Inc, Walkersville, MD). Protein concentration of the endotoxin free granzyme was determined by Coomassie stain. The efficiency of the EndoTrap-Blue kit was verified by applying LPS to the EndoTrap column examining the flow-through for residual endotoxin activity using the Kinetic QCL kit (Cambrex Bioscience Walkersville Inc,). Efficiency of endotoxin depletion was shown to be >98%.
Preparation of and monocyte infection by mycobacteria
BCG was grown in Middlebrook 7H9 media supplemented with 10% albumin, dextrose, catalase (ADC)+0.05% tween. Aliquoted stocks were frozen and the concentration determined after thawing. Thawed aliquots were sonicated to generate single-cell suspensions before dilution and infection of monocytes. Mycobacterium tuberculosis H37Rv (Mtb, from BEI Resources, Manassas, VA) was grown in Middlebrook 7H9 media containing ADC enrichment and 0.1% glycerol. Aliquoted stocks were frozen and the concentration determined after thawing. Thawed aliquots were vortexed for 5 minutes to disrupt clumps before dilution and infection of monocytes in the presence of 200 nM granzyme A. Plastic adherent PBMCs were either used fresh or differentiated for 6 days before infection with a mycobacterial multiplicity of infection (MOI) of 3 as previously described [18]. Following removal of extracellular mycobacteria, infected monocytes were either cultured in medium alone or with γ9δ2 T cells (E∶T 10∶1) or soluble granzyme A at the indicated concentrations.
Assay of γδ T cell-mediated inhibition of intracellular mycobacterial growth
Two densities of adherent monocyte targets (∼1.5×104 and ∼1.2×105/well) were used as previously described [18]. Briefly, BCG - or Mtb-infected (MOI = 3) monocytes were cultured for 3 days with γ9δ2 T cell lines at an effector to target ratio of 10∶1. In assays with lower density of monocytes, medium-rested PBMC (1.5×105/well) were added to support optimal intracellular mycobacterial growth as previously described [18]. Residual intracellular BCG were measured by incorporation of H3-uridine and colony forming unit (CFU) counting on 7H10 Middlebrook agar plates. Percentages of mycobacterial growth inhibition were determined as follows: % inhibition = 100 - [100×(CFU or DPM in the presence of γ9δ2 T cells/CFU or disintegrations per minute (DPM) in the absence of γ9δ2 T cells)]. In transwell experiments, additional BCG-infected macrophages (∼4×104) were seeded in the upper chambers of the transwell inserts (0.4 µm HTS-96 from Corning, Acton, MA or 8 well strips from Nunc, Rochester, NY). γ9δ2 T cells were added at a 10∶1 E∶T ratio either to the top or bottom layers of infected monocytes/macrophages and the bottom layers harvested 3 days later for viable BCG/Mtb quantification.
Construction and use of lentivirus expressing shRNA for knockdown experiments
siRNAs were purchased from Applied Biosystems (Foster City, CA) targeting TNF-α, IFN-γ, perforin, and GAPDH, and cloned into the pSilencer adeno 1.0 CMV shuttle plasmid (Applied Biosystems). To evaluate effectiveness of siRNA-induced knockdown, plasmid DNA was transfected into THP-1 monocytes (ATCC, Manassas, MO) using Nucleofection (Amaxxa, Walkersville, MD) (Fig. S1). Shuttle vectors and adenovirus backbone were co-transfected into 293T cells and viral particles purified using the Adeno-X purification system (Clontech, Mountain View, CA). Viral titers were determined in 293T cells. γ9δ2 T cells or macrophages were infected with siRNA-expressing adenovirus for 4 hours 1 day prior to co-culture. For silencing of Granzyme A, three siRNA sequences for human GzmA (NM_006144.3) were selected with use of computer-assisted programs. Oligonucleotides containing a siRNA sequence, 8 nucleotide spacers, and a polyT terminator sequence were annealed and then cloned into the HapI and XhoI sites of GFP-expressing pLentilox3.7 vector [19]. siRNA was under the control of a U6 promoter. After screening, only one siRNA generating construct (GzmA2-siRNA: GATTGGAATGAATATGGTTTG) effectively silenced GzmA expression in γ9δ2 T cells. 293T cells were transfected with 13 µg siRNA lentiviral DNA [19], 6 µg VSV-G plasmid DNA [19] and 9 µg packaging viral CMV delta 8.9 plasmid [19], for 8 hours using Lipofectamine 2000 (Invitrogen). Viral supernatants were harvested 2–3 days after transfection, filtered and centrifuged at 20,000 rpm for 2 h. Virus pellets were resuspended and viral titers were determined in 293T cells. For transduction of γ9δ2 T cells, T cells were pre-activated with OKT3 (2 µg/ml)-coated plates, mixed with lentiviral supernatant at an MOI of 20 in medium containing 8 µg/ml polybrene (Sigma), and centrifuged at 1100×g for 1 h at room temperature. Fresh medium was added to each well after 16 h and transduction was repeated two days later. Transduction efficiency was analyzed 3–4 days post-transduction (Fig. S5). Transduced cells and control T cells were then used in the mycobacterial growth inhibition assay.
Statistical analysis
Microsoft Excel, GraphPad Prism or StatSoft Statistica were used for analysis. Mann-Whitney U, ANOVA Friedman test with Dunn's multiple comparisons test or Wilcoxon matched-pairs tests were used as appropriate.
Results
Direct inhibition of intracellular mycobacterial growth by mycobacteria-specific γ9δ2 T cells is dependent on soluble factors including TNF-α
Addition of BCG-specific γ9δ2 T cell lines or clones (data not shown) to BCG-infected macrophages resulted in intracellular mycobacterial growth inhibition (Fig. 1A; *p<0.05 by Wilcoxon matched pairs test, n = 5), as previously reported [7]. This inhibition did not require cell-to-cell contact as activated BCG-specific γ9δ2 T cells in the upper transwell chambers could suppress intracellular mycobacterial growth in monocytes plated in the lower chambers. However, stimulation with both BCG-infected macrophages and soluble 4-hydroxy-but-2-enyl pyrophosphate (HMB-PP) in the upper transwell chambers resulted in maximal inhibition of mycobacterial growth in the lower chambers (Fig. 1B), probably due to optimal production of soluble inhibitory factors mediating inhibitory effects. These combined results show that the effector mechanism that inhibits intracellular mycobacteria does not require contact between the γ9δ2 T cells producing the soluble effector molecules and the target infected macrophages, although the triggering of the protective response is contact dependent. Effector molecules produced by γ9δ2 T cells placed above the transwell inserts can pass through the membrane and induce macrophages placed below the membrane to better control intracellular mycobacterial growth. However, the γ9δ2 T cells above the membrane must be stimulated by cell contact with APCs presenting BCG antigens or HMBPP to produce the soluble effector molecules that can pass through the membrane and induce the enhanced control of intracellular mycobacteria in macrophages below the membrane. In any case, it is clear that soluble molecules secreted by activated γ9δ2 T cells can inhibit the survival of intracellular mycobacteria.
Fig. 1. TNF-α is critical for γ9δ2 T cell-mediated inhibition of intracellular mycobacterial growth. 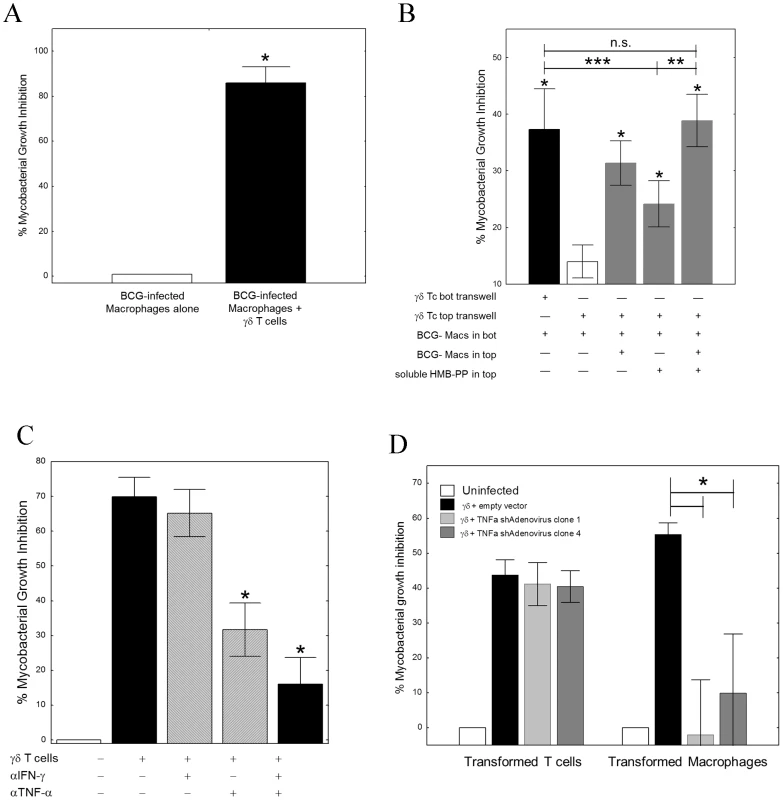
A, γ9δ2 T cell lines significantly suppressed intracellular growth of mycobacteria (n = 5; *p<0.05 by Wilcoxon matched-pairs test). γ9δ2 T cells were co-cultured with BCG-infected macrophages for 3 d. Surviving bacteria were quantified by H3-uridine incorporation and compared with cultures absent of γ9δ2 T cells. B, The effector mechanism responsible for γ9δ2 T cell-mediated mycobacterial growth inhibition involves soluble factors. γ9δ2 T cells were stimulated in the top chambers of semi-permeable transwell membrane (0.4 µm pores) systems with BCG-infected macrophages and/or soluble HMB-PP. The levels of intracellular growth inhibition were assayed in the bottom chambers and compared to the levels of inhibition achieved by direct co-culture of γ9δ2 T cells with the bottom layer of BCG-infected macrophages (*p<0.05, n = 5 by Wilcoxon matched pairs tests compared with unstimulated γ9δ2 T cells separated by transwells; **p<0.02, n = 7 by Wilcoxon matched pairs tests compared with HMB-PP stimulated γδ T cells separated by transwells; ***p<0.003, n = 15 by Wilcoxon matched pairs tests compared with HMB-PP stimulated γδ T cells separated by transwells). C, Neutralization of TNF-α alone prevents γ9δ2 T cell-mediated inhibition while neutralization of IFN-γ alone did not (*p<0.02, n = 7 by Wilcoxon matched pairs tests compared with no antibody control; **p<0.02, n = 6 by Wilcoxon matched pairs tests compared with no antibody control; n.s. – not significantly different). γ9δ2 T cells were co-cultured with BCG-infected macrophages in the presence or absence of the indicated neutralizing antibody and the surviving bacteria quantified by H3-uridine incorporation. D, shRNA-mediated knockdown of TNF-α in macrophages, but not γ9δ2 T cells, eliminates γ9δ2 T cell-mediated mycobacterial growth inhibition. (*p<0.02, n = 7 by Wilcoxon matched pairs tests compared with adenovirus expressing the empty vector). γ9δ2 T cells were co-cultured with BCG-infected macrophages. Prior to co-culture, either the T cells or uninfected macrophages were infected with shRNA expressing Adenovirus. Surviving bacteria were quantified by H3-uridine incorporation. IFN-γ and TNF-α have previously been implicated in the control of mycobacterial infections. Therefore, we next studied the effects of neutralization of these cytokines on γ9δ2 T cell-mediated inhibition of intracellular mycobacteria. Neutralization of IFN-γ alone did not affect mycobacterial growth inhibition. In contrast, neutralization of TNF-α significantly reduced γ9δ2 T cell-mediated inhibitory effects (Fig. 1C, S2; *p<0.03 by Wilcoxon matched pairs test, n = 7). These results demonstrate that TNF-α is critical for suppression of mycobacterial growth, consistent with the observed clinical effects of TNF-α blockade increasing the risks of TB disease reactivation. However, we were surprised to find in siRNA knockdown experiments that only the production of TNF-α by BCG-infected macrophages and not by γ9δ2 T cells was required for inhibition of intracellular mycobacterial growth (Fig. 1D; *p = 0.018 by Wilcoxon matched pairs test for both siRNA sequences, n = 7). Further studies indicated that autophagy, apoptosis, cytolysis, IFN-β and nitric oxide production were not involved in γ9δ2 T cell-mediated inhibition of intracellular mycobacteria (Fig. S3, S4). Our combined results indicate that inhibitory γ9δ2 T cells produce soluble factors that by some novel pathway activate BCG-infected macrophages to produce TNF-α and inhibit intracellular mycobacterial growth.
Classical cell-to-cell contact mechanisms are not involved in γδ T cell inhibitory activity
We considered the possibility that cell-to-cell contact mechanisms may also be involved in mycobacterial suppression. Antibody blockade of the Fas/FasL interaction did not interfere with the ability of γ9δ2 T cells to inhibit intracellular mycobacterial growth (Fig. 2; *p = 0.23 by Wilcoxon matched pairs test, n = 7). Inactivation of the cytolytic granule pathway (known to destroy target cells engaged via the immunological synapse) in γ9δ2 T cells by strontium pre-treatment, completely eliminated the capacity of these cells to suppress intracellular mycobacterial growth (*p<0.05 by Wilcoxon matched pairs test, n = 5). However, a perforin-specific neutralizing antibody previously shown to inhibit cytolysis [9] did not reverse γ9δ2 T cell-mediated mycobacterial growth suppression (p = 0.28 by Wilcoxon matched pairs test, n = 3). Overall, these results suggest that the cytolytic granule cargo secreted by activated γ9δ2 T cells contain soluble factors which stimulate infected macrophages to inhibit intracellular mycobacterial growth even in the absence of perforin.
Fig. 2. Classical effector mechanisms involving direct contact of γ9δ2 T cells with BCG-infected macrophages are not required for mycobacterial suppression. 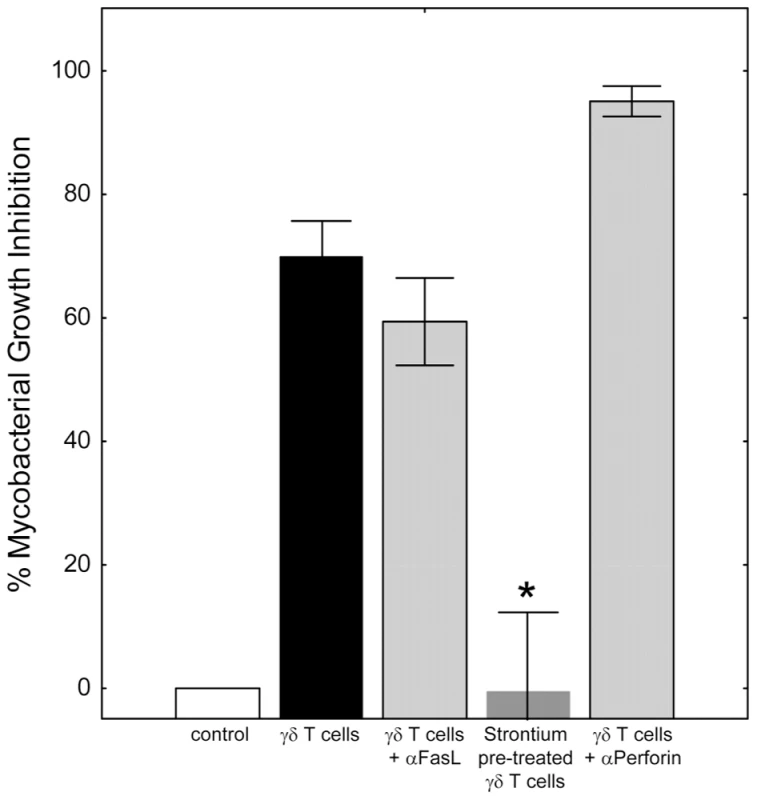
γ9δ2 T cells were co-cultured with BCG-infected macrophages in the presence or absence of the indicated treatment and surviving bacteria quantified by H3-uridine incorporation. Blocking of Fas/FasL interactions did not prevent γ9δ2 T cell-mediated inhibition (p = 0.23, n = 7). In contrast, γ9δ2 T cells pre-treated for 16 hours with 25 mM strontium chloride were unable to mediate mycobacterial inhibition (*p<0.05, n = 5 by Wilcoxon matched pairs test compared with untreated γ9δ2 T cells). Despite strontium depletion indicating the importance of γ9δ2 T cell cytolytic granules for mycobacterial inhibitory effects, antibody neutralization of perforin had no effect (p = 0.28, n = 3). Mycobacteria-specific γ9δ2 T cells utilize granzyme A to trigger pro-inflammatory TNF-α
Granzyme A, a component of cytolytic granules, recently has been shown to induce numerous pro-inflammatory cytokines including TNF-α [11]. We hypothesized that granzyme A produced by γ9δ2 T cells (Fig. S5A) may trigger macrophage TNF-α production and subsequent mycobacterial inhibition. Indeed, the levels of granzyme A in γ9δ2 T cell culture supernatants were found to be highly correlated with inhibition of intracellular mycobacteria (Fig. 3A; r = 0.7564, p<0.0001). In contrast, the supernatant levels of granzyme B, another component of cytolytic granules, weakly correlated with levels of inhibition (data not shown, r = 0.2298). Furthermore, purified human granzyme A induced dose-dependent increases in TNF-α and IL-1β by BCG-infected macrophages (Fig. 3B; p<0.05). More importantly, addition of highly purified, endotoxin-free granzyme A preparation alone to BCG-infected (Fig. 3C) or M. tuberculosis H37Rv-infected (Fig. 3D) macrophages resulted in highly significant inhibition of intracellular mycobacterial growth (p<0.03 by Wilcoxon matched pairs test, n = 6–12). Conversely, purified granzyme A had no direct effects on extracellular mycobacterial growth (data not shown). These data demonstrate for the first time that granzyme A alone can induce immunity protective against an intracellular pathogen.
Fig. 3. Granzyme A secretion by γ9δ2 T cells mediates inhibition of intracellular mycobacteria by induction of inflammatory responses in mycobacteria-infected macrophages. 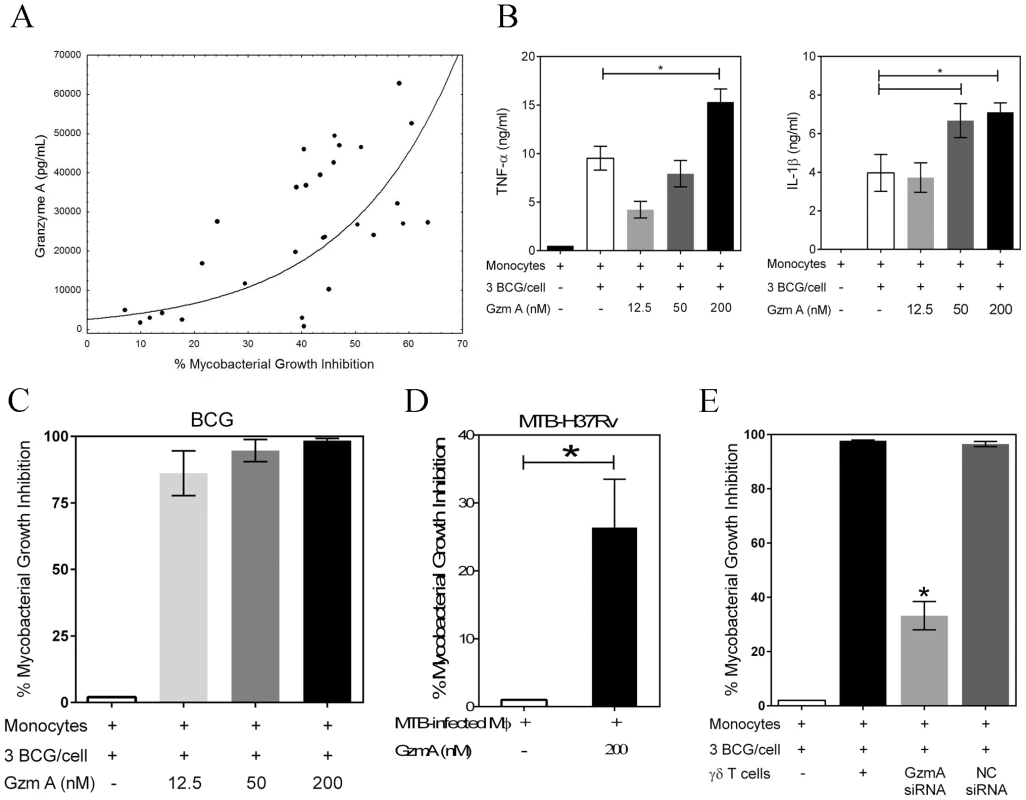
A, The levels of granzyme A produced by γ9δ2 T cells are directly and highly correlated with inhibition of intracellular mycobacterial growth (r = 0.7564; p<0.0001). Supernatant levels of granzyme A, measured by CBA, were correlated with the level of mycobacterial growth inhibition observed in γ9δ2 T cell co-cultures with BCG-infected macrophages. B, Purified granzyme A induces the production of pro-inflammatory cytokines TNF-α and IL-1β by BCG-infected macrophages (*p<0.05, n = 4; statistical comparisons performed using Friedman's test). The indicated concentrations of purified granzyme A were added to BCG-infected monocytes for 3 days and the levels of TNF-α and IL-1β in the culture supernatants determined by CBA. C-D, Purified granzyme A alone can induce inhibition of intracellular mycobacterial growth in the absence of perforin. The indicated concentrations of granzyme A were added to BCG-infected (C) or M. tuberculosis H37Rv-infected (D) co-cultures for 3 days and the surviving bacteria quantified by H3-uridine incorporation and CFU counting. (*p<0.03; n = 6–12 by Wilcoxon matched pairs test comparing levels of mycobacterial intracellular growth in cultures with and without added purified granzyme A). Purified granzyme A had no direct effects on extracellular mycobacterial growth (data not shown). E, siRNA knockdown of granzyme A in γ9δ2 T cells reduces the inhibitory activity of γ9δ2 T cells for mycobacterial growth (*p<0.05; n = 3 by one-way ANOVA comparing levels of intracellular mycobacterial growth inhibition in cultures with or without γ9δ2 T cells producing granzyme A). γ9δ2 T cells transduced with the indicated siRNA-lentivirus targeting GzmA or a negative control (NC) were co-cultured with BCG-infected macrophages for 3 days and surviving bacteria quantified by H3-uridine incorporation. In order to demonstrate that γ9δ2 T cells utilize this mechanism to suppress intracellular mycobacterial growth, lentiviral vectors containing shRNA constructs were generated to express siRNAs targeting granzyme A. γ9δ2 T cell lines were transduced with GzmA targeting or negative control (NC) siRNA lentivirus prior to co-culture with BCG-infected macrophages. Transduction of γ9δ2 T cells with the siRNA targeting granzyme A resulted in 50% decreases in the intracellular levels of granzyme A (Fig. S5). Associated with the GzmA knockdown was a significant reversal of the inhibitory effects of γ9δ2 for mycobacterial intracellular growth (Fig. 3E; p<0.05). These latter data confirm that granzyme A production represents a critical mechanism responsible for the ability of γ9δ2 T cells to inhibit intracellular mycobacterial growth.
These data lead us to propose the following model for γ9δ2 T cell-mediated control of mycobacterial replication (Fig. 4). Mycobacteria-infected macrophages present pathogen-specific or induced self antigens to γ9δ2 T cells. Activated γ9δ2 T cells then secrete granzyme A which acts upon the macrophages to produce TNF-α. It is unclear as yet whether bystander uninfected macrophages are similarly triggered to produce TNF-α or whether TNF-α is soluble vs membrane bound. TNF-α then acts in an autocrine and/or paracrine mechanism to induce infected macrophages to control mycobacterial replication. The intracellular details by which TNF-α causes this repression require further investigation. It is also likely that intracellular molecular events in addition to TNF-α production are activated by granzyme A and contribute to the mycobacterial inhibitory effects.
Fig. 4. Proposed model of granzyme A-mediated suppression of intracellular mycobacterial growth by γ9δ2 T cells. 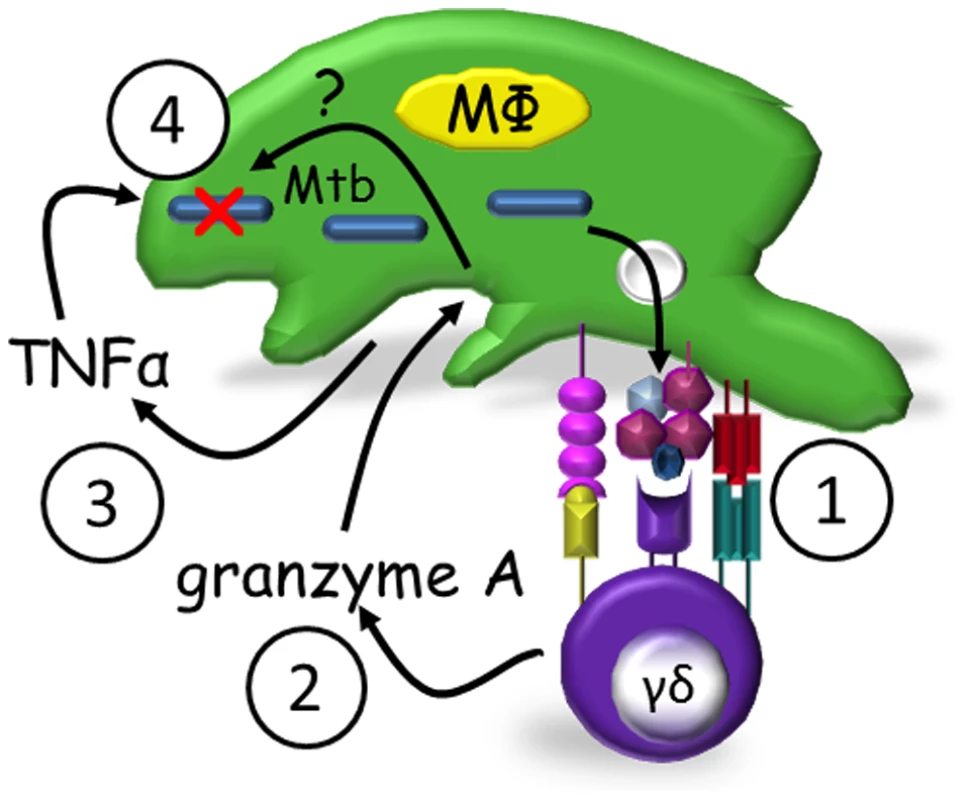
Mycobacteria-infected macrophages present antigens to γ9δ2 T cells (1) which secrete granzyme A upon activation (2). Granzyme A in turn induces TNF-α production by infected and/or bystander macrophages (3) apparently independent of perforin. TNF-α activates intracellular mechanisms which alone or in concert with other unknown granzyme A-induced intracellular mechanisms suppress mycobacterial growth (4). Discussion
γ9δ2 T cells have certain unique characteristics potentially important for the development of new vaccines capable of inducing protective immunity against several important human pathogens. Antigen recognition by these cells is not MHC restricted [20]. Therefore, the same antigens could induce protective γ9δ2 T cell responses in individuals from diverse genetic backgrounds. In addition, γ9δ2 T cells tend to accumulate in mucosal epithelial tissues, including the lung, where they can serve as a potent first line of defense against pathogenic microbes – e.g. mycobacteria [21], [22]. These properties make γ9δ2 T cells well-suited as early responders to infection. Furthermore, unlike conventional αβ T cells, which respond to peptide antigens, γ9δ2 T cells are activated by naturally occurring prenyl pyrophosphate metabolites (e.g. - isopentenyl pyrophosphate, IPP, and HMB-PP) [23], [24] expressed by actively replicating mycobacteria and other pathogens [3]. Thus, the stimulation of γ9δ2 T cells with prenyl pyrophosphates could represent a novel vaccine strategy. However, the effector mechanisms utilized by γ9δ2 T cells to inhibit intracellular mycobacterial replication have been unclear. Therefore, we sought to identify the key effector pathways of mycobacteria-stimulated γ9δ2 T cells that specifically act to inhibit the intracellular growth of mycobacteria.
Activated γ9δ2 T cells express numerous effector molecules including IFN-γ, TNF-α, perforin, granzyme and granulysin, all implicated in control of infectious microbes [8]–[10], [25]–[27]. Indeed, γ9δ2 T cells have been shown to be more potent producers of IFN-γ than CD4+ and CD8+ αβ T cells after re-stimulation in vitro with M. tuberculosis-infected APC [28], [29]. However, in addition to displaying cytotoxic activity, γ9δ2 T cells also stimulate other cell types to produce pro-inflammatory cytokines such as IFN-γ and TNF-α [30]–[33]. Our results provide further evidence indicating that γ9δ2 T cells both stimulate as well as express protective pro-inflammatory responses.
To explore the importance of soluble effector molecules in γ9δ2 T cell-mediated mycobacterial control, we neutralized the biological activity of IFN-γ, TNF-α and perforin in co-cultures of mycobacteria-protective γ9δ2 T cells and infected macrophages. Only the neutralization of TNF-α blocked the ability of mycobacteria-protective γ9δ2 T cells to control mycobacterial growth. These results indicate that TNF-α is critical for the suppression of mycobacterial growth, an observation confirmed by the clinical effects of TNF-α blockade leading to reactivation of latent TB infection. However, as demonstrated by siRNA knockdown experiments, macrophages and not γ9δ2 T cells were found to be the critical source of TNF-α for inhibition of intracellular mycobacterial growth in BCG-infected co-cultures.
The delivery of granzyme A to monocyte/macrophage cells, either as the isolated protein or by cytotoxic cells, has been shown to induce numerous pro-inflammatory cytokines including TNF-α [34]. Mycobacterial growth inhibition in our experiments strongly correlated with the levels of granzyme A in γ9δ2 T cell culture supernatants, while neutralization of perforin had no effect on mycobacterial growth inhibition. Our data support the hypothesis that granzyme A secreted by BCG-specific γ9δ2 T cells induces target macrophages to produce TNF-α which alone or in combination with unidentified factors inhibits intracellular mycobacterial growth. To our knowledge this is the first demonstration that the pro-inflammatory response elicited by granzyme A contributes to anti-pathogen host defense through microbial elimination. Furthermore, we present evidence that granzyme A triggers this protective response through a pathway that does not involve nitric oxide, type I interferons, autophagy and/or apoptosis (supplementary data).
We believe that the mechanism responsible for how granzyme A induces inhibition of intracellular mycobacteria is complex. TNF-α production by the macrophage is clearly necessary; anti-TNF neutralizing antibody prevents γ9δ2 T cell inhibitory activity and macrophage TNF knockdown prevents inhibition of intracellular mycobacterial growth. However, TNF-α is a pleiotropic cytokine contributing to both protective immunity and pathologic responses depending upon concentration and duration of production (e.g. TB reactivation occurs after TNF-α blockade, while cachexia or extreme wasting is caused by chronic excessive TNF-α production). In addition, we have shown previously [18] that high levels of TNF production by macrophages can be a marker of uncontrolled intracellular mycobacterial growth, and others have reported similar phenomena. Therefore, we believe that granzyme A-mediated induction of intracellular mycobacteria involves more than TNF-α production. TNF is required for the protective response, but there is not a simple quantitative relationship between the levels of TNF induced and the magnitude of inhibition of intracellular mycobacterial growth. We are currently trying to identify the more complete molecular signature responsible for granzyme A-mediated protective responses active against intracellular mycobacteria using a microarray approach.
Nevertheless, TNF-α production by infected or bystander macrophages may be necessary for the complete induction of γ9δ2 T cell effector functions mediating mycobacterial suppression [34]. Thus, future TB vaccine or therapeutic strategies should maximize the induction of mycobacteria-specific, granzyme A-producing γ9δ2 T cells and thereby favor the early and enhanced production of TNF-α in mycobacteria-infected and bystander macrophages in response to TB challenge.
Supporting Information
Zdroje
1. BeetzS, MarischenL, KabelitzD, WeschD (2007) Human γδ T cells: candidates for the development of immunotherapeutic strategies. Immunology Research 37 : 97–111.
2. MoritaCT, JinC, SarikondaG, WangH (2007) Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunological Reviews 215 : 59–76.
3. ConstantP, DavodeauF, PeyratMA, PoquetY, PuzoG, et al. (1994) Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264 : 267–270.
4. AbateG, EslickJ, NewmanFK, FreySE, BelsheRB, et al. (2005) Flow-cytometric detection of vaccinia-induced memory effector CD4+, CD8+, and γδ TCR+ T cells capable of antigen-specific expansion and effector functions. Journal of Infectious Diseases 192 : 1362–1371.
5. HoftDF, BabusisE, WorkuS, SpencerCT, LottenbachK, et al. (2011) Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T cell responses in young children. Journal of Infectious Diseases 204 : 845–853.
6. HoftDF, BrownRM, RoodmanST (1998) Bacille Calmette-Guérin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. Journal of Immunology 161 : 1045–1054.
7. SpencerCT, AbateG, BlazevicA, HoftDF (2008) Only a subset of phosphoantigen-responsive γ9δ2 T cells mediate protective tuberculosis immunity. Journal of Immunology 181 : 4471–4484.
8. DieliF, Troye-BlombergM, IvanyiJ, FournieJJ, BonnevilleM, et al. (2000) Vg9Vd2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. European Journal of Immunology 30 : 1512–1519.
9. DieliF, Troye-BlombergM, IvanyiJ, FournieJJ, KrenskyAM, et al. (2001) Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vg9Vd2 T lymphocytes. Journal of Infectious Diseases 184 : 1082–1085.
10. OliaroJ, DudalS, LiautardJ, AndraultJB, LiautardJP, et al. (2005) Vg9Vd2 T cells use a combination of mechanisms to limit the spread of the pathogenic bacteria Brucella. Journal of Leukocyte Biology 77 : 652–660.
11. MetkarSS, MenaaC, PardoJ, WangB, WallichR, et al. (2008) Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity 29 : 720–733.
12. ThurnerB, RoderC, DieckmannD, HeuerM, KruseM, et al. (1999) Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. Journal of Immunological Methods 223 : 1–15.
13. NeighbourPA, HubermanHS (1982) Sr++-induced inhibition of human natural killer (NK) cell-mediated cytotoxicity. Journal of Immunology 128 : 1236–1240.
14. BlommaartEF, KrauseU, SchellensJP, Vreeling-SindelarovaH, MeijerAJ (1997) The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. European Journal of Biochemistry 243 : 240–246.
15. SeglenPO, GordonPB (1982) 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proceedings of the National Academy of Sciences 79 : 1889–1892.
16. ZhuH, FearnheadHO, CohenGM (1995) An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Letters 374 : 303–308.
17. HannaWL, ZhangX, TurbovJ, WinklerU, HudigD, et al. (1993) Rapid purification of cationic granule proteases: application to human granzymes. Protein Expression and Purification 4 : 398–404.
18. WorkuS, HoftDF (2003) Differential effects of control and antigen-specific T cells on intracellular mycobacterial growth. Infection and Immunity 71 : 1763–1773.
19. PengG, GuoZ, KiniwaY, VooKs, PengW, et al. (2005) Toll-Like Receptor 8-Mediated Reversal of CD4+ Regulatory T Cell Function. Science 309 : 1380–1384.
20. AllisonTJ, WinterCC, FournieJJ, BonnevilleM, GarbocziDN (2001) Structure of a human γδ-cell antigen receptor. Nature 411 : 820–824.
21. BoomWH, ChervenakKA, MincekMA, EllnerJJ (1992) Role of the mononuclear phagocyte as an antigen-presenting cell for human γ/δ T cells activated by live Mycobacterium tuberculosis. Infection and Immunity 60 : 3480–3488.
22. RojasRE, TorresM, FournieJJ, HardingCV, BoomWH (2002) Phosphoantigen presentation by macrophages to Mycobacterium tuberculosis-reactive Vγ9Vδ2+ T cells: modulation by chloroquine. Infection and Immunity 70 : 4019–4027.
23. BurkMR, MoriL, De LiberoG (1995) Human Vγ9Vδ2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. European Journal of Immunology 25 : 2052–2058.
24. EvansPS, EndersPJ, YinC, RuckwardtTJ, MalkovskyM, et al. (2001) In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jδ1.2/Vδ2 T-cell receptors. Immunology 104 : 19–27.
25. MartinoA, CasettiR, SacchiA, PocciaF (2007) Central memory Vg9Vd2 T lymphocytes primed and expanded by Bacille Calmette-Guérin-infected dendritic cells kill mycobacterial-infected monocytes. Journal of Immunology 179 : 3057–3064.
26. OttonesF, DornandJ, NaroeniA, LiautardJP, FaveroJ (2000) Vγ9Vδ2 T cells impair intracellular multiplication of Brucella suis in autologous monocytes through soluble factor release and contact-dependent cytotoxic effect. Journal of Immunology 165 : 7133–7139.
27. WangL, KamathA, DasH, LiL, BukowskiJF (2001) Antibacterial effect of human Vg2Vd2 T cells in vivo. Journal of Clinical Investigation 108 : 1349–1357.
28. LeeJ, ChoiK, OlinMR, ChoSN, MolitorTW (2004) γδ T cells in immunity induced by Mycobacterium bovis bacillus Calmette-Guérin vaccination. Infection and Immunity 72 : 1504–1511.
29. TsukaguchiK, BalajiKN, BoomWH (1995) CD4+ αβ T cell and γδ T cell responses to Mycobacterium tuberculosis. Journal of Immunology 154 : 1786–1796.
30. BodnarKA, SerbinaNV, FlynnJL (2001) Fate of Mycobacterium tuberculosis within murine dendritic cells. Infection and Immunity 69 : 800–809.
31. CooperAM, DaltonDK, StewartTA, GriffinJP, RussellDG, et al. (1993) Disseminated tuberculosis in IFN-γ gene-disrupted mice. Journal of Experimental Medicine 178 : 2243–2247.
32. FlynnJL, ChanJ, TrieboldKJ, DaltonDK, StewartTA, et al. (1993) An essential role for IFN-gamma in resistance to Mycobacterium tuberculosis infection. Journal of Experimental Medicine 178 : 2249–2254.
33. OttenhoffTH, KumararatneD, CasanovaJL (1998) Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunology Today 19 : 491–494.
34. LiH, LuoK, PauzaCD (2008) TNF-alpha is a positive regulatory factor for human Vγ2 Vδ2 T cells. Journal of Immunolgy 181 : 7131–7137.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and ResearchČlánek Transcription of a -acting, Noncoding, Small RNA Is Required for Pilin Antigenic Variation inČlánek Structural Basis for Feed-Forward Transcriptional Regulation of Membrane Lipid Homeostasis inČlánek The Tomato Prf Complex Is a Molecular Trap for Bacterial Effectors Based on Pto TransphosphorylationČlánek Schmallenberg Virus Pathogenesis, Tropism and Interaction with the Innate Immune System of the HostČlánek The Importance of Prions
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Preclinical Therapy of Disseminated HER-2 Ovarian and Breast Carcinomas with a HER-2-Retargeted Oncolytic Herpesvirus
- Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and Research
- Glucose Phosphorylation Is Required for Persistence in Mice
- and Host Determinants of Susceptibility to Invasive Candidiasis
- TNFα-Mediated Liver Destruction by Kupffer Cells and Ly6C Monocytes during Infection
- Transcription of a -acting, Noncoding, Small RNA Is Required for Pilin Antigenic Variation in
- Structural Basis for Feed-Forward Transcriptional Regulation of Membrane Lipid Homeostasis in
- Intravital Placenta Imaging Reveals Microcirculatory Dynamics Impact on Sequestration and Phagocytosis of -Infected Erythrocytes
- The Tomato Prf Complex Is a Molecular Trap for Bacterial Effectors Based on Pto Transphosphorylation
- Schmallenberg Virus Pathogenesis, Tropism and Interaction with the Innate Immune System of the Host
- Make It, Take It, or Leave It: Heme Metabolism of Parasites
- Viral and Bacterial Interactions in the Upper Respiratory Tract
- The Importance of Prions
- Loss and Retention of RNA Interference in Fungi and Parasites
- Granzyme A Produced by γδ T Cells Induces Human Macrophages to Inhibit Growth of an Intracellular Pathogen
- Innate Sensing of Chitin and Chitosan
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and Research
- Loss and Retention of RNA Interference in Fungi and Parasites
- Make It, Take It, or Leave It: Heme Metabolism of Parasites
- Innate Sensing of Chitin and Chitosan
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



