-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
Reklamaand Host Determinants of Susceptibility to Invasive Candidiasis
article has not abstract
Published in the journal: . PLoS Pathog 9(1): e32767. doi:10.1371/journal.ppat.1003079
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003079Summary
article has not abstract
Introduction
Candida is the most common human fungal pathogen and the cause of invasive candidiasis, the fourth leading cause of nosocomial bloodstream infection in the United States with an estimated annual cost of ∼US$2 billion and mortality that exceeds 40% despite administration of antifungal therapy in modern intensive care unit facilities [1]. Hence, invasive candidiasis is an unmet medical condition for which better understanding of its pathogenesis at the host–pathogen interface is essential to improve patient outcomes. To that end, a mouse model of the infection, which introduces Candida yeast cells intravenously and mimics skin-derived bloodstream human candidiasis, has been successfully employed to study fungal and host factors that regulate susceptibility to the infection [2].
Which Candida Virulence Factors Influence the Outcome of Invasive Candidiasis?
Candida expresses a variety of virulence factors that contribute to its pathogenesis and could be exploited for development of vaccines and targeted therapeutic strategies. Firstly, Candida albicans filaments' virulence factors, including secreted aspartyl proteases and phospholipases, are thought to be important for Candida invasion in infected organs and, probably, for mediating fungus-induced tissue immunopathology [3]. Secondly, Candida is able to efficiently adhere to and invade epithelial and endothelial cells via induced endocytosis and active penetration; both adhesion and invasion facilitate Candida dissemination [4]. Effective adhesion also enables Candida to form biofilms on implanted medical devices such as central venous catheters, which are a frequent portal of entry for invasive infection in humans [5]. Among the known Candida factors that promote its adhesion and invasion, the agglutinin-like sequence (Als) family has attracted significant attention; Als3 in particular, a C. albicans–specific virulence factor, was recently shown to mediate brain-specific Candida endothelial invasion and tissue penetration [6]. Specifically, increased surface expression of Als3 in the vps51Δ/Δ C. albicans mutant was shown to be responsible for its increased ability to invade brain endothelial cells in vitro and traffic to the brain in vivo via binding to the gp96 heat shock protein, which is expressed specifically on brain endothelium [6]. Als3 has formed the basis for the development of a cell wall protein-based vaccine strategy against candidiasis, which was recently tested safely in humans in a Phase I clinical trial [7]. Studies in mice revealed that IFN-γ and IL-17α produced by Th1 and Th17 lymphocytes were essential for vaccine-induced protection, via Ccl3 - and Cxcl1-mediated neutrophil recruitment to sites of infection, which resulted in decreased Candida tissue burden [8].
Moreover, a fundamental C. albicans virulence factor is its ability to transition between unicellular yeast cells and filamentous growth during infection; in fact, it is the interchange between these morphotypes that is critical for pathogenesis, as strains locked either in the yeast or the filamentous forms have attenuated virulence in vivo [9], [10]. Of note, this morphogenic transition is not a prerequisite for virulence in non-albicans Candida species such as Candida glabrata, which is an important cause of invasive candidiasis in humans even though it does not form hyphae. Strikingly, the ability of C. albicans to form filaments in vivo is tissue-specific [2]. Hence, organs that successfully inhibit Candida growth in mice such as the liver and spleen prevent Candida filamentation, whereas hyphal formation is abundant in the kidney, the target organ of murine disseminated candidiasis, where Candida proliferation is inexorable [2]. Therefore, identifying tissue-specific immunological factors and environmental cues that restrict Candida filamentation could lead to the discovery of novel therapeutic interventions.
How Does the Innate Immune System Recognize Candida?
The first step in mounting an effective anti-Candida immune response is fungal recognition by the innate immune system. Over the past decade there has been an explosion in our understanding of how soluble and membrane-bound pattern recognition receptors (PRRs) recognize various pathogen-associated molecular patterns (PAMPs) of Candida yeast and filamentous forms (Text S1) (reviewed in [11], [12]). In brief, the complement components C3 and C5, the complement receptor 3 (CR3), the Toll-like receptors (TLR)-2 (in interaction with TLR1 and TLR6), TLR4, TLR7, and TLR9, and the C-type lectins (CLRs) dectin-1, dectin-2, mannose receptor, DC-SIGN, and Mincle are among the PRRs shown to recognize different fungal PAMPs including mannan, β-glucan, RNA, and DNA (Figure 1); several of these PRRs are indispensable for host defense in vivo by inducing the secretion of pro-inflammatory cytokines and chemokines and modulating innate and adaptive antifungal immune responses (Text S1) [11], [12]. In fact, synergistic interactions between different PRRs resulting in augmented downstream immune activation have been demonstrated, such as between TLRs and CLRs or C5a and TLRs [11], [12]. Candida also activates the inflammasome; both the Nlrp3/caspase-1 pathway and the non-canonical caspase-8 pathway have been implicated in IL-1β production via dectin-1/syk activation by β-glucans (Figure 1) [12], [13].
Fig. 1. The principal cell surface pattern recognition receptors involved in Candida recognition. 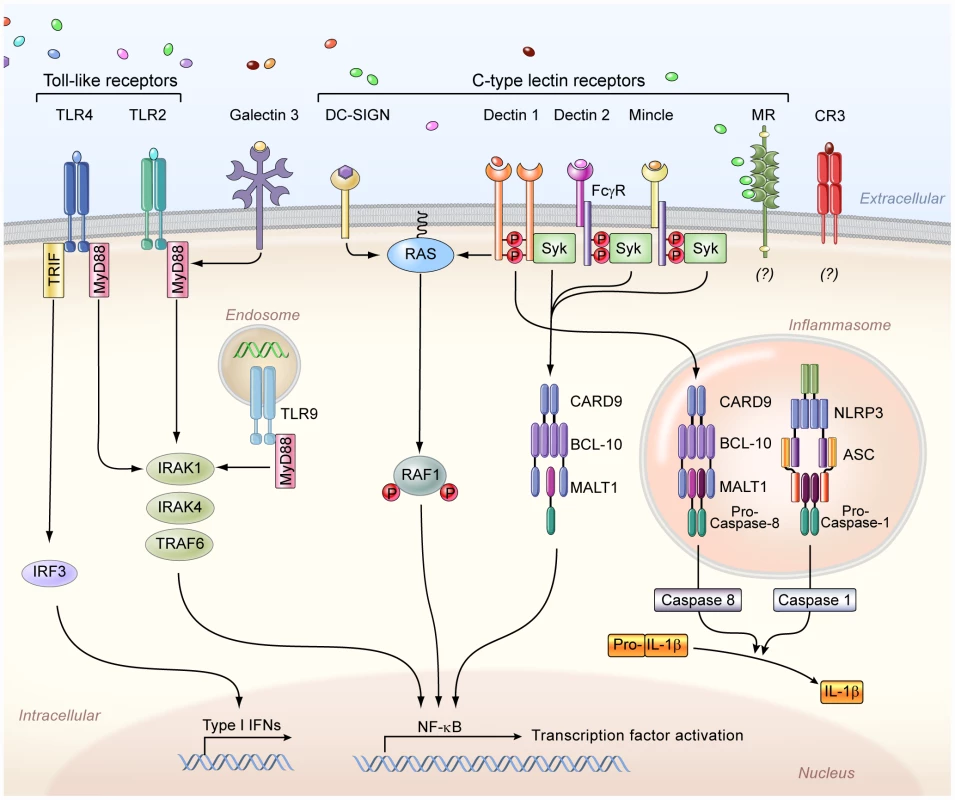
The Toll-like receptor 2 (TLR2) and TLR4 recognize phospholipomannans and O-linked mannans, respectively, whereas TLR9 within the cytosol recognizes fungal DNA, and intracellular TLR7 (not depicted) recognizes fungal RNA. TLR2 forms heterodimers with TLR1 or TLR6 for downstream signaling (not depicted), whereas TLR4 forms homodimers. Galectin-3, together with TLR2, recognizes β-mannosides. The membrane-bound C-type lectins dendritic cell–specific ICAM3-grabbing non-integrin (DC-SIGN), macrophage-inducible C-type lectin (Mincle), and macrophage mannose receptor (MR) recognize mannose-rich Candida structures. In addition, dectin-1 recognizes β-glucans, and dectin 2, together with the Fcγ receptor (FcγR), recognizes mannans. The complement receptor 3 (CR3) on neutrophils also recognizes β-glucans. Moreover, the NOD-like receptor NLRP3 (nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3) forms an inflammasome complex with ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and caspase 1, which leads to interleukin-1β (IL-1β) production. In addition, downstream dectin-1 signaling through caspase recruitment domain-containing protein 9 (CARD9) leads to non-canonical inflammasome activation and IL-1β production via caspase 8. IFNs, interferons; NF-κB, nuclear factor-κB; Syk, spleen tyrosine kinase; IRF3, interferon regulatory factor 3; TRIF, TIR-domain-containing adapter-inducing interferon-β; MyD88, myeloid differentiation primary response gene 88; BCL-10, B-cell lymphoma/leukemia 10; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; TRAF6, TNF receptor–associated factor 6; IRAK, interleukin-1 receptor-associated kinase. The challenge for future research will be to define how Candida recognition is integrated by the array of different PRRs in vivo and to determine what is the relative contribution of individual PRRs on different myeloid cells (e.g., dectin-1 or CR3 as β-glucan receptors on neutrophils versus monocytes/macrophages versus dendritic cells) in modulating downstream anti-Candida immune responses. In addition, more studies are needed to understand how Candida influences its recognition in vivo by employing immune evasion strategies; for example, β-glucan exposure on the Candida surface occurs in infected mouse tissues only late after infection [14], thus preventing CLR-mediated pathogen recognition during the early phase of invasive infection, when recruitment of effector immune cells is critical for survival [15].
In addition, the notable diversity in PAMP structure among various Candida experimental strains impedes on drawing definite conclusions about the in vivo role of certain PRRs, as apparently discrepant results have been reported for some TLRs and CLRs with different fungal strains [11], [12], [16]. To that end, the logistical and economical constraints associated with testing large numbers of Candida strains in mammals could potentially be ameliorated by employing non-vertebrate (e.g., Drosophila melanogaster) and/or mini-vertebrate model hosts (e.g., zebrafish) that have evolutionarily conserved innate immune pathways (e.g., TLR signaling), and could allow for facile and inexpensive high-throughput screening of greater numbers of Candida strains [17]. Not surprisingly, as C. albicans is the most common agent of human invasive candidiasis, research performed until now has predominantly focused on the recognition of this species, and much less is known about the interaction of other Candida species with the immune system. It is expected that research aiming to gain more insight on the recognition of emerging non-albicans Candida species will represent an important area of research in the coming years.
What Are the Divergent Roles of Neutrophils during Invasive Candidiasis?
Neutrophils are indispensable for host defense against invasive candidiasis, and neutropenia is a well-recognized risk factor for development of and adverse outcome after infection in humans [1]. The protective effects of neutrophils are mediated via oxidative and non-oxidative mechanisms that result in efficient Candida killing [12]. Specifically, Candida ingestion is followed by assembly of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex at the phagosomal membrane and oxidative burst, which leads to generation of candidacidal reactive oxygen species and K+-influx–induced activation of neutrophil candidacidal granular proteases [12]. Neutrophils are the only effector cells able to inhibit Candida yeast-to-hyphae conversion, a process dependent on the oxidative burst [12]. The oxidative burst is also important for generation of neutrophil extracellular traps, which ensnare Candida yeasts and hyphae and appear important for anti-Candida host defense in vivo [18].
Nonetheless, neutrophils have differential effects in invasive candidiasis in vivo depending on the phase of the infection in mice. Specifically, early neutrophil presence after infection is protective, whereas neutrophil presence late after infection is pathogenic [15]. The requirement of early neutrophil accumulation for effective host defense appears to correlate with the organ-specific microbiological progression of invasive candidiasis [2]; thus, liver and spleen, which recruit large numbers of neutrophils early post-infection, effectively control fungal growth. Conversely, sluggish early accumulation of neutrophils in the kidney is associated with unabated fungal proliferation [2]. With regard to the pathogenic role of neutrophils late after infection, we recently reported that the chemokine receptor Ccr1 drives neutrophil-induced tissue immunopathology and mortality in invasive candidiasis by mediating neutrophil trafficking from the blood into the kidney during the late phase of the infection [19]. In addition, late neutrophil accumulation into the infected kidney was shown to depend on type I interferon signaling, which mediated tissue immunopathology and mortality [20]. Future research will be required to define the role of regulatory T cells and anti-inflammatory mediators including cytokines in restricting neutrophil-mediated tissue injury in invasive candidiasis. Of interest, in a subset of patients with invasive candidiasis, neutrophils exhibit pathogenic effects, as recovery from neutropenia is associated with worsening symptoms that requires corticosteroid administration [21]. Hence, identification of Ccr1, Ifnar1, and other molecular factors that mediate pathogenic neutrophil effects in invasive candidiasis could potentially lead to targeted therapeutic interventions in selected patients.
What Is the Role of Other Immune Cell Types in Host Defense against Invasive Candidiasis?
Besides neutrophils, monocytes/macrophages are key phagocytic cells for protection against invasive candidiasis, as their depletion in mice leads to increased mortality [22]. Monocytes/macrophages are very effective in Candida phagocytosis and secretion of a variety of pro-inflammatory cytokines and chemokines that orchestrate the antifungal innate immune response [12]. Nevertheless, macrophages are significantly less able to inhibit yeast germination and kill Candida compared to neutrophils; the lack of macrophage myeloperoxidase and extracellular trap formation may, at least in part, account for this deficit [12]. On the other hand, the concomitant release of superoxide and nitric oxide, with the subsequent formation of peroxinitrite, has been suggested to mediate the macrophage anti-Candida effects in mice; yet, the importance of Candida-induced nitric oxide formation in human phagocytes is unclear [12]. Several studies have demonstrated the priming role of Th1 cytokines and the inhibitory role of Th2 cytokines on the macrophage killing capacity, but more research is required to elucidate the molecular mechanisms of macrophage activation and effector function in vivo at the sites of Candida infection. In addition, future studies should aim to shed light on the relative role of recruited versus resident monocytes/macrophages in host defense against invasive candidiasis.
Intriguingly, besides the protective role of monocytes/macrophages in host defense against primary Candida challenge, monocytes were recently shown to also confer protection following systemic Candida re-infection via functional reprogramming that involves the dectin-1/Raf-1 non-canonical signaling pathway and results in enhanced cytokine production [23]. This monocyte/macrophage reprogramming that results in innate immune memory (termed “trained immunity”) appears to involve epigenetic mechanisms mediated via stable changes in histone trimethylation at H3K4 [23]. Thus, induction of trained immunity shows promise for the potential design of novel vaccination strategies against candidiasis.
The role of other hematopoietic cells in host defense against invasive candidiasis merits further investigation. For example, dendritic cells are able to phagocytize Candida yeast and hyphal forms and respond differentially to the Candida morphotypes by priming the production of distinct cytokines [24]. In addition, CD1d+ dendritic cells were recently shown to activate invariant natural killer T cells to mediate innate immune responses against fungi (including Candida) via dectin-1 - and MyD88-dependent mechanisms without apparent requirement for fungal lipid antigen presentation [25]. However, the in vivo role of different dendritic cell subsets remains unclear, partly owing to technical difficulties in achieving specific dendritic cell depletion. Moreover, consistent with the lack of enhanced susceptibility of patients with acquired immunodeficiency syndrome, severe combined immunodeficiency, X-linked agammaglobulinemia, and common variable immunodeficiency to invasive candidiasis, T and B lymphocytes are dispensable for host defense in the mouse model [26]. Nonetheless, T lymphocytes play a critical role in protection against mucosal candidiasis [26]. In addition, the recent demonstration of direct IgA-mediated anti-Candida effects after systemic infection [27] and of protective Th17-mediated responses by Candida-specific CD4+ T cells after systemic infection of mice vaccinated with the Als-derived peptide pALS [28] suggest that exploiting antibody-mediated and cell-mediated anti-Candida immunity could lead to the design of effective vaccination and immunomodulatory strategies against invasive and mucosal candidiasis in patients.
What Host Factors Mediate Protection against Invasive Candidiasis in Humans?
In agreement with the requirement of innate immunity for effective host defense in the mouse model of invasive candidiasis, certain innate immune factors have been associated with protection against the infection in humans [26]. Consistent with the crucial role of the NADPH oxidase in phagocyte killing and the heightened susceptibility of NADPH oxidase-deficient mice to invasive candidiasis [8], [12], patients with chronic granulomatous disease are at increased risk for development of the infection [26]. In addition, in line with the enhanced susceptibility of myeloperoxidase-deficient mice to invasive candidiasis and the impaired anti-Candida killing capacity of human myeloperoxidase-deficient phagocytes, invasive candidiasis occurs in patients with myeloperoxidase deficiency, the most common inherited phagocytic disorder [26]. Yet, the majority of myeloperoxidase-deficient patients are asymptomatic, and invasive candidiasis develops only in patients with autosomal-recessive complete myeloperoxidase deficiency who also have concomitant disorders that adversely affect phagocyte function such as diabetes [26].
On the other hand, there is no universal concordance between humans and mice in regard to the importance of fungal PRRs and pro-inflammatory cytokines in host defense against invasive candidiasis. Hence, complement deficiencies and MyD88 mutations do not appear to confer a significant risk for invasive candidiasis in humans as opposed to mice [26]; however, a recent large cohort study demonstrated that TLR1 single nucleotide polymorphisms in Caucasian patients were associated with heightened risk for development of invasive candidiasis, suggesting that TLR signaling may contribute to optimal anti-Candida immunity in humans [29]. Furthermore, mutations in the adaptor molecule CARD9 predispose to both invasive and chronic mucocutaneous candidiasis (Text S1) [26], whereas dectin-1 mutations appear to be associated with chronic mucocutaneous but not invasive candidiasis in humans; instead, Card9−/− and dectin-1−/− mice are both susceptible to invasive candidiasis (Text S1) [26] Finally, Johnson and colleagues recently examined the impact of cytokine polymorphisms on host susceptibility to invasive candidiasis and reported that polymorphisms in IL-10 and IL-12B, but not IL-1β or IFN-γ, were associated with persistent fungemia in patients with candidemia [30]. Future studies will be required to identify additional genetic risk factors for development of and/or adverse outcome after invasive candidiasis, as such knowledge could eventually lead to individualized risk stratification and prognostication strategies for patients.
Perspective
Candida is a commensal organism that colonizes 50% of individuals of a population at any given time, but in conditions leading to weakening of host defense mechanisms it can convert to an opportunistic pathogen causing localized mucosal disease or life-threatening invasive infections with high mortality rate despite antifungal therapy. In recent years we have witnessed a surge of studies of Candida pathogenesis at the host–pathogen interface. Dissecting the fungal virulence factors that foster the transition of Candida from a commensal to an opportunistic pathogen, and deepening our understanding of the molecular and cellular basis of effective antifungal immunity should lead to novel risk stratification, prognostication, vaccination, and therapeutic strategies in patients.
Supporting Information
Zdroje
1. HornDL, NeofytosD, AnaissieEJ, FishmanJA, SteinbachWJ, et al. (2009) Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48 : 1695–1703.
2. LionakisMS, LimJK, LeeCC, MurphyPM (2011) Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 3 : 180–199.
3. FelkA, KretschmarM, AlbrechtA, SchallerM, BeinhauerS, et al. (2002) Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect Immun 70 : 3689–3700.
4. FillerSG, SheppardDC (2006) Fungal invasion of normally non-phagocytic host cells. PLoS Pathog 2: e129 doi:10.1371/journal.ppat.0020129.
5. FinkelJS, MitchellAP (2011) Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9 : 109–118.
6. LiuY, MittalR, SolisNV, PrasadaraoNV, FillerSG (2011) Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog 7: e1002305 doi:10.1371/journal.ppat.1002305.
7. Hennessey JP Jr, Schmidt CS, Ibrahim A, Filler S, White CJ, et al.. (2011). A Phase 1 clinical evaluation of NDV3, a vaccine to prevent disease caused by Candida spp. and Staphylococcus aureus. In 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago. Washington, DC: American Society for Microbiology.
8. LinL, IbrahimAS, XuX, FarberJM, AvanesianV, et al. (2009) Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 5: e1000703 doi:10.1371/journal.ppat.1000703.
9. LoHJ, KöhlerJR, DiDomenicoB, LoebenbergD, CacciapuotiA, et al. (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90 : 939–949.
10. MuradAM, LengP, StraffonM, WishartJ, MacaskillS, et al. (2001) NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J 20 : 4742–4752.
11. NeteaMG, BrownGD, KullbergBJ, GowNA (2008) An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6 : 67–78.
12. BrownGD (2011) Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 29 : 1–21.
13. GringhuisSI, KapteinTM, WeversBA, TheelenB, van der VlistM, et al. (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1β via a noncanonical caspase-8 inflammasome. Nat Immunol 13 : 246–254.
14. WheelerRT, KombeD, AgarwalaSD, FinkGR (2008) Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog 4: e1000227 doi:10.1371/journal.ppat.1000227.
15. RomaniL, MencacciA, CenciE, Del SeroG, BistoniF, et al. (1997) An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol 158 : 2356–2362.
16. NeteaMG, GowNA, JoostenLA, VerschuerenI, van der MeerJW, et al. (2010) Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol 48 : 897–903.
17. ChamilosG, LionakisMS, LewisRE, KontoyiannisDP (2007) Role of mini-host models in the study of medically important fungi. Lancet Infect Dis 7 : 42–55.
18. UrbanCF, ErmertD, SchmidM, Abu-AbedU, GoosmannC, et al. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 5: e1000639 doi:10.1371/journal.ppat.1000639.
19. LionakisMS, FischerBS, LimJK, SwamydasM, WanW, et al. (2012) Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog 8: e1002865 doi:10.1371/journal.ppat.1002865.
20. MajerO, BourgeoisC, ZwolanekF, LassnigC, KerjaschkiD, et al. (2012) Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog 8: e1002811 doi:10.1371/journal.ppat.1002811.
21. LegrandF, LecuitM, DupontB, BellatonE, HuerreM, et al. (2008) Adjuvant corticosteroid therapy for chronic disseminated candidiasis. Clin Infect Dis 46 : 696–702.
22. QianQ, JutilaMA, Van RooijenN, CutlerJE (1994) Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J Immunol 152 : 5000–5008.
23. QuintinJ, SaeedS, MartensJH, Giamarellos-BourboulisEJ, IfrimDC, et al. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12 : 223–232.
24. d'OstianiCF, Del SeroG, BacciA, MontagnoliC, SprecaA, et al. (2000) Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med 191 : 1661–1674.
25. CohenNR, TatituriRV, RiveraA, WattsGF, KimEY, et al. (2011) Innate recognition of cell wall β-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe 10 : 437–450.
26. LionakisMS (2012) Genetic susceptibility to fungal infections in humans. Curr Fungal Infect Rep 6 : 11–22.
27. van SprielAB, SofiM, GartlanKH, van der SchaafA, VerschuerenI, et al. (2009) The tetraspanin protein CD37 regulates IgA responses and anti-fungal immunity. PLoS Pathog 5: e1000338 doi:10.1371/journal.ppat.1000338.
28. BärE, GladiatorA, BastidasS, RoschitzkiB, Acha-OrbeaH, et al. (2012) A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol 188 : 5636–5643.
29. PlantingaTS, JohnsonMD, ScottWK, van de VosseE, Velez EdwardsDR, et al. (2012) Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis 205 : 934–943.
30. JohnsonMD, PlantingaTS, van de VosseE, Velez EdwardsDR, SmithPB, et al. (2012) Cytokine gene polymorphisms and the outcome of invasive candidiasis: a prospective cohort study. Clin Infect Dis 54 : 502–510.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and ResearchČlánek Transcription of a -acting, Noncoding, Small RNA Is Required for Pilin Antigenic Variation inČlánek Structural Basis for Feed-Forward Transcriptional Regulation of Membrane Lipid Homeostasis inČlánek The Tomato Prf Complex Is a Molecular Trap for Bacterial Effectors Based on Pto TransphosphorylationČlánek Schmallenberg Virus Pathogenesis, Tropism and Interaction with the Innate Immune System of the HostČlánek The Importance of Prions
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Preclinical Therapy of Disseminated HER-2 Ovarian and Breast Carcinomas with a HER-2-Retargeted Oncolytic Herpesvirus
- Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and Research
- Glucose Phosphorylation Is Required for Persistence in Mice
- and Host Determinants of Susceptibility to Invasive Candidiasis
- TNFα-Mediated Liver Destruction by Kupffer Cells and Ly6C Monocytes during Infection
- Transcription of a -acting, Noncoding, Small RNA Is Required for Pilin Antigenic Variation in
- Structural Basis for Feed-Forward Transcriptional Regulation of Membrane Lipid Homeostasis in
- Intravital Placenta Imaging Reveals Microcirculatory Dynamics Impact on Sequestration and Phagocytosis of -Infected Erythrocytes
- The Tomato Prf Complex Is a Molecular Trap for Bacterial Effectors Based on Pto Transphosphorylation
- Schmallenberg Virus Pathogenesis, Tropism and Interaction with the Innate Immune System of the Host
- Make It, Take It, or Leave It: Heme Metabolism of Parasites
- Viral and Bacterial Interactions in the Upper Respiratory Tract
- The Importance of Prions
- Loss and Retention of RNA Interference in Fungi and Parasites
- Granzyme A Produced by γδ T Cells Induces Human Macrophages to Inhibit Growth of an Intracellular Pathogen
- Innate Sensing of Chitin and Chitosan
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and Research
- Loss and Retention of RNA Interference in Fungi and Parasites
- Make It, Take It, or Leave It: Heme Metabolism of Parasites
- Innate Sensing of Chitin and Chitosan
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



