-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaManipulation of Costimulatory Molecules by Intracellular Pathogens: Veni, Vidi, Vici!!
Some of the most successful pathogens of human, such as Mycobacterium tuberculosis (Mtb), HIV, and Leishmania donovani not only establish chronic infections but also remain a grave global threat. These pathogens have developed innovative strategies to evade immune responses such as antigenic shift and drift, interference with antigen processing/presentation, subversion of phagocytosis, induction of immune regulatory pathways, and manipulation of the costimulatory molecules. Costimulatory molecules expressed on the surface of various cells play a decisive role in the initiation and sustenance of immunity. Exploitation of the “code of conduct” of costimulation pathways provides evolutionary incentive to the pathogens and thereby abates the functioning of the immune system. Here we review how Mtb, HIV, Leishmania sp., and other pathogens manipulate costimulatory molecules to establish chronic infection. Impairment by pathogens in the signaling events delivered by costimulatory molecules may be responsible for defective T-cell responses; consequently organisms grow unhindered in the host cells. This review summarizes the convergent devices that pathogens employ to tune and tame the immune system using costimulatory molecules. Studying host-pathogen interaction in context with costimulatory signals may unveil the molecular mechanism that will help in understanding the survival/death of the pathogens. We emphasize that the very same pathways can potentially be exploited to develop immunotherapeutic strategies to eliminate intracellular pathogens.
Published in the journal: . PLoS Pathog 8(6): e32767. doi:10.1371/journal.ppat.1002676
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1002676Summary
Some of the most successful pathogens of human, such as Mycobacterium tuberculosis (Mtb), HIV, and Leishmania donovani not only establish chronic infections but also remain a grave global threat. These pathogens have developed innovative strategies to evade immune responses such as antigenic shift and drift, interference with antigen processing/presentation, subversion of phagocytosis, induction of immune regulatory pathways, and manipulation of the costimulatory molecules. Costimulatory molecules expressed on the surface of various cells play a decisive role in the initiation and sustenance of immunity. Exploitation of the “code of conduct” of costimulation pathways provides evolutionary incentive to the pathogens and thereby abates the functioning of the immune system. Here we review how Mtb, HIV, Leishmania sp., and other pathogens manipulate costimulatory molecules to establish chronic infection. Impairment by pathogens in the signaling events delivered by costimulatory molecules may be responsible for defective T-cell responses; consequently organisms grow unhindered in the host cells. This review summarizes the convergent devices that pathogens employ to tune and tame the immune system using costimulatory molecules. Studying host-pathogen interaction in context with costimulatory signals may unveil the molecular mechanism that will help in understanding the survival/death of the pathogens. We emphasize that the very same pathways can potentially be exploited to develop immunotherapeutic strategies to eliminate intracellular pathogens.
Introduction
The immune system is highly evolved to combat and eliminate pathogens. However, some pathogens can successfully subvert the host immune system to establish their intracellular survival via strategies such as the disguise or sequestration of antigens, molecular mimicry, immunosuppression, circumvention of complements and cytokines cascade, blockade of antigen presentation, escape from apoptosis and autophagy, and modulation of costimulatory signals [1].
T cells and antigen-presenting cells (APCs) play crucial roles in eliminating intracellular pathogens. The optimal activation of naive T cells is achieved by occupancy of T-cell receptor (TCR) by the peptide-MHC complex displayed on the surface of APCs, delivery of costimulatory signals, and the presence of proinflammatory cytokines [2]. The expression of costimulatory molecules on APCs is critical in shaping the extent and nature of the immune response. Thus, an encounter of T cells with peptide-MHC can result in two discrete events: (a) T-cell proliferation and differentiation into effector cells; (b) or anergy or apoptosis. The question of which of these outcomes transpires is determined by the delivery of appropriate costimulatory signals [3]. An array of costimulatory molecules is displayed on the surface of APCs (CD80/B7-1, CD86/B7-2, CD83, CD40, PDL-1, DC-SIGN, 4-1BBL, etc.) and T cells (CD28, CTLA-4, CD40L, PD-1, OX40, 4-1BB, etc). The level of the expression of the costimulatory molecules may play an important role during the course of acute disease and its remission or relapse. Hence, modulation of these molecules by pathogens can help them to establish their existence in the host.
Here we elaborate the mechanism by which pathogens such as Mtb, HIV, and Leishmania sp., employ molecules viz. CD28, CD40, CD40L, CD80, CD86, CTLA-4, PD1, PDL-1, etc., to break the code of costimulation to establish chronic infection. The organisms in consideration are representatives of successful pathogens from the class of bacteria, virus, and parasites. It appears that these pathogens adopt a common evolutionarily convergent mechanism to evade host immune reaction. This review provides insights into the mechanism by which pathogens suppress host immunity by modulating the expression of costimulatory molecules. We also suggest avenues of therapeutic intervention by exploiting costimulatory pathways for treating infections.
The Paradigm Shift in the Biology of Costimulation
The unilateral “help to T-cell” lymphocentric paradigm of costimulatory pathways has currently evolved into a bilateral signaling model that influences the activity of both T cells and APCs during their interaction (Figure 1) [4], [5]. Costimulatory molecules of CD80/CD28, tumor necrosis factor (TNF)/TNFR, and TIM superfamilies have unmasked the plethora of the possible ligand-receptor interactions that has expanded the understanding of regulation of the immune responses mediated by APCs and T cells. For example, a positive regulator like CD40L (on T cells) when associated with CD40 (on APCs), not only activates T cells but also results in the activation of dendritic cells (DCs); a process that is popularly called “T-cell licensing” [6]. Similarly, ligation of CD28 with CD80 and CD86 is known to induce the secretion of interleukin-6 (IL-6) and interferon-γ (IFN-γ) by DCs and activation, proliferation, and differentiation of B cells [5], [7], [8]. It is reported that 4-1BBL expressed on DCs, binds to 4-1BB on T cells, to bolster DCs help to T cells [9]. Many reports have highlighted the inhibitory roles of CTLA-4 (CD152) and PD-1 (expressed on T cells) with ligands CD80/CD86 and PDL-1/PDL-2 (on APCs), respectively [10], [11]. It clearly suggests that costimulation not only amplifies the magnitude of the activation of T cells and APCs, but fine tunes the immune response as well, thereby controlling the hyperactivation.
Fig. 1. Immune response against intracellular pathogens. 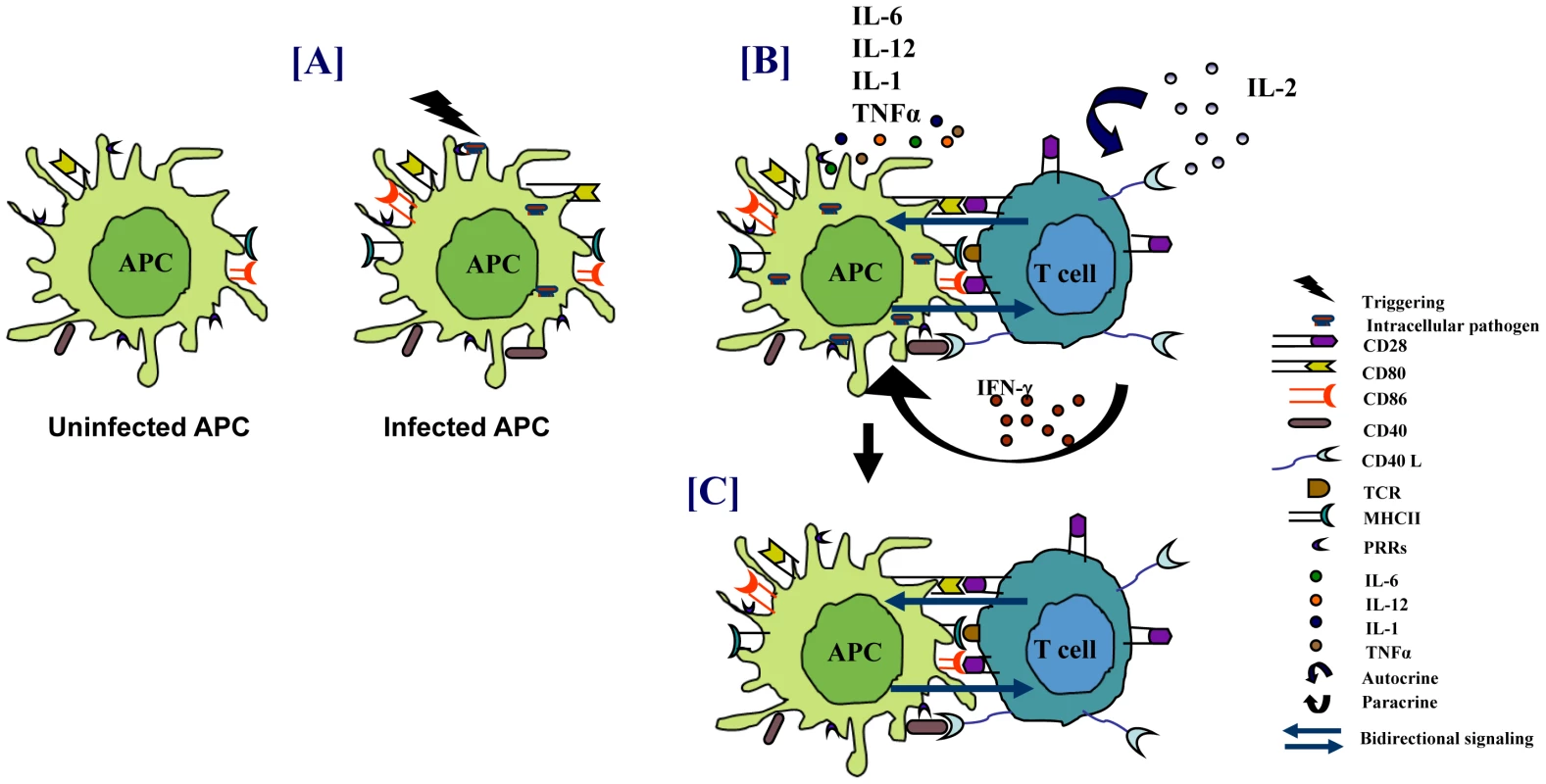
(A) PRRs of APCs sense pathogens that result in the activation of APCs. (B) This leads to enhanced antigen presentation, upregulation of costimulatory molecules, and secretion of proinflammatory cytokines that promote the activation of T cells. The activated T cells help in elimination of the pathogens. (C) Engagement of costimulatory molecules on APCs by T cells also results in “bidirectional signaling” that activates APCs to restrict the growth of pathogens. Modulation of Costimulatory Molecules by Bacteria
Intracellular pathogens like Mtb, Mycobacteria avium, M. leprae, Salmonella typhi (S. typhi), Helicobacter pylori (H. pylori), etc., infect both macrophages and DCs. These cells recognize bacterial components known as pathogen-associated molecular patterns (PAMPs) through their pathogen recognition receptors (PRRs). This triggers innate immunity that initiates antimicrobial defense mechanisms involving autophagy, apoptosis, release of antimicrobial compounds like IFN-γ and TNF-α, etc. [12]. It is followed by activation of adaptive immunity. The adaptive immune response, personified by specificity and memory, involves the clonal selection of B cells and T cells that confer humoral immunity (HI) and cell-mediated immunity (CMI) to pathogens, respectively. Pathogen-specific Th1 cells release cytokines, especially IFN-γ and TNF-α, which plays an imperative role in activating infected macrophages and restraining the microbial growth [13]. Further, ligation of CD80/CD86 with CD28 is essential for preventing apoptosis in T cells [7], [14]. Moreover, CD40/CD40L interaction results in efficient IL-12 production together with an upregulation of costimulatory molecules, in addition to enhanced antigen presentation by APCs thereby leading to a boosted T-cell response (Figure 1) [6], [15]. Hence, interference in the down-modulation of any of these pathways by the pathogens would be detrimental to the host.
Mtb is one of the most successful pathogens in the history of mankind. There are numerous reports indicating the role of mycobacteria in downregulating the expression of CD80, CD86, and CD40 on APCs [16], [17]. A recent study showed, albeit for BCG, that MHC-II, CD80, CD86, and CD40 are down-tuned during chronic phase of infection [16]. In such studies, it is important to delineate the expression of these molecules on infected versus non-infected cells, as by-stander inflammation could interfere in the interpretation of results. An elegant study demonstrated that the expression of costimulatory molecules and MHC are downregulated in macrophages infected with fluorescent reporter bacteria [16], [18]. In contrast, others suggested the augmentation of costimulatory molecules upon infection [19]. This discrepancy may be primarily dependent on the strain, system, or time-point of the study.
Downregulation of CD80/CD86 or upregulation of CTLA-4 by bacteria on APCs may induce anergy/apoptosis of interacting T cells [20], [21]. Defect in this signaling pathway is known to paralyze the release of IL-2, which may compromise the generation of T-cell memory [22]. Impediment in CD28 signaling interferes in IFN-γ production and hence promotes the survival of pathogens. It has been reported that M. leprae obstructs CD28/B7 signaling pathway for rendering antigen-specific T cell unresponsive in lepromatous leprosy patients [21]. Recently, the importance of CD80/CD86 in controlling mycobacterial infection has been demonstrated in CD80/CD86 double knockout mice [23]. The down-modulation of CD80/CD86 in chronic phase of the infection suggests that mycobacteria may actively exploit this pathway to anergize the T cells (Figure 2). Protective CMI is always associated with the release of chemokines and migration of immune cells to the site of infection. Mtb impair chemokines secretion by interfering with the CD28-B7 signaling pathway thereby obstructing the surveillance of immune cells and enhancing the propagation of the bacterium [24], [25]. It has been shown that the most abundant cell wall lipid trehalose 6, 6′-dimycolate (TDM) of Mtb and MTSA-10 inhibit the expression of costimulatory molecules on the surface of the macrophages [26], [27]. Similarly, CD40-CD40L interaction is very important in mediating efficient protection against mycobacteria [28]. Indeed, it has been shown in vitro and in lepromatous patients that CD40 is downregulated by M. leprae [29], [30]. There is indirect evidence indicating the involvement of Mtb manipulating CD40/CD40L expression. In humans, CD40L expression on Th1 cells of tuberculosis (TB) patients has been correlated with the intensity of IFN-γ secretion [31]. However, CD40 but not CD40L knockout mice are susceptible to Mtb infection [32], [33]. It is reported that the heat shock protein 70 (HSP70) of Mtb acts as an alternate ligand for CD40. Corroboratively, over-expression of HSP70 interferes with long-time persistence of Mtb and allows its clearance [34]. Interestingly, in chronic mycobacterial infections, CD40 is suppressed on infected cells [16]. Therefore, it is intriguing to speculate that in the chronic phase of infection, mycobacteria may hamper CD40 expression or manipulate CD40L signaling through binding with HSP70 instead of CD40L in order to evade host defense mechanisms.
Fig. 2. Pathogens modulate the expression of costimulatory molecules for their survival. 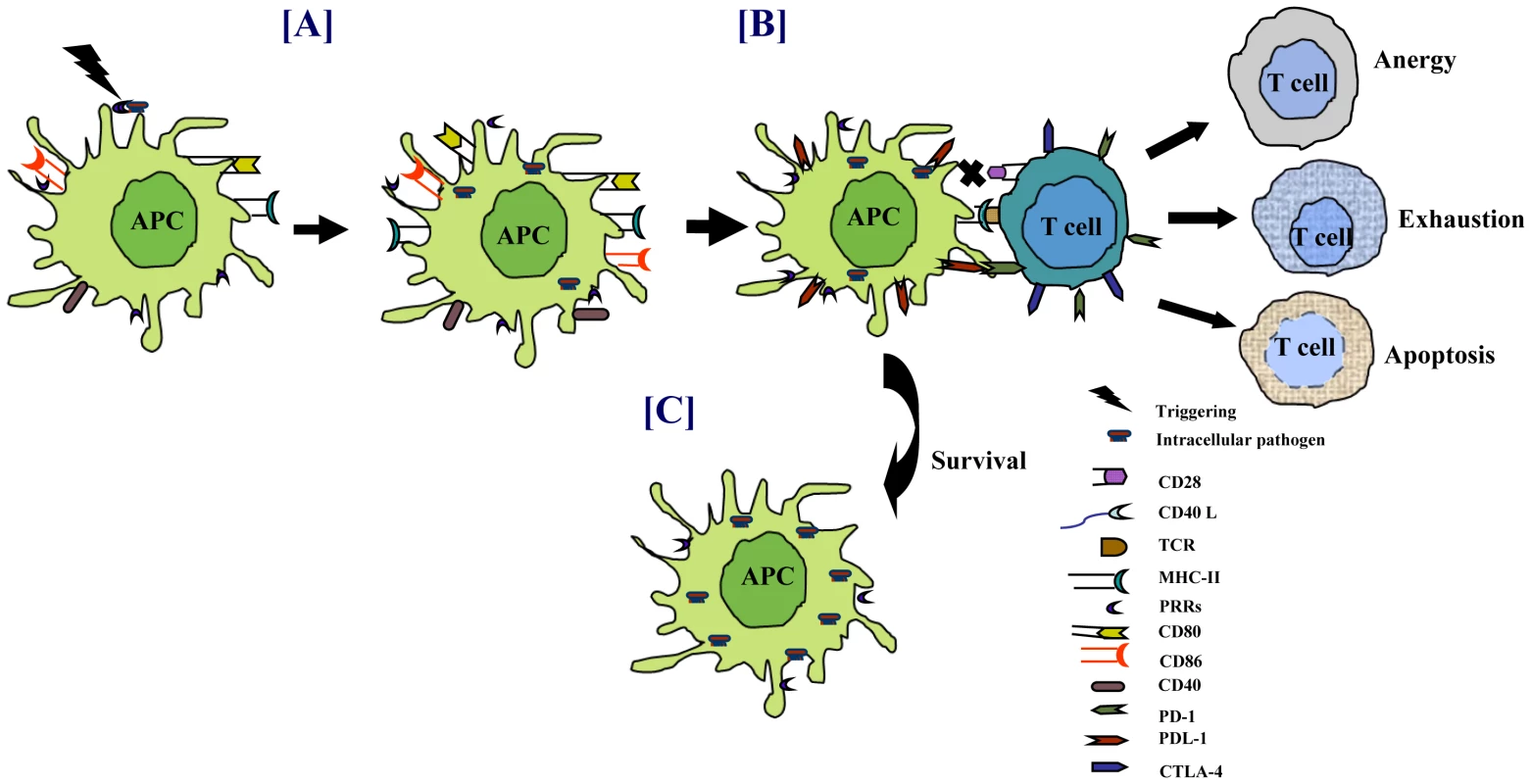
Sensing of pathogens through PRRs triggers the activation of APCs. (A) Costimulatory molecules, which act as the second signal for T-cell activation, are upregulated on infected cells. Persistence of intracellular pathogens modulates the expression of costimulatory molecules, such as downregulation of CD40/CD80/CD86 and upregulation of PDL-1 on infected APCs. Similarly, retarding the exhibition of CD28/CD40L augments PD-1/CTLA-4 on T cells. (B) Interaction of T cells with the infected APCs impairs the function of T cells by inducing anergy, apoptosis, or exhaustion. (C) Lack of T-cell help impedes the activity of APCs, eventually enhancing the survival of pathogens. Mycobacteria can upregulate the expression of PD-1 and its ligands PDL-1/PDL-2 [35]. Several studies have shown that T cells and natural killer (NK) cells from TB patients have increased PD-1 expression [36], [37]. Blockade of PD-1 interaction with PDL-1/PDL-2 enhances immune response. These observations suggest that mycobacteria may exploit PD-1 and PDL-1/PDL-2 pathways to dampen the host immune responses. In contrast, some studies have suggested that these pathways may be involved in controlling exuberant T-cell responses in TB and hence may be beneficial for both the host and the pathogen [36], [38], [39]. Mtb retards the development and maturation of monocyte-derived DCs to limit the immune response [40]. These immature DCs have a tolerogenic effect in vivo that can result in the generation of regulatory T cells (Tregs). Tregs can restrain the proliferation of naive and memory T cells, thereby suppressing pre-existing T-cell immunity [41], [42].
Besides mycobacteria, many other pathogens can exploit CD80/CD86-CD28/CTLA-4 pathways for their persistence (Table 1). H. pylori causes chronic infection in the gut resulting in peptic ulcers. Further, it is known to induce the expression of CTLA-4, resulting in the anergy of T cells and poor clearance of the bacteria [43]. Yersinia pseudotuberculosis decreases CD86 expression on B cells and impedes the function of both B cells and T cells [44]. S. typhi is known to suppress ICAM-1 and as a consequence reduces the antigen uptake by APCs and inadequate T-cell response [45], [46]. H. pylori diminishes the expression of CD40L on T cells and therefore employs CD40/CD40L pathway for its survival. Furthermore, it upregulates PDL-1 expression on gastric epithelial cells and inhibits the activation of T cells recruited to gastric mucosa [47]. In addition, it has been reported that PDL-1 upregulation not only blocks T-cell proliferation and IL-2 secretion, but also promotes the development of Tregs [48]. Bordetella pertussis and B. bronchiseptica decrease the manifestation of CD40 and ICAM-1 on DCs, and subsequently, render DCs tolerogenic and promote chiefly Tregs but not Th1 cells [49], [50]. Hence, costimulatory molecules could serve as attractive targets for bacteria to modulate in order to prolong their own survival.
Tab. 1. Exploitation of costimulatory molecules by intracellular pathogens. 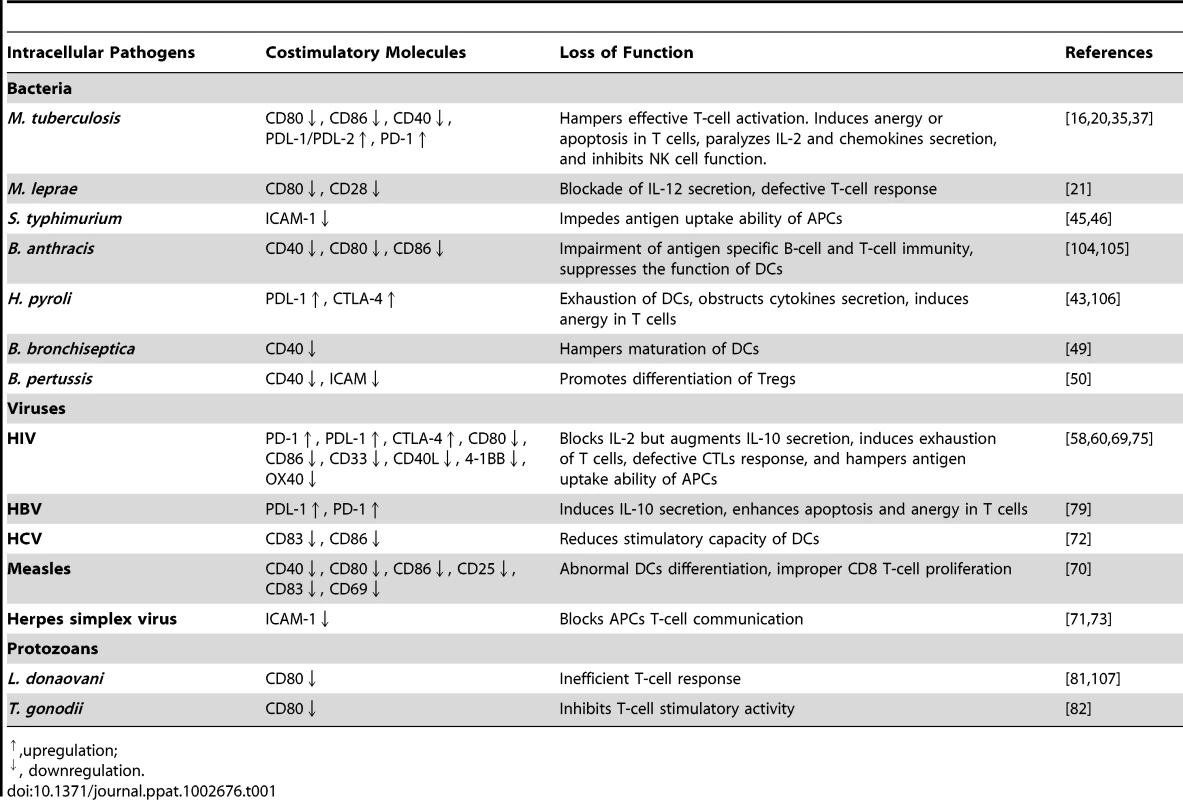
,upregulation; Modulation of Costimulatory Molecules by Viruses
To counter viral infestation, the vertebrate immune system has evolved complex antiviral innate and adaptive immune mechanisms. Some of the spectacular examples to limit viral replication by the host cells are the production of interferons, NK cell-mediated lysis, and apoptosis of the infected cells [51], [52]. Adaptive antiviral immunity relies greatly on the lysis of infected cells by cytotoxic CD8+ T cells and neutralizing antibodies secreted by B cells.
HIV is a retrovirus that is responsible for 1.9 million deaths annually [53]. It predominantly infects CD4+ T cells. Further, it invades DCs, monocytes, and macrophages expressing CD4 and one of its coreceptors CCR5 or CXCR4 [54]. Additionally, HIV can bind to DC-SIGN and mannose receptors [55]. However, the virus preferentially replicates in the activated HIV-specific CD4+ T cells [56]. Indeed, excessive loss of CD4+ T cells is the hallmark of HIV infection [57]. Like many other intracellular pathogens, HIV efficiently exploits the costimulatory molecules to override the immune responses. Its infection is associated with decreased expression of CD40L on CD4+ T cells [58]. Upon activation, CD4+ T cells from individuals with progressive disease show very little upregulation of CD40L, which corroborates with their inability to help APCs and failure to induce IL-12 in DCs [59]. Further, CD40-CD40L interaction has been demonstrated to be important in engendering a robust HIV-specific CD8+ T-cell response. Furthermore, HIV interferes in the CD40 signaling pathway in B cells and hinders T cell help, thereby impairing the secretion of IgG and IgA antibodies [60], [61]. AIDS patients suffer from a defective humoral immunity, which may be due to loss of T-cell function, or B-cell intrinsic defects [62], [63]. HIV upregulates Fas and FasL (members of TNF superfamily) on CD8+ T cells and APCs, respectively, which leads to the apoptosis of the interacting CD8+ T cells [64], [65]. Thus, in HIV infections, the hunter becomes the hunted!
During viral infections, continual expression of CD80/CD86 on DCs is decisive to maintain the effector function of CD8+ T cells [66]. Intriguingly, HIV downregulates the expression of CD80/CD86 and their ligand CD28 on infected APCs and T cells, respectively [67], [68]. The expression of costimulatory molecules such as 4-1BBL, CD70, OX40, and OX40L is affected during HIV-1 infection [9], [69]. Measles, herpes, and hepatitis C viruses (HCV) retard the expression of CD80, CD86, CD25, CD83, and CD40 that leads to poor CD8+ T-cell priming [70]–[72]. In addition, Herpes virus suppresses ICAM-1 on APCs, thereby obstructing immunological synapse with T cells [73].
Chronic viral infections are associated with loss of function in T cells, a phenomenon popularly known as T-cell exhaustion. Exhausted T cells highly express PD-1 and have poor effector function [74]. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression [75]. PD-1 is known to make CD8+ T cells more susceptible to Fas-mediated lysis [76]. Signaling through PD-1 can suppress IL-2 secretion by CD8+ T cells. IL-2 is known to rescue T cells from anergy and boost the memory response [77]. In addition, upregulation of PDL-1 on APCs during HIV or hepatitis B virus (HBV) infection supports the survival of pathogens (Table 1) [78], [79]. In essence, viruses can exploit costimulatory molecules in restraining the function of both T cells and APCs.
Modulation of the Expression of Costimulatory Molecules by Intracellular Protozoan Parasites
T cells play a significant role in controlling the protozoa-inflicted diseases like malaria, visceral leishmaniasis, and trypanosomiasis. Humoral responses play a less important role. However, complement-mediated killing or opsonization is responsible for control of pathogen during the intermittent extracellular phase of its replication cycle within the vertebrate host [80].
Parasites like Leishmania donovoni, L. chagasi, Toxoplasma gonodii (T. gonodii), T. cruzi, and Plasmodium falciparum can manipulate the costimulatory molecules for evading immune system. L. donovani and L. chagasi infect macrophages and are reported to downregulate both CD80 and ICAM-1 [21], [81]. Such APCs fail to optimally activate T cells. T. gondii and T. cruzi, selectively dampen the exhibition of CD80 and CD28, respectively to impair the function of T cells [82]. CD40-CD40L signaling is crucial for Th1 immunity against L. major, because CD40L/CD40 knockout mice sparsely secrete IL-12, favoring Th2-biased response [83], [84]. Interestingly, L. major can differentially modulate the expression of CD40 and thus anergizes T cells and promotes Tregs population [83]. Plasmodium interferes in the signaling mechanism of CD40 in DCs [85]. CD40 deficient mice succumb to plasmodium infection. This signifies that this pathway is detrimental in providing sterilizing immunity to parasites, as it activates APCs and protective Th1 responses. In conclusion, parasites can efficiently empower the immune system by dampening the expression of costimulatory molecules.
Mechanism Involved in Immunomodulation of Costimulatory Molecules
Induction of the expression of costimulatory molecules on exposure to various inflammatory cytokines (IL-6, IL-12, TNF-α, IFN-γ) or PAMPS/DAMPs is regulated by transcription factors such as NF-κB, IRF-3, AP-1, NFAT [86], [87]. Activation of these transcription factors is tightly regulated by various kinases or phosphatases that include mitogen-activated protein kinases (MAPKs), TNF receptor-associated factor proteins (TRAF), IL-1 receptor-associated kinase 4 (IRAK4), phosphoinositide 3-kinase (PI3K), and Janus-kinase. For example, triggering of TLR-4 with lipopolysaccharides (LPS) elicits pathways dependent on myeloid differentiation primary response gene 88 (MyD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF). Both the molecules induce downstream signaling to activate MAPKs, NF-κB, and IRF family proteins, which are responsible for the enhanced expression of CD40 and CD86 [86], [88]. Further, T-cell interaction with APC involving TCR and costimulatory molecules activates a plethora of downstream signaling molecules, leading to the induction of the expression of CD40L, PD-1, and CD28 [89], [90].
Intracellular pathogens utilize an array of mechanisms to manipulate costimulatory molecules. Upon infection, pathogens trigger IL-10 secretion by inhibiting p38MAP kinases and promoting ERK phosphorylation [91]. IL-10 blocks the degradation of IκB-α that inhibits the NF-κB activation eventually inhibiting the expression of costimulatory molecules [92]. Interference in TLRs signaling by the intracellular pathogens is considered to be a foremost event in the suppression of costimulatory molecules. Mannosylated lipoarabinomannan (ManLAM) of Mtb binds to DC-SIGN and compromises the LPS-induced activation of DCs by interfering in the TLRs' signalling [93]. Binding of Mtb early secreted antigenic target protein 6 (ESAT-6) to TLR-2 activates AKT and prevents interaction between the MyD88 and IRAK4, consequently abrogating NF-κB activation that suppresses CD80 expression [94]. Similarly, HIV upregulates PDL-1 on DCs and monocytes by attenuating the signaling of TLR-7 and TLR-8 [95]. In addition, HIV-mediated PI3K activation upregulates PDL-1 on APCs and thus suppresses the activation of HIV-specific CD8+ T cells [96]. Rapid endocytosis of costimulatory molecules upon infection is suggested as one of the mechanisms to interrupt the function of APCs. For instance, Nef protein of HIV modulates the actin-dependent trafficking mechanism to remove CD80/CD86 from the monocytes surface, making them inefficient to activate T cells [97].
TCR signaling is central for the optimum expression of costimulatory molecules and effector function of T cells. Therefore, it may serve as an important target for pathogens to paralyze T-cell activity [98]. Mtb glycolipids interfere with TCR signaling and block the activation of CD4+ T cells [99]. HIV gp120 interacts with CD4 (a coreceptor for TCR) that inhibits intracellular signal transduction through TCR, leading to decreased hydrolysis of polyphosphoinositide (PI), Ca2+ influx, activation of protein kinase C (PKC), and eventually failure of NFAT translocation. These events culminate in the inhibition of CD40L expression. Decline of CD40L on T cells abrogates the bidirectional signaling and reduces the exhibition of CD80 on APCs [100]. In contrast, Nef activates NFAT and stimulates IL-2 release to overcome the exogenous requirement of IL-2 to promote T-cell proliferation, consequently disseminating the infection [101]. However, Nef retards CD28 and CD4 expression, thereby making T cells incapable of promoting CMI [98]. Corroborative with these observations, Nef-mutated HIV is unable to cause persistent infection. Similarly, core protein of HCV inhibits TCR signaling and upregulates PD-1 [102]. The above-mentioned mechanisms imply that pathogens can effectively utilize various costimulatory pathways to subvert immune response to persist in the host.
Therapeutic Implications
The noncompliance with the relatively high dose and extended therapeutic regime reduces the effectiveness of current drugs, leading to global emergence of MDR/XDR/TDR pathogenic strains. Interestingly, costimulatory molecules have been suggested for therapeutic intervention to treat lymphoma patients [4], [103]. To develop alternative or adjunct (with drugs) therapies, an intensive effort has been undertaken in last decade to understand how intracellular pathogens exploit costimulatory molecules, which are the tour de force of the immune system [27], [31], [68], [78]. The potent role of costimulatory molecules is aptly established in the optimum activation of T cells and APCs; the cells that play a cardinal role in curbing the infections. Hence, immunotherapy involving costimulatory molecules can be a breakthrough strategy to treat various diseases, minimizing side effects inflicted by drug therapies and in restricting the emergence of drug resistance.
Zdroje
1. FinlayBBMcFaddenG 2006 Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell 124 767 782
2. CurtsingerJMMescherMF 2010 Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22 333 340
3. JenkinsMK 1994 The ups and downs of T cell costimulation. Immunity 1 443 446
4. SuvasSSinghVSahdevSVohraHAgrewalaJN 2002 Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem 277 7766 7775
5. GowthamanUChodisettiSBAgrewalaJN 2010 T cell help to B cells in germinal centers: putting the jigsaw together. Int Rev Immunol 29 403 420
6. CellaMScheideggerDPalmer-LehmannKLanePLanzavecchiaA 1996 Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 184 747 752
7. OrabonaCGrohmannUBelladonnaMLFallarinoFVaccaC 2004 CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol 5 1134 1142
8. BanchereauJBazanFBlanchardDBriereFGalizziJP 1994 The CD40 antigen and its ligand. Annu Rev Immunol 12 881 922
9. De KeersmaeckerBHeirmanCCorthalsJEmpsenCvan GrunsvenLA 2011 The combination of 4-1BBL and CD40L strongly enhances the capacity of dendritic cells to stimulate HIV-specific T cell responses. J Leukoc Biol 89 989 999
10. WalunasTLLenschowDJBakkerCYLinsleyPSFreemanGJ 1994 CTLA-4 can function as a negative regulator of T cell activation. Immunity 1 405 413
11. KeirMEButteMJFreemanGJSharpeAH 2008 PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26 677 704
12. KrutzikSRSielingPAModlinRL 2001 The role of Toll-like receptors in host defense against microbial infection. Curr Opin Immunol 13 104 108
13. CooperAM 2009 Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27 393 422
14. BoiseLHMinnAJNoelPJJuneCHAccavittiMA 1995 CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 3 87 98
15. GrewalISFlavellRA 1996 The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev 153 85 106
16. SchreiberHAHulsebergPDLeeJPrechlJBartaP 2010 Dendritic cells in chronic mycobacterial granulomas restrict local anti-bacterial T cell response in a murine model. PLoS One 5 e11453 doi:10.1371/journal.pone.0011453
17. BonatoVLMedeirosAILimaVMDiasARFacciolitiLH 2001 Downmodulation of CD18 and CD86 on macrophages and VLA-4 on lymphocytes in experimental tuberculosis. Scand J Immunol 54 564 573
18. PecoraNDFultonSARebaSMDrageMGSimmonsDP 2009 Mycobacterium bovis BCG decreases MHC-II expression in vivo on murine lung macrophages and dendritic cells during aerosol infection. Cell Immunol 254 94 104
19. HendersonRAWatkinsSCFlynnJL 1997 Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol 159 635 643
20. SahaBDasGVohraHGangulyNKMishraGC 1994 Macrophage-T cell interaction in experimental mycobacterial infection. Selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol 24 2618 2624
21. AgrewalaJNKumarBVohraH 1998 Potential role of B7-1 and CD28 molecules in immunosuppression in leprosy. Clin Exp Immunol 111 56 63
22. FuseSZhangWUsherwoodEJ 2008 Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol 180 1148 1157
23. BhattKUzelacAMathurSMcBrideAPotianJ 2009 B7 costimulation is critical for host control of chronic Mycobacterium tuberculosis infection. J Immunol 182 3793 3800
24. HeroldKCLuJRulifsonIVezysVTaubD 1997 Regulation of C-C chemokine production by murine T cells by CD28/B7 costimulation. J Immunol 159 4150 4153
25. AriasMAJaramilloGLopezYPMejiaNMejiaC 2007 Mycobacterium tuberculosis antigens specifically modulate CCR2 and MCP-1/CCL2 on lymphoid cells from human pulmonary hilar lymph nodes. J Immunol 179 8381 8391
26. Kan-SuttonCJagannathCHunterRLJr 2009 Trehalose 6,6′-dimycolate on the surface of Mycobacterium tuberculosis modulates surface marker expression for antigen presentation and costimulation in murine macrophages. Microbes Infect 11 40 48
27. SinghBSinghGTrajkovicVSharmaP 2003 Intracellular expression of Mycobacterium tuberculosis-specific 10-kDa antigen down-regulates macrophage B7.1 expression and nitric oxide release. Clin Exp Immunol 134 70 77
28. GrewalISBorrowPPamerEGOldstoneMBFlavellRA 1997 The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol 9 491 497
29. YamauchiPSBleharskiJRUyemuraKKimJSielingPA 2000 A role for CD40-CD40 ligand interactions in the generation of type 1 cytokine responses in human leprosy. J Immunol 165 1506 1512
30. MurrayRASiddiquiMRMendilloMKrahenbuhlJKaplanG 2007 Mycobacterium leprae inhibits dendritic cell activation and maturation. J Immunol 178 338 344
31. SamtenBThomasEKGongJBarnesPF 2000 Depressed CD40 ligand expression contributes to reduced gamma interferon production in human tuberculosis. Infect Immun 68 3002 3006
32. Campos-NetoAOvendalePBementTKoppiTAFanslowWC 1998 CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol 160 2037 2041
33. LazarevicVMyersAJScangaCAFlynnJL 2003 CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity 19 823 835
34. StewartGRSnewinVAWalzlGHussellTTormayP 2001 Overexpression of heat-shock proteins reduces survival of Mycobacterium tuberculosis in the chronic phase of infection. Nat Med 7 732 737
35. SakaiSKawamuraIOkazakiTTsuchiyaKUchiyamaR 2010 PD-1-PD-L1 pathway impairs T(h)1 immune response in the late stage of infection with Mycobacterium bovis bacillus Calmette-Guerin. Int Immunol 22 915 925
36. JuradoJOAlvarezIBPasquinelliVMartinezGJQuirogaMF 2008 Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol 181 116 125
37. AlvarezIBPasquinelliVJuradoJOAbbateEMusellaRM 2010 Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J Infect Dis 202 524 532
38. Lazar-MolnarEChenBSweeneyKAWangEJLiuW 2010 Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A 107 13402 13407
39. BarberDLMayer-BarberKDFengCGSharpeAHSherA 2010 CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol 186 1598 1607
40. HanekomWAMendilloMMancaCHaslettPASiddiquiMR 2003 Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J Infect Dis 188 257 266
41. ShortmanKHeathWR 2001 Immunity or tolerance? That is the question for dendritic cells. Nat Immunol 2 988 989
42. LevingsMKSangregorioRRoncaroloMG 2001 Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med 193 1295 1302
43. AndersonKMCzinnSJRedlineRWBlanchardTG 2006 Induction of CTLA-4-mediated anergy contributes to persistent colonization in the murine model of gastric Helicobacter pylori infection. J Immunol 176 5306 5313
44. YaoTMecsasJHealyJIFalkowSChienY 1999 Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J Exp Med 190 1343 1350
45. PryjmaJBaranJErnstMWoloszynMFladHD 1994 Altered antigen-presenting capacity of human monocytes after phagocytosis of bacteria. Infect Immun 62 1961 1967
46. WyantTLTannerMKSzteinMB 1999 Potent immunoregulatory effects of Salmonella typhi flagella on antigenic stimulation of human peripheral blood mononuclear cells. Infect Immun 67 1338 1346
47. DasSSuarezGBeswickEJSierraJCGrahamDY 2006 Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J Immunol 176 3000 3009
48. BeswickEJPinchukIVDasSPowellDWReyesVE 2007 Expression of the programmed death ligand 1, B7-H1, on gastric epithelial cells after Helicobacter pylori exposure promotes development of CD4+ CD25+ FoxP3+ regulatory T cells. Infect Immun 75 4334 4341
49. SkinnerJAReissingerAShenHYukMH 2004 Bordetella type III secretion and adenylate cyclase toxin synergize to drive dendritic cells into a semimature state. J Immunol 173 1934 1940
50. RossPJLavelleECMillsKHBoydAP 2004 Adenylate cyclase toxin from Bordetella pertussis synergizes with lipopolysaccharide to promote innate interleukin-10 production and enhances the induction of Th2 and regulatory T cells. Infect Immun 72 1568 1579
51. EverettHMcFaddenG 1999 Apoptosis: an innate immune response to virus infection. Trends Microbiol 7 160 165
52. PriceDAKlenermanPBoothBLPhillipsRESewellAK 1999 Cytotoxic T lymphocytes, chemokines and antiviral immunity. Immunol Today 20 212 216
53. World Health Organization 2010 Global health observatory (GHO). Available: http://www.who.int/gho/hiv/en/index.html Accessed 5 June 2012
54. DavisCBDikicIUnutmazDHillCMArthosJ 1997 Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med 186 1793 1798
55. GeijtenbeekTBvan KooykY 2003 DC-SIGN: a novel HIV receptor on DCs that mediates HIV-1 transmission. Curr Top Microbiol Immunol 276 31 54
56. DouekDCBrenchleyJMBettsMRAmbrozakDRHillBJ 2002 HIV preferentially infects HIV-specific CD4+ T cells. Nature 417 95 98
57. Rowland-JonesS 1999 HIV infection: where have all the T cells gone? Lancet 354 5 7
58. KornbluthRS 2002 An expanding role for CD40L and other tumor necrosis factor superfamily ligands in HIV infection. J Hematother Stem Cell Res 11 787 801
59. Smed-SorensenALoreKWalther-JallowLAnderssonJSpetzAL 2004 HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood 104 2810 2817
60. KornbluthRS 2000 The emerging role of CD40 ligand in HIV infection. J Leukoc Biol 68 373 382
61. QiaoXHeBChiuAKnowlesDMChadburnA 2006 Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat Immunol 7 302 310
62. CagigiANilssonAPensierosoSChiodiF 2010 Dysfunctional B-cell responses during HIV-1 infection: implication for influenza vaccination and highly active antiretroviral therapy. Lancet Infect Dis 10 499 503
63. De MilitoANilssonATitanjiKThorstenssonRReizensteinE 2004 Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103 2180 2186
64. BoudetFLecoeurHGougeonML 1996 Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J Immunol 156 2282 2293
65. GehriRHahnSRothenMSteuerwaldMNueschR 1996 The Fas receptor in HIV infection: expression on peripheral blood lymphocytes and role in the depletion of T cells. Aids 10 9 16
66. DolfiDVDuttaguptaPABoesteanuACMuellerYMOliaiCH 2011 Dendritic cells and CD28 costimulation are required to sustain virus-specific CD8+ T cell responses during the effector phase in vivo. J Immunol 186 4599 4608
67. ChaudhryADasSRHussainAMayorSGeorgeA 2005 The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J Immunol 175 4566 4574
68. VenkatachariNJMajumderBAyyavooV 2007 Human immunodeficiency virus (HIV) type 1 Vpr induces differential regulation of T cell costimulatory molecules: direct effect of Vpr on T cell activation and immune function. Virology 358 347 356
69. De KeersmaeckerBThielemansKAertsJL 2011 Fighting with the enemy's weapons? the role of costimulatory molecules in HIV. Curr Mol Med 11 172 196
70. Servet-DelpratCVidalainPOBausingerHManieSLe DeistF 2000 Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J Immunol 164 1753 1760
71. SalioMCellaMSuterMLanzavecchiaA 1999 Inhibition of dendritic cell maturation by herpes simplex virus. Eur J Immunol 29 3245 3253
72. Auffermann-GretzingerSKeeffeEBLevyS 2001 Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97 3171 3176
73. CoscoyLGanemD 2001 A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J Clin Invest 107 1599 1606
74. WherryEJ 2011 T cell exhaustion. Nat Immunol 12 492 499
75. DayCLKaufmannDEKiepielaPBrownJAMoodleyES 2006 PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443 350 354
76. PetrovasCCasazzaJPBrenchleyJMPriceDAGostickE 2006 PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203 2281 2292
77. ChikumaSTerawakiSHayashiTNabeshimaRYoshidaT 2009 PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol 182 6682 6689
78. TrabattoniDSaresellaMBiasinMBoassoAPiacentiniL 2003 B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood 101 2514 2520
79. ChenLZhangZChenWZhangZLiY 2007 B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol 178 6634 6641
80. HallBFJoinerKA 1991 Strategies of obligate intracellular parasites for evading host defences. Immunol Today 12 A22 27
81. KayePMRogersNJCurryAJScottJC 1994 Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol 24 2850 2854
82. FischerHGDorflerRSchadeBHaddingU 1999 Differential CD86/B7-2 expression and cytokine secretion induced by Toxoplasma gondii in macrophages from resistant or susceptible BALB H-2 congenic mice. Int Immunol 11 341 349
83. CampbellKAOvendalePJKennedyMKFanslowWCReedSG 1996 CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4 283 289
84. MartinSAgarwalRMurugaiyanGSahaB 2010 CD40 expression levels modulate regulatory T cells in Leishmania donovani infection. J Immunol 185 551 559
85. MukherjeePChauhanVS 2008 Plasmodium falciparum-free merozoites and infected RBCs distinctly affect soluble CD40 ligand-mediated maturation of immature monocyte-derived dendritic cells. J Leukoc Biol 84 244 254
86. QinHWilsonCALeeSJZhaoXBenvenisteEN 2005 LPS induces CD40 gene expression through the activation of NF-kappaB and STAT-1alpha in macrophages and microglia. Blood 106 3114 3122
87. TerawakiSChikumaSShibayamaSHayashiTYoshidaT 2011 IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol 186 2772 2779
88. LimWGeeKMishraSKumarA 2005 Regulation of B7.1 costimulatory molecule is mediated by the IFN regulatory factor-7 through the activation of JNK in lipopolysaccharide-stimulated human monocytic cells. J Immunol 175 5690 5700
89. JaiswalAIDubeyCSwainSLCroftM 1996 Regulation of CD40 ligand expression on naive CD4 T cells: a role for TCR but not co-stimulatory signals. Int Immunol 8 275 285
90. LenschowDJWalunasTLBluestoneJA 1996 CD28/B7 system of T cell costimulation. Annu Rev Immunol 14 233 258
91. RubADeyRJadhavMKamatRChakkaramakkilS 2009 Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat Immunol 10 273 280
92. DingLLinsleyPSHuangLYGermainRNShevachEM 1993 IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol 151 1224 1234
93. GeijtenbeekTBVan VlietSJKoppelEASanchez-HernandezMVandenbroucke-GraulsCM 2003 Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 197 7 17
94. PathakSKBasuSBasuKKBanerjeeAPathakS 2007 Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 8 610 618
95. MeierABagchiASidhuHKAlterGSuscovichTJ 2008 Upregulation of PD-L1 on monocytes and dendritic cells by HIV-1 derived TLR ligands. AIDS 22 655 658
96. MuthumaniKShedlockDJChooDKFagonePKawalekarOU 2010 HIV-mediated phosphatidylinositol 3-kinase/serine-threonine kinase activation in APCs leads to programmed death-1 ligand upregulation and suppression of HIV-specific CD8 T cells. J Immunol 187 2932 2943
97. ChaudhryADasSRJameelSGeorgeABalV 2007 A two-pronged mechanism for HIV-1 Nef-mediated endocytosis of immune costimulatory molecules CD80 and CD86. Cell Host Microbe 1 37 49
98. SwigutTShohdyNSkowronskiJ 2001 Mechanism for down-regulation of CD28 by Nef. Embo J 20 1593 1604
99. MahonRNRojasREFultonSAFrankoJLHardingCV 2009 Mycobacterium tuberculosis cell wall glycolipids directly inhibit CD4+ T-cell activation by interfering with proximal T-cell-receptor signaling. Infect Immun 77 4574 4583
100. ChirmuleNMcCloskeyTWHuRKalyanaramanVSPahwaS 1995 HIV gp120 inhibits T cell activation by interfering with expression of costimulatory molecules CD40 ligand and CD80 (B71). J Immunol 155 917 924
101. ManninenARenkemaGHSakselaK 2000 Synergistic activation of NFAT by HIV-1 nef and the Ras/MAPK pathway. J Biol Chem 275 16513 16517
102. YaoZQKingEPraytherDYinDMoormanJ 2007 T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral Immunol 20 276 287
103. LeonardJPFriedbergJWYounesAFisherDGordonLI 2007 A phase I/II study of galiximab (an anti-CD80 monoclonal antibody) in combination with rituximab for relapsed or refractory, follicular lymphoma. Ann Oncol 18 1216 1223
104. AgrawalALingappaJLepplaSHAgrawalSJabbarA 2003 Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424 329 334
105. ChouPJNewtonCAPerkinsIFriedmanHKleinTW 2008 Suppression of dendritic cell activation by anthrax lethal toxin and edema toxin depends on multiple factors including cell source, stimulus used, and function tested. DNA Cell Biol 27 637 648
106. MitchellPGermainCFioriPLKhamriWFosterGR 2007 Chronic exposure to Helicobacter pylori impairs dendritic cell function and inhibits Th1 development. Infect Immun 75 810 819
107. SahaBDasGVohraHGangulyNKMishraGC 1995 Macrophage-T cell interaction in experimental visceral leishmaniasis: failure to express costimulatory molecules on Leishmania-infected macrophages and its implication in the suppression of cell-mediated immunity. Eur J Immunol 25 2492 2498
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- A Highly Intensified ART Regimen Induces Long-Term Viral Suppression and Restriction of the Viral Reservoir in a Simian AIDS Model
- An Endogenous Foamy-like Viral Element in the Coelacanth Genome
- Evidence for Induction of Integron-Based Antibiotic Resistance by the SOS Response in a Clinical Setting
- Manipulation of Costimulatory Molecules by Intracellular Pathogens: Veni, Vidi, Vici!!
- Highly Efficient Prion Transmission by Blood Transfusion
- Scavenges Host Zinc via Pra1 during Endothelial Invasion
- Protecting against Pneumococcal Disease: Critical Interactions between Probiotics and the Airway Microbiome
- How Do Viruses Interact with Stress-Associated RNA Granules?
- The Interdomain Linker of AAV-2 Rep68 Is an Integral Part of Its Oligomerization Domain: Role of a Conserved SF3 Helicase Residue in Oligomerization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Protecting against Pneumococcal Disease: Critical Interactions between Probiotics and the Airway Microbiome
- Manipulation of Costimulatory Molecules by Intracellular Pathogens: Veni, Vidi, Vici!!
- A Highly Intensified ART Regimen Induces Long-Term Viral Suppression and Restriction of the Viral Reservoir in a Simian AIDS Model
- An Endogenous Foamy-like Viral Element in the Coelacanth Genome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



