-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHydrophobins—Unique Fungal Proteins
article has not abstract
Published in the journal: . PLoS Pathog 8(5): e32767. doi:10.1371/journal.ppat.1002700
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002700Summary
article has not abstract
Microorganisms are often covered by a proteinaceous surface layer that serves as a sieve for external molecular influx, as a shield to protect microbes from external aggression, or as an aid to help microbial dispersion. In bacteria, the latter is called the S-layer, in Actinomycetes, the rod-like fibrillar layer, and in fungi, the rodlet layer [1]. The self-assembly properties and remarkable structural and physicochemical characteristics of hydrophobin proteins underlie the multiple roles played by these unique proteins in fungal biology.
What Are Hydrophobins?
Hydrophobins, low molecular mass (≤20 kDa) secreted proteins of fungi, are characterized by moderate to high levels of hydrophobicity and the presence of eight conserved cysteine (Cys) residues. These proteins are able to assemble spontaneously into amphipathic monolayers at hydrophobic–hydrophilic interfaces. Although functional homologues are reported in Streptomyces (chaplins, SapB, and SapT for aerial morphogenesis; [2]), hydrophobins are unique to the fungal kingdom. Fungal genome analyses have indicated that hydrophobins generally exist as small gene families with two to ten members, although certain species contain more members (e.g., Coprinus cinereus displays 33 members; http://www.broadinstitute.org) [3], [4]. Hydrophobins show very little sequence conservation in general, apart from the idiosyncratic pattern of eight Cys residues implicated in the formation of four disulfide bridges (Cys1–Cys6, Cys2–Cys5, Cys3–Cys4, Cys7–Cys8) [5] (Figure 1). Based on hydropathy plots, solubility and the type of layer they form, hydrophobins are divided into two classes [6, Reference S1 in Text S1], although recent bioinformatics studies suggest that intermediate/different forms can also exist and that many hydrophobins with distinct physicochemical characteristics may have been overlooked in the past [4], [7]. In class I, considerable variation is seen in the inter-Cys-spacing; these hydrophobins assemble into highly insoluble polymeric monolayers composed of fibrillar structures known as rodlets. The rodlets are extremely stable, can only be solubilized with harsh acid treatments, and the soluble forms can polymerize back into rodlets under appropriate conditions. Despite the low sequence similarity, class I hydrophobins from different fungal species could partially complement a Magnaporthe grisea class I hydrophobin gene (MPG1) deletion mutant, suggesting that hydrophobins constitute a closely related group of morphogenetic proteins [8]. The sequence and the inter-Cys spacing are more conserved in class II; the monolayers formed by class II hydrophobins lack the fibrillar rodlet morphology and can be solubilized with organic solvents and detergents.
Fig. 1. Fungal hydrophobins. 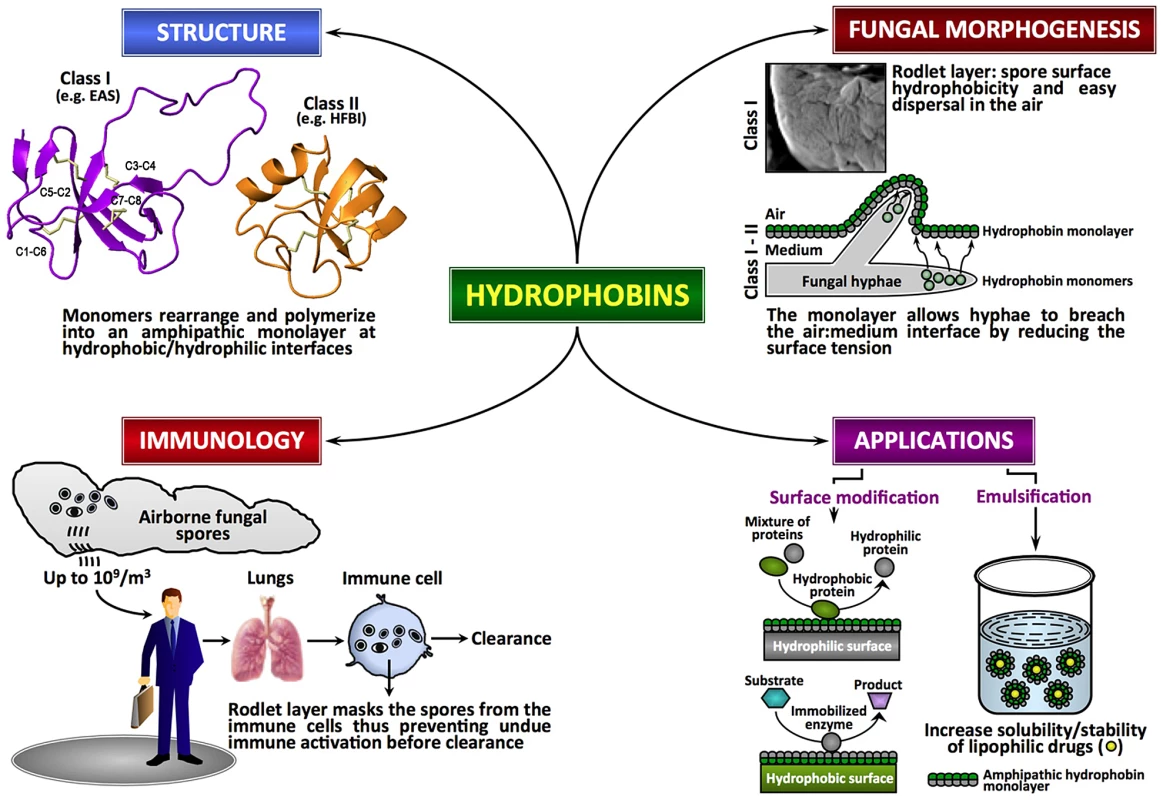
Fungal hydrophobins are unique amphipathic proteins with multiple roles in the fungal life cycle and in mediating interactions between fungus and host. There is diversity in the primary sequences of hydrophobins but they share a similar core three-dimensional structure and a pattern of four disulfide bonds (shown in amber) that stabilize the structures. Increasingly, these proteins show potential for modification of hydrophobic nanomaterials and in solubilizing lipophilic drugs. Hydrophobins at the Interface in the Fungal Life Cycle
Fungi are heterotrophic terrestrial eukaryotes, showing two types of growth morphologies: unicellular yeast and multicellular filamentous forms. Yeasts are hydrophilic and they lack hydrophobins. The vegetative hyphae of filamentous fungi growing on moist environments are also hydrophilic and do not show the presence of rodlets on their surface. In contrast, the aerial hyphae and the asexual spores (conidia) are hydrophobic, due to the presence of hydrophobins. The functions of hydrophobins are related to their high surfactant activity, which results from their self-assembly at hydrophilic–hydrophobic interfaces to form an amphipathic monolayer. The hydrophobin layer reduces the surface tension of the medium or the substratum in/on which fungi grow, allowing them to breach the air–water interface or preventing water-logging while maintaining permeability to gaseous exchange [9]. Spores produced on the aerial structures of filamentous fungi are covered by a hydrophobin rodlet layer that renders the conidial surface hydrophobic and wet-resistant, thus facilitating spore-dispersal in the air. The rodlet-forming hydrophobins are essential for these fungi to complete their biological cycle. In many “wet” fungi (e.g., Conidiobolus obscurus), the rodlet-layer is covered by a mucilaginous extracellular matrix that helps the conidia to bind to the substrate, and once the spores are bound to the host, the rodlet-layer is unmasked for better resistance to the environment [10]. In the basidiomycete Agaricus bisporus, the hydrophobin HypA, found in the peel tissue of the mushroom cap, is suggested to form a protective layer during fruiting body development [11]. In Cryphonectria parasitica, the deletion of the gene coding the class II hydrophobin cryparin generated a mutant incapable of erupting through the bark of the tree [12]. Hydrophobins are also reported to play a role in the surface interaction during infection-related development of M. grisea [13, Reference S2 in Text S1]. In the symbiotic phenotypes of lichen-forming ascomycetes Xanthoria spp., the continuous rodlet-layer seals the apoplast continuum [14].
Structure of Hydrophobins
Hydrophobins from both classes have been studied in vitro and have been shown to be highly surface active and to form amphipathic monolayers on hydrophobic/hydrophilic surfaces. The crystal structures of the class II hydrophobins HFBI and HFBII from Trichoderma reesei have been solved [15], [16]. In addition, the structure of the class I EAS protein from Neurospora crassa has been determined by NMR [5]. These studies indicate that all hydrophobins share a similar small β-structured core that is dictated by the presence of the four disulfide bonds and that the proteins have large exposed hydrophobic surface regions that give rise to their high surface activity. The structures of the class I hydrophobins DewA (Aspergillus nidulans) and Mpg1 (M. grisea) and the class II hydrophobin from N. crassa, as well as the secondary structure of the class I hydrophobins RodA and RodB from Aspergillus fumigatus obtained through the analysis of their backbone NMR chemical shifts, are consistent with this (J. I. Guijarro and M. Sunde, unpublished data). Monolayer formation by class II hydrophobins does not appear to be associated with major conformational changes. In contrast, biophysical analysis of SC3 from S. commune and EAS indicate that rodlet formation is associated with significant structural rearrangements, in some cases involving helical intermediates, but always to a final rodlet form with high β-sheet content and amyloid characteristics [5], [17, Reference S3 in Text S1]. Digestion and hydrogen-deuterium exchange experiments with SC3 [18] indicated that the Cys3–Cys4 loop is important for adhesion to hydrophobic surfaces and may directly participate in the formation of rodlets. However, truncation [19] and systematic site-directed mutagenesis [20] experiments with EAS have shown that the Cys3–Cys4 loop is not involved in rodlet formation and that the Cys7–Cys8 loop region is crucial for auto-assembly, suggesting that the variability of the sequences of class I hydrophobins may translate into different mechanisms of rodlet formation [18]. Nevertheless, the surface tension seems to be the driving force to recruit class I hydrophobins to the air–water interface where the structural changes from the soluble form to the rodlet conformation take place [21].
Hydrophobins and Fungus–Host Interactions
The surface rodlet-layer has a critical role in masking the immunogenicity of airborne fungal spores [22]. By covering the spore surface, the rodlet-layer imparts immunological inertness to the spores and ensures that pathogen-associated molecular patterns (PAMPs) are not recognized by innate and adaptive immune cells, thus preventing the activation of host immune system, inflammation, and tissue damage [22], [23], [24], [25, Reference S4 in Text S1]. Several lines of evidence suggest that the rodlet-layer, which covers the spores of both pathogenic and non-pathogenic fungal species, prevents immune recognition [22], [23], [25] (Figure 1). In opportunistic pathogen A. fumigatus, the rodlet-layer made up of RodA imparts resistance to NETosis (a process associated with disruption of neutrophil-membranes and release of a mixture of nuclear DNA with a granular content that acts as a neutrophil extracellular trap [NET]) and killing by alveolar macrophages [23], [26]. However, removal of RODA and RODB did not affect pathogenicity of A. fumigatus [Reference S5 in Text S1].
In plant-/entomo-pathogenic fungi, hydrophobins are also described as pathogenicity factors, but their precise role in fungal virulence remains to be understood. In the rice blast fungus M. grisea, the hydrophobin Mpg1 is suggested to function as a developmental sensor for appresorium formation, since it is involved in the interaction with hydrophobic leaf surfaces necessary for establishing the pathogenicity [13]. Deletion of the MPG1 gene resulted in a mutant of M. grisea with reduced virulence; the deletion of another hydrophobin gene in M. grisea, MHP1, led also to a loss of viability and a reduced capacity to infect and colonize a susceptible rice cultivar [27]. In Beauveria bassiana, the non-specific hydrophobic interaction between the fungal spore coat hydrophobin and the insect epicuticle is involved in establishing the pathogenicity of the fungus [28].
Prospective Applications of Hydrophobins
The potential applications of hydrophobins rely on their ability to reverse the hydrophilic-hydrophobic character of a surface and/or their surfactant capacity. Several biotechnological applications of hydrophobins have been proposed [29, Reference S6–S12 in Text S1]. However, the large-scale applications of hydrophobins might be difficult to implement due to the production cost of recombinant proteins and/or the large-scale requirements of the proteins. In contrast, in the pharmaceutical or in the nanotechnology industry, where the returns of investment are high, it is possible to envisage a potential development for these proteins. For example, the foam and air-/oil-filled emulsion-forming capacity of hydrophobins has been exploited in protecting nanoparticles and drug formulations [30, Reference S13–S16 in Text S1] (Figure 1). From a therapeutic point of view, the degradation-resistance and immunologically inert properties of hydrophobins could be used to generate hydrophobin-based nanoparticles with embedded therapeutic proteins and molecules that have to be slowly released within the host or transported to a specific body location without being recognized by the host immune system.
Many questions, however, remain unsolved in the study of hydrophobins: for instance, how is the 3D rodlet-structure organized? How are hydrophobins transported to the cell surface? How is the rodlet-layer attached to the spore surface? What are the signals that trigger germination of the spores covered by a rodlet layer? Addressing these questions will reveal the mechanism by which hydrophobins accomplish their multiple roles in the fungal life cycle.
Supporting Information
Zdroje
1. WesselsJDe VriesOAsgeirsdottirSASchurenF 1991 Hydrophobin Genes Involved in Formation of Aerial Hyphae and Fruit Bodies in Schizophyllum. Plant Cell 3 793 799
2. KodaniSLodatoMADurrantMCPicartFWilleyJM 2005 SapT, a lanthionine-containing peptide involved in aerial hyphae formation in the streptomycetes. Mol Microbiol 58 1368 1380
3. SundeMKwanAHTempletonMDBeeverREMackayJP 2008 Structural analysis of hydrophobins. Micron 39 773 784
4. LittlejohnaKAHooleybPCoxPW 2012 Bioinformatics predicts diverse Aspergillus hydrophobins with novel properties. Food Hydrocolloids 27 503 516
5. KwanAHWinefieldRDSundeMMatthewsJMHaverkampRG 2006 Structural basis for rodlet assembly in fungal hydrophobins. Proc Natl Acad Sci U S A 103 3621 3626
6. WesselsJG 1997 Hydrophobins: proteins that change the nature of the fungal surface. Adv Microb Physiol 38 1 45
7. JensenBGAndersenMRPedersenMHFrisvadJCSondergaardI 2010 Hydrophobins from Aspergillus species cannot be clearly divided into two classes. BMC Res Notes 3 344
8. KershawMJWakleyGTalbotNJ 1998 Complementation of the mpg1 mutant phenotype in Magnaporthe grisea reveals functional relationships between fungal hydrophobins. EMBO J 17 3838 3849
9. WangXShiFWostenHAHektorHPoolmanB 2005 The SC3 hydrophobin self-assembles into a membrane with distinct mass transfer properties. Biophys J 88 3434 3443
10. LatgéJPColeGTHoris bergerMPrévostMC 1986 Ultrastructure and chemical composition of the ballistospore wall of Conidiobolus obscurus. Exp Mycol 10 99 113
11. De GrootPWSchaapPJSonnenbergASVisserJVan GriensvenLJ 1996 The Agaricus bisporus hypA gene encodes a hydrophobin and specifically accumulates in peel tissue of mushroom caps during fruit body development. J Mol Biol 257 1008 1018
12. KazmierczakPKimDHTurinaMVan AlfenNK 2005 A hydrophobin of the chestnut blight fungus, Cryphonectria parasitica, is required for stromal pustule eruption. Eukaryot Cell 4 931 936
13. TalbotNJKershawMJWakleyGEDe VriesOWesselsJ 1996 MPG1 Encodes a fungal hydrophobin involved in surface interactions during infection-related development of Magnaporthe grisea. Plant Cell 8 985 999
14. ScherrerSDe VriesOMDudlerRWesselsJGHoneggerR 2000 Interfacial self-assembly of fungal hydrophobins of the lichen-forming ascomycetes Xanthoria parietina and X. ectaneoides. Fungal Genet Biol 30 81 93
15. HakanpaaJPaananenAAskolinSNakari-SetalaTParkkinenT 2004 Atomic resolution structure of the HFBII hydrophobin, a self-assembling amphiphile. J Biol Chem 279 534 539
16. HakanpaaJSzilvayGRKaljunenHMaksimainenMLinderM 2006 Two crystal structures of Trichoderma reesei hydrophobin HFBI–the structure of a protein amphiphile with and without detergent interaction. Protein Sci 15 2129 2140
17. de VochtMLReviakineIUlrichWPBergsma-SchutterWWostenHA 2002 Self-assembly of the hydrophobin SC3 proceeds via two structural intermediates. Protein Sci 11 1199 1205
18. WangXPermentierHPRinkRKruijtzerJALiskampRM 2004 Probing the self-assembly and the accompanying structural changes of hydrophobin SC3 on a hydrophobic surface by mass spectrometry. Biophys J 87 1919 1928
19. KwanAHMacindoeIVukasinPVMorrisVKKassI 2008 The Cys3–Cys4 loop of the hydrophobin EAS is not required for rodlet formation and surface activity. J Mol Biol 382 708 720
20. MacindoeIKwanAHRenQMorrisVKYangW 2012 Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin EAS. Proc Natl Acad Sci U S A 109 E804 E811
21. MorrisVKRenQMacindoeIKwanAHByrneN 2011 Recruitment of class I hydrophobins to the air:water interface initiates a multi-step process of functional amyloid formation. J Biol Chem 286 15955 15963
22. AimaniandaVBayryJBozzaSKniemeyerOPerruccioK 2009 Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460 1117 1121
23. BrunsSKniemeyerOHasenbergMAimaniandaVNietzscheS 2010 Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog 6 e1000873 doi:10.1371/journal.ppat.1000873
24. HohlTMVan EppsHLRiveraAMorganLAChenPL 2005 Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog 1 e30 doi:10.1371/journal.ppat.0010030
25. DagenaisTRGilesSSAimaniandaVLatgeJPHullCM 2010 Aspergillus fumigatus LaeA-mediated phagocytosis is associated with a decreased hydrophobin layer. Infect Immun 78 823 829
26. ParisSDebeaupuisJPCrameriRCareyMCharlesF 2003 Conidial hydrophobins of Aspergillus fumigatus. Appl Environ Microbiol 69 1581 1588
27. KimSAhnIPRhoHSLeeYH 2005 MHP1, a Magnaporthe grisea hydrophobin gene, is required for fungal development and plant colonization. Mol Microbiol 57 1224 1237
28. ZhangSXiaYXKimBKeyhaniNO 2011 Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol Microbiol 80 811 826
29. LinderMBSzilvayGRNakari-SetalaTPenttilaME 2005 Hydrophobins: the protein-amphiphiles of filamentous fungi. FEMS Microbiol Rev 29 877 896
30. ValoHKLaaksonenPHPeltonenLJLinderMBHirvonenJT 2010 Multifunctional hydrophobin: toward functional coatings for drug nanoparticles. ACS Nano 4 1750 1758
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 5- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Five Questions on Prion Diseases
- Type III Secretion in : Injectisome or Not?
- In Vitro and In Vivo Isolation and Characterization of Duvenhage Virus
- CD200 Receptor Controls Sex-Specific TLR7 Responses to Viral Infection
- From Molecular Genetics to Phylodynamics: Evolutionary Relevance of Mutation Rates Across Viruses
- Evolution of an Eurasian Avian-like Influenza Virus in Naïve and Vaccinated Pigs
- Vitamin D Inhibits Human Immunodeficiency Virus Type 1 and Infection in Macrophages through the Induction of Autophagy
- Influence of Microbiota on Viral Infections
- Hydrophobins—Unique Fungal Proteins
- Interferon-Induced Protects Mice from Lethal VSV Neuropathogenesis
- A New Evolutionary Model for Hepatitis C Virus Chronic Infection
- The [Het-s] Prion, an Amyloid Fold as a Cell Death Activation Trigger
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Type III Secretion in : Injectisome or Not?
- Hydrophobins—Unique Fungal Proteins
- In Vitro and In Vivo Isolation and Characterization of Duvenhage Virus
- The [Het-s] Prion, an Amyloid Fold as a Cell Death Activation Trigger
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



