-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSexual Development in : Lessons from Functional Analyses
article has not abstract
Published in the journal: . PLoS Pathog 8(1): e32767. doi:10.1371/journal.ppat.1002404
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002404Summary
article has not abstract
Malaria is a devastating global disease with several hundred million clinical cases and just under 1 million deaths each year (http://www.who.int/topics/malaria/). It is caused by protozoan parasites of the genus Plasmodium, which have a complex life cycle in a vertebrate host and a mosquito vector. Malaria parasites are haploid throughout most of this life cycle, replicating by asexual multiplication twice in a mammalian host: in liver hepatocytes (pre-erythrocytic schizogony) and within red blood cells (blood stage schizogony), and once in the mosquito (sporogony). The essential sexual stage occurs at the transmission from vertebrate to insect. Some asexual blood stage parasites develop into either male or female gametocytes (the precursor sex cells) and following ingestion in a mosquito blood meal differentiate further into gametes in the lumen of the mosquito's gut, where fertilization takes place. The core processes of gametocytogenesis, gamete activation, exflagellation, fertilization, and zygote formation are conserved across the species from the human parasite Plasmodium falciparum to the rodent parasite Plasmodium berghei, which is an attractive laboratory model, in part because of its ease of genetic manipulation and the shorter time for the maturation and differentiation of the sexual stages. Here, we focus largely on recent functional studies using reverse genetics that have uncovered many aspects of the parasite's sexual development.
The Onset of the Sexual Phase
In the asexual blood stage of multiplication, merozoites invade erythrocytes, mitotic division produces a multinucleate syncitium (the schizont), and then cytokinesis produces daughter merozoites that burst out and invade fresh erythrocytes. However, a sub-population of intracellular parasites that forego mitosis undergoes gametocytogenesis (the production of gametocytes) in preparation for the sexual phase. The master regulator(s) of this commitment are completely unknown, but gametocytogenesis can be induced chemically or by culture conditions in vitro. The process involves environmental stress responses, depends on parasite density and genetic variation, and is mediated by a number of signaling pathways [1], [2]. Immune factors and the male:female gametocyte allocation as a fitness trait are important in reproduction [3].
At the molecular level, little is known of the mechanisms by which an individual parasite is triggered to differentiate into a gametocyte. The current consensus is that all merozoites derived from a single schizont are committed to becoming either male or female gametocytes, suggesting that commitment is determined in this previous asexual cycle. Studies using reporters (such as green fluorescent protein [GFP]) controlled by gametocyte-specific promoters (including those for PF14_748, pfs16, pfg27, and SET) have all implicated the previous schizont stage in commitment to gametocytogenesis [4]. Gender-specific markers introduced using reverse genetics have been observed in sibling progeny from single schizonts that have committed to gametocytogenesis, including pfg377, expressed only in females and with a role in egress from the erythrocyte, and α-tubulin II, expressed only in males. Gene disruption studies of other putative gametocyte developmental markers in P. falciparum (the most important cause of human malaria) include gene implicated in gametocytogenesis (pfgig) (deletion resulted in reduced gametocyte production) and male development gene 1 (pfmdv-1) (deletion resulted in reduced gametocyte production and defects in male gamete exflagellation) (as reviewed in [5]). More recently, an RNA binding protein, pfpuf2, and a novel transporter, npt1, were proposed to regulate gametocytogenesis and sexual differentiation [6], [7] (Figure 1). Together, all these studies suggested that there is a gender-specific commitment to gametocytogenesis determined at an early stage in asexual replication. Since sexual development is essential for transmission to the mosquito and completion of the life cycle, this stage is considered to be a major focus for drug and vaccine development to prevent transmission and disrupt the cycle [1], [2].
Fig. 1. Regulators of gametocytogenesis and the sexual stage of the life cycle. 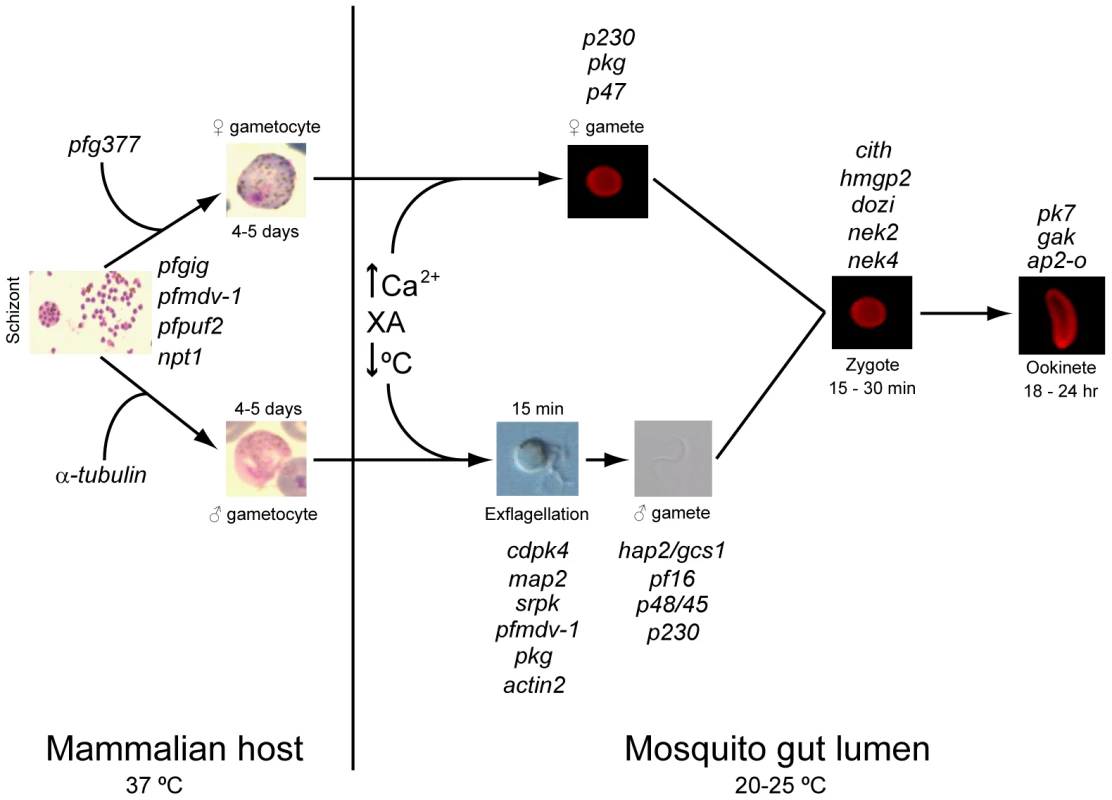
Differentiation of asexual blood stage parasites into gametocytes occurs in the vertebrate host. Ingestion in a mosquito blood meal results in activation of the gametocytes in the lumen of the gut, stimulated by an increase in Ca2+, a drop in temperature, and the presence of the mosquito-derived xanthurenic acid, leading to the production of male and female gametes. Fertilization and fusion of these gametes results in zygote formation. Eventual elongation of the zygote leads to the final stage of the sexual cycle, a highly motile ookinete. Genes involved at each stage of the process (as suggested by a number of functional analyses) and time spans for each stage are indicated. The Sexual Stages: Advances in Global “-omic” Studies
Global “-omic” studies of stage-specific gene expression have come a long way. Expression profiling studies (at both transcriptome and proteome levels) have identified many genes expressed in sexual stages, and many of these have a function specific to these stages (see Table 1 of [8] and [5] for details and associated references). These studies identified 200–300 transcripts and corresponding proteins used by Plasmodium for and during the production of gametocytes, with a smaller subset of transcripts stored for use after gametogenesis and fertilization. However, although these studies identified stage-specific genes, the unique features that determine the sex of a gametocyte remained obscure.
A proteomic analysis of separate male and female P. berghei gametocytes revealed that the two sexual populations are very different at the molecular level [9]. Of the 406 gametocyte-specific proteins identified, only 69 were shared by both sexes. The highest percentage of sex-specific proteins (69%) was found in male gametocytes with large numbers of proteins involved in axoneme assembly and flagellar-based motility, and DNA replication, whereas many of the proteins specific to female gametocytes were for mitochondrial and ribosomal function.
Lessons from Functional Studies
The advances in genomics, transcriptomics, and proteomics have been complemented by much improved gene targeting technology, providing the potential to undertake systematic functional studies [10]. This approach, together with other functional studies [5], [8], has given us a detailed knowledge of the role of some genes and may provide crucial understanding of the regulation of sexual development in the malaria parasite (Figure 1).
Male Gametogenesis
Ingestion of the blood meal by the mosquito activates the gametocytes to produce gametes in the midgut. Activation of both male and female gametocytes is due to a drop in temperature, a rise in pH and calcium concentration, and the presence of the mosquito-derived metabolic intermediate xanthurenic acid [11]. For the male gametocyte, activation results in three rounds of rapid DNA replication (within 15 min) and the assembly of flagella, leading to the formation of eight flagellated and highly motile microgametes on the surface of the male gametocyte, a process called exflagellation. Factors important in induction of exflagellation include a rapid rise in intracellular calcium and activation of phospholipase C (PLC) and guanylyl cyclases, leading to increased intracellular cyclic guanosine monophosphate (cGMP), which activates a cGMP-dependent protein kinase (PKG) (reviewed in [12]). Gene disruption studies of the P. berghei kinome have revealed a number of essential kinase regulators of male gamete formation [9]–[11]. Calcium-dependent protein kinase 4 (cdpk4), mitogen-activated protein kinase 2 (map2), and SR protein kinase (srpk) have vital roles in DNA replication and axoneme assembly, cytokinesis and axoneme-mediated motility, and exflagellation, respectively [10], [11], [13]. However, in P. falciparum, map2 is essential for asexual replication in the bloodstream, so there may be species-specific differences in the roles of different kinases [14].
Gene disruption studies have implicated other specific proteins in male gametogenesis and gamete motility and fertility, including those coded by the genes p48/45, pf16, hap2/gcs1, and actin II. Deletion of pf16, a gene coding for an armadillo repeat motif (ARM) protein of the flagellum central apparatus, results in abnormal movement and reduced fertility, but does not lead to complete sterility [15]. In contrast, deletion of either p48/45 coding for a 6-Cys repeat domain protein, or hap2/gcs1 coding for a conserved plant-sterility gene, results in sterility due to the male gamete being unable either to attach (p48/45) [16] or fuse (hap2/gcs1) [17] to fertile female gametes. Disruption of actin II is shown to affect egress of the male gamete from the host cell [18] (Figure 1).
Female Gametogenesis, and Zygote and Ookinete Development
As with the male, the female gametocyte is activated by exposure to mosquito factors. DNA replication does not occur but the gamete exits the erythrocyte, allowing the male gamete to attach and fuse. Nuclear fusion in the zygote is followed by DNA replication and meiosis [19], with the zygote developing into a motile ookinete that penetrates the gut wall.
Gene deletion studies have defined a number of genes that are vital to zygote and ookinete development [20]. One major mechanism regulating zygote development is translational repression. Deletion of two components of the ribonucleoprotein complex (which withholds certain mRNA species from translation to provide coding potential for proteins during early post-fertilization development) called dozi (development of zygote inhibited) and cith (homolog of worm CAR-I and fly Trailer Hitch) resulted in hundreds of normally translationally repressed transcripts to be targeted for degradation, with deleterious effects on zygote formation [21], [22]. Two Never in mitosis/Aspergillus (NIMA)-related protein kinases (nek2 and nek4) have also been shown to be vital for meiosis in zygote development and ookinete maturation. Other protein kinases that play a role in ookinete development and maturation include those coded by the genes gak and pk7 [10]; however, their mechanism of action is unknown (Figure 1).
Sexual Stages: Possible Targets for Therapeutic Development?
Functional studies have uncovered a number of candidate targets for drug and vaccine development; however, parasite resistance to current antimalarial drugs will develop, and the first potential malaria vaccine is only in Phase 3 trials. Nonetheless, the sexual stage of parasite development is increasingly being considered to be a key component in future campaigns to block transmission, and to eliminate and eventually to eradicate malaria.
Potential drug targets include proteins coded by genes described in this review, such as the kinase regulators of exflagellation (cdpk4 and map2), regulators of DNA replication prior to meiosis (nek2 and nek4), or PKG. Proteins on the gamete surface such as p48/45 or hap2/gcs1 may be vaccine candidates, since antibodies to them induced by immunization could interfere with fertilization. The studies reviewed here are part of the ongoing intensive research to understand sexual development in the malaria parasite with the potential to make a significant contribution in the fight against malaria through the design of intervention strategies for blocking malaria parasite transmission.
Zdroje
1. BakerDA 2010 Malaria gametocytogenesis. Mol Biochem Parasitol 172 57 65
2. BousemaTDrakeleyC 2011 Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 24 377 410
3. RamiroRSAlpedrinhaJCarterLGardnerAReeceSE 2011 Sex and death: the effects of innate immune factors on the sexual reproduction of malaria parasites. PLoS Pathog 7 e1001309 doi:10.1371/journal.ppat.1001309
4. AlanoP 2007 Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol Microbiol 66 291 302
5. AlyASVaughanAMKappeSH 2009 Malaria parasite development in the mosquito and infection of the mammalian host. Annu Rev Microbiol 63 195 221
6. BoissonBLacroixCBischoffEGueirardPBargieriDY 2011 The novel putative transporter NPT1 plays a critical role in early stages of Plasmodium berghei sexual development. Mol Microbiol 81 1343 1357
7. MiaoJLiJFanQLiXCuiL 2010 The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J Cell Sci 123 1039 1049
8. KooijTWMatuschewskiK 2007 Triggers and tricks of Plasmodium sexual development. Curr Opin Microbiol 10 547 553
9. KhanSMFranke-FayardBMairGRLasonderEJanseCJ 2005 Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121 675 687
10. TewariRStraschilUBatemanABohmeUCherevachI 2010 The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8 377 387
11. BillkerODechampsSTewariRWenigGFranke-FayardB 2004 Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117 503 514
12. SindenRETalmanAMarquesSRWassMNSternbergMJ 2010 The flagellum in malarial parasites. Curr Opin Microbiol 13 491 500
13. TewariRDorinDMoonRDoerigCBillkerO 2005 An atypical mitogen-activated protein kinase controls cytokinesis and flagellar motility during male gamete formation in a malaria parasite. Mol Microbiol 58 1253 1263
14. Dorin-SemblatDQuashieNHalbertJSicardADoerigC 2007 Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Mol Microbiol 65 1170 1180
15. StraschilUTalmanAMFergusonDJBuntingKAXuZ 2010 The Armadillo repeat protein PF16 is essential for flagellar structure and function in Plasmodium male gametes. PLoS ONE 5 e12901 doi:10.1371/journal.pone.0012901
16. van DijkMRvan SchaijkBCKhanSMvan DoorenMWRamesarJ 2010 Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog 6 e1000853 doi:10.1371/journal.ppat.1000853
17. LiuYTewariRNingJBlagboroughAMGarbomS 2008 The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22 1051 1068
18. DeligianniEMorganRNBertucciniLKooijTWLaforgeA 2011 Critical role for a stage-specific actin in male exflagellation of the malaria parasite. Cell Microbiol 13 1714 1730
19. JanseCJvan der KloosterPFvan der KaayHJvan der PloegMOverdulveJP 1986 DNA synthesis in Plasmodium berghei during asexual and sexual development. Mol Biochem Parasitol 20 173 182
20. EckerABushellESTewariRSindenRE 2008 Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Mol Microbiol 70 209 220
21. MairGRBraksJAGarverLSWiegantJCHallN 2006 Regulation of sexual development of Plasmodium by translational repression. Science 313 667 669
22. MairGRLasonderEGarverLSFranke-FayardBMDCarretCK 2010 Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog 6 e1000767 doi:10.1371/journal.ppat.1000767
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- How “Humane” Is Your Endpoint?—Refining the Science-Driven Approach for Termination of Animal Studies of Chronic Infection
- Sexual Development in : Lessons from Functional Analyses
- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Historical Contingencies Modulate the Adaptability of
- Human-like PB2 627K Influenza Virus Polymerase Activity Is Regulated by Importin-α1 and -α7
- -Sialidase in Complex with a Neutralizing Antibody: Structure/Function Studies towards the Rational Design of Inhibitors
- Zebrafish: A See-Through Host and a Fluorescent Toolbox to Probe Host–Pathogen Interaction
- How Do Bacteria Know They Are on a Surface and Regulate Their Response to an Adhering State?
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- The Murine Coronavirus Hemagglutinin-esterase Receptor-binding Site: A Major Shift in Ligand Specificity through Modest Changes in Architecture
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Type 1 Interferons and Antiviral CD8 T-Cell Responses
- Sequence Divergent RXLR Effectors Share a Structural Fold Conserved across Plant Pathogenic Oomycete Species
- Temporal Expression of Bacterial Proteins Instructs Host CD4 T Cell Expansion and Th17 Development
- Sexual Development in : Lessons from Functional Analyses
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



