-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
Arenaviridae synthesize viral mRNAs using short capped primers presumably acquired from cellular transcripts by a ‘cap-snatching’ mechanism. Here, we report the crystal structure and functional characterization of the N-terminal 196 residues (NL1) of the L protein from the prototypic arenavirus: lymphocytic choriomeningitis virus. The NL1 domain is able to bind and cleave RNA. The 2.13 Å resolution crystal structure of NL1 reveals a type II endonuclease α/β architecture similar to the N-terminal end of the influenza virus PA protein. Superimposition of both structures, mutagenesis and reverse genetics studies reveal a unique spatial arrangement of key active site residues related to the PD…(D/E)XK type II endonuclease signature sequence. We show that this endonuclease domain is conserved and active across the virus families Arenaviridae, Bunyaviridae and Orthomyxoviridae and propose that the arenavirus NL1 domain is the Arenaviridae cap-snatching endonuclease.
Published in the journal: . PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001038
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001038Summary
Arenaviridae synthesize viral mRNAs using short capped primers presumably acquired from cellular transcripts by a ‘cap-snatching’ mechanism. Here, we report the crystal structure and functional characterization of the N-terminal 196 residues (NL1) of the L protein from the prototypic arenavirus: lymphocytic choriomeningitis virus. The NL1 domain is able to bind and cleave RNA. The 2.13 Å resolution crystal structure of NL1 reveals a type II endonuclease α/β architecture similar to the N-terminal end of the influenza virus PA protein. Superimposition of both structures, mutagenesis and reverse genetics studies reveal a unique spatial arrangement of key active site residues related to the PD…(D/E)XK type II endonuclease signature sequence. We show that this endonuclease domain is conserved and active across the virus families Arenaviridae, Bunyaviridae and Orthomyxoviridae and propose that the arenavirus NL1 domain is the Arenaviridae cap-snatching endonuclease.
Introduction
The Arenaviridae family includes 22 viral species into a single genus Arenavirus, with new species awaiting classification [1], [2]. They cause chronic and asymptomatic infections in rodents, and occasional transmission to man may result in life-threatening meningitis and/or hemorrhagic fever. Lymphocytic choriomeningitis virus (LCMV) is the prototypic species and first arenavirus isolated in 1933. Because its natural host is the common house mouse (Mus musculus), LCMV is the only known arenavirus presumably exhibiting a worldwide distribution. LCMV is a human pathogen of significant clinical relevance, causing central nervous system disease, congenital malformation, choriomeningitis, and systemic and highly fatal infection in immuno-compromised, organt transplant recipient patients [3], [4], [5], [6]. Humans are generally infected through the respiratory tract after exposure to aerosols, or by direct contact with infectious material.
Arenaviruses are enveloped viruses with a bisegmented negative single-strand RNA genome. Each RNA segment, called large (L; ∼7.2 kb) and short (S; ∼3.5 kb), contains two open reading frames in mutually opposite orientations and use an ambisense coding strategy to direct the synthesis of two polypeptides [7]. Between the two open reading frames of each segment resides a non-coding intergenic region (IGR), composed of a sequence predicted to form a stable hairpin structure [8]. The S RNA encodes the viral nucleoprotein (NP; ∼63 kDa) and glycoprotein precursor (GPC; ∼75 kDa), whereas the L RNA encodes a small RING finger protein (Z; ∼11 kDa) and a large protein (L; ∼250 kDa) which is the viral RNA-dependent RNA polymerase (RdRp). The two RNA genomes are encapsidated by the NP, which is the most abundant protein in virions and infected cells, and act as templates for two fundamentally different processes, RNA replication and transcription. During RNA replication, the L protein first binds to the 3′-end of RNA templates and reads them from end to end to direct the synthesis of encapsidated full-length anti-genomes. During transcription, the RdRp stops RNA synthesis at a pause site located near the IGR [7]. The newly synthesized mRNA molecules have a non-polyadenylated 3′-end with a heterogeneous sequence mapped within the predicted hairpin in the IGR [9]. Furthermore, non template-directed sequences have been identified at the 5′-end of the subgenomic mRNA [10]. These sequences are variable in length [9], [10], [11] and terminate with a 5′-cap structure, which suggests the presence of a cap-snatching mechanism for arenaviruses. In this process, originally described for influenza viruses [12], [13] and bunyaviruses [14], the viral RdRp binds cellular mRNAs caps and ‘steals’ them using an endonuclease activity, located in the influenza PA subunit [15], [16], and presumably in L protein of bunyaviruses. These short capped RNAs are then used as primers for mRNA synthesis. The arenavirus L protein is an essential element in genome replication and transcription [17]. It is the largest viral protein composed of approximately 2200 amino-acid (aa) residues, and sequence analysis using homologous proteins led to the prediction of several conserved domains [18], [19]. A biological function can be inferred for the L3 domain containing conserved and typical RdRp signature sequence motifs [19], [20]. For Tacaribe virus, both domains L1 and L3 interact with the Z protein [21]. By analogy with influenza and bunyaviruses, the L protein may also carry activities and domains responsible for a cap-snatching mechanism that would account for the sequence diversity found at the 5′-end of RNA transcripts. The expression and purification of such a large viral polymerase is problematic and has not been documented.
We report here the first crystal structure of an Arenaviridae L protein domain at 2.13 Å resolution, that of the N-terminus domain of the LCMV L protein. We show that this domain is able to bind nucleotides, with a preference for UTP, and RNA. Structural comparison with the N-terminal part of the influenza virus PA protein characterizes unambiguously the domain as an endonuclease. Sequence and secondary structure analysis of L proteins from various Bunyaviridae family members predict that their N-terminal end carries a similar endonuclease activity, that we demonstrate for Toscana virus (TOSV) (genus Phlebovirus, family Bunyaviridae). Activity assays and mutagenesis show that the arenavirus endonuclease exhibits sequence-specificity with a preference for uracil-containing substrates. Lastly, reverse genetics studies correlate expression of endonuclease activity with the selective production of mRNA, making the N-terminus domain of the L protein a likely candidate to be involved in the cap-snatching mechanism of arenaviruses.
Results
Delineation of an Arenavirus L Protein Domain and its Crystal Structure
Based on aa sequence conservation across arenaviruses and on the presence of a potential nucleotide-binding site, we designed cDNA constructs encoding aa residues 1 to ∼250 for the N-terminal end of four arenavirus (Pirital virus (PIRV), Lassa fever virus (LASV), Parana virus (PARV), and LCMV) L proteins. All four domains were expressed as soluble recombinant proteins. We observed a self-limited proteolysis of the Parana arenavirus N-terminus L domain which prompted us to refine boundaries into a shorter 196 aa form, hereafter named “NL1”, fully included in the previously predicted arenavirus L1 domain (1–250 aa) [19]. The construct was expressed in E.coli and purified, but yielded crystals diffracting to 8 Å. However, the homologous 196 residues domain of LCMV yielded well-diffracting crystals. The atomic structure of NL1 was first determined by the SAD technique with seleno-methionylated crystals that diffracted to 3.4 Å. The structure was refined using a native data set at 2.13 Å resolution (Table 1). Two NL1 molecules are present within the asymmetric unit. Residues 1–191 are visible for one molecule whilst only 1–175 could be modelled for the other NL1 molecule owing to high mobility of the C-terminal end of helix α7.
Tab. 1. Data Collection and Refinement Statistics. 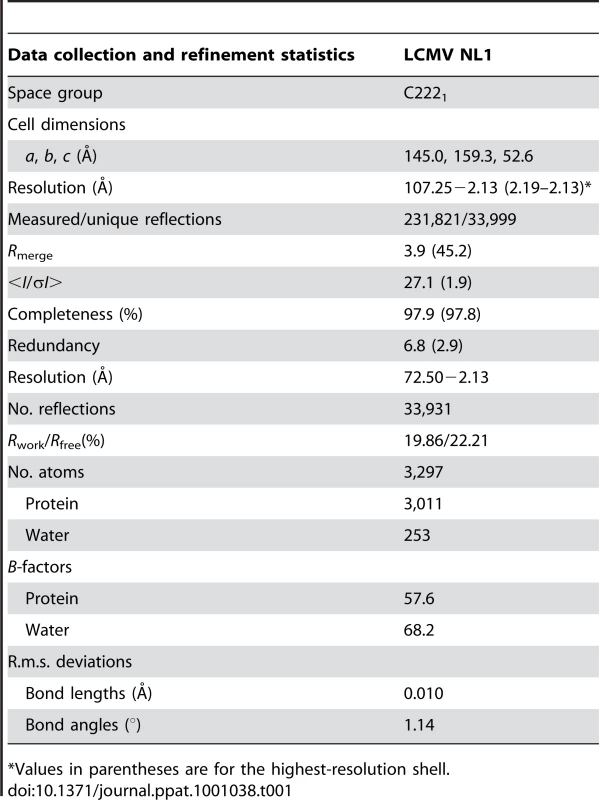
*Values in parentheses are for the highest-resolution shell. The LCMV NL1 Domain Exhibits a Type II Endonuclease Fold
The LCMV NL1 monomer structure has approximate dimensions of 59 Å ×37 Å ×27 Å. It features four mixed β-strands forming a twisted plane surrounded by seven α-helices (Figure 1A). The two anti-parallel strands β1 and β2 are connected by helix α4, whereas the two parallel strands β3 and β4 are connected by the long helix α5. These two helices run parallel to the central β-sheet and are disposed at the same side of the latter. On the opposite side of the β-sheet, helix α3 is surrounded at its extremity by two N-terminal (α1 and α2) and C-terminal helices (α6 and α7). A search for similar protein folds using the DALI server [22] returned the PA N-terminal domain structure that was recently identified as a type II endonuclease domain [15], [16]. The structural match with published molecular structures of the influenza PA N-terminal domains (PAN) returns a Z-score of 5.7 and an r.s.m.d. of 3.9 Å for 121 superposed aa (PDB code 3EBJ) and Z-score 5.2, r.m.s.d. 4 Å for 122 aa (PDB code 2W69). As was the case for PAN, other type II endonuclease proteins are also recovered: the Tt1808 hypothetical protein from Thermus Thermophilus HB88 (PDB code 1WDJ, Z-score 3.8, r.m.s.d. 3.4 Å for 81 aa), and the restriction endonuclease SdaI (PDB code 2IXS, Z-score 3.6, r.m.s.d. 6.3 Å for 104 aa).
Fig. 1. NL1 structure and comparisons with the influenza PAN structure. 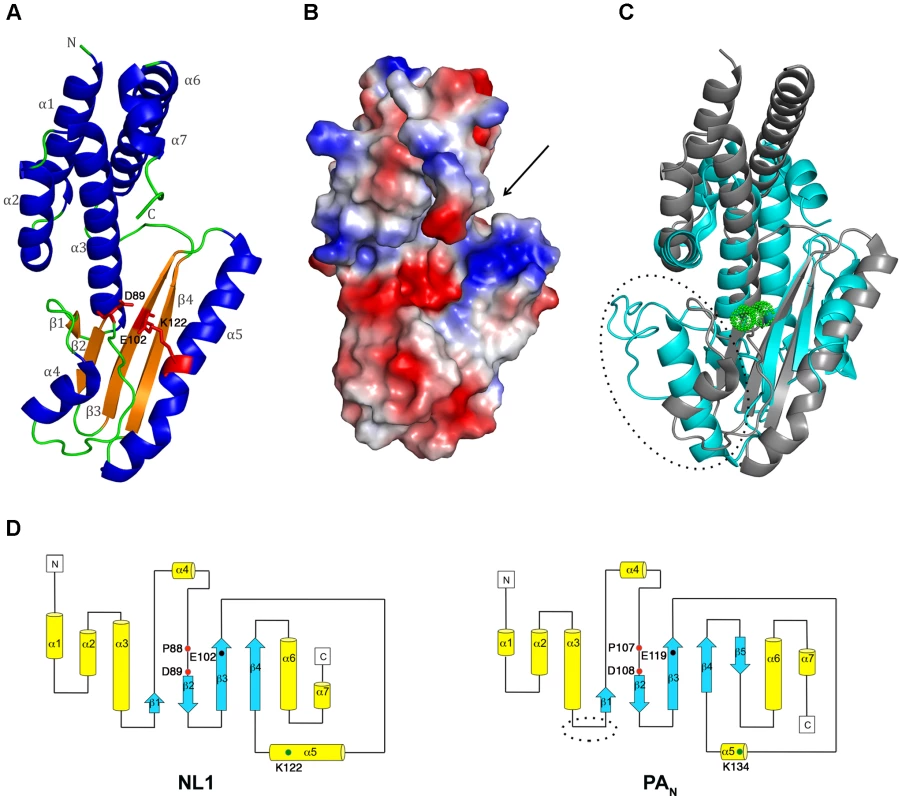
A, Cartoon-representation of the NL1 structure. Secondary-structure elements are labelled and colored as follows: α-helices, blue, β-strands, orange, and loops, green. Side-chains for residues within the NL1 endonuclease active site are shown as red sticks and labelled. B, Electrostatic surface representation showing NL1 in the same orientation as in panel (A). The arrow indicates the putative RNA binding groove and the active site crevice. Negative charges are in red and positive charges in blue and neutral in white. C, Superimposition (view in the same orientation as in A) of the structures of NL1 (grey) and PAN (PDB code:2W69, cyan) highlighting their shared structural core as well as variations in the form of an extra loop only present in the PAN structure (circled). The two Mn2+ ions in the PAN structure active site are depicted as green spheres. D, Topology diagrams of the NL1 (left) and PAN (right) structures. α-helices are represented as yellow tubes and β-strands are blue arrows. The extra-loop of PAN protein is circled as in panel C. Key residues from the endonuclease active site (PD, E/D, and K), are schematically depicted by colored dots and labelled, highlighting the fact that they project from conserved structural elements between the influenza PAN protein and the arenavirus NL1 domain. The β-sheet forms a negatively charged cavity creating a binding site for divalent cations, whilst above that cavity, the C-terminal end of helix α5 forms a positively charged patch and a concave surface that is likely to accommodate the RNA substrate (Figure 1B, arrow). The PA protein constitutes one subunit that associates with PB1 and PB2 to form the heterotrimeric influenza virus polymerase. Its N-terminal domain PAN hosts the RNA cap-snatching endonuclease activity [15], [16]. Both NL1 and PAN share a similar core structure. Except for the absence of a fifth β-strand in NL1, all other secondary structure elements are conserved (Figure 1C) and the overall topology of these two structures is very similar (Figure 1D), albeit with interesting differences in the vicinity of the PAN active site (discussed below). At the aa sequence level, NL1 shares the conserved active site sequence motif characteristic of type II endonucleases: PD…(D/E)XK. In NL1, the corresponding residues are P88, D89…E102, and either K115 or K122 (Figure S1A, B). The identity of the distal lysine is not certain since it is found at different positions in the primary sequence, as is the case for influenza virus. The influenza PAN domain was crystallized either in the presence of magnesium or manganese ions in the active site which comprises five conserved catalytic residues: H41, E80, D108, E119 and K134. A structural superimposition of the arenavirus NL1 and influenza PAN active sites shows that the side-chains of three evolutionary-conserved residues within arenaviruses (P88, D89 and E102) closely superimpose with P107, D108 and E119 of the influenza virus PAN protein, pointing to a common function for these residues (Figure 2A and Figure S1B). Upon superimposition with PAN, one Mn2+ ion needed for the enzymatic reaction coordinated by D108 in the PAN active site, falls at right distances to be coordinated by the carboxylate side-chains of D89 and E102. NL1 was crystallized without metal ions and a water molecule is found close to the position that should be occupied by the divalent metal. Interestingly, no close structural match is found neither for H41 nor K134 of the influenza virus PAN. This points to differences between the two active sites since His41 was proposed to play a catalytic role in the influenza PAN. However, we note that another possible contributor could be NL1 C103 main-chain carbonyl as it superimposes quite well with PAN I120 main-chain carbonyl (Figure 2B). The triad made of K115, D119, and K122 in NL1 is spatially equivalent to K134 in PAN. In summary, despite no aa sequence homology, the active site structures of the influenza PAN and LCMV NL1 domains are clearly related but not identical (Figure 1C, 2), strongly suggesting that these two domains exhibit closely related enzymatic activities (see below).
Fig. 2. The endonuclease active site. 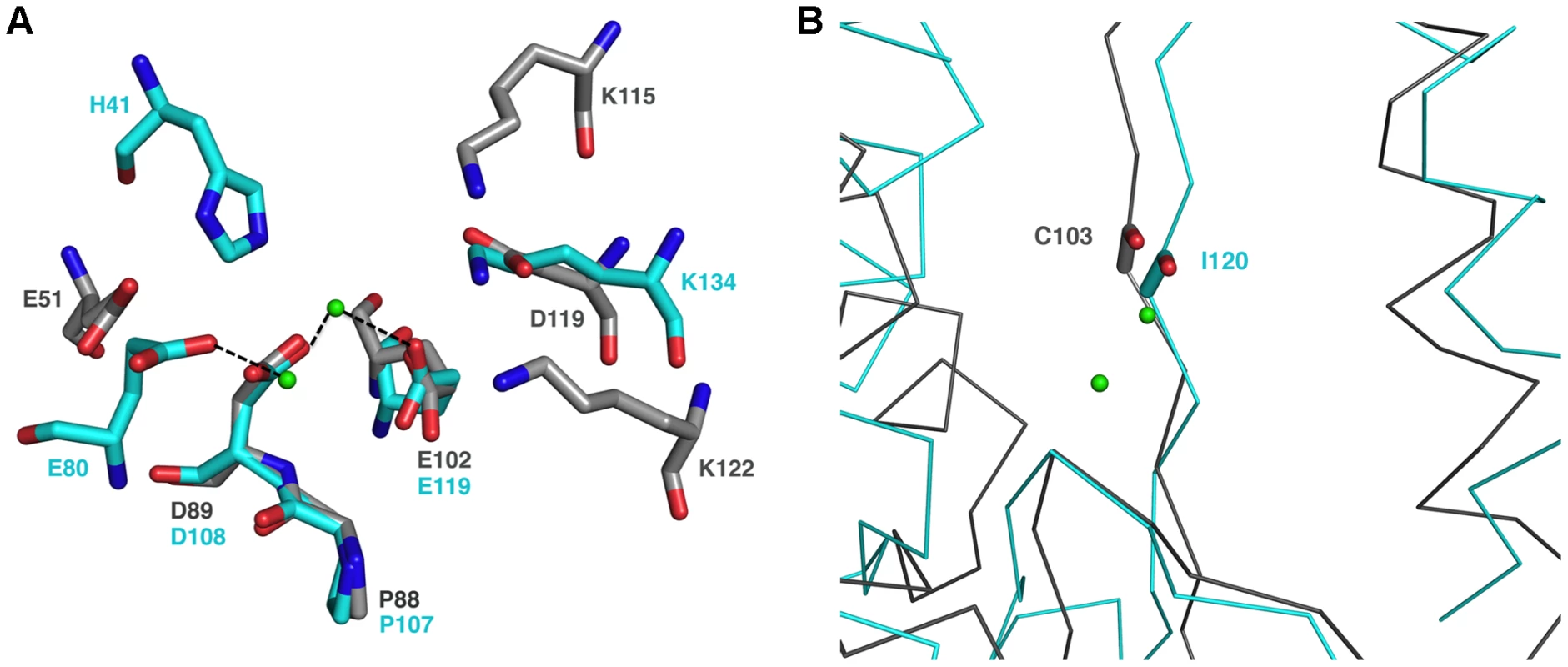
A, Structure-based superimposition of the endonuclease active site from the influenza PAN protein and the arenavirus NL1 domain. Putative active site residues of NL1 are shown as grey sticks and the active site of PAN (PDB code 2W69) in cyan. The two Mn2+ ions present in the PAN structure (but not in the present NL1 domain crystal structure) are shown as light green spheres with their closest ligand indicated by a dashed line. B, C-alpha trace ribbon-representation of the superimposition of the endonuclease active site from the influenza PAN (cyan) protein and the arenavirus NL1 domain (grey). The carbonyl main-chain of PAN I120 and NL1 C103 are shown in sticks. The metal ions are shown as light green spheres. The NL1 Endonuclease Fold is Conserved Amongst Bunyaviridae
In addition to Arenaviridae and Orthomyxoviridae, Bunyaviridae is the other family of virus to possess a segmented negative-strand RNA genome. It contains four genera of animal viruses (Orthobunyavirus, Phlebovirus, Nairovirus, Hantavirus) and one genus of plant virus (Tospovirus) [23]. Although the genomic organisation differs between these three virus families, Bunyaviridae are also thought to use a cap-snatching mechanism to prime mRNA synthesis [24]. Arenaviruses, and Bunyaviridae share a conserved RdRp motif within their large L protein, as well as a conserved N-terminus domain [18]. Amino-acid sequence alignments, assisted by secondary structured prediction, of the N-terminal part of LCMV and Bunyaviridae L protein reveal that the latter also possesses the conserved active site motifs characteristic of type II endonucleases (Figure S2A). However, we could identify the catalytic motif within the L protein N-terminal end for only four out of the five bunyavirus genera: Orthobunyavirus, Phlebovirus, Hantavirus and Tospovirus. The L protein of Nairovirus is much larger (∼4000 aa) than the L protein of other members of the Bunyaviridae family (∼2200 aa). The putative endonuclease catalytic motif was located after aa ∼700, the N-terminal of Nairovirus L protein being assigned as a so-called OTU-like domain [25].
Secondary structure predictions were used to draw the topology diagram of the NL1-like domain for each genera (Figure S2B). As expected from the sequence alignment, each genus seems to share a β-sheet with a variable number of β-strands. Furthermore, the PD catalytic motifs are in each case located in a loop before a β-strand, as expected. The PUMV, HLCV and RVFV NL1-like domains are more closely related to LCMV NL1 than are the TOMV and CCGV. The TOMV NL1-like domain contains 6 β-strands and shares the PD motif just upstream the first β-strand, whereas it is just upstream the second β-strand in the case of NL1 and PAN. Finally, the structural organization of the putative CCGV endonuclease domain seems to diverge even further from the others. Indeed, whereas the conserved lysine is shared by the same helix for all the domains, that of Nairovirus may be located at the end of the β4 strand (Figure S2B). Thus we conclude that the endonuclease motif is conserved across four animal virus genera Orthobunyavirus, Phlebovirus, Nairovirus and Hantavirus.
NL1 is a Mn2+-Dependent RNA Endonuclease
Recent crystal structures of complexes of PAN with three different nucleoside monophosphates show that PAN binds nucleotides [26]. The ability of NL1 to bind nucleotides was investigated using UV-crosslink experiments. We observe that NL1 binds NTPs, preferably UTP and GTP, whereas ATP and CTP show a weaker association (Figure 3A). The PAN structures were determined in complex with ATP, CTP and UTP but not GTP [26] whereas NL1 bind GTP in a stronger fashion than ATP or CTP. The crystal structure relatedness to the endonuclease fold would suggest that the NL1 domain is able to bind RNA rather than nucleotides. We tested RNA binding by NL1, and found that indeed, NL1 binds RNA (Figure 3B). The band shift assay is also suggestive that the RNA substrate is cleaved under the assay conditions, as judged by degradation products at the bottom of the gel under the labeled RNA oligo (Figure 3B). Therefore, we surmise that nucleotide binding properties observed here reflect the ability of NL1 to bind RNA with some sequence specificity in the cap-snatching pathway (see below).
Fig. 3. Nucleotide and RNA binding assays of LCMV NL1 domain. 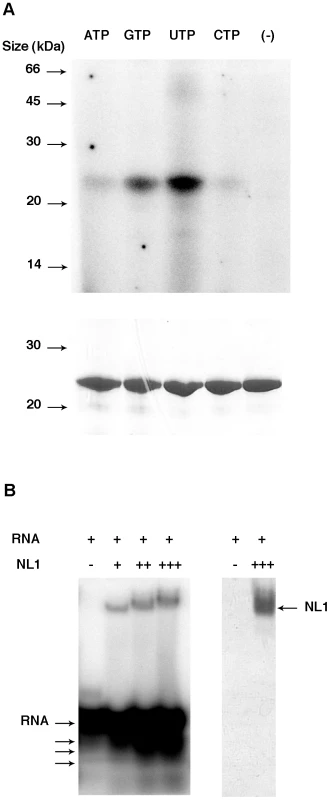
A, Cross-linking assay. 7 µg of purified protein were incubated in the absence (−) or presence of each indicated radiolabelled NTP. The mixture was then UV-irradiated and loaded onto a denaturing polyacrylamide gel. The latter was analyzed by autoradiography (top) and Coomassie blue staining (bottom). B, Band shift assay. Radiolabelled RNA was incubated with increasing quantities (1.4 µg (+), 4.2 µg (++) and 7 µg (+++)) of NL1 protein. Reaction mixture was then analyzed by PAGE, and the gel was visualized by autoradiography (left) and Coomassie blue staining (right). Apparent degradation products are indicated by arrows under the RNA input arrow. Several synthetic RNA oligonucleotides were used to characterize the endonuclease activity (Figure 4). NL1 is able to cleave ssRNA having no stable secondary structure at specific sites indicating a preference for the presence of uracil (Figure 4A, B), and adenosine to a lesser extent. Likewise, a moderately stable RNA hairpin containing uracil (ΔG = −3.4 kcal/mole) is cleaved down to a 14/15-mer product whereas a stable (ΔG = −14.7 kcal/mole) RNA hairpin devoid of uracil remains unattacked even in its single stranded regions (Figure 4A, B). PolyU RNA is cleaved randomly down to a 8-mer product with a better efficiency than polyA, whereas polyC is not a substrate for NL1 (not shown). A 5′-terminal nucleoside uracil or adenosine 5′-monophosphate is also cleaved and the 5′-monophosphate RNA end apparently competes for internal cleavage. A 5′-capped RNA of 264 nucleotides in length also acts as a substrate. It is cleaved at several specific positions indicated by the sequential appearance of band products over time (Figure 4B). This indicates that the cap structure does not seem to be a direct RNA binding determinant. A Phlebovirus (Toscana) virus endonuclease domain was prepared according to bio-informatic predictions described above. Its endonuclease activity was compared to both that of arenavirus NL1 and the influenza H5N1 endonuclease [16]. The enzymes were equally active using short RNA substrates, although it is apparent that sequence-specific cleavage is different for each enzyme: the influenza enzymes prefers cleavage at puric sites, Toscana virus and LCMV enzymes prefer adenosine - and uracil-containing sites (Figure 4B). NL1 is ∼90-fold more active in the presence of Mn2+ than Mg2+, and shows background activity with Ca2+ and Zn2+(Figure 4C and not shown). The Mn2+ ion has also a significant stabilizing effect as judged by thermostability studies, whereas Zn2+ has a deleterious effect.
Fig. 4. Endonuclease activity of NL1. 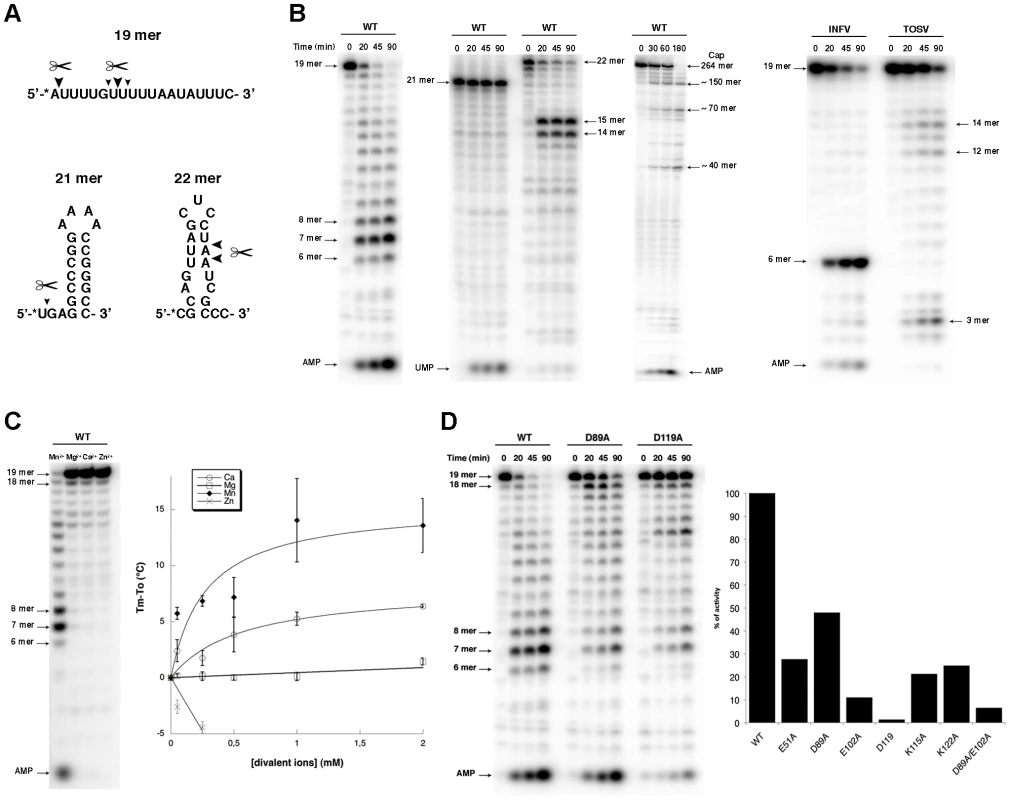
A, Nucleotide sequence of the radiolabelled RNA used for the activity assays. The * indicates the radiolabelled nucleotide. Big and small triangles indicates the primary and secondary cleavage site by wild-type (WT) NL1, respectively. B, Kinetics of endonuclease activity of WT NL1 on different substrates (left), and for Influenza and Toscana virus (INFV, TOSV) endonuclease domain (right). Activity assays were performed as described in Materials and Methods, using 3.3 µM of RNA and 1 µM of protein. Reactions were quenched by the addition of EDTA/formamide, and analyzed using 20% polyacrylamide/7M urea gel. Substrate and degradation product sizes are indicated. C, Divalent cations effect on the NL1 activity. The reaction was allowed to proceed during 45 min as described in Materials and Methods. The divalent cation assay (left) was run during 45 min without intermediate points. Titration of divalent ions on NL1 by thermal shift assay (right). Tm is the melting temperature of NL1 with the divalent ions ; To is the melting temperature of the protein alone. D, Mutational analysis of NL1 domain on the endonuclease activity. Kinetics were performed as described above, with WT, D89A and D119A mutants (Left). Graph showing the % of endonuclease activity determined using FujiImager normalized quantitation for the different mutants (right). Mutagenesis analysis of most residues identified as part of the active site (Figure 2A) impaired the endonuclease activity. The most drastic effect was observed for D119, but residual activity was scored for E51, D89, and less for E102 (Figure 4D). As these three residues might coordinate metal ions as proposed above, defective metal-binding due to a point mutation might be compensated by the presence of the remaining two adjacent acidic residues. A double mutant D89A/E102A shows further reduced but not abolished activity. Mutations K115A and K122A generated strongly altered activity, but the similar level of residual activity does not allow the identification of which lysine is predominant in catalysis.
The Endonuclease Activity is Essential for RNA Transcription, not Replication
The effect of 33 mutations in L1 on virus RNA and protein expression was studied in a cell-based mini-replicon system. The LCMV L protein mediates the synthesis of two RNA species: first, capped mRNA terminating within the intergenic region, and second, antigenomic RNA being a full-length copy of the genomic RNA template [9], [27]. This dual role in RNA synthesis is recapitulated in the mini-replicon system. It contains all trans-acting factors (L protein and NP) required for transcription and replication of a genome analogue containing Renilla luciferase as a reporter gene (mini-genome). Reporter gene expression was measured in luciferase assay (Table 2), while RNA synthesis was measured in Northern blot (Figure 5), in which luciferase mRNA and antigenome can easily be distinguished due to their size difference. Wild-type (WT) L protein led to expression of high levels of Renilla luciferase (2–3 log units signal-to-noise ratio) as well as Renilla luciferase mRNA and antigenome in a ratio of about 1∶1. Expression of mutant L protein was verified by immunoblotting (Figure S3).
Fig. 5. Mutational analysis of the L protein in the context of the LCMV replicon system. 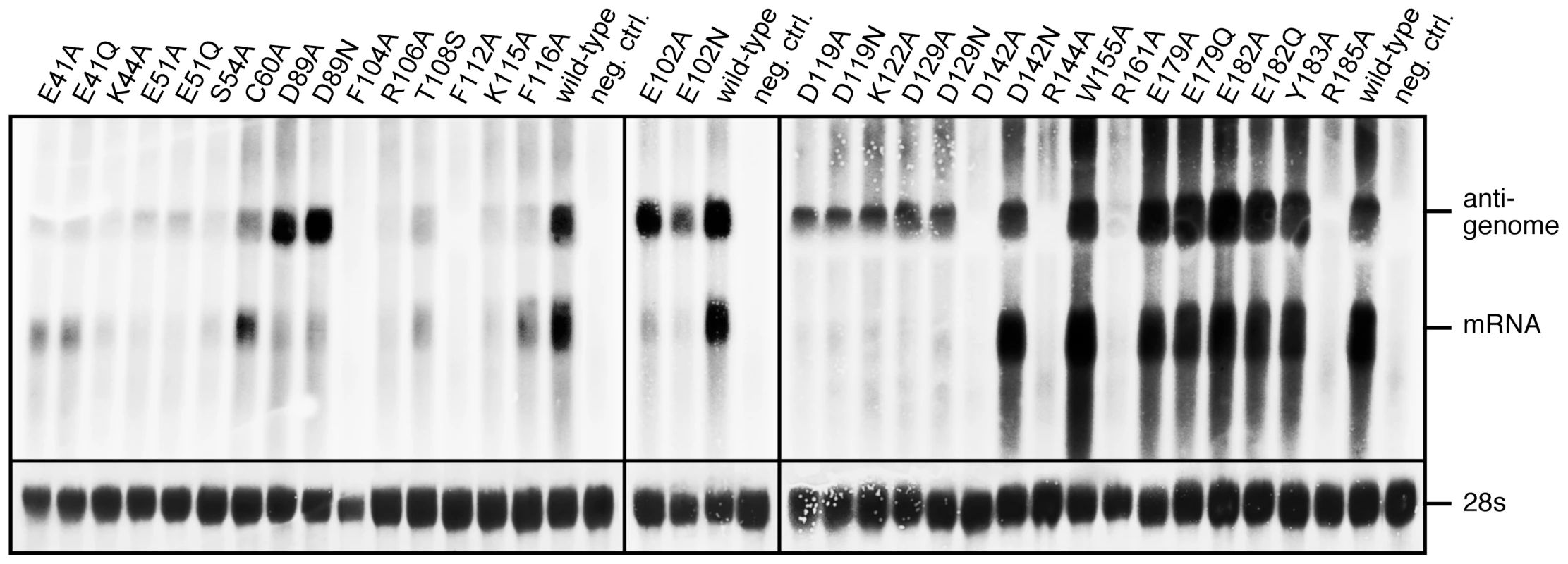
Synthesis of antigenomic RNA and Renilla luciferase mRNA was analyzed by Northern blotting. Negative control cells (neg. ctrl.) expressed mini-genome, NP, and an L protein mutant with a mutation in the catalytic site of the RNA-dependent RNA polymerase. The methylene blue-stained 28S rRNA is shown below the blots as a marker for gel loading and RNA transfer. Each panel represents an independent experiment with separate controls. Careful examination of the blots revealed residual signals at the mRNA position for some mutants negative in Renilla luciferase assay. Thus, these signals do not correspond to functional mRNA, but may be prematurely terminated antigenome. Tab. 2. Functional Analysis of L Protein Mutants in LCMV Mini-Replicon System. 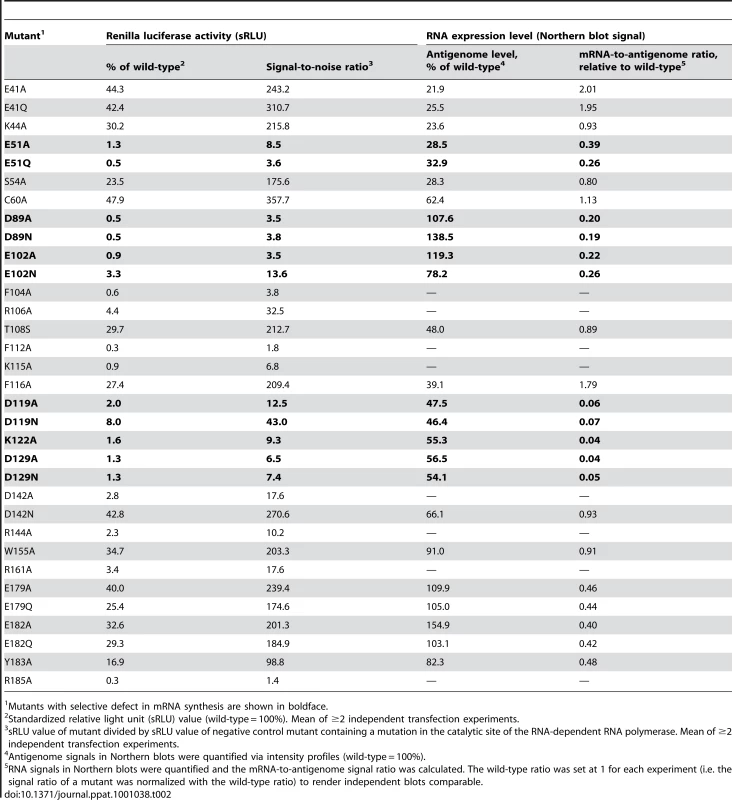
Mutants with selective defect in mRNA synthesis are shown in boldface. The phenotype of mutants E41A, E41Q, K44A, S54A, C60A, T108S, F116A, D142N, and W155A is similar to that of wild-type. Mutants E179A, E179Q, E182A, E182Q, and Y183A also express luciferase and RNA at high level, but the steady-state level of mRNA relative to that of antigenome is reduced by about 50%. Mutants F104A, R106A, F112A, K115A, D142A, R144A, R161A, R185A neither express Renilla luciferase nor any RNA species, indicating that global functions of L protein are affected.
The most interesting phenotype is observed with mutants D89A, D89N, E102A, E102N, D119A, D119N, K122A, D129A, and D129N. They synthesize antigenome close or equal to wild-type level, but are defective in mRNA and, thus, reporter gene expression (Figure 5 and Table 2, shown in boldface). A similar phenotype is seen with mutants E51A and E51Q, though associated with reduced antigenome level. These data indicate that residues E51, D89, E102, D119, K122, and D129A are essential for viral mRNA synthesis, but not required for expression of uncapped RNA species. With the exception of the D129 residue located at the surface of the protein remote from the endonuclease active site, it is remarkable that these transcription-null mutants form the catalytic site (Figure 2) and match precisely those of the PD…(D/E)XK endonuclease type II signature sequence.
Discussion
The structural and functional results presented here show that the LCMV NL1 domain is an RNA endonuclease. The uncoupling of RNA replication from transcription and selective disappearance of mRNA when NL1 active site residues are mutated strongly suggests that this activity is involved in cap-snatching.
The identification of the arenavirus endonuclease is in line with the recent discovery of the PAN endonuclease domain of influenza virus. Whereas the active site of influenza virus features a cluster of three acidic residues, the active site of arenavirus contains four acidic residues (E51, D89, E102 and D119), as well as two important lysine residues K115 and K122 neighboring D119 (Figure 2A). The NL1 active site resembles but is clearly distinct from that of influenza PAN. Indeed, there is no histidine in the catalytic center, and the arenavirus NL1 nuclease has some specific features both upstream and downstream of the PD signature sequence. We define the arenavirus endonuclease motif as E-X38-P-D-X(11,13)-E-X12-K-X3-D-X2-K. The most obvious difference with the only known related RNA endonuclease, that of influenza virus PAN, is a divergence upstream the PD motif in structural elements carrying the E51 residue (Figure 1C), and the presence of a triad K…D…K at the distal side of the latter signature sequence (Figure 2A). Contrary to PAN which shares a conserved and essential histidine involved in the binding of both the metal ion and a nucleotide onto helix α3 [15], [16], [26], NL1 does not possess this conserved histidine residue. Instead, NL1 has a glutamic acid residue E51, which might reflect a different nucleobase specificity as detected in our nuclease assays (Figure 4). Likewise, residues downstream the PD motifs are distinct from the consensus sequence, and differently organized into a triad including two lysines. The presence of water molecules and previous structural models for influenza PAN allows to propose putative positions of metal ions, coordinated by D89 and E102.
The first step in the general mechanism for phosphodiester hydrolysis is the preparation of the attacking nucleophile by deprotonation, usually involving a general base deprotonating a water molecule. Lysine is often considered as this general base candidate in endonucleases but is not strictly conserved [28], [29]. Here, there are no indications against D119 being this general base. Alternately, it could well be either lysine K115 or K122. Both are oriented towards the active site, and they could well have their pKa lowered by D119 in order to initiate the reaction. Reverse genetic studies provide evidence for K122, not K115. Indeed, mRNA production is selectively abolished and clearly uncoupled from RNA synthesis in the case of K122A mutant, while the K115A mutant was completely defective preventing interpretation of its role in the endonuclease catalytic site. Although it is not known if uncapped mRNAs are synthesized and degraded for the transcription-null mutants, the most plausible scenario is that primer shortage prevents significant capped mRNA synthesis. Overall, the replicon data presented here closely match those obtained on the closely related Lassa arenavirus using a similar replicon system [30]. Arenaviruses may thus use two clearly independent and distinct RNA synthesis priming mechanisms: one is dependent on an active endonuclease carried by the N-terminus of the L protein, and the other might be linked to the observation that an extra G residue is found at the 5′-end of arenavirus genomes and antigenomes. The latter G bases would thus reflect a yet-uncharacterized priming mechanism unrelated to the U/A cleavage sequence preference of NL1.
NL1 also binds nucleotides, but the NTP binding site should differ from that of PAN. Indeed, the influenza PAN histidine 41 is involved in binding the nucleobase of the presumed incoming RNA substrate. The NL1 endonuclease does not share the same sequence specificity, and E51 is positioned at a spatially equivalent position.
The cap structure does not seem to be a direct RNA binding determinant (Figure 4B), as endonucleolytic cleavage is not directed to cleavage sites preferentially in the vicinity of the cap. We thus infer that an independent cap-binding site way exist elsewhere in viral proteins to bind and select cellular mRNAs, a possibility reminiscent of influenza for which PA carries the endonuclease activity and PB2 the cap binding site [15], [16], [31].
Structure and sequence alignment studies show that the N-terminal endonuclease domain of the L protein is also conserved in the Bunyaviridae family, although the Nairovirus endonuclease domain is not located into the N-terminal end of the protein. These findings were confirmed by the endonuclease activity of the N-terminal end of the L protein of TOSV (Figure 4B). Thus, we provide evidence that all three segmented negative single-strand RNA virus species share an endonuclease domain probably involved in the cap-snatching process during the viral life cycle. These data raise the question of a possible common ancestor for these viruses. Indeed, these three virus families use a cap-snatching mechanism involving binding and cleavage of cellular mRNA caps subsequently used by a large primer-dependent RNA-dependent RNA polymerase. It seems more plausible that the L gene has evolved by divergence over time, rather than by multiple acquisitions of several activities converging into a common structure, at least in the case of the endonuclease. Furthermore, our study raises the interesting possibility that other activities involved in RNA replication/transcrition might be discovered by comparative analysis of Orthomyxoviridae PB1, PB2, PA and Arenaviridae/Bunyaviridae L proteins.
To our knowledge, a single crystal structure of a functional arenavirus protein is currently available, that of the Machupo virus glycoprotein GP1 in complex with its human receptor, TfR1 [32]. Our results provide an arenavirus L domain structure, with a role consistent with the hypothesis of a cap-snatching mechanism suggested for arenaviruses [9], [10]. The strategy used here to produce individually active domains might be useful to further characterize the Arenaviridae/Bunyaviridae large L protein which had so far resisted all biochemical characterization attempts.
The influenza, Arenaviridae and Bunyaviridae endonucleases are so far the only three examples of RNA endonucleases similar to type II DNA restriction endonucleases. The presence of such an endonuclease suggests that it could serve as a fruitful target for antiviral strategies against these two families, since such kind of inhibitors have been reported in the case of the influenza virus [33], [34], [35].
Materials and Methods
Cloning, Expression and Purification of LCMV NL1 Domain
The LCMV NL1 cDNA (Armstrong strain, aa 1 to 196) was cloned into pDest14 with a N-terminus hexa-histidine tag and expressed in E.coli Rosetta (DE3) pLysS (Novagen), at 17°C in 2YT medium overnight after induction with 500 µM IPTG. Cell pellets from harvested cultures were resuspended in 50 mM Tris buffer, pH 8.0, 300 mM NaCl, 10 mM imidazole, 0.1% Triton, 5% Glycerol. Lysozyme (0.25 mg/ml), PMSF (1 mM), DNase I (2 µg/ml), and EDTA free protease cocktail (Roche) were added before sonication. IMAC chromatography of clarified lysates was performed on a 5 ml His prep column (Akta Xpress FPLC system, GE Healthcare) eluted with imidazole. Size exclusion chromatography was performed on preparative Superdex 200 column (GE Healthcare) pre-equilibrated in 10 mM Imidazole, pH 8.0, 50 mM NaCl, 2 mM DTT. Protein was concentrated (28 mg/ml) using a centrifugal concentrator. For enzymatic studies, WT and mutants were express in the E.coli BL21 star strain (Invitrogen) and further purified on HiTrap Q sepharose 1 ml column (GE Healthcare) to remove E. coli RNase contaminants. Proteins eluted in a linear gradient from 50 mM to 1 M NaCl in 10 mM Hepes buffer, pH 7.5, 2 mM DTT. A synthetic gene of the H5N1 PAN endonuclease was designed as described [16]. The Toscana virus (strain France AR_2005, aa 2 to 233) cDNA was obtained from infected cell cultures. Both ORFs were cloned as a N-terminal Thioredoxin-Hexahistidine fusion in pETG20A. The tag was cleaved using TEV protease before a final gel filtration.
Crystallization
Crystals grew in LiSO4 250 mM, citrate 50 mM, isopropanol 5.5%, using the hanging drop vapor diffusion method in Linbro plates by mixing 1 µl of protein solution with 1 µl of reservoir solution. Crystals were cryoprotected by dipping in a solution containing 65% of crystallization buffer and 35% of a buffer made of size exclusion chromatography buffer/glycerol (50/50). Crystals were cryo-cooled in liquid N2. The crystals belong to space group C2221 and have two molecules per asymmetric unit. Despite repeated attempts, crystal soaked into the above buffer supplemented with various concentrations of MnCl2 yielded crystals diffracting to >4 Å.
Data Collection and Structure Determination
Diffraction intensities were recorded on the ID14-4 beamline at the European Synchrotron Radiation facility, Grenoble, France. Data were processed and integrated with MOSFLM [36]. Scaling and merging of the intensities was performed with SCALA and programs from the Collaborative Computational Project, No. 4 (CCP4) suite [37]. The structure was determined using SAD data from one selenomethionylated protein crystal diffracting to 3.4 Å resolution with SHARP/autoSHARP, followed by density modification with SOLOMON and DM. An initial model was built using BUCCANEER and completed in COOT, followed by refinement using BUSTER (see Text S1). Details of structure determination are given as supplemental material. Data from a native crystals diffracting to a 2.13-Å resolution were collected on an ADSC QUANTUM 315r at a wavelength of 0.9835 Å. The structure was refined with BUSTER and COOT using this data set (Table 1) [38]. The atomic coordinates have been deposited at the PDB (3JSB).
Sequence Retrieval
A PHI-BLAST search using the sequence corresponding to the L1 domain and the signature of the Arenaviridae endonuclease motif i.e. P-D-x(11,13)-E-x(12)-K-x(3)-D-x(2)-K ; was performed against non-redundant databases [39]. After 3 iterations, Batai and Kairi viruses both belonging to Orthomyxoviridae, appears in the section with an E-Value below threshold. A fourth iteration including these two sequences allows retrieving the entire family of orthomyxoviruses, with E-value comprised between 3e−18 and 2e−4.
A standard CDD search from the sequence of Tensaw virus allows retrieving all the L of the Bunyaviridae family hitting the pfam 04196 [40].
Sequence Comparison
A multiple sequence alignment of the N-terminal end of the L protein from LCMV, HLCV, BUNV, HANV, PUMV, RVFV, TOSV, TOMV, WTMV, CCGV, DUGV, was first performed with the T-coffee algorithm (http://tcoffee.vital-it.ch/cgi-bin/Tcoffee/tcoffee_cgi/index.cgi). Using the secondary structure prediction of the endonuclease domain of L proteins, the putative conserved active site residues were identified and placed correctly in the alignment.
UV-Crosslink Experiments
7 µg of purified protein were incubated for 15 min at 25°C, with 0.5 µl of the various α-32P NTP (0.4 µCi/µl) in 10 µl of reaction buffer containing 10 mM Imidazole, pH 8.0, 50 mM NaCl, 2 mM DTT. The reaction mixtures were then exposed to UV light (254 nm) for 6 min at 5 mm distance. The crosslinked species were separated in a 15% polyacrylamide denaturing gel and visualized by autoradiography using photo-stimulated plates and a Fujilmager (Fuji).
RNA Binding Experiments
The RNA 5′-AUUUUGUUUUUAAUAUUUC-3′ (Ambion) was [32P] 5′-end labeled, and 0.4 µM of radiolabelled RNA was incubated 20 min at 25°C without and with 1.4 µg, 4.2 µg and 7 µg of protein in 10 µl of 10 mM Imidazole, pH 8.0, 50 mM NaCl, 2 mM DTT. Reaction mixtures was analyzed by PAGE and visualized by autoradiography.
Ion Binding Assays
Titration curves with CaCl2, MnCl2, MgCl2 and ZnCl2 were performed at 1 mg/ml protein in gel filtration buffer using thermal shift assay. Technical details can be be found in [41].
Endonuclease Assays
Endonuclease activity was assayed using 4 different heteromeric RNA substrates: an unstructured 19 mer as described above, a 21 mer stable hairpin (5′-UGAGGCCCGGAAACCGGGGCC-3′ (Ambion), ΔG = −14.7 Kcal/mole), a 22 mer moderately stable hairpin (5′ - CGCAGUUAGCUCCUAAUCGCCC-3′ (Ambion), ΔG = −3.4 Kcal/mole), and a long 264 mer RNA corresponding to the SARS-CoV 5′-genome sequence. The latter was radiolabelled with a cap structure at its 5′-end using the ScriptCap m7G Capping System (Epicentre Biotechnologies) with [α32P]GTP. Endonuclease assays were carried out using 3.3 µM of radio-labeled RNA in a buffer containing 40 mM Tris-base, pH 7.5, 100 mM NaCl, 10 mM β-Mercaptoethanol and 2 mM MnCl2. Reactions were initiated by the addition of 1 µM of protein and incubated at 37°C, and stopped by the addition of EDTA/formamide. Reactions products were analyzed using denaturing polyacrylamide gel electrophoresis (20% polyacrylamide, 7 M urea in TTE buffer (89 mM Tris, 28 mM taurine, 0.5 mM EDTA) and analyzed by autoradiography.
Mutagenesis and Reverse Genetics Assays Using a LCMV Mini-Replicon System
The LCMV replicon system is based on strain Armstrong clone 13 and has been established in analogy to the Lassa virus replicon described previously [42]. BSR T7/5 cells constitutively expressing T7 RNA polymerase [43] were transiently transfected with T7 promoter-driven expression constructs for L protein, nucleoprotein (NP), mini-genome (MG) containing Renilla luciferase reporter gene, and firefly luciferase as a transfection control. L protein mutants were generated as described [44]. One day after transfection, total RNA was prepared for Northern blotting and cell lysate was assayed for firefly and Renilla luciferase activity. Renilla luciferase levels were normalised with firefly luciferase levels resulting in standardized relative light units (sRLU). Northern blot was performed using an antisense 32P-labeled riboprobe targeting the Renilla luciferase gene. Autoradiography was quantified on a PhosphorImager (Amersham Biosciences). To verify protein expression, hemagglutinin (HA)-tagged L protein was expressed in BSR T7/5 cells inoculated with modified vaccinia virus Ankara expressing T7 RNA polymerase (MVA - T7) [45] and detected in immunoblot using anti-HA antibody.
Supporting Information
Zdroje
1. BrieseT
PaweskaJT
McMullanLK
HutchisonSK
StreetC
2009 Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog 5 e1000455
2. CharrelRN
de LamballerieX
EmonetS
2008 Phylogeny of the genus Arenavirus. Curr Opin Microbiol 11 362 368
3. BartonLL
BuddSC
MorfittWS
PetersCJ
KsiazekTG
1993 Congenital lymphocytic choriomeningitis virus infection in twins. Pediatr Infect Dis J 12 942 946
4. BartonLL
HyndmanNJ
2000 Lymphocytic choriomeningitis virus: reemerging central nervous system pathogen. Pediatrics 105 E35
5. FischerSA
GrahamMB
KuehnertMJ
KottonCN
SrinivasanA
2006 Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med 354 2235 2249
6. PalaciosG
DruceJ
DuL
TranT
BirchC
2008 A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med 358 991 998
7. MeyerBJ
de la TorreJC
SouthernPJ
2002 Arenaviruses: genomic RNAs, transcription, and replication. Curr Top Microbiol Immunol 262 139 157
8. SalvatoMS
ShimomayeEM
1989 The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology 173 1 10
9. MeyerBJ
SouthernPJ
1993 Concurrent sequence analysis of 5′ and 3′ RNA termini by intramolecular circularization reveals 5′ nontemplated bases and 3′ terminal heterogeneity for lymphocytic choriomeningitis virus mRNAs. J Virol 67 2621 2627
10. RajuR
RajuL
HackerD
GarcinD
CompansR
1990 Nontemplated bases at the 5′ ends of Tacaribe virus mRNAs. Virology 174 53 59
11. PolyakSJ
ZhengS
HarnishDG
1995 5′ termini of Pichinde arenavirus S RNAs and mRNAs contain nontemplated nucleotides. J Virol 69 3211 3215
12. PlotchSJ
BouloyM
KrugRM
1979 Transfer of 5′-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A 76 1618 1622
13. PlotchSJ
BouloyM
UlmanenI
KrugRM
1981 A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell 23 847 858
14. BishopDH
GayME
MatsuokoY
1983 Nonviral heterogeneous sequences are present at the 5′ ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res 11 6409 6418
15. DiasA
BouvierD
CrepinT
McCarthyAA
HartDJ
2009 The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458 914 918
16. YuanP
BartlamM
LouZ
ChenS
ZhouJ
2009 Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 458 909 913
17. LopezN
JacamoR
Franze-FernandezMT
2001 Transcription and RNA replication of tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J Virol 75 12241 12251
18. MullerR
PochO
DelarueM
BishopDH
BouloyM
1994 Rift Valley fever virus L segment: correction of the sequence and possible functional role of newly identified regions conserved in RNA-dependent polymerases. J Gen Virol 75 Pt 6 1345 1352
19. ViethS
TordaAE
AsperM
SchmitzH
GuntherS
2004 Sequence analysis of L RNA of Lassa virus. Virology 318 153 168
20. LukashevichIS
DjavaniM
ShapiroK
SanchezA
RavkovE
1997 The Lassa fever virus L gene: nucleotide sequence, comparison, and precipitation of a predicted 250 kDa protein with monospecific antiserum. J Gen Virol 78 Pt 3 547 551
21. WildaM
LopezN
CasabonaJC
Franze-FernandezMT
2008 Mapping of the tacaribe arenavirus Z-protein binding sites on the L protein identified both amino acids within the putative polymerase domain and a region at the N terminus of L that are critically involved in binding. J Virol 82 11454 11460
22. HolmL
KaariainenS
RosenstromP
SchenkelA
2008 Searching protein structure databases with DaliLite v.3. Bioinformatics 24 2780 2781
23. NicholSTBBJ
ElliottRM
2005 Virus Taxonomy, VIIIth Report of the ICTV. Fauquet CM, Mayo AM, Maniloff J et al eds London: Elsevier Academic Press 695 716
24. GroMC
Di BonitoP
AccardiL
GiorgiC
1992 Analysis of 3′ and 5′ ends of N and NSs messenger RNAs of Toscana Phlebovirus. Virology 191 435 438
25. Frias-StaheliN
GiannakopoulosNV
KikkertM
TaylorSL
BridgenA
2007 Ovarian tumor domain-containing viral proteases evade ubiquitin - and ISG15-dependent innate immune responses. Cell Host Microbe 2 404 416
26. ZhaoC
LouZ
GuoY
MaM
ChenY
2009 Nucleoside monophosphate complex structures of the endonuclease domain from the influenza virus polymerase PA subunit reveal the substrate binding site inside the catalytic center. J Virol 83 9024 9030
27. GarcinD
KolakofskyD
1990 A novel mechanism for the initiation of Tacaribe arenavirus genome replication. J Virol 64 6196 6203
28. NewmanM
StrzeleckaT
DornerLF
SchildkrautI
AggarwalAK
1994 Structure of restriction endonuclease BamHI and its relationship to EcoRI. Nature 368 660 664
29. PingoudA
FuxreiterM
PingoudV
WendeW
2005 Type II restriction endonucleases: structure and mechanism. Cell Mol Life Sci 62 685 707
30. LelkeM
BrunotteL
BuschC
GuntherS
2010 An N-terminal region of Lassa virus L protein plays a critical role in transcription but not replication of the virus genome. J Virol 84 1934 1944
31. GuilligayD
TarendeauF
Resa-InfanteP
ColomaR
CrepinT
2008 The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol 15 500 506
32. AbrahamJ
CorbettKD
FarzanM
ChoeH
HarrisonSC
Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat Struct Mol Biol
33. De ClercqE
NeytsJ
2007 Avian influenza A (H5N1) infection: targets and strategies for chemotherapeutic intervention. Trends Pharmacol Sci 28 280 285
34. HsiehHP
HsuJT
2007 Strategies of development of antiviral agents directed against influenza virus replication. Curr Pharm Des 13 3531 3542
35. ParkesKE
ErmertP
FasslerJ
IvesJ
MartinJA
2003 Use of a pharmacophore model to discover a new class of influenza endonuclease inhibitors. J Med Chem 46 1153 1164
36. PowellHR
1999 The Rossmann Fourier autoindexing algorithm in MOSFLM. Acta Crystallogr D Biol Crystallogr 55 1690 1695
37. 1994 The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50 760 763
38. EmsleyP
CowtanK
2004 Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126 2132
39. AltschulSF
MaddenTL
SchafferAA
ZhangJ
ZhangZ
1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389 3402
40. FinnRD
MistryJ
TateJ
CoggillP
HegerA
The Pfam protein families database. Nucleic Acids Res 38 D211 222
41. MaletH
CoutardB
JamalS
DutartreH
PapageorgiouN
2009 The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol 83 6534 6545
42. HassM
GölnitzU
MüllerS
Becker-ZiajaB
GüntherS
2004 Replicon system for Lassa virus. J Virol 78 13793 13803
43. BuchholzUJ
FinkeS
ConzelmannKK
1999 Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol 73 251 259
44. HassM
LelkeM
BuschC
Becker-ZiajaB
GüntherS
2008 Mutational evidence for a structural model of the Lassa virus RNA polymerase domain and identification of two residues, Gly1394 and Asp1395, that are critical for transcription but not replication of the genome. J Virol 82 10207 10217
45. SutterG
OhlmannM
ErfleV
1995 Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett 371 9 12
46. Kabsch W Xds. Acta Crystallogr D Biol Crystallogr 66 125 132
47. EvansP
2006 Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr 62 72 82
48. VonrheinC
BlancE
RoversiP
BricogneG
2007 Automated structure solution with autoSHARP. Methods Mol Biol 364 215 230
49. SchneiderTR
SheldrickGM
2002 Substructure solution with SHELXD. Acta Crystallogr D Biol Crystallogr 58 1772 1779
50. BricogneG
VonrheinC
FlensburgC
SchiltzM
PaciorekW
2003 Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr D Biol Crystallogr 59 2023 2030
51. CowtanK
1994 An automated procedure for phase improvement by density modification. Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography 31 34 38
52. CowtanK
2006 The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr D Biol Crystallogr 62 1002 1011
53. AbrahamsJP
LeslieAG
1996 Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr D Biol Crystallogr 52 30 42
54. EmsleyP
LohkampB
ScottWG
CowtanK
Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66 486 501
55. BricogneG
BlancE
BrandlM
FlensburgC
KellerP
2010 BUSTER version 2.X. Global Phasing Ltd Cambridge, United Kingdom
56. LeslieAG
1992 MOSFLM - Recent changes and future developments. Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography 35 18 19
57. MurshudovGN
VaginAA
DodsonEJ
1997 Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53 240 255
58. SmartO
BrandlM
FlensburgC
KellerP
PaciorekW
2008 Refinement with Local Structure Similarity Restraints (LSSR) Enables Exploitation of Information from Related Structures and Facilitates use of NCS. Annual Meeting of the American Crystallographic Association
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral LoadČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



