-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTransforming Growth Factor-β: Activation by Neuraminidase and Role in Highly Pathogenic H5N1 Influenza Pathogenesis
Transforming growth factor-beta (TGF-β), a multifunctional cytokine regulating several immunologic processes, is expressed by virtually all cells as a biologically inactive molecule termed latent TGF-β (LTGF-β). We have previously shown that TGF-β activity increases during influenza virus infection in mice and suggested that the neuraminidase (NA) protein mediates this activation. In the current study, we determined the mechanism of activation of LTGF-β by NA from the influenza virus A/Gray Teal/Australia/2/1979 by mobility shift and enzyme inhibition assays. We also investigated whether exogenous TGF-β administered via a replication-deficient adenovirus vector provides protection from H5N1 influenza pathogenesis and whether depletion of TGF-β during virus infection increases morbidity in mice. We found that both the influenza and bacterial NA activate LTGF-β by removing sialic acid motifs from LTGF-β, each NA being specific for the sialic acid linkages cleaved. Further, NA likely activates LTGF-β primarily via its enzymatic activity, but proteases might also play a role in this process. Several influenza A virus subtypes (H1N1, H1N2, H3N2, H5N9, H6N1, and H7N3) except the highly pathogenic H5N1 strains activated LTGF-β in vitro and in vivo. Addition of exogenous TGF-β to H5N1 influenza virus–infected mice delayed mortality and reduced viral titers whereas neutralization of TGF-β during H5N1 and pandemic 2009 H1N1 infection increased morbidity. Together, these data show that microbe-associated NAs can directly activate LTGF-β and that TGF-β plays a pivotal role protecting the host from influenza pathogenesis.
Published in the journal: . PLoS Pathog 6(10): e32767. doi:10.1371/journal.ppat.1001136
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001136Summary
Transforming growth factor-beta (TGF-β), a multifunctional cytokine regulating several immunologic processes, is expressed by virtually all cells as a biologically inactive molecule termed latent TGF-β (LTGF-β). We have previously shown that TGF-β activity increases during influenza virus infection in mice and suggested that the neuraminidase (NA) protein mediates this activation. In the current study, we determined the mechanism of activation of LTGF-β by NA from the influenza virus A/Gray Teal/Australia/2/1979 by mobility shift and enzyme inhibition assays. We also investigated whether exogenous TGF-β administered via a replication-deficient adenovirus vector provides protection from H5N1 influenza pathogenesis and whether depletion of TGF-β during virus infection increases morbidity in mice. We found that both the influenza and bacterial NA activate LTGF-β by removing sialic acid motifs from LTGF-β, each NA being specific for the sialic acid linkages cleaved. Further, NA likely activates LTGF-β primarily via its enzymatic activity, but proteases might also play a role in this process. Several influenza A virus subtypes (H1N1, H1N2, H3N2, H5N9, H6N1, and H7N3) except the highly pathogenic H5N1 strains activated LTGF-β in vitro and in vivo. Addition of exogenous TGF-β to H5N1 influenza virus–infected mice delayed mortality and reduced viral titers whereas neutralization of TGF-β during H5N1 and pandemic 2009 H1N1 infection increased morbidity. Together, these data show that microbe-associated NAs can directly activate LTGF-β and that TGF-β plays a pivotal role protecting the host from influenza pathogenesis.
Introduction
Transforming growth factor-β1 (TGF-β) is the prototypic member of a family of multifunctional cytokines that modulate diverse cellular, developmental, and immunological processes (reviewed in [1]–[3]). TGF-β is secreted by virtually all cells as a biologically inactive molecule termed latent TGF-β (LTGF-β) [4], [5]. The latent complex consists of an N-terminal latency-associated peptide (LAP) and the mature TGF-β domain. LAP and TGF-β are products of a single gene, which after posttranslational modifications such as glycosylation and phosphorylation and cleavage by furin remain noncovalently associated, forming the small latent complex [6]. The small latent complex is secreted by cells as an inactive complex, and in some cases is linked by a disulfide bond to the latent TGF-β-binding protein to form the large latent complex.
The non-covalent association of LAP with the mature domain is critical for latency. The molecular mechanism by which LAP confers latency to mature TGF-β is largely unknown. However, recent studies suggest that amino acids 50–85, several of which are glycosylated and contain terminal sialic acid residues, are critical for proper formation and function of the LTGF-β complex [7]. Mutations in this region reduce the binding of LAP to the mature domain and significantly impair the ability of LAP to confer latency to mature TGF-β [8]. Agents that activate the latent complex can disrupt the association of LAP with the mature domain either by proteolysis or denaturing the LAP or by altering its folding [6]. Given the abundance of LTGF-β and the prevalence of high-affinity receptors on most cell types, the activation of LTGF-β is recognized as a crucial step in TGF-β function (reviewed in [9], [10]).
Chaotropic agents, heat, reactive oxygen species [11], [12], and extreme pH [13], [14] can activate LTGF-β. In vitro studies have identified proteases, which degrade the LAP (reviewed in [15]), and molecules such as thrombospondin-1, which alter the conformation of the LAP [16], [17], [18], [19], [20], as putative physiological TGF-β activators. Less is known about activation in vivo, although integrins appear to be the primary LTGF-β activators in the lung [10], [21], [22].
Little is known about the direct activation of LTGF-β by microbes. Several parasites such as Trypanosoma cruzi, Leishmania spp., and Plasmodium spp. and the bacteria Mycobacterium tuberculosis activate LTGF-β through proteolysis, using either host-derived plasmin or microbe-encoded proteases [23]–[26]. In a previous study, we have shown that influenza viruses activate TGF-β in vitro and in vivo [27]. Antibodies to the viral neuraminidase (NA) protein inhibited viral-induced LTGF-β activation, suggesting that NA plays a role in LTGF-β activation, but the precise mechanism of activation remains to be identified and the role of TGF-β in influenza disease is unknown. In this study, we determined the mechanism of activation of rLTGF-β by viral and bacterial NA. Since NA is essential for viral replication, we tested a panel of influenza virus subtypes (including two 2009 H1N1 pandemic strains) for their ability to activate LTGF-β in vitro. For strains that failed to activate LTGF-β, we used reverse genetic studies to determine whether these viruses had deficient NA activity. We also investigated whether exogenous TGF-β provides protection from H5N1 influenza pathogenesis and whether depletion of TGF-β during virus infection increases morbidity in mice.
Results
Purified NA activates LTGF-β
To begin defining the mechanism of NA-mediated activation of LTGF-β, we first asked if NA purified from the virion was sufficient for activation. Thus, recombinant LTGF-β (rLTGF-β) was incubated with buffer alone, purified A/Gray Teal/Australia/2/1979 virus (N4 virus), purified Gray Teal NA (N4 NA, BEI Resources, Manassas, VA), or low-protease-content NA purified from Clostridium perfringens (Roche), which was used as a non-viral NA control (bNA). All the samples were standardized to equivalent NA enzymatic activity and rLTGF-β activation was monitored by two different assays; the PAI/L bioassay, which monitors the activation of a TGF-β-specific reporter construct expressed in a stable cell line, or a sandwich ELISA specifically recognizing an epitope on the active TGF-β protein. All of the samples activated rLTGF-β in both assays in a dose-dependent manner. In the PAI/L bioassay, the concentration of active TGF-β increased with increasing amounts of NA (Fig. 1A). However, at the highest concentration of N4 virus (180,000 RFU) there was no TGF-β activity and the cells appeared dead. In the ELISA, both the N4 virus and purified NAs had low, but detectable levels of TGF-β activity at the lowest dose tested (10,000 RFU), that increased at 30,000 RFU, and then remained steady at the higher NA concentrations (Fig. 1B). Overall, these studies demonstrate that both influenza viral and a bacterial NA can activate LTGF-β.
Fig. 1. NA activates LTGF-β. 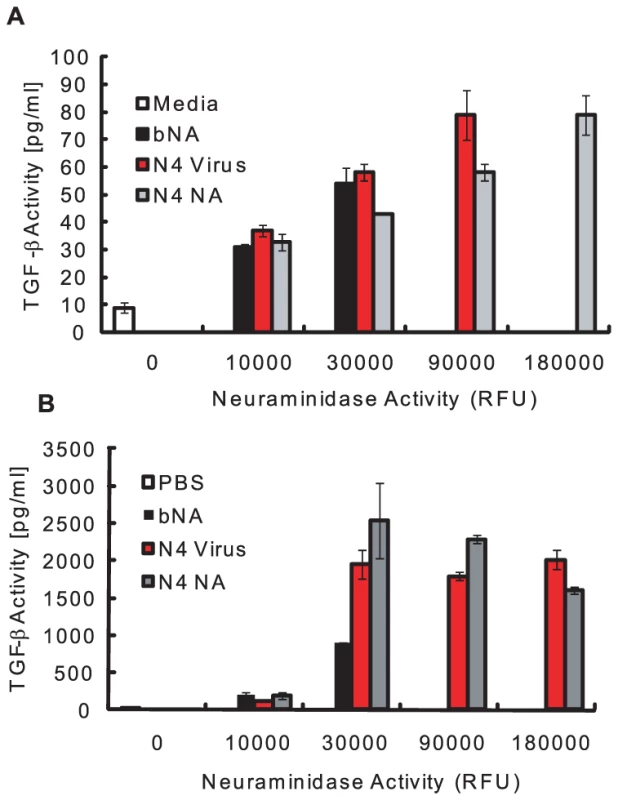
rLTGF-β (10 ng/ml) was incubated alone (white bar) or with increasing amounts of enzymatically equivalent amounts of bacterial NA (bNA, black bar), N4 virus (red bar), or purified Gray Teal NA (gray bar) for 1 h at 37°C and TGF-β activity (pg/ml) measured by the PAI/L assay (A) or ELISA (B). Error bars represent standard error of the mean. NA removes sialic acids on the LAP
To determine if NA-mediated activation involved removal of the sialic acid motifs on the LAP, rLTGF-β was incubated with PBS, bNA, N4 virus, or purified N4 NA, and the size of the LAP was determined by Western blot. HCl was used as a control for non-enzymatic-mediated activation of rLTGF-β. The rLTGF-β incubated with bNA, N4 virus, and N4 NA showed a slight shift in mobility of the LAP as compared to that incubated with PBS or HCl (Fig. 2A). There was no significant difference in the mobility between the bNA, N4 virus, and N4 NA. This shift in mobility was not evident when N4 NA was incubated with rLTGF-β purified from insect cells (Fig. 2B). The rLTGF-β produced by insect cells is unsialylated, as insect cells have no detectable sialyltransferase activity [28]. Thus, the lack of size change upon incubation with N4 NA suggests that the increased mobility of LTGF-β treated with N4 virus, bNA, or N4 NA is due to removal of sialic acid moieties. To confirm this, rLTGF-β was incubated with PBS, bNA, or N4 NA, proteins separated on a reducing SDS-PAGE, and sialic acid expression monitored by Western blot analysis using digoxigenin (DIG)–labeled lectins Maackia amurensis agglutinin (MAA; recognizes α2-3 sialic acid linkages, Fig. 2C) or Sambucus nigra agglutinin (SNA; recognizes α2-6 sialic acid linkages, Fig. S1). Blots were also probed with anti-LAP to confirm that the protein analyzed was the LAP (Fig. 2D). rLTGF-β incubated with PBS was detected by both SNA and MAA, suggesting the presence of both α2-6 and α2-3–linked sialic acids on the LAP (Fig. 2C and Fig. S1). In contrast, MAA and SNA failed to recognize bNA-treated rLTGF-β. Similarly, N4 NA–treated rLTGF-β was not recognized by MAA (Fig. 2C), but was detected by SNA (Fig. S1). However, this does not imply that the N4 NA fails to cleave α2-6 linkages. Hence, the mobility shift observed when rLTGF-β was incubated with NA is likely because of the loss of LAP-associated α2,3-linked sialic acids, but the specific sialic acid linkages removed may depend on the NA, as seen in the case of bNA-treated rLTGF-β.
Fig. 2. NA induces a shift in the size of LAP and removes sialic acids from the LAP. 
rLTGF-β (0.4 µg) was incubated with PBS, HCl (pH 2), bacterial NA (bNA), purified Gray Teal virus (GT Virus, 2 µg), or purified Gray Teal NA (GT NA, 0.5 µg) in the presence or absence of 1 µM oseltamivir carboxylate (NAi) or 1× protease inhibitor cocktail (PI) for 1 h at 37°C. Proteins were separated by SDS-PAGE under reducing conditions, transferred to nitrocellulose, and probed with anti-LAP antibody (A and D) or DIG-labeled MAA lectin (C) by Western blot analysis. (B) Insect cell–derived rLAP (0.5 µg) was incubated as described above and probed with anti-LAP antibody by Western blot analysis. bNA and GT NA alone were run as controls for (C). NA enzymatic activity is involved in rLTGF-β activation, but proteases may also play a role in this process
To determine whether the enzymatic activity of NA was required for loss of specific sialic acid motifs and TGF-β activation, N4 virus or NA was pre-incubated with the influenza-specific inhibitor (NAi) oseltamivir carboxylate (10 nM) before incubation with rLTGF-β. Pre-incubation with NAi inhibited the mobility shift in the LAP (Fig. 2A), the loss of sialic acid (Fig. 2C), and TGF-β activation (Fig. 3A and B). N4 virus and NA–induced activation was completely inhibited with 10 nM NAi in the PAI/L assay (Fig. 3A) and to a lesser degree (75–95%) in the ELISA (Fig. 3B). Increasing the concentration of NAi up to 10 µM failed to completely inhibit activation in the ELISA assay (data not shown). Because the NAi is specific for influenza NA, it did not inhibit bNA-induced activation of rLTGF-β (Fig. 3A and 3B).
Fig. 3. Role for NA activity and proteases in LTGF-β activation by influenza virus. 
(A) rLTGF-β (10 ng/ml) was incubated alone (black bar) or with bNA (30,000 RFU NA activity), purified N4 virus (90,000 RFU NA activity), or purified N4 NA (90,000 RFU NA activity) in the presence or absence of 1× PI cocktail (red bars) or 10 nM oseltamivir carboxylate (NAi, gray bars) for 1 h at 37°C. TGF-β levels were measured by (A) PAI/L assay or (B) ELISA. (C) rLTGF-β (10 ng/ml) was incubated with purified Tk/WI virus (106 TCID50 units/ml) alone or in the presence of increasing concentrations of bestatin (▪, nM), leupeptin (•, µM) or GM1489 (Δ, nM), and TGF-β activity measured by ELISA. Error bars represent standard error of the mean. Asterisk (*) indicates significant inhibition as compared with virus/NA-treated LTGF-β in the absence of inhibitor. Proteases are established activators of LTGF-β [15] and can be contaminants of viral preparations or even components of the viral membrane [29], [30]. To examine the role for proteases, the LAP shift and activation assays were performed in the presence of a broad-spectrum protease inhibitor (PI) cocktail. The PI cocktail used in these studies had no effect on sialidase activity of either the virus or NAs and did not inhibit active TGF-β detection in either assay (data not shown). Unlike NAi, pre-incubation with PI had no effect on the LAP mobility shift (Fig. 2A). However, the PI inhibited the N4 virus and NA-induced activation of LTGF-β in the ELISA assay (Fig. 3B) and to some extent in the PAI/L assay (Fig. 3A), although the inhibition was not as much as that seen with NAi treatment. To determine the specific class of proteases causing the inhibition, virus was pretreated with increasing concentrations of individual protease inhibitors within their effective inhibitory ranges and incubated with rLTGF-β, and TGF-β activity was determined by ELISA (Fig. 3C). None of the protease inhibitors blocked rLTGF-β activation by the N4 virus. Further, when incubated with substrate for 1 h, all reagents tested protease-free (negative for trypsin, chymotrypsin, thrombin, plasmin, elastase, subtilisin, papain, cathepsin B, thermolysin, and pepsin) in a fluorescein thiocarbamoyl-casein derivative-based assay kit (data not shown). Even when incubations were extended to 24 h, only few viral stocks were positive for proteases (Fig. S2). Together, these data suggest that NA activates LTGF-β primarily via a mechanism involving enzymatic activity. However, a role for proteases cannot be discounted especially during infection in vivo.
Most influenza strains activate LTGF-β
As NA is essential for viral replication, we hypothesized that all influenza strains could activate LTGF-β. rLTGF-β was incubated with a panel of influenza virus subtypes, including two 2009 H1N1 pandemic strains, and several highly pathogenic avian influenza viruses (H5N1 and H5N9), and TGF-β activity was measured by the PAI/L assay (Fig. 4A) or ELISA (Fig. 4B). Although most of the strains activated LTGF-β, the levels of activation differed despite having equivalent NA activity. Surprisingly, several of the H5N1 influenza viruses failed to activate rLTGF-β; only A/Hong Kong/486/1997 (HK/486) consistently activated rLTGF-β (Fig. 4A and 4B). Incubation of rLTGF-β with a representative non-activating H5N1 virus, A/Hong Kong/483/1997 (HK/483), did not cause the expected mobility shift in the LAP (Fig. 4C), suggesting that the NA from viruses that did not activate rLTGF-β may also not cleave sialic acids from the LAP.
Fig. 4. Influenza viruses differentially activate LTGF-β. 
rLTGF-β (10 ng/ml) was incubated alone (white bar) or with different influenza virus subtypes (90,000 RFU) for 1 h at 37°C and TGF-β activity measured by PAI/L assay (A) or ELISA (B). (C) rLTGF-β (0.4 µg) was incubated with PBS, bNA, N4 Virus (2 µg), or HK/483 virus (HK/483, 2 µg) in the presence or absence of 1 µM oseltamivir carboxylate (NAi) for 1 h at 37°C. Proteins were separated by SDS-PAGE under reducing conditions, transferred to nitrocellulose, and probed with anti-LAP antibody by Western blot analysis. Error bars represent standard error of the mean. There is no intrinsic defect in the H5N1 viral NA
To determine whether the failure of H5N1 viruses to activate rLTGF-β was due to an intrinsic defect in the H5 NA protein, we first examined rLTGF-β activation by A/Teal/Hong Kong/W312/97 (Teal/HK) H6N1 virus. Teal/HK NA shares 97% sequence nucleotide homology with the H5N1 NA including a 19-amino-acid deletion in the stalk region and is the proposed donor of the NA and the internal genes of the H5N1 viruses [31]. Unlike the H5N1 viruses, Teal/HK virus activated rLTGF-β in both assays (Fig. 4A and 4B) suggesting that the deletion in the NA stalk domain has no effect on TGF-β activation.
To further assess the H5N1 NA, two H1N1 influenza viruses (A/California/04/09 and A/Puerto Rico/8/34) expressing the HK/483 NA were generated (CA/09+HK/483 NA and PR8+HK/483 NA) and tested for rLTGF-β activation. Both the parental viruses and the reassortant viruses containing the HK/483 NA activated rLTGF-β in the PAI/L (Fig. 5A) and ELISA (Fig. 5B) assays. Further, activation was inhibited by NAi but not the PI cocktail (Fig. 5C), confirming that the HK/483 NA can activate rLTGF-β in a NA-dependent manner.
Fig. 5. Reassortant viruses differentially activate rLTGF-β. 
rLTGF-β (10 ng/ml) was incubated alone (white bar), or with equivalent levels (90,000 RFU NA activity) of parental CA/09 (red bars), parental PR8 (dark gray bars) or parental HK/486 (light gray) viruses, or reassortant viruses expressing the HK/483 NA (hatched bars) for 1 h at 37°C and TGF-β activity measured by PAI/L assay (A) or ELISA (B). (C) rLTGF-β (10 ng/ml) was incubated with bNA, parental PR8 virus, or the PR8+HK/483 NA viruses alone (black bars) or in the presence or absence of 1× PI cocktail (red bars) or 10 nM oseltamivir carboxylate (gray bars) for 1 h at 37°C and TGF-β activity measured by ELISA. (D) BALB/c mice (4–6 weeks old) were inoculated i.n. with PBS (control, n = 8), or 104 TCID50 units of the parental or reassortant viruses (n = 10) and lungs collected at 2, 4, and 7 dpi. Total (acid-activated samples) and active TGF-β levels were monitored in the lung homogenates by the mouse TGF-β–specific ELISA. Error bars represent standard error of the mean. H5N1 viral NA fails to activate rLTGF-β on an H5 virus in vitro and in vivo
To construct an H5N1 virus that activated rLTGF-β, HK/483 virus expressing the HK/486 NA was generated. Unfortunately, this virus was unable to activate rLTGF-β (Fig. 5A and 5B). Further, expressing the HK/483 NA on the HK/486 virus led to reduced activation as compared to the parental HK/486 virus. To evaluate LTGF-β activation in vivo, BALB/c mice were intranasally inoculated with PBS (control, n = 8) or 104 TCID50 units of the different reassortant viruses (n = 10) and lungs collected at 2, 4, and 7 days post-infection (dpi). Active or total (determined by acid activation of the sample) levels of TGF-β in the lung homogenates were determined by ELISA (Fig. 5D). Similar to the in vitro results, only HK/486 increased TGF-β activity in the lungs of infected mice as compared to PBS-inoculated mice. Levels of active TGF-β were increased >5-fold within 2 dpi, remained elevated at 4 dpi, before returning to control levels at 7 dpi (Fig. 5D). These kinetics were similar to those observed in mice infected with PR8 virus [27] and other highly pathogenic avian influenza viruses [32].
The total TGF-β levels in the lungs remained constant for all the viruses except for a significant decrease (∼60%) in the HK/483 infected mice at 2 dpi (Fig. 5D). This decline was not seen in mice infected with the HK/483+HK/486 NA reassortant virus, which remained at control levels at 2 dpi. These findings suggest that the NA may influence the total levels of TGF-β in the lungs of infected mice through an undefined mechanism.
Exogenous TGF-β provides partial protection during HK/483 infection
Because we were unable to construct an HK/483 H5N1 virus that activates TGF-β in vivo, active TGF-β1 was administered to HK/483-infected mice by using a replication-deficient adenovirus vector. Twenty-four hours after HK/483 infection (104 TCID50), 108 PFUs of control adenovirus vector (AdDL70, n = 12), TGF-β-expressing vector AdTGFβ223/225 (n = 12), or PBS (n = 12) were administered intranasally. Lung TGF-β levels were measured at 2, 4, and 7 dpi. By 2 dpi, TGF-β levels in the lung increased to approximately 450 pg/ml in mice treated with the TGF-β–expressing adenovirus and remained above control levels even at 7 dpi (Fig. 6A). Mice treated with the control virus AdDL70 showed a transient increase in lung TGF-β activity at 2 dpi (100 pg/ml), which returned to control levels by 4 dpi. By 4 dpi with the HK/483 virus, all infected mice lost approximately 15% (p<0.01) of their initial body weight, which increased to more than 20% by 7 dpi in the HK/483 and +AdDL70 groups (Fig. 6B), at which time mice either succumbed to infection or were euthanized (Fig. 6C). Mice inoculated with AdTGFβ223/225 showed delayed weight loss and prolonged survival. At 7 dpi, weight loss remained at approximately 15% (p<0.01), but increased to 25% by 9 dpi (Fig. 6B). This was associated with a significant delay in mortality: AdTGFβ223/225 - infected mice survived until 10 dpi (p<0.05, Fig. 6C). These mice also had significantly lower viral titers than HK/483-infected mice (Fig. 6D). By 2 dpi (1 day post AdTGFβ223/225 inoculation), viral titers decreased from approximately 107.5 TCID50 to 105.5 TCID50 (p<0.05) in the HK/483 alone and AdDL70 groups. Similar decreases in titers were observed at 4 and 7 dpi (p<0.05) in the HK/483 alone group. However, there was no significant difference in titers between the AdDL70 and AdTGFβ223/225 groups at 4 dpi.
Fig. 6. Exogenous TGF-β delays mortality in HK/483-infected mice. 
BALB/c mice (4–6 weeks old) were inoculated i.n. with PBS (control, n = 10), or 104 TCID50 units of HK/483 virus and 24 hpi inoculated with 108 PFU/mouse of a TGF-β-expressing adenovirus (AdTGFβ223/225) or a control adenovirus vector (AdDL70) (infected groups, n = 12). Mice inoculated with AdDL70 or AdTGFβ223/225 alone served as controls (n = 10). At 2, 4, and 7 days post HK/483 infection (1, 3, and 6 day post-adenovirus administration), lung homogenates were monitored for TGF-β levels by a mouse-specific ELISA (A) and viral titers by TCID50 analysis on MDCK cells (D). For titers, red bars, day 2pi; dark gray bars, day 4 pi; and gray bars, day 7 pi. Weights (B) and survival (C) were monitored for 10 dpi. Error bars represent standard error of the mean. Asterisk (*) indicates significant increase in TGF-β levels as compared with other groups (A); difference in weight loss (B) or mortality (C) as compared with HK/483 virus and AdDL70-treated mice; and decrease in viral titers as compared with HK/483 infected mice (D). Given the increased survival of mice infected with AdTGFβ223/225, we tested whether pretreatment with TGF-β afforded additional protection to mice. Mice (n = 12) were administered 108 PFUs AdDL70 control or the AdTGFβ223/225 virus 48 h before HK/483 infection. Pretreatment with AdTGFβ223/225 provided no added protection; all the HK/483-infected mice succumbed to infection by 8 dpi (Fig. S3B). Both the uninfected and HK/483-infected mice pretreated with AdTGFβ223/225 lost significantly more weight by 4 dpi than mice in other groups (10% vs. 0%, p<0.01, Fig. S3A), suggesting that increased TGF-β activity before H5N1 influenza infection can be detrimental to mice.
Depletion of TGF-β during HK/486 or pandemic 2009 H1N1 infection increases morbidity
We then examined the effect of removing TGF-β during HK/486 infection by depleting TGF-β using a pan-TGF-β neutralizing antibody. Briefly, 1D11 antibody or isotype-control IgG was administered and total TGF-β levels in the lungs were monitored by ELISA (Fig. 7A). Total TGF-β levels in HK/486-infected mice were significantly (3 times) lower (p = 0.0003) by 24 hpi than in the HK/486-infected mice receiving isotype IgG. Two doses of the neutralizing antibody decreased TGF-β levels to control levels; by 7 dpi, levels were significantly (3 times) lower (p = 0.04) than control levels. By 8 dpi, all HK/486-infected mice (105 TCID50, n = 15) lost approximately 20% of their starting weight whereas HK/486-alone mice lost only 10% (Fig. 7B, p<0.01). By 9 dpi, 40% of mice in the TGF-β–depleted group succumbed to infection, and all died by 10 dpi (Fig. 7C), whereas those in the HK/486 and isotype IgG groups began to recover and gain weight. The increased mortality in the TGF-β–depleted group was not associated with a significant increase in viral replication. HK/486-infected mice with and without isotype IgG had peak lung titers of approximately 104.6 TCID50 by 2 dpi, which decreased to 101.5 TCID50 by 10 dpi (Fig. 7D). In contrast, the TGF-β–depleted group had a slight, although not significant, increase in viral titers at 2 and 4 dpi. At 8 dpi, 1D11-treated mice had a 15-fold increase in viral titers over HK/486 and isotype IgG–treated mice (p<0.02). No virus was detected in control tissues or outside the lungs of infected mice.
Fig. 7. Depletion of TGF-β alters morbidity in HK/486-infected mice. 
Six hours before infection, BALB/c mice were treated with a TGF-β neutralizing antibody (1D11) or an isotype control antibody (IgG) at a dose of 0.5 mg/mouse and subsequently inoculated with PBS (control) or 105 TCID50 units of HK/486 virus (n = 15). Antibodies were readministered every 48 hpi. At 0, 1, 4, and 7 days pi, total TGF-β levels were analyzed in lung homogenates by a mouse TGF-β-specific ELISA (A). Weights (B) and survival (C) were monitored for 14 dpi. On days 2, 4, 8, and 10 pi, lung homogenates were monitored viral titers by TCID50 analysis on MDCK cells (D). Error bars represent standard error of the mean. Asterisk (*) indicates significant decrease in TGF-β levels as compared with infected group treated with IgG (A), and increase in viral titers as compared with HK/486-infected mice with and without IgG treatment (D). To determine if these findings were specific to the highly pathogenic H5N1 influenza viruses, mice (n = 6) were pre-treated with PBS, the 1D11 antibody or isotype-control IgG as described, infected with A/California/04/09 (CA/09, 105 TCID50), and monitored for morbidity. The CA/09-infected mice treated with PBS or receiving the isotype IgG lost approximately 20–25% of their starting weight by 6 to 8 dpi before returning to day 0 weights by 12 dpi (Fig. 8A). Clinically the mice had ruffled fur and were shivering. The 1D11-treated mice followed a similar pattern but lost significantly more weight by 6 dpi (p = 0.047) and had a delayed recovery with significantly more weight loss still evident at 12 dpi (p = 0.029). The 1D11-treated mice had more significant clinical signs of infection including rear-leg paralysis and had 20% mortality by 8 dpi reaching 67% by 12 dpi (Fig. 8B). Taken together, the data suggest that TGF-β is modulated by the virus, and this modulation during infection may be important in disease outcome.
Fig. 8. Depletion of TGF-β alters morbidity in HK/486-infected mice. 
Forty-eight hours before infection, BALB/c mice were treated with a TGF-β neutralizing antibody (1D11) or an isotype control antibody (IgG) at a dose of 0.5 mg/mouse and subsequently inoculated with PBS (control) or 105 TCID50 units of CA/09 virus (n = 6). Antibodies were re-administered every 48 hpi. Mice were monitored daily for weight loss (A) and mortality (B). Error bars represent standard error of the mean. Asterisk (*) indicates significant weight loss as compared to HK/486 infected mice. Discussion
These studies establish NA as a direct activator of LTGF-β and demonstrate a role for TGF-β in protection against influenza virus pathogenesis. We have previously shown that purified influenza virus activates TGF-β [27] and that antibodies to the viral NA but not the HA inhibit viral-mediated LTGF-β activation. In this study, we demonstrate that purified NA alone can convert the biologically latent form of TGF-β to its active form and that TGF-β plays an important role in protection against influenza virus pathogenesis. Activation of LTGF-β by viral NA involves removal of sialic acid moieties to release the active TGF-β molecule from the latent complex or expose other residues for cell surface interactions. To our knowledge, these are the first studies demonstrating that microbe-associated sialidases can directly activate LTGF-β.
Our study also shows that NA-mediated LTGF-β activation is not specific to influenza virus. As the topology of the NA catalytic domain is well conserved and the active sites share many structural features [33], NAs from diverse pathogens may activate LTGF-β. In our study, Clostridium perfringens–derived bNA also activated LTGF-β, which is consistent with previous studies showing LTGF-β activation by bNA, although sialidase activity was not explicitly identified as the means of activation [34], [35]. Paramyxoviruses, which also have a functional NA protein, directly activate LTGF-β (unpublished data). A study by Zou and Sun demonstrated that LTGF-β2 and LTGF-β3 were also activated by NA [36], suggesting that NA may be a biological activator of numerous types of LTGF-β.
Despite the functional conservation among NAs, some highly pathogenic avian (HPAI) H5N1 influenza viruses failed to activate LTGF-β in vitro and in vivo [32], [37]. The NA from these viruses has a 19-amino-acid deletion in the stalk [31], [38] that could contribute to the decreased ability to activate LTGF-β. To test this possibility, we assessed the activation of rLTGF-β by the Teal/HK H6N1 virus. Hoffmann et al., proposed that this virus may have donated the NA gene to the H5N1 viruses given the high degree of nucleotide homology [31]. In spite of the stalk deletion, Teal/HK activated rLTGF-β unlike the H5N1 viruses. Further, expressing the HK/483 NA on either PR8 or CA/09 virus had no effect on rLTGF-β activation suggesting that there is no intrinsic defect in the NA.
However, expressing the HK/483 NA on the H5 HK/486 virus led to an inability to activate LTGF-β in vitro and in vivo. In addition the HK/486 NA failed to rescue the activation phenotype with the HK/483 virus. A recent study by Matsuoka et al demonstrated that short-stalk NAs from the H5N1 viruses are more virulent in mice and chickens. Intriguingly, the NA-mediated virulence can be affected by HA glycosylation. Virulence in mice conferred by a short stalk NA was most evident when the HA had no glycosylation [38]. Although virulence in vivo is much more complicated than LTGF-β activation, studies are underway to examine the role of HA in LTGF-β. We hypothesize that although HA will not be directly involved in activation, it may influence the ability of NA to activate.
The question remains whether NA-mediated activation has an important biological role in TGF-β activation during influenza infection in vivo. At this time we can't definitively answer that question. What is intriguing is that the NA may influence the total levels of lung TGF-β during infection. Mice infected with HK/483 had a dramatic decrease in total LTGF-β levels by 2 dpi (from ∼2000 pg/ml to ∼1000 pg/ml). This phenotype was reversed with the HK/483 virus expressing the HK/486 NA. A similar trend was seen when HK/483 NA was expressed on HK/486; a significant decrease in total TGF-β levels. Studies are on-going to determine if this is due to a change in the cells in the lung associated with TGF-β secretion or if the viruses differentially regulate the known physiologic activators.
There are numerous physiologic TGF-β activators in the lung: the infected epithelium could release thrombospondin-1, proteases and matrix metalloproteases, or even reactive oxygen species (reviewed in [15], [21], [39]). Virus-induced injury to the epithelium can directly activate LTGF-β through the induction of apoptosis [40] or the upregulation of integrins [41]–[44], and immune cells have high levels of active TGF-β [45], [46]. Proteases may play a role in influenza virus-induced LTGF-β activation, especially during influenza infection in vivo, wherein cellular proteases are essential for influenza virus replication (reviewed in [47]–[50]). Proteases can be contaminants of viral preparations or even components of the viral membrane [29], [30]. Thus, only protease-free reagents were used in our assays, and a broad-spectrum PI cocktail partially blocked influenza virus and NA-mediated LTGF-β activation. Further studies confirmed that the broad-spectrum PI cocktail had no effect on either sialidase activity (as measured in the MUNANA assay) or directly on TGF-β detection in either assay (data not shown). However, we have not been able to identify the specific class of proteases or a potential cleavage site within LTGF-β by mass spectrometry (data not shown).
Since our initial attempts to construct H5 viruses that can activate TGF-β in vivo were unsuccessful, we evaluated the role of TGF-β in influenza pathogenesis by using a neutralizing antibody and administering exogenous TGF-β via an adenovirus vector. Mice administered TGF-β neutralizing antibody during HK/486 H5N1 infection had higher morbidity and mortality than mice treated with control virus or isotype IgG. This finding was not specific to H5N1 influenza viruses; inhibiting TGF-β activity during 2009 H1N1 infection also increased morbidity. Although mice exhibited clinical signs of illness, administration of exogenous TGF-β to HK/483-infected mice once at 24 hpi delayed morbidity and mortality. Administration of exogenous TGF-β 48 h pre-infection did not affect survival, and TGF-β–treated infected and non-infected mice had increased weight loss by 4 dpi, suggesting that the timing of TGF-β activation may be important.
Although we are still investigating the specific protective role of TGF-β during influenza infection, we did find that mice administered exogenous TGF-β had significantly lower titers within 2 dpi than untreated infected and AdDL70-treated mice. This decrease in viral load may contribute to the delayed morbidity observed, but was not sufficient to protect mice from severe infection. In contrast, depleting TGF-β during HK/486 infection had little to no significant effect on viral load until 8 dpi. Thus, mechanisms other than reduction of viral load may be involved in TGF-β–mediated modulation of influenza pathogenesis.
TGF-β serves as a global regulator of immunity by controlling the initiation and resolution of inflammatory responses (reviewed in [39], [46]). Thus, a pathogen that can regulate TGF-β activation could promote an immune-privileged state for itself within its host, as has been seen in the case of multiple parasitic, bacterial, and fungal pathogens (reviewed in [46], [51]–[55]). We postulate that failure of certain H5N1 influenza viruses to activate TGF-β [56], [57] may result in improper immune stimulation and resolution, contributing to exacerbated immunopathology for the host. Further investigation into specific immune cell activities and cytokine profiles during TGF-β modulation is required to fully elucidate the mechanisms of TGF-β regulation of influenza virus replication.
Materials and Methods
Ethics statement
All procedures involving animals were approved by the Southeast Poultry Research Laboratory (USDA-ARS), University of Wisconsin-Madison School of Medicine and Public Health, and the St. Jude Children's Research Hospital IACUCs and were in compliance with the Guide for the Care and Use of Laboratory Animals. These guidelines were established by the Institute of Laboratory Animal Resources and approved by the Governing Board of the U.S. National Research Council.
Laboratory facility
All experiments in which H5N1 viruses were used were conducted in a Biosafety level 3 enhanced containment laboratory [58]. Investigators were required to wear appropriate respirator equipment (RACAL, Health and Safety Inc., Frederick, MD). Mice were housed in HEPA-filtered, negative pressure, vented isolation containers (M.I.C.E. ®, Animal Care Systems, Littleton, CO).
Cell and virus propagation
A/Turkey/Wisconsin/68 (Tk/WI, H5N9), A/Gray Teal/Australia/2/79 (N4, H4N4), A/Swine/Nebraska/2/92 (Sw/NB, H1N1), A/Turkey/England/69 (Tk/Eng, H3N2), A/Turkey/Oregon/71 (Tk/Oreg, H7N3), A/Turkey/Ontario/6528/67 (Tk/Ont, H5N9), A/Mallard/Wisconsin/8/76 (Mal/WI, H1N1), A/Teal/Hong Kong/W312/97(Teal/HK, H6N1), and the 2009 H1N1 A/California/04/09 (CA/09) and A/Wisconsin/054/09 viruses were propagated in the allantoic cavity of 10-day-old specific pathogen-free embryonated chicken eggs (Sunnyside Farms, Beaver Dam, WI) at 37°C. Allantoic fluid was harvested, clarified by centrifugation and stored at −70°C. A/Puerto Rico/8 (PR8, H1N1), A/WSN/33 (WSN, H1N1), A/New Caledonia/20/99 (New Cal, H1N1), A/Hawaii/10/2002 (Hawaii, H1N2), A/Wyoming/3/2003 (Wyom, H3N2), A/Korea/770/2002 (Korea, H3N2), A/Aichi/2/68 (Aichi, H3N2), and the H5N1 viruses A/Hong Kong/156/97 (HK/156), A/Hong Kong/486/1997 (HK/486), A/Hong Kong/483/1997 (HK/483), A/Vietnam/1203/2004 (VN/1203), and A/Vietnam/1194/2004 (VN/1194) were propagated in Madin-Darby canine kidney (MDCK) cells as described previously [59]. Culture supernatants were harvested, clarified by centrifugation, and stored at −70°C. All viral titers were determined by 50% tissue culture infective dose (TCID50) analysis in MDCK cells and evaluated by the method of Reed and Muench [60]. MDCK cells were cultured in Eagle's minimal essential medium (MEM) supplemented with 2 mM glutamine (Mediatech, Manassas, VA), and 10% fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, CA). Mink lung epithelial cells stably transfected with the TGF-β-sensitive plasminogen activator inhibitor reporter construct (Mv1Lu-PAI cells, generous gift of Dr. Daniel Rifkin, New York University) were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM glutamine, 7% FBS, and 400 µg/ml Geneticin (G418, Calbiochem, La Jolla, CA).
Purification of influenza virus and NA
Gray Teal influenza virus was purified by sucrose gradient ultracentrifugation [61]. Purified Gray Teal NA was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: N4 Neuraminidase (NA) Protein from Influenza Virus, A/grey teal/Australia/2/79 (H4N4), Recombinant from baculovirus, NR-656 (BEI Resources, Manassas, VA). Briefly, it was expressed in Sf9 cells using a baculovirus expression vector system and purified using conventional chromatographic techniques.
NA activity assay
NA enzymatic activity was determined by the MUNANA (2-(4-methylumbelliferyl)-α-d-N-acetylneuraminic acid) assay as described previously [62]. NA inhibition was assayed with purified NA, and virus was standardized to equivalent NA enzyme activity and incubated for 1 h at 37°C with oseltamivir carboxylate (0–1000 nM, generous gift of Hoffman La-Roche, Inc., Nutley, NJ).
TGF-β assays
TGF-β activity was assessed by the plasminogen activator inhibitor-luciferase (PAI/L) bioassay or by a TGF-β-specific ELISA following manufacturer's instructions (R&D Systems, Minneapolis, MN). The ELISA is a quantitative sandwich immunoassay where a monoclonal antibody specific for the active region of TGF-β1 is coated onto a microplate and any bound TGF-β1 is detected with an enzyme-linked polyclonal antibody specific for TGF-β1. It will not detect the latent form of TGF-β1. The PAI/L bioassay was performed as previously described [63], with several modifications. Briefly, 2×104 Mv1Lu-PAI cells per well of 96-well plates were incubated overnight, washed with PBS, and incubated with 100 µl/well test sample for 5 h at 37°C, 5% CO2. After washing, cells were lysed and luciferase activity measured by using a luciferase assay substrate (Promega, Madison, WI) on a Turner Biosystems 20/20n luminometer (Turner Biosystems Instruments, Sunnyvale, CA). Test samples included 10 ng/ml recombinant LTGF-β1 (rLTGF-β1, R&D Systems) incubated with different concentrations of low-protease-content Clostridium perfringens-purified NA (Roche Applied Sciences, Indianapolis, IN), purified NA, or influenza virus in serum-free DMEM containing 0.1% BSA for 1 h at 37°C. To generate TGF-β1 standard curves, 2-fold dilutions of active TGF-β1 (0–1000 pg/ml, R&D Systems) in DMEM containing 0.1% BSA were added to Mv1Lu-PAI cells. To determine the role of NA activity or proteases, rLTGF-β1 was pre-incubated for 1 h at 37°C with different NA activities of bNA, purified influenza virus, or viral NA (as noted in figures and figure legends) in the presence of oseltamivir carboxylate (10 nM) or 1× EDTA-free PI cocktail (Pierce, Rockford, IL).
Protease assays
To examine the role of individual proteases, 106 TCID50 units/ml of purified Tk/WI influenza virus was pre-incubated for 1 h at 37°C with bestatin (20–1000 nM; Sigma), leupeptin (10–100 µM; Sigma), or GM 1489 (1–500 nM; Calbiochem), followed by incubation with 10 ng/ml rLTGF-β1 for 1 h at 37°C. LTGF-β1 activation was determined by the PAI/L assay. All protease inhibitors were used within their effective inhibitory concentrations, as determined by the manufacturer. To test for the presence of proteases in experimental reagents, including purified virus, proteins, and inhibitors, a thiocarbamoyl-casein derivative-based assay was used as per manufacturer's instructions (Calbiochem) in the presence or absence of 1× protease inhibitor cocktail (Pierce).
LTGF-β Western blot and sialic acid content
rLTGF-β (0.4 µg) or rLAP (0.5 µg, R&D Systems) was incubated with PBS, HCl (final pH of 2), purified Gray Teal virus (2 µg), purified Gray Teal NA (0.5 µg), or bNA either alone or pre-incubated with NAi (1 µM) or 1× PI for 1 h at 37°C as described previously. Samples were then separated on a 5%–20% SDS-PAGE gel under reducing conditions. After transferring to nitrocellulose, blots were blocked in 2% non-fat dry milk in Tris-buffered saline plus 1% Tween 20 (TTBS) for 1 h at room temperature and probed for LAP with mouse-anti-LAP (1∶500, R&D Systems) in TTBS for 1 h at room temperature. Blots were washed and incubated with goat anti-mouse-HRP (1∶5000, Jackson Laboratories, Bar Harbor, ME). To examine the sialic acids present on LAP, TGF-β samples were prepared as described above and blots were probed with DIG-labeled MAA (1∶200), which recognizes α2,3-linked sialic acids, or SNA (1∶1000), which recognizes α2,6-linked sialic acids, for 1 h at room temperature (DIG glycan differentiation kit, Roche Applied Science). Lectins were visualized by staining with anti-DIG-AP. After detection, blots were analyzed by Western blotting for LAP, as described above.
Reverse genetics
H5N1 reverse genetic viruses were generated by the RNA polymerase I reverse genetics system [64]. The PR8 and CA/09 virus expressing HK/483 NA was constructed by using the eight-plasmid system as previously described [65]. P1 viral stocks were generated, the NA genes sequenced to ensure that no spurious mutations arose during viral propagation, stiters determined by TCID50 analysis in MDCK cells, and NA activity quantitated by the MUNANA assay as described above. To determine TGF-β levels with the reassortant viruses, BALB/c mice were lightly anesthetized and inoculated with 104 TCID50 units of the different reassortant viruses or PBS alone, as described below.
TGF-β modulation in vivo
To deplete TGF-β activity during HK/486 and CA/09 virus infection, 4 - to 6-week-old BALB/c mice (Charles River Laboratories, Wilmington, MA) were intraperitoneally (i.p.) inoculated with PBS, anti-TGF-β neutralizing antibody 1D11, or isotype-matched mouse IgG (0.5 mg per mouse, Sigma) in PBS 6 to 48 h pre-infection and then every 48 hpi. Mice were then intranasally (i.n.) inoculated with 25 µl PBS or 105 TCID50 virus. 1D11 was either purchased (R&D Systems) or purified from the 1D11 hybridoma (ATCC# 1D11.16.8). 1D11 produces IgG1 antibodies that neutralize all 3 mammalian TGF-β isoforms (β1, β2, β3) [66]. IgG was purified from cell culture supernatants by T-Gel (Pierce), potential endotoxin contaminations removed by Detoxi Gel Endotoxin Removing Gel (Pierce), and purified IgG concentrated and buffer exchanged with Amicon Ultra-15 concentrators (Millipore, Bedford, MA). The endotoxin levels were less than 0.2 EU/mg as measured by the Biowhittaker QCL-1000 assay (Biowhittaker, Walkersville, MD).
Exogenous active TGF-β1 was administered to mice by infection with a replication-defective adenovirus expressing active TGF-β1 (AdTGFβ223/225) or the control vector (AdDL70) as described previously [67], [68]. Briefly, full-length porcine TGFββ1 cDNA (differing from murine TGF-β1 by 1 amino acid) was mutated at serine 223 and 225 (TGF-β223/225) to render the protein constitutively active and expressed in a recombinant, replication-deficient type-5 adenovirus. The replication-deficient virus (AdTGFβ223/225) was purified by cesium chloride (CsCl) gradient centrifugation and concentrated by using a Sephadex PB-10 chromatography column. Mice were i.n. inoculated with 108 PFUs of active AdTGFβ223/225 or AdDL70 either 48 h pre - or 24 h post-infection with 104 TCID50 units of HK/483 virus. Mice were monitored daily and weighed every 48 h post-infection. At different time points post-infection, 2 mice from the control group and 3 mice from the experimental group were euthanized and lungs collected. Tissues were homogenized in cold PBS, and clarified tissue homogenates were tested for TGF-β levels, using a mouse-specific ELISA (R&D Systems) or viral titers by TCID50 analysis on MDCK cells.
Statistical analyses
Statistical significance of data was determined by using analysis of variance (ANOVA) or Student's t - test on GraphPad Prism (San Diego, CA). All assays were run in triplicate and are representative of at least 2 separate experiments. Error bars represent standard deviation, and statistical significance was defined as a p value of less than 0.05.
Supporting Information
Zdroje
1. MortyRE
KonigshoffM
EickelbergO
2009 Transforming Growth Factor-{beta} Signaling across Ages: From Distorted Lung Development to Chronic Obstructive Pulmonary Disease. Proc Am Thorac Soc 6 607 613
2. TaipaleJ
MiyazonoK
HeldinCH
Keski-OjaJ
1994 Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol 124 171 181
3. PiekE
HeldinCH
Ten DijkeP
1999 Specificity, diversity, and regulation in TGF-beta superfamily signaling. Faseb J 13 2105 2124
4. GentryLE
LioubinMN
PurchioAF
MarquardtH
1988 Molecular events in the processing of recombinant type 1 pre-pro - transforming growth factor beta to the mature polypeptide. Mol Cell Biol 8 4162 4168
5. LawrenceDA
PircherR
Kryceve-MartinerieC
JullienP
1984 Normal embryo fibroblasts release transforming growth factors in a latent form. J Cell Physiol 121 184 188
6. MungerJS
HarpelJG
GleizesPE
MazzieriR
NunesI
1997 Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int 51 1376 1382
7. ShaX
YangL
GentryLE
1991 Identification and analysis of discrete functional domains in the pro region of pre-pro-transforming growth factor beta 1. J Cell Biol 114 827 839
8. YoungGD
Murphy-UllrichJE
2004 Molecular Interactions That Confer Latency to Transforming Growth Factor-{beta}. J Biol Chem 279 38032 38039
9. AnnesJP
MungerJS
RifkinDB
2003 Making sense of latent TGFbeta activation. J Cell Sci 116 217 224
10. AluwihareP
MungerJS
2008 What the lung has taught us about latent TGF-beta activation. Am J Respir Cell Mol Biol 39 499 502
11. Barcellos-HoffMH
DixTA
1996 Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10 1077 1083
12. VodovotzY
CheslerL
ChongH
KimSJ
SimpsonJT
1999 Regulation of transforming growth factor beta1 by nitric oxide. Cancer Res 59 2142 2149
13. BrownPD
WakefieldLM
LevinsonAD
SpornMB
1990 Physicochemical activation of recombinant latent transforming growth factor-beta's 1, 2, and 3. Growth Factors 3 35 43
14. LawrenceDA
PircherR
JullienP
1985 Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun 133 1026 1034
15. JenkinsG
2008 The role of proteases in transforming growth factor-beta activation. Int J Biochem Cell Biol 40 1068 1078
16. Schultz-CherryS
ChenH
MosherDF
MisenheimerTM
KrutzschHC
1995 Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem 270 7304 7310
17. Schultz-CherryS
LawlerJ
Murphy-UllrichJE
1994 The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J Biol Chem 269 26783 26788
18. Schultz-CherryS
RibeiroS
GentryL
Murphy-UllrichJE
1994 Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J Biol Chem 269 26775 26782
19. Schultz-CherryS
Murphy-UllrichJE
1993 Thrombospondin causes activation of latent transforming growth factor - beta secreted by endothelial cells by a novel mechanism [published erratum appears in J Cell Biol 1993 Sep;122(5):following 1143]. J Cell Biol 122 923 932
20. Murphy-UllrichJE
PoczatekM
2000 Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 11 59 69
21. TaylorAW
2009 Review of the activation of TGF-{beta} in immunity. J Leukoc Biol 85 29 33
22. KothLL
AlexB
HawgoodS
NeadMA
SheppardD
2007 Integrin beta6 mediates phospholipid and collectin homeostasis by activation of latent TGF-beta1. Am J Respir Cell Mol Biol 37 651 659
23. GanttKR
Schultz-CherryS
RodriguezN
JeronimoSM
NascimentoET
2003 Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J Immunol 170 2613 2620
24. OmerFM
de SouzaJB
CorranPH
SultanAA
RileyEM
2003 Activation of transforming growth factor beta by malaria parasite-derived metalloproteinases and a thrombospondin-like molecule. J Exp Med 198 1817 1827
25. AungH
WuM
JohnsonJL
HirschCS
ToossiZ
2005 Bioactivation of latent transforming growth factor beta1 by Mycobacterium tuberculosis in human mononuclear phagocytes. Scand J Immunol 61 558 565
26. WaghabiMC
KeramidasM
FeigeJJ
Araujo-JorgeTC
BaillyS
2005 Activation of transforming growth factor beta by Trypanosoma cruzi. Cell Microbiol 7 511 517
27. Schultz-CherryS
HinshawVS
1996 Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol 70 8624 8629
28. KimNY
KimHG
KimYH
ChungIS
YangJM
2008 Expression and characterization of human N-acetylglucosaminyltransferases and alpha2,3-sialyltransferase in insect cells for in vitro glycosylation of recombinant erythropoietin. J Microbiol Biotechnol 18 383 391
29. BenureauY
HuetJC
CharpilienneA
PoncetD
CohenJ
2005 Trypsin is associated with the rotavirus capsid and is activated by solubilization of outer capsid proteins. J Gen Virol 86 3143 3151
30. ShawML
StoneKL
ColangeloCM
GulcicekEE
PaleseP
2008 Cellular proteins in influenza virus particles. PLoS Pathog 4 e1000085
31. HoffmannE
StechJ
LenevaI
KraussS
ScholtissekC
2000 Characterization of the influenza A virus gene pool in avian species in southern China: was H6N1 a derivative or a precursor of H5N1? J Virol 74 6309 6315
32. DybingJK
Schultz-CherryS
SwayneDE
SuarezDL
PerdueML
2000 Distinct pathogenesis of hong kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J Virol 74 1443 1450
33. TaylorG
1996 Sialidases: structures, biological significance and therapeutic potential. Current Opinion in Structural Biology 6 830 837
34. MiyazonoK
HeldinCH
1989 Role for carbohydrate structures in TGF-beta 1 latency. Nature 338 158 160
35. WakefieldLM
WinokurTS
HollandsRS
ChristophersonK
LevinsonAD
1990 Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J Clin Invest 86 1976 1984
36. ZouZ
SunPD
2006 An improved recombinant mammalian cell expression system for human transforming growth factor-[beta]2 and -[beta]3 preparations. Protein Expression and Purification 50 9 17
37. CauthenAN
SwayneDE
Schultz-CherryS
PerdueML
SuarezDL
2000 Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J Virol 74 6592 6599
38. MatsuokaY
SwayneDE
ThomasC
Rameix-WeltiMA
NaffakhN
2009 Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J Virol 83 4704 4708
39. KoliK
MyllarniemiM
Keski-OjaJ
KinnulaVL
2008 Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal 10 333 342
40. VictorT
SolovyanJK-O
2006 Proteolytic activation of latent TGF-? precedes caspase-3 activation and enhances apoptotic death of lung epithelial cells. J Cell Phys 207 445 453
41. MungerJS
HuangX
KawakatsuH
GriffithsMJ
DaltonSL
1999 The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96 319 328
42. PittetJF
GriffithsMJ
GeiserT
KaminskiN
DaltonSL
2001 TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107 1537 1544
43. SheppardD
2001 Integrin-mediated activation of transforming growth factor-beta(1) in pulmonary fibrosis. Chest 120 49S 53S
44. SheppardD
2005 Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev 24 395 402
45. BosseY
Rola-PleszczynskiM
2007 Controversy surrounding the increased expression of TGF beta 1 in asthma. Respir Res 8 66
46. LiMO
WanYY
SanjabiS
RobertsonAK
FlavellRA
2006 Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 24 99 146
47. KidoH
OkumuraY
YamadaH
LeTQ
YanoM
2007 Proteases essential for human influenza virus entry into cells and their inhibitors as potential therapeutic agents. Curr Pharm Des 13 405 414
48. ZhangL
KatzJM
GwinnM
DowlingNF
KhouryMJ
2009 Systems-based candidate genes for human response to influenza infection. Infection, Genetics and Evolution 9 1148 1157
49. KidoH
MurakamiM
ObaK
ChenY
TowatariT
1999 Cellular proteinases trigger the infectivity of the influenza A and Sendai viruses. Mol Cells 9 235 244
50. RoweRK
BrodySL
PekoszA
2004 Differentiated cultures of primary hamster tracheal airway epithelial cells. In Vitro Cell Dev Biol Anim 40 303 311
51. GordonKJ
BlobeGC
2008 Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 1782 197 228
52. FitzpatrickDR
Bielefeldt-OhmannH
1999 Transforming growth factor beta in infectious disease: always there for the host and the pathogen. Trends Microbiol 7 232 236
53. ReedSG
1999 TGF-beta in infections and infectious diseases. Microbes Infect 1 1313 1325
54. OmerFM
KurtzhalsJA
RileyEM
2000 Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol Today 16 18 23
55. OmerFM
RileyEM
1998 Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med 188 39 48
56. BaigentSJ
McCauleyJW
2001 Glycosylation of haemagglutinin and stalk-length of neuraminidase combine to regulate the growth of avian influenza viruses in tissue culture. Virus Res 79 177 185
57. ShtyryaY
MochalovaL
VoznovaG
RudnevaI
ShilovA
2009 Adjustment of receptor-binding and neuraminidase substrate specificities in avian-human reassortant influenza viruses. Glycoconj J 26 99 109
58. RichmondJY
McKinneyRWIII
1993 Biosafety in microbiological and biomedical laboratories Atlanta, GA U.S. Department of Health and Human Services, Centers for Disease Control 26 36
59. JonesJC
TurpinEA
BultmannH
BrandtCR
Schultz-CherryS
2006 Inhibition of Influenza Virus Infection by a Novel Antiviral Peptide That Targets Viral Attachment to Cells. J Virol 80 11960 11967
60. ReedLJ
MuenchH
1938 A simple method of estimating fifty percent endpoints. Am J Hyg 27 493 497
61. JohanssonBE
BucherDJ
KilbourneED
1989 Purified influenza virus hemagglutinin and neuraminidase are equivalent in stimulation of antibody response but induce contrasting types of immunity to infection. J Virol 63 1239 1246
62. PotierM
MameliL
BelisleM
DallaireL
MelanconSB
1979 Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Analytical Biochemistry 94 287 296
63. AbeM
HarpelJG
MetzCN
NunesI
LoskutoffDJ
1994 An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216 276 284
64. NeumannG
WatanabeT
ItoH
WatanabeS
GotoH
1999 Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A 96 9345 9350
65. HoffmannE
KraussS
PerezD
WebbyR
WebsterRG
2002 Eight-plasmid system for rapid generation of influenza virus vaccines. Vaccine 20 3165 3170
66. DaschJ
PaceD
WaegellW
InenagaD
EllingsworthL
1989 Monoclonal antibodies recognizing transforming growth factor-beta. Bioactivity neutralization and transforming growth factor beta 2 affinity purification. J Immunol 142 1536 1541
67. GauldieJ
GaltT
BonniaudP
RobbinsC
KellyM
2003 Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol 163 2575 2584
68. RobertsonJV
NathuZ
NajjarA
DwivediD
GauldieJ
2007 Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Mol Vis 13 457 469
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA RecombinantsČlánek Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral BuddingČlánek Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral ReplicationČlánek Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Social Media and Microbiology Education
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Phylodynamics and Human-Mediated Dispersal of a Zoonotic Virus
- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Distinct Clones of Caused the Black Death
- Strain-Specific Differences in the Genetic Control of Two Closely Related Mycobacteria
- The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA Recombinants
- MHC Class I Bound to an Immunodominant Epitope Demonstrates Unconventional Presentation to T Cell Receptors
- Stimulates Immune Gene Expression and Inhibits Development in
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
- Cytomegalovirus microRNAs Facilitate Persistent Virus Infection in Salivary Glands
- Strategies to Avoid Killing by Human Neutrophils
- Transforming Growth Factor-β: Activation by Neuraminidase and Role in Highly Pathogenic H5N1 Influenza Pathogenesis
- Autoimmunity in Arabidopsis Is Mediated by Epigenetic Regulation of an Immune Receptor
- Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral Budding
- Dengue Virus Ensures Its Fusion in Late Endosomes Using Compartment-Specific Lipids
- Nucleocapsid Promotes Localization of HIV-1 Gag to Uropods That Participate in Virological Synapses between T Cells
- Host Genetics and HIV-1: The Final Phase?
- Variations in the Hemagglutinin of the 2009 H1N1 Pandemic Virus: Potential for Strains with Altered Virulence Phenotype?
- High-Resolution Functional Mapping of the Venezuelan Equine Encephalitis Virus Genome by Insertional Mutagenesis and Massively Parallel Sequencing
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Calcineurin Inhibition at the Clinical Phase of Prion Disease Reduces Neurodegeneration, Improves Behavioral Alterations and Increases Animal Survival
- Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral Replication
- -Induced Inactivation of the Macrophage Transcription Factor AP-1 Is Mediated by the Parasite Metalloprotease GP63
- Epstein Barr Virus-Encoded EBNA1 Interference with MHC Class I Antigen Presentation Reveals a Close Correlation between mRNA Translation Initiation and Antigen Presentation
- Fidelity Variants of RNA Dependent RNA Polymerases Uncover an Indirect, Mutagenic Activity of Amiloride Compounds
- The Spread of Tomato Yellow Leaf Curl Virus from the Middle East to the World
- Concerted Action of Two Formins in Gliding Motility and Host Cell Invasion by
- Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X
- Release of Intracellular Calcium Stores Facilitates Coxsackievirus Entry into Polarized Endothelial Cells
- Gene Annotation and Drug Target Discovery in with a Tagged Transposon Mutant Collection
- Nuclear Export and Import of Human Hepatitis B Virus Capsid Protein and Particles
- Retention and Loss of RNA Interference Pathways in Trypanosomatid Protozoans
- Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite
- Controlling Cellular P-TEFb Activity by the HIV-1 Transcriptional Transactivator Tat
- Direct Visualization of Peptide/MHC Complexes at the Surface and in the Intracellular Compartments of Cells Infected by
- Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
- In Vitro and In Vivo Studies Identify Important Features of Dengue Virus pr-E Protein Interactions
- Inhibition of Nipah Virus Infection In Vivo: Targeting an Early Stage of Paramyxovirus Fusion Activation during Viral Entry
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



