-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMultimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort
Using latent class analysis, Marlous Hall and colleagues examine the associations between mulitmorbidity and survival in patients with acute myocardial infarction.
Published in the journal: . PLoS Med 15(3): e32767. doi:10.1371/journal.pmed.1002501
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002501Summary
Using latent class analysis, Marlous Hall and colleagues examine the associations between mulitmorbidity and survival in patients with acute myocardial infarction.
Introduction
The increasing prevalence of long-term health conditions, and consequent growing prevalence of multimorbidity (the presence of multiple co-morbidities), is a major global challenge facing healthcare systems [1,2]. Presently, around two-thirds of patients with cardiovascular disease are estimated to have at least 1 long-term health condition [3]. With improved survival rates following acute myocardial infarction (AMI) as well as an ageing population [4–6], there are more patients living longer with multimorbidity, which is associated with reduced quality of life, increased healthcare burden, and increased mortality [3,7,8].
Although many prior studies have assessed the association between individual co-morbidities—including diabetes [9,10], chronic obstructive pulmonary disease (COPD) [11–15], and heart failure [16]—and survival in patients with AMI, few have quantified the burden of multimorbidity—in particular how the complex patterns of multiple conditions simultaneously associate with mortality. Where data do exist on multimorbidity clusters, studies are limited to regional rather than national data and have relied on basic analytical techniques that consider composite additive [17,18] or weighted [19–22] co-morbidity scores, or focus on all possible combinations of conditions [18,23,24]. Moreover, previous data are mostly limited to short - (30 days) and medium-term (1 year) outcomes, with the exception of the study by Di Angelantonio et al. [24] (12.8 years of follow-up), even though such conditions are lifelong diseases and, therefore, warrant investigation of outcomes over the longer term. More sophisticated methods, such as latent class analysis, enable insights into multidimensional disease patterns based on probabilistic modelling of specific conditions without the aforementioned limitations [25]. Furthermore, insights into the association of multimorbidity with survival following AMI from latent class analysis may help define and target therapeutic strategies to specific groups of patients in an attempt to reduce premature death [1,26,27].
Therefore, this study aimed to investigate which multimorbidity phenotype clusters exist across a range of pre-existing long-term health conditions and study their association with long-term survival for patients hospitalised with AMI. We hypothesise that the presence of multimorbidity confers an increased long-term risk of death for patients with AMI. In addressing this hypothesis, we provide a greater understanding of the clustering of pre-existing conditions and their simultaneous burden on survival.
Methods
Ethical approval
The National Institute for Cardiovascular Outcomes Research, which includes the Myocardial Ischaemia National Audit Project (MINAP) database (Ref: NIGB ECC 1-06(d)/2011), has support under section 251 of the National Health Service Act 2006 to use patient information for medical research without informed consent. Further ethical approval, or patient consent, was not required under current National Health Service research governance arrangements, and all data analysed in the study were anonymised. This study is reported as per RECORD guidelines (S1 Checklist).
Data and patients
Data for this study were obtained from MINAP, representing all hospitals in a single health system (the National Health Service in England and Wales). MINAP offers the opportunity to undertake population-based observational studies of an array of multimorbid conditions and their association with survival following AMI. Full details of MINAP have been published elsewhere [28]. The analytical cohort (n = 693,388) was drawn from 693,633 patients with AMI admitted to 1 of 247 hospitals between 1 January 2003 and 30 June 2013 (S1 Text; S1 Fig). Patients were eligible for the study if they were aged 18 years or over; where multiple admissions for AMI were recorded per person, only the first admission was included. We excluded 245 (0.04%) patients due to missing mortality data. Patients were defined as having multimorbidity if they had a history of 1 or more of the following conditions when admitted to hospital with AMI: diabetes mellitus, COPD or asthma, chronic heart failure, chronic renal failure (defined as creatinine chronically >200 μmol/l [>2.26 mg/dl]), cerebrovascular disease, peripheral vascular disease, or hypertension (defined as a patient already receiving treatment [drug, dietary, or lifestyle] for hypertension or with recorded blood pressure >140/90 mm Hg on at least 2 occasions prior to admission).
Patient-level data included baseline ischaemic risk (calculated using the Global Registry of Acute Coronary Events [GRACE] risk score parameters: age, cardiac arrest, electrocardiographic ST segment deviation, elevated cardiac enzymes, systolic blood pressure, heart rate on admission to hospital, prescription of a loop diuretic [substituted for Killip class] and creatinine [29,30]), patient demographics (sex and index of multiple deprivation [31]), type of AMI (ST-elevated myocardial infarction [STEMI] and non-ST-elevated myocardial infarction [NSTEMI]), medical history (smoking status, family history of coronary heart disease, hypertension, total cholesterol, previous AMI, angina, percutaneous coronary intervention, and coronary artery bypass graft surgery), pharmacological therapies at the time of discharge from hospital (aspirin, β-blockers, HMG-CoA reductase inhibitors [statins], ACE inhibitors/angiotensin receptor blockers, P2Y12 inhibitors, and aldosterone antagonists), revascularisation strategy (thrombolysis or coronary intervention [percutaneous coronary intervention or coronary artery bypass graft surgery] or both), and all-cause mortality (through patient-level linkage to the United Kingdom Office for National Statistics). Patients were followed up for mortality status to a censoring date of 30 April 2011 for those diagnosed between 2003 and 2009, and a final censoring date of 31 December 2013 for those diagnosed from 2010 onwards. This resulted in a maximum observed follow-up time of 8.4 years (median and interquartile range: 2.3, 0.9–4.0 years), representing 1,872,468 person-years at risk.
Statistical analyses
While no formal analysis plan exists, all analytical methods described here were planned prospectively prior to analyses. Where data-driven approaches were used for model selection, or sensitivity analyses conducted as a result of peer review, this has been clearly indicated below.
Latent class analysis was performed to assimilate individual patient data for multiple long-term health conditions into multimorbidity classes. The resultant classes represented probabilistic groups of patients with similar combinations of conditions, and as such depicted complex patterns of higher order interactions between multiple conditions (S3 Text; S3 Fig; S4 Table). The latent class analysis was based only on the probability distributions of the baseline long-term health conditions and did not take outcomes into account. Latent class analysis was preferred over simpler techniques such as creating an additive score of long-term health conditions, through which the granularity of specific combinations of conditions would be lost, or analysing all possible combinations of conditions, which is subject to high false positive rates (type I errors) and can lead to low study power.
Baseline characteristics were described according to each multimorbidity phenotype cluster using numbers and percentages for categorical data and means and standard deviations and medians and interquartile ranges for normally and non-normally distributed continuous variables, respectively. The differences in baseline characteristics between multimorbidity phenotype clusters were summarised using chi-squared tests, t tests, and Wilcoxon rank-sum tests appropriate to the data type and distribution. Furthermore, differences in receipt of guideline-recommended therapies were compared according to multimorbidity phenotype cluster.
Royston–Parmar flexible parametric survival models [32] based on all-cause mortality were fitted to determine the association of multimorbidity phenotype clusters with long-term survival (8.4 years). In addition, the association of individual pre-existing conditions and the accumulation of multimorbid conditions (grouped into 0, 1, and 2 or more conditions) with long-term survival was assessed using the same flexible parametric survival models. Left ventricular ejection fraction (LVEF; categorised as good, ≥50%; moderate, 30%–49%; and poor, <30%) and estimated glomerular filtration rate (eGFR; categorised as normal or mild impairment, ≥60 ml/min per 1.73 m2; moderate impairment, 30–59 ml/min per 1.73 m2; or severe/very severe impairment, <30 ml/min per 1.73 m2) were used to model the association of the severity of chronic heart failure and chronic renal failure, respectively, with survival. The models were adjusted for known confounders based on clinical consideration and previous research [27,33] including baseline ischaemic risk, demographic variables, type of AMI, medical history, revascularisation strategy, and pharmacological therapies at discharge as defined earlier. We selected flexible parametric models a priori in favour of standard Cox regression to allow for modelling of non-proportional hazards as well as extension to a relative survival framework for calculation of loss in life expectancy (more details below). Further sensitivity analyses were conducted following peer review in order to assess the impact of the increase in recorded multimorbidity between 2003 and 2004 upon the results (S3 Table).
Finally, we estimated the loss of life expectancy in years compared with the age-, sex-, and year-matched populace of the UK [34] that was due to multimorbidity phenotype cluster as well as according to individual and cumulative conditions. Mortality data for England and Wales were obtained from Office for National Statistics life tables [35] (S4 Text).
Multiple imputation by chained equations was used to produce 10 imputed datasets to minimise potential bias due to missing data (S2 Text; S1 Table), using previously defined methods for imputation of the MINAP data [36]. Pooled estimates and accompanying 95% confidence intervals were generated according to Rubin’s rules [37]. The scale (proportional hazards, proportional odds, or normal) and complexity (number of degrees of freedom) for flexible parametric survival models were determined by minimising the Akaike information criterion and the Bayesian information criterion for the complete case analysis as well as for each individual imputation. According to good practice guidelines for multiple imputation, a sensitivity analysis comparing the main imputed analysis with a complete case analysis was conducted (S2 Table; S2 Fig). All tests were 2-sided, and statistical significance was considered as P < 0.05. Statistical analyses were performed in Stata MP version 14 (http://www.stata.com/), R version 3.1.2 (https://cran.r-project.org/), and Mplus version 7.3 (https://www.statmodel.com/).
Results
There were a total of 693,388 patients included (median age 70.7 years; 452,896 [65.5%] men) and 1,872,468 person-years follow-up. There were 412,809 (59.5%) patients with AMI who had at least 1 pre-existing co-morbid condition. The majority of these had 1 condition (238,302, 57.7%), whereas 120,693 (29.2%) had 2, and 53,814 (13.0%) had 3 or more, up to a maximum of 7 (63, 0.02%). The most prevalent conditions were hypertension (n = 302,388, 45.9%), diabetes mellitus (n = 122,228, 18.6%), and COPD or asthma (n = 89,211, 13.6%). Whilst chronic renal failure, chronic heart failure, and peripheral vascular disease were the least prevalent, patients with these conditions most frequently had additional conditions (27,812 [89.6%], 28,445 [84.1%], and 23,201 [84.0%], respectively) (Fig 1).
Fig. 1. Time trends and prevalence of multimorbid conditions for patients hospitalised with acute myocardial infarction in England and Wales, 2003–2013. 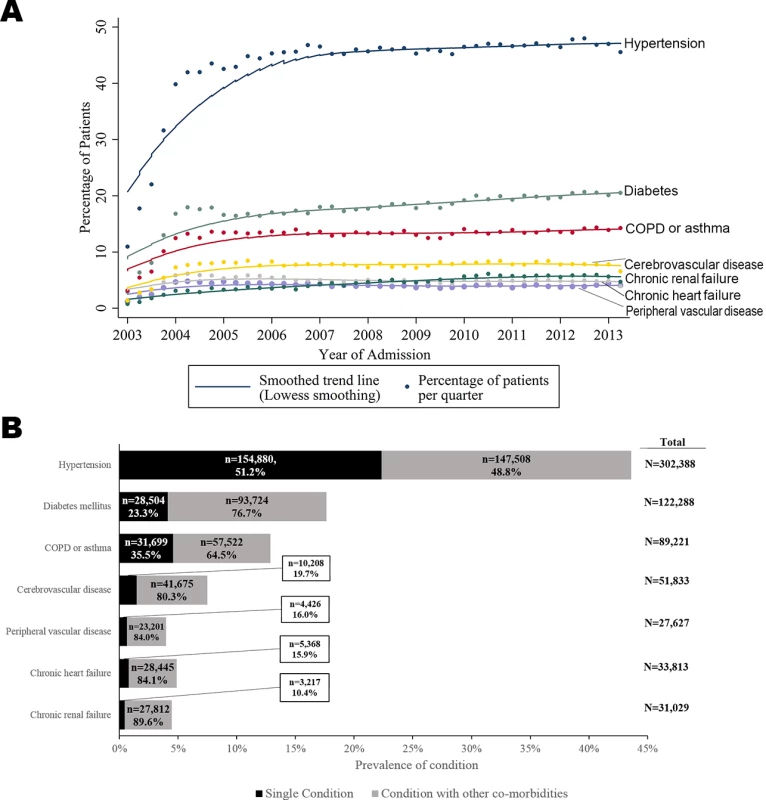
(A) Time trends. (B) Prevalence. COPD, chronic obstructive pulmonary disease. Latent class analysis revealed 3 distinct groups of patients in terms of their multimorbidity profile: class 1, a high multimorbidity cluster, with concomitant heart failure, peripheral vascular disease, and hypertension; class 2, a medium multimorbidity cluster, with peripheral vascular disease and hypertension; and class 3, with low levels of multimorbidity overall but with peripheral vascular disease (Fig 2; S5 Table).
Fig. 2. Probabilistic distribution of multimorbid conditions within each multimorbidity phenotype cluster. 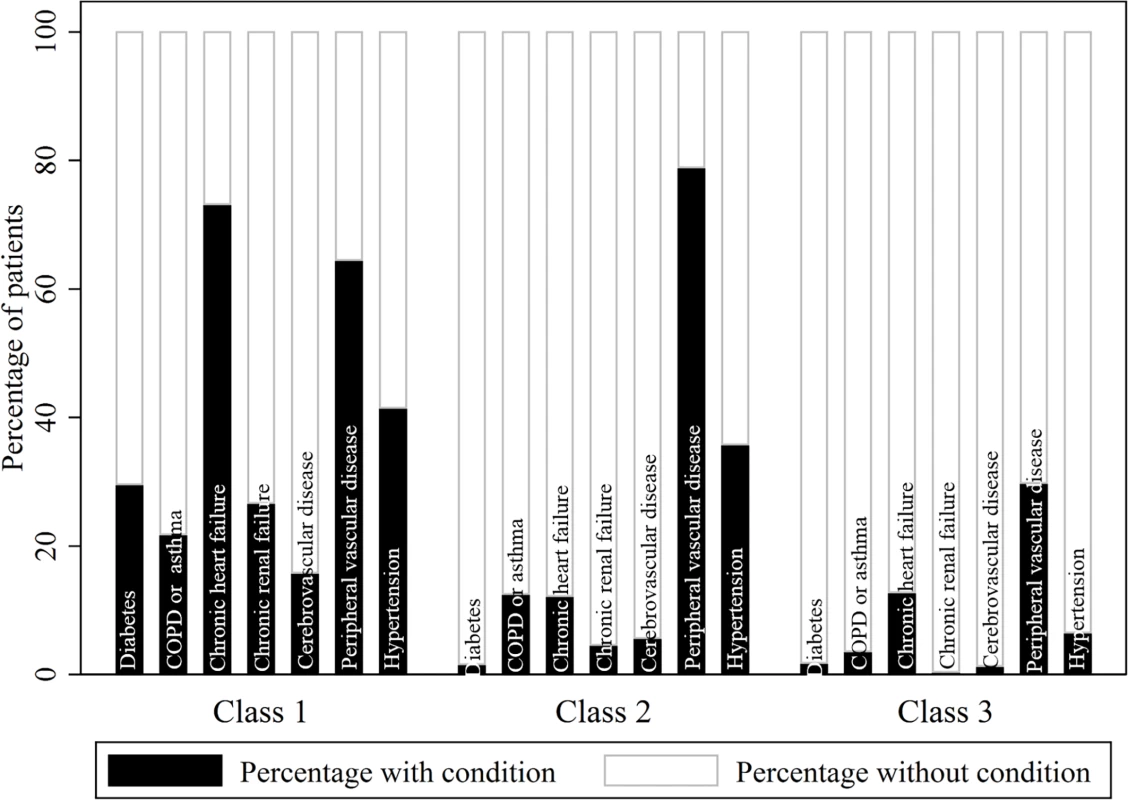
S5 Table provides the full probabilistic latent class structure. The observed proportions of patients with and without each condition per class indicate that class 1 characterises patients with high multimorbidity, especially including chronic heart failure, peripheral vascular disease, and hypertension; class 2 characterises patients with medium multimorbidity, especially including peripheral vascular disease and hypertension; and class 3 characterises patients with low multimorbidity but with peripheral vascular disease. COPD, chronic obstructive pulmonary disease. Patients in multimorbidity class 1 (high multimorbidity with concomitant heart failure, peripheral vascular disease, and hypertension) tended to be older (median age 78.9 years; IQR 71.0–84.8) than those in multimorbidity class 2 (medium multimorbidity with concomitant peripheral vascular disease and hypertension; median age 74.0 years; IQR 64.8–81.3) and multimorbidity class 3 (low levels of multimorbidity overall but with peripheral vascular disease; median age 68.5 years; IQR 57.5–79.0). Class 1 patients also tended to be female (40.5% versus 38.5% and 33.1%, respectively) and more often had NSTEMI (83.2%) than STEMI compared with class 2 and 3 patients (71.6% and 57.6%, respectively). Furthermore, patients in class 1 were less likely to receive pharmacological therapies after accounting for eligibility compared with class 2 and 3 patients (Table 1). Of note, there were more people in the high and medium multimorbidity phenotype clusters (class 1 and 2) in the latest period of the study compared with the earliest period (class 1 : 9.0% in 2011–2013 versus 7.9% in 2003–2006; class 2 : 16.6% in 2011–2013 versus 13.9% in 2003–2006). There was an associated 2.0% (95% CI 1.9%–2.3%) increase in the number of conditions per year (Fig 1).
Tab. 1. Baseline characteristics stratified according to multimorbidity phenotype cluster at the time of acute myocardial infarction hospitalisation, 2003–2013 (prior to multiple imputation for missing data). 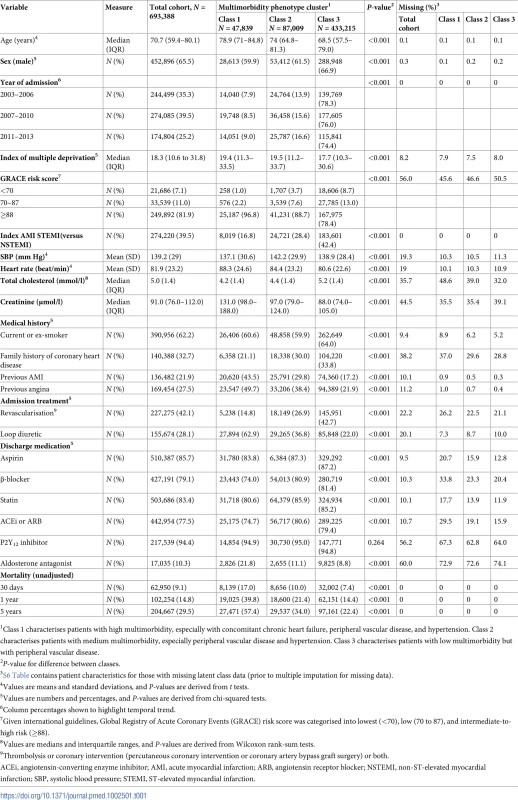
1Class 1 characterises patients with high multimorbidity, especially with concomitant chronic heart failure, peripheral vascular disease, and hypertension. Class 2 characterises patients with medium multimorbidity, especially peripheral vascular disease and hypertension. Class 3 characterises patients with low multimorbidity but with peripheral vascular disease. Outcomes
Unadjusted all-cause mortality was higher for those in class 1 than for those in classes 2 and 3 at 30 days (17.0% [95% CI 16.7%–17.4%] versus 10% [9.7%–10.1%] and 7.4% [7.3%–7.5%], respectively; P < 0.001), 1 year (39.8% [39.3%–40.2%] versus 21.4% [21.1%–21.6%] and 14.4% [14.2%–14.5%]; P < 0.001), and 5 years (57.4% [57.0%–57.9%] versus 34.0% [33.6%–34.3%] and 22.4% [22.3%–22.6%]; P < 0.001).
Those in the high multimorbidity cluster with chronic heart failure, peripheral vascular disease, and hypertension (class 1) had a 2.4-fold increased hazard of death compared with low-multimorbidity patients (class 3) (hazard ratio [HR] 2.40; 95% CI 2.33–2.47) over the 8.4-year follow-up period. Those who were in the medium multimorbidity cluster with peripheral vascular disease and hypertension (class 2) had a 1.5-fold increased hazard compared with those with low levels of multimorbidity (class 3) (HR 1.45; 95% CI 1.41–1.48) (Table 2).
Tab. 2. Unadjusted and adjusted HRs for long-term survival according to multimorbidity class or health condition obtained from flexible parametric survival models after multiple imputation (5 degrees of freedom, odds scale). 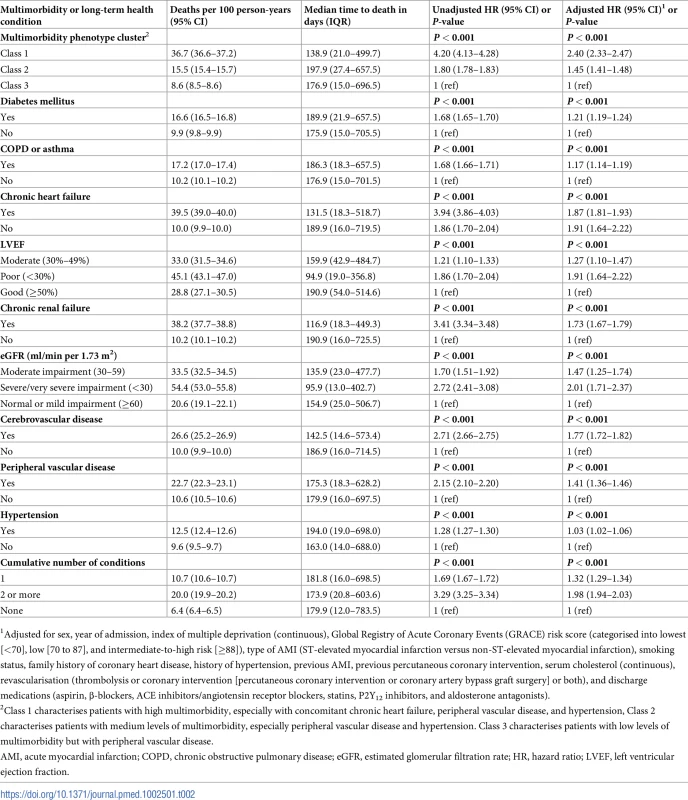
1Adjusted for sex, year of admission, index of multiple deprivation (continuous), Global Registry of Acute Coronary Events (GRACE) risk score (categorised into lowest [<70], low [70 to 87], and intermediate-to-high risk [≥88]), type of AMI (ST-elevated myocardial infarction versus non-ST-elevated myocardial infarction), smoking status, family history of coronary heart disease, history of hypertension, previous AMI, previous percutaneous coronary intervention, serum cholesterol (continuous), revascularisation (thrombolysis or coronary intervention [percutaneous coronary intervention or coronary artery bypass graft surgery] or both), and discharge medications (aspirin, β-blockers, ACE inhibitors/angiotensin receptor blockers, statins, P2Y12 inhibitors, and aldosterone antagonists). For individual conditions, the worst outcomes were observed in patients with chronic heart failure (8.4-year mortality rate 63.5%, 95% CI 63.0%–64.1%; adjusted HR 1.87, 95% CI 1.81–1.93; median time to death 131.49 days, IQR 18.3–518.7; 39.5 deaths per 100 person years [/100 py]), chronic renal failure (8.4-year mortality rate 57.2%, 95% CI 56.7%–57.8%; adjusted HR 1.73, 95% CI 1.67–1.79; median time to death 116.9 days, IQR 18.3–449.3; 38.2 deaths/100 py), and cerebrovascular disease (8.4-year mortality rate 50.7%, 95% CI 50.3%–51.1%; adjusted HR 1.77, 95% CI 1.72–1.82; median time to death 142.5 days, IQR 14.6–573.4; 26.6 deaths/100 py) (Fig 3; Table 2). There was a corresponding decline in prognosis with decreasing LVEF (HR 1.27 [95% CI 1.10–1.47] and 1.91 [1.64–2.22] for moderate and poor LVEF, respectively, compared with good LVEF) and eGFR (HR 1.47 [1.25–1.74] and 2.01 [1.71–2.37] for moderate and severe/very severe impairment, respectively, compared with mild impairment or normal eGFR). Moreover, after adjustment for confounders, the accumulation of multimorbid conditions for patients with AMI was significantly associated with worse survival (HR 1.32 [95% CI 1.29–1.34] for 1 condition and HR 1.98 [1.94–2.03] for 2 or more conditions versus no multimorbidity; P-value for trend < 0.001).
Fig. 3. Long-term survival according to multimorbidity phenotype. 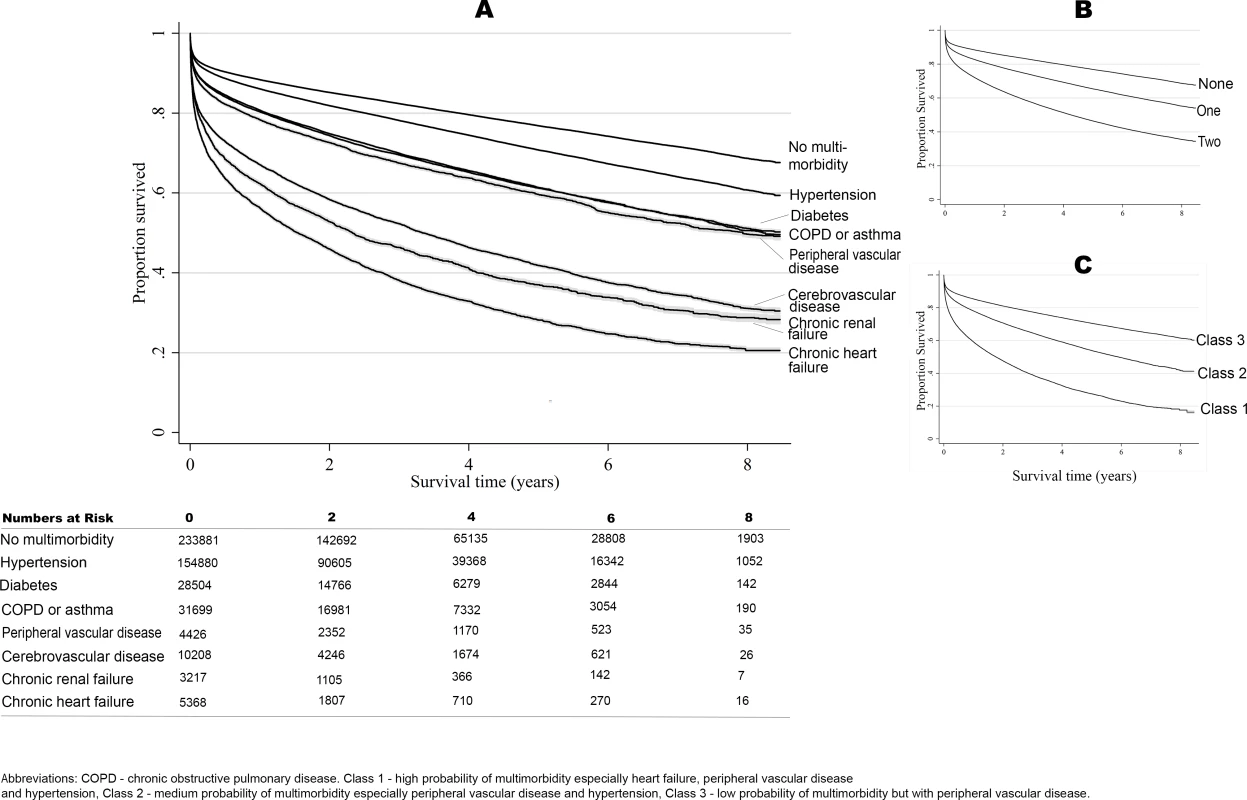
Unadjusted Kaplan–Meier curves according to individual conditions (A), cumulative number of conditions (B), and multimorbidity phenotype cluster of multimorbid conditions (C). The association between individual long-term health conditions and survival translated into a significant loss in life expectancy, such that those with chronic heart failure, chronic renal failure, and peripheral vascular disease had a loss in life expectancy of 2.91 (95% CI 2.58–3.25), 2.78 (95% CI 2.47–3.10), and 2.14 (95% CI 1.86–2.42) years, respectively, compared to those without long-term conditions (S7 Table). Moreover, multimorbidity was also associated with significant loss in life expectancy, such that those in class 1 had a loss in life expectancy of 2.89 years (95% CI 2.59–3.19), and those in class 2 a loss in life expectancy of 1.52 years (95% CI 1.33–1.71), compared with class 3 patients.
Discussion
In this nationwide study of patients hospitalised with AMI, almost two-thirds had multimorbidity, most commonly with hypertension and diabetes mellitus. Those with 1 long-term health condition in addition to AMI were 32% more likely to die over the 8.4-year follow-up period, whereas those with 2 or more long-term health conditions were twice as likely to die, compared with those without multimorbidity. Each condition was associated with a unique and significant loss of life expectancy, which was greatest for those with chronic heart failure. Using latent class analysis, we identified 3 patient groups based on their probability of long-term health conditions that had distinct survival trajectories and may be considered as novel post-AMI survival phenotypes.
Global population demographics have changed such that nowadays patients with AMI are older and have more co-morbidities [38,39]. This and the fact that mortality rates from AMI have declined have created a new cohort of patients with multimorbidity who are now living with the aftermath of AMI [27]. In the United States, this amounts to over 85 million people (and 7 million in the United Kingdom) living with cardiovascular disease, of whom the majority are elderly or have co-existent long-term health conditions [40,41]. The resultant healthcare utilisation and associated direct costs are high and escalate according to multimorbidity [42,43]. A key international healthcare priority, therefore, is the reduction in multimorbidity and improvement in disease-free survival. To date, however, studies reporting multimorbidity have either focused on individual conditions [9–11,13,16] or have been largely limited to regional data with short-term outcomes [18,23]. Our study clearly depicts, in a modern healthcare system, the considerable burden of multimorbidity among patients with AMI and, importantly, identified clear patient classes for clusters of such conditions, such that multidisciplinary interventions may be targeted towards those in greatest need.
We noted that patients with AMI who also had a history of chronic heart failure, peripheral vascular disease, and hypertension had the worst prognosis. Such patients lived, on average, for about 4.5 months after hospitalisation and, after standardisation to population mortality rates, were estimated to have lost up to 5.5 years of life. Others have reported the detrimental effects of heart failure, yet the paucity of evidence for heart failure and multimorbidity is acknowledged [44]. Few have quantified the association of heart failure with prognosis after AMI [16,45], and, to our knowledge, none have identified the poor prognosis after AMI in combination with other co-morbidities within a national and contemporary dataset. Patients with cerebrovascular disease and chronic renal failure also had very poor outcomes, living on average 3 to 4 months after hospitalisation with AMI. Moreover, there was a cumulative deficit in survival among patients with more than 1 long-term health condition, such that those with 2 or more conditions in addition to AMI were twice as likely to die as those with none of the pre-existing conditions, and this level of multimorbidity was associated with between 1.9 and 2.6 expected years of life lost.
The identification of specific survival trajectories for phenotype clusters of multimorbidity has potentially important repercussions. This is because the proportion of patients with only 1 condition was lower than that for multiple conditions, and multimorbidity was more prevalent in the later years of the study. Future healthcare demands are therefore likely to arise from post-AMI patients with multimorbidity. To account for all possible multimorbid disease combinations and to investigate whether unique multimorbid phenotypes existed, we employed latent class analysis. This data-driven technique identified 3 significantly different disease-determined prognostic groups (so called ‘computational phenotypes’) [46]. Understanding the components of these classes of disease survivorship may help refine approaches to healthcare and stimulate the development of innovative health technologies aimed at improving clinical outcomes.
Although we found that patients with multimorbidity were less likely to receive guideline-indicated care, after adjustment for this, as well as patient demographics, these patients continued to have a significantly poorer prognosis. This suggests that even though outcomes could be improved to an extent through the greater provision of evidence-based in-hospital care, additional and/or novel interventions are warranted in this vulnerable population. For example, for heart failure and AMI, the design of new pharmacotherapies or greater use of community-based interventions such as homecare and follow-up visits may be important. It is worth noting that medical research designs such as randomised trials are optimised to focus on single diseases and single disease pathways: our findings suggest the importance of developing appropriate research designs for people with multiple diseases.
Our analyses are likely to have underestimated the association of multimorbidity with survival following AMI. This is because (1) some long-term health conditions may have been under-recorded in MINAP and (2) we did not have information about other diseases such as chronic arthritis, mental illness, dementia, obesity, cancer, and inflammatory bowel disease. However, in the calculation of years of life lost, we standardised by age, sex, and year to population mortality rates to ascertain relative survival. This technique allowed estimation of the association of index AMI with survival. On the other hand, it is possible that some patients may have had end-stage diseases, multiple intractable conditions, or frailty, whereby it may not have been appropriate to escalate care [47], and end-of-life decisions may have been commenced.
Strengths and limitations
To our knowledge, MINAP is the largest nationwide single healthcare system database covering a prospective cohort of acute coronary syndromes. MINAP is designed to be representative of the management of acute coronary syndromes in a clinical setting, and our previous work has shown results consistent with those produced by randomised clinical trial data replicated in a real-world clinical setting [27]. Previous studies assessing a number of conditions have tended to rely on basic analytical techniques that either consider conditions independently or investigate all possible combinations of conditions [18,23]. Such techniques result in high rates of false positives (type I errors) and suffer from low statistical power. This study used latent class analysis to provide further insight into multidimensional disease patterns using a data-driven, probabilistic modelling approach. This allowed us to model the complex disease interactions of multiple conditions and their association with survival in a more sophisticated manner and without the aforementioned limitations. Despite these strengths, there were other study limitations. First, the study was reliant upon the accurate recording of data, and MINAP does not have 100% case ascertainment. Second, missing data, in particular missing data for each of the multimorbid conditions, could have biased the estimates. However, a thorough imputation strategy, including for multimorbid conditions, was implemented to minimise bias following a previous comprehensive study of the nature of missing data within MINAP [36]. Third, the study was limited to all-cause mortality due to the lack of available cause-specific mortality data. However, it has been shown that cause-specific mortality data may not always be reliable for cardiovascular-related causes of death [48]. Fourth, the study included historical data ranging from 2003 to 2013, which may therefore underestimate the most recent survival rates, due to improved treatments over time. Moreover, there was an apparent increase in the number of multimorbid conditions from 2003 to 2004, which may be a result of improved recording following the introduction of the quality outcomes framework in 2004, which incentivised general practitioners to screen for and identify co-morbid conditions, or a result of improved case ascertainment in this time period for patients who had NSTEMI, amongst whom multimorbidity tends to be more common. Fifth, the observational nature of the study means that we cannot demonstrate causation, though adjustment was made for confounders based on a rich set of available information in the study dataset and informed by external information from other studies.
Conclusions
Among patients hospitalised with AMI, pre-existing multimorbid conditions were common and significantly associated with reduced survival. In particular, the presence of chronic heart failure, cerebrovascular disease, and chronic renal failure independently conferred the greatest risk of mortality and highest expected years of life lost. Three novel computational phenotypes of survivorship according to multimorbidity were identified, which may direct future research into the development of new pharmacotherapies and health service interventions for those in greatest need. Addressing multimorbidity among patients hospitalised with AMI is a necessary step in the international effort to reduce the burden of cardiovascular disease.
Supporting Information
Zdroje
1. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2 22579043
2. World Health Organization. Global status report on noncommunicable diseases 2010: description of the global burden of NCDs, their risk factors and determinants. Geneva: World Health Organization; 2011 [cited 2011 Dec 22]. Available from: http://www.who.int/nmh/publications/ncd_report2010/en/.
3. Rashid M, Kwok CS, Gale CP, Doherty P, Olier I, Sperrin M, et al. Impact of co-morbid burden on mortality in patients with coronary heart disease, heart failure and cerebrovascular accident: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2017;3(1):20–36. doi: 10.1093/ehjqcco/qcw025 28927187
4. Fox K, Steg P, Eagle K, Goodman S, Anderson F, Granger C, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297(17):1892–900. doi: 10.1001/jama.297.17.1892 17473299
5. Alzuhairi K, Søgaard P, Ravkilde J, Gislason G, Køber L, Torp-Pedersen C. Incidence and outcome of first myocardial infarction according to gender and age in Denmark over a 35-year period (1978–2012). Eur Heart J Qual Care Clin Outcomes. 2015;1 : 72–8.
6. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389(10076):1323–35. doi: 10.1016/S0140-6736(16)32381-9 28236464
7. Collerton J, Jagger C, Yadegarfar ME, Davies K, Parker SG, Robinson L, et al. Deconstructing complex multimorbidity in the very old: findings from the Newcastle 85+ Study. BioMed Res Int. 2016;2016 : 8745670. doi: 10.1155/2016/8745670 26885519
8. Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–9. doi: 10.1016/j.arr.2011.03.003 21402176
9. Norhammar A, Lindbäck J, Rydén L, Wallentin L, Stenestrand U. Improved but still high short-and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart. 2007;93(12):1577–83. doi: 10.1136/hrt.2006.097956 17237125
10. Alabas O, Hall M, Dondo T, Rutherford MJ, Timmis A, Batin P, et al. Long term excess mortality associated with diabetes following acute myocardial infarction: a population-based cohort study. J Epidemiol Community Health. 2017;71 : 25–32. doi: 10.1136/jech-2016-207402 27307468
11. Hawkins NM, Huang Z, Pieper KS, Solomon SD, Kober L, Velazquez EJ, et al. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur J Heart Fail. 2009;11(3):292–8. doi: 10.1093/eurjhf/hfp001 19176539
12. Salisbury AC, Reid KJ, Spertus JA. Impact of chronic obstructive pulmonary disease on post-myocardial infarction outcomes. Am J Cardiol. 2007;99(5):636–41. doi: 10.1016/j.amjcard.2006.09.112 17317363
13. Lazzeri C, Valente S, Attanà P, Chiostri M, Picariello C, Gensini GF. The prognostic role of chronic obstructive pulmonary disease in ST-elevation myocardial infarction after primary angioplasty. Eur J Prev Cardiol. 2013;20(3):392–8. doi: 10.1177/1741826711428243 22023803
14. Sidney S, Sorel M, Quesenberry CP, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128(4):2068–75. doi: 10.1378/chest.128.4.2068 16236856
15. Kjøller E, Køber L, Iversen K, Torp‐Pedersen C. Importance of chronic obstructive pulmonary disease for prognosis and diagnosis of congestive heart failure in patients with acute myocardial infarction. Eur J Heart Fail. 2004;6(1):71–7. doi: 10.1016/j.ejheart.2003.09.008 15012921
16. Juillière Y, Cambou JP, Bataille V, Mulak G, Galinier M, Gibelin P, et al. Heart failure in acute myocardial infarction: a comparison between patients with or without heart failure criteria from the FAST-MI registry. Rev Esp Cardiol (Engl Ed). 2012;65(4):326–33.
17. Lazzeri C, Valente S, Chiostri M, Attana P, Mattesini A, Dini CS, et al. Comorbidities in STEMI patients submitted to primary PCI: temporal trends and impact on mortality: a 6-year single center experience. Int J Cardiol. 2013;167(6):3042–4. doi: 10.1016/j.ijcard.2012.11.091 23200270
18. Chen H-Y, Saczynski JS, McManus DD, Lessard DM, Yarzebski JL, Lapane KL, et al. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: a population-based perspective. Clin Epidemiol. 2013;5 : 439–48. doi: 10.2147/CLEP.S49485 24235847
19. Gili M, Sala J, Lopez J, Carrion A, Bejar L, Moreno J, et al. [Impact of comorbidities on in-hospital mortality from acute myocardial infarction, 2003–2009.] Rev Esp Cardiol. 2011;64(12):1130–7. doi: 10.1016/j.recesp.2011.07.010 22018686
20. Sanchis J, Nunez J, Bodi V, Nunez E, Garcia-Alvarez A, Bonanad C, et al. Influence of comorbid conditions on one-year outcomes in non-ST-segment elevation acute coronary syndrome. Mayo Clin Proc. 2011;86(4):291–6. doi: 10.4065/mcp.2010.0702 21346247
21. Schelde AB, Schmidt M, Madsen M, Petersen KL, Nielsen SS, Frokiar J, et al. Impact of co-morbidity on the risk of first-time myocardial infarction, stroke, or death after single-photon emission computed tomography myocardial perfusion imaging. Am J Cardiol. 2014;114(4):510–5. doi: 10.1016/j.amjcard.2014.05.031 25015696
22. Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344:e356. doi: 10.1136/bmj.e356 22279115
23. McManus DD, Nguyen HL, Saczynski JS, Tisminetzky M, Bourell P, Goldberg RJ. Multiple cardiovascular comorbidities and acute myocardial infarction: temporal trends (1990–2007) and impact on death rates at 30 days and 1 year. Clin Epidemiol. 2012;4 : 115–23. doi: 10.2147/CLEP.S30883 22701091
24. Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314(1):52–60. doi: 10.1001/jama.2015.7008 26151266
25. Hagenaars JA, McCutcheon AL. Applied latent class analysis. Cambridge: Cambridge University Press; 2002.
26. National Institute for Health and Care Excellence. Multimorbidity: clinical assessment and management. London: National Institute for Health and Care Excellence; 2016 [cited 2018 Jan 10]. Available from: https://www.nice.org.uk/guidance/NG56.
27. Hall M, Dondo TB, Yan AT, Goodman SG, Bueno H, Chew DP, et al. Association of clinical factors and therapeutic strategies with improvements in survival following non–ST-elevation myocardial infarction, 2003–2013. JAMA. 2016;316(10):1073–82. doi: 10.1001/jama.2016.10766 27574717
28. Herrett E, Smeeth L, Walker L, Weston C. The Myocardial Ischaemia National Audit Project (MINAP). Heart. 2010;96(16):1264–7. doi: 10.1136/hrt.2009.192328 20659944
29. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. doi: 10.1093/eurheartj/ehv320 26320110
30. National Institute for Health and Care Excellence. Unstable angina and NSTEMI: early management. London: National Institute for Health and Care Excellence; 2013 [cited 2015 Jul 13]. Available from: http://www.nice.org.uk/guidance/CG94.
31. Ministry of Housing, Communities & Local Government. English indices of deprivation 2010. London: Ministry of Housing, Communities & Local Government; 2011 [cited 2018 Jan 10]. Available from: https://www.gov.uk/government/statistics/english-indices-of-deprivation-2010.
32. Royston P, Lambert PC. Flexible parametric survival analysis using Stata: beyond the Cox model. College Station (Texas): Stata Press; 2011.
33. Dondo T, Hall M, Timmis A, Gilthorpe M, Alabas O, Batin P, et al. Excess mortality and guideline-indicated care following non ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2016;6(5):412–20. doi: 10.1177/2048872616647705 27142174
34. Andersson TML, Dickman PW, Eloranta S, Lambe M, Lambert PC. Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Stat Med. 2013;32(30):5286–300. doi: 10.1002/sim.5943 24038155
35. Government Actuary’s Department. Interim life tables of mortality rates for England and Wales. London: Government Actuary’s Department; 2014.
36. Cattle B, Baxter P, Greenwood D, Gale C, West R. Multiple imputation for completion of a national clinical audit dataset. Stat Med. 2011;30(22):2736–53. doi: 10.1002/sim.4314 21786284
37. Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–92.
38. Alfredsson J, Alexander KP. Multiple chronic conditions in older adults with acute coronary syndromes. Clin Geriatr Med. 2016;32(2):291–303. doi: 10.1016/j.cger.2016.01.009 27113147
39. Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel J-P, et al. The world report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387(10033):2145–54. doi: 10.1016/S0140-6736(15)00516-4 26520231
40. British Heart Foundation. CVD statistics—BHF UK factsheet. Birmingham: British Heart Foundation; 2017 [cited 2018 Jan 12]. Available from: https://www.bhf.org.uk/-/media/files/research/heart-statistics/bhf-cvd-statistics—uk-factsheet.pdf?la=en.
41. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131(4):434–41.
42. Asaria M, Walker S, Palmer S, Gale CP, Shah AD, Abrams KR, et al. Using electronic health records to predict costs and outcomes in stable coronary artery disease. Heart. 2016;102(10):755–62. doi: 10.1136/heartjnl-2015-308850 26864674
43. Walker S, Asaria M, Manca A, Palmer S, Gale CP, Shah AD, et al. Long-term healthcare use and costs in patients with stable coronary artery disease: a population-based cohort using linked health records (CALIBER). Eur Heart J Qual Care Clin Outcomes. 2016;2(2):125–40. doi: 10.1093/ehjqcco/qcw003 27042338
44. Ertl G, Ruschitzka F. The year in cardiology 2013: heart failure. Eur Heart J. 2014;35(7):470–3. doi: 10.1093/eurheartj/eht555 24385377
45. Gho JM, Schmidt A, Koudstaal S, Pujades-Rodriguez M, Denaxas S, Gale C, et al. Heart failure following myocardial infarction: a cohort study of incidence and prognostic factors in 24 745 patients using linked electronic records. Eur J Heart Fail. 2016;18 : 442.
46. Lasko TA, Denny JC, Levy MA. Computational phenotype discovery using unsupervised feature learning over noisy, sparse, and irregular clinical data. PLoS ONE. 2013;8(6):e66341. doi: 10.1371/journal.pone.0066341 23826094
47. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9 23395245
48. Hall M, Alabas OA, Dondo TB, Jernberg T, Gale CP. Use of relative survival to evaluate non-ST-elevation myocardial infarction quality of care and clinical outcomes. Eur Heart J Qual Care Clin Outcomes. 2015;1(2):85–91.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- What is the value of multidisciplinary care for chronic kidney disease?
- 2017 Reviewer and Editorial Board Thank You
- Delays in completion and results reporting of clinical trials under the Paediatric Regulation in the European Union: A cohort study
- Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance
- Causes of death and infant mortality rates among full-term births in the United States between 2010 and 2012: An observational study
- Time for high-burden countries to lead the tuberculosis research agenda
- The importance and challenges of shared decision making in older people with multimorbidity
- Trajectories of functional decline in older adults with neuropsychiatric and cardiovascular multimorbidity: A Swedish cohort study
- Mortality, ethnicity, and country of birth on a national scale, 2001–2013: A retrospective cohort (Scottish Health and Ethnicity Linkage Study)
- Validation of a genetic risk score for atrial fibrillation: A prospective multicenter cohort study
- Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort
- Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial
- Cost-effectiveness of multidisciplinary care in mild to moderate chronic kidney disease in the United States: A modeling study
- Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: A preclinical experimental study
- Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014: A population-based cohort study
- Global child and adolescent mental health: The orphan of development assistance for health
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Primary prevention of cardiovascular disease: The past, present, and future of blood pressure- and cholesterol-lowering treatments
- Physical activity levels in adults and older adults 3–4 years after pedometer-based walking interventions: Long-term follow-up of participants from two randomised controlled trials in UK primary care
- Effect and cost-effectiveness of educating mothers about childhood DPT vaccination on immunisation uptake, knowledge, and perceptions in Uttar Pradesh, India: A randomised controlled trial
- Comorbidity health pathways in heart failure patients: A sequences-of-regressions analysis using cross-sectional data from 10,575 patients in the Swedish Heart Failure Registry
- Multimorbidity in patients with heart failure from 11 Asian regions: A prospective cohort study using the ASIAN-HF registry
- Antiviral efficacy of favipiravir against Ebola virus: A translational study in cynomolgus macaques
- Transmission of HIV-1 drug resistance mutations within partner-pairs: A cross-sectional study of a primary HIV infection cohort
- A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study)
- Integrating HIV and hypertension management in low-resource settings: Lessons from Malawi
- The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- Blood pressure-lowering treatment strategies based on cardiovascular risk versus blood pressure: A meta-analysis of individual participant data
- Cerebral white matter disease and functional decline in older adults from the Northern Manhattan Study: A longitudinal cohort study
- HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease: The rise of the genetic risk score
- Comparative analysis of the association between 35 frailty scores and cardiovascular events, cancer, and total mortality in an elderly general population in England: An observational study
- Progression of diabetes, heart disease, and stroke multimorbidity in middle-aged women: A 20-year cohort study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Forced anal examinations to ascertain sexual orientation and sexual behavior: An abusive and medically unsound practice
- Polycystic ovary syndrome, androgen excess, and the risk of nonalcoholic fatty liver disease in women: A longitudinal study based on a United Kingdom primary care database
- The current and potential health benefits of the National Health Service Health Check cardiovascular disease prevention programme in England: A microsimulation study
- Cardiovascular disease and multimorbidity: A call for interdisciplinary research and personalized cardiovascular care
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



