-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCancer trials in sub-Saharan Africa: Aligning research and care
Satish Gopal discusses the challenges of deliverable cancer care and cancer trials in sub-Saharan Africa as well as a potential framework for overcoming these challenges.
Published in the journal: . PLoS Med 14(7): e32767. doi:10.1371/journal.pmed.1002351
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.1002351Summary
Satish Gopal discusses the challenges of deliverable cancer care and cancer trials in sub-Saharan Africa as well as a potential framework for overcoming these challenges.
Summary points
There is an extreme scarcity of evidence to guide cancer treatment in sub-Saharan Africa (SSA), as well as major differences between SSA and resource-rich settings regarding cancer treatment infrastructure.
A possible framework for conceptualizing cancer clinical trials in SSA is proposed, and key issues related to equipoise, innovation, and efficiency are addressed within the SSA context.
Strongly aligned cancer care and research agendas can generate forward progress for cancer treatment in the region and globally impactful clinical science that can change treatment paradigms even in resource-rich settings.
Introduction
Cancer burden is increasing in sub-Saharan Africa (SSA), with more than 600,000 estimated new cancer cases in 2012 and age-standardized incidence increases of 10%–20% in most countries between 2005 and 2015 [1,2]. Major regional limitations in pathology, surgery, medical oncology, radiation, and palliation have been extensively described and contribute directly to worse cancer outcomes in SSA than in high-income countries [3]. However, even when treatment is available, there is a remarkable and unacceptable scarcity of high-grade evidence to guide the application of cancer treatment for the 1 billion people living in SSA (Table 1). Interest, investment, and infrastructure are gathering to address this problem. Two cooperative groups sponsored by the United States National Cancer Institute (NCI) have initiated activities in the region, the AIDS Malignancy Consortium (AMC) for human immunodeficiency virus (HIV)-associated malignancies and a second network for pediatric Burkitt lymphoma. Other similar efforts are ongoing. However, conceptualizing cancer treatment trials that are sufficiently informative and appropriate to pursue in SSA continues to prove challenging, even for experienced investigators in the region. This Essay, based on experience in Malawi and broader participation in regional clinical trial groups, elucidates some of these challenges and possible ways forward.
Tab. 1. Key completed and ongoing clinical trials evaluating specific cancer treatment interventions among adults in sub-Saharan Africa. 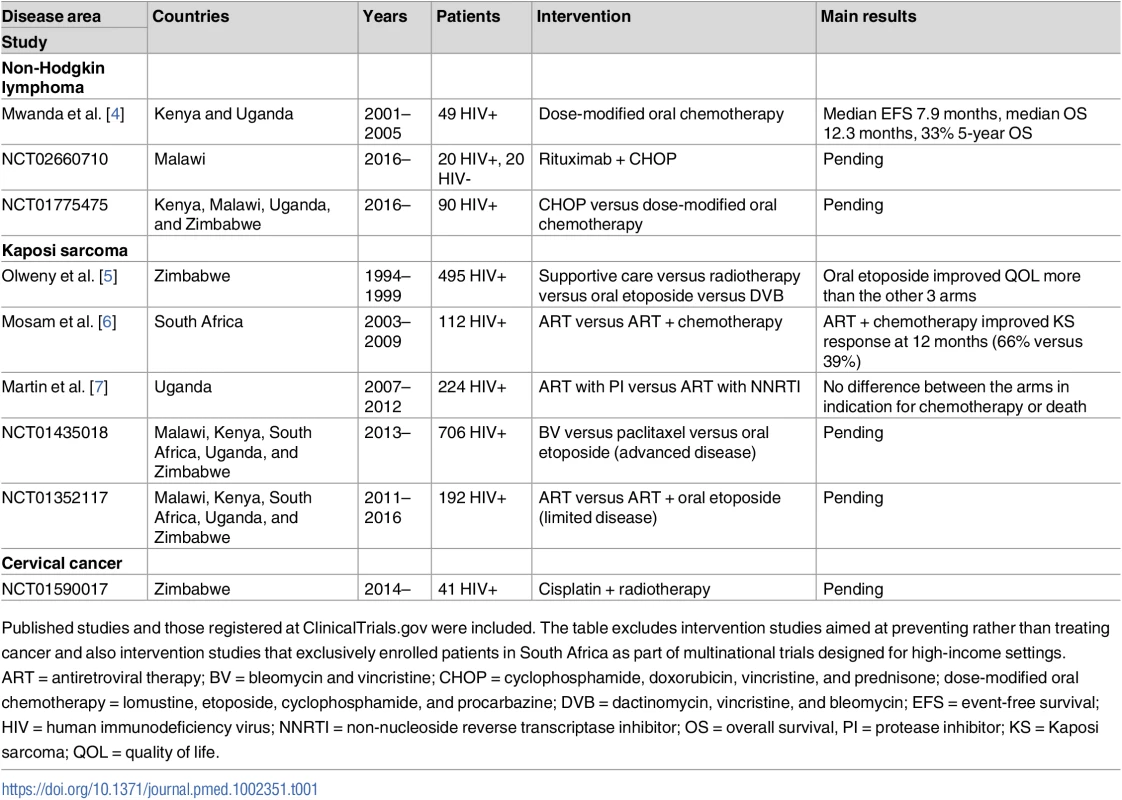
Published studies and those registered at ClinicalTrials.gov were included. The table excludes intervention studies aimed at preventing rather than treating cancer and also intervention studies that exclusively enrolled patients in South Africa as part of multinational trials designed for high-income settings. ART = antiretroviral therapy; BV = bleomycin and vincristine; CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone; dose-modified oral chemotherapy = lomustine, etoposide, cyclophosphamide, and procarbazine; DVB = dactinomycin, vincristine, and bleomycin; EFS = event-free survival; HIV = human immunodeficiency virus; NNRTI = non-nucleoside reverse transcriptase inhibitor; OS = overall survival, PI = protease inhibitor; KS = Kaposi sarcoma; QOL = quality of life. What is equipoise for cancer trials in SSA?
One difficulty is deciding which established treatments in resource-rich settings can be straightforwardly generalized to SSA and which require specific demonstrations of safety and efficacy in SSA. There is no established framework for such decisions, and there are significant differences of opinion regarding this issue. Uncertainty results from scarce regional data and important SSA differences from resource-rich settings where cancer treatments have been studied. These differences include host genetics and metabolism; tumor biology; endemic burden of infectious pathogens, including HIV; and profound differences in general healthcare infrastructure, any of which could dramatically alter how specific treatments perform relative to high-income countries.
As a result, one might argue that SSA is sufficiently different from resource-rich settings where existing evidence for cancer treatment has been generated and that all studies should be redone in the region. Indeed, for an arguably much less intensive intervention, antiretroviral therapy (ART), the HIV community first undertook proof-of-principle studies as a foundation for larger scale-up, to mitigate concerns that poor adherence and rampant HIV resistance would result without intensive monitoring [8,9]. Additionally, even in high-income countries, real-world effectiveness is often very different from within-trial efficacy [10]. Thus, extrapolating not only from trials to routine care settings but from resource-rich settings to SSA may be too much to ask of SSA ministries of health and medicine licensing boards without first building additional evidence, as these agencies are tasked with applying scarce resources to optimally benefit public health. Alternatively, some experts argue that no further evidence is needed to apply international standards of care for cancer patients in SSA. This is admirable, but in countries like Malawi, such dogmatic “universality” arguments are unrealistic and possibly even harmful—for example, if high-intensity cancer treatments from high-income countries are applied without suitable levels of support. Despite billions of dollars in donor assistance over many decades, the fact remains that the Malawi public sector does not have remotely comparable capacity to the United States for delivering high-intensity cancer treatment.
Therefore, a nuanced middle approach is likely most sensible, with clearer articulation of evidentiary standards for including antineoplastic essential medicines in World Health Organization (WHO) lists [11]. For instance, the uniquely favorable therapeutic ratio for imatinib in chronic myelogenous leukemia (CML) led to the Novartis Glivec International Patient Assistance Program (GIPAP), perhaps the most far-reaching global oncology pharmaceutical access program ever attempted, without first redemonstrating efficacy in low-income countries benefitting from GIPAP [12]. Similar arguments could be made for trastuzumab for HER2-positive breast cancer, for which worldwide access remains limited, and possibly rituximab for CD20-positive non-Hodgkin lymphoma (NHL). Both medicines are notable for having available biosimilars with published evidence for equivalence [13,14], although even these biosimilars remain economically out of reach for many SSA countries. For rituximab, we have argued in Malawi that high opportunistic infection burden, high HIV prevalence among adult NHL patients, and limited infusion and supportive care including absent hematopoietic growth factors warrant rituximab introduction within defined patient populations in the context of a prospective clinical trial [15]. For our study, we are using the commercially available biosimilar, with research ethics committee approval and data safety monitoring board oversight to ensure patients are not harmed by excessive unanticipated hypersensitivity, neutropenia, lymphodepletion, or infectious complications, before advocating more widespread application. Developing a robust regional framework to prioritize cancer interventions requiring further study, through consensus generated in SSA, would be valuable.
What is innovation in SSA, and can SSA cancer trials have global importance?
Given uncertainties about clinical equipoise in SSA, a related question is what constitutes cancer innovation in SSA, as well as whether SSA cancer trials can have global rather than regional importance. Indeed, the global cancer research community was remarkably fortunate with its first SSA activities focused on endemic Burkitt lymphoma, which led to the discovery of Epstein-Barr virus (EBV), the MYC oncogene, and modern principles of combination chemotherapy [16]. Similar discoveries from contemporary efforts might not be so readily forthcoming. Indeed, given implicit justifications for many cancer studies in the region, it may be worth stating that SSA cancer patients do not exist primarily to discover new viruses or gene mutations and that the overarching goal of trials must be to generate knowledge that extends life and reduces morbidity for cancer patients in SSA. Especially when modern sequencing and bioinformatics are increasingly available in more economically advanced countries, like China, India, or Brazil, where cancer pathogenesis may at least partially resemble SSA, there may be limited future opportunities in SSA to discover entirely new fundamental biologic insights for cancer. This is particularly true when many experiments require shipping tissues to high-income countries, raising important and complex ethical, regulatory, and cultural considerations that must first be addressed. Indeed, we have undertaken among the first molecular profiling studies in SSA for 3 high-burden cancers in Malawi (Kaposi sarcoma, esophageal squamous cell carcinoma, and diffuse large B-cell lymphoma), which have often reproduced findings from other parts of the world, though in our view this makes them no less important for SSA [17–19].
Moreover, in pursuit of innovation, the willingness to invest in incremental advances for cancer patients in high-income countries has often not translated to SSA. New “blockbuster” cancer therapies, like brentuximab vedotin for Hodgkin lymphoma or immune checkpoint inhibitors for many cancer types, are appropriately heralded with incremental efforts to define their utility across a range of clinical niches in high-income countries. In SSA, among all cancers, pediatric Burkitt lymphoma has benefitted from the strongest commitment to achieving rigorous incremental advances under local conditions, as in other international pediatric oncology groups [20–24]. Nevertheless, the appropriate first-line chemotherapy regimen for this disease in SSA remains uncertain half a century after its discovery in Uganda, with substantial regional variation in treatment approaches and reported outcomes despite often quite similar patients and settings.
Whatever the difficulties encountered in SSA, several lines of inquiry could lead to globally impactful and innovative clinical science. First, certain cancers occur with unique frequency in SSA and can only be studied in large numbers if patients are included from this region. Examples include endemic Burkitt lymphoma, Kaposi sarcoma, and conjunctival squamous cell carcinoma. Second, with the availability of highly effective noncytotoxic treatments for cancer and the identification of patient subgroups with particularly favorable prognoses like head and neck squamous cell carcinoma caused by human papillomavirus (HPV), there is increasing interest in treatment de-escalation in high-income countries. De-escalated treatment, however, may be difficult to accept in resource-rich settings, where there is a natural reluctance to deviate substantially from aggressive treatment protocols proven to result in excellent outcomes and where undertreatment is typically viewed as a graver error than overtreatment. Given real-world barriers to universally applying regimens from high-income countries as articulated above, de-escalated strategies may be highly appropriate and even necessary in SSA, presenting opportunities for “leapfrog” advances by evaluating regimens that could be applicable in high-income countries. Finally, development of noninvasive biomarkers for cancer diagnosis, prognosis, and response assessment would be particularly valuable in SSA where pathology and diagnostic imaging are limited, especially when these have realistic potential for implementation in SSA. As an example, we have pursued evaluation of quantitative plasma EBV DNA in endemic Burkitt lymphoma, using instruments already in place for HIV RNA monitoring, to guide risk-adapted and response-guided therapy analogous to fluorodeoxyglucose positron emission tomography (FDG-PET) in high-income countries [25–27].
How can clinical trial efficiency be optimized in SSA?
In addition to biologic heterogeneity, cancer trials in SSA will have to cope with marked heterogeneity in populations and health infrastructure across settings. Moreover, health infrastructure in SSA is changing constantly and at times quite rapidly. Trials predicated on absent radiotherapy or hematopoietic growth factors, for instance, might be undermined by the sudden introduction of these adjunctive treatments within relatively short periods and with this introduction typically not achieved in a uniform manner. Given these complicating factors, plus the enormous evidence deficit for cancer treatment in SSA, as well as the enormous need to build trust and prove to SSA stakeholders that cancer research unequivocally benefits public health, there is no region of the world in greater need of flexible, adaptive trial designs that appropriately respond to accumulating evidence or changing conditions on the ground, ensure maximum therapeutic benefit for trial participants, and minimize financial and time expenditures. Again, this is an opportunity for true leapfrog advances within nascent cancer trial networks in the region, which do not have to be unduly constrained by traditional protocol development approaches that may be too rigid and slow to drive oncology care forward in SSA.
A way forward
Taking these issues into consideration, a possible framework for conceptualizing cancer clinical trials in SSA is proposed in Table 2. This scheme is not intended to address more fundamental aspects of clinical trial design, larger economic considerations related to global cancer care, or complex ethical issues in global health, but rather, it is meant to highlight key attributes for an otherwise well-designed study that might make it particularly apt for SSA. There is admittedly tremendous subjectivity in judging a given study according to these traits, and ideally, such judgments would reside within groups of cancer investigators in SSA rather than meetings outside SSA, where a deficit of high-grade evidence from the region makes it easy to distort what may or may not be appropriate.
Tab. 2. Ideal attributes of a cancer treatment trial in sub-Saharan Africa. 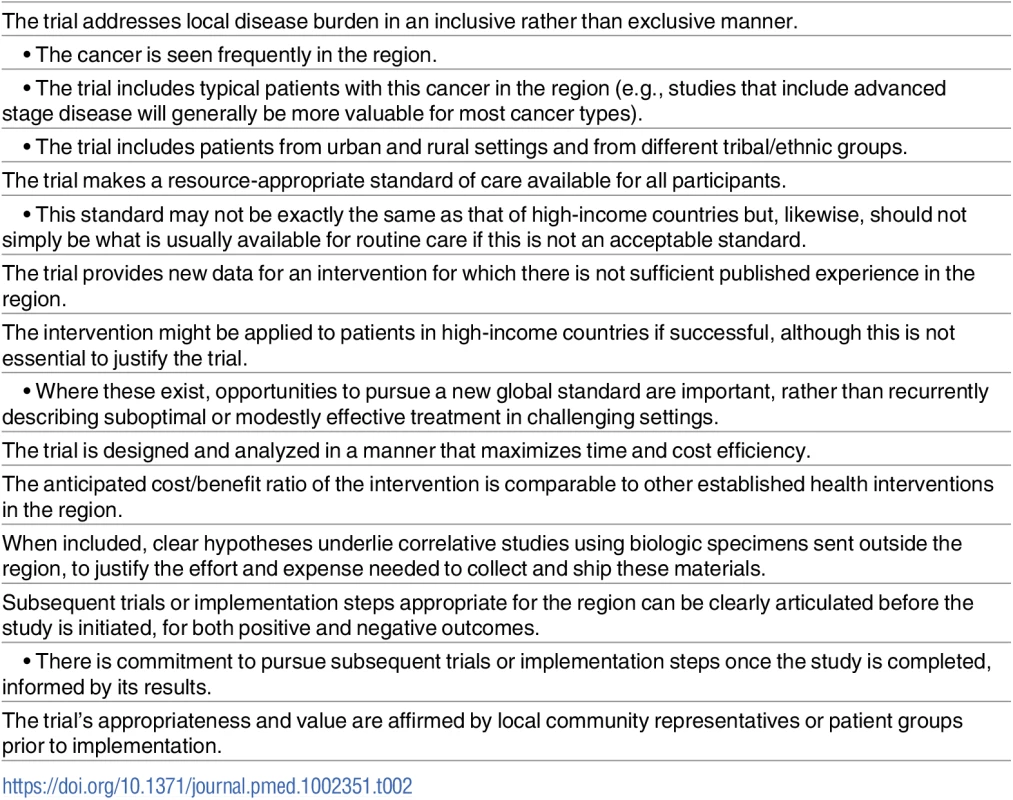
Conclusions
There is a growing recognition in high-income countries that care and research agendas for cancer must be brought closer together, for clinical, scientific, and economic reasons. In SSA, there are simply not sufficient resources to allow these agendas to diverge and/or compete, a luxury born of having excess resources in the first place. Care and research must be aligned and even considered the same—for example, as in international pediatric oncology groups, in which there has been a decades-long commitment to treating cancer on harmonized protocols across centers, to drive clinical science forward for specific diseases and individual children [28]. A potential framework for achieving similar progress in SSA is proposed, to avoid excessive external investment in studies that do not substantially inform or improve care in SSA. With these challenges at the forefront, continued regional efforts and momentum to generate forward progress are eagerly anticipated, by cancer policy makers, clinicians, and patients above all.
Zdroje
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. http://globocan.iarc.fr.
2. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3 : 524–48. doi: 10.1001/jamaoncol.2016.5688 27918777
3. Kingham TP, Alatise OI, Vanderpuye V, Casper C, Abantanga FA, Kamara TB, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2013;14: e158–67. doi: 10.1016/S1470-2045(12)70472-2 23561747
4. Mwanda WO, Orem J, Fu P, Banura C, Kakembo J, Onyango CA, et al. Dose-modified oral chemotherapy in the treatment of AIDS-related non-Hodgkin's lymphoma in East Africa. J Clin Oncol. 2009;27 : 3480–8. doi: 10.1200/JCO.2008.18.7641 19470940
5. Olweny CL, Borok M, Gudza I, Clinch J, Cheang M, Kiire CF, et al. Treatment of AIDS-associated Kaposi's sarcoma in Zimbabwe: results of a randomized quality of life focused clinical trial. Int J Cancer. 2005;113 : 632–9. doi: 10.1002/ijc.20606 15472910
6. Mosam A, Shaik F, Uldrick TS, Esterhuizen T, Friedland GH, Scadden DT, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr. 2012;60 : 150–7. doi: 10.1097/QAI.0b013e318251aedd 22395672
7. Martin J, Laker-Oketta M, Walusansa V, et al. Randomized Trial of Protease Inhibitor-Based Antiretroviral Therapy for Kaposi Sarcoma in Africa. Paper presented at the Conference on Retroviruses and Opportunistic Infections; March 2014; Boston, MA.
8. Farmer P, Leandre F, Mukherjee JS, Claude M, Nevil P, Smith-Fawzi MC, et al. Community-based approaches to HIV treatment in resource-poor settings. Lancet. 2001;358 : 404–9. 11502340
9. Weidle PJ, Malamba S, Mwebaze R, Sozi C, Rukundo G, Downing R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients' response, survival, and drug resistance. Lancet. 2002;360 : 34–40. doi: 10.1016/S0140-6736(02)09330-3 12114039
10. Sanoff HK, Chang Y, Lund JL, O'Neil BH, Dusetzina SB. Sorafenib Effectiveness in Advanced Hepatocellular Carcinoma. Oncologist. 2016;21 : 1113–20. doi: 10.1634/theoncologist.2015-0478 27185615
11. Shulman LN, Wagner CM, Barr R, Lopes G, Longo G, Robertson J, et al. Proposing Essential Medicines to Treat Cancer: Methodologies, Processes, and Outcomes. J Clin Oncol. 2016;34 : 69–75. doi: 10.1200/JCO.2015.61.8736 26578613
12. Kanavos P, Vandoros S, Garcia-Gonzalez P. Benefits of global partnerships to facilitate access to medicines in developing countries: a multi-country analysis of patients and patient outcomes in GIPAP. Global Health. 2009;5 : 19. doi: 10.1186/1744-8603-5-19 20043820
13. Gota V, Karanam A, Rath S, Yadav A, Tembhare P, Subramanian P, et al. Population pharmacokinetics of Reditux, a biosimilar Rituximab, in diffuse large B-cell lymphoma. Cancer Chemother Pharmacol. 2016;78 : 353–9. doi: 10.1007/s00280-016-3083-x 27329361
14. Rugo HS, Barve A, Waller CF, Hernandez-Bronchud M, Herson J, Yuan J, et al. Effect of a Proposed Trastuzumab Biosimilar Compared With Trastuzumab on Overall Response Rate in Patients With ERBB2 (HER2)-Positive Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA. 2016.
15. ClinicalTrials.gov. Rituximab Plus CHOP Chemotherapy for Diffuse Large B-cell Lymphoma in Malawi. https://clinicaltrials.gov/ct2/show/NCT02660710.
16. Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, et al. Burkitt's lymphoma. Lancet. 2012;379 : 1234–44. doi: 10.1016/S0140-6736(11)61177-X 22333947
17. Hosseinipour MC, Sweet KM, Xiong J, Namarika D, Mwafongo A, Nyirenda M, et al. Viral profiling identifies multiple subtypes of Kaposi's sarcoma. MBio. 2014;5: e01633–14. doi: 10.1128/mBio.01633-14 25249280
18. Liu W, Snell JM, Jeck WR, Hoadley KA, Wilkerson MD, Parker JS, et al. Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight. 2016;1: e88755. doi: 10.1172/jci.insight.88755 27734031
19. Fedoriw Y, Montgomery N, Parker J et al. Immunophenotypic and Molecular Profiling of HIV-Associated Diffuse Large B-cell Lymphomas from sub-Saharan Africa. Paper presented at annual meeting of the United States & Canadian Academy of Pathology Annual Meeting; March 2016; Seattle, WA.
20. Molyneux E, Schwalbe E, Chagaluka G, Banda K, Israels T, Depani S, et al. The use of anthracyclines in the treatment of endemic Burkitt lymphoma. Br J Haematol. 2016 Nov 28. doi: 10.1111/bjh.14440. [Epub ahead of print] 27891583
21. Buckle G, Maranda L, Skiles J, Ong'echa JM, Foley J, Epstein M, et al. Factors influencing survival among Kenyan children diagnosed with endemic Burkitt lymphoma between 2003 and 2011: A historical cohort study. Int J Cancer. 2016;139 : 1231–40. doi: 10.1002/ijc.30170 27136063
22. Stanley CC, Westmoreland KD, Heimlich BJ, El-Mallawany NK, Wasswa P, Mtete I, et al. Outcomes for paediatric Burkitt lymphoma treated with anthracycline-based therapy in Malawi. Br J Haematol. 2016;173 : 705–12. doi: 10.1111/bjh.13986 26914979
23. Harif M, Barsaoui S, Benchekroun S, Bouhas R, Doumbe P, Khattab M, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa—report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer. 2008;50 : 1138–42. doi: 10.1002/pbc.21452 18213709
24. Traore F, Coze C, Atteby JJ, Andre N, Moreira C, Doumbe P, et al. Cyclophosphamide monotherapy in children with Burkitt lymphoma: a study from the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer. 2011;56 : 70–6. doi: 10.1002/pbc.22746 21058286
25. Westmoreland KD, Montgomery ND, Stanley CC, El-Mallawany NK, Wasswa P, van der Gronde T, et al. Plasma Epstein-Barr virus DNA for pediatric Burkitt lymphoma diagnosis, prognosis, and response assessment in Malawi. Int J Cancer. 2017;140 : 2509–16 doi: 10.1002/ijc.30682 28268254
26. Press OW, Li H, Schoder H, Straus DJ, Moskowitz CH, LeBlanc M, et al. US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose-Positron Emission Tomography Imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34 : 2020–7. doi: 10.1200/JCO.2015.63.1119 27069074
27. Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin's Lymphoma. N Engl J Med. 2016;374 : 2419–29. doi: 10.1056/NEJMoa1510093 27332902
28. Rodriguez-Galindo C, Friedrich P, Alcasabas P, Antillon F, Banavali S, Castillo L, et al. Toward the Cure of All Children With Cancer Through Collaborative Efforts: Pediatric Oncology As a Global Challenge. J Clin Oncol. 2015;33 : 3065–73. doi: 10.1200/JCO.2014.60.6376 26304881
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Time for trauma immunology
- Translational approaches to coagulopathy after trauma: Towards targeted treatment
- The new survivors and a new era for trauma research
- Research questions in pre-hospital trauma care
- Major scientific lessons learned in the trauma field over the last two decades
- The science of rapid start—From the when to the how of antiretroviral initiation
- Reducing undiagnosed HIV infection among adolescents in sub-Saharan Africa: Provider-initiated and opt-out testing are not enough
- Community and health system intervention to reduce disrespect and abuse during childbirth in Tanga Region, Tanzania: A comparative before-and-after study
- Prescription medicine use by pedestrians and the risk of injurious road traffic crashes: A case-crossover study
- Trauma care: Finding a better way
- Risk of surgical site infection, acute kidney injury, and infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: A national propensity-score-adjusted retrospective cohort study
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
- Years of life lost due to traumatic brain injury in Europe: A cross-sectional analysis of 16 countries
- Antimicrobial resistance in : Global surveillance and a call for international collaborative action
- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Cellular therapies in trauma and critical care medicine: Looking towards the future
- Leveraging peer-based support to facilitate HIV care in Kenya
- Trends in traumatic brain injury mortality in China, 2006–2013: A population-based longitudinal study
- Temporal profile of intracranial pressure and cerebrovascular reactivity in severe traumatic brain injury and association with fatal outcome: An observational study
- Community health promotion and medical provision for neonatal health—CHAMPION cluster randomised trial in Nagarkurnool district, Telangana (formerly Andhra Pradesh), India
- A comparison of Selective Aortic Arch Perfusion and Resuscitative Endovascular Balloon Occlusion of the Aorta for the management of hemorrhage-induced traumatic cardiac arrest: A translational model in large swine
- Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: A retrospective cohort study using the Finnish national health registries
- Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: A prospective observational study
- Cancer trials in sub-Saharan Africa: Aligning research and care
- Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: A retrospective study
- Validation of the sensitivity of the National Emergency X-Radiography Utilization Study (NEXUS) Head computed tomographic (CT) decision instrument for selective imaging of blunt head injury patients: An observational study
- Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial
- Community burden of undiagnosed HIV infection among adolescents in Zimbabwe following primary healthcare-based provider-initiated HIV testing and counselling: A cross-sectional survey
- Timing of femoral shaft fracture fixation following major trauma: A retrospective cohort study of United States trauma centers
- IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model
- Long-term health status and trajectories of seriously injured patients: A population-based longitudinal study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study
- Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines
- Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale–29
- Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



