-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaClinical applicability and cost of a 46-gene panel for genomic analysis of solid tumours: Retrospective validation and prospective audit in the UK National Health Service
Sarah Wordsworth and colleagues study the feasibility of employing next-generation sequencing of tumor DNA in clinical practice.
Published in the journal: . PLoS Med 14(2): e32767. doi:10.1371/journal.pmed.1002230
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002230Summary
Sarah Wordsworth and colleagues study the feasibility of employing next-generation sequencing of tumor DNA in clinical practice.
Introduction
Historically, the standard approach to testing for somatic mutations in cancers has been single gene testing using methods such as Sanger sequencing. With such methods, candidate genes are examined for mutations, and, as a result, patients may become eligible to enter a clinical trial or receive targeted drug therapies [1–3]. Advances in sequencing technology, collectively referred to as next generation sequencing (NGS), mean that the entire cancer genome (whole genome sequencing [WGS]) or parts of it (via targeted panels or whole exome sequencing [WES]) can now be sequenced in hours and at great depth and increasing sensitivity. However, while NGS offers high-throughput, rapid, and accurate testing of multiple genes, it remains to be proven whether it also leads to more appropriate use of targeted drug therapies and an enhanced ability to identify patients who are more likely to benefit from treatment compared with single gene sequencing.
An increasing number of primarily privately funded laboratories are already using NGS to profile tumours for mutations in multiple cancer genes simultaneously, with designs ranging from hotspot panels to a 287-gene panel covering all exons of constituent genes and selected introns (those involved in translocations). DNA requirements vary from 10 to 750 ng, and sample types evaluated include fresh frozen tissue, formalin-fixed paraffin-embedded (FFPE) samples, and fine needle aspirate specimens [4–7]. Many NGS technologies have demonstrated good sensitivity and excellent correlation with standard genetic techniques, as well as providing potentially clinically actionable information. However, the widespread translation of NGS technologies into routine cancer diagnostics has been slow due to technical obstacles (e.g., problems with robust bioinformatics) compromising clinical-grade validation, challenges with clinical interpretation (e.g., paucity of functional data), the absence of genotype–phenotype databases for cancer [8], a lack of demonstrable clinical utility surpassing that of single gene testing, and concern over the costs of NGS to healthcare payers.
Healthcare systems are now recognising the need to understand how to efficiently use genomic technologies in the context of precision medicine and verify their safety and effectiveness with timely evidence [9]. However, there is limited empirical evidence on whether results obtained from NGS technology direct clinical management and/or improve patient outcomes and whether they represent an efficient use of healthcare resources or are just an expensive addition to cancer care.
The aim of our study was to investigate whether a targeted hotspot NGS cancer panel could be translated into routine patient care in the UK National Health Service (NHS). This study involved a clinical-grade optimisation and validation of the panel and bioinformatics pipeline for diagnostics, an assessment of the panel’s impact on clinical management, and a cost analysis of the panel compared with single gene testing.
Methods
Study ethics
Clinical consent was obtained for all samples prior to genetic panel testing. The validation cohort included samples from the VICTOR trial that were consented for genetic analysis (approval obtained from Oxford Research Ethics Committee B [approval number 05\Q1605\66]). For the prospective cohort analysis, we used anonymised diagnostic samples for which ethical approval for service development was not required.
Study design
The NGS technology we assessed was the Ion AmpliSeq Cancer Hotspot Panel (Thermo Fisher Scientific; 46 genes, 189 amplicons). This study was completed in two stages. Stage 1 involved technical validation of the panel using an anonymised retrospective cohort of previously genotyped tumour samples (undertaken as a service development) and comparative costings of the assay with existing technologies in use. Stage 2 was clinical implementation of the validated panel with an accompanying prospective audit of the clinical impact of this assay on treatment choice. Study design, including the genes partially covered by the panel, is presented in Fig 1.
Fig. 1. Study design. 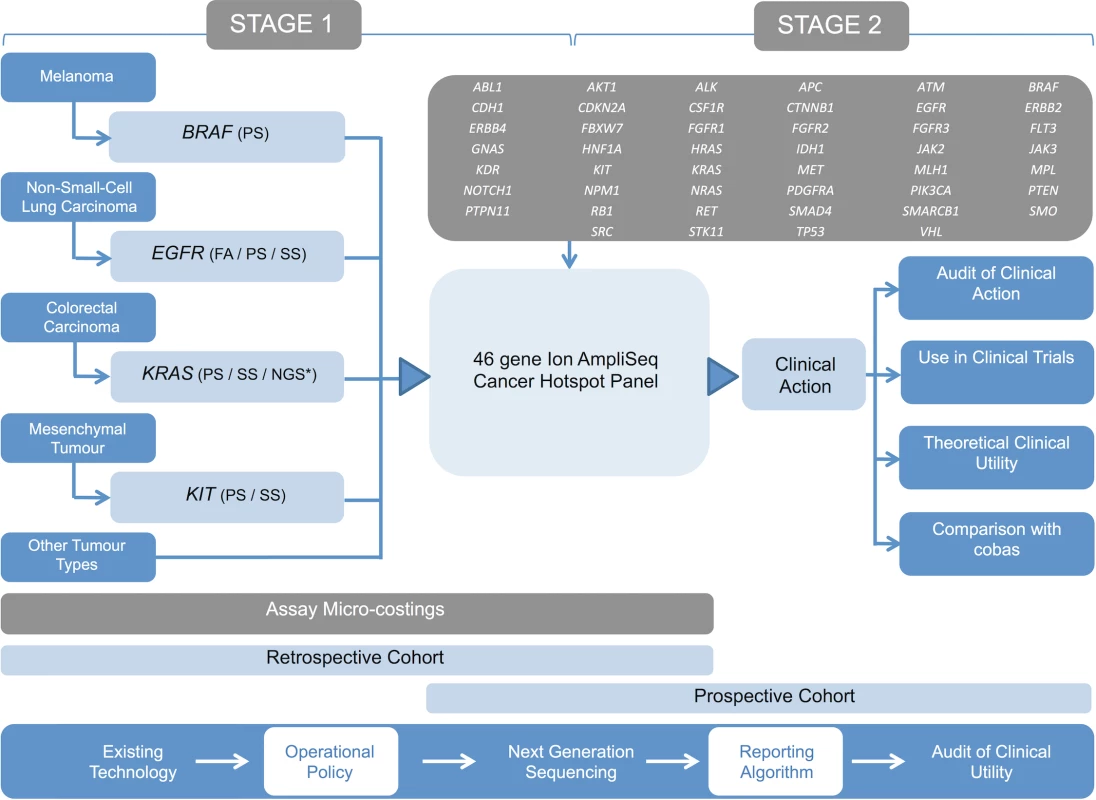
Outline of the study design demonstrating the existing genetic testing repertoire of the laboratory and the proposed NGS assay (cancer panel). Stage 1 involved the technical validation of the panel using a retrospective cohort of samples and performance of micro-costings. Stage 2 involved the panel’s introduction into diagnostic pathways using a prospective patient cohort. An operational policy was developed to select samples for routine analysis using the panel, and comprehensive phenotypic data were obtained in order to assess impact on clinical management. Tumour-appropriate tandem analysis of BRAF, EGFR, and KRAS was performed using cobas assays. FA, fragment analysis; NGS*, alternative next generation sequencing; PS, pyrosequencing; SS, Sanger sequencing. Patient cohorts and data collection
The retrospective cohort used for technical validation (n = 108) was composed of two sequentially tested groups; cohort 1 (n = 63) and cohort 2 (n = 45). Cohort 1 included samples from colorectal carcinoma (CRC), non-small-cell lung cancer (NSCLC), melanoma, and gastrointestinal stromal tumour (GIST) patients that were tested in tandem with standard diagnostic assays (S1 Text; S1 Table). Cohort 2 included previously sequenced CRC samples from the VICTOR (Vioxx In Colorectal cancer Therapy: definition of Optimal Regime) trial [10] as well as NSCLC and GIST specimens. Cohort 1 provided a more diverse range of tumour types, whilst cohort 2 provided mutational information on a wider range of genes than was available from standard diagnostic assays.
The prospective cohort (n = 351) consisted of malignant specimens (predominantly mesenchymal tumours, melanoma, NSCLC, and CRC) consecutively submitted to the Oxford Molecular Diagnostics Centre over a 12-mo period. The decision to submit a tumour sample for testing was made at the weekly multidisciplinary team meeting, with input from the treating oncologist and reporting histopathologist. An operational policy with sample testing algorithms, designed to ensure that testing was restricted to those patients with the potential to benefit from the acquisition of more comprehensive sequencing data, was available to provide guidance on appropriate samples for testing (S1 Fig). This cohort was also used to inform the translation of the panel into routine NHS care.
Phenotypic data were obtained for patients in the prospective cohort. In addition to tumour type, number of mutations, and drugs given, socio-demographic data were collected according to the best practice guidance for clinical audit [11]. In order to evaluate turnaround times, the date of assay request was also collected.
Sample preparation and conventional analysis
FFPE samples from both patient cohorts were macrodissected and DNA extraction performed as described in S1 Text. All retrospective cohort samples had conventional diagnostic testing performed in tandem, e.g., Sanger sequencing, pyrosequencing, and fragment analysis depending on sample type and gene under investigation. Among the prospective cohort, 278/351 samples had tandem cobas (Roche Diagnostics) analysis performed as per DNA availability and referring clinician preference, allowing comparison of the two alternative technologies. Cohort design and testing strategy are outlined in Fig 2 and S1 Table.
Fig. 2. Patient cohort details. 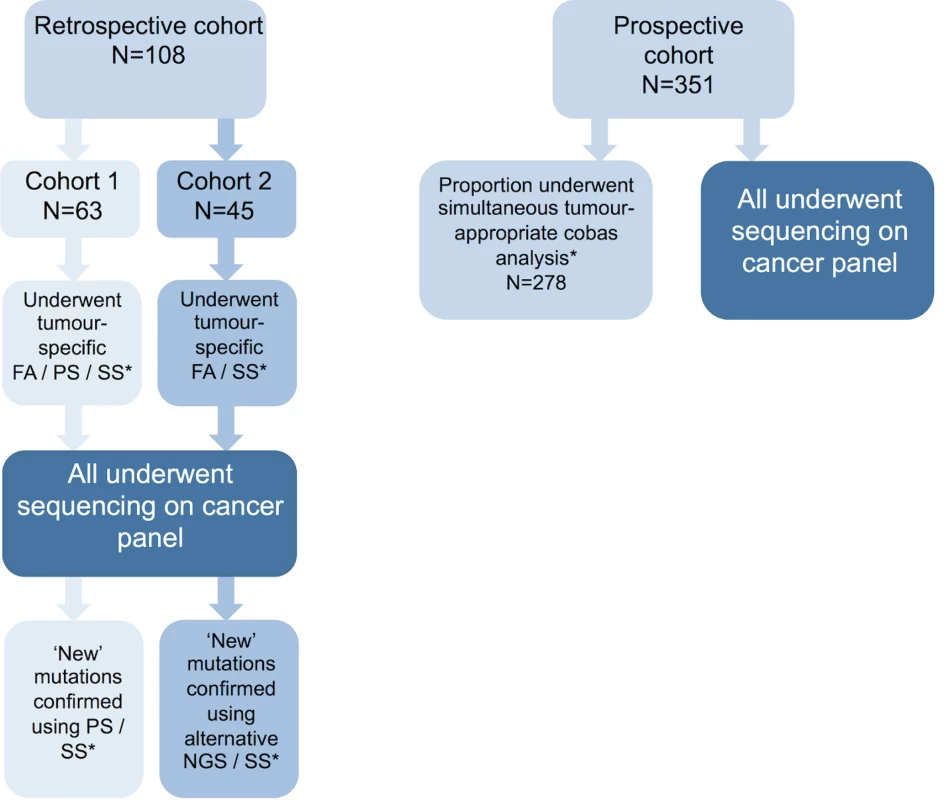
Description of the patient cohorts used in this study. Retrospective cohorts 1 and 2 were used in stage 1 of this study, while the prospective patient cohort was used for stage 2. Retrospective cohort samples had tumour-type-appropriate conventional diagnostic mutation screening prior to testing with the panel. Any novel variants detected by the panel were confirmed using an alternative assay. Prospective cohort samples had tandem analysis of tumour-appropriate genes using cobas assays. *See S1 Table for details of assay and genes tested in each cohort. FA, fragment analysis; NGS; next generation sequencing; PS, pyrosequencing; SS, Sanger sequencing. Next generation sequencing
The design of the panel (i.e., which genes, exons, and codons were included) was based on variants for which there were potential therapeutic, prognostic, or diagnostic implications (for full details see S2 Table). Variants with therapeutic options included both those with established treatments approved by the US Food and Drug Administration/European Medicines Agency (e.g., BRAF, KRAS, and EGFR) and those potentially targetable (e.g., IDH1). The breadth of variants covered by the assay in any gene was designed to extend that available via single gene tests (e.g., inclusion of KRAS exon 5 to cover codon [12]). The overall scope (i.e., number of targets covered by the panel) was a balance between providing maximal data of clinical utility (i.e., potentially actionable) and permitting sufficient multiplexing of samples on a single sequencing chip to render the assay affordable.
DNA from all samples in both cohorts underwent NGS using the Ion AmpliSeq Cancer Hotspot Panel (Thermo Fisher Scientific). Ten nanograms of tumour DNA was amplified using the Ion AmpliSeq Library Kit 2.0 and Ion AmpliSeq Cancer Primer Pool, designed to detect mutations in hotspots in 46 genes (Fig 1), and indexed using the Ion Xpress DNA Barcode Adaptor 1–96 Kit (all Thermo Fisher Scientific) according to the manufacturer’s instructions. Libraries were purified using the AxyPrep Mag PCR Clean-Up Kit (Axygen Biosciences) and quantified using either an Agilent 2100 Bioanalyzer with the DNA High Sensitivity Kit (both Agilent Technologies) or quantitative PCR with the Ion Library Quantitation Kit (Thermo Fisher Scientific). Individual amplified libraries were diluted to 20 pM, and four or eight libraries were multiplexed to give a final concentration of 20 pM. Template-positive Ion Sphere Particles containing clonally amplified DNA were prepared using the Ion OneTouch Template Kit v2 and enriched using the Ion OneTouch ES as per manufacturer’s instructions. Libraries were sequenced on the Ion Torrent Personal Genome Machine with four and eight barcoded samples multiplexed on 316 and 318 chips, respectively (all Thermo Fisher Scientific).
Next generation sequencing data analysis and clinical reporting
Sequencing data were analysed using Torrent Suite software, optimised in an iterative fashion (S1 Text). In a similar fashion to other investigators, we used a tier system to classify variants [13,14] (Fig 3), with all being reported to clinicians in an integrated molecular and histopathological report overseen by a senior clinical scientist and histopathologist.
Fig. 3. Variant classification tiers. 
Tier system used to classify variants detected using the cancer panel. aMy Cancer Genome: http://www.mycancergenome.org. bCOSMIC (Catalogue of Somatic Mutations in Cancer): http://cancer.sanger.ac.uk/cancergenome/projects/cosmic. ccBioPortal for Cancer Genomics: http://www.cbioportal.org/public-portal/. The panel was integrated into diagnostic laboratory workflows to enable reporting within a clinically relevant timescale (Fig 4).
Fig. 4. Next generation sequencing laboratory workflow. 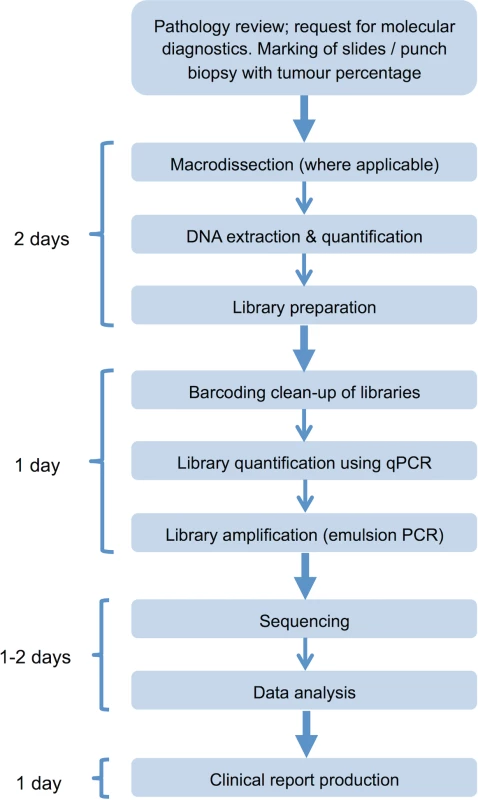
Assay workflow indicating time requirement for each stage of the process; clinical reports can be produced within 5–6 d of receipt of the tumour sample in the laboratory. qPCR, quantitative PCR. Cost analysis
We undertook a detailed micro-costing of both the panel and the cobas system at the Oxford Molecular Diagnostics Centre to determine whether NGS was likely to be sufficiently affordable to translate into routine care. Micro-costing is a highly detailed costing approach that identifies all the underlying resources required for an intervention/activity, such as equipment, consumables, and staff time, and then calculates costs for these resources. The standard operating procedures for the alternative technologies were used to develop costing questionnaires to collect the resource use information (S2 Text). The questionnaires covered each stage in the experimental protocol from sample preparation to data interpretation and reporting. Resource information on staff time, consumables, and equipment was derived from the questionnaires. We accounted for the expected cost of errors during the testing processes. For most equipment items, the cost was spread over the item’s predicted lifetime and depreciated using equivalent annual costing with a discount rate of 3.5%. The cost of the cobas z 480 Analyzer is covered in a combined cost with the mutation kits by the machine manufacturer Roche Diagnostics. The costs of reagents were obtained from prices reported by the diagnostics laboratory and also by contacting reagent manufacturers. Commercially available, rather than any discounted prices, were used where possible. Price per sample was based on the measured yearly throughput of the sequencing platforms, which was 832 (clinical and research samples, based on sequencing 16 samples per week for 52 wk) for the Ion AmpliSeq panel and 2,340 (45 samples per week) for the cobas.
To compare the panel costs with single gene test costs, we used NSCLC, melanoma, and CRC as examples, because these cancers made up the majority of cases in our clinical study. Several alternative costing scenarios were costed, based on clinical practice and the UK National External Quality Assessment Service (UK NEQAS) 2013 guidelines for molecular pathology [15]. For example, for NSCLC we compared the following testing scenarios: (a) the Ion AmpliSeq panel, (b) cobas with single and multiple mutation kits (EGFR, BRAF, and KRAS), and (c) cobas with an EGFR mutation kit, followed by the Ion AmpliSeq panel. Scenario (c) was included to confirm with the Ion AmpliSeq panel whether a lung cancer that was EGFR negative was also KRAS and BRAF negative: if all three genes tested negative, patients went on to have ALK testing, as these mutations have been found to be mutually exclusive [16].
Results
Validation of the cancer panel
Detailed information concerning the technical validation of the panel is provided in S1 Text. Among the prospective cohort, comparative failure rates of the panel and cobas were examined, as were real world turnaround times. The overall panel failure rate in this cohort was 2.6% (9/351). Among those samples analysed using both the panel and cobas, the failure rates were comparable: 0.7% (2/278) and 1.1% (3/278). Fig 5 demonstrates the range of turnaround times (in working days) observed for the panel (n = 342). The median turnaround time was seven working days, with an interquartile range of 6–9 d.
Fig. 5. Turnaround times. 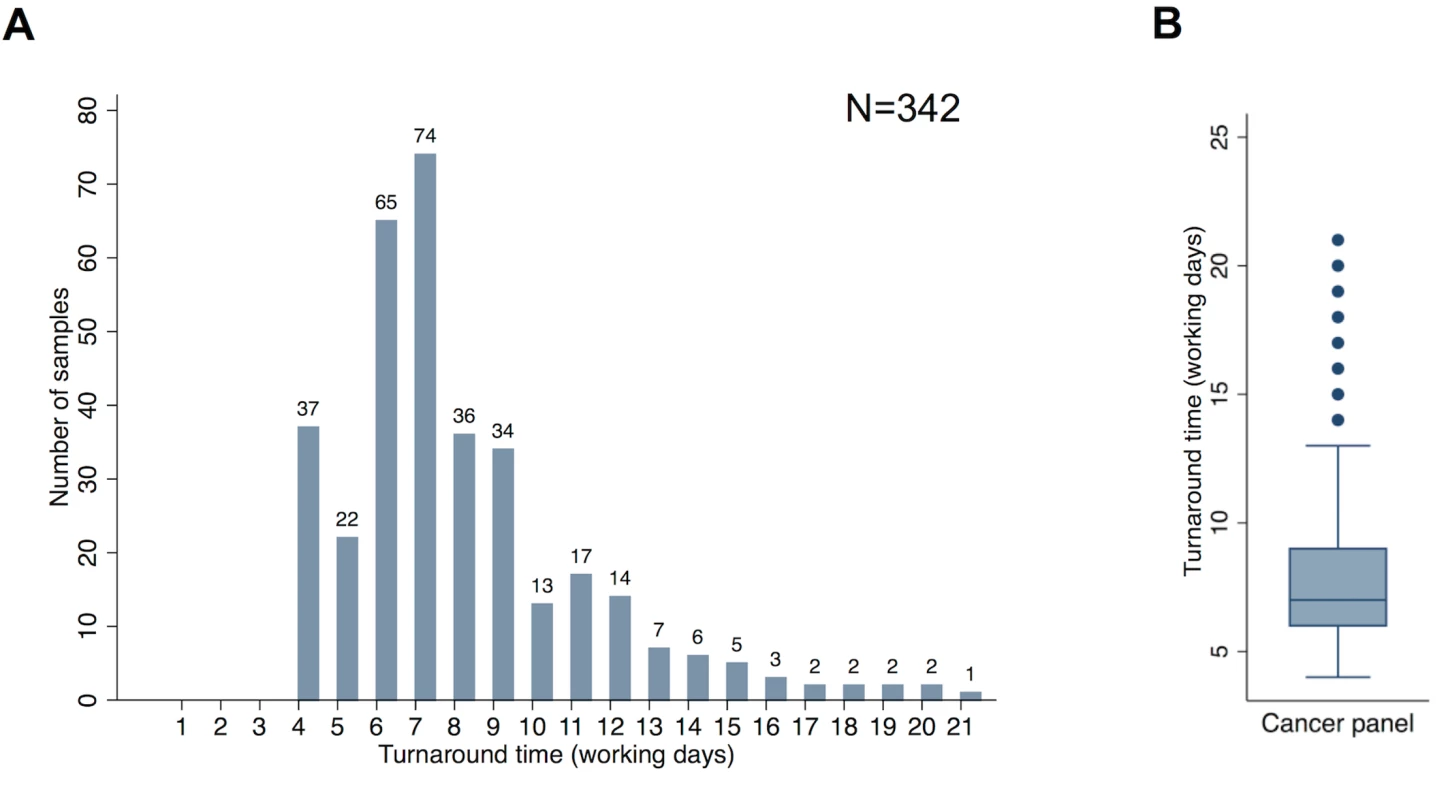
(A) Distribution of turnaround time from request of the panel assay to production of a clinical report for samples in the prospective cohort. (B) Median, interquartile range, and outlying turnaround times from request of the panel assay to production of a clinical report for samples in the prospective cohort. Analysis and figure generation performed using Stata version 13 (StataCorp). Turnaround time represents the timespan from assay request in the laboratory to report generation. It should be noted that this does not include the time taken to make the original histopathological diagnosis or produce sections or punches of the tumour suitable for DNA extraction. Turnaround times of 4 d were observed when extracted DNA was already available within the laboratory (due to prior single gene tests having been previously requested). Longer turnaround times could be accounted for by occasional failed assays being repeated, the need to multiplex samples on the sequencing chip, and the fact that the assay was performed only once a week, meaning that if a sample arrived immediately after the assay was initiated, the sample had a wait of five working days before library preparation was commenced.
Although turnaround times of 2–3 working days from sample receipt in the laboratory to report generation were possible for single gene cobas tests, the laboratory practice of batching samples for this analysis and performing assays on a weekly basis meant three sequential single gene tests had a turnaround time in excess of ten working days.
Patient demographics and mutations detected
The 351 prospective cohort samples sequenced had comprehensive phenotype data, a summary of which is given in Table 1; 52% were female, and the median age was 68 y (range 9–95) at the time of tumour sampling. The predominant tumour types tested were melanoma (31.1%), NSCLC (30.8%), and CRC (25.1%), all of which should have been metastatic or unresectable, correlating with the malignancies for which there are targeted therapies approved by the National Institute for Health and Care Excellence (NICE) (S7 Table) [1–3,17]. The remaining samples were mostly mesenchymal tumours, e.g., GISTs, (for which tyrosine kinase inhibitors [TKIs] may be appropriate), or were submitted to allow assessment for clinical trial entry [18].
Tab. 1. Prospective cohort patient characteristics (n = 351 patients). 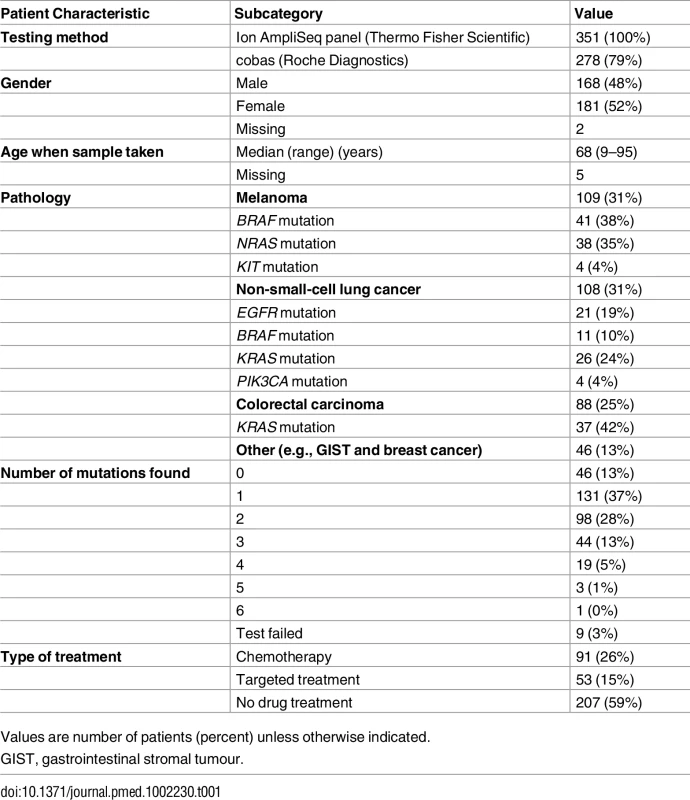
Values are number of patients (percent) unless otherwise indicated. In all, 144/351 (41.0%) patients received pharmacological therapy, either chemotherapy or targeted molecular therapy. Treatment decisions were determined at the tumour-specific multidisciplinary team meeting and followed locally endorsed guidelines in line with national and international recommendations for each tumour type. Targeted therapies were administered in accordance with NICE guidelines [1–3,17] or in the context of a clinical trial. Fig 6 demonstrates the timing of the assay in the treatment pathway of these 144 patients. All patients presented in this study had a single sample analysed once using the panel; most often, the assay was performed prior to any treatment, with only a few patients having the test done after three or more lines of therapy, usually to facilitate clinical trial entry (e.g., a trial of a PI3K inhibitor in breast cancer [19]). The proportions of these 144 pharmacologically treated patients receiving targeted and non-targeted therapies were 36.8% (53/144) and 63.2% (91/144), respectively.
Fig. 6. Timing of the cancer panel in the patient care pathway. 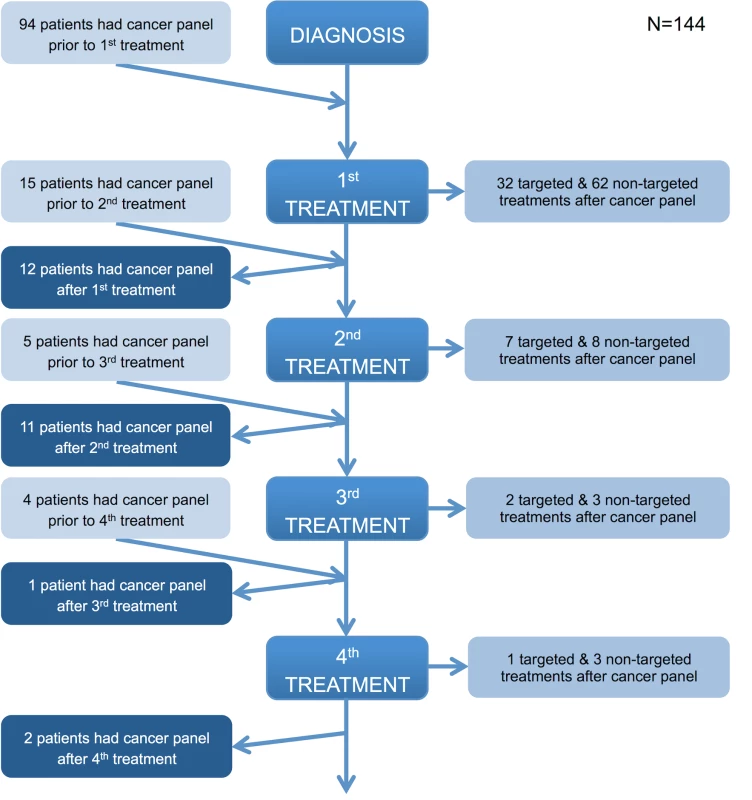
The flowchart illustrates when the panel was performed in the patient journey relative to diagnosis and lines of treatment and the type of treatment (if any) subsequently given. If a patient received a line(s) of treatment after the panel was performed, they were considered to have had the panel prior to a treatment. If a patient did not receive any further lines of treatment after the panel was performed, they were considered to have had the panel after a treatment. Those patients who did not receive any treatment or for whom there are no details of any treatment received are not represented in the flowchart. All patients in the prospective cohort had their tumour analysed on a single occasion. Fig 7 demonstrates the number of mutations detected per sample for the various tumour types tested (Fig 7A), as well as the distribution of mutations across the genes on the panel (Fig 7B). Among the successfully sequenced samples (97.4%, i.e., 342/351), at least one mutation was detected in 86.5% (296/342) of the samples, while 48.2% (165/342) of the samples had two or more mutations identified. In keeping with published studies, there were frequent mutations of BRAF and NRAS in melanoma; BRAF, EGFR, KRAS, and STK11 in NSCLC; APC, BRAF, KRAS, and PIK3CA in CRC; KIT and PDGFR3A in GIST; and CTNNB1 in other tumours (desmoid fibromatosis). TP53 was the most frequently mutated gene across the cohort, with mutations in all tumour types.
Fig. 7. Distribution of mutations. 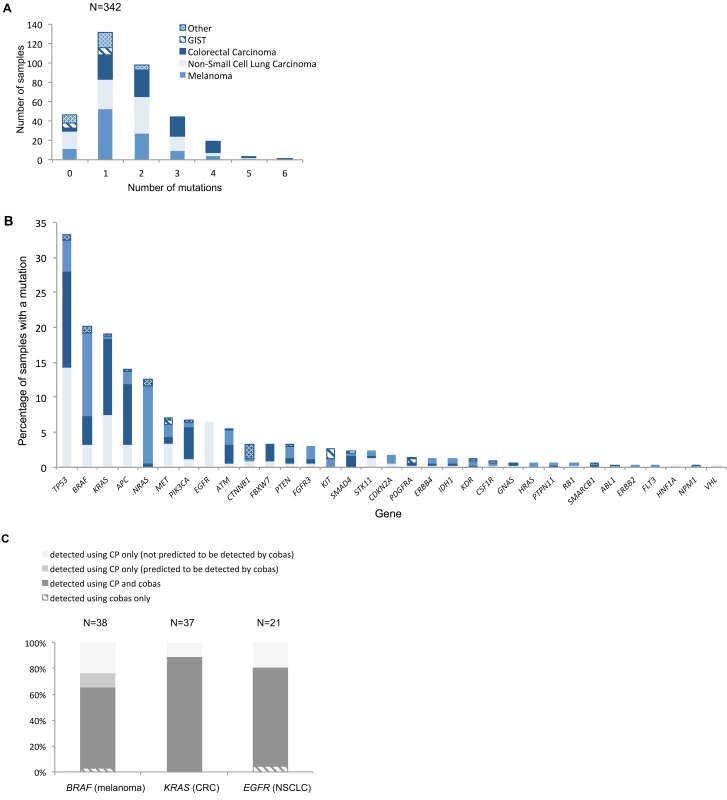
(A) Number of mutations per histological sample by tumour type. Across all tumour types, mean and median numbers of mutations detected were 1.63 and 1, respectively. The hierarchy of least to most mutated tumour type was GIST, other, melanoma, NSCLC, and CRC, with mean/median numbers of mutations of 0.71/1, 0.97/1, 1.49/1, 1.61/2, and 2.17/2, respectively. (B) Distribution of mutations across the different genes represented on the panel for the different tumour types. The key in (A) applies to both charts. For genes that are not displayed in (B), no mutations were detected. (C) Percentage of mutations in key clinically actionable genes detected by standard diagnostic methods and the panel in the prospective cohort (N relates to number of mutations). Only samples that had tandem tumour-appropriate cobas analysis are included in this comparison. CP, cancer panel; CRC, colorectal carcinoma; GIST, gastrointestinal stromal tumour; NSCLC, non-small-cell lung carcinoma. The mutation rates detected with the panel were similar for many genes to those found in publicly available WES/WGS studies (S4 Fig) despite different portions of the genome being examined. A Fisher’s exact test was applied to these data for each tumour type on a per-gene basis, and the resulting p-values were corrected for multiplicity using a Bonferroni–Hochberg procedure [20] (S1 Data). This analysis demonstrated no statistically significant difference in the rates of mutation observed using the two different sequencing approaches for the majority of genes across all tumour types (only p-values < 0.05 were considered to be statistically significant).
Genes where a statistically significant difference in mutation rate was observed were APC (p = 9 × 10−7) in CRC, STK11 (p = 0.008) in NSCLC, and CDKN2A (p = 0.02), ERBB4 (p = 9 × 10−4), FLT3 (p = 0.02), and KDR (p = 0.01) in melanoma. This finding suggests that although the panel covers only small amounts of the genome, it is very well targeted to the most frequently mutated regions: those genes where significantly more mutations were seen using WES/WGS compared to the panel have variants distributed across the gene rather than targeted on a few codons. This means that, despite its limited scope compared to WES/WGS, the panel is likely to capture most mutations in these genes, indicating its potential utility.
Full details of the mutations detected in the prospective cohort are given in S2 Data.
Changes in patient management following cancer panel testing
Standard genetic testing of tumours is usually limited to situations where variant information will influence a specific intervention. In order to justify the cost of more extensive mutation analysis, it is necessary to demonstrate clinical utility, e.g., by analysing treatment decisions and patient outcomes based on data in electronic patient records. Given that the prescription of targeted therapies in the UK is regulated by NICE, evidence regarding clinical utility beyond NICE-approved indications is anecdotal.
Fig 7C demonstrates that the panel provided additional mutation information not detected by the cobas technology for the three “most actionable” genes: 34.2% (13/38) of BRAF mutations in melanoma, 19.0% (4/19) of EGFR mutations in NSCLC, and 11.1% (4/36) of KRAS mutations in CRC (only samples tested using both the panel and the cobas assay were included in this comparison). These mostly pertained to codons or variants outside the scope of the cobas assay, some of which are actionable. However, four additional BRAF V600E mutations in melanoma specimens were identified using the panel that should have been detected by the cobas (two failures and two false negatives). In contrast, cases where standard diagnostic assays provided additional information are entirely accounted for by panel sequencing failures.
Table 2 lists variants in the prospective patient cohort that did change, or could have changed, patient management given local availability of targeted therapeutics (assuming clinical criteria were met). In melanoma, guidance from NICE permits vemurafenib use for BRAF V600 mutated tumours, meaning one patient with a V600R mutation received the drug and others with V600G/M and V600K mutations were eligible. One patient with melanoma with a KIT V560D mutation entered a clinical trial of the TKI nilotinib, while another received the multi-targeted receptor TKI pazopanib after NRAS mutation status was determined (clinical trial entry required only knowledge of NRAS mutation status, not the detection of a mutation). NICE guidance regarding eligibility for vemurafenib in BRAF V600 mutated melanoma stipulates that patients have metastatic disease, but not all patients tested met this criterion, accounting for some of the discrepancy between actionable mutations and subsequent changes to patient management.
Tab. 2. Potentially actionable mutations detected in the prospective cohort using the cancer panel. 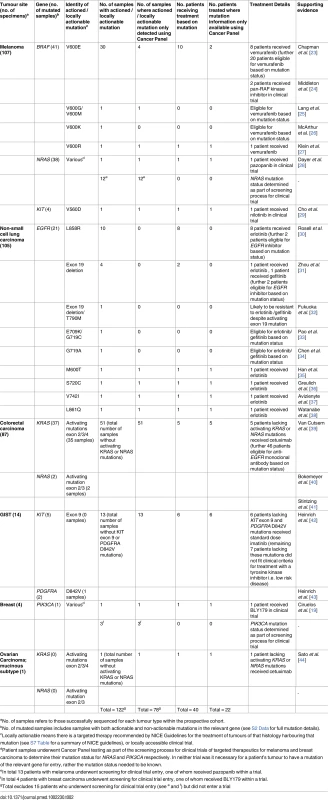
aNo. of samples refers to those successfully sequenced for each tumour type within the prospective cohort. NICE guidance requires only the presence of activating EGFR mutations [17,21] for NSCLC patients to be eligible for EGFR inhibitors, meaning four patients with unusual EGFR mutations (M600T, S720C, V742I, and L861Q) received erlotinib as a result of the panel. Evidence for the efficacy of EGFR inhibition in these mutations is scant due to their low frequency. There is some evidence that L861Q is an activating mutation although in vitro studies suggest it is sensitive to WZ-4002 (irreversible second-generation EGFR inhibitor) rather than erlotinib [22].
During this study, eligibility for anti-EGFR monoclonal antibody therapy (cetuximab or panitumumab) in CRC evolved from requiring wild-type KRAS to requiring wild-type RAS, necessitating mutation testing of NRAS in addition to KRAS. The only method of assessing NRAS status within our laboratory was the panel, and 51/88 (58.0%) patients with CRC were found to have tumours with wild-type RAS. The audit of subsequent clinical action revealed that 5/51 (9.8%) of these patients received anti-EGFR therapy (cetuximab). Further examination revealed that many of the 46 patients who on the basis of RAS mutation status would have been eligible for the treatment did not meet the clinical criteria (i.e., did not have metastatic disease; see S7 Table). Among CRC patients with mutated RAS tumours (37/88; 42.0%), four tumours would have been classed as wild-type RAS using conventional diagnostics: two had NRAS codon 61 mutations and two had KRAS codon 146 mutations (outside the scope of the cobas assay).
The panel was also the only mutation assay available for GIST patients at the time of analysis: although use of TKIs is dictated by clinical factors (moderate/high-risk or metastatic disease), certain mutations cause tumour resistance or require a higher TKI dose [45]. Six GIST patients received standard dose imatinib due to the absence of KIT exon 9 (requires higher dose) or PDGFRA D842V mutations (confers imatinib resistance). Four breast cancer patients had their PIK3CA mutation status determined, with one receiving BLY719 (PI3K inhibitor) in a clinical trial as a result, while a patient with a wild-type RAS mucinous ovarian carcinoma received cetuximab.
In total, 122/351 prospective cohort patients (34.8%) had a mutation for which there was either a NICE-approved targeted therapy or locally available clinical trial of a targeted therapy, 40 (32.8%) of whom received a targeted therapy. Fifty-five percent (22/40) of these patients received a targeted treatment only as a result of novel information from the panel. The additional 13 patients who received targeted therapies were NSCLC patients who received EGFR inhibitors second line in the absence of a previously detected activating EGFR mutation and patients with a variety of solid tumours who received targeted inhibitors in the context of a clinical trial where demonstration of a particular mutation was unnecessary.
A concern with testing progressively larger quantities of the tumour genome is that multiple potentially actionable mutations may be detected with conflicting recommended actions. Owing to the targeted nature of this panel centred around well-characterised hotspots and the paucity of targeted agents available in the UK, variant interpretation in this study was not impacted by this possibility. Where more than one mutation was identified in an actionable gene, there was either a clear hierarchy of action—e.g., NSCLC specimen G150739T had both an exon 19 deletion and p.T790M mutation in EGFR, meaning the patient would not respond to first generation EGFR TKIs but rather may benefit from a third generation drug [46]—or the same action was appropriate for both mutations, e.g., melanoma specimen G151372L had BRAF p.V600M and p.V600G mutations, both of which are likely to benefit from a BRAF inhibitor [25,47].
In order to investigate what the potential impact on clinical management of the panel might be in the future, we analysed the mutation data from the NSCLC and melanoma samples in the prospective cohort for theoretically actionable mutations as described by Meador et al. [48] (S5 Fig). A mutation was classified as theoretically actionable if there was peer-reviewed data at any level (from in vitro cell line to phase III randomised controlled trial [RCT] data) that indicated efficacy of an available treatment (predominantly targeted inhibitors). Assuming unrestricted access to these targeted therapies, 39.0% (41/105 successfully sequenced) of NSCLC and 66.4% (71/107 successfully sequenced) of melanoma patients had a potentially actionable mutation, in contrast to 20.0% (21/105) and 32.7% (35/107) of NSCLC and melanoma patients, respectively, who had a locally actionable mutation. This suggests that, in the future, far more of the variant information generated will lead to clinical management changes.
Cost analysis
As shown in Table 3, the total cost for testing 46 genes using the panel was £339 (US$449) per sample (patient). This is compared to the cost of mutation testing with the single gene approach (cobas): £71 (US$94) for BRAF, £104 (US$138) for EGFR, and £141(US$187) for KRAS. Table 4 shows the cost by resource category for the different tests and for the combinations of these tests for different malignancies. For all tests, most costs are attributed to consumables, followed by staff and overheads. For example, the consumable cost is £185 per sample for the panel and between £34 and £93 for the cobas, depending on the gene tested.
Tab. 3. Test cost results. 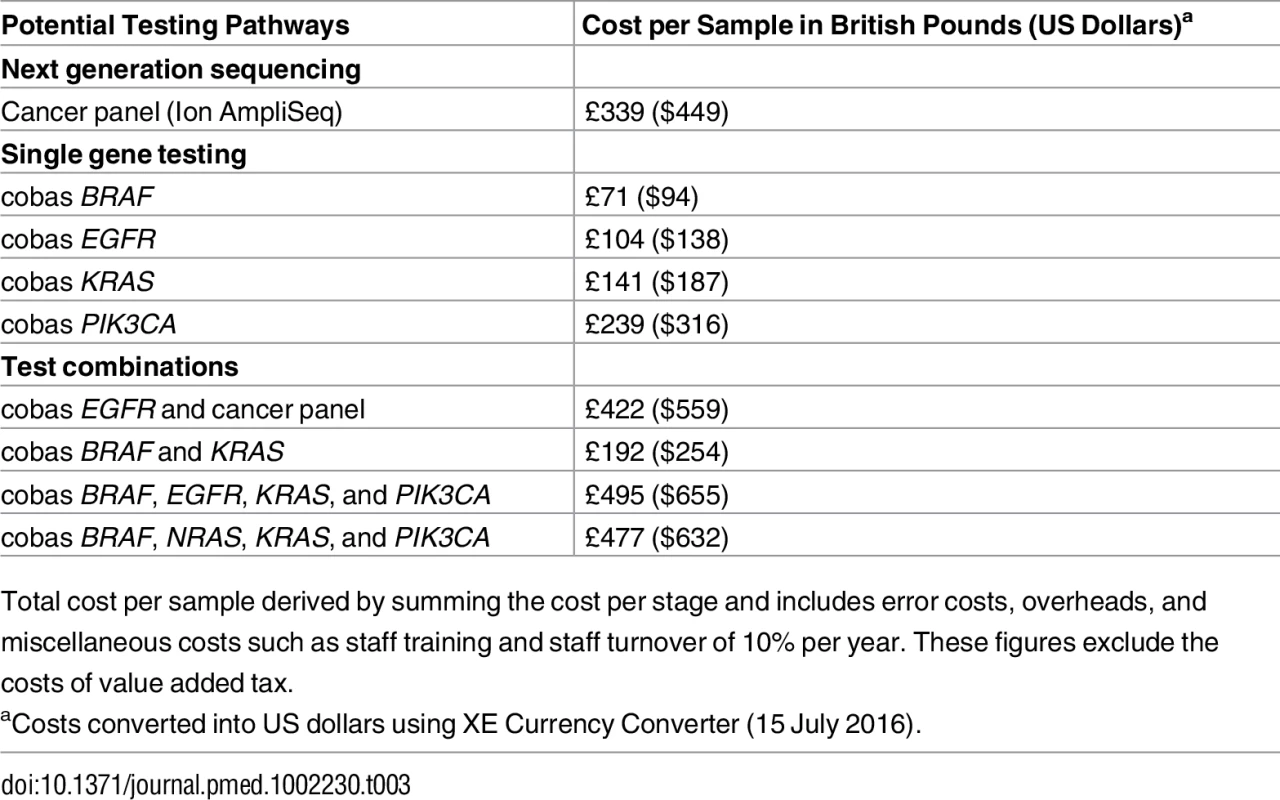
Total cost per sample derived by summing the cost per stage and includes error costs, overheads, and miscellaneous costs such as staff training and staff turnover of 10% per year. These figures exclude the costs of value added tax. Tab. 4. Total cost per sample and type of malignancy by resource category. 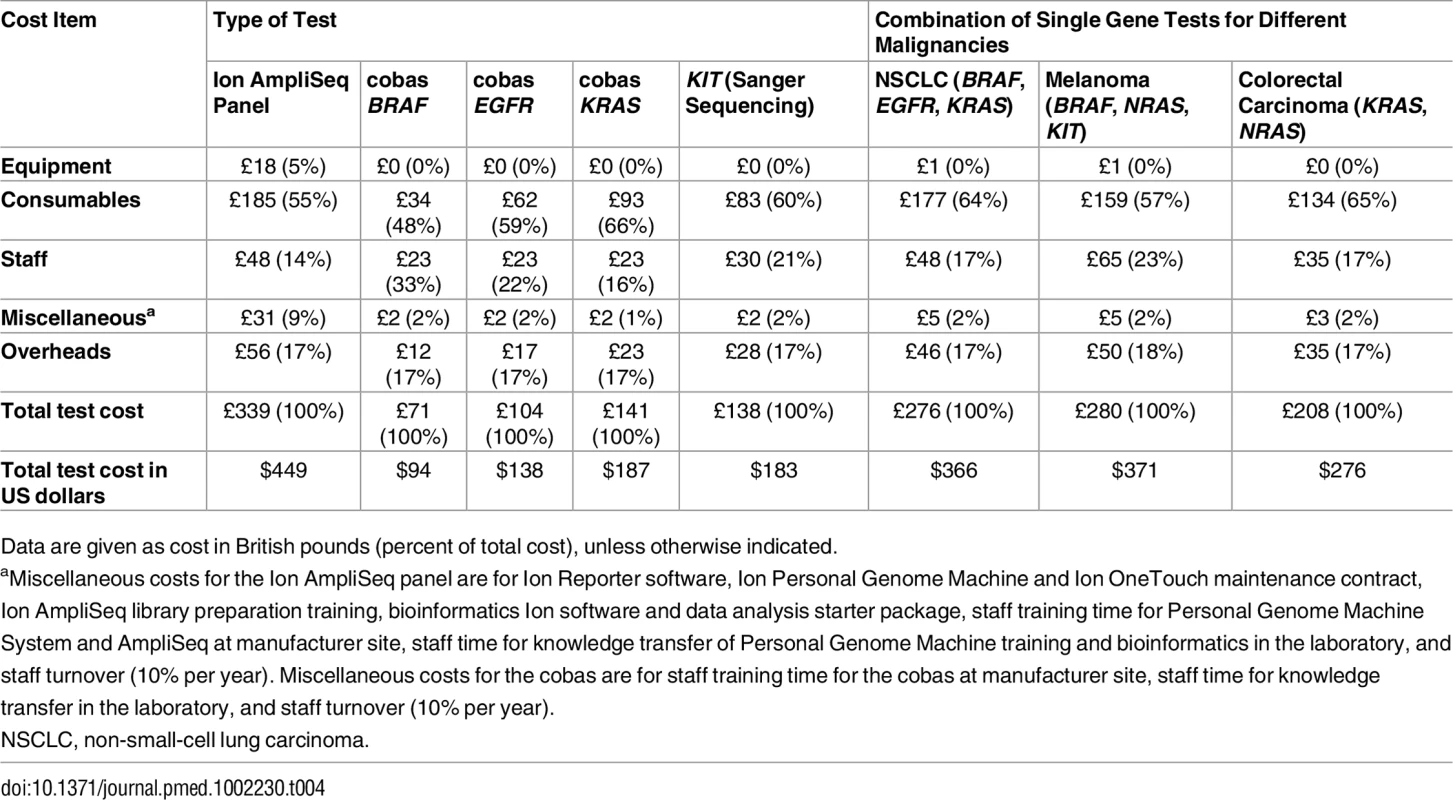
Data are given as cost in British pounds (percent of total cost), unless otherwise indicated. Discussion
We have validated and clinically implemented an NGS assay that detects relevant mutations across mutational hotspots of 46 genes from minimal quantities of FFPE-derived highly fragmented tumour DNA, allowing routine testing of multiple genes from small biopsies. Its performance has been validated across a variety of tumour types for single nucleotide variants and indels and has been shown to have an enhanced sensitivity compared with conventional diagnostic techniques. Treatment data revealed that surprisingly few patients (~40%) received any pharmacological treatment, targeted or otherwise, confounding the fact that for most patients the indication for mutation analysis should be to inform whether treatment should be conventional chemotherapy or targeted agents. Examination of individual cases showed that, in contravention of operational policy, many samples were not from metastatic or unresectable malignancies. Whilst early testing in the diagnostic pathway may be desirable, many targeted therapies are indicated only for metastatic or unresectable disease, such as erlotinib in NSCLC. Additionally, differences in side effects between conventional chemotherapy and targeted agents are such that some elderly patients may be deemed suitable for the latter but not the former, meaning that if no actionable mutation is identified there is no appropriate treatment.
Over a third of the patients in our prospective cohort (122/351) had a locally actionable mutation, with 40 patients receiving a targeted therapy, of which 22 received this therapy only because of tumour testing with the panel (three patients were able to access a clinical trial, 12 patients had access to NICE-sanctioned therapies, six patients had changes to their management in line with European Societal Guidelines, and one patient was able to access a therapy via local funding arrangements). Whilst these numbers are modest, they reflect the limited availability of targeted therapies approved by NICE. For example, significant numbers of patients within the prospective cohort could potentially have benefited from targeted therapies when data from just 7/46 genes were considered (EGFR, KRAS, NRAS, BRAF, KIT, PDGFRA, PIK3CA). Although not all these patients required treatment at this stage, others were unable to access drugs due to lack of either licensing for their specific mutation and tumour type or NICE approval, reflecting a lack of prospective phase III RCT data.
The panel provides flexibility to rapidly introduce new testing as novel therapies are licensed or eligibility criteria are updated, as was demonstrated with the introduction of NRAS testing in our cohort. The panel also enables patients’ eligibility for novel agents in clinical trials to be assessed, which provides a significant contribution to available treatment options.
It is important to note that the panel testing also highlights patients who should not receive treatment due to the presence of confounding activating mutations, in particular, CRC patients with activating mutations in KRAS or NRAS, for whom treatment with anti-EGFR monoclonal antibody is not indicated, and GIST patients with activating mutations in KIT or PDGFRA, who are not eligible for imatinib. This accounted for 38/351 patients (10.8%) in the prospective cohort (37 CRC patients with activating KRAS or NRAS mutations and one GIST patient with a PDGFRA D842V mutation) and is also an important outcome of tumour testing.
The panel described here enables parallel testing of multiple genes, in contrast to conventional diagnostic techniques. However, it must be noted that the panel covers hotspot mutations only, not full gene sequencing, and in its current format does not allow testing for larger copy number variants such as HER2 gene amplification in breast cancer [49].
Furthermore, it should be recognised that although RCTs increasingly use mutation status in treatment stratification decisions, e.g., the Medical Research Council FOCUS4 trial [50], for rare mutations in frequently mutated genes, combinations of mutations, and any mutations in rarely mutated genes, it is unlikely that phase III RCTs will ever recruit sufficient patients to determine the most appropriate treatment option, particularly with ever-increasing numbers of inhibitors.
The alternative is to develop and join up worldwide repositories of genotype–phenotype data so all anecdotal experiences can be collated and statistically mined for commonality. For this approach to be informative, more molecularly directed access to novel treatment modalities of proven efficacy is required. In addition, clinical drug development has to systematically include genomic characterisation of patient samples to detect differential responses due to mutation signatures. An example of this is the National Lung Matrix trial, currently recruiting in the UK, which consists of parallel, multi-centre, single-arm phase II trials, each arm testing an experimental targeted drug in a population stratified by multiple prespecified target biomarkers employing a Bayesian adaptive design [51].
In terms of the affordability of NGS technologies for healthcare payers, Cancer Research UK has set several requirements for tumour profiling tests. One of these requirements is that the cost for such tests must be less than £300 [52]. Our cost analysis shows that the panel cost is only slightly higher than this, at £339 (US$449) per sample, and that for certain gene test combinations, such as testing for BRAF, EGFR, KRAS, and PIK3CA (Table 3), sequential single gene testing is actually more expensive than testing several genes at the same time using the panel (£27 for NSCLC and £32 for melanoma). In the context of other costing studies in this area, estimates show that there is considerable variation across studies [53]. For example, Gallego et al. reported a cost of £1,703 (US$2,700) for using a cancer panel in the diagnosis of CRC and polyposis syndromes [54], while Yorczyk et al. estimated the average cost for a single-tier hereditary 25-gene panel test using the MyRisk platform at £2,581 (US$4,099) per person [55]. Ghemlas et al. reported that the cost of NGS was £297 (US$470) per patient for genetic testing of inherited bone marrow failure [56]. There is also variation in what these studies include in the cost analysis, with some including only the costs of consumables. However, a common theme is that most of the cost estimates are substantially higher than the Cancer Research UK £300 target.
Further, our results suggest that, depending on the combination of genes tested, the panel can be less expensive than single gene testing. For example, if testing for melanoma is done using a combination of three single gene tests for BRAF, NRAS, and KIT, this costs less than the panel. However, in other contexts, if KRAS and PIK3CA or EGFR and PIK3CA were tested, then testing for only two genes would be more expensive than the cancer panel.
This study has a number of potential limitations, some of which are common to most NGS studies, and others of which are specific to our study. First, correct identification of variants requires access to high-quality tumour material; as with other studies, we used FFPE biopsies. Although sequencing was usually successful using this NGS assay, the formalin fixation process itself can cause alterations in the sequence due to deamination of cytosine to uracil [57]. Equally, detection of all the relevant variants in a tumour assumes a representative biopsy, which may not be the case given the phenomenon of clonal heterogeneity [58]. Second, as with most sequencing studies, assessment of the pathogenicity of variants whose clinical significance is not understood is challenging, and in the absence of biological data, many in silico variant effect prediction algorithms have limitations.
Specific study limitations relate to our use of real word data: submission of cases for sequencing did not always follow the suggested algorithms (see S1 Fig) and therefore, regardless of the variants detected, the patients were not eligible for targeted treatments on clinical grounds. Owing to some cases being submitted from other institutions, not all clinical information was available for all patients, reducing the overall power of the study. Finally, limited access to some targeted therapies within the UK NHS meant that even if there was a potential drug indicated for a variant in a particular tumour type, often this drug would not be funded for use. In terms of our cost analysis, due to the limited availability of single gene cost data, the comparison between the Ion AmpliSeq panel and cobas was based on only three genes, whereas the comparison should ideally be made on the cost of the nine genes that have known clinical value. At the same time, we believe that the use of real-life data in this setting was the most appropriate way to study the true clinical utility and cost-effectiveness of the panel in a given healthcare system.
Conclusions
In conclusion, in the context of the UK NHS, we validated and translated a cancer panel that reports clinically actionable results back to clinicians. This led to actionable mutations being identified in 122/351 (34.8%) patients in our cohort and allowed 15.3% (22/144) of those receiving pharmacological treatment to have access to targeted therapies not indicated using conventional single gene testing, thereby demonstrating the ability of the panel to impact clinical management. Our results also show that providing a mutation analysis service using a cancer panel for cancer diagnostics and treatment would provide value for money for the NHS, because if several genes are tested individually, which is often the case, then a one-stop test would require less resources (and time) and be less expensive than sequential gene testing. Using cancer panels for molecular testing will help molecular diagnostic laboratories deal with the increased demand for genetic testing and rapidly and reliably detect relevant mutations, even for a limited amount of tumour tissue, at a relatively low cost. Currently, only 9/46 genes on the panel are recognised to have clinical utility in the UK system, but as additional genes are clinically validated as targets, greater potential of NGS technologies could be realised.
Supporting Information
Zdroje
1. National Institute for Health and Care Excellence. Erlotinib for the first-line treatment of locally advanced or metastatic EGFR-TK mutation-positive non-small-cell lung cancer. NICE technology appraisal guidance 258. London: National Institute for Health and Care Excellence; 2012 [cited 2017 Jan 17]. Available from: https://www.nice.org.uk/guidance/ta258.
2. National Institute for Health and Care Excellence. Cetuximab for the first-line treatment of metastatic colorectal cancer. NICE technology appraisal guidance 176. London: National Institute for Health and Care Excellence; 2009 [cited 2017 Jan 17]. Available from: https://www.nice.org.uk/Guidance/TA176.
3. National Institute for Health and Care Excellence. Vemurafenib for treating locally advanced or metastatic BRAF V600 mutation-positive malignant melanoma. NICE technology appraisal guidance 269. London: National Institute for Health and Care Excellence; 2012 [cited 2017 Jan 17]. Available from: https://www.nice.org.uk/Guidance/TA269.
4. Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–31. doi: 10.1038/nbt.2696 24142049
5. Cottrell CE, Al-Kateb H, Bredemeyer AJ, Duncavage EJ, Spencer DH, Abel HJ, et al. Validation of a next-generation sequencing assay for clinical molecular oncology. J Mol Diagn. 2014;16(1):89–105. doi: 10.1016/j.jmoldx.2013.10.002 24211365
6. Kanagal-Shamanna R, Portier BP, Singh RR, Routbort MJ, Aldape KD, Handal BA, et al. Next-generation sequencing-based multi-gene mutation profiling of solid tumors using fine needle aspiration samples: promises and challenges for routine clinical diagnostics. Mod Pathol. 2014;27(2):314–27. doi: 10.1038/modpathol.2013.122 23907151
7. Tsongalis GJ, Peterson JD, de Abreu FB, Tunkey CD, Gallagher TL, Strausbaugh LD, et al. Routine use of the Ion Torrent AmpliSeq (TM) Cancer Hotspot Panel for identification of clinically actionable somatic mutations. Clin Chem Lab Med. 2014;52(5):707–14. doi: 10.1515/cclm-2013-0883 24334431
8. Gottlieb B, Beitel LK, Trifiro M. Changing genetic paradigms: creating next-generation genetic databases as tools to understand the emerging complexities of genotype/phenotype relationships. Hum Genomics. 2014;8 : 9. doi: 10.1186/1479-7364-8-9 24885908
9. Fiore LD, Brophy MT, Turek S, Kudesia V, Ramnath N, Shannon C, et al. The VA Point-of-Care Precision Oncology Program: balancing access with rapid learning in molecular cancer medicine. Biomark Cancer. 2016;8 : 9–16. doi: 10.4137/BIC.S37548 26949343
10. Midgley RS, McConkey CC, Johnstone EC, Dunn JA, Smith JL, Grumett SA, et al. Phase III randomized trial assessing rofecoxib in the adjuvant setting of colorectal cancer: final results of the VICTOR trial. J Clin Oncol. 2010;28(30):4575–80. doi: 10.1200/JCO.2010.29.6244 20837956
11. Shaw CD. Principles for best practice in clinical audit. Int J Qual Health Care. 2003;15(1):87.
12. Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101(4):715–21. doi: 10.1038/sj.bjc.6605177 19603018
13. Dienstmann R, Dong F, Borger D, Dias-Santagata D, Ellisen LW, Le LP, et al. Standardized decision support in next generation sequencing reports of somatic cancer variants. Mol Oncol. 2014;8(5):859–73. doi: 10.1016/j.molonc.2014.03.021 24768039
14. Wagle N, Berger MF, Davis MJ, Blumenstiel B, Defelice M, Pochanard P, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2(1):82–93. doi: 10.1158/2159-8290.CD-11-0184 22585170
15. Dubbink HJ, Deans ZC, Tops BB, van Kemenade FJ, Koljenovic S, van Krieken HJ, et al. Next generation diagnostic molecular pathology: critical appraisal of quality assurance in Europe. Mol Oncol. 2014;8(4):830–9. doi: 10.1016/j.molonc.2014.03.004 24704265
16. Wong DW-S, Leung EL-H, So KK-T, Tam IY-S, Sihoe AD-L, Cheng L-C, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723–33. doi: 10.1002/cncr.24181 19170230
17. National Institute for Health and Care Excellence. Gefitinib for the first-line treatment of locally advanced or metastatic non-small-cell lung cancer. Technology appraisal guidance 192. London: National Institute for Health and Care Excellence; 2010. Available from: https://www.nice.org.uk/guidance/ta192.
18. Gupta A, Love S, Schuh A, Shanyinde M, Larkin JM, Plummer R, et al. DOC-MEK: a double-blind randomized phase II trial of docetaxel with or without selumetinib in wild-type BRAF advanced melanoma. Ann Oncol. 2014;25(5):968–74. doi: 10.1093/annonc/mdu054 24567366
19. Ciruelos Gil EM. Targeting the PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer. Cancer Treat Rev. 2014;40(7):862–71. doi: 10.1016/j.ctrv.2014.03.004 24774538
20. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–2.
21. National Institute for Health and Care Excellence. EGFR-TK mutation testing in adults with locally advanced or metastatic non-small-cell lung cancer. Diagnostics guidance 9. London: National Institute for Health and Care Excellence; 2013. Available from: https://www.nice.org.uk/guidance/dg9.
22. Kancha RK, Peschel C, Duyster J. The epidermal growth factor receptor-L861Q mutation increases kinase activity without leading to enhanced sensitivity toward epidermal growth factor receptor kinase inhibitors. J Thorac Oncol. 2011;6(2):387–92. doi: 10.1097/JTO.0b013e3182021f3e 21252719
23. Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16. doi: 10.1056/NEJMoa1103782 21639808
24. Middleton M, Rasco DW, Olszanski AJ, Corrie P, Lorigan P, Plummer R, et al. First-in-human phase 1 study of MLN2480, an investigational oral pan-RAF kinase inhibitor, in patients (pts) with relapsed or refractory solid tumors, including BRAF/NRAS-mutant melanoma. Eur J Cancer. 2014;50(Suppl 6):117.
25. Lang N, Weisser A, Enk A, Hassel JC. Vemurafenib therapy for stage IV melanoma with V600G-mutation. J Dtsch Dermatol Ges. 2013;11(12):1198–9. doi: 10.1111/ddg.12200 24016000
26. McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–32. doi: 10.1016/S1470-2045(14)70012-9 24508103
27. Klein O, Clements A, Menzies AM, O’Toole S, Kefford RF, Long GV. BRAF inhibitor activity in V600R metastatic melanoma. Eur J Cancer. 2013;49(5):1073–9. doi: 10.1016/j.ejca.2012.11.004 23237741
28. Dayer LE, Hutchins LF, Johnson JT. Treatment of metastatic melanoma with pazopanib: a report of five patient cases. J Oncol Pharm Pract. 2015;21(3):224–31. doi: 10.1177/1078155214524084 24576945
29. Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs. 2012;30(5):2008–14. doi: 10.1007/s10637-011-9763-9 22068222
30. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X 22285168
31. Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42. doi: 10.1016/S1470-2045(11)70184-X 21783417
32. Fukuoka M, Wu Y-L, Thongprasert S, Sunpaweravong P, Leong S-S, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non–small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–74. doi: 10.1200/JCO.2010.33.4235 21670455
33. Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073 15737014
34. Chen Z, Feng J, Saldivar JS, Gu D, Bockholt A, Sommer SS. EGFR somatic doublets in lung cancer are frequent and generally arise from a pair of driver mutations uncommonly seen as singlet mutations: one-third of doublets occur at five pairs of amino acids. Oncogene. 2008;27(31):4336–43. doi: 10.1038/onc.2008.71 18372921
35. Han SW, Kim TY, Hwang PG, Jeong S, Kim J, Choi IS, et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2005;23(11):2493–501. doi: 10.1200/JCO.2005.01.388 15710947
36. Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2(11):e313. doi: 10.1371/journal.pmed.0020313 16187797
37. Avizienyte E, Ward RA, Garner AP. Comparison of the EGFR resistance mutation profiles generated by EGFR-targeted tyrosine kinase inhibitors and the impact of drug combinations. Biochem J. 2008;415(2):197–206. doi: 10.1042/BJ20080728 18588508
38. Watanabe S, Minegishi Y, Yoshizawa H, Maemondo M, Inoue A, Sugawara S, et al. Effectiveness of gefitinib against non-small-cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol. 2014;9(2):189–94. doi: 10.1097/JTO.0000000000000048 24419415
39. Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17. doi: 10.1056/NEJMoa0805019 19339720
40. Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–71. doi: 10.1200/JCO.2008.20.8397 19114683
41. Stintzing S, Jung A, Rossius L, Modest D, von Weikersthal LF, Decker T, et al. Analysis of KRAS/NRAS and BRAF mutations in FIRE-3: a randomized phase III study of FOLFIRI plus cetuximab or bevacizumab as first-line treatment for wild-type (WT) KRAS (exon 2) metastatic colorectal cancer (mCRC) patients. Eur J Cancer. 2013;49(Suppl 3):Abstract 17.
42. Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24(29):4764–74. doi: 10.1200/JCO.2006.06.2265 16954519
43. Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26(33):5352–9. doi: 10.1200/JCO.2007.15.7461 18955458
44. Sato N, Saga Y, Mizukami H, Wang D, Fujiwara H, Takei Y, et al. Cetuximab inhibits the growth of mucinous ovarian carcinoma tumor cells lacking KRAS gene mutations. Oncol Rep. 2012;27(5):1336–40. doi: 10.3892/or.2012.1626 22246397
45. Casali PG, Blay JY, Bertuzzi A, Bielack S, Bjerkehagen B, Bonvalot S, et al. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 : 21–6.
46. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–61. doi: 10.1158/2159-8290.CD-14-0337 24893891
47. Ponti G, Tomasi A, Pellacani G. Overwhelming response to Dabrafenib in a patient with double BRAF mutation (V600E; V600M) metastatic malignant melanoma. J Hematol Oncol. 2012;5 : 60. doi: 10.1186/1756-8722-5-60 23031422
48. Meador CB, Micheel CM, Levy MA, Lovly CM, Horn L, Warner JL, et al. Beyond histology: translating tumor genotypes into clinically effective targeted therapies. Clin Cancer Res. 2014;20(9):2264–75. doi: 10.1158/1078-0432.CCR-13-1591 24599935
49. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. 3798106
50. FOCUS4. Welcome to FOCUS4. London: Medical Research Council Clinical Trials Unit; 2015 [cited 10 Jan 2017]. Available from: http://www.focus4trial.org.
51. Torjesen I. Large personalised medicine trial in lung cancer heralds new research partnership. BMJ. 2014;348:g2837. doi: 10.1136/bmj.g2837 24742663
52. Technology Strategy Board. Stratified medicines programme: tumour profiling and data capture to improve cancer care. Competition for collaborative R&D funding. London: Technology Strategy Board; 2011 Jan [cited 10 Jan 2017]. Available from: http://webarchive.nationalarchives.gov.uk/20130221185318/www.innovateuk.org/_assets/pdf/competition-documents/briefs/tsb_stratmedtumourprofilingcompetition.pdf.
53. Lennerz JK, McLaughlin HM, Baron JM, Rasmussen D, Sumbada Shin M, Berners-Lee N, et al. Health care infrastructure for financially sustainable clinical genomics. J Mol Diagn. 2016;18(5):697–706. doi: 10.1016/j.jmoldx.2016.04.003 27471182
54. Gallego CJ, Shirts BH, Bennette CS, Guzauskas G, Amendola LM, Horike-Pyne M, et al. Next-generation sequencing panels for the diagnosis of colorectal cancer and polyposis syndromes: a cost-effectiveness analysis. J Clin Oncol. 2015;33(18):2084–91. doi: 10.1200/JCO.2014.59.3665 25940718
55. Yorczyk A, Robinson LS, Ross TS. Use of panel tests in place of single gene tests in the cancer genetics clinic. Clin Genet. 2015;88(3):278–82. doi: 10.1111/cge.12488 25318351
56. Ghemlas I, Li H, Zlateska B, Klaassen R, Fernandez CV, Yanofsky RA, et al. Improving diagnostic precision, care and syndrome definitions using comprehensive next-generation sequencing for the inherited bone marrow failure syndromes. J Med Genet. 2015;52(9):575–84. doi: 10.1136/jmedgenet-2015-103270 26136524
57. Do H, Wong SQ, Li J, Dobrovic A. Reducing sequence artifacts in amplicon-based massively parallel sequencing of formalin-fixed paraffin-embedded DNA by enzymatic depletion of uracil-containing templates. Clin Chem. 2013;59(9):1376–83. doi: 10.1373/clinchem.2012.202390 23649127
58. Sankin A, Hakimi AA, Mikkilineni N, Ostrovnaya I, Silk MT, Liang Y, et al. The impact of genetic heterogeneity on biomarker development in kidney cancer assessed by multiregional sampling. Cancer Med. 2014;3(6):1485–92. doi: 10.1002/cam4.293 25124064
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 2- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Clinical Action against Drunk Driving
- Evidence for scaling up HIV treatment in sub-Saharan Africa: A call for incorporating health system constraints
- Performance and Cost-Effectiveness of Computed Tomography Lung Cancer Screening Scenarios in a Population-Based Setting: A Microsimulation Modeling Analysis in Ontario, Canada
- Clinical applicability and cost of a 46-gene panel for genomic analysis of solid tumours: Retrospective validation and prospective audit in the UK National Health Service
- Precision oncology: Charting a path forward to broader deployment of genomic profiling
- Taxes and Subsidies for Improving Diet and Population Health in Australia: A Cost-Effectiveness Modelling Study
- Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: A retrospective cohort study
- Haemolysis in G6PD Heterozygous Females Treated with Primaquine for Malaria: A Nested Cohort in a Trial of Radical Curative Regimens
- Housing Improvements and Malaria Risk in Sub-Saharan Africa: A Multi-Country Analysis of Survey Data
- Estimated impact on birth weight of scaling up intermittent preventive treatment of malaria in pregnancy given sulphadoxine-pyrimethamine resistance in Africa: A mathematical model
- Serotonin transporter gene polymorphism and susceptibility to a home-visiting maternal-infant attachment intervention delivered by community health workers in South Africa: Reanalysis of a randomized controlled trial
- Serelaxin as a potential treatment for renal dysfunction in cirrhosis: Preclinical evaluation and results of a randomized phase 2 trial
- Refining Lung Cancer Screening Criteria in the Era of Value-Based Medicine
- Renewing ’s editorial board in times of global distraction
- Hope for HIV control in southern Africa: The continued quest for a vaccine
- The potential benefit of scaling up malaria prevention to reduce low birth weight in Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Precision oncology: Charting a path forward to broader deployment of genomic profiling
- Taxes and Subsidies for Improving Diet and Population Health in Australia: A Cost-Effectiveness Modelling Study
- Refining Lung Cancer Screening Criteria in the Era of Value-Based Medicine
- Renewing ’s editorial board in times of global distraction
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



