-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV Treatment-As-Prevention Research: Taking the Right Road at the Crossroads
article has not abstract
Published in the journal: . PLoS Med 12(3): e32767. doi:10.1371/journal.pmed.1001800
Category: Formal Comment
doi: https://doi.org/10.1371/journal.pmed.1001800Summary
article has not abstract
Trials of Treatment-As-Prevention
Despite recent reductions in HIV incidence in several countries in sub-Saharan Africa, incidence still remains at unacceptably high levels [1]. Effective control of the epidemic requires more intensive prevention efforts. Two important approaches to address this goal are “combination prevention” [2], in which a number of partially effective interventions are combined to achieve a substantial reduction in HIV incidence, and “treatment-as-prevention” (TasP), offering antiretroviral therapy (ART) to all HIV-infected adults irrespective of CD4+ T-cell count to prevent onward transmission of HIV [3].
Mathematical models of the effects of TasP on population-level HIV incidence have produced a wide range of projections [4–7]. There remain many uncertainties in model assumptions and, critically, the impact of TasP at a population level depends on the coverage and uptake of HIV-testing, linkage to care, treatment initiation and adherence—the “cascade of care” [8]. Rigorous empirical studies are needed to determine whether TasP programmes can be implemented successfully in practice; measure these important process indicators; assess the balance between harms, costs, and benefits; and evaluate the impact of TasP on HIV incidence at population level.
Four large community trials are currently underway in South Africa [9,10], Zambia [10], Botswana [11], and Kenya and Uganda [12]. The trials are studying a range of intervention strategies with important differences in study design, but all four are measuring the impact of TasP on HIV incidence using a community-randomised design.
In June 2014, Till Bärnighausen and colleagues [13] presented their views on the implications of the 2013 change in WHO ART guidelines for the TasP studies. Their main conclusions were that as WHO guidelines are implemented [14], it will become unethical to continue the trials because the new guidelines cannot be withheld in control communities; that if the new guidelines are adopted in the control communities, the trials will no longer be adequately powered; and that alternative approaches such as pooling of data or adoption of stepped-wedge study designs should be considered.
We believe that the article by Bärnighausen and colleagues contains a number of inaccurate statements that compromise their conclusions. We discuss these issues in relation to the HPTN 071 (PopART) trial that our study team is carrying out in 21 communities in Zambia and South Africa to measure the impact of a combination prevention package, including universal HIV testing and treatment, on population-level HIV incidence [10]. The HPTN 071 (PopART) trial has three study arms (Fig. 1).
Fig. 1. Design of HPTN 071 (PopART) study showing the three study arms. 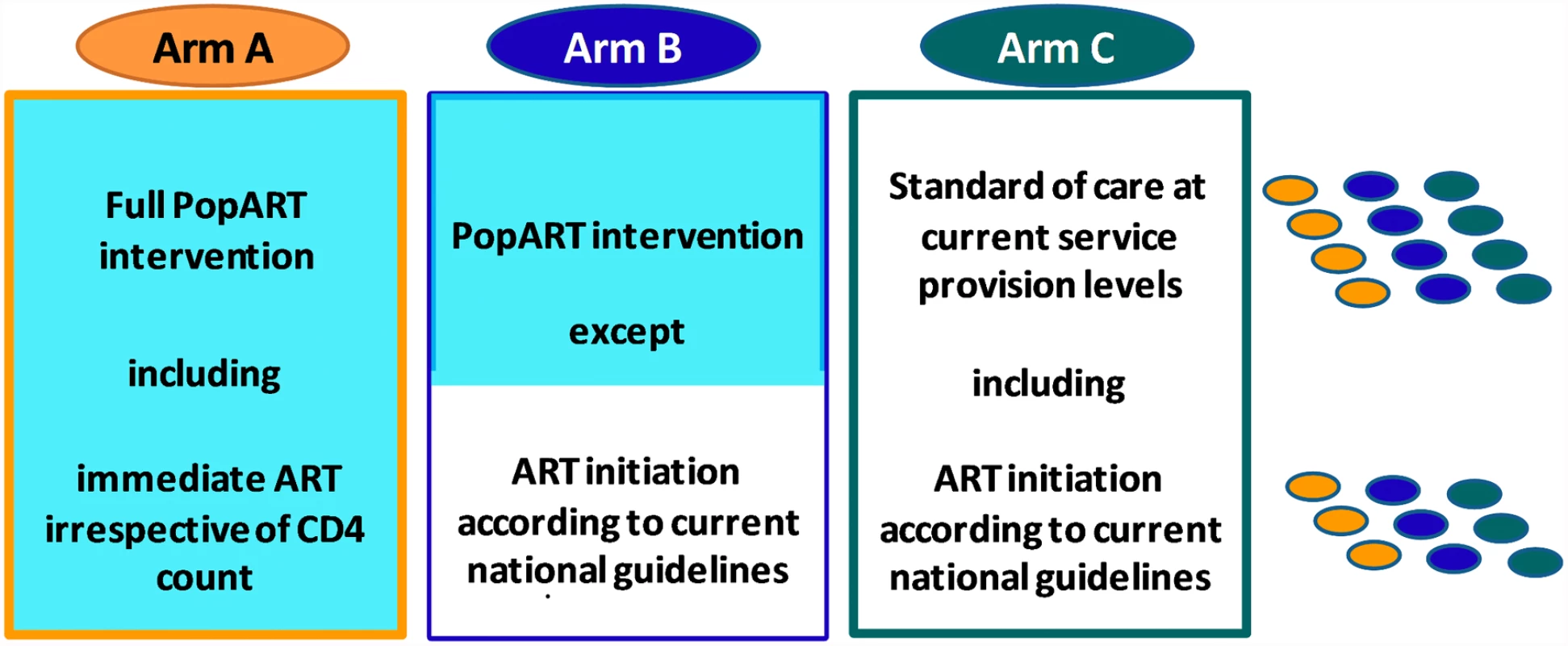
This is a three-arm cluster-randomised trial with 21 communities (n ≈ 1.2 million total population). The three matched triplets (three in South Africa, four in Zambia) are illustrated on the right of the diagram, with one community per study arm in each triplet. The communities in Arm A will receive the full “PopART” combination prevention programme, including the offer of immediate initiation of ART for all HIV-infected adults irrespective of CD4 count. The PopART package includes home-based provision of HIV testing and counselling, for all household members, referral of HIV-negative men for voluntary male medical circumcision, screening for symptoms of tuberculosis and sexually transmitted infections, and referral of all HIV-infected individuals to the local health facility with active linkage, follow-up, and ART adherence support. Arm B communities will receive the full PopART package except that ART will be provided according to the prevailing local treatment guidelines. Arm C communities will continue to receive current standard of care.
Ethics of Trial Continuation
Bärnighausen and colleagues imply that when the study countries adopt the 2013 WHO treatment guidelines, the TasP trial protocols would require the control communities to continue receiving ART according to previous guidelines, with ART initiated at a CD4+ count of <350 cells/mm3. However, this assumption does not apply to HPTN 071 (PopART). The HPTN 071 trial protocol [10] states that any changes in treatment guidelines during the course of the trial will be implemented in Arms B and C. Zambia has already adopted and is introducing the 2013 WHO guidelines into communities in Arms B and C, and South Africa is now following.
There is therefore no ethical concern with the continuation of the HPTN 071 trial with adoption of the new WHO guidelines.
Inadequate Study Power
Bärnighausen and colleagues state that if the control arms of the TasP trials do switch to providing ART according to the 2013 WHO guidelines, the studies will be underpowered for answering the primary study question, because of much smaller differences between the study arms.
With respect to HPTN 071 (PopART), this is incorrect. The impact of HIV treatment on transmission at a population level critically depends not only on the eligibility criteria for ART initiation but also on several other key variables, including the uptake and coverage of HIV testing, linkage to care, treatment initiation, and adherence. At each step in this cascade of care there are substantial challenges in achieving high coverage and, without effective measures to increase service uptake, the impact of any treatment programme (and of changes in ART eligibility) will be limited.
The design of HPTN 071 (PopART) was informed by the results of mathematical modelling of the projected impact of the interventions in Arms A and B under a range of assumptions about uptake and coverage of the various components of the intervention [10,15]. At the request of the study’s Data and Safety Monitoring Board (DSMB), the model was used to evaluate the effects on study power if the study countries were to adopt the 2013 WHO guidelines (S1 Text). In brief, the projections show only a small reduction in impact when Arm A is compared with Arm C. This is because the effects of the change in eligibility criteria in Arm C will have only a limited effect without substantial increases in uptake and coverage of testing and linkage. The study power for the Arm A versus C comparison, the main study comparison, remains very high. The difference between Arms B and C will increase following adoption of the new guidelines, leading to higher power for this comparison. This is because more patients will be eligible for treatment under the 2013 guidelines, but uptake and coverage will be higher in Arm B due to the PopART home-based services. Finally, the difference between Arms A and B will decrease. Both of these study arms benefit from the increased uptake and coverage achieved by the PopART home-based services. Following adoption of the 2013 guidelines, there will be a smaller number of HIV-infected individuals offered treatment in Arm A who would not be eligible for treatment if in Arm B communities, reducing the power to demonstrate a difference between Arms A and B.
Importance of Implementation Science to Determine Effectiveness and Inform Policy on TasP
We agree with Bärnighausen and colleagues that an important objective of the TasP trials, in addition to measuring effects on HIV incidence, is to provide useful data on the implementation of TasP to guide future policy and practice. TasP will only reach its potential effect on HIV transmission if the uptake and coverage of HIV services is substantially expanded. Changing treatment guidelines alone is not sufficient to assure the population level benefits of TasP. For optimal impact, a large proportion of the population needs to know their HIV status (through regular testing and re-testing), with effective linkage to appropriate treatment and care. We therefore prefer the term “Universal Testing and Treatment” (UTT), which emphasises the importance of the entire cascade of care, and not just treatment provision [16]. There remains an urgent need for implementation science to provide information on how such HIV services can most effectively be delivered in resource-poor settings, if TasP is found to be effective in reducing population level HIV incidence.
HPTN 071 (PopART) will provide valuable data on a wide range of process indicators. Social science research will investigate the acceptability of the intervention to local communities, and case-control studies will explore factors related to uptake of the different steps of the cascade with detailed costing exercises to determine overall cost-effectiveness. It cannot be assumed that TasP carries no risks; the study will also measure behavioural risk disinhibition, ART toxicity, stigma, and drug resistance and balance these against effects on HIV incidence.
Alternative Approaches
In their article, Bärnighausen and colleagues propose two alternative approaches to take forward the evaluation of TasP interventions.
They suggest first that it may be possible to pool data from the TasP trials to gain a more reliable overall measure of impact. This will be difficult, in practice, because of the substantial differences in the interventions being tested and the study designs, as well as differences in HIV transmission dynamics in different populations. We agree, however, that it will be important to bring together the data from the four studies in careful analyses, supported by mathematical modelling, to learn what we can from the findings. The investigators of the TasP trials have resolved to collaborate closely, facilitating future joint analyses.
Their second proposal is that if the trials cannot proceed to planned completion, it may be possible for the researchers to work with Ministries of Health to agree on a phased introduction of the new treatment guidelines according to a randomised stepped-wedge design. While this is an interesting proposal, it is unlikely that countries would be willing to delay initiation of new guidelines long enough for such a strategy to be feasible.
Conclusions
In summary, we believe that the conclusions of Bärnighausen and colleagues are based on misunderstandings about the design of the HPTN 071 (PopART) trial. Because this trial is committed to providing care and treatment in Arms B and C according to the prevailing national guidelines, there are no ethical concerns with the continuation of the trial. We also show that the study, with its three-arm design, will remain highly powered for its main comparisons even with adoption of the 2013 WHO guidelines in the study countries. We do not consider the implications for other ongoing TasP trials in this article, but there may be value in exploring the effects of changing guidelines on the power of those studies. Ultimately, there is an urgent need to demonstrate effectiveness of the UTT approach at a population level and to rigorously evaluate how best to safely and effectively deliver such an approach, which can then inform international policy decisions.
Supporting Information
Zdroje
1. UNAIDS Global AIDS response Progress report (2014) http://www.unaids.org/en/media/unaids/contentassets/documents/document/2014/GARPR_2014_guidelines_en.pdf. Accessed 17 July 2014.
2. Jones A, Cremin I, Abdullah F, Idoko J, Cherutich P, Kilonzo N, Rees H, Hallett T, O'Reilly K, Koechlin F, Schwartlander B, de Zalduondo B, Kim S, Jay J, Huh J, Piot P, Dybul M. Transformation of HIV from pandemic to low-endemic levels: a public health approach to combination prevention. Lancet 384 : 272–279. 2014 Apr 11. doi: 10.1016/S0140-6736(13)62230-8 24740087
3. McNairy ML, Cohen M, El-Sadr WM. Antiretroviral therapy for prevention is a combination strategy. Curr HIV/AIDS Rep. 2013 Jun;10(2):152–8. doi: 10.1007/s11904-013-0152-1 23371351
4. Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9 19038438
5. Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by 'test and treat' in hyperendemic settings. AIDS. 2010 Mar 13;24(5):729–35. doi: 10.1097/QAD.0b013e32833433fe 20154580
6. Celum C, Hallett TB, Baeten JM. HIV-1 prevention with ART and PrEP: mathematical modeling insights into resistance, effectiveness, and public health impact. J Infect Dis. 2013 Jul 15;208(2):189–91. doi: 10.1093/infdis/jit154 23570851
7. Cremin I, Alsallaq R, Dybul M, Piot P, Garnett G, Hallett TB. The new role of antiretrovirals in combination HIV prevention: a mathematical modelling analysis. AIDS. 2013 Jan 28;27(3):447–58 doi: 10.1097/QAD.0b013e32835ca2dd 23296196
8. Gardner EM, Young B. The HIV care cascade through time. Lancet Infect Dis. 2014 Jan;14(1):5–6. doi: 10.1016/S1473-3099(13)70272-X 24076276
9. Iwuji CC, Orne-Gliemann J, Tanser F, Boyer S, Lessells RJ, Lert F, Imrie J, Bärnighausen T, Rekacewicz C, Bazin B, Newell ML, Dabis F; ANRS 12249 TasP Study Group. Evaluation of the impact of immediate versus WHO recommendations-guided antiretroviral therapy initiation on HIV incidence: the ANRS 12249 TasP (Treatment as Prevention) trial in Hlabisa sub-district, KwaZulu-Natal, South Africa: study protocol for a cluster randomised controlled trial. Trials. 2013 Jul 23;14 : 230. doi: 10.1186/1745-6215-14-230 23880306
10. Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, Shanaube K, Bock P, Griffith S, Moore A, Watson-Jones D, Fraser C, Vermund SH, Fidler S; HPTN 071 (PopART) Study Team. HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials. 2014 Feb 13;15 : 57. doi: 10.1186/1745-6215-15-57 24524229
11. Botswana Combination Prevention Project (BCPP). http://clinicaltrials.gov/show/NCT01965470. Accessed 17 July 2014.
12. Sustainable East Africa Research in Community Health (SEARCH). http://clinicaltrials.gov/show/NCT01864603. Accessed 17 July 2014.
13. Bärnighausen T, Eyal N, Wikler D. HIV treatment-as-prevention research at a crossroads. PLoS Med. 2014 Jun 3;11(6):e1001654. doi: 10.1371/journal.pmed.1001654 24892694
14. WHO (2014) Developing the 2013 WHOconsolidated antiretroviral guidelines. http://www.who.int/hiv/pub/journal_articles/arv2013-supplement-june2014/en/. Accessed 17 July 2014.
15. Cori A, Ayles H, Beyers N, Schaap A, Floyd S, Sabapathy K, Eaton JW, Hauck K, Smith P, Griffith S, Moore A, Donnell D, Vermund SH, Fidler S, Hayes R, Fraser C; HPTN 071 PopART Study Team. HPTN 071 (PopART): a cluster-randomized trial of the population impact of an HIV combination prevention intervention including universal testing and treatment: mathematical model. PLoS ONE. 2014 Jan 15;9(1):e84511. doi: 10.1371/journal.pone.0084511 24454728
16. McNairy ML, El-Sadr WM. The HIV Care Continuum: No Partial Credit Given. AIDS. 2012 Sep 10;26(14):1735–8 doi: 10.1097/QAD.0b013e328355d67b 22614888
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Testing and Treating the Missing Millions with Tuberculosis
- UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age
- Association between Traffic-Related Air Pollution in Schools and Cognitive Development in Primary School Children: A Prospective Cohort Study
- Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial
- Strengthening the Detection of and Early Response to Public Health Emergencies: Lessons from the West African Ebola Epidemic
- HIV Treatment-As-Prevention Research: Authors’ Reply
- Role of Acute HIV Infection in Driving HIV Transmission: Implications for HIV Treatment as Prevention
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- HIV Treatment-As-Prevention Research: Taking the Right Road at the Crossroads
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
- A Public Health Approach to Hepatitis C Control in Low- and Middle-Income Countries
- Development and Validation of a Risk Score for Chronic Kidney Disease in HIV Infection Using Prospective Cohort Data from the D:A:D Study
- Reassessment of HIV-1 Acute Phase Infectivity: Accounting for Heterogeneity and Study Design with Simulated Cohorts
- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



