-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV Treatment-As-Prevention Research: Authors’ Reply
article has not abstract
Published in the journal: . PLoS Med 12(3): e32767. doi:10.1371/journal.pmed.1001799
Category: Formal Comment
doi: https://doi.org/10.1371/journal.pmed.1001799Summary
article has not abstract
We thank Richard Hayes and colleagues for their commentary [1] on our PLOS Medicine article [2]. We share the desire to learn through rigorous research whether HIV treatment-as-prevention (TasP) works in population-wide implementation. Our article pointed out that the 2013 WHO antiretroviral treatment (ART) guidelines put the on-going TasP trials at risk of failing “in their primary aim to establish the effectiveness of TasP in general populations in sub-Saharan Africa because of insufficient power” [2]. The 2013 WHO guidelines recommend substantially expanded ART eligibility. The host countries of the TasP trials are adopting the guidelines as national policy, and currently accepted ethical standards for clinical trials oblige the TasP trials to do likewise for patients enrolled in all trial arms. The problem is that the trials were designed and originally powered under the more restricted, previous ART eligibility standards and may thus become insufficiently powered to test the TasP hypothesis [2].
Hayes and colleagues assert that the HPTN071 (PopART) trial does not face this risk because “[t]he study power for the Arm A versus C comparison, the main study comparison, remains very high” when all PopART arms offer ART under the expanded eligibility standards [1]. But this comparison of Arms A versus C does not test the TasP hypothesis. Rather, it tests the very different hypothesis that an extensive HIV combination prevention package can reduce HIV incidence.
To explain, TasP aims to achieve substantial HIV incidence reductions through immediate ART initiation in all HIV-infected individuals [3–5]. In contrast, HIV combination prevention packages aim to achieve the same goal through implementation of many different interventions believed to be effective in preventing HIV [6–8]. PopART is to test both strategies. Our concern regards only the former; i.e., its test of TasP.
The PopART protocol published in Trials [9] in 2014 makes clear why PopART faces the risk of failing to test the TasP hypothesis because of insufficient power, just like the other trials. PopART was designed and powered “to detect a difference in incidence between Arms A and C (reflecting the full impact of the intervention), as well as the difference in intervention effect between Arms A and B (reflecting the additional effect of immediate HIV treatment compared with current national guidelines)” [9].
The additional effect of immediate HIV treatment (Arm A versus B) is the effect of TasP, namely, offering immediate HIV treatment when the “uptake and coverage of [other] HIV services is substantially expanded” [1]. Regarding this test, Hayes and colleagues write that “[f]ollowing adoption of 2013 guidelines, there will be a smaller number of HIV-infected individuals offered treatment in Arm A who would not be eligible for treatment if in Arm B communities, reducing the power to demonstrate a difference between Arms A and B” [1]. It is precisely this comparison that tests the TasP hypothesis in PopART and that is now threatened to fail because of insufficient power (Table 1). If WHO follows the United States [10,11] in recommending immediate ART initiation for all HIV-infected people in the 2015 WHO ART guidelines, PopART will face the even larger threat of completely losing the Arm A versus B comparison [12].
Tab. 1. The two interventions the HPTN071 (PopART) trial aims to test. 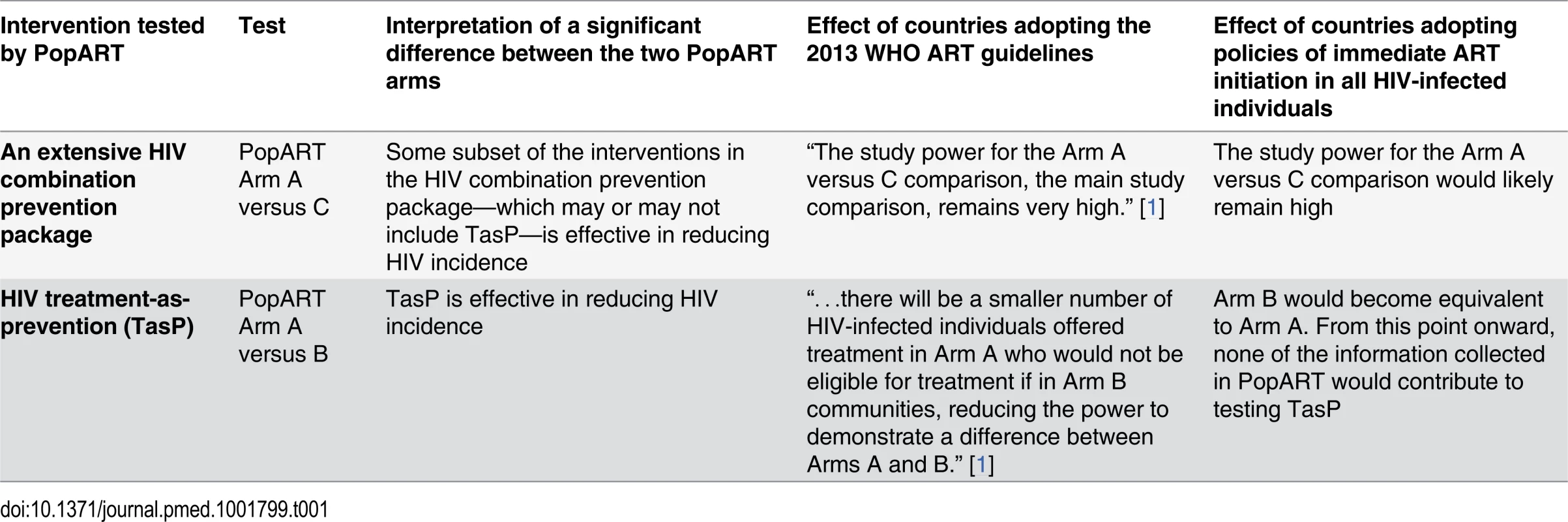
In contrast to the Arm A versus B comparison, the difference between Arms A and C in PopART is the effect of an extensive HIV combination prevention package (“the full intervention” [9]), which includes immediate HIV treatment but also many other interventions: male circumcision, condom promotion, home-based behavioural HIV risk-reduction counselling, home-based HIV testing and referral to HIV treatment and care, home-based screening for sexually transmitted infections and referral for treatment, home-based screening for tuberculosis and referral for treatment, home-based identification of pregnant women and encouragement to attend antenatal care, and encouragement to access prevention of mother-to-child transmission services for pregnant women who test HIV-positive [9].
If the PopART combination prevention package is shown to be effective, any subset of interventions in the package—which may or may not include TasP—could be responsible for the effect, and we cannot know which. Importantly, based on this comparison it is impossible to rule out that any significant effect is due entirely to those interventions in the package that have already been firmly established to be effective in preventing HIV, such as male circumcision [13–15]. The comparison of PopART Arm A versus C is thus not a valid test of the TasP hypothesis (Table 1).
Given our shared interest in testing TasP, we are glad to read that Hayes and colleagues broadly endorse two of our proposals: to increase the power to test the TasP hypothesis by pooling data across trials and to consider randomised stepped-wedge scale-up of TasP as an additional strategy to establish TasP effectiveness.
Zdroje
1. Hayes R, Fidler S, Cori A, Fraser C, Floyd S, et al. (2015) HIV treatment-as-prevention research: taking the right road at the crossroads. PLoS Med 12(3): e1001800. doi: 10.1371/journal.pmed.1001800
2. Bärnighausen T, Eyal N, Wikler D (2014) HIV treatment-as-prevention research at a crossroads. PLoS Med 11: e1001654. doi: 10.1371/journal.pmed.1001654 24892694
3. The HIV Modelling Consortium Treatment as Prevention Editorial Writing Group (2012) HIV treatment as prevention: models, data, and questions—towards evidence-based decision-making. PLoS Med 9 e1001259. doi: 10.1371/journal.pmed.1001259 22802739
4. Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG (2009) Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373 : 48–57. doi: 10.1016/S0140-6736(08)61697-9 19038438
5. Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, et al. (2012) HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 9: e1001245. doi: 10.1371/journal.pmed.1001245 22802730
6. Vermund SH, Fidler SJ, Ayles H, Beyers N, Hayes RJ (2013) Can combination prevention strategies reduce HIV transmission in generalized epidemic settings in Africa? The HPTN 071 (PopART) study plan in South Africa and Zambia. J Acquir Immune Defic Syndr 63 Suppl 2: S221–227. doi: 10.1097/QAI.0b013e318299c3f4 23764639
7. Kurth AE, Celum C, Baeten JM, Vermund SH, Wasserheit JN (2011) Combination HIV prevention: significance, challenges, and opportunities. Curr HIV/AIDS Rep 8 : 62–72. doi: 10.1007/s11904-010-0063-3 20941553
8. Merson M, Padian N, Coates TJ, Gupta GR, Bertozzi SM, et al. (2008) Combination HIV prevention. Lancet 372 : 1805–1806. doi: 10.1016/S0140-6736(08)61752-3 19027478
9. Hayes R, Ayles H, Beyers N, Sabapathy K, Floyd S, et al. (2014) HPTN 071 (PopART): rationale and design of a cluster-randomised trial of the population impact of an HIV combination prevention intervention including universal testing and treatment—a study protocol for a cluster randomised trial. Trials 15 : 57. doi: 10.1186/1745-6215-15-57 24524229
10. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents (2013) Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
11. Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, et al. (2012) Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA 308 : 387–402. doi: 10.1001/jama.2012.7961 22820792
12. WHO (2014) Systematic reviews to support the antiretroviral therapy (ART) consolidated guidelines Geneva: WHO.
13. Bailey RC, Moses S, Parker CB, Agot K, Maclean I, et al. (2007) Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 369 : 643–656. 17321310
14. Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, et al. (2007) Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369 : 657–666. 17321311
15. Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, et al. (2005) Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med 2: e298. 16231970
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Testing and Treating the Missing Millions with Tuberculosis
- UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age
- Association between Traffic-Related Air Pollution in Schools and Cognitive Development in Primary School Children: A Prospective Cohort Study
- Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial
- Strengthening the Detection of and Early Response to Public Health Emergencies: Lessons from the West African Ebola Epidemic
- HIV Treatment-As-Prevention Research: Authors’ Reply
- Role of Acute HIV Infection in Driving HIV Transmission: Implications for HIV Treatment as Prevention
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- HIV Treatment-As-Prevention Research: Taking the Right Road at the Crossroads
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
- A Public Health Approach to Hepatitis C Control in Low- and Middle-Income Countries
- Development and Validation of a Risk Score for Chronic Kidney Disease in HIV Infection Using Prospective Cohort Data from the D:A:D Study
- Reassessment of HIV-1 Acute Phase Infectivity: Accounting for Heterogeneity and Study Design with Simulated Cohorts
- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- CD47 Agonist Peptides Induce Programmed Cell Death in Refractory Chronic Lymphocytic Leukemia B Cells via PLCγ1 Activation: Evidence from Mice and Humans
- Paying Physicians to Prescribe Generic Drugs and Follow-On Biologics in the United States
- Ultra-Sensitive Detection of by Amplification of Multi-Copy Subtelomeric Targets
- Sugar Industry Influence on the Scientific Agenda of the National Institute of Dental Research’s 1971 National Caries Program: A Historical Analysis of Internal Documents
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



