-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDiversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled
Esteban Gonzalez Burchard and colleagues explore how making medical research more diverse would aid not only social justice but scientific quality and clinical effectiveness, too.
Published in the journal: . PLoS Med 12(12): e32767. doi:10.1371/journal.pmed.1001918
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001918Summary
Esteban Gonzalez Burchard and colleagues explore how making medical research more diverse would aid not only social justice but scientific quality and clinical effectiveness, too.
Summary Points
Health disparities persist across race/ethnicity for the majority of Healthy People 2010 health indicators.
Most physicians and scientists are informed by research extrapolated from a largely homogenous population, usually white and male.
A growing proportion of Americans are not fully benefiting from clinical and biomedical advances since racial and ethnic minorities make up nearly 40% of the United States population.
Ignoring the racial/ethnic diversity of the US population is a missed scientific opportunity to fully understand the factors that lead to disease or health.
US biomedical research and study populations must better reflect the country’s changing demographics. Adequate representation of diverse populations in scientific research is imperative as a matter of social justice, economics, and science.
In 1993, the National Institutes of Health (NIH) Revitalization Act was passed by United States Congress and signed into law by President Clinton. The Act called for the NIH to require that all federally funded clinical research prioritize the inclusion of women and minorities and that research participant characteristics be disclosed in research documentation [1]. When pivotal NIH-funded studies included large proportions of women by design, they made important, clinically relevant scientific contributions by identifying sex-specific differences in symptoms, pathologies, and treatment response [2–4]. In continuation of this effort, the NIH announced new measures to enhance gender equity [5]. Herein, we evaluate the impact of the Revitalization Act’s other stated aim: diversifying study populations by race/ethnicity. We also make suggestions on what we believe will bolster the Revitalization Act’s effect in shaping clinical and biomedical research and thereby provide guidance for President Obama’s new Precision Medicine Initiative (PMI) [6].
Disease Pattern, Clinical Presentation, and Therapeutic Response Can Vary Dramatically by Race/Ethnicity and Ancestral Background
Race is a social construct rooted in cultural identity and shaped by historic and current events, which influence an individual’s behavior and place of residence. Genetic variation correlates with self-identified race [7], and this genetic variation also correlates with clinical presentation and therapeutic response. Thus, while not every study needs to examine racial differences or include all racial/ethnic groups, we feel that the group(s) included should be representative of their larger population(s) such that including an adequate proportion of racially/ethnically diverse groups in clinical and biomedical research can provide meaningful opportunities to examine the complex relationship of ancestral influences, environmental exposures, and social factors. In turn, understanding the interaction between the social and environmental milieu with an individual’s genomic profile and genetic ancestry can extend our understanding of disease pathology and expand therapeutic options for everyone [8]. For example, up to 75% of Pacific Islanders are unable to convert the antiplatelet drug clopidogrel into its active form and are at higher risk for adverse outcomes following angioplasty [9,10]. Other examples are listed in Table 1.
Tab. 1. Insights from studies conducted in diverse race/ethnic groups. 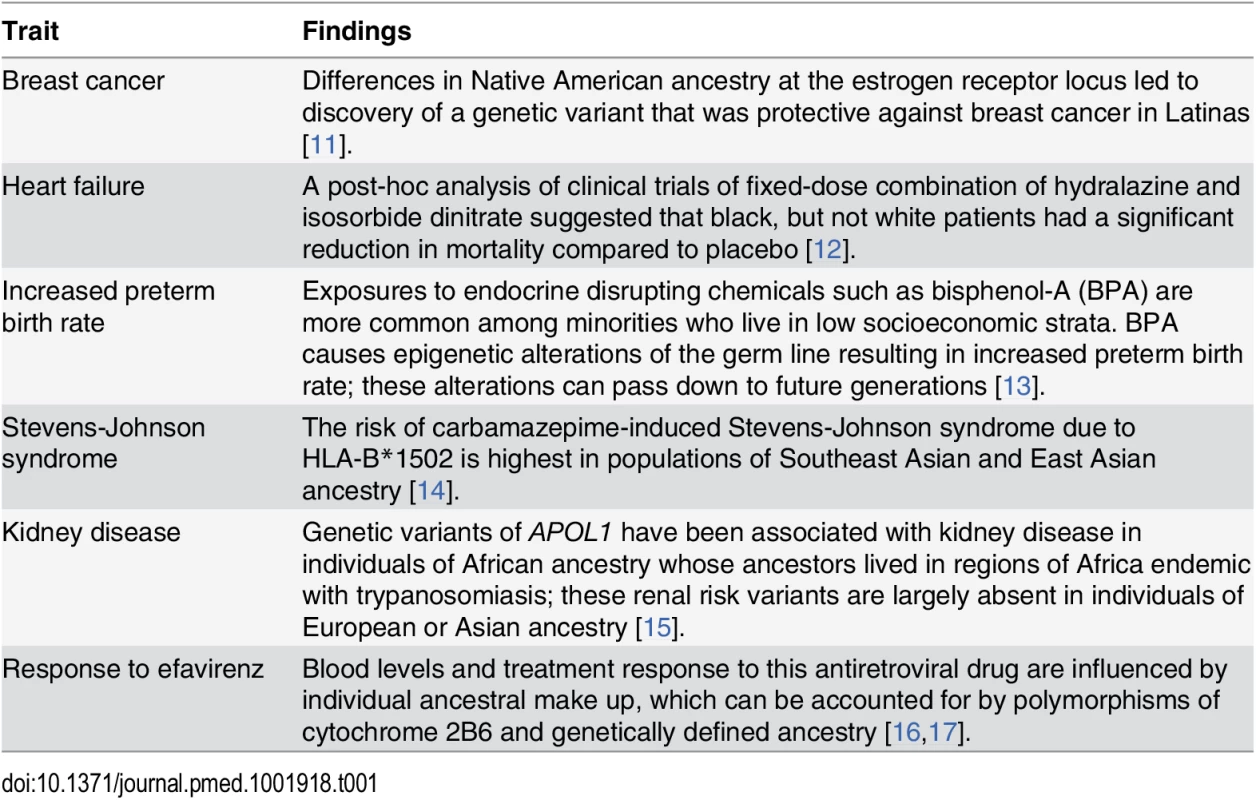
Past Research Has Under-Studied Minorities
The US has been regarded as a “global lead” and “exemplar” in biomedical and clinical health research since the end of the Cold War [18]. Yet, few US biomedical studies focus recruitment efforts on attaining adequate minority representation, nor do they focus their research attention to factors most relevant to minority health [19]. Since the passage of Revitalization Act in 1993, less than 2% of more than 10,000 cancer clinical trials funded by the National Cancer Institute included enough minority participants to meet the NIH’s own criteria and goals [20]. Moreover, less than 5% of NIH-funded respiratory research reported inclusion of racial/ethnic minorities [21]. Minority enrollment in cancer clinical trials remains inadequate despite striking racial/ethnic disparities in cancer incidence and mortality [22,23]. Similar incongruities between disease burden and representation in biomedical research exist for cardiovascular diseases and diabetes [24,25]. These disparities have economic consequences: eliminating racial/ethnic health disparities would have reduced total medical costs during 2003–2006 by more than $1.2 trillion [26]. Some NIH reviewers have argued that the inclusion of diverse groups will increase the financial costs of clinical and biomedical research. However, it is generally agreed upon that the long-term financial benefits outweigh short-term expenses [27]. The social, biomedical, and economic costs of inaction are ameliorated by a new appreciation for the clinical and biomedical benefits achieved through precision medicine when applied to all populations [6]. The proportion of taxpayers who have not gained optimal benefit from scientific discoveries they are funding continues to grow with the changing US demographics. Therefore, ensuring that diverse populations are adequately included in scientific research is imperative not only in terms of scientific integrity and fiduciary responsibility but also as a matter of social justice.
Barriers to Diversify Research Need Concerted Attention
While US minorities may be as willing to participate in health research as non-Hispanic whites [28], barriers to participation among minority populations must be addressed and will require buy-in from stakeholders: funders, academic institutions, investigators, and potential research participants [29]. Minority populations often have limited access to specialty care centers that serve as referral sources for clinical studies, resulting in a lack of an effective referral base [30]. Other barriers include, but are not limited to, fears of exploitation in medical research [31], financial constraints [32], competing demands of time, lack of access to information and comprehension about research, unique cultural and linguistic differences, fears of unintended outcomes, stigmatization, and health care discrimination [31].
Highly feasible changes can increase minority participation despite the challenges described. Ideally, investigators would reflect the communities being studied. Given the tremendous disparities in our biomedical workforce, we must seek out other realistic solutions. For example, some participants prefer studies that include research staff who share their same culture and with whom they can communicate in their own language [31]. Potential contributors are also more likely to partake when recruited by research staff they personally know or with whom they identify [33,34]. Town hall meetings and study newsletters can be adapted to the language and reading level requirements of target groups; these can describe how collected data will be used, ensuring transparency and allaying fears stemming from lack of information [29]. Challenges of transportation, childcare, work hour considerations, and meals can be addressed via payment, travel support, flexible recruitment hours and locations, provision of food during study visits, and positioning study sites in areas with diverse residents. To compensate for the limited internal referral base, tertiary care centers can partner with community health care providers. Targeted advertising (e.g., on public transportation) can reach potential participants at a moderate cost. Nonetheless, outreach and external partnerships introduce costs and effort that can raise recruitment budgets. The Revitalization Act specifically prohibits cost considerations from being a reason to exclude minorities, and NIH study sections are instructed to disregard budgetary requests in evaluating a project’s scientific merit. However, our experience in grant reviewing has been that in practice, the size of budgetary requests can bias reviewers. Grant applicants, in turn, react by submitting proposals with inadequate budgets to recruit minority participants so as not to “raise eyebrows” of reviewers.
Minorities would likely to be as willing to be involved in research as whites if problems of diversity could be better addressed. Some of these problems may stem from issues within the research community and its own profound diversity gap. Minority physicians and scientists are more likely to conduct research in minority populations and are often best suited to gain the trust of minority communities, but they are also significantly underrepresented in medical and scientific communities [35]. For example, blacks or African Americans and Hispanics, respectively, represented only 4.3% and 7.2% of doctorate degree awardees in biomedical sciences in 2013, although they represented 13.9% and 17.2% of the US population during the same period [36]. Moreover, less than 2% of NIH principal investigators on research project grants are black [37], a proportion much lower than in the general US population (10.2%) [38]. Similar disparities were observed for Latinos (3.4% versus 12.5%), American Indians and Alaska Natives (0.4% versus 0.7%), and Native Hawaiians and other Pacific Islanders (1.2% versus 10.2%).
To further complicate the picture, an NIH study of research grant awards found that the proportion of applications funded was 13% lower for blacks or African Americans and 4% lower for Asians than among whites [39]. According to demographic information provided by the NIH’s Office of Extramural Research under the Freedom of Information Act, the award rate for R01 or equivalent grants has been consistently lower among non-white applicants (Pacific Islander, Native Hawaiian, African American, American Indian, and Asian) than white applicants (42.1% versus 48.6% in 1985 and 19.3% versus 23.3% in 2013) [40].
Contributors to funding disparities arise throughout the research application review process [41]. The NIH has commented on reviewer bias [42], acknowledging that the probability of funding after peer review does not differ by race, but that minority investigators tend to receive lower priority scores from peer review, indicating that the review process is biased against applications from minority investigators. The relative absence of minority participants throughout the research application evaluation process may contribute to this problem, since underrepresented minorities comprised 10% of NIH study section reviewers in 2000 and only 10.9% in 2013 [40]. Increasing minority representation within the research community could in itself promote better science. Diverse research teams are more likely to have diverse ideas [43], which may explain why manuscripts authored by multi-ethnic research teams are more likely to be cited than publications authored by authors of the same ethnicity [44]. However, since study section members are drawn from the pool of successfully funded researchers, funding disparities have a self-perpetuating effect [45] and functionally eliminate scientists best suited to respond to the call to action we describe.
How Can the NIH (Re)catalyze Diversity in Research?
The Revitalization Act intended to re-catalyze diversity in biomedical research by increasing minority representation. President Obama’s Precision Medicine Initiative plans to enroll a cohort of 1 million or more Americans that will provide the platform for expanding our knowledge and benefit the nation for many years to come. It is time to heed the President’s call to action, given the changing US demographics. The NIH should be empowered to set and enforce recruitment of diverse research populations as the default and require scientific justification for limited or selected study population enrollment, as they have just created policies to do for sex balance [5]. Other US government agencies (e.g., Centers for Disease Control and Prevention, Food and Drug Administration, Agency for Healthcare Research and Quality, Patient-Centered Outcomes Research Institute, Department of Defense) should be similarly empowered. Recruitment approaches should be formally included as criteria for scientific merit scoring, rather than the current application of such criteria after scoring.
In this vein, the NIH should include race/ethnicity as a criterion for assigning priority scores to ensure that well-characterized cohorts and clinical trials not only answer questions relevant to the growing diversity of the US population but are also appropriately statistically powered. The same techniques for monitoring sex/gender inclusion [5] should be used to explicitly review minority accruals over the course of the award, and adjust funding levels accordingly. We believe this would prompt researchers of all racial/ethnic and cultural backgrounds to incorporate understudied populations in their research studies.
To their credit, the NIH is actively addressing many of the issues we have mentioned. Following President Obama’s PMI announcement during his 2015 State of the Union address, the NIH has actively solicited feedback [46] to help guide creation of a diverse research cohort of 1 million or more Americans [47]. The NIH has since hosted several workshops to develop a vision for building the national PMI cohort, and maximizing cohort diversity (across socioeconomic standing, geography, sexual orientation, education, and age, in addition to race/ethnicity) has been an ongoing topic at these workshops [48]. In particular, participant and public engagement, diversity and inclusion, and health disparities considerations for the development of a national research cohort were among the topics discussed at a workshop dedicated to participant engagement and health equity [49].
We applaud and encourage the NIH’s focus on diversifying the makeup of the forthcoming PMI cohort. To build on these efforts, an administrative supplement for currently funded research to investigate racial/ethnic differences in health and therapeutics should be created, similar to efforts by the Office of Research on Women’s Health to promote discovery of sex differences [50]. This supplement would be hypothesis-generating and show the NIH’s commitment to diversify study populations throughout all Institutes. The NIH should also incentivize collaboration amongst groups with similar approaches and data elements so that adequately powered analyses can examine racial/ethnic differences.
Applications from minority-serving institutions should be judged on their capacity to conduct research rather than relying on the institutions’ research track records. In our experience, applications from institutions with strong community ties are better equipped to enroll and retain subjects in clinical and biomedical research. The importance and novelty of studies focused primarily or solely on minority populations should be recognized for their validity and worth, as these may be the only studies to recruit sufficient minority participants to determine whether research findings can be generalized to these populations.
Given the systemic bias against minority scientists, the solution does not lie in simply increasing the number of competitive applicants. To this end, the NIH is actively funding investigations to understand and eliminate discrepancies for minority investigators in the peer review process [51]. In September 2014, the NIH announced [52] winners of two competitions on increasing the fairness and impartiality of the scientific review process and for novel methods of identifying bias. A program assessing the complete anonymization of grant applications is also being piloted [53]. These efforts are part of a larger campaign to identify and root out unconscious bias in peer review [41,51]. The NIH must act on these data to ensure a just and fair voice for all stakeholders.
NIH proposals passing scientific peer review are forwarded to a second level of review, conducted by Institute and Center (IC) National Advisory Councils or Boards (henceforth referred to as “Councils”). NIH Councils make funding decisions based on the priority score and the priorities of the IC, which have varying levels of discretionary funds. A reasonable way to fund meritorious applications that reflect the diversity priorities of the ICs is to use the discretion of the Councils. Other NIH efforts to increase support for the diversity pipeline (e.g., NIH’s Building Infrastructure Leading to Diversity (BUILD) Initiative [54]) and for diversity-related scientific initiatives are commendable, but in the absence of strong changes throughout the review process, research will continue to suffer.
Inclusive Research Needs the Support of the Entire Country
Efforts by the NIH and other agencies to address disparities in research priorities will have limited impact unless broader themes of political and economic inequality are addressed. The most important changes in our approach to science will only come when we consider inclusion and diversity important by default—not just in biomedical science, but in all aspects of society. Homogeneity in study populations will cease when racial/ethnic and socioeconomic diversity are considered socially desirable and social norms [55], be it in study populations, academic faculty, NIH study sections, or boardrooms and classrooms.
We have suggested a number of measures for the NIH to build upon the Revitalization Act. Despite the Act’s stipulation that cost not be used as justification for failure to enroll diverse populations, no discussion of new mandates for NIH-funded research can take place without addressing the crisis of declining inflation-adjusted NIH budgets. Society and patients will benefit when the NIH exercises the full scope of power provided under the 1993 Act: a call for the inclusion of historically under-represented communities in clinical research. The NIH alone will not be able to correct the disparities or inequities of the health care system, but it can send a powerful message that may promote changes in our health care and health science systems. There must be a collective will to prioritize diversifying our study populations, rallied by outreach to the lay community to educate voters who can exercise their franchise to their own best health care interests.
Fulfilling the promise of the Revitalization Act does not pit a future of precision medicine and the advancement of science against the realization of social justice for under-represented communities. Rather, the choice to study diverse populations is itself a promising path toward sound science. By reprioritizing our approach to clinical research and recruitment, we may accomplish an even greater goal: to usher in a new era of scientific discovery and health prosperity for all citizens of the world.
Zdroje
1. US Congress. National Institutes of Health Revitalization Act of 1993: Act to Amend the Public Health Service Act to Revise and Extend the Programs of the National Institutes of Health, and for Other Purposes. Public Law Washington, DC; 1993 pp. 103–143.
2. Canto JG, Goldberg RJ, Hand MM, Bonow RO, Sopko G, Pepine CJ, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Archives of internal medicine. 2007;167 : 2405–2413. doi: 10.1001/archinte.167.22.2405 18071161
3. Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44 : 499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453 14744256
4. Rathore SS, Wang Y, Krumholz HM. Sex-based differences in the effect of digoxin for the treatment of heart failure. N Engl J Med. 2002;347 : 1403–1411. doi: 10.1056/NEJMoa021266 12409542
5. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509 : 282–283. 24834516
6. Remarks by the President in State of the Union Address | January 20, 2015. 2015. https://www.whitehouse.gov/the-press-office/2015/01/20/remarks-president-state-union-address-january-20-2015
7. Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348 : 1170–1175. doi: 10.1056/NEJMsb025007 12646676
8. Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8 : 621–628. 9345660
9. Wu AH, White MJ, Oh SS, Burchard E. The Hawaii clopidogrel lawsuit: the possible effect on clinical laboratory testing. Per Med. 2015;12 : 179–181. doi: 10.2217/pme.15.4
10. Mega JL, Simon T, Collet J-P, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA: The Journal of the American Medical Association. 2010;304 : 1821–1830. doi: 10.1001/jama.2010.1543 20978260
11. Fejerman L, Ahmadiyeh N, Hu D, Huntsman S, Beckman KB, Caswell JL, et al. Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun. 2014;5 : 5260. doi: 10.1038/ncomms6260 25327703
12. Carson P, Ziesche S, Johnson G, Cohn JN. Racial differences in response to therapy for heart failure: analysis of the vasodilator-heart failure trials. Vasodilator-Heart Failure Trial Study Group. J Card Fail. 1999;5 : 178–187. 10496190
13. Ranjit N, Siefert K, Padmanabhan V. Bisphenol-A and disparities in birth outcomes: a review and directions for future research. J Perinatol. 2010;30 : 2–9. doi: 10.1038/jp.2009.90 19587689
14. Chung W-H, Hung S-I, Hong H-S, Hsih M-S, Yang L-C, Ho H-C, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428 : 486–486. doi: 10.1038/428486a 15057820
15. Genovese G, Friedman DJ, Ross MD, Lecordier L. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010 Aug 13;329(5993):841–5. doi: 10.1126/science.1193032 20647424
16. Gandhi M, Greenblatt RM, Bacchetti P, Jin C, Huang Y, Anastos K, et al. A single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. The Journal of infectious diseases. 2012;206 : 1453–1461. doi: 10.1093/infdis/jis508 22927450
17. Frasco MA, Mack WJ, Van Den Berg D, Aouizerat BE, Anastos K, Cohen M, et al. Underlying genetic structure impacts the association between CYP2B6 polymorphisms and response to efavirenz and nevirapine. AIDS. 2012;26 : 2097–2106. doi: 10.1097/QAD.0b013e3283593602 22951632
18. Bhopal RS. Migration, Ethnicity, Race, and Health in Multicultural Societies. 2nd ed. Oxford University Press; 2014.
19. Institute of Medicine (US) Committee on the Review and Assessment of the NIH’s Strategic Research Plan and Budget to Reduce and Ultimately Eliminate Health Disparities. Examining the Health Disparities Research Plan of the National Institutes of Health: Unfinished Business. Thomson GE, Mitchell F, Williams MB, editors. Washington (DC): National Academies Press (US); 2006.
20. Chen MS, Lara PN, Dang JHT, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120 Suppl 7 : 1091–1096. doi: 10.1002/cncr.28575 24643646
21. Burchard EG, Oh SS, Foreman MG, Celedón JC. Moving toward True Inclusion of Racial/Ethnic Minorities in Federally Funded Studies. A Key Step for Achieving Respiratory Health Equality in the United States. Am J Respir Crit Care Med. 2015;191 : 514–521. doi: 10.1164/rccm.201410-1944PP 25584658
22. American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014.
23. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA: The Journal of the American Medical Association. 2004;291 : 2720–2726. doi: 10.1001/jama.291.22.2720 15187053
24. Chow EA, Foster H, Gonzalez V, McIver L. The Disparate Impact of Diabetes on Racial/Ethnic Minority Populations. Clinical Diabetes. 2012;30 : 130–133. doi: 10.2337/diaclin.30.3.130
25. Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. American Medical Association; 2014;174 : 1868–1870. doi: 10.1001/jamainternmed.2014.4758 25264856
26. LaVeist TA, Gaskin D, Richard P. Estimating the economic burden of racial health inequalities in the United States. Int J Health Serv. 2011;41 : 231–238. doi: 10.2190/HS.41.2.c 21563622
27. National Prevention Council. National Prevention Strategy. Washington, DC: US Department of Health and Human Services, Office of the Surgeon General; 2011.
28. Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3: e19. doi: 10.1371/journal.pmed.0030019 16318411
29. Powe NR, Gary TL. Clinical Trials. In: Beech BM, Goodman M, editors. Race & Research: Perspectives on Minority Participation in Health Studies. Washington, DC: American Public Health Association Press; 2004. pp. 61–78.
30. Durant RW, Wenzel JA, Scarinci IC, Paterniti DA, Fouad MN, Hurd TC, et al. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT). Cancer. 2014;120 Suppl 7 : 1097–1105. doi: 10.1002/cncr.28574 24643647
31. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104: e16–31. doi: 10.2105/AJPH.2013.301706 24328648
32. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111 : 1684–1687. doi: 10.1038/bjc.2014.108 25093493
33. Maxwell AE, Bastani R, Vida P, Warda US. Strategies to recruit and retain older Filipino-American immigrants for a cancer screening study. J Community Health. 2005;30 : 167–179. doi: 10.1007/s10900-004-1956-0 15847243
34. Johnson VA, Edwards KA, Sherman SL, Stephens LD, Williams W, Adair A, et al. Decisions to participate in fragile X and other genomics-related research: Native American and African American voices. J Cult Divers. 2009;16 : 127–135. 19824292
35. Association of American Medical Colleges. The Diversity Research Forum: The Importance and Benefits of Diverse Faculty in Academic Medicine: Implications for Recruitment, Retention, and Promotion. Washington, DC; 2009.
36. National Science Foundation. Doctorate recipients from US colleges and universities [Internet]. [cited 11 Sep 2015]. http://www.nsf.gov/statistics/sed/2013/data/tab24.pdf
37. Rockey S. Diversity—Looking at the Numbers [Internet]. [cited 24 Sep 2014]. http://nexus.od.nih.gov/all/2012/07/20/diversity-looking-at-the-numbers
38. Rockey S. New NIH Study on Diversity [Internet]. [cited 23 Sep 2014]. http://nexus.od.nih.gov/all/2011/08/18/new-nih-study-on-diversity/
39. Ginther DK, Schaffer WT, Schnell J, Masimore B, Liu F, Haak LL, et al. Race, ethnicity, and NIH research awards. Science. 2011;333 : 1015–1019. doi: 10.1126/science.1196783 21852498
40. Freedom of Information Act Request. NIH/Office of Extramural Research. 2014.
41. Reardon S. NIH to probe racial disparity in grant awards. Nature. 2014;512 : 243–243. doi: 10.1038/512243a 25143096
42. NIH VideoCast—Advisory Committee to the Director—December 2014 (Day 1) [Internet]. [cited 18 Dec 2014]. http://videocast.nih.gov/summary.asp?live=15269&bhcp=1
43. National Research Council, Committee on the Science of Team Science. Enhancing the Effectiveness of Team Science. Cooke NJ, Hilton ML, editors. Washington (DC): National Academies Press (US); 2015.
44. Freeman RB, Huang W. Collaboration: Strength in diversity. Nature. 2014;513 : 305. doi: 10.1038/513305a 25230634
45. Tabak LA, Collins FS. Weaving a richer tapestry in biomedical science. Science. 2011;333 : 940–941. doi: 10.1126/science.1211704 21852476
46. Request for Information: NIH Precision Medicine Cohort. 2015. http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-096.html
47. Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372 : 793–795. doi: 10.1056/NEJMp1500523 25635347
48. NIH Precision Medicine Initiative [Internet]. [cited 1 Sep 2015]. http://www.nih.gov/precisionmedicine/events.htm
49. ACD Precision Medicine Initiative Working Group Public Workshop. Participant Engagement and Health Equity Workshop Summary [Internet]. [cited 1 Sep 2015]. http://www.nih.gov/precisionmedicine/2015-07-01-workshop-summary.pdf
50. PA-15-034: Administrative Supplements for Research on Sex/Gender Differences (Admin Supp) [Internet]. [cited 30 Dec 2014]. http://grants.nih.gov/grants/guide/pa-files/PA-15-034.html
51. Rockey S. New Efforts to Maximize Fairness in NIH Peer Review [Internet]. [cited 23 Sep 2014]. http://nexus.od.nih.gov/all/2014/05/29/new-efforts-to-maximize-fairness-in-nih-peer-review/
52. CSR Announces Winners of its America COMPETES Challenges to Maximize Fairness in NIH Peer Review, [Internet]. [cited 14 Dec 2014]. http://public.csr.nih.gov/Documents/LearnMoreabouttheWinningIdeas.pdf
53. NIH proposes critical initiatives to sustain future of US biomedical research [Internet]. [cited 1 Sep 2015]. http://www.nih.gov/news/health/dec2012/od-07.htm
54. Enhancing the Diversity of the NIH-Funded Workforce [Internet]. [cited 16 Dec 2014]. http://commonfund.nih.gov/diversity/Initiatives
55. King G. Institutional racism and the medical/health complex: a conceptual analysis. Ethn Dis. 1996 Winter-Spring;6(1–2):30–46.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 12- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials
- Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled
- Police Killings and Police Deaths Are Public Health Data and Can Be Counted
- A Successful Failure: Missing the MDG4 Target for Under-Five Mortality in South Africa
- The Ebola Vaccine, Iatrogenic Injuries, and Legal Liability
- Progress in Medicine: Experts Take Stock
- Use of Viremia to Evaluate the Baseline Case Fatality Ratio of Ebola Virus Disease and Inform Treatment Studies: A Retrospective Cohort Study
- Acute Cardiovascular Events after Herpes Zoster: A Self-Controlled Case Series Analysis in Vaccinated and Unvaccinated Older Residents of the United States
- Moving Beyond “Food Deserts”: Reorienting United States Policies to Reduce Disparities in Diet Quality
- Public Health and International Partnerships in the Democratic People’s Republic of Korea
- Self-Administered Outpatient Antimicrobial Infusion by Uninsured Patients Discharged from a Safety-Net Hospital: A Propensity-Score-Balanced Retrospective Cohort Study
- Association between Regimen Composition and Treatment Response in Patients with Multidrug-Resistant Tuberculosis: A Prospective Cohort Study
- 10-y Risks of Death and Emergency Re-admission in Adolescents Hospitalised with Violent, Drug- or Alcohol-Related, or Self-Inflicted Injury: A Population-Based Cohort Study
- World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010
- Bariatric Surgery in the United Kingdom: A Cohort Study of Weight Loss and Clinical Outcomes in Routine Clinical Care
- Traditional and Emerging Lifestyle Risk Behaviors and All-Cause Mortality in Middle-Aged and Older Adults: Evidence from a Large Population-Based Australian Cohort
- Inequalities in Alcohol-Related Mortality in 17 European Countries: A Retrospective Analysis of Mortality Registers
- World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis
- A Molecular Host Response Assay to Discriminate Between Sepsis and Infection-Negative Systemic Inflammation in Critically Ill Patients: Discovery and Validation in Independent Cohorts
- World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Self-Administered Outpatient Antimicrobial Infusion by Uninsured Patients Discharged from a Safety-Net Hospital: A Propensity-Score-Balanced Retrospective Cohort Study
- Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials
- World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010
- Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



