-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation between Class III Obesity (BMI of 40–59 kg/m) and Mortality: A Pooled Analysis of 20 Prospective Studies
Background:
The prevalence of class III obesity (body mass index [BMI]≥40 kg/m2) has increased dramatically in several countries and currently affects 6% of adults in the US, with uncertain impact on the risks of illness and death. Using data from a large pooled study, we evaluated the risk of death, overall and due to a wide range of causes, and years of life expectancy lost associated with class III obesity.Methods and Findings:
In a pooled analysis of 20 prospective studies from the United States, Sweden, and Australia, we estimated sex - and age-adjusted total and cause-specific mortality rates (deaths per 100,000 persons per year) and multivariable-adjusted hazard ratios for adults, aged 19–83 y at baseline, classified as obese class III (BMI 40.0–59.9 kg/m2) compared with those classified as normal weight (BMI 18.5–24.9 kg/m2). Participants reporting ever smoking cigarettes or a history of chronic disease (heart disease, cancer, stroke, or emphysema) on baseline questionnaires were excluded. Among 9,564 class III obesity participants, mortality rates were 856.0 in men and 663.0 in women during the study period (1976–2009). Among 304,011 normal-weight participants, rates were 346.7 and 280.5 in men and women, respectively. Deaths from heart disease contributed largely to the excess rates in the class III obesity group (rate differences = 238.9 and 132.8 in men and women, respectively), followed by deaths from cancer (rate differences = 36.7 and 62.3 in men and women, respectively) and diabetes (rate differences = 51.2 and 29.2 in men and women, respectively). Within the class III obesity range, multivariable-adjusted hazard ratios for total deaths and deaths due to heart disease, cancer, diabetes, nephritis/nephrotic syndrome/nephrosis, chronic lower respiratory disease, and influenza/pneumonia increased with increasing BMI. Compared with normal-weight BMI, a BMI of 40–44.9, 45–49.9, 50–54.9, and 55–59.9 kg/m2 was associated with an estimated 6.5 (95% CI: 5.7–7.3), 8.9 (95% CI: 7.4–10.4), 9.8 (95% CI: 7.4–12.2), and 13.7 (95% CI: 10.5–16.9) y of life lost. A limitation was that BMI was mainly ascertained by self-report.Conclusions:
Class III obesity is associated with substantially elevated rates of total mortality, with most of the excess deaths due to heart disease, cancer, and diabetes, and major reductions in life expectancy compared with normal weight.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(7): e32767. doi:10.1371/journal.pmed.1001673
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001673Summary
Background:
The prevalence of class III obesity (body mass index [BMI]≥40 kg/m2) has increased dramatically in several countries and currently affects 6% of adults in the US, with uncertain impact on the risks of illness and death. Using data from a large pooled study, we evaluated the risk of death, overall and due to a wide range of causes, and years of life expectancy lost associated with class III obesity.Methods and Findings:
In a pooled analysis of 20 prospective studies from the United States, Sweden, and Australia, we estimated sex - and age-adjusted total and cause-specific mortality rates (deaths per 100,000 persons per year) and multivariable-adjusted hazard ratios for adults, aged 19–83 y at baseline, classified as obese class III (BMI 40.0–59.9 kg/m2) compared with those classified as normal weight (BMI 18.5–24.9 kg/m2). Participants reporting ever smoking cigarettes or a history of chronic disease (heart disease, cancer, stroke, or emphysema) on baseline questionnaires were excluded. Among 9,564 class III obesity participants, mortality rates were 856.0 in men and 663.0 in women during the study period (1976–2009). Among 304,011 normal-weight participants, rates were 346.7 and 280.5 in men and women, respectively. Deaths from heart disease contributed largely to the excess rates in the class III obesity group (rate differences = 238.9 and 132.8 in men and women, respectively), followed by deaths from cancer (rate differences = 36.7 and 62.3 in men and women, respectively) and diabetes (rate differences = 51.2 and 29.2 in men and women, respectively). Within the class III obesity range, multivariable-adjusted hazard ratios for total deaths and deaths due to heart disease, cancer, diabetes, nephritis/nephrotic syndrome/nephrosis, chronic lower respiratory disease, and influenza/pneumonia increased with increasing BMI. Compared with normal-weight BMI, a BMI of 40–44.9, 45–49.9, 50–54.9, and 55–59.9 kg/m2 was associated with an estimated 6.5 (95% CI: 5.7–7.3), 8.9 (95% CI: 7.4–10.4), 9.8 (95% CI: 7.4–12.2), and 13.7 (95% CI: 10.5–16.9) y of life lost. A limitation was that BMI was mainly ascertained by self-report.Conclusions:
Class III obesity is associated with substantially elevated rates of total mortality, with most of the excess deaths due to heart disease, cancer, and diabetes, and major reductions in life expectancy compared with normal weight.
Please see later in the article for the Editors' SummaryIntroduction
Obesity (body mass index [BMI]≥30 kg/m2) increases the risk for numerous adverse health outcomes, including most major chronic diseases [1]–[3]. Class III, or extreme, obesity (BMI≥40 kg/m2) [4] is emerging as a major public health problem in several developed countries [5]–[7], most notably in the US, where BMI>30, 40, or 50 kg/m2 among adults has increased more than 2-, 4-, and 10-fold, respectively, since the mid-1980s [8]. While class III obesity currently affects 6% of the US adult population [8],[9], those belonging to this group accounted for 20% of the total per capita health-care expenditures in 2000 [10].
Effective interventions and accurate projections of future health-care costs requires a better understanding of the health risks associated with class III obesity, but the necessary data are scarce. Because class III obesity was relatively uncommon in the recent past, the few studies that have specifically evaluated mortality rates associated with class III obesity generally had limited sample sizes (e.g., fewer than 400 deaths [2],[11]) or did not separately evaluate risks of death for BMI of 50 kg/m2 or higher [2],[3]. As such, there is little quantitative information about the burden of disease, including total and cause-specific mortality rates, for individuals with BMI values of 40 kg/m2 and above.
We combined original data from 20 prospective studies from the United States, Sweden, and Australia to evaluate—in what is to our knowledge the largest study on this topic to date—the excess rates of death overall and due to a wide range of specific causes, as well as the expected number of years of life lost attributable to class III obesity.
Methods
Study Population
The study sample included participants from the 20 cohort studies in the National Cancer Institute Cohort Consortium that met the eligibility criteria (>5 y of follow-up, >1,000 deaths among non-Hispanic white participants, baseline year 1970 or later) and had the ability to submit data for a previous pooled analysis of BMI and mortality [3], including the Adventist Health Study–I [12], Agricultural Health Study [13], Breast Cancer Detection Demonstration Project [14], California Teachers Study [15], Cancer Prevention Study–II [16], CLUE–I and –II [17], Cohort of Swedish Men [18], Health Professionals Follow-Up Study [19], Iowa Women's Health Study [20], Melbourne Collaborative Cohort Study [21], New York University Women's Health Study [22], National Institutes of Health–American Association of Retired Persons Diet and Health Study (NIH-AARP) [23], Nurses' Health Study–I [24], Physicians' Health Study–I and –II [25], Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial [26], Swedish Mammography Cohort [27], Swedish Women's Lifestyle and Health Study [28], United States Radiologic Technologists Study [29], VITamins and Lifestyle Study [30], and the Women's Health Study [31]. Baseline was defined as the date of completion of the first questionnaire that collected information on key variables (e.g., height, weight, smoking, and personal history of chronic diseases). Personal history of chronic diseases and other important covariates, including race/ethnicity, education, marital status, alcohol consumption, and physical activity level, were self-reported. Height and weight information was self-reported in all but one cohort [21]. Individual-level data were formatted uniformly across studies and combined.
Participants who were younger than 18 y or older than 85 y old at baseline, participants with less than 1 y of follow-up, and participants with BMI≥60 kg/m2 were excluded. We also excluded participants who reported having ever smoked cigarettes and/or a history of heart disease, cancer, stroke, or emphysema, as tobacco use and these preexisting illnesses have been demonstrated to confound the association between BMI and mortality [3]. The final sample consisted of 9,564 individuals with a BMI of 40.0 to 59.9 kg/m2 (1,575 men and 7,989 women) and 304,011 with a BMI of 18.5 to 24.9 kg/m2 (75,680 men and 228,331 women).
Follow-Up
Participants were followed from the date of completion of the baseline questionnaire to death, loss to follow-up, or administrative end date, whichever occurred first. Causes of death were ascertained from death records or registries and coded according to the Surveillance, Epidemiology, and End Results Program recodes [32] based on cause of death codes defined by the International Classification of Diseases, eighth, ninth, or tenth revisions [33]–[35], and categorized according to the 2002 National Vital Statistics Report of the US Centers for Disease Control and Prevention [36].
Statistical Methods
We calculated age-adjusted mortality rates (number of deaths per 100,000 persons per year) for the class III obesity (BMI 40.0–59.9 kg/m2) and normal-weight (BMI 18.5–24.9 kg/m2) groups using direct standardization for age [37], in which rates were weighted according to the age distribution of the total US population in 2000, restricted to ages 20–84 y, using 5-y categories of attained age. We used the US standard to most closely approximate the age distributions of the countries from which the study populations were selected. Specific causes of death were chosen from among the top leading causes of death in the US population in 2000 [36], including (but not limited to) heart disease, cerebrovascular disease, malignant neoplasms, chronic lower respiratory diseases, accidents, diabetes mellitus, influenza and pneumonia, kidney disease (nephritis, nephrotic syndrome, and nephrosis), septicemia, and chronic liver disease and cirrhosis. Mortality rates from suicide and homicide and from major causes of death resulting in fewer than five events in both men and women with BMI values of 40.0 kg/m2 and above (e.g., Alzheimer disease) are not shown. Within the major categories of heart disease, malignant neoplasms, and cerebrovascular disease, where the sample size was sufficient, we additionally calculated mortality rates due to more specific causes (e.g., ischemic heart disease). Rate differences were calculated as the difference in age-adjusted mortality rates between the two BMI groups, and two-sided p-values for these differences were calculated using a two-sample Z test with the variance calculated under the assumption that the number of deaths in each group followed an independent Poisson distribution.
We used proportional hazards regression models, with attained age as the underlying time metric, to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for total mortality and mortality due to major causes in relation to class III obesity (BMI 40.0–59.9 kg/m2) compared with normal weight (BMI 18.5–24.9 kg/m2). All models were adjusted for sex, race/ethnicity, education, alcohol intake, level of physical activity, and study. In separate models, we additionally adjusted for self-reported history of diabetes mellitus and hypertension to evaluate the potential for mediation by these co-morbid conditions. In separate models restricted to participants with BMI values between 40.0 and 59.9 kg/m2, BMI was included as a continuous variable to evaluate HRs and 95% CIs per 5 kg/m2 BMI and to calculate tests for trend. We observed no evidence of violation of the proportional hazards assumption based on graphical assessment of cumulative hazards plots by BMI category.
To calculate years of life lost, we calculated direct adjusted survival curves for each BMI category [38],[39]. Proportional hazards regression models were used to estimate probabilities of survival at each age for each individual, which were then averaged to obtain an overall summary curve. Curves for each BMI category were estimated by counterfactual (i.e., applying the beta for each BMI category in turn to estimate individual and overall survival curves). Years of life lost were calculated as the difference between the adjusted median life expectancy for a given BMI category and the reference category of BMI 18.5–24.9 kg/m2. Models were restricted to participants who were 40 y and older at baseline.
As a secondary analysis and alternative approach to combining results across studies, we additionally calculated pooled HRs for class III obesity and total mortality using a random effects meta-analysis model. Heterogeneity between studies was calculated using the I2 index [40]. Confidence intervals for the I2 index were calculated according to the method described by Thorlund et al. [41]. We conducted subgroup analyses within the aggregate dataset to evaluate potential sources of between-study heterogeneity. For instance, because the association between BMI and mortality has been shown to differ by age at BMI measurement/report and sex [3], and medical care for extremely obese individuals may have improved over calendar time, we calculated HRs and 95% CIs within strata of these factors. We also evaluated geographic location (US versus non-US cohorts) as another potential source of between-study heterogeneity. Tests for interaction by sex, geographic location (US versus non-US cohorts), attained age, and calendar period of follow-up (before and after age 65 y, and before and after the year 2000) were conducted by including a cross-product term in the model. Models evaluating interactions by attained age and calendar period were time-dependent, in which person-time for each participant was split before and after age 65 y or the year 2000.
Results
A description of participants with BMI in the class III obesity range from each of the 20 cohorts is shown in Table 1. In total, 1,036 of the 9,564 participants died during the follow-up period (median = 9 y, maximum = 27 y).
Tab. 1. Description of participants with BMI in the normal-weight (18.5–24.9 kg/m<sup>2</sup>) and class III obesity (40.0–59.0 kg/m<sup>2</sup>) range, by cohort. 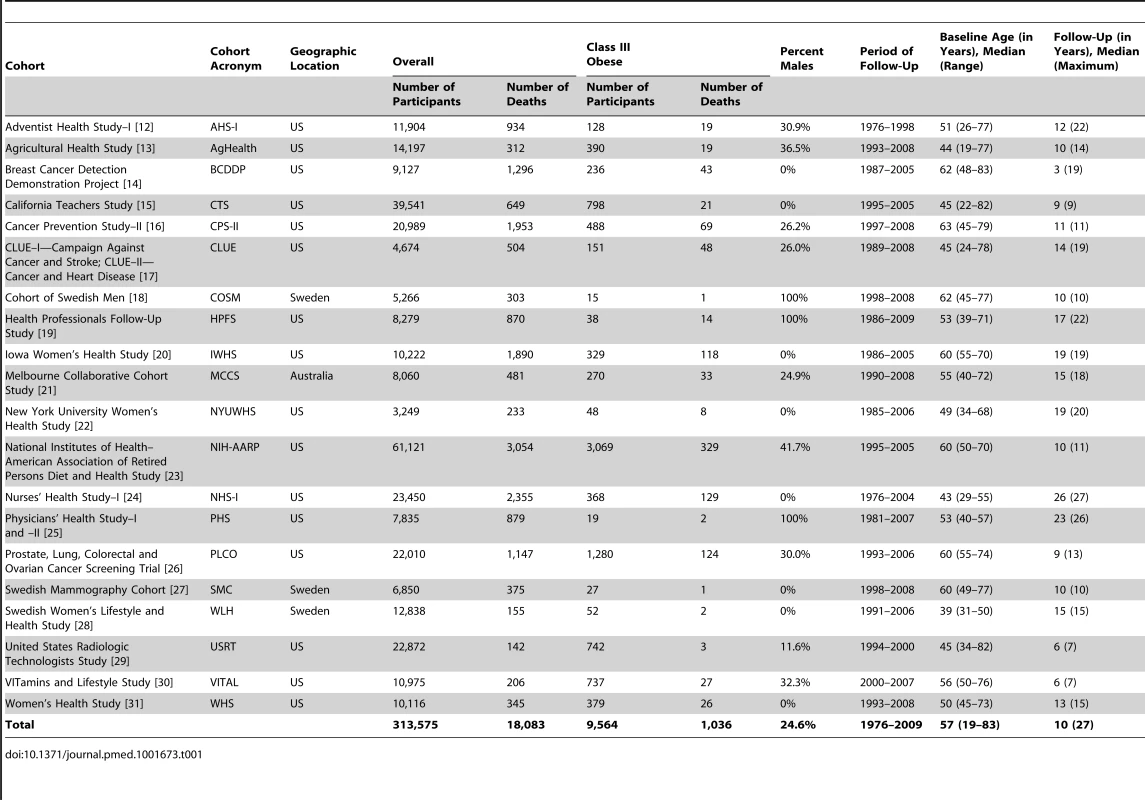
Compared with participants in the normal-weight range at baseline, those in the class III obesity range included a higher proportion of women, more individuals with baseline age 50 to 69 y (as opposed to younger [19–49 y] or older [70–83 y] individuals), fewer non-Hispanic white individuals, fewer individuals with a college education, more individuals with lower alcohol intake, and more individuals with lower levels of physical activity (Table 2). Among participants within the range of class III obesity, we observed little variation in baseline age, sex, education, and alcohol intake. By contrast, the prevalence of non-Hispanic black race/ethnicity, low physical activity, and history of diabetes, as well as median waist circumference, was generally higher for the top BMI categories.
Tab. 2. Baseline characteristics of participants in the pooled dataset by BMI category. 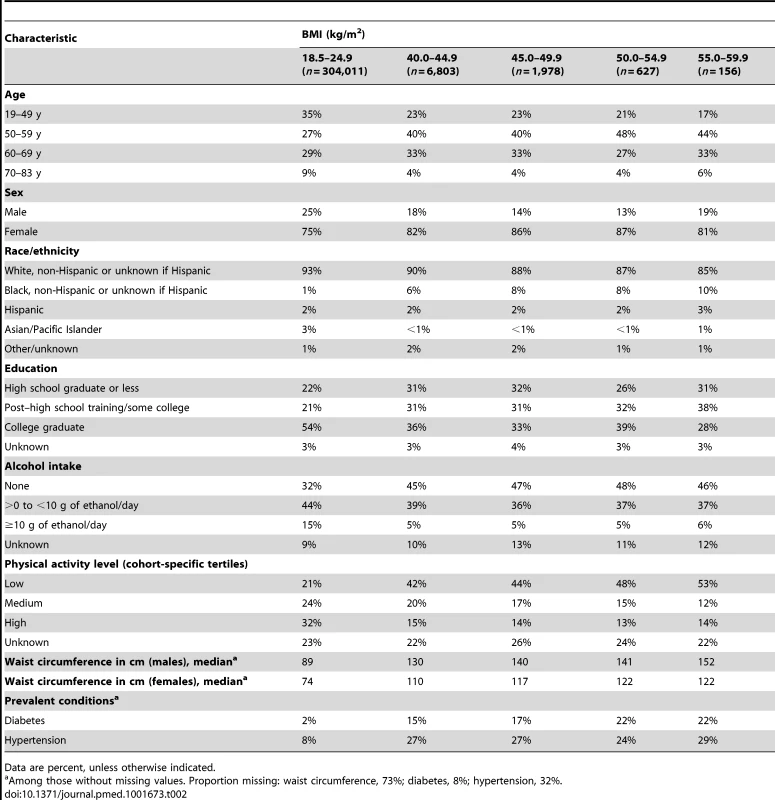
Data are percent, unless otherwise indicated. Table 3 compares total and cause-specific mortality rates (deaths per 100,000 persons per year) for the class III obesity and normal-weight groups separately by sex. Total mortality rates for class III obesity participants were 856.0 in men and 663.0 in women. The differences in mortality rates for the two BMI groups were 509.3 and 382.5 in men and women, respectively. Heart disease was the most common underlying cause of death for the class III obesity group (mortality rate differences were 238.9 and 132.8 in men and women, respectively), followed by malignant neoplasms (mortality rate differences were 36.7 and 62.3 in men and women, respectively), and diabetes (mortality rate differences were 51.2 and 29.2 in men and women, respectively). Higher rates of death were also observed for nearly every other major cause of death that we examined, apart from cerebrovascular disease in men and malignant neoplasms of respiratory and intrathoracic organs and diseases of the arteries, arterioles, and capillaries in women, although not all of these differences were statistically significant because of the relatively small numbers of deaths in the class III obesity group for some of the major causes (e.g., malignant neoplasms, chronic lower respiratory disease, and nephritis/nephrotic syndrome/nephrosis deaths in men). The negative rate differences that were observed were also based on small numbers of deaths in the class III obesity group (five, eight, and five deaths due to cerebrovascular disease, malignant neoplasms of respiratory and intrathoracic organs, and diseases of the arteries, arterioles, and capillaries, respectively).
Tab. 3. Age-adjusted cause-specific mortality rates (number of deaths per 100,000 persons per year) by BMI category. 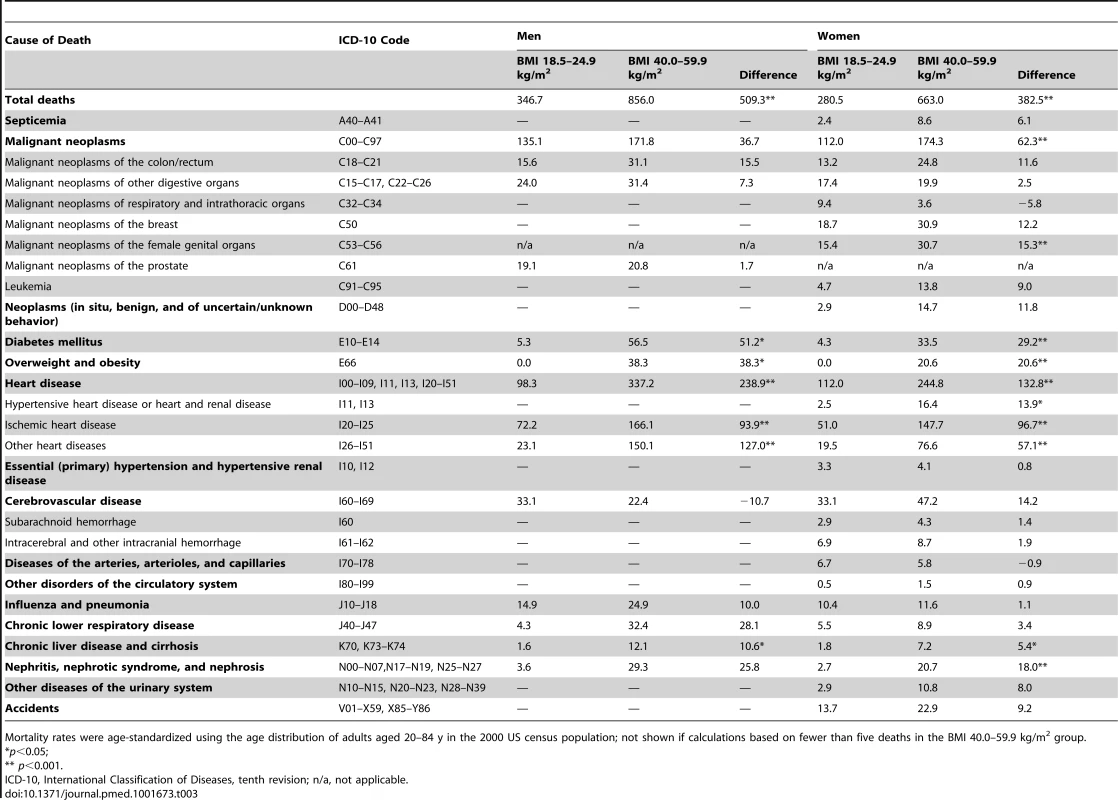
Mortality rates were age-standardized using the age distribution of adults aged 20–84 y in the 2000 US census population; not shown if calculations based on fewer than five deaths in the BMI 40.0–59.9 kg/m2 group. As an internal comparison, in the subset of participants in the pooled dataset without heart disease, cancer, stroke, or emphysema and having BMI values between 18.5 and 24.9 kg/m2, differences in mortality rates for current versus never smokers were 721.0 and 519.6 in men and women, respectively.
Table 4 shows the minimally adjusted and multivariable-adjusted HRs for death across categories of BMI and for continuous (per 5 kg/m2) values of BMI restricted to the BMI 40.0–59.9 kg/m2 range. In minimally adjusted models, we observed a sharp gradient in relative risk with higher values of BMI across the class III obesity levels that was consistent with a linear increase. The HRs from models of BMI categories were slightly, but not fully, attenuated with additional adjustment for race/ethnicity, education, alcohol intake, and physical activity level. Compared with adults with BMI in the 18.5–24.9 kg/m2 range, multivariable-adjusted HRs (95% CIs) for those with BMI of 40.0–44.9, 45.0–49.9, 50.0–54.9, and 55.0–59.9 kg/m2 were 2.25 (2.07–2.43), 3.32 (2.92–3.77), 3.48 (2.82–4.31), and 5.91 (4.24–8.24), respectively. This trend of increasing risk of death with increasing level of BMI was apparent in categorical and continuous models (HR per 5 kg/m2 = 1.40, 95% CI: 1.31–1.51) after restricting to the BMI 40.0–59.9 kg/m2 range.
Tab. 4. Hazard ratios and 95% confidence intervals for risk of death by BMI category. 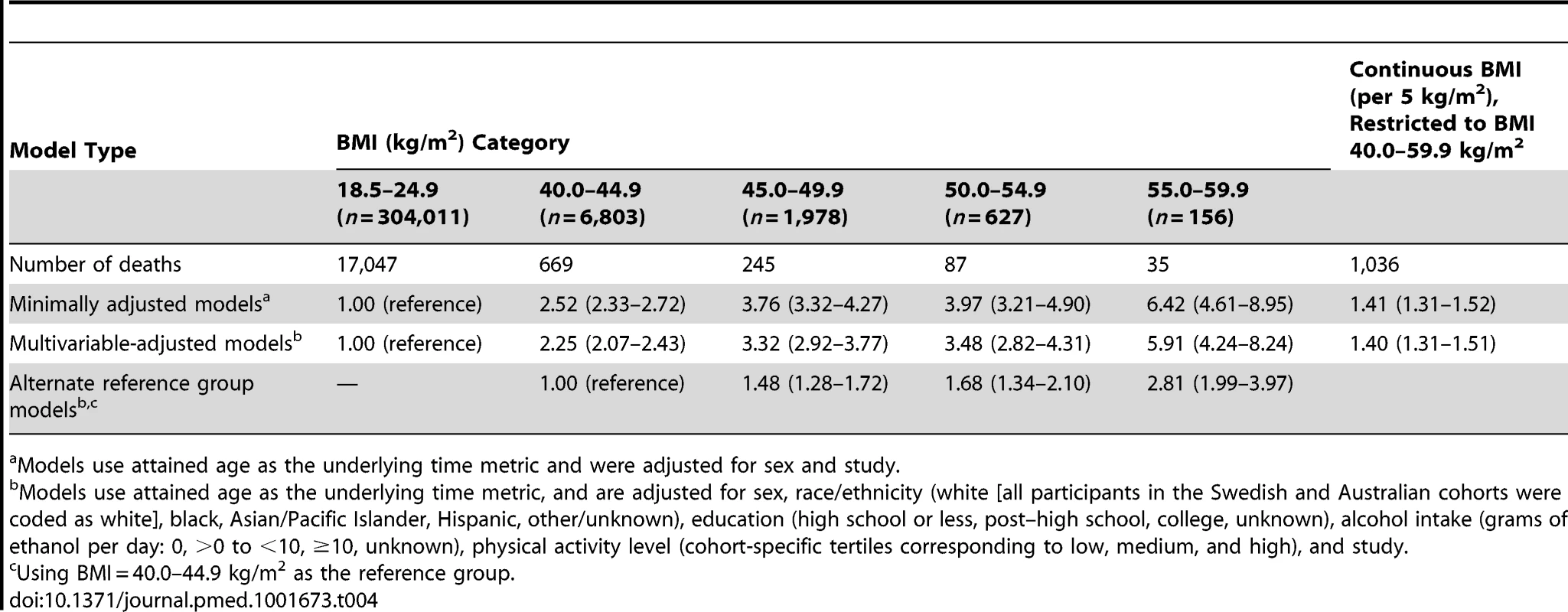
Models use attained age as the underlying time metric and were adjusted for sex and study. Although the pooled HRs calculated from random effects meta-analysis models were similar to those based on the aggregate dataset (BMI 40.0–59.9 versus 18.5–24.9 kg/m2, HR = 2.57, 95% CI: 2.23–2.96; per 5 kg/m2 BMI, HR = 1.42, 95% CI: 1.25–1.62), the heterogeneity estimates for differences in the associations by cohort were statistically significant (Figures 1 and 2). We evaluated potential sources of heterogeneity in Table 5, which shows the multivariable-adjusted HRs conducted overall and by population subgroup. Overall, we observed a 2.57-fold (95% CI: 2.41–2.74) increased risk of death in the BMI 40.0–59.9 kg/m2 versus the 18.5–24.9 kg/m2 group, and the association increased linearly for BMI values above 40 kg/m2 (HR per 5 kg/m2 = 1.40, 95% CI: 1.31–1.51). As observed in studies of BMI across the entire range of values in relation to mortality [3], these associations were significantly modified by sex (p-interaction = 0.03), by geographic location (US versus non-US, p-interaction = 0.03), and follow-up time before and after age 65 y (p-interaction <0.001), with stronger associations observed for men (HR = 2.83, 95% CI: 2.45–3.26), for US cohorts (HR = 2.60, 95% CI: 2.44–2.78), and for follow-up before age 65 y (HR = 3.10, 95% CI: 2.77–3.47). The interaction by geographic location may have arisen due to chance, as there were only 37 deaths in the class III obesity group among non-US cohorts. No differences were observed for follow-up time occurring before versus after the year 2000. The HR for BMI 40.0–59.9 kg/m2 versus 18.5–24.9 kg/m2 did not change importantly after excluding the largest cohort, NIH-AARP, though the continuous HR for BMI values over 40 kg/m2 was slightly attenuated after this exclusion (HR = 1.32, 95% CI: 1.20–1.45). Exclusion of participants who reported a history of diabetes at baseline slightly attenuated the HR for BMI 40.0–59.9 kg/m2 versus 18.5–24.9 kg/m2 (HR = 2.27, 95% CI: 2.10–2.45). Exclusion of the first 2 y of follow-up did not appreciably change the HRs (<5% change). Although physical activity could confound or mediate these associations, removal of the physical activity variable also had little influence on the HRs (<5% change).
#other:1#
#other:2#
Tab. 5. Hazard ratios and 95% confidence intervals for risk of death according to BMI category (40.0–59.9 versus 18.5–24.9 kg/m2) or continuous values of BMI between 40.0 and 59.9 kg/m2 (per 5 kg/m2): subgroup analyses. 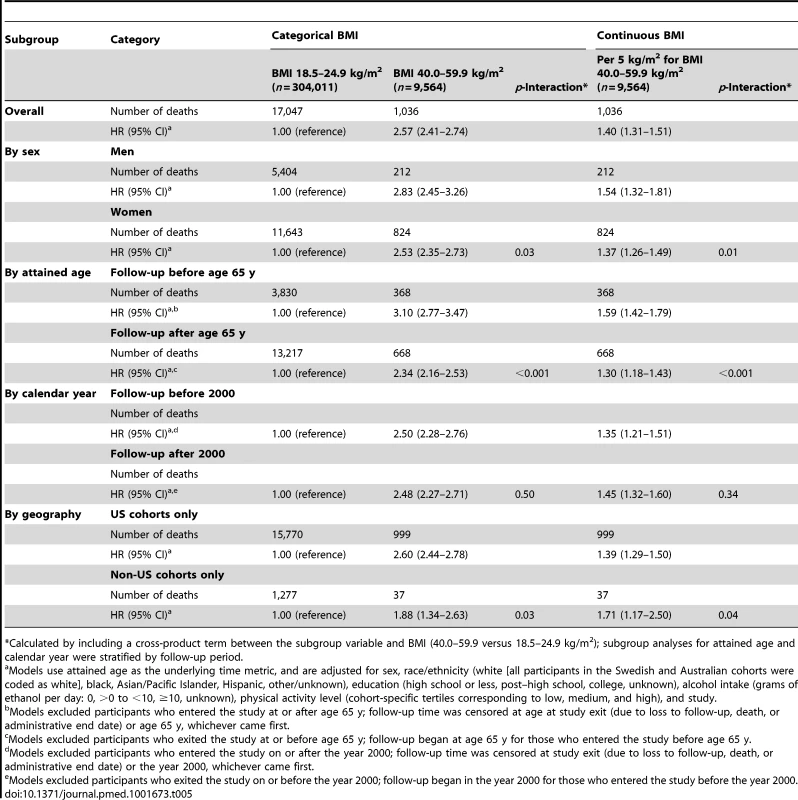
*Calculated by including a cross-product term between the subgroup variable and BMI (40.0–59.9 versus 18.5–24.9 kg/m2); subgroup analyses for attained age and calendar year were stratified by follow-up period. Multivariable-adjusted HRs for select major causes of death for BMI 40.0–59.9 kg/m2 compared with 18.5–24.9 kg/m2 are shown in Table 6. HRs were significantly elevated for deaths due to heart disease, malignant neoplasms, cerebrovascular disease, diabetes mellitus, nephritis/nephritic syndrome/nephrosis, chronic lower respiratory disease, influenza/pneumonia, accidents, and septicemia, ranging from 1.69 for deaths due to malignant neoplasms to 9.57 for deaths due to nephritis/nephrotic syndrome/nephrosis. Additional adjustment for self-reported history of diabetes and hypertension attenuated the HRs for all specific causes of death apart from malignant neoplasms, though all remained statistically significant. Within the BMI 40.0–59.9 kg/m2 range, greater BMI values were linearly associated with increasing HRs for all of these major causes of death apart from cerebrovascular disease, accidents, and septicemia; additional adjustment for history of diabetes and hypertension had little influence on these continuous HRs.
Tab. 6. Hazard ratios and 95% confidence intervals for select major causes of death by BMI category. 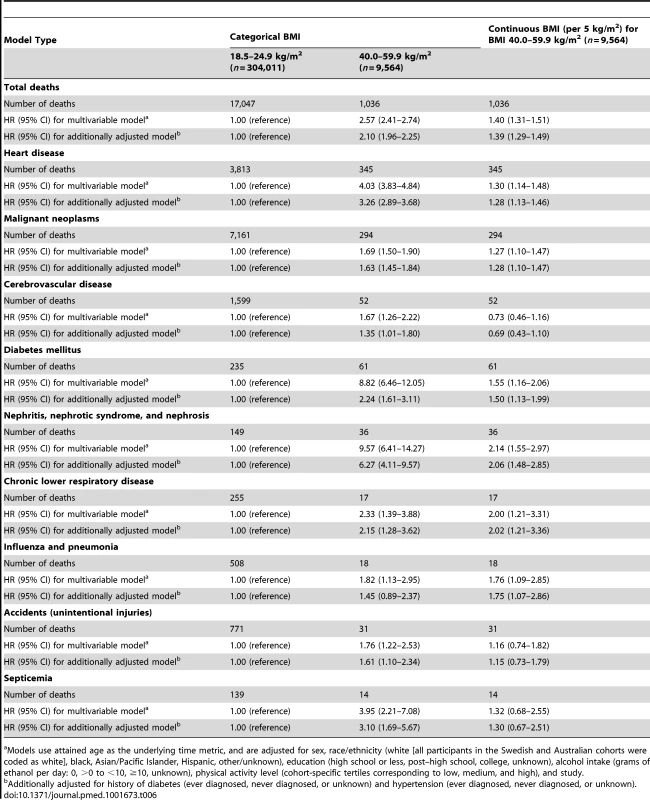
Models use attained age as the underlying time metric, and are adjusted for sex, race/ethnicity (white [all participants in the Swedish and Australian cohorts were coded as white], black, Asian/Pacific Islander, Hispanic, other/unknown), education (high school or less, post–high school, college, unknown), alcohol intake (grams of ethanol per day: 0, >0 to <10, ≥10, unknown), physical activity level (cohort-specific tertiles corresponding to low, medium, and high), and study. We estimated that BMI categories of 40–44.9, 45–49.9, 50–54.9, and 55–59.9 kg/m2 were associated with an estimated 6.5 (95% CI: 5.7–7.3), 8.9 (95% CI: 7.4–10.4), 9.8 (95% CI: 7.4–12.2), and 13.7 (95% CI: 10.5–16.9) y of life lost (Table 7). These estimates (95% CIs) were 6.5 (5.6–7.4), 10.8 (9.3–12.3), 10.6 (7.6–13.6), and 17.0 (12.9–21.1) in men and 6.9 (6.1–7.7), 9.1 (7.9–10.3), 10.3 (8.3–12.3), and 13.1 (9.0–17.2) in women. By comparison, in the subset of participants in the pooled dataset without heart disease, cancer, stroke, or emphysema and having BMI values between 18.5 and 24.9 kg/m2, current versus never cigarette smoking was associated with an estimated 8.9 y of life lost (95% CI: 8.6–9.3).
Tab. 7. Years of life lost and 95% confidence intervals by BMI category. 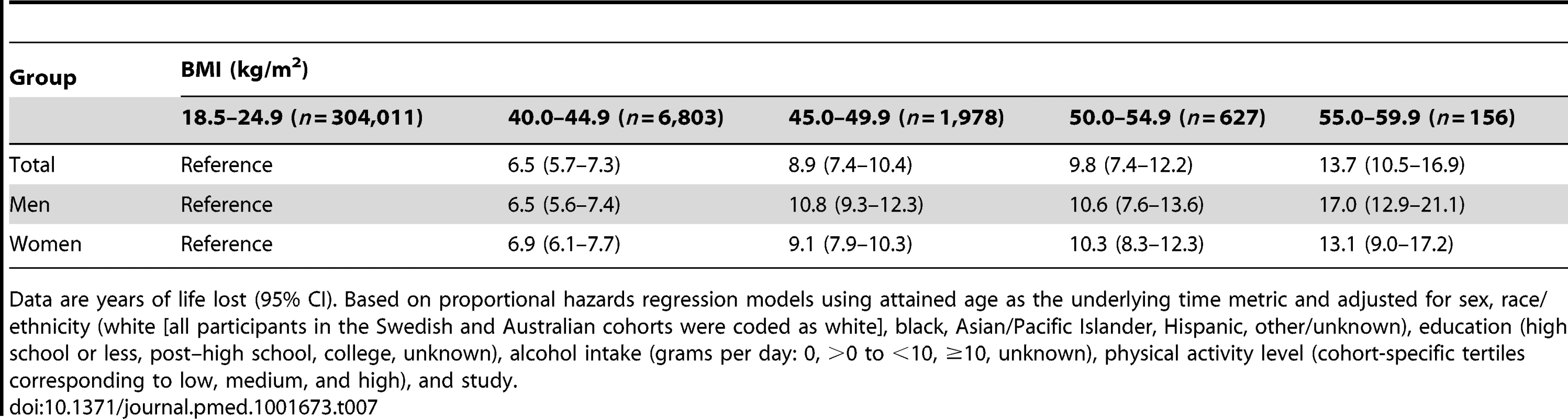
Data are years of life lost (95% CI). Based on proportional hazards regression models using attained age as the underlying time metric and adjusted for sex, race/ethnicity (white [all participants in the Swedish and Australian cohorts were coded as white], black, Asian/Pacific Islander, Hispanic, other/unknown), education (high school or less, post–high school, college, unknown), alcohol intake (grams per day: 0, >0 to <10, ≥10, unknown), physical activity level (cohort-specific tertiles corresponding to low, medium, and high), and study. Discussion
To our knowledge, this is the largest study to date on the association between class III obesity and mortality, and the first to show that class III obesity is associated with excess rates of total mortality and mortality due to a wide range of causes, particularly heart disease, cancer, and diabetes, and that the risk of death overall and from these specific causes continues to rise with increasing values of BMI. The accumulating excess risk resulted in major reductions in life expectancy after the age of 40 y that were comparable to those of cigarette smoking.
The estimated 6.5 to 13.7 y of life lost for BMI values between 40 and 59 kg/m2 versus 18.5–24.9 kg/m2 were in line with those of a previous pooled analysis that found that individuals with BMI values of 35–50 kg/m2 had a median 8–10 fewer years of life than those with BMI 22.5–24.9 kg/m2 [2]. Our study further demonstrates that the expected number of years of life lost continued to increase for BMI values beyond 50 kg/m2, at which point the loss in life expectancy (9.8 y) exceeded that observed for current versus never smoking (8.9 y) in this study. These results have great relevance to the current era, during which class III obesity rates have increased dramatically at the same time that smoking rates have declined. That we observed a 7.2-y decrease in life expectancy from BMI 40.0–44.9 to 55.0–59.9 kg/m2 suggests that otherwise healthy, non-smoking adults having BMI values within the class III obesity range may considerably expand their life expectancy by avoiding additional weight gain.
We observed significant differences in the relative risks for class III obesity and total mortality by cohort, which may reflect differences in the ages, sex distribution, and geographical location of the participants, as these factors were shown to be significant effect modifiers in our study. Similar to observations from previous studies based on lower values of BMI [3], these results suggest that the risks associated with class III obesity may be even greater for men than women, and for younger compared to older adults. These results could be considered in the development of public health interventions aimed at reducing health risks associated with extreme obesity, particularly those that are targeted toward susceptible populations.
The clear finding of excess risk for nearly every major cause of death suggests that excess weight can have a wide range of physiological effects. In particular, the results of our study suggest that diabetes and hypertension are common mechanisms that might explain the majority of the excess mortality burden in the class III obesity group. Most of this excess was attributable to heart disease, diabetes, cancer, and kidney and liver diseases, and risks of death due to these causes were largely attenuated with additional adjustment for diabetes and hypertension. Obesity is strongly associated with metabolic abnormalities, including insulin resistance, type 2 diabetes, hypertension, and dyslipidemia, mediated in part by the chronic inflammatory state induced by the secretion of adipocytokines, such as angiotensinogen, transforming growth factor–beta, tumor necrosis factor–alpha, and interleukin-six [42]–[45]. The severity of these conditions has been shown to increase with increasing BMI [44] and improve after weight loss either due to lifestyle modification or bariatric surgery [46]–[49]. Diabetes, hypertension, and other obesity-related aspects of the metabolic syndrome have been shown to play an important role in the development of heart disease, chronic kidney disease, and end-stage renal disease [42],[45], among other major causes of death observed to be elevated in the class III obesity group. Thus, these metabolic conditions could be effect targets in efforts to reduce the burden of morbidity and mortality in individuals with extreme obesity. Individuals with extreme obesity may experience other physiological impairments that could explain the higher rates of death from other causes. Specifically, deaths due to chronic lower respiratory disease were shown in this and the much smaller National Health and Nutrition Examination Survey III study to be elevated at extreme levels of BMI due to reduced lung capacity and airway obstruction [50]. It is possible that excess risks associated with class III obesity may decline as treatments for these co-morbid conditions improve.
An important strength of this pooling study is its large size. With sufficient numbers, we were able to quantify the risks of total and cause-specific deaths associated with class III obesity with greater precision than has been possible in previous studies. Furthermore, our results were based on a population of never smokers without prevalent disease, thereby reducing the potential for confounding due to major preexisting illness and smoking. Our results were also based on original, as opposed to published, data, which allowed for standardization of the variables and statistical methods across studies, and thereby reduced the potential for methodological heterogeneity between studies.
Some limitations of this study include the use of mostly self-reported, as opposed to measured, height and weight. Objectively measured values are ideal, but epidemiologic studies have reported high correlations between self-reported and measured height and weight [51],[52]. Nonetheless, misclassification across BMI categories due to self-reported height and weight has been shown to yield stronger relative risks than those based on measured values [53]. This potential source of bias may have inflated our relative risk estimates, although this effect is likely to have been modest [3]. Another limitation is that height and weight were ascertained only once at the beginning of follow-up, which precluded us from examining risks associated with weight change. Although BMI is the most commonly used indicator of adiposity in epidemiologic studies, it does not directly measure the amount of adipose tissue and cannot distinguish between fat and lean body mass [54],[55]. Nonetheless, BMI has been shown to be just as strongly correlated with obesity-related metabolic indicators as more accurate methods for measuring total fat mass and body fat percent, such as dual-energy X-ray absorptiometry [55]. BMI also does not provide information on central versus peripheral body fat distribution, each posing different risks in relation to the metabolic syndrome and related chronic diseases [56]. Studies that combine BMI with measures of central adiposity may yield more accurate estimates of disease risks associated with high levels BMI [57],[58]. For instance, in a pooled analysis based on data from the same 20 cohort studies, waist circumference was positively associated with all-cause mortality, even among individuals at the highest range of BMI (35.0–49.9 kg/m2) [58]. There has been some concern about the accuracy of cause of death reports [59], which may be differential by BMI level. The excess deaths due to both diabetes mellitus and overweight and obesity that we observed in the class III obesity group may reflect greater difficulty in identifying the primary cause of death among individuals with multiple co-morbid conditions. Our results may not be generalizable to all populations, as the pooled dataset comprised mostly non-Hispanic white individuals from affluent countries (the majority being from the US). Also, our estimates rely on prospective cohort studies, whose volunteer participants typically are healthier than those in the general population.
In this large pooled analysis of 20 prospective studies, participants with BMI in the class III obesity range (40.0–59.9 kg/m2) experienced substantially higher rates of death compared with those in the normal BMI range (18.5–24.9 kg/m2), with most of the excess due to deaths from heart disease, cancer, and diabetes. These higher rates appear to be largely attributable to metabolic abnormalities associated with excess adiposity, including diabetes and hypertension. We found that the reduction in life expectancy associated with class III obesity was similar to (and, for BMI values above 50 kg/m2, even greater than) that observed for current smoking. If current global trends in obesity continue, we must expect to see substantially increased rates of mortality due to these major causes of death, as well as rising health-care costs. These results underscore the need to develop more effective interventions to combat this growing public health problem.
Zdroje
1. World Health Organization (2003) Controlling the global obesity epidemic. Available: http://www.who.int/nutrition/topics/obesity/en/. Accessed 3 June 2014.
2. Prospective Studies Collaboration (2009) WhitlockG, LewingtonS, SherlikerP, ClarkeR, et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373 : 1083–1096.
3. Berrington de GonzalezA, HartgeP, CerhanJR, et al. (2010) Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363 : 2211–2219.
4. PoirierP, CornierMA, MazzoneT, StilesS, CummingsS, et al. (2011) Bariatric surgery and cardiovascular risk factors: a scientific statement from the American Heart Association. Circulation 123 : 1683–1701.
5. PascoJA, BrennanSL, KotowiczMA (2013) Morbid obesity in women on the rise: an observational, population-based study. BMC Public Health 13 : 290.
6. KortelainenML, PorvariK (2011) Extreme obesity and associated cardiovascular disease verified at autopsy. Am J Forensic Med Pathol 32 : 372–377.
7. Health and Social Care Information Centre (2011) Health survey for England—2010, trend tables. Available: http://www.ic.nhs.uk/pubs/hse10trends. Accessed 3 June 2014.
8. SturmR, HattoriA (2013) Morbid obesity rates continue to rise rapidly in the United States. Int J Obes (Lond) 37 : 889–891.
9. FlegalKM, CarrollMD, KitBK, OgdenCL (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307 : 491–497.
10. ArterburnDE, MaciejewskiML, TsevatJ (2005) Impact of morbid obesity on medical expenditures in adults. Int J Obes (Lond) 29 : 334–339.
11. McTigueK, LarsonJC, ValoskiA, BurkeG, KotchenJ, et al. (2006) Mortality and cardiac and vascular outcomes in extremely obese women. JAMA 296 : 79–86.
12. BeesonWL, MillsPK, PhillipsRL, AndressM, FraserGE (1989) Chronic disease among Seventh-day Adventists, a low-risk group: rationale, methodology, and description of the population. Cancer 64 : 570–581.
13. AlavanjaMC, SandlerDP, McMasterSB, ZahmSH, McDonnellCJ, et al. (1996) The Agricultural Health Study. Environ Health Perspect 104 : 362–369.
14. SchairerC, ByrneC, KeylPM, BrintonLA, SturgeonSR, et al. (1994) Menopausal estrogen and estrogen-progestin replacement therapy and risk of breast cancer (United States). Cancer Causes Control 5 : 491–500.
15. BernsteinL, AllenM, Anton-CulverH, DeapenD, Horn-RossPL, et al. (2002) High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States). Cancer Causes Control 13 : 625–635.
16. CalleE, RodriguezC, JacobsEJ, AlmonML, ChaoA, et al. (2002) The American Cancer Society Cancer Prevention Study II Nutrition Cohort—rationale, study design and baseline characteristics. Cancer 94 : 2490–2501.
17. ComstockGW, AlbergAJ, HuangHY, WuK, BurkeAE, et al. (1997) The risk of developing lung cancer associated with antioxidants in the blood: ascorbic acid, carotenoids, alpha-tocopherol, selenium, and total peroxyl radical absorbing capacity. Cancer Epidemiol Biomarkers Prev 6 : 907–916.
18. OrsiniN, BelloccoR, BottaiM, PaganoM, MichaelssonK, et al. (2008) Combined effects of obesity and physical activity in predicting mortality among men. J Intern Med 264 : 442–451.
19. BaikI, AscherioA, RimmEB, GiovannucciE, SpiegelmanD, et al. (2000) Adiposity and mortality in men. Am J Epidemiol 152 : 264–271.
20. FolsomAR, KayeSA, SellersTA, HongCP, CerhanJR, et al. (1993) Body fat distribution and 5-year risk of death in older women. JAMA 269 : 483–487.
21. GilesGG, EnglishDR (2002) The Melbourne Collaborative Cohort Study. IARC Sci Publ 156 : 69–70.
22. TonioloPG, LevitzM, Zeleniuch-JacquotteA, BanerjeeS, KoenigKL, et al. (1995) A prospective study of endogenous estrogens and breast cancer in postmenopausal women. J Natl Cancer Inst 87 : 190–197.
23. SchatzkinA, SubarAF, ThompsonFE, HarlanLC, TangreaJ, et al. (2001) Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 154 : 1119–1125.
24. HuFB, WillettWC, LiT, StampferMJ, ColditzGA, et al. (2004) Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 351 : 2694–2703.
25. ChristenWG, GazianoJM, HennekensCH (2000) Design of Physicians' Health Study II—a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol 10 : 125–134.
26. HayesRB, RedingD, KoppW, SubarAF, BhatN, et al. (2000) Etiologic and early marker studies in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 21 : 349S–355S.
27. WolkA, LarssonSC, JohanssonJE, EkmanP (2006) Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA 296 : 1371–1376.
28. WeiderpassE, BraatenT, MagnussonC, KumleM, VainioH, et al. (2004) A prospective study of body size in different periods of life and risk of premenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 13 : 1121–1127.
29. FreedmanDM, RonE, Ballard-BarbashR, DoodyMM, LinetMS (2006) Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 30 : 822–829.
30. WhiteE, PattersonRE, KristalAR, ThornquistM, KingI, et al. (2004) VITamins and Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol 159 : 83–93.
31. RexrodeKM, LeeIM, CookNR, HennekensCH, BuringJE (2000) Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med 9 : 19–27.
32. Surveillance Epidemiology, and End Results Program (2012) SEER cause of death recode 1969+ (04/16/2012). Available: http://seer.cancer.gov/codrecode/1969+_d04162012/index.html. Accessed 3 June 2014.
33. World Health Organization (1967) International classification of diseases, 1965 revision (ICD-8). Geneva: World Health Organization.
34. World Health Organization (1977) International classification of diseases, 1975 revision (ICD-9). Geneva: World Health Organization.
35. World Health Organization (2010) International statistical classification of diseases and health related problems, tenth revision. Geneva: World Health Organization.
36. MiniñoAM, AriasE, KochanekKD, MurphySL, SmithBL (2002) Deaths: final data for 2000. Natl Vital Stat Rep 50 : 1–119.
37. CurtinLR, KleinRJ (1995) Direct standardization (age-adjusted death rates). Healthy People 2000 Stat Notes 6 : 1–10.
38. GhaliWA, QuanH, BrantR, van MelleG, NorrisCM, et al. (2001) Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA 286 : 1494–1497.
39. MakuchRW (1982) Adjusted survival curve estimation using covariates. J Chronic Dis 35 : 437–443.
40. Huedo-MedinaTB, Sánchez-MecaJ, Marín-MartínezF, BotellaJ (2006) Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 11 : 193–206.
41. ThorlundK, ImbergerG, JohnstonBC, WalshM, AwadT, et al. (2012) Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS ONE 7: e39471.
42. NikolopoulouA, KadoglouNP (2012) Obesity and metabolic syndrome as related to cardiovascular disease. Expert Rev Cardiovasc Ther 10 : 933–939.
43. CottamDR, MattarSG, Barinas-MitchellE, EidG, KullerL, et al. (2004) The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg 14 : 589–600.
44. VinciguerraF, BarattaR, Grazia FarinaM, PadovaG, VigneriR, et al. (2013) Very severely obese patients have a high prevalence of type 2 diabetes mellitus and cardiovascular disease. Acta Diabetol 50 : 443–449.
45. Maric-BilkanC (2013) Obesity and diabetic kidney disease. Med Clin North Am 97 : 59–74.
46. HofsøD, NordstrandN, JohnsonLK, KarlsenTI, HagerH, et al. (2010) Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol 163 : 735–745.
47. GoodpasterBH, DelanyJP, OttoAD, KullerL, VockleyJ, et al. (2010) Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 304 : 1795–1802.
48. RyanDH, JohnsonWD, MyersVH, PratherTL, McGloneMM, et al. (2010) Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana Obese Subjects Study. Arch Intern Med 170 : 146–154.
49. UnickJL, BeaversD, JakicicJM, KitabchiAE, KnowlerWC, et al. (2011) Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: results from the Look AHEAD trial. Diabetes Care 34 : 2152–2157.
50. JordanJGJr, MannJR (2010) Obesity and mortality in persons with obstructive lung disease using data from the NHANES III. South Med J 103 : 323–330.
51. SpencerEA, ApplebyPN, DaveyGK, KeyTJ (2002) Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr 5 : 561–565.
52. McAdamsMA, Van DamRM, HuFB (2007) Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring) 15 : 188–196.
53. ShieldsM, GorberSC, TremblayMS (2008) Effects of measurement on obesity and morbidity. Health Rep 19 : 77–84.
54. PrenticeAM, JebbSA (2001) Beyond body mass index. Obes Rev 2 : 141–147.
55. SunQ, van DamRM, SpiegelmanD, HeymsfieldSB, WillettWC, et al. (2010) Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol 172 : 1442–1454.
56. HamdyO, PorramatikulS, Al-OzairiE (2006) Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2 : 367–373.
57. PischonT, BoeingH, HoffmannK, BergmannM, SchulzeMB, et al. (2008) General and abdominal adiposity and risk of death in Europe. N Engl J Med 359 : 2105–2120.
58. CerhanJR, MooreSC, JacobsEJ, KitaharaCM, RosenbergPS, et al. (2014) A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc 89 : 335–345.
59. Al-SamarraiT, MadsenA, ZimmermanR, MaduroG, LiW, et al. (2013) Impact of a hospital-level intervention to reduce heart disease overreporting on leading causes of death. Prev Chronic Dis 10: E77.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Blue Marble Health: A Call for Papers
- Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites
- Association of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis
- Urbanicity and Lifestyle Risk Factors for Cardiometabolic Diseases in Rural Uganda: A Cross-Sectional Study
- The Importance of Implementation Strategy in Scaling Up Xpert MTB/RIF for Diagnosis of Tuberculosis in the Indian Health-Care System: A Transmission Model
- Severe Maternal Sepsis in the UK, 2011–2012: A National Case-Control Study
- Association between Class III Obesity (BMI of 40–59 kg/m) and Mortality: A Pooled Analysis of 20 Prospective Studies
- Using Evidence to Combat Overdiagnosis and Overtreatment: Evaluating Treatments, Tests, and Disease Definitions in the Time of Too Much
- Improving the Transparency of Prognosis Research: The Role of Reporting, Data Sharing, Registration, and Protocols
- Does Diagnosing Fatty Liver and Chronic Kidney Disease Do More Good Than Harm?
- Urban Development in Sub-Saharan Africa: Bearer of Goods and Risks
- Mortality after Parental Death in Childhood: A Nationwide Cohort Study from Three Nordic Countries
- Defining Catastrophic Costs and Comparing Their Importance for Adverse Tuberculosis Outcome with Multi-Drug Resistance: A Prospective Cohort Study, Peru
- Cesarean Section and Rate of Subsequent Stillbirth, Miscarriage, and Ectopic Pregnancy: A Danish Register-Based Cohort Study
- Effects of BMI, Fat Mass, and Lean Mass on Asthma in Childhood: A Mendelian Randomization Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Association of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis
- Using Evidence to Combat Overdiagnosis and Overtreatment: Evaluating Treatments, Tests, and Disease Definitions in the Time of Too Much
- Association between Class III Obesity (BMI of 40–59 kg/m) and Mortality: A Pooled Analysis of 20 Prospective Studies
- Blue Marble Health: A Call for Papers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



