-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis
Background:
Chronic kidney disease (CKD) is a frequent, under-recognized condition and a risk factor for renal failure and cardiovascular disease. Increasing evidence connects non-alcoholic fatty liver disease (NAFLD) to CKD. We conducted a meta-analysis to determine whether the presence and severity of NAFLD are associated with the presence and severity of CKD.Methods and Findings:
English and non-English articles from international online databases from 1980 through January 31, 2014 were searched. Observational studies assessing NAFLD by histology, imaging, or biochemistry and defining CKD as either estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 or proteinuria were included. Two reviewers extracted studies independently and in duplicate. Individual participant data (IPD) were solicited from all selected studies. Studies providing IPD were combined with studies providing only aggregate data with the two-stage method. Main outcomes were pooled using random-effects models. Sensitivity and subgroup analyses were used to explore sources of heterogeneity and the effect of potential confounders. The influences of age, whole-body/abdominal obesity, homeostasis model of insulin resistance (HOMA-IR), and duration of follow-up on effect estimates were assessed by meta-regression. Thirty-three studies (63,902 participants, 16 population-based and 17 hospital-based, 20 cross-sectional, and 13 longitudinal) were included. For 20 studies (61% of included studies, 11 cross-sectional and nine longitudinal, 29,282 participants), we obtained IPD. NAFLD was associated with an increased risk of prevalent (odds ratio [OR] 2.12, 95% CI 1.69–2.66) and incident (hazard ratio [HR] 1.79, 95% CI 1.65–1.95) CKD. Non-alcoholic steatohepatitis (NASH) was associated with a higher prevalence (OR 2.53, 95% CI 1.58–4.05) and incidence (HR 2.12, 95% CI 1.42–3.17) of CKD than simple steatosis. Advanced fibrosis was associated with a higher prevalence (OR 5.20, 95% CI 3.14–8.61) and incidence (HR 3.29, 95% CI 2.30–4.71) of CKD than non-advanced fibrosis. In all analyses, the magnitude and direction of effects remained unaffected by diabetes status, after adjustment for other risk factors, and in other subgroup and meta-regression analyses. In cross-sectional and longitudinal studies, the severity of NAFLD was positively associated with CKD stages. Limitations of analysis are the relatively small size of studies utilizing liver histology and the suboptimal sensitivity of ultrasound and biochemistry for NAFLD detection in population-based studies.Conclusion:
The presence and severity of NAFLD are associated with an increased risk and severity of CKD.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(7): e32767. doi:10.1371/journal.pmed.1001680
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001680Summary
Background:
Chronic kidney disease (CKD) is a frequent, under-recognized condition and a risk factor for renal failure and cardiovascular disease. Increasing evidence connects non-alcoholic fatty liver disease (NAFLD) to CKD. We conducted a meta-analysis to determine whether the presence and severity of NAFLD are associated with the presence and severity of CKD.Methods and Findings:
English and non-English articles from international online databases from 1980 through January 31, 2014 were searched. Observational studies assessing NAFLD by histology, imaging, or biochemistry and defining CKD as either estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 or proteinuria were included. Two reviewers extracted studies independently and in duplicate. Individual participant data (IPD) were solicited from all selected studies. Studies providing IPD were combined with studies providing only aggregate data with the two-stage method. Main outcomes were pooled using random-effects models. Sensitivity and subgroup analyses were used to explore sources of heterogeneity and the effect of potential confounders. The influences of age, whole-body/abdominal obesity, homeostasis model of insulin resistance (HOMA-IR), and duration of follow-up on effect estimates were assessed by meta-regression. Thirty-three studies (63,902 participants, 16 population-based and 17 hospital-based, 20 cross-sectional, and 13 longitudinal) were included. For 20 studies (61% of included studies, 11 cross-sectional and nine longitudinal, 29,282 participants), we obtained IPD. NAFLD was associated with an increased risk of prevalent (odds ratio [OR] 2.12, 95% CI 1.69–2.66) and incident (hazard ratio [HR] 1.79, 95% CI 1.65–1.95) CKD. Non-alcoholic steatohepatitis (NASH) was associated with a higher prevalence (OR 2.53, 95% CI 1.58–4.05) and incidence (HR 2.12, 95% CI 1.42–3.17) of CKD than simple steatosis. Advanced fibrosis was associated with a higher prevalence (OR 5.20, 95% CI 3.14–8.61) and incidence (HR 3.29, 95% CI 2.30–4.71) of CKD than non-advanced fibrosis. In all analyses, the magnitude and direction of effects remained unaffected by diabetes status, after adjustment for other risk factors, and in other subgroup and meta-regression analyses. In cross-sectional and longitudinal studies, the severity of NAFLD was positively associated with CKD stages. Limitations of analysis are the relatively small size of studies utilizing liver histology and the suboptimal sensitivity of ultrasound and biochemistry for NAFLD detection in population-based studies.Conclusion:
The presence and severity of NAFLD are associated with an increased risk and severity of CKD.
Please see later in the article for the Editors' SummaryIntroduction
Chronic kidney disease (CKD) affects 4%–13% of the Western adult population and over 25% of individuals older than 65 years [1]. CKD prevalence is continuously rising in concert with the rising epidemic of its risk factors including ageing, diabetes, obesity, metabolic syndrome, smoking, and hypertension [2]–[4]. In the United States, over 400,000 people currently receive some form of renal replacement therapy, and this number is expected to reach 2.2 million by 2030 [2]. Beside being a risk factor for end-stage renal disease (ESRD), CKD is an important cardiovascular disease (CVD) risk factor, and most patients with CKD die from CVD before any renal replacement therapy is initiated [5].
Early recognition and treatment of CKD aimed at reducing renal disease progression and CVD complications may limit its health-related burden [4]. In particular, patients with stage 3 CKD benefit the most from early referral strategies [6]. Despite these premises, CKD often goes unrecognized: in the Third National Health and Nutrition Survey (NHANES III), among all individuals with stage 3 CKD, the awareness was only 8.2% [7].
The high morbidity, mortality, and health care costs associated with CKD have led investigators to seek novel modifiable risk factors. Non-alcoholic fatty liver disease (NAFLD), the hepatic manifestation of the metabolic syndrome, affects 30% of the general adult population and up to 60%–70% of diabetic and obese patients [8]. NAFLD encompasses a histological spectrum ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), the latter with or without advanced fibrosis. NAFLD confers an increased risk of cirrhosis, largely limited to NASH, and of CVD, independently of metabolic syndrome and traditional risk factors and through mechanisms which remain unclear [9]. Growing experimental and epidemiological evidence suggests that NAFLD and CKD share common pathogenic mechanisms and interactions [10]. However, evidence of a link between NAFLD and CKD is uncertain due to the small study populations and the borderline associations between NAFLD and traditional risk factors for CKD in the published literature. A meta-analysis on the association of NAFLD and CKD has not been conducted to date. We therefore analysed the evidence regarding two research questions: (1) Does NAFLD affect the risk of CKD independent of major confounders? (2) Is NAFLD severity associated with the severity of CKD?
Methods
Data Sources and Searches
We searched English and non-English language publications on MEDLINE, Ovid MEDLINE In-Process, EMBASE, ISI Web of Science, and Cochrane Library, and abstracts from the annual American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), the European Association for the Study of the Liver (EASL), the Digestive Disease Week (DDW), and the American Society of Nephrology (ASN) Kidney Week meetings from 1980 through January 31, 2014. Search terms were: chronic kidney disease OR CKD OR kidney function OR kidney failure OR renal disease OR renal insufficiency OR renal failure OR glomerular filtration rate (GFR) OR estimated glomerular filtration rate (eGFR) OR creatinine OR albuminuria OR microalbuminuria OR macroalbuminuria OR proteinuria OR kidney injury AND NASH OR NAFLD OR non-alcoholic steatohepatitis OR non-alcoholic fatty liver disease OR fatty liver OR liver fat OR steatosis OR liver enzymes OR transaminase OR ALT OR AST OR GGT OR severity of liver disease OR fibrosis. A full list of the search strategies in different databases is reported in Text S2.
Study Selection
Inclusion criteria
Criteria were observational studies including adult (age ≥18 y) populations of any sex or ethnicity, with a diagnosis of NAFLD and CKD. NAFLD had to be diagnosed by (1) liver histology, (2) imaging (ultrasound, computer tomography, magnetic resonance imaging, or spectroscopy), or (3) biochemistry (elevations in serum AST, ALT, or GGT). Competing causes of steatosis, including alcohol consumption and viral hepatitis infection had to be excluded according to standard guidelines [8]. The presence of CKD had to be defined by (1) persistent (>3 months) GFR<60 ml/min/1.73 m2, as estimated using the creatinine-based Modified Diet in Renal Disease (MDRD) or Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations [11],[12] or cystatin C–based equation [13], (2) by creatinine clearance <60 ml/min per 1.73 m2 using 24-hour urinary studies, (3) persistent (>3 months) kidney damage (regardless of GFR), as defined by proteinuria (microalbuminuria or macroalbuminuria using albumin-to-creatinine ratio, 24-h albumin excretion rate, or proteinuria on fresh morning urine dipstick), (4) other abnormalities due to tubular disorders or structural abnormalities detected by electrolyte or urinary sediment alterations, histology, imaging, or (5) history of kidney transplantation [2].
Exclusion criteria
Excluded from the meta-analysis were nnon-human studies, letters/case reports, studies including fewer than ten individuals, articles not reporting outcomes of interest or primary data (editorials, reviews), or using inadequate case definitions. In particular, studies were excluded that did not adequately consider competing causes of hepatic steatosis including alcohol, or viral hepatitis, or that enrolled a mixed population of cirrhotic and non-cirrhotic individuals (due to the potential confounding effects of cirrhosis per se on GFR).
Outcome Measures
Primary outcome measures
Primary outcome measures were differences in the prevalence or incidence of CKD. We compared the risk of primary outcomes between individuals with NAFLD and without NAFLD as well as across the main histological subtypes of NAFLD, since NASH and advanced fibrosis carry a significantly worse prognosis than steatosis and milder fibrosis stages, respectively [9]. The impact of NAFLD and of NAFLD histological subtypes (NASH, advanced fibrosis) on eGFR, treated as a continuous variable, and on proteinuria, was also examined.
Secondary outcome measures
The severity of CKD was the secondary outcome measure. We estimated the effect of the severity of liver disease in NAFLD, as defined by NASH or advanced fibrosis, on the stage of CKD. CKD stage was categorized by GFR according to recent guidelines into CKD stage 3b (eGFR 30–44 ml/min/1.73 m2, CKD stage 4 (eGFR 15–29 ml/min/1.73 m2), and CKD stage 5 (eGFR<15 ml/min/1.73 m2) [2].
Data Extraction and Quality Assessment
Data were extracted from each study independently and in duplicate by two authors (GM, RG), using a predefined protocol (supplied in Text S2) and a data extraction sheet based on the Cochrane Handbook for Systematic Reviews of Intervention [14]. The analysis was carried out in concordance with the Cochrane Handbook of Systematic Reviews and reported according to PRISMA guidelines (Text S1) [15]. The initial agreement between the two reviewers for selection and validity assessment of the studies was evaluated by the Kappa coefficient. Discrepancies between the reviewers were resolved by joint discussion and mutual agreement.
Methodological quality of studies was assessed by the 22-item STROBE score [16], with two items additionally incorporated into the checklist. For imaging assessment of NAFLD, examiners had to be blinded to clinical data, and the exam had to follow pre-specified, standardized criteria to detect steatosis [17]. For histological assessment of NAFLD, adequate biopsy specimens with a fragment length ≥1.5 cm with more than six portal tracts had to be obtained and scored by a blinded pathologist according to standard criteria [8].
Data Synthesis and Analysis
For all included studies, individual participant data (IPD) was solicited from principal investigators (PIs). PIs were asked to provide the most complete and updated data, even if the follow-up was longer than that used for their respective publications. The quality of the submitted IPD was assessed using pre-specified methods (see protocol in Text S2), and any inconsistencies were clarified with the PIs.
Data not available upon database closure, either because the IPD had not been provided or because full manuscripts had not been published, were not included in our analyses.
For all analyses, we combined studies providing IPD and studies providing aggregate data (AD) into a pooled effect measure using the two-stage method [18],[19]: first, the available IPD were reduced to AD in each study, then these AD (from the IPD studies) were combined with the existing AD (from the AD studies) using standard meta-analysis techniques.
In reducing IPD to AD, for dichotomous outcomes we used multivariate logistic regression in cross-sectional studies to obtain log odds ratio (OR) with its standard error (SE), and Cox proportional hazard model in longitudinal studies (all providing time-to-event data) to obtain log hazard ratio (HR) and its SE separately for each study. We then combined individual ORs (for cross-sectional studies) and HRs (for longitudinal studies) and their 95% CIs from all included studies. Associations with continuous outcome variables were expressed as weighted mean differences (WMD) with 95% CI. Only the most adjusted risk estimates that were reported in the studies were included in the analysis. All measures of dispersion were converted to standard deviations (SDs).
The study-specific risk estimates were pooled using random-effects model, because this approach provides a more conservative assessment of the average effect size than fixed-effects model. Significance was set at p = 0.05.
The I2 statistic and its 95% CI [20]were calculated to assess statistical heterogeneity across studies: 0% suggests no heterogeneity, 0%–25% very low heterogeneity, 25%–50% low heterogeneity, 50%–75% moderate heterogeneity, and a value of >75% high heterogeneity [14]. In case of I2 values ≥50%, we explored individual study characteristics and those of subgroups of the main body of evidence.
We separately analyzed cross-sectional and longitudinal studies. Furthermore, for each outcome, the results of studies defining NAFLD by histology, imaging, or liver enzyme elevation are presented separately.
Sensitivity analysis was performed by repeating the meta-analysis after one study at a time was removed to assess whether any one study significantly affected pooled estimates. Additionally, a number of subgroup analyses were planned a priori. These subgroup analyses included repeated analysis after excluding studies not fulfilling each STROBE item, and separate analyses for the following items: diabetes: we examined the effect of NAFLD on CKD in non-diabetic versus diabetic individuals, to assess if the presence of diabetes affects the association of NAFLD with CKD; studies simultaneously adjusting versus studies not adjusting for all the following risk factors for CKD: age, body mass index (BMI), metabolic syndrome (overall or each of its components), hypertension, smoking status; study design: population-based versus hospital-based; ethnicity (Asian versus non-Asian population), as defined by the investigators. As highlighted by the recent report of the Third Asian Forum of Chronic Kidney Disease Initiatives, there are striking differences in risk factors for CKD between Asian ancestry and the remaining ethnicities: as an example, chronic glomerulonephritis due to IgA nephropathy is among the three leading causes of CKD in Asians, while it is far less common in the rest of the world. Different susceptibility loci involved in innate and adaptive immunity have been identified in recent genome-wide association studies (GWAS), but also environmental factors like infections and regular consumption of herbal remedies, may underlie these epidemiological differences [21]–[23]. Similarly, in Asians NAFLD is often encountered in the absence of obesity and metabolic syndrome and a different genetic background (including different Apolipoprotein C3 gene variants as an example), has been proposed to account for these ethnic differences [24].
We therefore explored whether differences in epidemiology of NAFLD and CKD between Asian and non-Asian populations affect the association of NAFLD with CKD studies including exclusively non-cirrhotic patients versus studies including exclusively cirrhotic patients; methods used to estimate GFR; outcomes related to CKD: studies assessing both eGFR and proteinuria versus studies assessing solely eGFR or proteinuria; study data availability: studies providing IPD versus studies providing exclusively AD.
When eight or more comparisons were available, the effect of continuous variables including age, whole-body and abdominal obesity (as estimated by BMI and by waist circumference, respectively) [25], insulin resistance (estimated by homeostasis model assessment of insulin resistance [HOMA-IR] index), and duration of follow-up (for longitudinal studies) on the association between NAFLD and CKD was evaluated by meta-regression analysis (random effects model, within-study variance estimated with the unrestricted maximum-likelihood method).
Small study bias was examined by constructing funnel plots and by performing the Egger's test and the trim-and-fill analysis [26].
Additionally, for the primary end-point we separately performed a one-stage meta-analysis of studies providing IPD, to examine how the association of NAFLD with CKD was altered when individual patient level covariates were accounted for [19],[20]. In this analysis, data from all studies providing IPD were pooled together into a single dataset and effect estimates were calculated using multivariate logistic regression (cross-sectional studies) or Cox proportional hazard models (longitudinal studies). In these models, studies were incorporated as cluster and treated as random-effect, while covariates were treated as fixed-effect. The covariates entered in the models were age, BMI, metabolic syndrome, diabetes, hypertension, smoking status, ethnicity (Asian versus non-Asian population), presence of cirrhosis, waist circumference, HOMA-index, duration of follow-up (for longitudinal studies. We first analyzed the influence of each single pre-specified covariate on the association of NAFLD with CKD with NAFLD and covariate as fixed-effect and the study as random-effects. In a second step, we did a complete case multivariable analysis with respect to NAFLD and all pre-specified covariates.
We used RevMan 5.2 (Nordic Cochrane Center) and SAS 9.2 (SAS Institute) for additional analyses that could not be done with RevMan. The trim-and-fill analysis was performed with Comprehensive Meta-analysis 2.0 (Biostat).
Results
The mean (standard deviation [SD]) agreement between the two reviewers for study selection and for quality assessment were 0.89 (0.02) and 0.91 (0.04), respectively. The flow of study selection is reported in Figure 1.
Fig. 1. Flow of study selection. 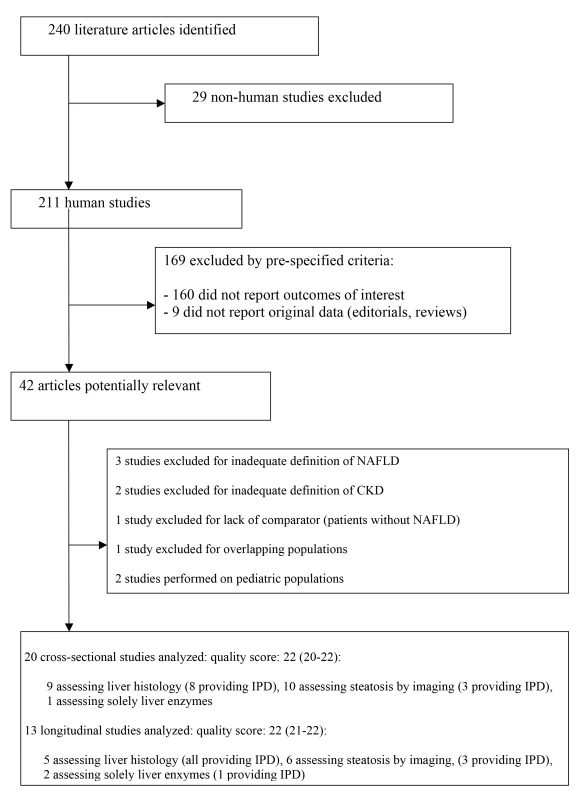
STROBE score of included studies is provided as median (range). Thirty-three studies (63,902 participants, 16 population-based and 17 hospital-based, 20 cross-sectional and 13 longitudinal) were included (Tables 1 and 2) [27]–[59]. Twenty studies (34,939 participants) were cross-sectional and evaluated the association of NAFLD with prevalent CKD [27]–[46]; 13 studies (28,963 participants) were longitudinal (mean duration of follow-up ranging three to 27 years) and evaluated the association of NAFLD with new-onset CKD [47]–[59].
Tab. 1. Cross-sectional studies connecting NAFLD to chronic kidney disease included in the meta-analysis. 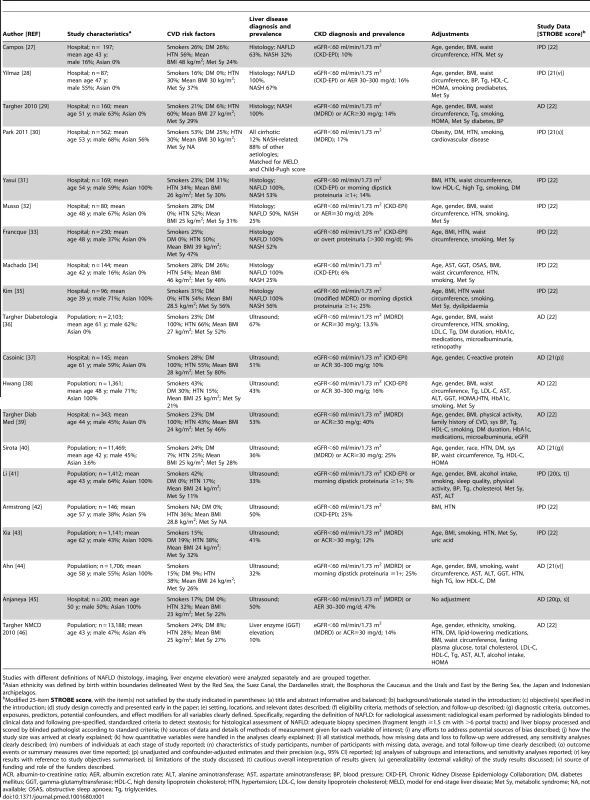
Studies with different definitions of NAFLD (histology, imaging, liver enzyme elevation) were analyzed separately and are grouped together. Tab. 2. Longitudinal studies connecting NAFLD to chronic kidney disease included in the meta-analysis. 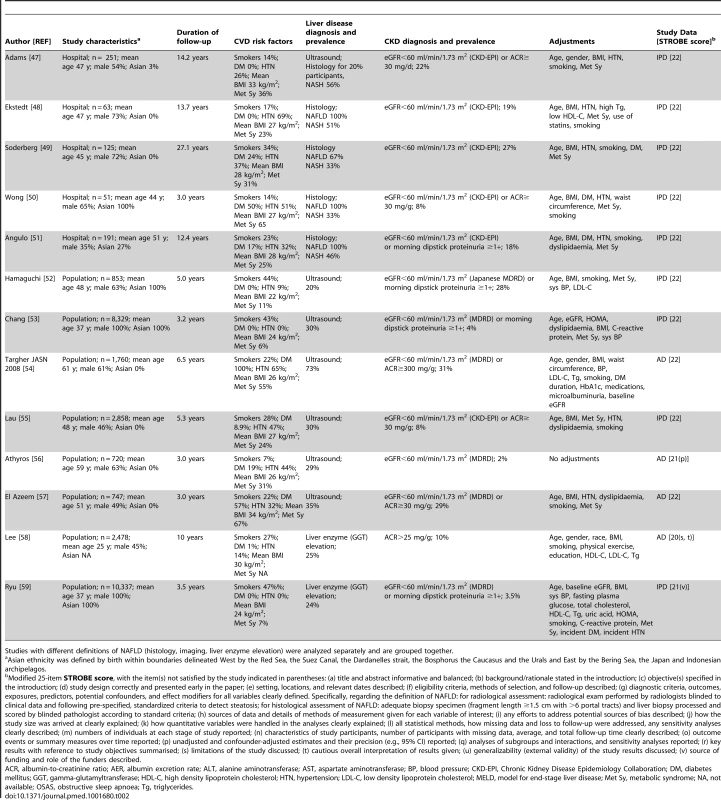
Studies with different definitions of NAFLD (histology, imaging, liver enzyme elevation) were analyzed separately and are grouped together. We obtained IPD for 20 studies (61% of included studies, 29,282 participants), including 11 cross-sectional studies (5,145 participants) (Table 1) [27],[28],[30]–[35],[41]–[43] and nine longitudinal studies (24,137 participants) (Table 2) [47]–[53],[55],[59].
NAFLD was defined by liver histology in 13 studies (2,205 participants) [27]–[35],[48]–[51], by ultrasound in 17 studies (35,694 participants) [36]–[45],[52]–[57], and exclusively by liver enzyme elevation in three studies (26,003 participants) (Tables 1 and 2) [46],[58],[59].
Overall, the methodological quality of the studies was good: the median (range) STROBE score was 21 (20–22). Three studies did not report confounder-adjusted estimates and their precision (STROBE item [p]) [37],[45],[56], four studies did not discuss their limitations (item [s]) [30],[41],[45],[58], three studies did not disclose funding sources and role of the funders (item [v]) [28],[44],[59], two studies did not give a cautious overall interpretation of results (item [t]) [41],[58], and one study set the diagnosis of steatosis retrospectively based on archived images of gallbladder ultrasound examinations (item [g]) (Tables 1 and 2; Figure S1 within Text S3) [40].
Twelve studies enrolled exclusively non-diabetic individuals [28],[32],[33],[35],[41],[42],[45],[47],[48],[52],[52,59], four studies enrolled exclusively diabetic patients [36],[37],[39],[54], 11 studies evaluated diabetic and non-diabetic participants separately [27],[30],[31],[34],[38],[43],[46],[49],[50],[51],[55]. Overall, separate risk estimates for diabetic and non-diabetic individuals were obtained in 27 studies (82%, 47342 participants).
Twenty-eight studies (85% of all studies, 97% of participants) adjusted for potential confounders, including all of the following: age, BMI, metabolic syndrome (overall and each component), hypertension, and smoking (Tables 1, 2, and 3) [27]–[29],[31]–[36],[38]–[41],[43],[44],[46]–[55],[57]–[59].
Tab. 3. Results of subgroup analysis for the outcome: chronic kidney disease. 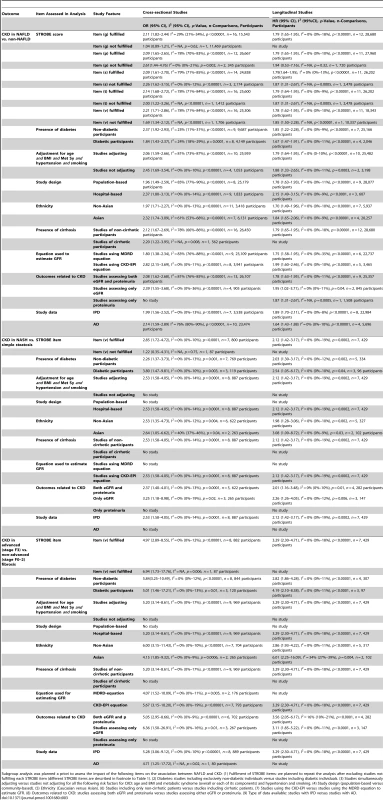
Subgroup analysis was planned a priori to assess the impact of the following items on the association between NAFLD and CKD: (1) Fulfilment of STROBE items: we planned to repeat the analysis after excluding studies not fulfilling each STROBE item (different STROBE items are described in footnote to Table 1). (2) Diabetes: studies including exclusively non-diabetic individuals versus studies including diabetic individuals. (3) Studies simultaneously adjusting versus studies not adjusting for all the following risk factors for CKD: age and BMI and metabolic syndrome (overall or each of its components) and hypertension and smoking. (4) Study design (population-based versus community-based). (5) Ethnicity (Caucasian versus Asian). (6) Studies including only non-cirrhotic patients versus studies including cirrhotic patients. (7) Studies using the CKD-EPI versus studies using the MDRD equation to estimate GFR. (8) Outcomes related to CKD: studies assessing both eGFR and proteinuria versus studies assessing either eGFR or proteinuria. (9) Type of data available: studies with IPD versus studies with AD. Eleven studies enrolled exclusively Asian populations [31],[35],[38],[41],[43],[44],[45],[50],[52],[53],[59], 15 studies enrolled exclusively non-Asian individuals [27]–[29],[32]–[34],[36],[37],[39],[48],[49],[54]–[57], four studies evaluated separately Asian and non-Asian participants [27],[30],[42],[47],[51]. Overall, separate risk estimates for Asian and non-Asian individuals were obtained in 30 studies (91%, 36,767 participants).
All studies included non-cirrhotic participants, except one cross-sectional study comparing NASH-related cirrhosis with cirrhosis of other aetiologies, matched for Child-Pugh and Model for End-stage Liver Disease-(MELD) scores [30].
GFR was estimated with the CKD-EPI equation in 16 studies [27],[28],[31]–[34],[37],[38],[41],[42],[47]–[51],[55] and with the MDRD equation in 17 studies [29],[30],[35],[36],[39],[40],[43]–[46],[52]–[54],[56],[57],[59]. One study assessed only proteinuria and not eGFR [58], while seven studies (21%) evaluated only eGFR and not proteinuria [27],[30],[34],[42],[48],[49],[56].
NAFLD and Prevalent/Incident CKD
In cross-sectional studies, pooled OR for the presence of CKD of NAFLD versus non-NAFLD was 2.12 (95% CI 1.69–2.66, I2 = 77% [95% CI 66%–84%], N-comparisons = 17, p<0.00001) (Figure 2). The magnitude and direction of the effect were similar across different NAFLD definitions (Figure 2). Heterogeneity was high, due to the high heterogeneity in studies assessing NAFLD by ultrasound, but fell after excluding one study [40], where the diagnosis of steatosis was made retrospectively on the basis of archived videotapes of gallbladder ultrasound examinations, while pooled OR remained similar in magnitude and direction of the overall effect (OR 2.11, 95% CI 1.82–2.44; I2 = 29% [95% CI 15%–35%], n = 16, p<0.00001) (Table 3).
Fig. 2. Forest plot of comparison. 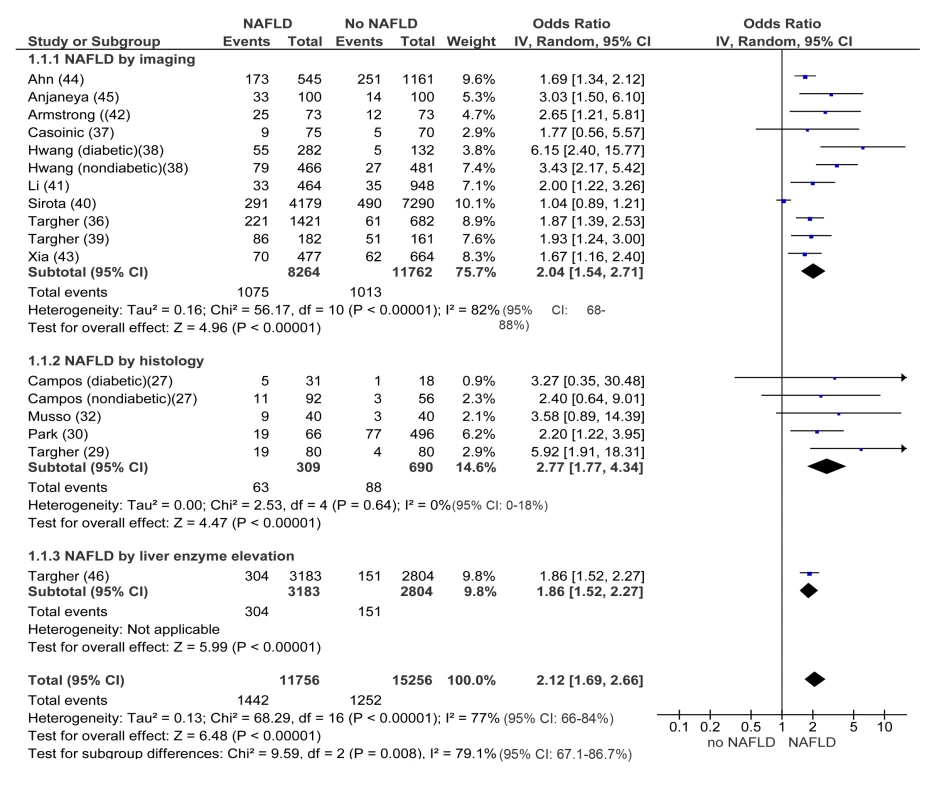
NAFLD versus non-NAFLD, outcome: prevalent chronic kidney disease in cross-sectional studies. Studies assessing NAFLD by imaging, histology or liver enzyme elevation were considered separately. In longitudinal studies, pooled HR for incident CKD of NAFLD versus non-NAFLD was 1.79 (95% CI 1.65–1.95, I2 = 0% [95% CI 0%–18%], n comparisons = 13, p<0.00001) (Figure 3). There was no heterogeneity in the meta-analysis of overall events, suggesting a consistent disease effect.
Fig. 3. Forest plot of comparison. 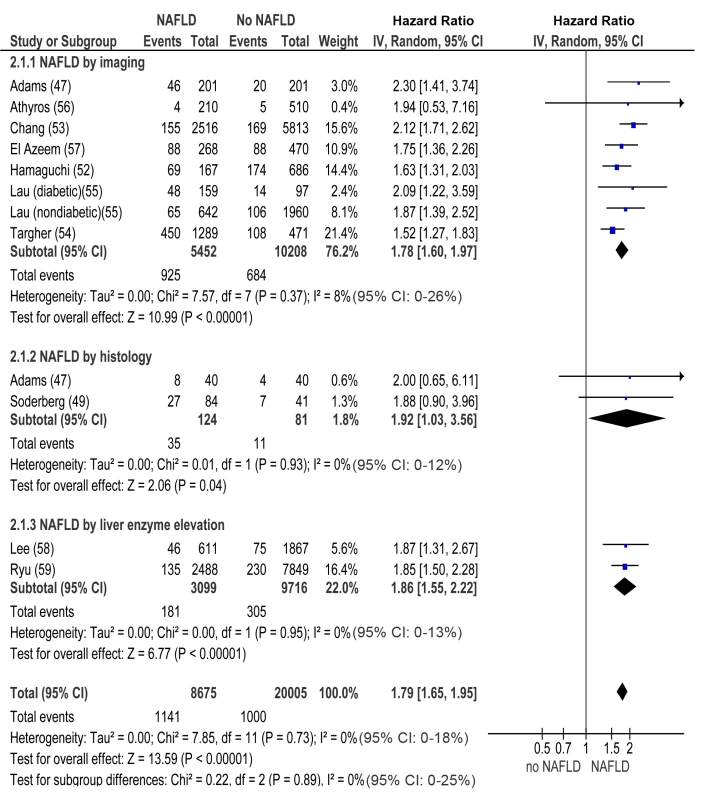
NAFLD versus non-NAFLD, outcome: incident chronic kidney disease in prospective studies. NAFLD was defined by imaging, histology, or liver enzyme elevation. Studies assessing NAFLD by imaging, histology, or liver enzyme elevation were considered separately. In both cross-sectional and longitudinal studies, the difference between NAFLD and non-NAFLD patients remained statistically significant even when considering eGFR as a continuous variable or when considering only proteinuria as outcome (Figures S2A, S2B, S3A, and S3B within Text S3).
In both cross-sectional and longitudinal studies, meta-regression analysis found no association between CKD and age (cross-sectional studies: β = 0.004, 95% CI −0.023 to 0.031, p = 0.772; longitudinal studies: β = 0.005, 95% CI −0.014 to 0.021, p = 0.207), BMI (cross-sectional studies: β = 0.013, 95% CI −0.034 to 0.059, p = 0.592; longitudinal studies: β = 0.003, 95% CI −0.019 to 0.026, p = 0.786), waist circumference (cross-sectional studies: β = −0.003, 95% CI −0.023 to 0.031, p = 0.772; longitudinal studies: β = −0.003, 95% CI −0.016 to 0.011, p = 0.686), HOMA-IR index (cross-sectional studies: β = 0.089, 95% CI −0.210 to 0.388, p = 0.559; longitudinal studies: β = −0.041, 95% CI −0.171 to 0.087, p = 0.524), and duration of follow-up (longitudinal studies: β = 0.002, 95% CI −0.022 to 0.026, p = 0.880).
The Egger's test found no strong evidence for small study bias and the trim-and-fill analysis did not appreciably attenuate the strength of the association (Figures S4A and S4B within Text S3).
NAFLD Histological Subtypes and the Risk of CKD in Non-cirrhotic NAFLD
Cross-sectional studies
In cross-sectional studies, pooled OR for CKD of NASH versus steatosis was 2.53 (95% CI 1.58–4.05, I2 = 0% [95% CI 0%–14%], n-comparisons = 8, p = 0.0001) (Figure 4). Pooled OR for CKD of advanced (stage F3) versus non-advanced (stage F0–F2) fibrosis was 5.20 (95% CI 3.14–8.61, I2 = 0% [95% CI 0%–17%], n = 9, p<0.00001) (Figure 4). There was no heterogeneity in the meta-analyses of overall events, suggesting a consistent disease effect.
Fig. 4. Forest plot of comparison. 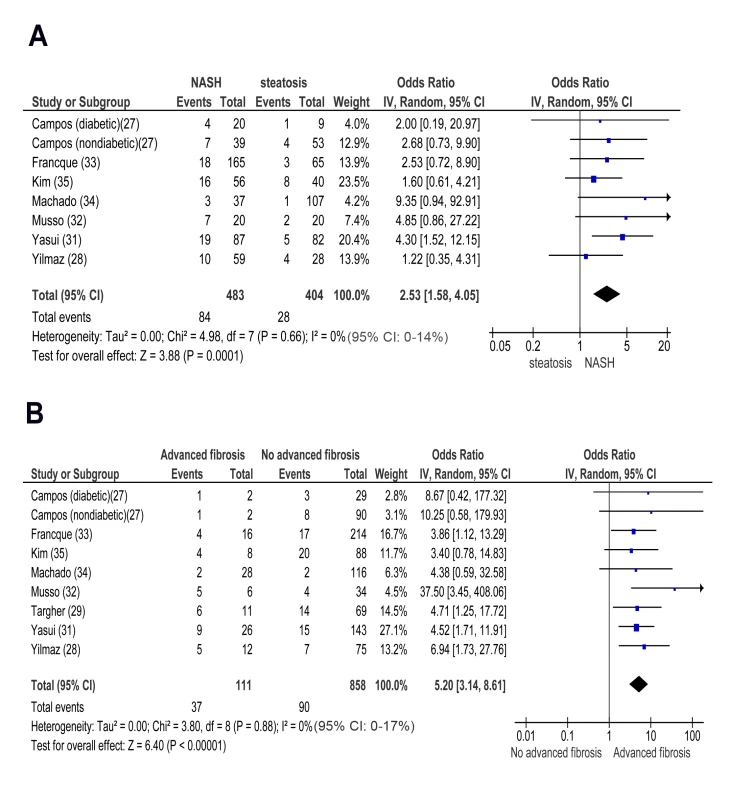
(A) NASH versus simple steatosis in biopsy-proven non-cirrhotic NAFLD; outcome: prevalent chronic kidney disease in cross-sectional studies. (B) Advanced (stage F3) fibrosis versus no-advanced (stage F0–F2) fibrosis in biopsy-proven non-cirrhotic NAFLD, outcome: prevalent CKD in cross-sectional studies. NASH and advanced fibrosis were also associated with higher ORs for proteinuria and with a lower eGFR than steatosis and non-advanced fibrosis, respectively (Figures S5A, S5B, S6A, and S6B within Text S3).
Meta-regression analysis found no association between CKD and age (for NASH: β = 0.050, 95% CI −0.039 to 0.140, p = 0.269; for advanced fibrosis: β = 0.002, 95% CI −0.101 to 0.105, p = 0.964), BMI (for NASH: β = 0.003, 95% CI −0.049 to 0.056, p = 0.896; for advanced fibrosis: β = 0.002, 95% CI −0.007 to 0.065, p = 0.949), waist circumference (for NASH: β = 0.004, 95% CI −0.031 to 0.040, p = 0.812; for advanced fibrosis: β = −0.004, 95% CI −0.043 to 0.034, p = 0.820), and HOMA-IR index (for NASH: β = −0.231, 95% CI −0.691 to 0.229, p = 0.324; for advanced fibrosis: β = −0.161, 95% CI −0.705 to 0.383, p = 0.562).
The Egger's test found no strong evidence for small study bias and the trim-and-fill analysis did not appreciably attenuate the strength of the association (Figures S4A–S4D within Text S3).
Longitudinal studies
In longitudinal studies, pooled HR for incident CKD of NASH versus simple steatosis was 2.12 (95% CI 1.42–3.17, I2 = 0% [95% CI 0%–19%, n-comparisons = 7, p = 0.0002) (Figure 5). Pooled HR for CKD of advanced fibrosis versus non-advanced fibrosis was 3.29 (95% CI 2.30–4.71, I2 = 0% [95% CI 0%–18%], n = 7, p<0.00001) (Figure 5). There was no heterogeneity in the meta-analyses of overall events, again suggesting a consistent disease effect.
Fig. 5. Forest plot of comparison. 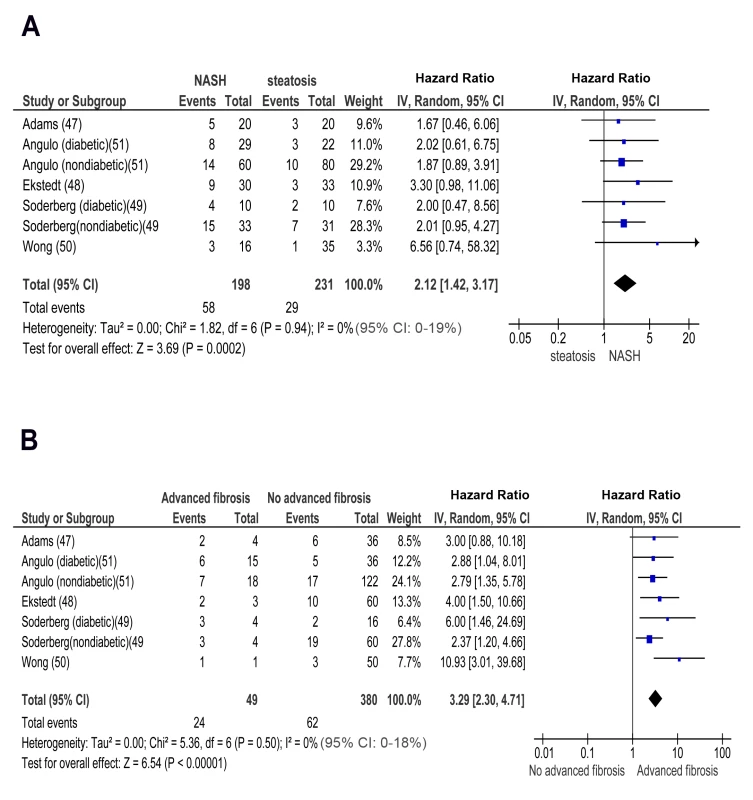
(A) NASH versus simple steatosis in biopsy-proven noncirrhotic NAFLD; outcome: incident CKD in prospective studies. (B) Advanced (stage F3) fibrosis versus no-advanced (stage F0–F2) fibrosis in biopsy-proven non-cirrhotic NAFLD, outcome: incident CKD in prospective studies. NASH and advanced fibrosis were also associated with a higher OR for incident proteinuria and with more severe eGFR reduction than steatosis and non-advanced fibrosis, respectively (Figures S7A, S7B, S8A, and S8B within Text S3).
Meta-regression analysis found no association between CKD and age (for NASH: β = −0.019, 95% CI −0.113 to 0.774, p = 0.681; for advanced fibrosis: β = −0.007, 95% CI −0.088 to 0.074, p = 0.868), BMI (for NASH: β = −0.106, 95% CI −0.366 to 0.154, p = 0.425; for advanced fibrosis: β = −0.075, 95% CI −0.307 to 0.158, p = 0.529), waist circumference (for NASH: β = −0.026, 95% CI −0.116 to 0.060, p = 0.559; for advanced fibrosis: β = −0.026, 95% CI −0.101 to 0.050, p = 0.508), HOMA-IR index (for NASH: β = 0.167, 95% CI −0.153 to 0.487, p = 0.306; for advanced fibrosis: β = 0.048, 95% CI −0.376 to 0.472, p = 0.825) and duration of follow-up (for NASH: β = −0.012, 95% CI −0.067 to 0.043, p = 0.675; for advanced fibrosis: β = −0.006, 95% CI −0.058 to 0.046, p = 0.817).
The Egger's test found no strong evidence for small study bias and the trim-and-fill analysis did not appreciably attenuate the strength of the association (Figures S4E and S4F within Text S3).
NAFLD Histological Subtypes and the Stage of CKD in Non-cirrhotic NAFLD
Cross-sectional studies
In cross-sectional studies, pooled OR for CKD stage 3b of NASH versus steatosis was 3.38 (95% CI 1.11–10.31, I2 = 0% [95% CI 0%–17%], n-comparisons = 8, p = 0.03) (Figure S9A within Text S3). Pooled OR for CKD stage 3b of advanced versus non-advanced fibrosis was 26.98 (95% CI 9.12–79.84, I2 = 0% [95% CI 0%–21%], n = 9 p<0.00001) (Figure S9B within Text S3). There was no heterogeneity in the meta-analysis of overall events, suggesting a consistent disease effect.
The presence of serum creatinine elevation, configuring severely decreased renal function (CKD stage 4) or renal failure (CKD stage 5), was an exclusion criterion in cross-sectional studies, which focused on the association of NAFLD with clinically unrecognized (stage 1–3) CKD.
Longitudinal studies
In longitudinal studies, pooled HR for CKD stage 3b, 4, and 5 (renal failure) was significantly higher in NASH versus steatosis: OR for CKD stage 3b: 2.49 (95% CI 1.21–5.13, I2 = 0% [95% CI 0%–21%], n-comparisons = 7, p = 0.01); OR for CKD stage 4 : 3.45 (95% CI 1.15–10.39, I2 = 0% [95% CI 0%–18%], n-comparisons = 6, p = 0.03); OR for CKD stage 5 : 3.87 (95% CI 1.10–13.58, I2 = 0% [95% CI 0%–16%], n-comparisons = 6, p = 0.03) (Figures 6 and 7).
Fig. 6. Forest plot of comparison. 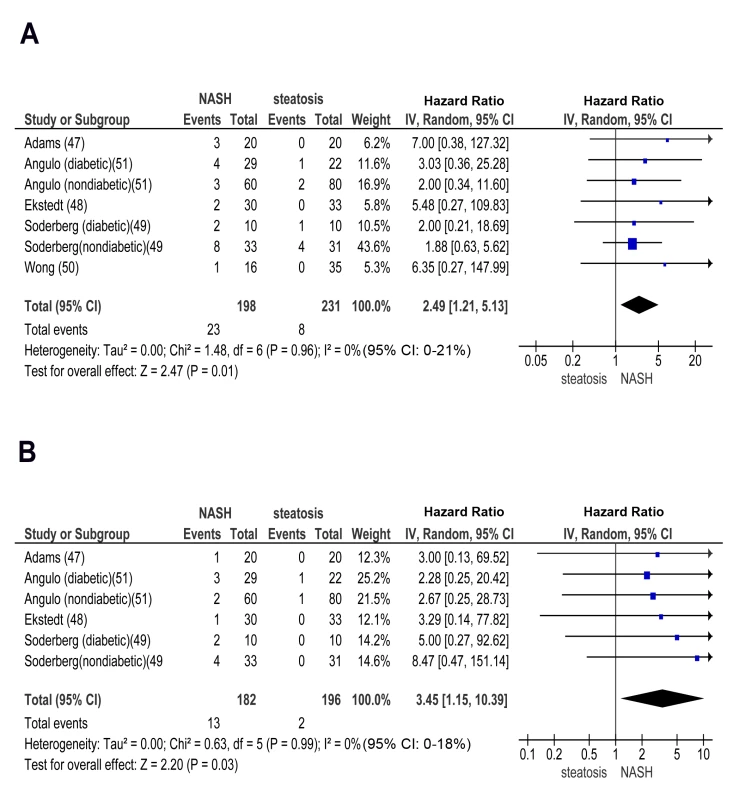
(A) NASH versus simple steatosis in biopsy-proven non-cirrhotic NAFLD; outcome: incident CKD stage 3b in prospective studies. (B) NASH versus simple steatosis in biopsy-proven non-cirrhotic NAFLD; outcome: incident CKD stage 4 in prospective studies. Fig. 7. Forest plot of comparison. 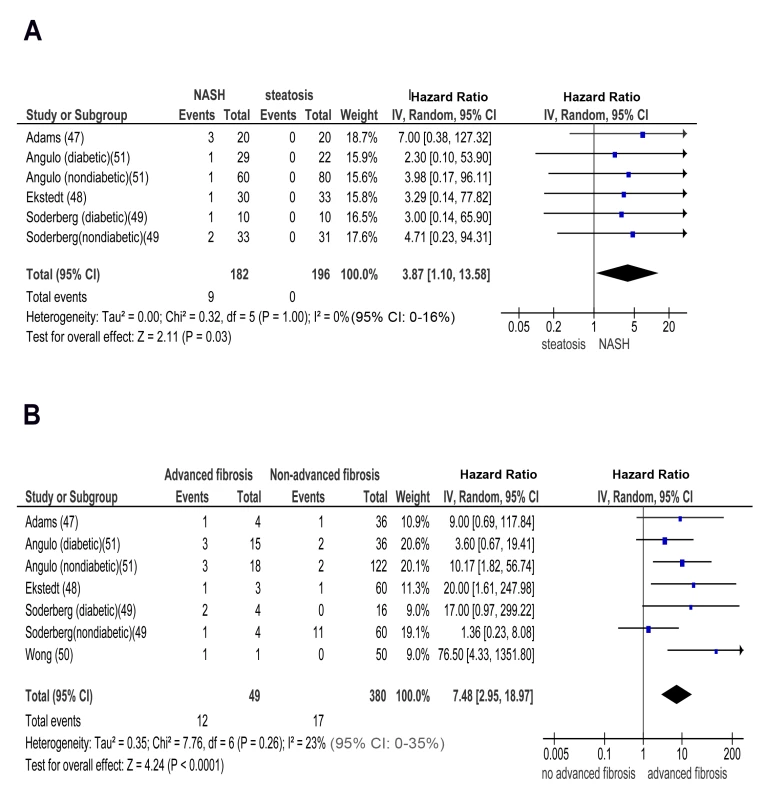
(A) NASH versus simple steatosis in biopsy-proven non-cirrhotic NAFLD; outcome: incident CKD stage 5 (renal failure) in prospective studies. (B) Advanced (stage F3) fibrosis versus no advanced (stage F0–F2) fibrosis in biopsy-proven non-cirrhotic NAFLD; outcome: incident CKD stage 3b in prospective studies. Similarly, pooled HR for CKD stage 3b, 4, and 5 (renal failure) was significantly higher in advanced versus non-advanced fibrosis: OR for CKD stage 3b: 7.48 (95% CI 2.95–18.97, I2 = 23% [95% CI 0%–35%], n-comparisons = 7, p<0.0001); OR for CKD stage 4 : 7.66 (95% CI 2.72–21.56, I2 = 0% [95% CI 0%–16%], n-comparisons = 6, p = 0.0001); OR for CKD stage 5 : 12.67 (95% CI 4.49–35.76, I2 = 0% [95% CI 0%–26%], n-comparisons = 6, p<0.00001) (Figures 7 and 8).
Fig. 8. Forest plot of comparison. 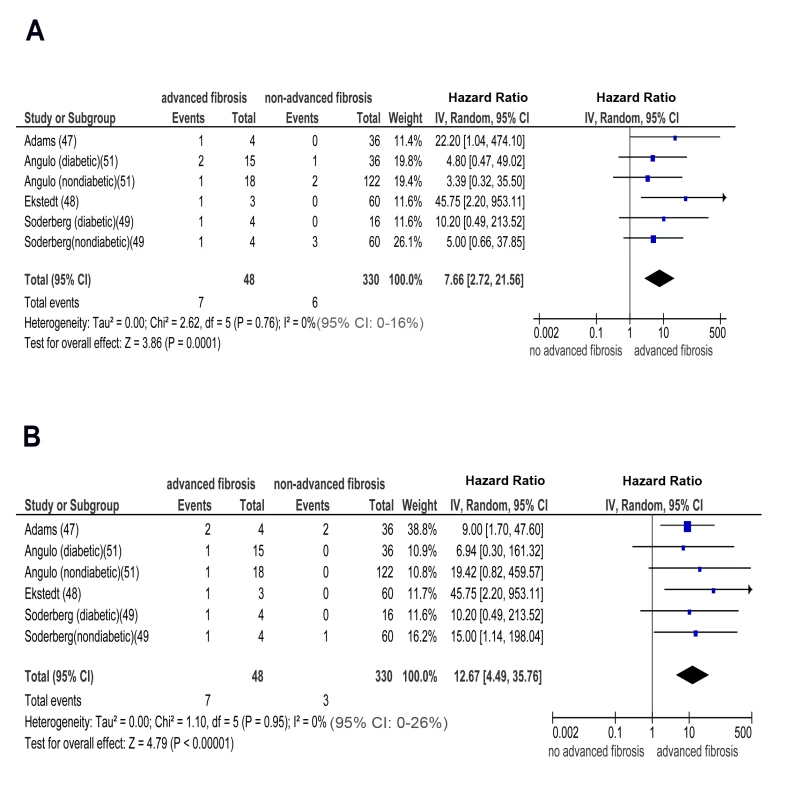
(A) advanced (stage F3) fibrosis versus no advanced (stage F0–F2) fibrosis in biopsy-proven non-cirrhotic NAFLD; outcome: incident CKD stage 4 in prospective studies. (B) Advanced (stage F3) fibrosis versus no advanced (stage F0–F2) fibrosis in biopsy-proven non-cirrhotic NAFLD; outcome: incident (CKD) stage 5 (renal failure) in prospective studies. There was no heterogeneity in the meta-analysis of overall events, suggesting a consistent disease effect.
Subgroup Analyses
NAFLD and prevalent/incident CKD
The magnitude and direction of the associations were unaltered across studies fulfilling different STROBE score items in non-diabetic individuals (Figures S10–S14 and S23–S26 within Text S3) versus diabetic individuals (Figures S15 and S27 within Text S3), when the analysis was restricted to studies adjusting for age and BMI and metabolic syndrome and hypertension and smoking status (Figures S16 and S28 within Text S3), in population-based versus hospital-based studies (Figures S17 and S29 within Text S3), in studies including Asian versus non-Asian individuals (Figures S18 and S30 within Text S3), in studies using CKD-EPI versus studies using the MDRD equation (Figures S20 and S31 within Text S3), after exclusion of studies assessing only eGFR or proteinuria (Figures S21 and S32 within Text S3), and in studies providing IPD versus studies providing exclusively AD (Figures S22 and S33 within Text S3). Furthermore, the main results remained largely unaltered after excluding the only cross-sectional study including cirrhotic individuals (Figure S19 within Text S3), while no prospective study enrolled subjects with cirrhosis at baseline. Subgroup analyses are summarized in Table 3.
NAFLD Histological Subtypes and the Risk of CKD
NASH/advanced fibrosis and prevalent CKD
The magnitude and direction of the effect were unaltered across studies fulfilling different STROBE score items (Figures S34 and S38 within Text S3) in non-diabetic versus diabetic individuals (Figures S35 and S39 within Text S3), in studies including Asian versus non-Asian individuals (Figures S36 and S40 within Text S3), in studies using CKD-EPI versus studies using the MDRD equation (Figure S41 within Text S3), after exclusion of studies assessing only eGFR or proteinuria (Figures S37 and S42 within Text S3), and in studies providing IPD versus studies providing exclusively AD (Figure S43 within Text S3).
All studies adjusted for traditional risk factors for CKD, were hospital-based and enrolled non-cirrhotic patients.
NASH/advanced fibrosis and incident CKD
The magnitude and direction of the effect remained unaltered in non-diabetic versus diabetic individuals (Figures S44 and S47 within Text S3), in Asian versus non-Asian individuals (Figures S45 and S48 within Text S3) and after exclusion of studies assessing only eGFR (Figures S46 and S49 within Text S3). All studies satisfied all STROBE score items, were hospital-based, enrolled non-cirrhotic individuals, adjusted for traditional risk factors for CKD, and used CKD-EPI equation to estimate GFR.
One-Stage Individual Participant Data Meta-analysis
Twenty studies (29,282 participants, 11 cross-sectional studies, nine longitudinal studies) were included in this analysis. We first analyzed the influence of each single pre-specified individual patient level covariate on the association of NAFLD with CKD with NAFLD and covariate as fixed-effect and the study as random-effects. In a second step, we did a complete case multivariable analysis with respect to NAFLD and all pre-specified covariates. The covariates entered in the models were age, BMI, metabolic syndrome (presence versus absence), diabetes (presence versus absence), hypertension (presence versus absence), smoking status (current smokers versus non-smokers), ethnicity (Asian versus non-Asian population), cirrhosis (presence versus absence), waist circumference, HOMA-index, duration of follow-up (for longitudinal studies).
The magnitude of the effect of NAFLD on CKD remained largely unaffected after adjusting for the covariates separately and in the fully adjusted models (Table 4).
Tab. 4. Adjusted effect estimates for non-alcoholic fatty liver disease, non-alcoholic steato-hepatitis, advanced (stage F3) fibrosis and prevalent/incident chronic kidney disease, based on individual participant data meta-analysis from 20 studies (29,282 participants). 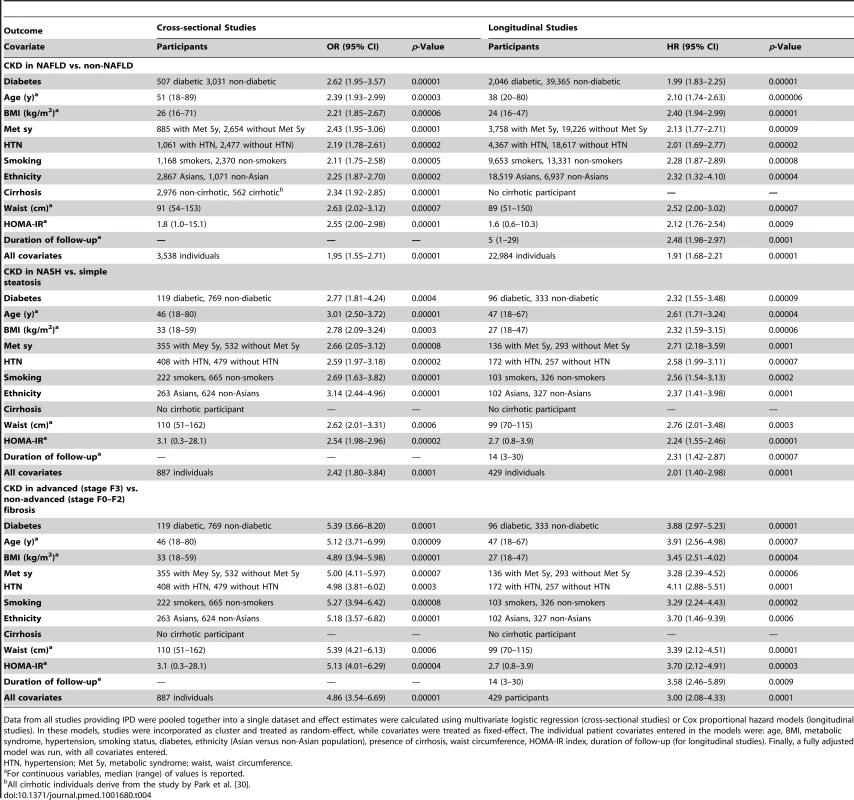
Data from all studies providing IPD were pooled together into a single dataset and effect estimates were calculated using multivariate logistic regression (cross-sectional studies) or Cox proportional hazard models (longitudinal studies). In these models, studies were incorporated as cluster and treated as random-effect, while covariates were treated as fixed-effect. The individual patient covariates entered in the models were: age, BMI, metabolic syndrome, hypertension, smoking status, diabetes, ethnicity (Asian versus non-Asian population), presence of cirrhosis, waist circumference, HOMA-IR index, duration of follow-up (for longitudinal studies). Finally, a fully adjusted model was run, with all covariates entered. Discussion
The main results of our analysis are the following: (1) NAFLD was associated with an increased prevalence and incidence of CKD; (2) liver disease severity in NAFLD was associated with an increased risk and severity of CKD; (3) these associations remained statistically significant in diabetic and non-diabetic individuals, as well as in studies adjusting for traditional risk factors for CKD, and were independent of whole body/abdominal obesity and insulin resistance.
The prevalence of CKD is rapidly growing and in the United States over 1.1 million individuals are estimated to have ESRD by the year 2015 [60]. In addition to progressing to ESRD, CKD is also a major risk factor for CVD, and most individuals with CKD die from CVD before they develop ESRD. Therefore, the search for modifiable risk factors for CKD is attracting much attention.
NAFLD is an emerging risk factor for end-stage liver disease and CVD: the frequency of NASH as the primary indication for liver transplantation has increased from 1.2% to 9.7% in the last decade, becoming the third most common indication for liver transplantation in the United States [61]. Furthermore, the number of combined liver and kidney transplants has been increasing exponentially in the last 5 years [62], thereby challenging cost-effective resource utilization in the treatment of end-stage organ disease. For these reasons, establishing a link between liver and kidney injury would enhance earlier identification of kidney disease and allow for the selection of treatments targeting both liver disease and CKD progression in individuals with NAFLD, with potentially relevant preventive and therapeutic implications. Our analysis disclosed an association between the presence and severity of NAFLD and the risk and severity of CKD. This association remained robust in cross-sectional and longitudinal studies, across different definitions (imaging, histology, biochemistry) of NAFLD and after taking different confounders into account. Notably, heterogeneity across cross-sectional studies evaluating NAFLD by ultrasound was abated after excluding data from analysis of the NHANES III 1988–1994 cohort, which failed to find an association between NAFLD and CKD [40]. This finding may be at least partially explained by the protocol used in that study: NHANES III was not originally designed to study hepatic steatosis and the authors diagnosed NAFLD retrospectively, on the basis of archived videotapes of gallbladder ultrasound examinations. In 2009–2010, trained ultrasound readers examined the protocol used in that study and found only modest intra - and inter-reliability for the presence of hepatic steatosis, i.e., 0.77 (95% CI 0.73–0.82) and 0.70 (95% CI 0.64–0.76), respectively [63]–[65]. This flaw may have further diluted the disease effect on CKD through misclassification of NAFLD cases as non-NAFLD, since mild steatosis, often present in progressive NASH and advanced fibrosis, is frequently missed by ultrasound.
Implications for Practice
Current guidelines do not recommend screening for CKD in the absence of traditional risk factors for CKD [66]. Our data suggest that individuals with NAFLD should be screened for CKD by estimation of GFR and urinalysis even in the absence of classical risk factors for CKD, particularly if NASH and/or advanced fibrosis are suspected. Early recognition of impaired kidney function in NAFLD may also allow drug dosage adjustment, thus preventing drug accumulation, especially in those being treated for obesity-associated comorbidities.
From a therapeutic standpoint, there is a considerable potential for improving the current care of NAFLD patients with CKD: with respect to lifestyle interventions, smoking cessation should be more vigorously pursued, as cigarette smoking is an established risk factor for CKD, and may also aggravate NAFLD [67],[68]. Among pharmacological options, preliminary data from the GREACE and FANTASY randomized trials suggest statins and angiotensin receptor blockers (ARBs) may improve both liver and kidney disease in NAFLD [56],[69]–[71]. Beside statins and ARBs, other agents, including pentoxifylline and ω-3 polyunsaturated fatty acids, improved surrogate markers of NAFLD and CKD in distinct NALFD-associated settings like obesity, diabetes, and hypertension, and their impact on CKD in NAFLD warrants future assessment [72]–[76].
Implications for Research
Further research is required to unravel the specific cascades linking NAFLD and kidney disease. NAFLD and CKD share common risk factors and therefore both liver and kidney injury may be driven by obesity-associated mechanisms of disease, including lipotoxicity, oxidative stress, enhanced pro-inflammatory cytokine, and renin-angiotensin-aldosterone system (RAAS) axis activation [10],[77]–[80]. However, our analysis of longitudinal studies suggests NAFLD may promote CKD independently of coexisting risk factors. Consistently, recent data suggest the steatotic and inflamed liver may be a relevant source of pro-inflammatory, pro-fibrogenic, and anti-fibrinolytic molecules, including fetuin-A, fibroblast growth factor (FGF)-21, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, and plasminogen activator inhibitor-1, all having the ability to promote kidney injury [81]–[85]. Furthermore, fatty liver may damage the kidney through VLDL lipoprotein over-secretion and induction of atherogenic dyslipidemia [86],[87], as triglyceride-rich lipoproteins and oxidized LDLs promote glomerular injury and mesangial cell proliferation [77].
We also found a cross-sectional association between NAFLD and CKD, implying that even mild renal dysfunction may promote liver disease, in a mutual negative loop with detrimental cardio-metabolic consequences Consistently, uni-nephrectomized rats developed body fat redistribution from adipose depots to non-adipose tissues, profound dysregulation of hepatic fatty acid metabolism, steatohepatitis, insulin resistance, hyperglycaemia, and dyslipidaemia early after uni-nephrectomy and long before glomerulosclerosis and chronic renal failure occur [88],[89]. Notably, all renal and metabolic changes were prevented by angiotensin converting enzyme (ACE) inhibitors in this experimental model, indicating the involvement of the RAAS [74].
Lastly, since NAFLD is an emerging risk factor for liver-related and cardiovascular complications, the hypothesis that the presence of CKD may represent a simple, cost-effective tool to predict increased liver-related and CVD risk in NAFLD is intriguing and warrants assessment in large-scale prospective studies. Currently, in fact, there is no validated non-invasive marker to predict the risk of both liver disease progression and future CVD, the main causes of death in NAFLD patients [9].
Our findings may also have therapeutic research implications. Future trials will need to evaluate the impact of experimental treatments on kidney-related outcomes. Notably, only two of the available randomized controlled trials in NAFLD reports the effect of drugs on eGFR and proteinuria and none has adequate size and duration to evaluate the impact of treatments on kidney-related clinical outcomes.
Our analysis has limitations, which are intrinsic to the nature of included studies and provide the basis for future research. Studies with biopsy-proven NAFLD were by their own nature less numerous and smaller than those adopting ultrasonographic/biochemical definitions of NAFLD, leaving the possibility of small study bias that is not detected by current tests. Furthermore, these studies were performed in tertiary centers with the possibility of selection bias. Conversely, ultrasound/liver enzyme elevations are relatively insensitive to detect NAFLD, with possible misclassification of individuals with NASH/advanced fibrosis as healthy controls and underestimation of the strength of the association between NAFLD and CKD. However, there was no heterogeneity between studies: disease effect on CKD.
Finally, despite our best efforts IPD were unavailable from as much as 39% of relevant studies, representing 54% of participant population. While meta-analysis of AD has several limitations, including ecological bias and study-level confounding, excluding such a substantial proportion of relevant literature from our analysis would have raised the concern of data availability bias [90]. Furthermore, the methodological quality of the 13 studies providing exclusive AD was generally good, 77% of them adjusted for all potential confounders, and 69% of them allowed separate risk estimates for individual patient level covariates (including diabetes, cirrhosis, ethnicity).
Balancing all these reasons, we presented all relevant literature evidence by combining IPD with AD in the main analysis, and separately analyzed studies providing IPD with a one-stage method: notably, the two analyses yielded similar results, further supporting the robustness of overall findings.
In conclusion, our analysis shows that the presence and severity of NAFLD are associated with an increased risk and severity of CKD and may be a target for the prevention and treatment of CKD. Future research should evaluate strategies and interventions to prevent renal disease progression in individuals with NAFLD.
Supporting Information
Zdroje
1. McCulloughK, SharmaP, AliT, KhanI, SmithWC, et al. (2012) Measuring the population burden of chronic kidney disease: a systematic literature review of the estimated prevalence of impaired kidney function. Nephrol Dial Transplant 27 : 1812–1821.
2. StevensPE, LevinA (2013) A Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158 : 825–830.
3. ThomasG, SehgalAR, KashyapSR, SrinivasTR, KirwanJP, et al. (2011) Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 6 : 2364–2373.
4. JamesMT, HemmelgarnBR, TonelliM (2010) Early recognition and prevention of chronic kidney disease. Lancet 375 : 1296–1309.
5. HerzogCA, AsingerRW, BergerAK, CharytanDM, DıezJ, et al. (2011) Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80 : 572–586.
6. BlackC, SharmaP, ScotlandG, McCulloughK, McGurnD, et al. (2010) Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess 14 : 1–184.
7. CoreshJ, Byrd-HoltD, AstorBC, BriggsJP, EggersPW, et al. (2005) Chronic kidney disease awareness, prevalence, and trends among US adults, 1999 to 2000. J Am Soc Nephrol 16 : 180–188.
8. ChalasaniN, YounossiZ, LavineJE, DiehlAM, BruntEM, CusiK, et al. (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 55 : 2005–2023.
9. MussoG, GambinoR, CassaderM, PaganoGF (2011) Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 43 : 617–649.
10. IxJH, SharmaK (2010) Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol 21 : 406–412.
11. LeveyAS, CoreshJ, GreeneT, StevensLA, ZhangYL, et al. (2006) Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med 145 : 247–254.
12. LeveyAS, StevensLA, SchmidCH, ZhangYL, CastroAF3rd, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150 : 604–612.
13. StevensLA, CoreshJ, SchmidCH, FeldmanHI, FroissartM, et al. (2008) Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51 : 395–406.
14. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions (version 5.1.0) Available: http://www.cochrane.org/training/cochrane-handbook. Accessed 31 March 2013.
15. LiberatiA, AltmanDG, TetzlaffJ, MulrowC, GøtzschePC, et al. (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol 62 : 1006–1012.
16. von ElmE, AltmanDG, EggerM, PocockSJ, GøtzschePC, et al. (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147 : 573–577.
17. HernaezR, LazoM, BonekampS (2011) Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 54 : 1082–1090.
18. RileyRD, SimmondsMC, LookMP (2007) Evidence synthesis combining individual patient data and aggregate data: a systematic review identified current practice and possible methods. J Clin Epidemiol 60 : 431–439.
19. RileyRD, SteyerbergEW (2010) Meta-analysis of a binary outcome using individual participant data and aggregate data. Research Syn Meth 1 : 2–19.
20. HigginsJP, ThompsonSG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21 : 1539–1558.
21. BögerCA, HeidIM (2011) Chronic kidney disease: novel insights from genome-wide association studies. Kidney Blood Press Res 34 : 225–234.
22. ChangJM, HwangSJ, TsukamotoY, ChenHC (2012) Chronic kidney disease prevention–a challenge for Asian countries: report of the Third Asian Forum of Chronic Kidney Disease Initiatives. Clin Exp Nephrol 16 : 187–194.
23. KirylukK, LiY, Sanna-CherchiS, RohanizadeganM, SuzukiH, et al. (2012) Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet 8: e1002765.
24. FarrellGC, WongVW, ChitturiS (2013) NAFLD in Asian–as common and important as in the West. Nat Rev Gastroenterol Hepatol 10 : 307–318.
25. KwakernaakAJ, ZelleDM, BakkerSJ, NavisG (2013) Central body fat distribution associates with unfavorable renal hemodynamics independent of body mass index. J Am Soc Nephrol 24 : 987–994.
26. DuvalS, TweedieR (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 : 455–463.
27. CamposGM, BambhaK, VittinghoffE, RablC, PosseltAM, et al. (2008) A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology 47 : 1916–1923.
28. YilmazY, AlahdabYO, YonalO, KurtR, KedrahAE, et al. (2010) Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism 59 : 1327–1330.
29. TargherG, BertoliniL, RodellaS, LippiG, ZoppiniG, et al. (2010) Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 5 : 2166–2171.
30. ParkCW, TsaiNT, WongLL (2011) Implications of worse renal dysfunction and medical comorbidities in patients with NASH undergoing liver transplant evaluation: impact on MELD and more. Clin Transplant 25: E606–E611.
31. YasuiK, SumidaY, MoriY, MitsuyoshiH, MinamiM, et al. (2011) Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism 60 : 735–739.
32. MussoG, CassaderM, De MichieliF, RosinaF, OrlandiF, et al. (2012) Nonalcoholic steatohepatitis versus steatosis: adipose tissue insulin resistance and dysfunctional response to fat ingestion predict liver injury and altered glucose and lipoprotein. Metabolism Hepatology 56 : 933–942.
33. FrancqueSM, VerrijkenA, MertensI, HubensG, Van MarckE, et al. (2012) Noninvasive assessment of nonalcoholic fatty liver disease in obese or overweight patients. Clin Gastroenterol Hepatol 10 : 1162–1168.
34. MachadoMV, GonçalvesS, CarepaF, CoutinhoJ, CostaA, et al. (2012) Impaired renal function in morbid obese patients with nonalcoholic fatty liver disease. Liver Int 32 : 241–248.
35. KimYS, JungES, HurW, BaeSH, ChoiJY, et al. (2013) Noninvasive predictors of nonalcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin Mol Hepatol 19 : 120–130.
36. TargherG, BertoliniL, RodellaS, ZoppiniG, LippiG, et al. (2008) Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 51 : 444–450.
37. CasoinicF, SâmpeleanD, BădăuC, PrunăL (2009) Nonalcoholic fatty liver disease–a risk factor for microalbuminuria in type 2 diabetic patients. Rom J Intern Med 47 : 55–59.
38. HwangST, ChoYK, YunJW, ParkJH, KimHJ, et al. (2010) Impact of non-alcoholic fatty liver disease on microalbuminuria in patients with prediabetes and diabetes. Intern Med J 40 : 437–442.
39. TargherG, PichiriI, ZoppiniG, TrombettaM, BonoraE (2012) Increased prevalence of chronic kidney disease in patients with Type 1 diabetes and non-alcoholic fatty liver. Diabet Med 29 : 220–226.
40. SirotaJC, McFannK, TargherG, ChoncholM, JalalDI (2012) Association between nonalcoholic liver disease and chronic kidney disease: an ultrasound analysis from NHANES 1988–1994. Am J Nephrol 36 : 466–471.
41. LiG, ShiW, HugH, ChenY, LiuL, et al. (2012) Nonalcoholic fatty liver disease associated with impairment of kidney function in nondiabetes population. Biochem Med (Zagreb) 22 : 92–99.
42. ArmstrongMJ, HoulihanDD, BenthamL, ShawJC, CrambR, et al. (2012) Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol 56 : 234–240.
43. XiaMF, LinHD, LiXM, YanHM, BianH, et al. (2012) Renal function-dependent association of serum uric acid with metabolic syndrome and hepatic fat content in a middle-aged and elderly Chinese population. Clin Exp Pharmacol Physiol 39 : 930–937.
44. AhnAL, ChoiJK, KimMN, KimSA, OhEJ, et al. (2013) Non-alcoholic fatty liver disease and chronic kidney disease in Koreans aged 50 years or older. Korean J Fam Med 34 : 199–205.
45. AnjaneyaV, SirishaY, Pradeep BabuKV (2013) Can Non-alcoholic fatty liver disease (NAFLD) as a marker for microalbuminuria in prediabetes group? Iosr Journal of Pharmacy 3 : 48–51.
46. TargherG, KendrickJ, SmitsG, ChoncholM (2010) Relationship between serum gamma-glutamyltransferase and chronic kidney disease in the United States adult population. Findings from the National Health and Nutrition Examination Survey 2001–2006. Nutr Metab Cardiovasc Dis 20 : 583–590.
47. AdamsLA, LympJF, St SauverJ, SandersonSO, LindorKD, et al. (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129 : 113–121.
48. EkstedtM, FranzénLE, MathiesenUL, ThoreliusL, HolmqvistM, et al. (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44 : 865–873.
49. SöderbergC, StålP, AsklingJ, GlaumannH, LindbergG, et al. (2010) Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 51 : 595–602.
50. WongVW, WongGL, ChoiPC, ChanAW, LiMK, et al. (2010) Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut 59 : 969–974.
51. AnguloP, BugianesiE, BjornssonES, CharatcharoenwitthayaP, MillsPR, et al. (2013) Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 145 : 782–789.
52. HamaguchiM, KojimaT, TakedaN, NagataC, TakedaJ, et al. (2007) Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 13 : 1579–1584.
53. ChangY, RyuS, SungE, WooHY, OhE, et al. (2008) Nonalcoholic fatty liver disease predicts chronic kidney disease in nonhypertensive and nondiabetic Korean men. Metabolism 57 : 569–576.
54. TargherG, ChoncholM, BertoliniL, RodellaS, ZenariL, et al. (2008) Increased risk of CKD among type 2 diabetics with nonalcoholic fatty liver disease. J Am Soc Nephrol 19 : 1564–1570.
55. LauK, LorbeerR, HaringR, SchmidtCO, WallaschofskiH, et al. (2010) The association between fatty liver disease and blood pressure in a population-based prospective longitudinal study. J Hypertens 28 : 1829–1835.
56. AthyrosVG, TziomalosK, GossiosTD, GrivaT, AnagnostisP, et al. (2010) Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet 376 : 1916–1922.
57. El AzeemHA, Khalekel-SA, El-AkabawyH, NaeimH, KhalikHA, et al. (2013) Association between nonalcoholic fatty liver disease and the incidence of cardiovascular and renal events. J Saudi Heart Assoc 25 : 239–246.
58. LeeDH, JacobsDRJr, GrossM, SteffesM (2005) Serum gamma-glutamyltransferase was differently associated with microalbuminuria by status of hypertension or diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem 51 : 1185–1191.
59. RyuS, ChangY, KimDI, KimWS, SuhBS (2007) Gamma-glutamyltransferase as a predictor of chronic kidney disease in nonhypertensive and nondiabetic Korean men. Clin Chem 53 : 71–77.
60. GilbertsonDT, LiuJ, XueJL, LouisTA, SolidCA, et al. (2005) Projecting the number of patients with endstage renal disease in the United States to the year 2015. J Am Soc Nephrol 16 : 3736–3741.
61. AgopianVG, KaldasFM, HongJC, WhittakerM, HoltC, et al. (2012) Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg 256 : 624–633.
62. Scientific Registry of Transplant Recipients. Available: http://www.ustransplant.org/. Accessed 30 September 2013.
63. TargherG (2011) NAFLD and adverse hepatic and extra-hepatic outcomes. BMJ 343 Available: www.bmj.com/rapid-response/2011/12/05.
64. AnguloP (2011) Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 343 Available: http://www.bmj.com/rapid-response/2011/12/12.
65. AnsteeQM A Flawed Study and an Opportunity Missed. BMJ 2011 : 343 at: http://www.bmj.com/rapid-response/2011/11/30.
66. QaseemA, HopkinsRHJr, SweetDE, StarkeyM, ShekelleP (2013) Screening, monitoring, and treatment of stage 1 to 3 chronic kidney disease: a clinical practice guideline from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med 159 : 835–847.
67. ZeinCO, UnalpA, ColvinR, LiuYC, McCulloughAJ (2011) Nonalcoholic Steatohepatitis Clinical Research Network. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol 54 : 753–759.
68. TsochatzisEA, PapatheodoridisGV (2010) Smoking is associated with histological severity in nonalcoholic steatohepatitis. Hepatology 52 : 1522–1523.
69. HirataT, TomitaK, KawaiT, YokoyamaH, ShimadaA, et al. (2013) Effect of telmisartan or losartan for treatment of nonalcoholic fatty liver disease: Fatty Liver Protection Trial by Telmisartan or Losartan Study (FANTASY). Int J Endocrinol 13 : 587140.
70. FogariR, MaffioliP, MugelliniA, ZoppiA, LazzariP, et al. (2012) Effects of losartan and amlodipine alone or combined with simvastatin in hypertensive patients with nonalcoholic hepatic steatosis. Eur J Gastroenterol Hepatol 24 : 164–171.
71. HanKH, RhaSW, KangHJ, BaeJW, ChoiBJ, et al. (2012) Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J Clin Lipidol 6 : 340–351.
72. MussoG, CassaderM, RosinaF, GambinoR (2012) Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 55 : 885–904.
73. PerkinsRM, AboudaraMC, UyAL, OlsonSM, CushnerHM, et al. (2009) Effect of pentoxifylline on GFR decline in CKD: a pilot, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis 53 : 606–616.
74. NakamuraT, FujiwaraN, KawagoeY, SugayaT, UedaY, et al. (2010) Effects of telmisartan and enalapril on renoprotection in patients with mild to moderate chronic kidney disease. Eur J Clin Invest 40 : 790–796.
75. NakamuraT, SatoE, FujiwaraN, KawagoeY, TakeuchiM, et al. (2010) Atorvastatin reduces proteinuria in non-diabetic chronic kidney disease patients partly via lowering serum levels of advanced glycation end products (AGEs). Oxid Med Cell Longev 3 : 304–307.
76. MillerER3rd, JuraschekSP, AndersonCA, GuallarE, Henoch-RyugoK, et al. (2013) The effects of n-3 long-chain polyunsaturated fatty acid supplementation on biomarkers of kidney injury in adults with diabetes: results of the GO-FISH trial. Diabetes Care 36 : 1462–1469.
77. GyebiL, SoltaniZ (2012) Reisin Lipid nephrotoxicity: new concept for an old disease. Curr Hypertens Rep 14 : 177–181.
78. RüsterC, WolfG (2013) The role of the renin-angiotensin-aldosterone system in obesity-related renal diseases. Semin Nephrol 33 : 44–53.
79. KalaitzidisRG, SiamopoulosKC (2011) The role of obesity in kidney disease: recent findings and potential mechanisms. Int Urol Nephrol 43 : 771–784.
80. YilmazY (2010) The AGEs-RAGE axis and nonalcoholic steatohepatitis: the evidence mounts. J Gastroenterol 45 : 782–783.
81. LiY, LiuL, WangB, WangJ, ChenD (2013) Simple steatosis is a more relevant source of serum inflammatory markers than omental adipose tissue. Clin Res Hepatol Gastroenterol 38 : 46–54.
82. DogruT, GencH, TapanS, AslanF, ErcinCN, et al. (2013) Plasma fetuin-A is associated with endothelial dysfunction and subclinical atherosclerosis in subjects with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf) 78 : 712–717.
83. LiY, SunX, YuY (2013) Serum fetuin-A levels related with microalbuminuria in diet-induced obese rats. Biomed Res Int 795103.
84. CrastoC, SembaRD, SunK, FerrucciL (2012) Serum fibroblast growth factor 21 is associated with renal function and chronic kidney disease in community-dwelling adults. J Am Geriatr Soc 60 : 792–793.
85. MałgorzewiczS, Skrzypczak-JankunE, JankunJ (2013) Plasminogen activator inhibitor-1 in kidney pathology. Int J Mol Med 31 : 503–510.
86. AdielsM, TaskinenMR, PackardC, CaslakeMJ, Soro-PaavonenA, et al. (2006) Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 49 : 755–765.
87. DeFilippisAP, BlahaMJ, MartinSS, ReedRM, JonesSR, et al. (2013) Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 227 : 429–436.
88. ZhaoHL, SuiY, HeL, GuanJ, XiaoSJ, et al. (2011) Lipid partitioning after uninephrectomy. Acta Diabetol 48 : 317–328.
89. JinK, NorrisK, VaziriND (2013) Dysregulation of hepatic fatty acid metabolism in chronic kidney disease. Nephrol Dial Transplant 28 : 313–320.
90. AhmedI, SuttonAJ, RileyRD (2012) Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ 344: d7762.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 7- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Blue Marble Health: A Call for Papers
- Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites
- Association of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis
- Urbanicity and Lifestyle Risk Factors for Cardiometabolic Diseases in Rural Uganda: A Cross-Sectional Study
- The Importance of Implementation Strategy in Scaling Up Xpert MTB/RIF for Diagnosis of Tuberculosis in the Indian Health-Care System: A Transmission Model
- Severe Maternal Sepsis in the UK, 2011–2012: A National Case-Control Study
- Association between Class III Obesity (BMI of 40–59 kg/m) and Mortality: A Pooled Analysis of 20 Prospective Studies
- Using Evidence to Combat Overdiagnosis and Overtreatment: Evaluating Treatments, Tests, and Disease Definitions in the Time of Too Much
- Improving the Transparency of Prognosis Research: The Role of Reporting, Data Sharing, Registration, and Protocols
- Does Diagnosing Fatty Liver and Chronic Kidney Disease Do More Good Than Harm?
- Urban Development in Sub-Saharan Africa: Bearer of Goods and Risks
- Mortality after Parental Death in Childhood: A Nationwide Cohort Study from Three Nordic Countries
- Defining Catastrophic Costs and Comparing Their Importance for Adverse Tuberculosis Outcome with Multi-Drug Resistance: A Prospective Cohort Study, Peru
- Cesarean Section and Rate of Subsequent Stillbirth, Miscarriage, and Ectopic Pregnancy: A Danish Register-Based Cohort Study
- Effects of BMI, Fat Mass, and Lean Mass on Asthma in Childhood: A Mendelian Randomization Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Association of Non-alcoholic Fatty Liver Disease with Chronic Kidney Disease: A Systematic Review and Meta-analysis
- Using Evidence to Combat Overdiagnosis and Overtreatment: Evaluating Treatments, Tests, and Disease Definitions in the Time of Too Much
- Association between Class III Obesity (BMI of 40–59 kg/m) and Mortality: A Pooled Analysis of 20 Prospective Studies
- Blue Marble Health: A Call for Papers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



