-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociation between Melanocytic Nevi and Risk of Breast Diseases: The French E3N Prospective Cohort
Background:
While melanocytic nevi have been associated with genetic factors and childhood sun exposure, several observations also suggest a potential hormonal influence on nevi. To test the hypothesis that nevi are associated with breast tumor risk, we explored the relationships between number of nevi and benign and malignant breast disease risk.Methods and Findings:
We prospectively analyzed data from E3N, a cohort of French women aged 40–65 y at inclusion in 1990. Number of nevi was collected at inclusion. Hazard ratios (HRs) for breast cancer and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression models. Associations of number of nevi with personal history of benign breast disease (BBD) and family history of breast cancer were estimated using logistic regression. Over the period 15 June 1990–15 June 2008, 5,956 incident breast cancer cases (including 5,245 invasive tumors) were ascertained among 89,902 women. In models adjusted for age, education, and known breast cancer risk factors, women with “very many” nevi had a significantly higher breast cancer risk (HR = 1.13, 95% CI = 1.01–1.27 versus “none”; ptrend = 0.04), although significance was lost after adjustment for personal history of BBD or family history of breast cancer. The 10-y absolute risk of invasive breast cancer increased from 3,749 per 100,000 women without nevi to 4,124 (95% CI = 3,674–4,649) per 100,000 women with “very many” nevi. The association was restricted to premenopausal women (HR = 1.40, ptrend = 0.01), even after full adjustment (HR = 1.34, ptrend = 0.03; phomogeneity = 0.04), but did not differ according to breast cancer type or hormone receptor status. In addition, we observed significantly positive dose–response relationships between number of nevi and history of biopsy-confirmed BBD (n = 5,169; ptrend<0.0001) and family history of breast cancer in first-degree relatives (n = 7,472; ptrend = 0.0003). The main limitations of our study include self-report of number of nevi using a qualitative scale, and self-reported history of biopsied BBD.Conclusions:
Our findings suggest associations between number of nevi and the risk of premenopausal breast cancer, BBD, and family history of breast cancer. More research is warranted to elucidate these relationships and to understand their underlying mechanisms.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(6): e32767. doi:10.1371/journal.pmed.1001660
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001660Summary
Background:
While melanocytic nevi have been associated with genetic factors and childhood sun exposure, several observations also suggest a potential hormonal influence on nevi. To test the hypothesis that nevi are associated with breast tumor risk, we explored the relationships between number of nevi and benign and malignant breast disease risk.Methods and Findings:
We prospectively analyzed data from E3N, a cohort of French women aged 40–65 y at inclusion in 1990. Number of nevi was collected at inclusion. Hazard ratios (HRs) for breast cancer and 95% confidence intervals (CIs) were calculated using Cox proportional hazards regression models. Associations of number of nevi with personal history of benign breast disease (BBD) and family history of breast cancer were estimated using logistic regression. Over the period 15 June 1990–15 June 2008, 5,956 incident breast cancer cases (including 5,245 invasive tumors) were ascertained among 89,902 women. In models adjusted for age, education, and known breast cancer risk factors, women with “very many” nevi had a significantly higher breast cancer risk (HR = 1.13, 95% CI = 1.01–1.27 versus “none”; ptrend = 0.04), although significance was lost after adjustment for personal history of BBD or family history of breast cancer. The 10-y absolute risk of invasive breast cancer increased from 3,749 per 100,000 women without nevi to 4,124 (95% CI = 3,674–4,649) per 100,000 women with “very many” nevi. The association was restricted to premenopausal women (HR = 1.40, ptrend = 0.01), even after full adjustment (HR = 1.34, ptrend = 0.03; phomogeneity = 0.04), but did not differ according to breast cancer type or hormone receptor status. In addition, we observed significantly positive dose–response relationships between number of nevi and history of biopsy-confirmed BBD (n = 5,169; ptrend<0.0001) and family history of breast cancer in first-degree relatives (n = 7,472; ptrend = 0.0003). The main limitations of our study include self-report of number of nevi using a qualitative scale, and self-reported history of biopsied BBD.Conclusions:
Our findings suggest associations between number of nevi and the risk of premenopausal breast cancer, BBD, and family history of breast cancer. More research is warranted to elucidate these relationships and to understand their underlying mechanisms.
Please see later in the article for the Editors' SummaryIntroduction
Melanocytic nevi (hereafter referred to as nevi) are benign skin tumors resulting from epidermal melanocyte proliferation. They occur more frequently in fair - than in dark-skinned individuals and can be either congenital or acquired later in life. Nevus acquisition starts early in childhood and peaks during puberty, then declines in adulthood, with progressive loss with age [1]. Twin studies have consistently demonstrated genetic heritability of number of nevi [2]–[4], with an estimated 40%–80% of the variance in nevus counts being attributable to genetic effects [2],[4]. Genome-wide association studies have indeed reported several genes involved in nevus count, including CDKN2A and MTAP, both located at the 9p21 locus, and PLA2G6, located at 22q13 [5],[6]. Childhood sun exposure is also likely to play an important role in nevus acquisition, as suggested by studies of site distribution of nevi in children, which have consistently shown higher nevus densities on habitually sun-exposed body sites [7]–[10]. Number of sunburns [8],[11]–[13] and low latitude [12],[14]–[18] have also been associated with increased nevus prevalence. Because number of nevi peaks during puberty [1] and nevi are reported to be darker and larger during pregnancy [19],[20], a hormonal influence on nevi may also be speculated. Consistently, melanocytes, the pigment-producing cells, have been shown to express estrogen and androgen receptors [21]. However, the role of sex hormones in the occurrence of nevi remains to be determined.
Number of nevi is the strongest known risk factor for cutaneous melanoma [22], and an association has been suggested between melanoma and breast cancer [23],[24]. In addition, number of nevi and melanoma risk have been associated with variants in the CDKN2A gene [6], which plays a role in cell cycle regulation, while CDKN2A inactivation has been shown to be common in breast cancer [25]. Number of nevi has also been found to be associated with several benign and malignant diseases, particularly hormone-related conditions, including endometriosis [26], leiomyoma [27], and thyroid diseases [28]. Whether these associations are explained by common hormonal or genetic pathways has not yet been clarified.
In order to investigate whether nevus count is associated with breast tumor risk, we explored the relationships between number of nevi and the risks of benign and malignant breast diseases and family history of breast cancer, in the French E3N prospective cohort.
Methods
Ethics Statement
The E3N cohort received ethical approval from the French National Commission for Computed Data and Individual Freedom (Commission Nationale de l'Informatique et des Libertés), and all participants in the study provided informed consent.
The E3N Cohort
E3N is a prospective cohort study involving 98,995 women born in 1925–1950, living in metropolitan France at inclusion and insured by the Mutuelle Générale de l'Éducation Nationale, a national health scheme primarily covering teachers. The cohort has been described in detail elsewhere [29]. Briefly, women were enrolled from February 1, 1989, through November 30, 1991, after returning a baseline self-administered questionnaire on their lifestyle and medical history. Follow-up questionnaires were sent every 2–3 y thereafter.
Breast Cancer Assessment
All cohort questionnaires inquired about the occurrence of cancer, including breast cancer, requesting contact details of the participants' physicians and permission to contact them. A small number of breast cancer cases were further identified from insurance files and death certificates. Pathology reports were obtained for 93% of incident cases. We also considered cases for which pathology reports had not been obtained, because the proportion of false-positive self-reports was low in our study population (<5%). Information on ascertained estrogen receptor (ER) and progesterone receptor (PR) status was extracted from pathology reports, and invasive breast cancer cases were classified accordingly into four categories: ER+/PR+, ER+/PR−, ER−/PR+, and ER−/PR−. Women with unknown receptor status—mostly with tumors diagnosed in the early years of follow-up, when determining hormone receptor status was not compulsory (n = 1,415, 27% of the tumors)—were excluded from the analyses stratified according to hormone receptor status.
Benign Breast Disease Ascertainment
The 1990 and 1992 questionnaires asked women to report if they had ever had a personal history of benign breast disease (BBD) (breast adenoma or fibroadenoma, fibrocystic breast disease, or other) before inclusion in the cohort (prevalent BBD, n = 19,742). Women also reported whether a biopsy had been performed for these diseases. This information was then collected prospectively in subsequent questionnaires (incident BBD, n = 11,600). To avoid the potential selection bias of including only late-diagnosed BBD, both prevalent and incident BBDs were included in the analyses. When history of BBD was analyzed as a potential confounder, both biopsied and non-biopsied BBDs were included in order to maximize statistical power; however, we performed a sensitivity analysis excluding non-biopsied BBDs and verified the stability of the findings. When history of BBD was analyzed as an outcome, only biopsy-confirmed BBDs were considered in order to minimize misclassification.
Assessment of Family History of Breast Cancer
Family history of breast cancer was collected in the inclusion questionnaire, where participants were asked to self-report whether their first-degree relatives had ever had a history of breast cancer.
Assessment of Exposure and Covariates
The inclusion questionnaire asked women to self-report their number of moles, with four possible answers: none, a few, many, or very many. The questionnaire also inquired about education level, skin complexion, height and weight, and physical activity. Data on age at menarche, parity, age at first full-term pregnancy, and breastfeeding were collected in the inclusion (1990) and 1992 questionnaires. Data on use of oral contraceptives (OCs), premenopausal progestogens, and menopausal hormone therapy (MHT) were collected in 1992 and updated in each follow-up questionnaire. Body mass index (BMI) was calculated as weight in kilograms, reported at inclusion and updated at each follow-up questionnaire, divided by height in meters squared. Menopausal status, age at menopause, and history of recent mammographic exam (in the period since the previous questionnaire) were collected at inclusion and at each follow-up questionnaire. To obtain average levels of residential sun exposure during childhood and adulthood, we linked data on county of birth and county of residence at inclusion to a database from the Joint Research Centre of the European Commission containing mean daily ultraviolet (UV) radiation dose in French counties [30]. Physical activity, height, and UV dose in county of birth and of residence at baseline were analyzed in tertiles. BMI was grouped using World Health Organization cutoff points of 18.5 and 25 kg/m2, further dividing those with BMI 18.5–25 kg/m2 into the categories 18.5–22.5 and 22.5–25 kg/m2, according to the median BMI in our study population. Age at menarche, age at first full-term pregnancy, and age at menopause were categorized according to the median for these variables in the cohort. Women were considered postmenopausal if they reported 12 consecutive months of amenorrhea (unless due to hysterectomy), bilateral oophorectomy, or use of MHT, or if they self-reported being postmenopausal. Date of menopause was defined as the date of last menstrual period (unless due to hysterectomy, and if the last menstrual period occurred before MHT use), date of bilateral oophorectomy, or, in decreasing order of priority, self-reported date of menopause, date MHT use began, or date menopausal symptoms began; if no information on date of menopause was available, date of menopause was defined as the date corresponding to age 47 y if menopause was known to be artificial, or age 51 y otherwise, i.e., the median ages for artificial and natural menopause in the cohort, respectively.
Statistical Analyses
Statistical analyses were performed using SAS software (version 9.3, SAS Institute). Hazard ratios (HRs) for breast cancer and 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression models with age as the timescale. We also calculated absolute risks of breast cancer associated with the number of nevi. The association between number of nevi and breast cancer risk was assessed using different adjustment models. We first used age-adjusted models stratified by birth cohort in 5-y categories (Model 1) and then built a model including as covariates only those factors whose inclusion resulted in a 10% change of the HR for number of nevi. Besides age and birth cohort, this model included education (<12, 12–14, or ≥15 y), use of premenopausal progestogens (ever/never), menopausal status (pre - or postmenopausal), age at menopause in postmenopausal women (<51 or ≥51 y), and use of MHT in postmenopausal women (ever/never) (Model 2). Because further adjustment for personal history of BBD or family history of breast cancer resulted in the largest changes in HRs for breast cancer, we evaluated their effect separately by creating two models, Model 2 further adjusted for personal history of BBD (Model 3) and Model 3 additionally adjusted for family history of breast cancer (Model 4). Finally, a fully adjusted model included all the preceding covariates along with the remaining potential confounders, including BMI, height, physical activity, age at menarche, parity, age at first full-term pregnancy, breastfeeding, use of OCs, personal history of mammographic exam, and UV dose in county of birth and of residence at cohort inclusion (Model 5). All models were generated both for in situ and invasive breast cancer cases combined, and for in situ and invasive cases separately, and homogeneity tests were used to compare estimates between in situ and invasive cases. We performed tests for linear trend across categories of number of nevi by assigning a numerical value to each category. Effect modification was evaluated using interaction tests. In addition, we stratified the analyses according to menopausal status, and we performed stratified analyses according to hormone receptor status or tumor histological type in invasive cases using competing-risk models [16]. We performed homogeneity tests to compare risk estimates across strata.
We additionally used a case–control design to explore the relationships between number of nevi and (1) personal history of BBD and (2) family history of breast cancer in first-degree relatives. For this analysis, unconditional logistic regression models were used to estimate odds ratios (ORs) and 95% CIs. For history of BBD, potential confounders were included only if their inclusion resulted in at least a 10% change in the OR. Final models included age at cohort inclusion, age at last returned questionnaire, education, premenopausal progestogens, menopausal status, age at menopause, use of MHT in postmenopausal women, and family history of breast cancer. For family history of breast cancer, models included education and age at cohort inclusion.
For all analyses, missing values in covariates were imputed to the modal category if values were missing in <5% of observations, otherwise a “missing” category was created for the covariate. Regarding our exposure of interest, women with no information on number of nevi (n = 2,211; 2.2% of the total cohort) were more likely to be older, to have a late menarche, to be younger at their first full-term pregnancy, and to be nulliparous, but were less likely to have a high education level or a high physical activity level at baseline; to be overweight or obese; to be tall; to have ever used OCs, premenopausal progestogens, or MHT; to have ever breastfed; to have ever had a history of BBD, a mammographic exam, or a family history of breast cancer; or to have a high UV dose in their county of residence at baseline compared to those with available data on this factor (Table S1). However, given the low rate of missing values in this variable (2.2%), we speculate that exclusion of missing data would have little impact on the findings; we thus excluded participants lacking information on number of nevi from all analyses.
Population for Analysis
From the original study population, we excluded women with missing data on number of nevi (n = 2,211), with a cancer history before inclusion (n = 5,024), lost to follow-up after they replied to the inclusion questionnaire (n = 1,931), or with primary amenorrhea (n = 27). Our final sample for analysis consisted of 89,802 women. Woman-years were computed from the date the first questionnaire was returned to the date of diagnosis of breast cancer or any other cancer, date of last questionnaire returned, or date of end of follow-up (June 25, 2008), whichever occurred first.
For the analyses exploring number of nevi in relation to history of BBD, we further excluded women with non-biopsied BBD (n = 26,173). For analyses exploring family history of breast cancer, we excluded from the original study population women with missing data on number of nevi (n = 2,211) and those with missing data on family history of breast cancer (n = 728). The final study populations for these analyses included 63,629 women for history of BBD and 96,056 women for family history of breast cancer.
Results
Over 1,385,970 woman-years and a median follow-up of 17.9 y, a total of 5,956 incident breast cancer cases (including 5,245 invasive and 711 in situ cases) were ascertained among the 89,902 included women. Table 1 describes the baseline characteristics of the participants according to number of nevi. Women with a high number of nevi were more likely to be from more recent birth cohorts; to have a high education level; to be tall (≥164 cm); to have an early menarche (<13 y of age); to have ever used OCs, premenopausal progestogens, or MHT; to have ever breastfed; to be premenopausal; and to have a history of BBD. In contrast, they were less likely to be physically active, to be overweight or obese, to have had three or more full-term pregnancies, and to have had their first live birth before the age of 30 y.
Tab. 1. Baseline characteristics of the study population. 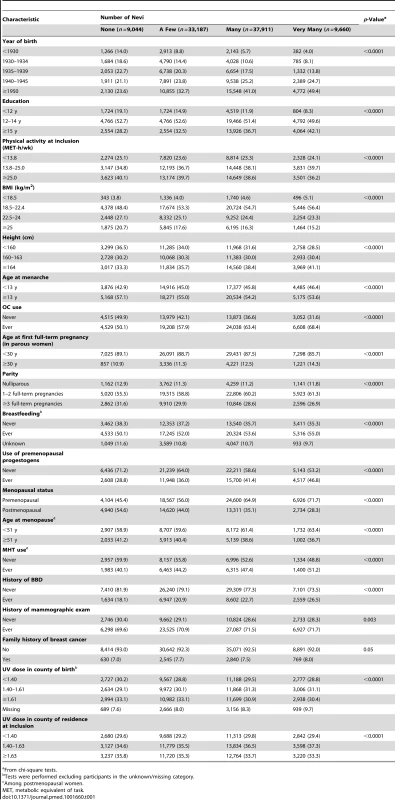
From chi-square tests. In age-adjusted models, women in the highest category of number of nevi had a significantly higher breast cancer risk (HR = 1.17, 95% CI = 1.05–1.31; ptrend = 0.006) compared to those in the lowest (Table 2). The association remained in Model 2; however, associations were reduced and were no longer statistically significant after additional adjustment for history of BBD (Model 3, HR = 1.10, 95% CI = 0.98–1.23) and family history of breast cancer (Model 4, HR = 1.09, 95% CI = 0.98–1.22). The association was observed for both in situ and invasive tumors, with no evidence of heterogeneity across these groups (phomogeneity = 0.27 in Model 2); however, the HR for number of nevi in invasive cases was statistically significant in the model adjusted only for age (Model 1). The 10-y absolute risk of invasive breast cancer increased from 3,749 per 100,000 women without nevi to 4,124 (95% CI = 3,674–4,649) per 100,000 women with “very many” nevi.
Tab. 2. Hazard ratios and 95% confidence intervals for risk of breast cancer in relation to number of nevi, E3N cohort (n = 89,802). 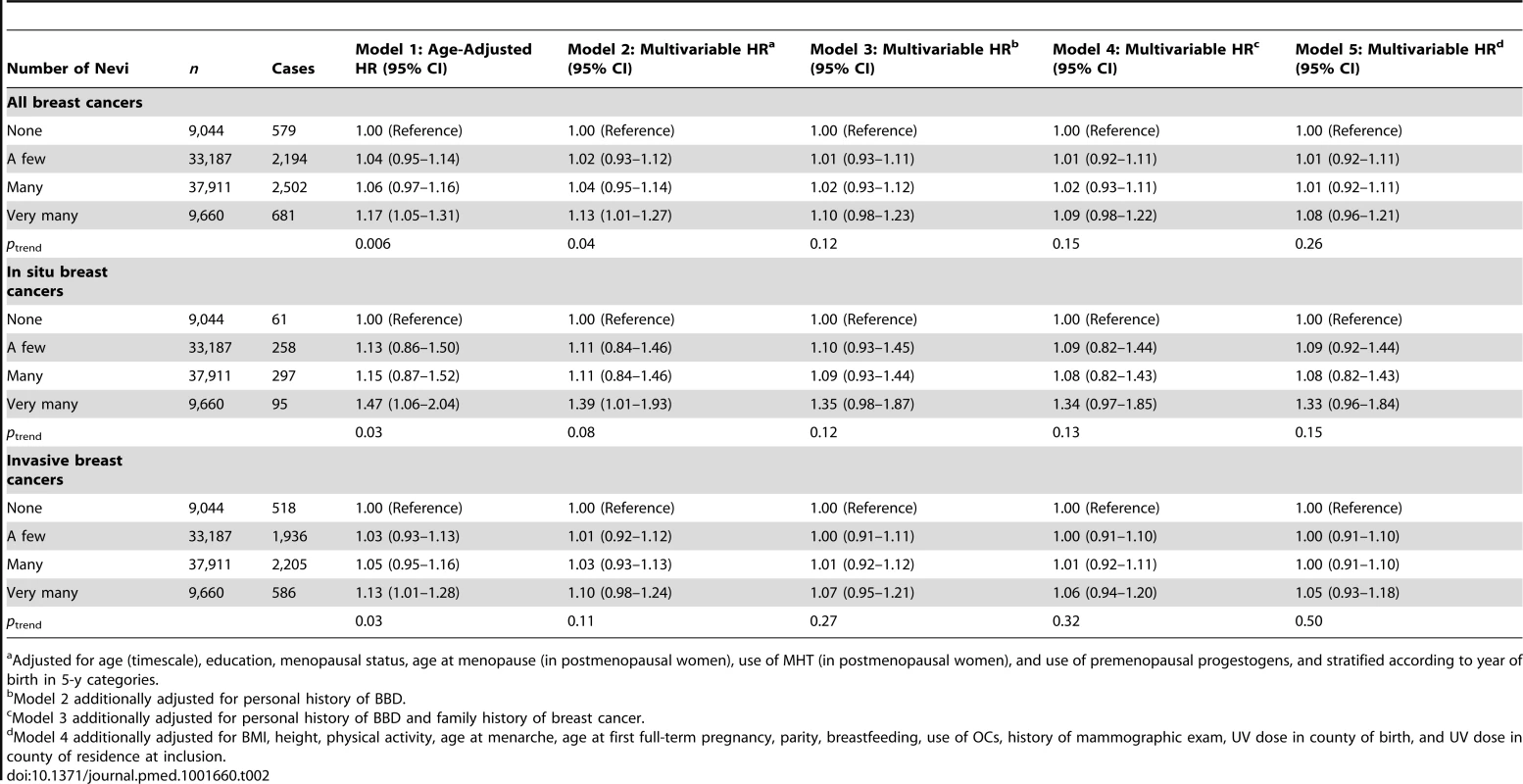
Adjusted for age (timescale), education, menopausal status, age at menopause (in postmenopausal women), use of MHT (in postmenopausal women), and use of premenopausal progestogens, and stratified according to year of birth in 5-y categories. Although “none” was the obvious reference category to explore number of nevi, it was the smallest category in our cohort, and it could be argued that this could inflate error estimates. However, since the “none” group was not small per se—it included over 9,000 participants—and since our findings were not substantially modified when testing different reference groups for this variable (“none/a few”: Table S2; “a few”: Table S3), we kept “none” as the reference category for number of nevi in all subsequent analyses.
No significant interactions were found between number of nevi and history of BBD (pinteraction = 0.27) or family history of breast cancer (pinteraction = 0.97) for the risk of breast cancer. We also detected no significant interaction of number of nevi with skin color (pinteraction = 0.73), physical activity at inclusion (pinteraction = 0.17), UV dose in county of birth (pinteraction = 0.07), or UV dose in county of residence at inclusion (pinteraction = 0.06). Since the p-values for the last two factors were close to statistical significance, we conducted stratified analyses according to the median of UV dose in county of birth (Table S4) and according to the median of UV dose in county of residence at baseline (Table S5); however, we observed no significant heterogeneity across these strata.
When stratifying the analysis according to menopausal status, we detected significant heterogeneity across strata (phomogeneity = 0.04). The association was restricted to premenopausal women, in whom the relation remained even in the multivariable model (HR = 1.34, 95% CI = 1.00–1.81 for “very many” versus “none”; ptrend = 0.03) (Table 3), corresponding to a 10-y absolute risk of invasive breast cancer of 2,515 per 100,000 women with no nevi versus 3,370 (95% CI = 2,515–4,552) per 100,000 women with “very many” nevi. Subgroup analyses according to histological type of breast cancer (ductal carcinoma, lobular carcinoma, or other) (Table 4) or ER/PR status (Table 5) yielded no significant heterogeneity.
Tab. 3. Hazard ratios and 95% confidence intervals for risk of breast cancer in relation to number of nevi, stratified by menopausal status, E3N cohort (n = 89,802). 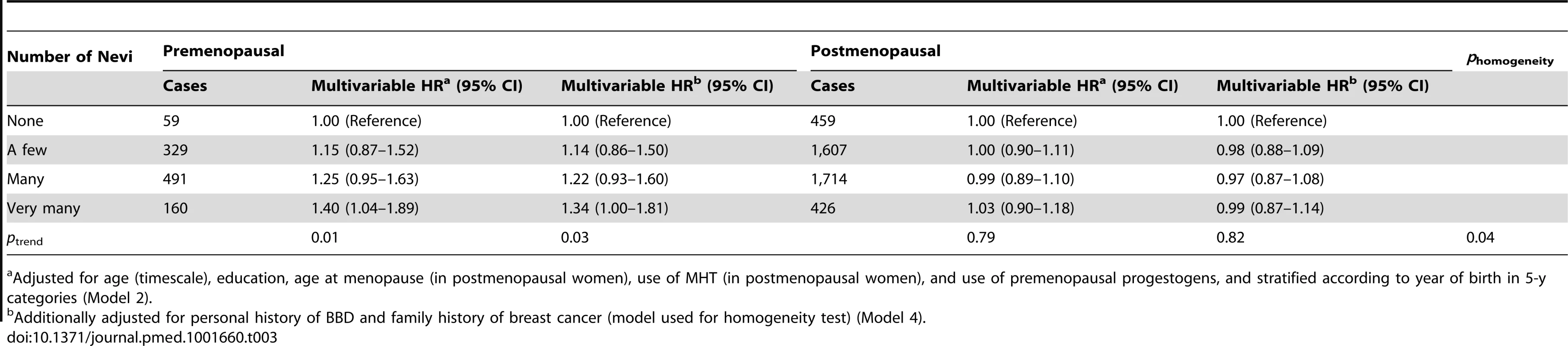
Adjusted for age (timescale), education, age at menopause (in postmenopausal women), use of MHT (in postmenopausal women), and use of premenopausal progestogens, and stratified according to year of birth in 5-y categories (Model 2). Tab. 4. Hazard ratios and 95% confidence intervals for risk of breast cancer in relation to number of nevi, stratified by histological type of breast cancer, E3N cohort (n = 89,429). 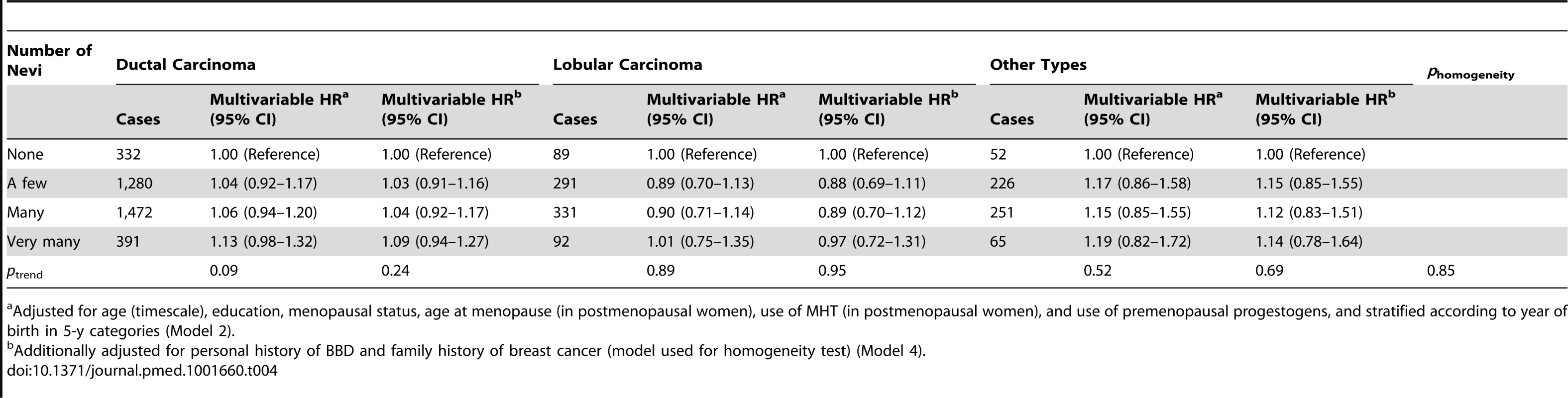
Adjusted for age (timescale), education, menopausal status, age at menopause (in postmenopausal women), use of MHT (in postmenopausal women), and use of premenopausal progestogens, and stratified according to year of birth in 5-y categories (Model 2). Tab. 5. Hazard ratios and 95% confidence intervals for risk of breast cancer in relation to number of nevi, stratified by hormonal receptor status, E3N cohort (n = 88,387). 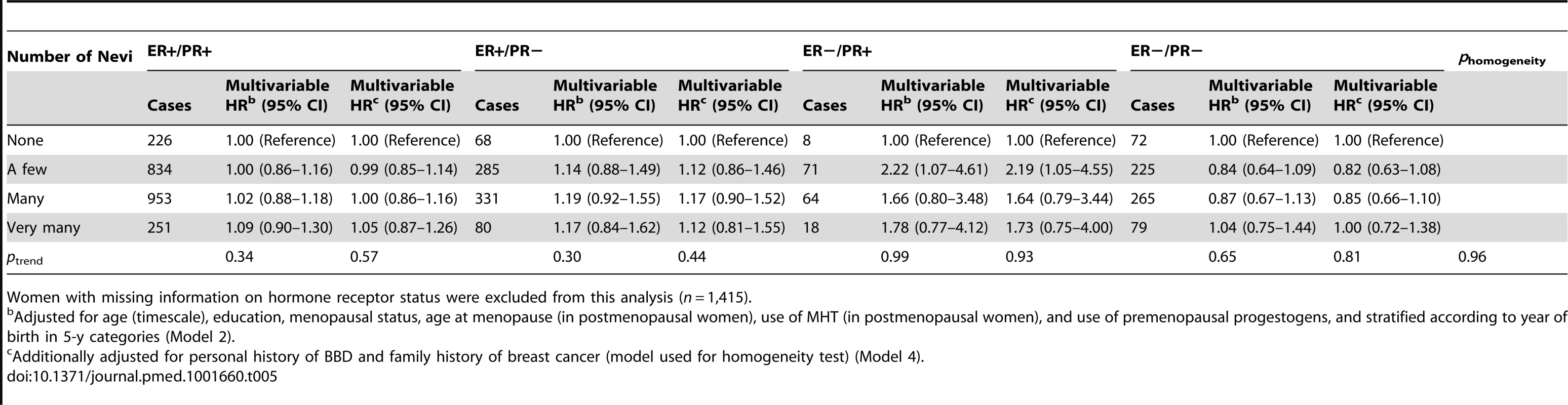
Women with missing information on hormone receptor status were excluded from this analysis (n = 1,415). Associations between number of nevi and BBD and family history of breast cancer are reported in Tables 6 and 7. We observed positive dose–response relationships between number of nevi and a personal history of biopsy-confirmed BBD (ORs of 1.14, 1.15, and 1.26 across increasing categories of number of nevi; ptrend<0.0001) and family history of breast cancer in first-degree relatives (ORs of 1.14, 1.15, and 1.25 across increasing categories of number of nevi; ptrend = 0.0003).
Tab. 6. Odds ratios and 95% confidence intervals for number of nevi in relation to history of benign breast disease, E3N cohort. 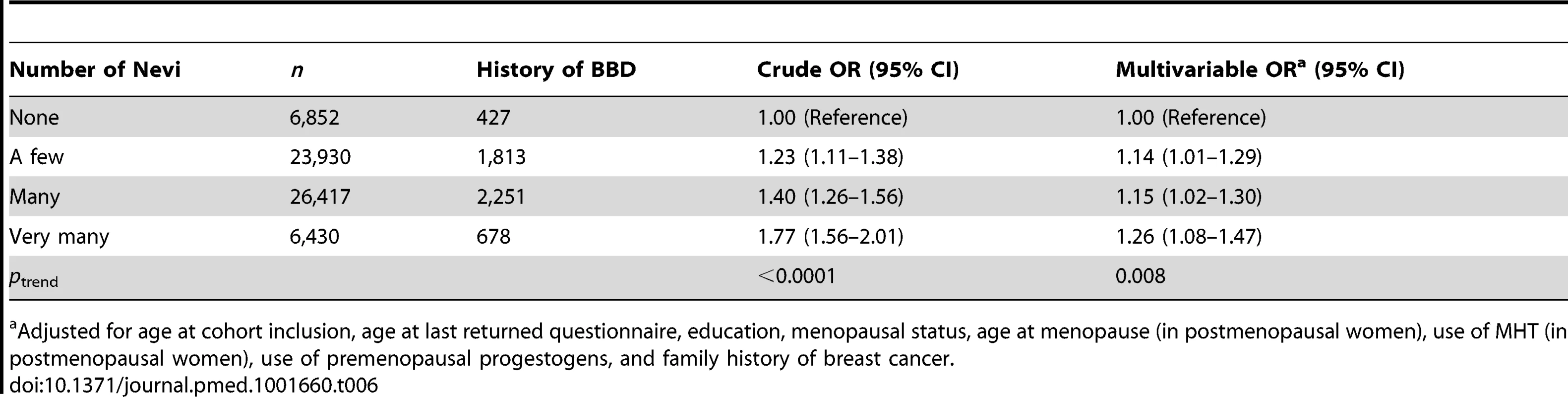
Adjusted for age at cohort inclusion, age at last returned questionnaire, education, menopausal status, age at menopause (in postmenopausal women), use of MHT (in postmenopausal women), use of premenopausal progestogens, and family history of breast cancer. Tab. 7. Odds ratios and 95% confidence intervals for number of nevi in relation to family history of breast cancer, E3N cohort. 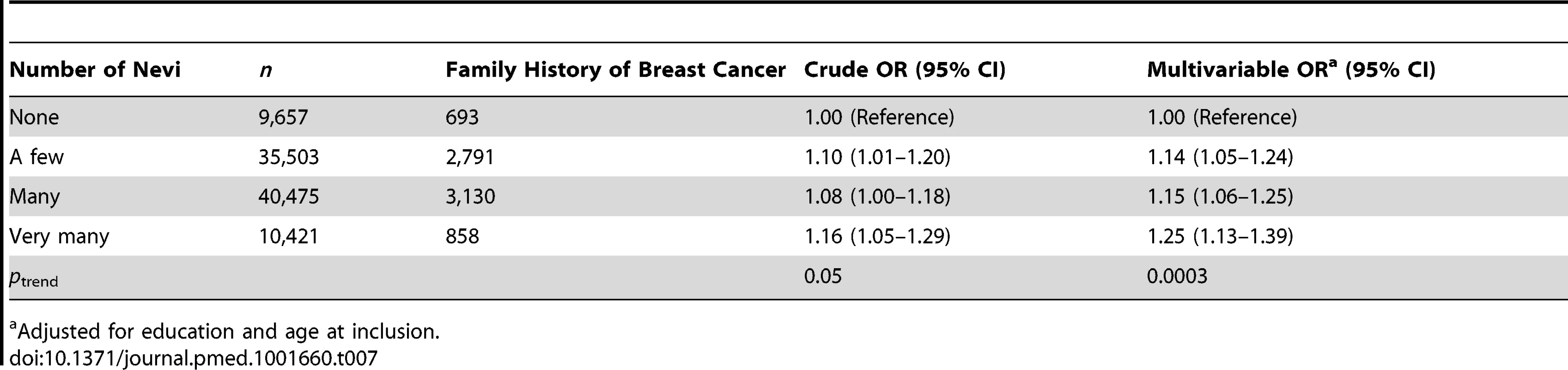
Adjusted for education and age at inclusion. Discussion
In the present study, we found a modest association between number of nevi and overall breast cancer risk, which was restricted to premenopausal women. In these women, the highest versus lowest category of number of nevi was associated with a HR of 1.34 for breast cancer (10-y absolute risk of invasive breast cancer of 2,515 per 100,000 women with no nevi versus 3,370 [95% CI = 2,515–4,552] per 100,000 women with “very many” nevi). A high number of nevi was also associated with the risk of biopsy-confirmed BBD and with family history of breast cancer in first-degree relatives. While adjustment for personal history of BBD and family history of breast cancer reduced the association between number of nevi and overall breast cancer risk, it did not substantially modify the association with premenopausal breast cancer risk.
While a causal relationship between number of nevi and breast disease risk seems unlikely, our observed associations between number of nevi and risk of premenopausal breast cancer, history of BBD, and family history of breast cancer may suggest at least two potential mechanisms.
One potential mechanism is that these relations could reflect a common hormonal influence on nevi and breast diseases. The fact that significant associations were restricted to premenopausal breast cancer and that number of nevi was associated with both BBD and breast cancer risk are consistent with this hypothesis, although we failed to find a stronger association with ER+ than with ER − tumors, possibly because of a lack of power in subgroup analyses. Consistent with this hypothesis, melanocytes are known to express estrogen and androgen receptors [21], melanogenesis is known to be influenced by endogenous sex hormones [21],[31], and a transient increase in nevi darkness and diameter has been observed during pregnancy [19],[20],[32]. Nonetheless, if this hypothesis were true, then an increase in nevus number should also be observed during pregnancy or MHT use, when endogenous female hormone levels are substantially increased. However, no previous study to our knowledge has made this observation to date.
A second potential mechanism is that nevi and breast diseases could share genetic factors, which is consistent with our observed association between number of nevi and family history of breast cancer. Interestingly, several studies have reported associations between melanoma and breast cancer, finding either a higher risk of breast cancer following melanoma diagnosis [33]–[37] or the opposite [23],[24],[34],[38]–[48], and a higher breast cancer risk was reported in melanoma-prone families carrying CDKN2A mutations [49]. Among genetic factors that could account for a common heritability between nevus count and breast cancer, one potential candidate is CDKN2A, a tumor suppressor gene encoding cyclin-dependent kinase inhibitors known to be frequently mutated or suppressed in a number of cancers. This gene has been identified as a high penetrance susceptibility gene for melanoma, with germline mutations occurring in 20%–40% of families with three or more melanoma cases [50], and it has also been associated with nevus count in recent genome-wide association studies [6],[51]. In addition, a SNP located in a block encompassing CDKN2A and CDKN2B at 9p21, rs1011970, was reported to be associated with breast cancer in a recent genome-wide scan [52]. The association was later confirmed in a pooled study, in which similar associations were reported in ER+ and ER − tumors [53].
CDKN2A codes for two proteins, p14 and p16 [54]. By competing with cyclin D1 for CDK4/6 binding, p16 inhibits the expression and transcription of cyclin D1, one of the main mediators of the proliferative action of estrogens [55]. Silencing of p16 protein expression through epigenetic mechanisms, or because of a germline mutation, has been suspected to play a crucial role in the progression of intraductal proliferative lesions [56] and has been associated with breast cancer risk, particularly in young women [57]. Moreover, estradiol-induced cell proliferation in the case of p16-enhanced cyclin D1 expression may be amplified in a highly estrogenic environment. This may be consistent with our finding that the association between number of nevi and breast cancer risk is restricted to premenopausal women.
However, because it is unclear whether the associations we found reflect common hormonal, genetic, or environmental pathways, more research is warranted to understand their underlying biological mechanisms.
Strengths of our study include the large sample size and prospective design of the E3N cohort; we also had detailed data on breast cancer cases, personal history of BBD, and family history of breast cancer.
The main limitation regarded self-report of nevi number, and use of a qualitative scale instead of counts. Repeatability studies of number of nevi indeed show a moderate reliability [58]–[60]. However, in this cohort of mostly educated women, self-reported features have demonstrated high reproducibility in several validation studies [61]–[63]. In addition, number of nevi showed a strong dose–response relationship with the risk of cutaneous melanoma in our cohort [64], which suggests satisfactory validity for this variable. Also, misclassification, if any, would be non-differential and independent from the studied outcomes, and would thus likely result in underestimating existing relationships.
Another limitation is that race information was not available in our cohort, as this type of question is not acceptable to French ethical committees. However, while E3N women did report their skin color, we detected no significant interaction in our findings according to this factor. Further, while the large sample size of our cohort makes it possible to detect small associations, our findings regarding breast cancer risk were of modest effect size and sensitive to adjustment, particularly for history of BBD and family history of breast cancer, for which we found an independent association with number of nevi. Also, given the number of interaction tests that we performed, some of our findings could be attributable to chance and should therefore be interpreted with caution. However, while a multiple testing issue can arise when a high number of hypothesis-free tests are performed [65], the significant interaction between menopausal status and nevi number with regard to breast cancer risk is based on known heterogeneity of breast cancer risk factors according to this characteristic.
Another limitation was that history of BBD was self-reported and could not always be confirmed through biopsy reports, which may have introduced some degree of misclassification. However, when we studied BBD as a potential confounder, our results were unchanged whether analyses included all BBDs or biopsied BBDs only, and when we studied history of BBD as an outcome, restricting the analyses to biopsy-confirmed BBD likely reduced this bias.
Another potential bias could arise from a complex relationship between UV exposure, number of nevi, and vitamin D levels. Indeed, exposure to UVB rays results in higher vitamin D synthesis, and normal versus insufficient or deficient vitamin D levels in adulthood have been associated with a reduced breast cancer risk [66]. Because number of nevi increases with sun exposure, UV exposure may act as a confounder in the association between number of nevi and breast cancer risk. Although our models were adjusted for residential UV dose, level of recreational sun exposure was not available, and we thus cannot rule out residual confounding, which would most likely result in reducing the strength of an association between number of nevi and breast cancer risk. Thus, it is unlikely that the observed associations between premenopausal breast cancer risk result from confounding, but regarding the absence of an association with postmenopausal breast cancer, we cannot rule out some residual negative confounding.
In conclusion, these data from a large prospective cohort study suggest associations between number of nevi and risk of premenopausal breast cancer, history of BBD, and family history of breast cancer. Because associations were modest and the results for breast cancer were sensitive to adjustment, we mostly consider our findings in terms of enhancement of our knowledge of pathophysiological mechanisms. More research is warranted before these findings could possibly be used in diagnosis or screening scores for breast cancer. If confirmed, these findings may suggest that nevi could be associated with other markers of breast cancer risk, such as mammographic density, which should warrant a specific study.
Supporting Information
Zdroje
1. GreenA, SwerdlowAJ (1989) Epidemiology of melanocytic nevi. Epidemiol Rev 11 : 204–221.
2. McGregorB, PfitznerJ, ZhuG, GraceM, EldridgeA, et al. (1999) Genetic and environmental contributions to size, color, shape, and other characteristics of melanocytic naevi in a sample of adolescent twins. Genet Epidemiol 16 : 40–53.
3. WachsmuthRC, GautRM, BarrettJH, SaundersCL, Randerson-MoorJA, et al. (2001) Heritability and gene-environment interactions for melanocytic nevus density examined in a U.K. adolescent twin study. J Invest Dermatol 117 : 348–352.
4. WachsmuthRC, TurnerF, BarrettJH, GautR, Randerson-MoorJA, et al. (2005) The effect of sun exposure in determining nevus density in UK adolescent twins. J Invest Dermatol 124 : 56–62.
5. FalchiM, BatailleV, HaywardNK, DuffyDL, BishopJA, et al. (2009) Genome-wide association study identifies variants at 9p21 and 22q13 associated with development of cutaneous nevi. Nat Genet 41 : 915–919.
6. ZhuG, MontgomeryGW, JamesMR, TrentJM, HaywardNK, et al. (2007) A genome-wide scan for naevus count: linkage to CDKN2A and to other chromosome regions. Eur J Hum Genet 15 : 94–102.
7. AutierP, BoniolM, SeveriG, GilesG, CattaruzzaMS, et al. (2001) The body site distribution of melanocytic naevi in 6-7 year old European children. Melanoma Res 11 : 123–131.
8. DoddAT, MorelliJ, MokrohiskyST, AsdigianN, ByersTE, et al. (2007) Melanocytic nevi and sun exposure in a cohort of Colorado children: anatomic distribution and site-specific sunburn. Cancer Epidemiol Biomarkers Prev 16 : 2136–2143.
9. HarrisonSL, BuettnerPG, MacLennanR (1999) Body-site distribution of melanocytic nevi in young Australian children. Arch Dermatol 135 : 47–52.
10. WhitemanDC, BrownRM, PurdieDM, HughesMC (2005) Melanocytic nevi in very young children: the role of phenotype, sun exposure, and sun protection. J Am Acad Dermatol 52 : 40–47.
11. DennisLK, WhiteE, LeeJA, KristalA, McKnightB, et al. (1996) Constitutional factors and sun exposure in relation to nevi: a population-based cross-sectional study. Am J Epidemiol 143 : 248–256.
12. KellyJW, RiversJK, MacLennanR, HarrisonS, LewisAE, et al. (1994) Sunlight: a major factor associated with the development of melanocytic nevi in Australian schoolchildren. J Am Acad Dermatol 30 : 40–48.
13. ValiukevicieneS, MisevicieneI, GollnickH (2005) The prevalence of common acquired melanocytic nevi and the relationship with skin type characteristics and sun exposure among children in Lithuania. Arch Dermatol 141 : 579–586.
14. DennisLK, WhiteE, McKnightB, KristalA, LeeJA, et al. (1996) Nevi and migration within the United States and Canada: a population-based cross-sectional study. Cancer Causes Control 7 : 464–473.
15. FritschiL, McHenryP, GreenA, MackieR, GreenL, et al. (1994) Naevi in schoolchildren in Scotland and Australia. Br J Dermatol 130 : 599–603.
16. NguyenTD, SiskindV, GreenL, FrostC, GreenA (1997) Ultraviolet radiation, melanocytic naevi and their dose-response relationship. Br J Dermatol 137 : 91–95.
17. RichardMA, GrobJJ, GouvernetJ, CulatJ, NormandP, et al. (1994) [Role of sun exposure on benign melanocytic nevi. A first study in populations controlled for age, sex and phenotype.]. Ann Dermatol Venereol 121 : 639–644.
18. RodvallY, WahlgrenCF, UllenH, WiklundK (2007) Common melanocytic nevi in 7-year-old schoolchildren residing at different latitudes in Sweden. Cancer Epidemiol Biomarkers Prev 16 : 122–127.
19. DriscollMS, Grant-KelsJM (2007) Hormones, nevi, and melanoma: an approach to the patient. J Am Acad Dermatol 57 : 919–931.
20. RubegniP, SbanoP, BurroniM, CeveniniG, BocchiC, et al. (2007) Melanocytic skin lesions and pregnancy: digital dermoscopy analysis. Skin Res Technol 13 : 143–147.
21. SlominskiA, TobinDJ, ShibaharaS, WortsmanJ (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84 : 1155–1228.
22. GandiniS, SeraF, CattaruzzaMS, PasquiniP, AbeniD, et al. (2005) Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer 41 : 28–44.
23. MellemkjaerL, ChristensenJ, FrederiksenK, PukkalaE, WeiderpassE, et al. (2011) Risk of primary non-breast cancer after female breast cancer by age at diagnosis. Cancer Epidemiol Biomarkers Prev 20 : 1784–1792.
24. YangGB, Barnholtz-SloanJS, ChenY, BordeauxJS (2011) Risk and survival of cutaneous melanoma diagnosed subsequent to a previous cancer. Arch Dermatol 147 : 1395–1402.
25. MitchellPJ, Perez-NadalesE, MalcolmDS, LloydAC (2003) Dissecting the contribution of p16(INK4A) and the Rb family to the Ras transformed phenotype. Mol Cell Biol 23 : 2530–2542.
26. KvaskoffM, MesrineS, Clavel-ChapelonF, Boutron-RuaultMC (2009) Endometriosis risk in relation to naevi, freckles and skin sensitivity to sun exposure: the French E3N cohort. Int J Epidemiol 38 : 1143–1153.
27. KvaskoffM, BijonA, MesrineS, Clavel-ChapelonF, Boutron-RuaultMC (2010) Pigmentary traits and risk of endometriosis. Hum Reprod 25 : 3157–3158.
28. RedondoP, IdoateM, De FelipeI (1998) Nevi related to thyroid diseases. Arch Intern Med 158 : 1577.
29. Clavel-ChapelonF, van LiereMJ, GiuboutC, NiravongMY, GoulardH, et al. (1997) E3N, a French cohort study on cancer risk factors. E3N Group. Etude Epidemiologique aupres de femmes de l'Education Nationale. Eur J Cancer Prev 6 : 473–478.
30. VerdeboutJ (2004) A European satellite-derived UV climatology available for impact studies. Radiat Prot Dosimetry 111 : 407–411.
31. ResnikS (1967) Melasma induced by oral contraceptive drugs. JAMA 199 : 601–605.
32. BorgesV, PuigS, MalvehyJ (2011) [Melanocytic nevi, melanoma, and pregnancy.]. Actas Dermosifiliogr 102 : 650–657.
33. BradfordPT, FreedmanDM, GoldsteinAM, TuckerMA (2010) Increased risk of second primary cancers after a diagnosis of melanoma. Arch Dermatol 146 : 265–272.
34. GogginsW, GaoW, TsaoH (2004) Association between female breast cancer and cutaneous melanoma. Int J Cancer 111 : 792–794.
35. SoerjomataramI, LouwmanWJ, LemmensVE, CoeberghJW, de VriesE (2008) Are patients with skin cancer at lower risk of developing colorectal or breast cancer? Am J Epidemiol 167 : 1421–1429.
36. SpanogleJP, ClarkeCA, AronerS, SwetterSM (2010) Risk of second primary malignancies following cutaneous melanoma diagnosis: a population-based study. J Am Acad Dermatol 62 : 757–767.
37. WassbergC, ThornM, YuenJ, HakulinenT, RingborgU (1999) Cancer risk in patients with earlier diagnosis of cutaneous melanoma in situ. Int J Cancer 83 : 314–317.
38. EwertzM, MouridsenHT (1985) Second cancer following cancer of the female breast in Denmark, 1943–80. Natl Cancer Inst Monogr 68 : 325–329.
39. GalperS, GelmanR, RechtA, SilverB, KohliA, et al. (2002) Second nonbreast malignancies after conservative surgery and radiation therapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys 52 : 406–414.
40. HarveyEB, BrintonLA (1985) Second cancer following cancer of the breast in Connecticut, 1935–82. Natl Cancer Inst Monogr 68 : 99–112.
41. KirovaYM, De RyckeY, GambottiL, PiergaJY, AsselainB, et al. (2008) Second malignancies after breast cancer: the impact of different treatment modalities. Br J Cancer 98 : 870–874.
42. MellemkjaerL, FriisS, OlsenJH, SceloG, HemminkiK, et al. (2006) Risk of second cancer among women with breast cancer. Int J Cancer 118 : 2285–2292.
43. ProchazkaM, HallP, GranathF, CzeneK (2006) Family history of breast cancer and young age at diagnosis of breast cancer increase risk of second primary malignancies in women: a population-based cohort study. Br J Cancer 95 : 1291–1295.
44. RubinoC, de VathaireF, DialloI, ShamsaldinA, LeMG (2000) Increased risk of second cancers following breast cancer: role of the initial treatment. Breast Cancer Res Treat 61 : 183–195.
45. SchaapveldM, VisserO, LouwmanMJ, de VriesEG, WillemsePH, et al. (2008) Risk of new primary nonbreast cancers after breast cancer treatment: a Dutch population-based study. J Clin Oncol 26 : 1239–1246.
46. SoerjomataramI, LouwmanWJ, de VriesE, LemmensVE, KlokmanWJ, et al. (2005) Primary malignancy after primary female breast cancer in the south of the Netherlands, 1972–2001. Breast Cancer Res Treat 93 : 91–95.
47. VolkN, Pompe-KirnV (1997) Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control 8 : 764–770.
48. YuGP, SchantzSP, NeugutAI, ZhangZF (2006) Incidences and trends of second cancers in female breast cancer patients: a fixed inception cohort-based analysis (United States). Cancer Causes Control 17 : 411–420.
49. BorgA, SandbergT, NilssonK, JohannssonO, KlinkerM, et al. (2000) High frequency of multiple melanomas and breast and pancreas carcinomas in CDKN2A mutation-positive melanoma families. J Natl Cancer Inst 92 : 1260–1266.
50. de SnooFA, HaywardNK (2005) Cutaneous melanoma susceptibility and progression genes. Cancer Lett 230 : 153–186.
51. BishopDT, DemenaisF, IlesMM, HarlandM, TaylorJC, et al. (2009) Genome-wide association study identifies three loci associated with melanoma risk. Nat Genet 41 : 920–925.
52. TurnbullC, AhmedS, MorrisonJ, PernetD, RenwickA, et al. (2010) Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 42 : 504–507.
53. NickelsS, TruongT, HeinR, StevensK, BuckK, et al. (2013) Evidence of gene-environment interactions between common breast cancer susceptibility loci and established environmental risk factors. PLoS Genet 9: e1003284.
54. Agarwal P, Lutful Kabir FM, DeInnocentes P, Bird RC (2012) Tumor suppressor gene p16/INK4A/CDKN2A and its role in cell cycle exit, differentiation, and determination of cell fate. In: Cheng Y, editor. Tumor suppressor genes. Rijeka (Croatia): InTech.
55. Doisneau-SixouSF, SergioCM, CarrollJS, HuiR, MusgroveEA, et al. (2003) Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer 10 : 179–186.
56. LiuT, NiuY, FengY, NiuR, YuY, et al. (2008) Methylation of CpG islands of p16(INK4a) and cyclinD1 overexpression associated with progression of intraductal proliferative lesions of the breast. Hum Pathol 39 : 1637–1646.
57. DebniakT, GorskiB, HuzarskiT, ByrskiT, CybulskiC, et al. (2005) A common variant of CDKN2A (p16) predisposes to breast cancer. J Med Genet 42 : 763–765.
58. BaxterAJ, HughesMC, KvaskoffM, SiskindV, ShekarS, et al. (2008) The Queensland Study of Melanoma: environmental and genetic associations (Q-MEGA); study design, baseline characteristics, and repeatability of phenotype and sun exposure measures. Twin Res Hum Genet 11 : 183–196.
59. GlanzK, SchoenfeldE, WeinstockMA, LayiG, KiddJ, et al. (2003) Development and reliability of a brief skin cancer risk assessment tool. Cancer Detect Prev 27 : 311–315.
60. WesterdahlJ, AndersonH, OlssonH, IngvarC (1996) Reproducibility of a self-administered questionnaire for assessment of melanoma risk. Int J Epidemiol 25 : 245–251.
61. Clavel-ChapelonF, Dormoy-MortierN (1998) A validation study on status and age of natural menopause reported in the E3N cohort. Maturitas 29 : 99–103.
62. RacineA, BijonA, FournierA, MesrineS, Clavel-ChapelonF, et al. (2013) Menopausal hormone therapy and risk of cholecystectomy: a prospective study based on the French E3N cohort. CMAJ 185 : 555–561.
63. TehardB, van LiereMJ, Com NougueC, Clavel-ChapelonF (2002) Anthropometric measurements and body silhouette of women: validity and perception. J Am Diet Assoc 102 : 1779–1784.
64. KvaskoffM, MesrineS, FournierA, Boutron-RuaultMC, Clavel-ChapelonF (2007) Personal history of endometriosis and risk of cutaneous melanoma in a large prospective cohort of French women. Arch Intern Med 167 : 2061–2065.
65. RothmanKJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1 : 43–46.
66. EngelP, FagherazziG, BouttenA, DupreT, MesrineS, et al. (2010) Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev 19 : 2341–2350.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials
- Health Care in Danger: Deliberate Attacks on Health Care during Armed Conflict
- Melanocytic Nevi as Biomarkers of Breast Cancer Risk
- Blood Transfusions following Trauma: Finding an Evidence-Based Vein
- Antiretroviral Therapy for Refugees and Internally Displaced Persons: A Call for Equity
- Association between Cutaneous Nevi and Breast Cancer in the Nurses' Health Study: A Prospective Cohort Study
- Efficacy of Pneumococcal Nontypable Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: A Double-Blind Randomized Controlled Trial
- Pediatric Oncology as the Next Global Child Health Priority: The Need for National Childhood Cancer Strategies in Low- and Middle-Income Countries
- Place and Cause of Death in Centenarians: A Population-Based Observational Study in England, 2001 to 2010
- HIV among People Who Inject Drugs in the Middle East and North Africa: Systematic Review and Data Synthesis
- Association between Melanocytic Nevi and Risk of Breast Diseases: The French E3N Prospective Cohort
- Patient-Safety-Related Hospital Deaths in England: Thematic Analysis of Incidents Reported to a National Database, 2010–2012
- Pushback: The Current Wave of Anti-Homosexuality Laws and Impacts on Health
- HIV Treatment-as-Prevention Research at a Crossroads
- Red Blood Cell Transfusion and Mortality in Trauma Patients: Risk-Stratified Analysis of an Observational Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Melanocytic Nevi as Biomarkers of Breast Cancer Risk
- Antiretroviral Therapy for Refugees and Internally Displaced Persons: A Call for Equity
- Efficacy of Pneumococcal Nontypable Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: A Double-Blind Randomized Controlled Trial
- Blood Transfusions following Trauma: Finding an Evidence-Based Vein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



