-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAntiretroviral Therapy for Refugees and Internally Displaced Persons: A Call for Equity
article has not abstract
Published in the journal: . PLoS Med 11(6): e32767. doi:10.1371/journal.pmed.1001643
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001643Summary
article has not abstract
Summary Points
-
Available evidence suggests that refugees and internally displaced persons (IDPs) in stable settings can sustain high levels of adherence and viral suppression.
-
Moral, legal, and public health principles and recent evidence strongly suggest that refugees and IDPs should have equitable access to HIV treatment and support.
-
Exclusion of refugees and IDPs from HIV National Strategic Plans suggests that they may not be included in future national funding proposals to major donors.
-
Levels of viral suppression among refugees and nationals documented in a stable refugee camp suggest that some settings require more intensive support for all population groups.
-
Detailed recommendations are provided for refugees and IDPs accessing antiretroviral therapy in stable settings.
Background
For people living with HIV and AIDS (PLHIV), treatment with antiretroviral therapy (ART) can result in viral suppression, prolonged life expectancy, and reduced HIV transmission [1]–[3]. To realize these benefits, PLHIV require regular access to medications and supportive services. Within conflict-affected settings, there are unique challenges to providing, accessing, and adhering to ART [4],[5]. An estimated 1.5 billion people live in countries impacted by violent conflict and 45.2 million are forcibly displaced as a result of persecution, conflict, violence, and/or human rights violations [6],[7]. In 2006, 1.8 million PLHIV were affected by conflict, disaster, or displacement. Half of countries (7/15) with the largest number of PLHIV were affected by a major conflict between 2002 and 2006 [8]. Conflict-affected persons reside in areas of recent or active conflict, or in a post-conflict camp, urban, or rural setting, and may be accorded an official status depending on their situation [9],[10]. Refugee status is available to individuals with “a well-founded fear of being persecuted for reasons of race, religion, nationality, membership of a particular social group or political opinion, is outside the country of his/her nationality and is unable or unwilling (owing to such fear) to avail him/herself of the protection of that country” [11]. Internally displaced persons (IDPs) are citizens who have “been forced or obliged to flee or to leave their homes or places of habitual residence, in particular as a result of or in order to avoid the effects of armed conflict, situations of generalized violence, violations of human rights or natural or human-made disasters, and who have not crossed an internationally recognized State border” [12]. A protracted refugee situation occurs when >25,000 refugees of the same nationality have been in exile for ≥5 years. At the end of 2012, 6.4 million refugees, or 61% of the global total of 10.5 million, lived in a protracted situation [7]. In the post-emergency phase of forced displacement, refugees and IDPs live in relatively “stable” settings, meaning that exceptional measures are no longer required to remove threats to life or well-being [13]. Here, we synthesize norms of practice with evidence on the impact of ART programs in stable settings, including our own experiences delivering and evaluating ART as part of the United Nations High Commissioner for Refugees (UNHCR) mandate. We then propose operational recommendations for improving HIV treatment outcomes among refugees and IDPs in stable settings. Moral, legal, and public health principles, in combination with recent evidence, provide a clear rationale for sustainable treatment provision and adequate support for refugees and IDPs.
Equity and Sustainability
Debates over the merits of providing ART to refugees and IDPs have echoed earlier discussions on treatment scale-up in resource-constrained settings [14]. Four main arguments support the provision of ART to these groups: (1) the evidence for clinical benefit, reduced transmission, and cost-effectiveness supports the scaling-up of treatment to all in need [15],[16]; (2) where scarcity exists, principles of fairness should be applied uniformly to allocation of treatment [17],[18]; (3) the right to health, including the principle of access to essential medicines, clearly articulates a rationale for providing access to life-saving interventions for PLHIV [19]–[21]; and (4) the Convention Relating to the Status of Refugees enshrines a principle of equity whereby hosting countries should provide refugees with a similar standard of medical care to the standard that is routinely available to host nationals [11],[12].
In practice, however, countries do not uniformly provide access to ART within their borders [22], and international assistance can be slow in reaching these groups. Compared with unaffected countries, conflict-affected countries received less than half of the funds provided through overseas development assistance (ODA) for reproductive health services in the period 2006–2008 [23]. Funding cycles are also temporary. Funding provided for refugees and local populations by the World Bank through the Great Lakes Initiative on AIDS for refugees and surrounding host populations recently ended, leading to a resource gap. Given budget pressures, national governments may give preferential treatment to nationals and/or non-minorities. Funds provided by the major donors such as Global Fund to Fight AIDS, Tuberculosis and Malaria (Global Fund), and the President's Emergency Plan for AIDS Relief (PEPFAR) are less likely to find groups who are excluded from national funding proposals, an omission that is more likely to occur when such marginalized groups are also excluded from HIV National Strategic Plans (NSPs). Of NSPs issued in the ten year period from 1998 to 2008 by African countries, only 52% and 43% mentioned refugees and IDPs, respectively [24],[25].
Evidence for Action
Evidence of effectiveness is needed to support arguments for scaling-up treatment and support. Findings from studies conducted among refugees and IDPs showed that 87%–99.5% of clients studied had achieved ≥95% adherence and positive treatment outcomes, as measured by survival and CD4 gains [26]. However, outside of high-income settings, very few published studies have used virologic outcomes to assess program performance. One study that compared citizens with “foreigners” who relocated to Johannesburg found higher levels of viral suppression among the displaced group (76% versus 58%, p = 0.02) [27].
Our direct experience comes from delivering and evaluating ART programs as part of UNHCR's mandate to protect refugees. We conducted two evaluations using virologic outcomes in two stable refugee settings (Table 1). In a stable urban setting (Kuala Lumpur, Malaysia), similar proportions of refugees and host nationals accessing treatment from a shared clinic achieved viral suppression (83% overall), while proportions adhering to treatment were similar according to pharmacy refill records and self-reports [28]. In a stable refugee camp (Kakuma, Kenya), considerably lower proportions (50% overall) of both groups on treatment for ≥25 weeks were virologically suppressed [29]. This discrepancy may have been due to unverified adherence lapses, background levels of drug resistance, or high ambient medication storage temperatures [29]. These evaluations, in combination with evidence from other settings, suggest that treatment outcomes among stable refugees or IDPs, and host nationals, are similar when treatment and support is accessed from a shared clinic. Therefore, similar levels of viral suppression should be expected, although more challenging settings will require more intensive and specialized support for all population groups. Even in stable settings, disruptions may still occur. During the 2008 post-election violence in Kenya, an otherwise stable setting, people on ART appeared to have difficulties locating and/or taking their treatment. During this period of instability, 16% of clients on ART interrupted their treatment, as compared with 10% during a stable comparison period, and mortality rates increased [30],[31]. In stable settings at increased risk for disruption, the potential negative effects of treatment interruptions may be lessened if the period of disruption is short, there are strong contingency plans in place for ensuring continuing access to treatment, and people on ART have been educated on how to best manage their treatment in these challenging circumstances.
Tab. 1. Results of UNHCR-sponsored evaluations conducted in Malaysia and Kenya. 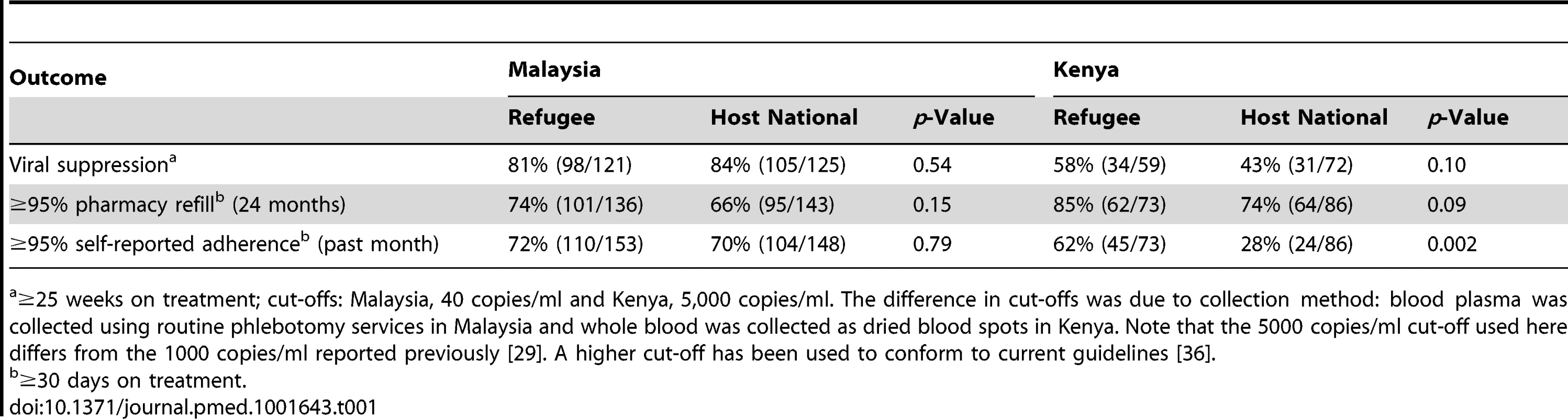
≥25 weeks on treatment; cut-offs: Malaysia, 40 copies/ml and Kenya, 5,000 copies/ml. The difference in cut-offs was due to collection method: blood plasma was collected using routine phlebotomy services in Malaysia and whole blood was collected as dried blood spots in Kenya. Note that the 5000 copies/ml cut-off used here differs from the 1000 copies/ml reported previously [29]. A higher cut-off has been used to conform to current guidelines [36]. Recommendations
Despite the challenges, there is now general agreement on the public health, humanitarian, and human rights arguments that support access to ART for refugees and IDPs when clinically indicated [32]–[35]. To this end, donors, health care workers, and hosting countries should collaborate on strategies for expanding access and providing the necessary supportive services. On the basis of our experiences implementing ART interventions for refugees and IDPs in stable post-conflict settings, our own rigorous evaluations of two such programs, and the experiences of others in this area, we have proposed a set of operational recommendations in support of ART programs for refugees and IDPs situated in stable settings (Table 2). These recommendations are intended to complement WHO's new consolidated guidelines on the use of antiretroviral drugs [36].
Tab. 2. Recommendations for provision of antiretroviral therapy to refugees and IDPs in stable settings. 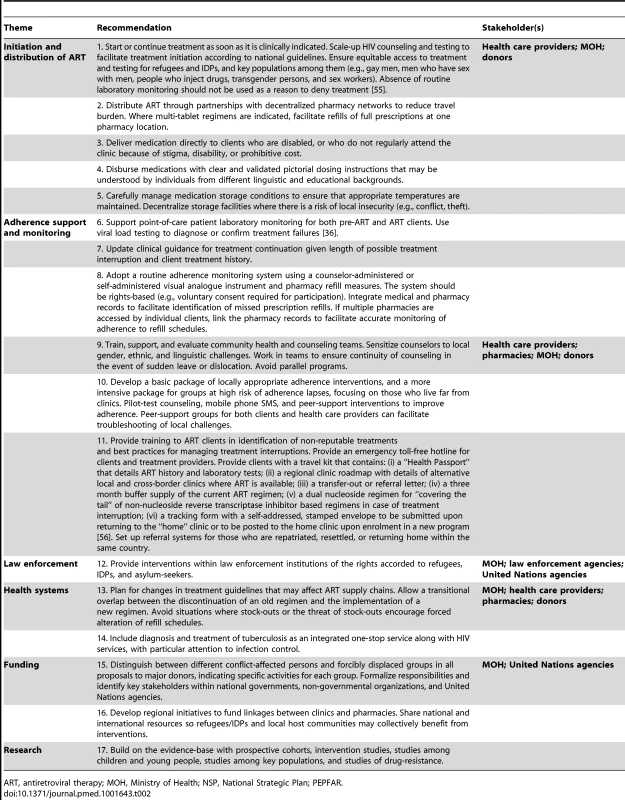
ART, antiretroviral therapy; MOH, Ministry of Health; NSP, National Strategic Plan; PEPFAR. First, treatment should be offered to all refugees and IDPs who meet national guidelines (Recommendation 1). Despite the known effectiveness of ART, some governments may be reluctant to provide it to these groups owing to a misplaced belief that starting or continuing them on treatment may make it difficult for them to return home and that ART may serve as a pull factor, drawing additional refugee claimants to the country. However, the majority of hosting countries (88%) provide equitable access to ART for refugees and IDPs, UNHCR has not noted such a pull factor [37], and international humanitarian law entitles refugees to equitable access. IDPs should clearly benefit from inclusion in their national HIV programs. Scaling-up of HIV counseling and testing so all who are eligible are given a chance to initiate ART at the optimal time will save lives and reduce costs by increasing survival and reducing HIV transmission [2],[3],[38]. Appropriate measures must be taken to ensure that key populations (e.g., men who have sex with men, people who inject drugs, transgender persons, and sex workers) within refugee, IDP, and host national groups also receive equitable access to treatment and testing.
ART should be available in convenient locations through decentralized networks of clinics and pharmacies (Recommendation 2) or delivered directly to clients who are unable to access the medications themselves (Recommendation 3). Dosing instructions that are designed and proven to be universally understood should be provided with each medication refill (Recommendation 4). In areas with extreme ambient temperatures, drug stocks should be stored carefully and within the temperature ranges suggested by manufacturers (Recommendation 5).
Adherence monitoring and support is crucial for achieving consistent treatment outcomes and can help to avoid costly treatment failures. Viral load testing should be used to diagnose or confirm treatment failures (Recommendation 6) and may be done on an annual basis or as often as resources will permit. Pharmacy-based and self-reported measures should also be used to routinely monitor adherence (Recommendation 8). Although adherence self-reports may overestimate adherence, they have diagnostic value, are easy to implement in clinical settings, and can help to complement pharmacy refill measures when the period between refills is lengthy or if the pharmacy data is inconsistently recorded. Both measures are consistent with previous recommendations and are associated with virologic outcomes [39]–[41].
Community health and counseling teams who are sensitized to gender, ethnic, and linguistic challenges should be mobilized (Recommendation 9). Interventions that employ mobile phone text messaging, enhanced counseling and peer-support should be pilot-tested among refugees and IDPs to assess acceptability and effectiveness [42]–[44]. Addressing travel distances to clinics and transportation costs are crucial as longer travel times and higher costs may increase the likelihood of treatment interruptions [45]–[47]. Peer-support groups for both clients and health care providers can facilitate troubleshooting of local challenges (Recommendation 10). Refugees and IDPs may travel to re-unite with family in host countries, repatriate to their home country, or resettle to a new country. Even in stable settings, proper preparations for the possibility of onward displacement are important to include in programming and will serve the dual purpose of preparing people on treatment for sudden, unexpected instability (Recommendation 11). [48] As barriers to accessing treatment and supportive services sometimes include fear of detention by law enforcement officials during routine transit in urban and rural settings, interventions are needed to increase awareness among law enforcement officials of the rights accorded to refugees, asylum-seekers, and IDPs (Recommendation 12) [49].
As treatment guidelines change, new regimens will need to be stocked and distributed. Measures should be taken to avoid situations where stock-outs or the threat of stock-outs lead to forced alteration of ART refill schedules. Remote pharmacies and clinics (e.g., refugee camps) are advised to allow a transitional overlap of between the discontinuation of an old regimen and the implementation of a new regimen (Recommendation 13). There is a lack of integration, in general, between HIV and other programs including TB services; clinical settings serving refugees and IDPs are no different [50]. Diagnosis and treatment of tuberculosis should therefore be integrated with HIV services (Recommendation 14).
Host countries are advised to include specific results-oriented interventions for refugees and IDPs in their NSPs, Global Fund, and PEPFAR proposals (Recommendation 15). These proposals should identify a plan for equitable access to ART and which agencies will be responsible for program delivery [51]. Regional initiatives can facilitate partnerships in treatment management by providing a framework for assisting those who move within a country or across an international border, while helping to monitor and distinguish clients who are successfully re-integrated into treatment programs elsewhere from those who have been lost to follow-up. By engaging in partnerships with humanitarian organizations, governments can leverage international funds for the benefit of their own citizens as well as the groups they are hosting (Recommendation 16) [52]. Finally, there is a continuing need for additional evidence. As the majority of research has been conducted among adult refugees and IDPs, there is a need for research among children and young people [53],[54]. As past studies among refugees and IDPs have largely focused on short-term outcomes, prospective cohort studies or intervention studies with virologic endpoints are needed. We are not aware of any data on rates of acquired or transmitted drug resistance among these groups (Recommendation 17).
Overall, this set of recommendations will face a range of implementation barriers including resource constraints and coordination challenges among stakeholders. However, these improvements will be necessary if sustainable treatment outcomes are to be achieved among refugees and IDPs who live in stable settings.
Conclusions
Given recent evidence and the moral, legal, and public health arguments, refugees and IDPs situated in stable settings should have equitable access to HIV treatment and supportive services. Despite encouraging treatment outcomes among these groups across a range of settings, considerable challenges persist. Programs that are not achieving high levels of success should serve as a call for intervention, not exclusion. Few would rationally argue that challenges to providing life-saving treatment should be addressed by denying access. Since antiretroviral therapy can help prevent onward transmission of HIV to sexual partners [2], it is in the enlightened self-interest of governments that host refugees and IDPs to support programs that serve all populations within their borders to the highest possible standard.
Zdroje
1. MartinM, Del CachoE, CodinaC, TusetM, De LazzariE, et al. (2008) Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses 24 : 1263–1268.
2. CohenMS, ChenYQ, McCauleyM, GambleT, HosseinipourMC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 : 493–505.
3. KitahataMM, GangeSJ, AbrahamAG, MerrimanB, SaagMS, et al. (2009) Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 360 : 1815–1826.
4. International Federation of Red Cross and Red Crescent Societies (2008) World Disaster Report 2008: Focus on HIV and AIDS. Geneva: International Federation of Red Cross and Red Crescent Societies. pp. 118–141.
5. PorterM, HaslamN (2005) Predisplacement and postdisplacement factors associated with mental health of refugees and internally displaced persons - a meta-analysis. JAMA 294 : 602–612.
6. World Bank (2013) Fragile and conflict-affected situations (FCS). Available: http://web.worldbank.org/WBSITE/EXTERNAL/NEWS/0,contentMDK:20042303~menuPK:34480~pagePK:64257043~piPK:437376~theSitePK:4607,00.html. Accessed 10 October 2012.
7. UNHCR (2012) Statistical Yearbook 2012. Geneva: UNHCR. Available: http://www.unhcr.org/statistics.
8. Lowicki-ZuccaM, SpiegelPB, KellyS, DehneKL, WalkerN, et al. (2008) Estimates of HIV burden in emergencies. Sex Transm Infect 84 Suppl 1i42–i48.
9. Uppsala University Department of Peace and Conflict Research (2012) Uppsala Conflict Data Program Conflict Encyclopedia. Available: http://www.pcr.uu.se/research/ucdp/definitions/#Active. Accessed 13 March 2012.
10. SpiegelPB, ChecchiF, ColomboS, PaikE (2010) Health-care needs of people affected by conflict: future trends and changing frameworks. Lancet 375 : 341–345.
11. United Nations General Assembly (1951) Convention Relating to the Status of Refugees, 28 July 1951, United Nations, Treaty Series, vol. 189, p. 137. Available: http://www.unhcr.org/refworld/docid/3be01b964.html.
12. Inter-agency Standing Committee (2010) Handbook for the protection of internally displaced persons. Geneva: UNHCR. Available: http://www.unhcr.org/refworld/docid/4790cbc02.html.
13. UNHCR (2007) Handbook for emergencies (3rd edition). Geneva: UNHCR. Available: http://www.refworld.org/pdfid/46a9e29a2.pdf.
14. FordN (2009) Treating AIDS in complex emergencies: the need for clear policy consensus. Progress in Development Studies 9 : 55–61.
15. NosykB, MontanerJS (2012) The evolving landscape of the economics of HIV treatment and prevention. PLoS Med 9: e1001174.
16. NachegaJB, MillsEJ, SchechterM (2010) Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: current status of knowledge and research priorities. Curr Opin HIV AIDS 5 : 70–77.
17. LeaningJ, SpiegelP, CrispJ (2011) Public health equity in refugee situations. Confl Health 5 : 6.
18. PersadG, WertheimerA, EmanuelEJ (2009) Principles for allocation of scarce medical interventions. Lancet 373 : 423–431.
19. PillayN (2008) Right to health and the Universal Declaration of Human Rights. Lancet 372 : 2005–2006.
20. United Nations General Assembly (1966) International Covenant on Economic, Social, and Cultural Rights, 16 December 1966, United Nations, Treaty Series, vol. 993, p. 3. New York. Available: http://www.refworld.org/docid/3ae6b36c0.html.
21. United Nations Commission on Human Rights (2003) Commission on Human Rights Resolution 2003/29: Access to Medication in the Context of Pandemics Such as HIV/AIDS, Tuberculosis and Malaria, 22 April 2003, E/CN.4/RES/2003/29. New York. Available: ttp://www.refworld.org/docid/43f3132ed1.html.
22. TrippayyaV (2005) Botswana: refugees not entitled to same services as citizens. HIV AIDS Policy Law Rev 10 : 27–28.
23. PatelP, RobertsB, GuyS, Lee-JonesL, ContehL (2009) Tracking official development assistance for reproductive health in conflict-affected countries. PLoS Med 6: e1000090.
24. SpiegelPB, HeringH, PaikE, SchilperoordM (2010) Conflict-affected displaced persons need to benefit more from HIV and malaria national strategic plans and Global Fund grants. Confl Health 4 : 2.
25. Burton A (2010) HIV, refugees and conflict-affected populations in Asia. Forced Migration Review Oct 2010 (Suppl.).
26. Mendelsohn JB, Schilperoord M, Spiegel P, Ross DA (2012) Adherence to antiretroviral therapy and treatment outcomes in conflict-affected and forcibly displaced populations: a systematic review. Confl Health 6 : 9.
27. McCarthyK, ChersichMF, VeareyJ, Meyer-RathG, JafferA, et al. (2009) Good treatment outcomes among foreigners receiving antiretroviral therapy in Johannesburg, South Africa. Int J STD AIDS 20 : 858–862.
28. Mendelsohn JB, Spiegel P, Schilperoord M, Balasundaram S, Radhakrishnan A, et al.. (2014) Is forced migration a barrier to treatment success? Similar HIV treatment outcomes among refugees and a surrounding host community in Kuala Lumpur, Malaysia. AIDS Behav 18: : 323–334.
29. Mendelsohn JB, Schilperoord M, Spiegel P, Burton JW, Okonji JA, et al.. (2012) Poor treatment outcomes among both refugees and host community accessing highly active antiretroviral therapy (HAART) from Kakuma refugee camp in northwestern Kenya (THPDD0102). In: Proceedings of the International AIDS Conference; 22–27 July 2012; Washington (D.C.), United States.
30. Pyne-MercierLD, John-StewartGC, RichardsonBA, KagonduNL, ThigaJ, et al. (2011) The consequences of post-election violence on antiretroviral HIV therapy in Kenya. AIDS Care 23 : 562–568.
31. FeikinDR, AdazuK, OborD, OgwangS, VululeJ, et al. (2010) Mortality and health among internally displaced persons in western Kenya following post-election violence, 2008: novel use of demographic surveillance. Bull World Health Organ 88 : 601–608.
32. The Sphere Project (2011) The Sphere Handbook: Humanitarian Charter and Minimum Standards in Humanitarian Response (3ed). Rugby, UK. Available: http://www.sphereproject.org/resources/download-publications/?search=1&keywords=&language=English&category=22.
33. The Sphere Project (2011) What is new in the Sphere Handbook 2011 edition. Available: http://www.sphereproject.org/silo/files/what-is-new-in-the-sphere-handbook-2011-edition.pdf. Accessed 16 April 2012.
34. Inter-agency Standing Working Group on Reproductive Health in Crises (2010) Inter-agency field manual on reproductive health in humanitarian settings - 2010 revision for field review. Geneva. Available: http://www.iawg.net/IAFM%202010.pdf.
35. Inter-agency Standing Committee (2010) Guidelines for addressing HIV in humanitarian settings. Geneva: UNAIDS. Available: http://www.humanitarianinfo.org/iasc/pageloader.aspx?page=content-products-products&productcatid=9.
36. World Health Organization (2013) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva. Available: http://www.who.int/hiv/pub/guidelines/arv2013/en/index.html.
37. UNHCR (2013) Global strategy for four public health sectors: public health: a UNHCR strategy 2014–2018. Geneva: UNHCR. p. 33–43
38. GarnettGP, BeckerS, BertozziS (2012) Treatment as prevention: translating efficacy trial results to population effectiveness. Curr Opin HIV AIDS 7 : 157–163.
39. ThompsonMA, MugaveroMJ, AmicoKR, CargillVA, ChangLW, et al. (2012) Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med 156 : 817–833.
40. SimoniJM, KurthAE, PearsonCR, PantaloneDW, MerrillJO, et al. (2006) Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 10 : 227–245.
41. McMahonJH, JordanMR, KelleyK, BertagnolioS, HongSY, et al. (2011) Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Clin Infect Dis 52 : 493–506.
42. HorvathT, AzmanH, KennedyGE, RutherfordGW (2012) Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev 3: CD009756.
43. ChungMH, RichardsonBA, TapiaK, Benki-NugentS, KiarieJN, et al. (2011) A randomized controlled trial comparing the effects of counseling and alarm device on HAART adherence and virologic outcomes. PLoS Med 8: e1000422.
44. SimoniJM, NelsonKM, FranksJC, YardSS, LehavotK (2011) Are peer interventions for HIV efficacious? A systematic review. AIDS Behav 15 : 1589–1595.
45. Olupot-OlupotP, KataweraA, CooperC, SmallW, AnemaA, et al. (2008) Adherence to antiretroviral therapy among a conflict-affected population in Northeastern Uganda: a qualitative study. AIDS 22 : 1882–1884.
46. TullerDM, BangsbergDR, SenkunguJ, WareNC, EmenyonuN, et al. (2010) Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav 14 : 778–784.
47. KibonekaA, NyatiaRJ, NabiryoC, AnemaA, CooperCL, et al. (2009) Combination antiretroviral therapy in population affected by conflict: outcomes from large cohort in northern Uganda. BMJ 338: b201.
48. VeenstraN, WhitesideA, LallooD, GibbsA (2010) Unplanned antiretroviral treatment interruptions in southern Africa: how should we be managing these? Global Health 6 : 4.
49. de WaalA (2010) Reframing governance, security and conflict in the light of HIV/AIDS: a synthesis of findings from the AIDS, security and conflict initiative. Soc Sci Med 70 : 114–120.
50. KwanCK, ErnstJD (2011) HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 24 : 351–376.
51. UNHCR (2006) Note on HIV/AIDS and the protection of refugees, IDPs and other persons of concern, 5 April 2006. Geneva: United Nations High Commissioner for Refugees. Available: http://www.refworld.org/docid/4444f0884.html.
52. UNAIDS UNHCR (2005) Strategies to support the HIV-related needs of refugees and host populations. Geneva. Available: http://www.unaids.org/en/media/unaids/contentassets/dataimport/publications/irc-pub06/jc1157-refugees_en.pdf.
53. VreemanRC, NyandikoWM, SangE, MusickBS, BraitsteinP, et al. (2009) Impact of the Kenya post-election crisis on clinic attendance and medication adherence for HIV-infected children in western Kenya. Confl Health 3 : 5.
54. KibonekaA, NyatiaRJ, NabiryoC, Olupot-OlupotP, AnemaA, et al. (2008) Pediatric HIV therapy in armed conflict. AIDS 22 : 1097–1098.
55. UNHCR, Southern African HIV Clinicians Society (2007) Clinical guidelines for antiretroviral therapy management for displaced populations. Geneva: UNHCR. Available: http://www.unhcr.org/4683b0522.html.
56. Médecins Sans Frontières (2012) Providing antiretroviral therapy for mobile populations: lessons learned from a cross border ARV program in Musina, South Africa. Cape Town: Médecins Sans Frontières. Available: http://www.msfaccess.org/sites/default/files/MSF_assets/HIV_AIDS/Docs/AIDS_report_ARTformobilepops_ENG_2012.pdf.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 6- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Evidence for the Selective Reporting of Analyses and Discrepancies in Clinical Trials: A Systematic Review of Cohort Studies of Clinical Trials
- Health Care in Danger: Deliberate Attacks on Health Care during Armed Conflict
- Melanocytic Nevi as Biomarkers of Breast Cancer Risk
- Blood Transfusions following Trauma: Finding an Evidence-Based Vein
- Antiretroviral Therapy for Refugees and Internally Displaced Persons: A Call for Equity
- Association between Cutaneous Nevi and Breast Cancer in the Nurses' Health Study: A Prospective Cohort Study
- Efficacy of Pneumococcal Nontypable Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: A Double-Blind Randomized Controlled Trial
- Pediatric Oncology as the Next Global Child Health Priority: The Need for National Childhood Cancer Strategies in Low- and Middle-Income Countries
- Place and Cause of Death in Centenarians: A Population-Based Observational Study in England, 2001 to 2010
- HIV among People Who Inject Drugs in the Middle East and North Africa: Systematic Review and Data Synthesis
- Association between Melanocytic Nevi and Risk of Breast Diseases: The French E3N Prospective Cohort
- Patient-Safety-Related Hospital Deaths in England: Thematic Analysis of Incidents Reported to a National Database, 2010–2012
- Pushback: The Current Wave of Anti-Homosexuality Laws and Impacts on Health
- HIV Treatment-as-Prevention Research at a Crossroads
- Red Blood Cell Transfusion and Mortality in Trauma Patients: Risk-Stratified Analysis of an Observational Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Melanocytic Nevi as Biomarkers of Breast Cancer Risk
- Antiretroviral Therapy for Refugees and Internally Displaced Persons: A Call for Equity
- Efficacy of Pneumococcal Nontypable Protein D Conjugate Vaccine (PHiD-CV) in Young Latin American Children: A Double-Blind Randomized Controlled Trial
- Blood Transfusions following Trauma: Finding an Evidence-Based Vein
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



