-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaObstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study
Background:
Obstructive sleep apnea (OSA) has been reported to be a risk factor for cardiovascular (CV) disease. Although the apnea-hypopnea index (AHI) is the most commonly used measure of OSA, other less well studied OSA-related variables may be more pathophysiologically relevant and offer better prediction. The objective of this study was to evaluate the relationship between OSA-related variables and risk of CV events.Methods and Findings:
A historical cohort study was conducted using clinical database and health administrative data. Adults referred for suspected OSA who underwent diagnostic polysomnography at the sleep laboratory at St Michael's Hospital (Toronto, Canada) between 1994 and 2010 were followed through provincial health administrative data (Ontario, Canada) until May 2011 to examine the occurrence of a composite outcome (myocardial infarction, stroke, congestive heart failure, revascularization procedures, or death from any cause). Cox regression models were used to investigate the association between baseline OSA-related variables and composite outcome controlling for traditional risk factors. The results were expressed as hazard ratios (HRs) and 95% CIs; for continuous variables, HRs compare the 75th and 25th percentiles. Over a median follow-up of 68 months, 1,172 (11.5%) of 10,149 participants experienced our composite outcome. In a fully adjusted model, other than AHI OSA-related variables were significant independent predictors: time spent with oxygen saturation <90% (9 minutes versus 0; HR = 1.50, 95% CI 1.25–1.79), sleep time (4.9 versus 6.4 hours; HR = 1.20, 95% CI 1.12–1.27), awakenings (35 versus 18; HR = 1.06, 95% CI 1.02–1.10), periodic leg movements (13 versus 0/hour; HR = 1.05, 95% CI 1.03–1.07), heart rate (70 versus 56 beats per minute [bpm]; HR = 1.28, 95% CI 1.19–1.37), and daytime sleepiness (HR = 1.13, 95% CI 1.01–1.28).The main study limitation was lack of information about continuous positive airway pressure (CPAP) adherence.Conclusion:
OSA-related factors other than AHI were shown as important predictors of composite CV outcome and should be considered in future studies and clinical practice.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(2): e32767. doi:10.1371/journal.pmed.1001599
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001599Summary
Background:
Obstructive sleep apnea (OSA) has been reported to be a risk factor for cardiovascular (CV) disease. Although the apnea-hypopnea index (AHI) is the most commonly used measure of OSA, other less well studied OSA-related variables may be more pathophysiologically relevant and offer better prediction. The objective of this study was to evaluate the relationship between OSA-related variables and risk of CV events.Methods and Findings:
A historical cohort study was conducted using clinical database and health administrative data. Adults referred for suspected OSA who underwent diagnostic polysomnography at the sleep laboratory at St Michael's Hospital (Toronto, Canada) between 1994 and 2010 were followed through provincial health administrative data (Ontario, Canada) until May 2011 to examine the occurrence of a composite outcome (myocardial infarction, stroke, congestive heart failure, revascularization procedures, or death from any cause). Cox regression models were used to investigate the association between baseline OSA-related variables and composite outcome controlling for traditional risk factors. The results were expressed as hazard ratios (HRs) and 95% CIs; for continuous variables, HRs compare the 75th and 25th percentiles. Over a median follow-up of 68 months, 1,172 (11.5%) of 10,149 participants experienced our composite outcome. In a fully adjusted model, other than AHI OSA-related variables were significant independent predictors: time spent with oxygen saturation <90% (9 minutes versus 0; HR = 1.50, 95% CI 1.25–1.79), sleep time (4.9 versus 6.4 hours; HR = 1.20, 95% CI 1.12–1.27), awakenings (35 versus 18; HR = 1.06, 95% CI 1.02–1.10), periodic leg movements (13 versus 0/hour; HR = 1.05, 95% CI 1.03–1.07), heart rate (70 versus 56 beats per minute [bpm]; HR = 1.28, 95% CI 1.19–1.37), and daytime sleepiness (HR = 1.13, 95% CI 1.01–1.28).The main study limitation was lack of information about continuous positive airway pressure (CPAP) adherence.Conclusion:
OSA-related factors other than AHI were shown as important predictors of composite CV outcome and should be considered in future studies and clinical practice.
Please see later in the article for the Editors' SummaryIntroduction
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder that is characterized by repeated episodes of upper airway occlusion during sleep. OSA afflicts 3%–9% of women and 10%–17% of men in the United States [1]. Sleep apnea can increase the risk of developing cardiovascular (CV) disease through a number of mechanisms, including intermittent hypoxia, sleep fragmentation, chronic sympathetic activation, and systemic inflammation [2]–[6].
Although evidence exists for relationships between OSA and both all-cause mortality and various CV events, uncertainty surrounds the magnitude of these associations, and the contributions of different OSA-related variables to the development of long-term adverse outcomes [7]. The apnea-hypopnea index (AHI) is the most often-reported statistically significant predictor; however, a variety of non-clinically determined AHI thresholds are arbitrarily used to diagnose and categorize severity of OSA [7],[8]. By focusing almost exclusively on AHI, clinicians and researchers may have missed opportunities to better risk-stratify patients using other OSA-related variables [9]. A number of less-studied variables may be more pathophysiologically relevant and offer better predictive ability than AHI, which is only a crude measure of breathing stoppages: intermittent hypoxemia (e.g., level of oxygen saturation [SaO2]), sleep fragmentation or sleep deprivation (e.g., total sleep time [TST] or number of awakenings), sympathetic activation (e.g., heart rate during sleep), symptoms (daytime sleepiness, snoring), family history of snoring or OSA, and findings from physical examination (neck circumference) [7],[10].
A number of large, well-designed community-based studies have examined the relationship between OSA and CV disease and mortality [11]–[14]. However, these have been limited by the small number of individuals with severe OSA. On the other hand, in clinically based studies with higher disease severity, small numbers of events limit the number of variables that can be included in statistical models, a weak definition of CV events is often used, women are usually underrepresented, and inconsistency in polysomnographic scoring criteria over time means long-term follow-up is not possible [7].
Our study addresses the weaknesses described above by following up a large number of individuals with a wide spectrum of severity of OSA and evaluating the relationship between a comprehensive set of OSA-related variables and the development of CV outcomes and all-cause mortality after controlling for traditional CV risk factors. Finally, our study aims to resolve conflicting evidence on the impact of gender, age, body mass index (BMI), daytime sleepiness (DS), and comorbid CV disease on the strength of association between OSA and development of CV outcomes.
We hypothesize that the AHI, currently used to establish severity of OSA, is not by itself sufficient to accurately predict CV outcomes in individuals with OSA. We also hypothesize that an expanded set of factors including patient demographic and clinical characteristics and physiologic indices will provide greater accuracy in predicting CV outcomes.
Methods
Study Design
A historical cohort study was conducted using a clinical sleep database and provincial health administrative data. All adults who underwent a first diagnostic sleep study at St. Michael's Hospital (Toronto, Ontario, Canada) between September 1, 1994 and December 31, 2010 and were diagnosed or referred with OSA were included. Their clinical data were linked to health administrative data at the Institute for Clinical Evaluative Sciences (ICES, Toronto, Ontario, Canada) from July 1, 1991 to March 31, 2011.
Ethics Statement
The ethics committees of all institutions involved (St. Michael's Hospital, ICES, University of Toronto) approved the study.
Data Sources
Clinical data
The St. Michael's Hospital database includes a large set of clinical, demographic, and polysomnographical (PSG) variables that have been collected consistently for research purposes since 1991 (Table S1). Each patient in the cohort underwent full in-laboratory PSG recording that was scored by a sleep technologist and reviewed by a sleep physician. Disease-specific symptoms and history were collected using standardized questionnaires; a physical examination was performed by sleep technicians.
Health administrative data
Residents of Ontario have universal public health insurance covering all medically necessary services. ICES houses high quality [15] administrative data on a wide variety of publicly funded services provided since 1991, including individual-level information on physician claims, acute care hospitalization, and emergency department visits within the province. For Ontario residents with diagnosed OSA, funding is provided for continuous positive airway pressure (CPAP) devices, and this funding is documented in the Assistive Devices Program (ADP) database from 2004 onwards [16]. Table S2 gives details of variables derived from administrative data sets.
Study Sample
Patients who had undergone a first diagnostic sleep study during the defined study period, and who had a diagnosis of OSA (AHI≥5), or suspected OSA (referred with sleep apnea, but with AHI<5) were extracted from the St. Michael's Hospital database. Patients were excluded if they had (i) more than 50% central events or (ii) AHI<5 and a diagnosis of another sleep disorder.
Predictors
The following OSA-related variables were derived from clinical data and considered as predictors in our statistical models: (i) PSG indexes—TST, the percentage of each sleep stage, AHI, apnea index, hypopnea index, mean duration of apnea and hypopnea, total arousals index, total number of awakenings; mean of SaO2 in TST, sleep time spent with SaO2<90% (TST90SaO2); periodic leg movements index; and mean heart rate; (ii) clinical symptoms—DS, identified by means of the Epworth Sleepiness Scale or a positive answer to the question “During the day, do you ever fall asleep unintentionally?”; self-reported snoring; and morning headache; (iii) neck circumference; (iv) self-reported family history of snoring or OSA.
The AHI was defined as the number of apneas and hypopneas per hour of sleep. The definition of hypopnea was consistent during the study period: (i) a decrease of more than 50% of the baseline amplitude of breathing lasting 10 seconds or longer; or (ii) a clear but smaller decrease in amplitude lasting for at least 10 seconds that is associated with either an SaO2 drop of ≥3% or an arousal [17]. OSA was classified as mild (AHI of 5 to 14.9), moderate (AHI of 15 to 30), or severe (AHI>30) [18].
We assumed that CPAP treatment started at the time of the claim in the ADP data set.
Outcome Variables
The primary composite outcome was defined using health administrative data as the first of (i) hospitalization due to myocardial infarction (MI), stroke, or exacerbation of congestive heart failure (CHF); (ii) a revascularization procedure (percutaneous coronary intervention, coronary artery bypass graft surgery); or (iii) all-cause death (Table S2). Hospitalization was chosen as a well-defined, validated, and standardized measure of interest to patients, doctors, and policy-makers. Participants were followed from their first diagnostic sleep study to the end of March 2011, or the occurrence of a primary outcome, whichever occurred first.
Potential Confounders and Risk Factors
The following potential confounders and risk factors were extracted from clinical data: age, sex, BMI, waist and hip circumferences, and self-reported smoking. Comorbidities at baseline (stroke, MI, CHF, hypertension [HTN], chronic obstructive pulmonary disease [COPD], depression, and diabetes) were identified from administrative data over a three-year period before the diagnostic sleep study. Neighbourhood income and rural status were derived from administrative data at the time of the diagnostic sleep study.
Statistical Analysis
Descriptive statistics were calculated for relevant data. Crude incidence rates of the composite end point per 100 person-years were calculated for each OSA severity group.
Since AHI is the standard for defining of OSA and its severity [2],[18], and to allow comparisons with other research in this area, event-free survival in OSA severity groups was estimated using the Kaplan–Meier method and compared between groups with the log-rank test.
We used univariate and multivariable Cox regression models to investigate the relationships between additional predictors and the CV outcome, and expressed the results as hazard ratios (HRs) and 95% CIs. To avoid choosing arbitrary cut points for PSG characteristics (e.g., AHI, TST), they were kept as continuous variables. We used restricted cubic spline transformations for continuous explanatory variables if non-linearity was observed (Figure S1), and the resulting standardized HRs compare the 75th and 25th percentiles, allowing comparison of the HRs on a common scale. The proportional hazards assumption for each variable was tested [19],[20].
Variables missing on more than 50% of individuals were excluded from all analyses (Table S1). For all others, we used multivariate imputation by chained equations to generate five complete data sets [21]. Eighty two variables including both the event status and the survival time were chosen for the imputation model. The following built-in imputation models were used in our analyses: for continuous variables, predictive mean matching; for binary variables, logistic regression; for unordered categorical variables, polytomous logistic regression; and for ordered categorical variables, proportional odds [22]. The separate estimates and standard errors from imputed data sets were pooled according to Rubin's rules [23]. For a unified presentation of all results and figures, the findings shown are for a single imputed data set. Pooled CIs across imputations were at most 2% wider than those presented.
A systematic review [7] and expert opinion found age, sex, smoking status, BMI, AHI, TST, and DS to be clinically important, so these variables were forced into the models. Other variables were chosen for inclusion in the final model if they were selected by backward step-down variable deletion [24] in at least three imputed data sets. We investigated a priori-defined interactions between AHI or SaO2 and DS, BMI, age, sex, and CVD at baseline [7]. The final model was compared to a model with only traditional CV risk factors (age, sex, smoking status, BMI, prior HTN, diabetes, MI, stroke, and CHF [25]) using a likelihood ratio test [19]. To identify which outcomes were driving results, the final model was refitted to each separate component of the composite CV outcome.
A clinical nomogram was constructed from the multivariable Cox model to estimate three - and five-year event-free survival probabilities and median survival times [26].
We used the bootstrap for internal validation (optimism for C-index and R2) and over-fitting-corrected calibration (predicted versus observed five-year survival). Discriminative ability was assessed using Harrell's C-index and predictive ability using the model likelihood ratio χ2 statistic [19].
Sensitivity analysis
In the post-2004 cohort with information on CPAP claims, the final model was refitted with the addition of a time-dependent CPAP treatment variable.
All statistical analyses were performed using R version 2.15.2 (http://www.r-project.org) and SAS 9.2.
Results
Sample Characteristics
Between January 1, 1994 and December 31, 2010, 11,596 individuals underwent a first diagnostic sleep study and 10,149 (88%) were linked to administrative data sets and included in our analyses (Figure 1). Table 1 shows baseline characteristics of included and excluded patients. Excluded patients had similar OSA severity and demographic characteristics, but fewer CV comorbidities and greater DS. The included sample had 62% males, a mean age of 50 years, and a mean AHI of 25. The amount of missing data ranged from 0.69% (AHI) to10.1% (TST90SaO2).
Fig. 1. Flow diagram of the final cohort. 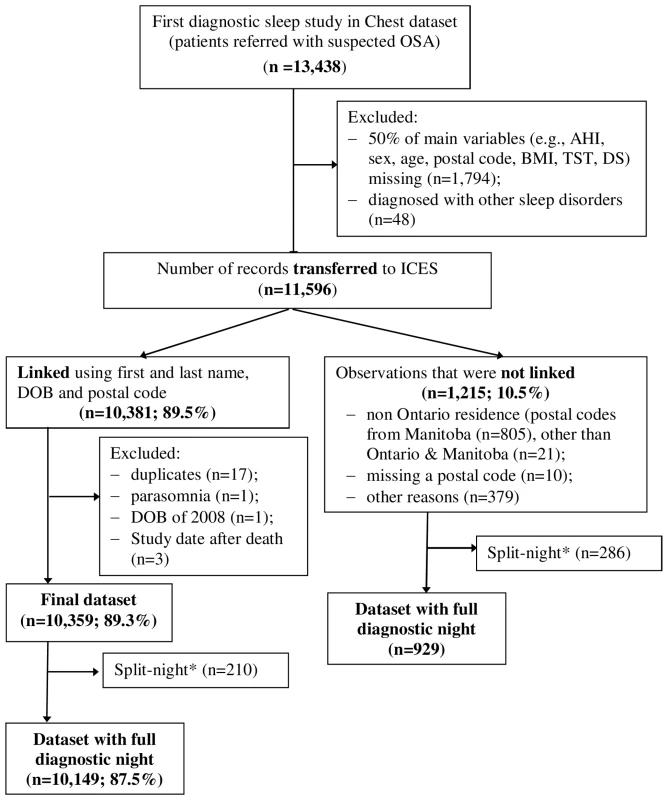
*Split night, diagnostic study night when treatment was initiated due to severe OSA. Tab. 1. Comparison of included and excluded samples for patients with a full-night diagnostic sleep study. 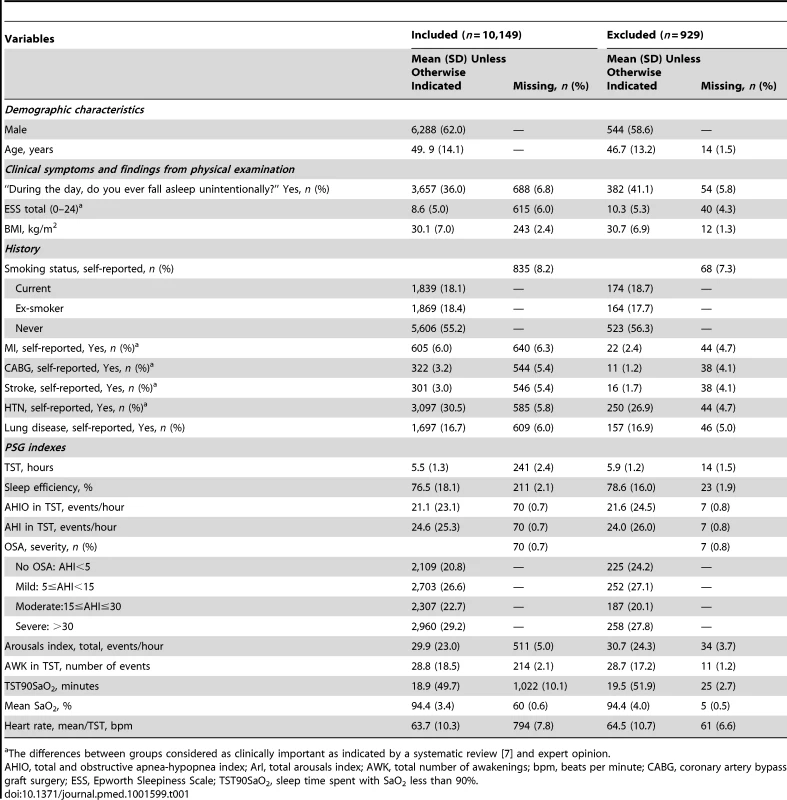
The differences between groups considered as clinically important as indicated by a systematic review [7] and expert opinion. Over a median follow-up of 68 months, 1,172 (11.5%) participants experienced a composite outcome, giving an incidence rate of 2 per 100 person-years. Event-free survival was 94% at three years and 90% at five years with CIs less than ±0.5%.
Analyses of AHI
AHI was significantly associated with event-free survival in univariate analyses when categorized (log-rank test; p<0.001; Figure 2) or treated as a continuous predictor in a Cox model (35 versus 6.3 events/hour: HR = 1.49, 95% CI 1.42–1.57; p<0.001). After controlling for traditional CV risk factors, the magnitude of association was attenuated: no significant difference was found across OSA severity groups (p>0.2) (Figure 3), although the association between AHI as a continuous variable and outcome of interest remained significant (HR = 1.12; 95% CI 1.05–1.2, p<0.001).
Fig. 2. Unadjusted Kaplan-Meier survival curves by obstructive sleep apnea severity as expressed by the apnea-hypopnea index. 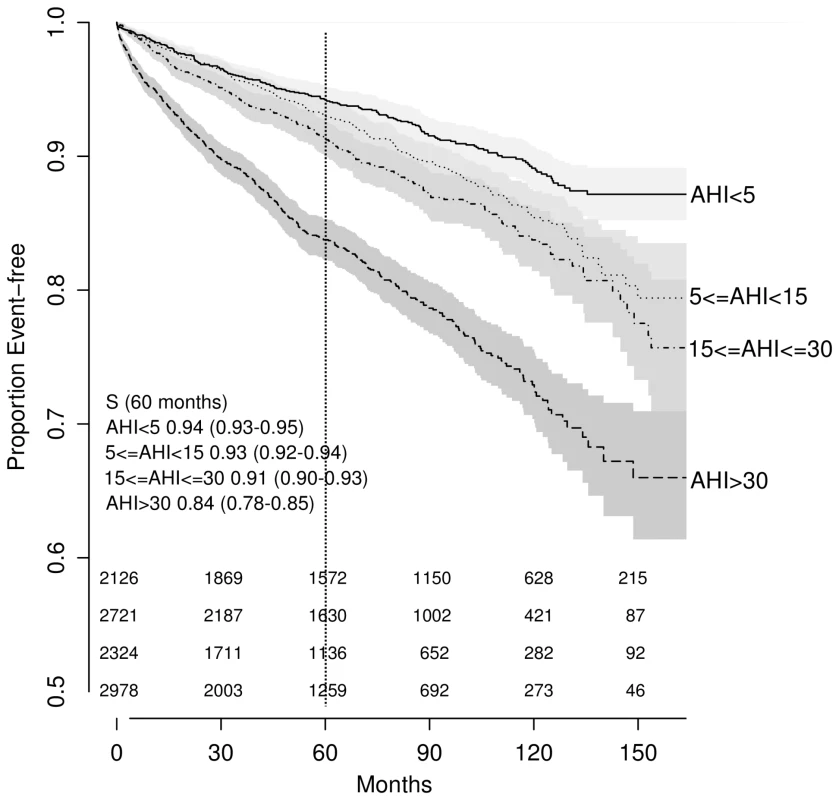
The numbers at risk are presented above the x-axis: from the top to the bottom, AHI<5; 5≤AHI<15; 15≤AHI<30; AHI>30. Fig. 3. Predicted survival by OSA severity, adjusted for traditional CV risk factors 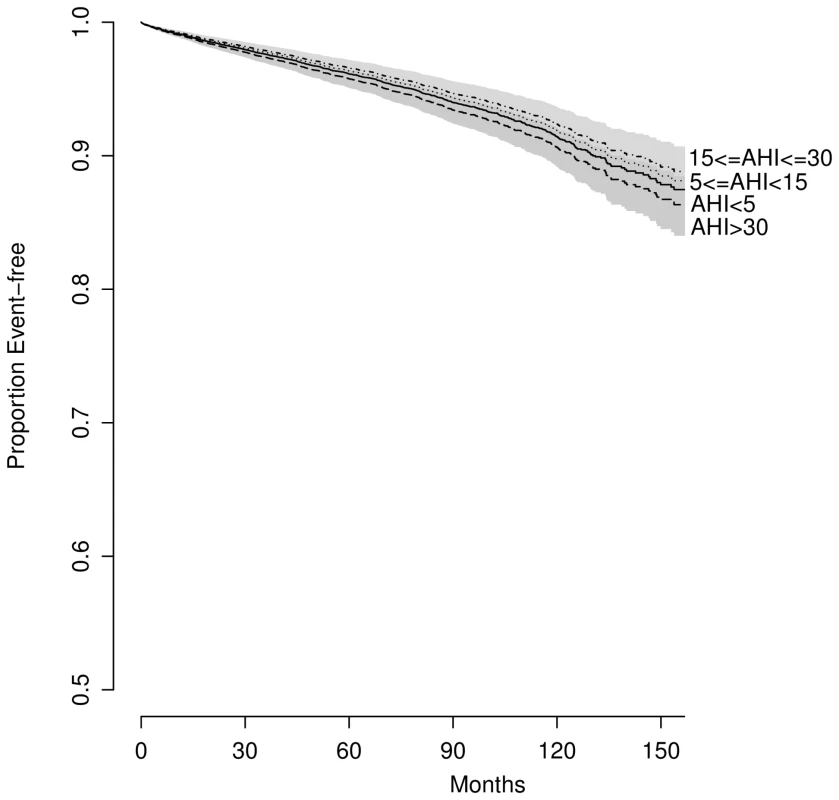
(BMI = 29, age = 50, sex = men, never smoked, without prior hypertension, diabetes, MI, stroke, or heart failure). Multivariable Cox Regression Models
In the fully adjusted model, the following OSA-related predictors were significantly associated with occurrence of a composite outcome: TST90SaO2, TST, awakenings, periodic leg movements, mean heart rate, and presence of unintentional DS (Figures 4 and 5; Table 2). AHI was no longer a significant predictor.
Fig. 4. Results from multivariable Cox regression model presented as hazard ratios with shading representing confidence levels (99%, 95%, 90%, 80%, and 70%). 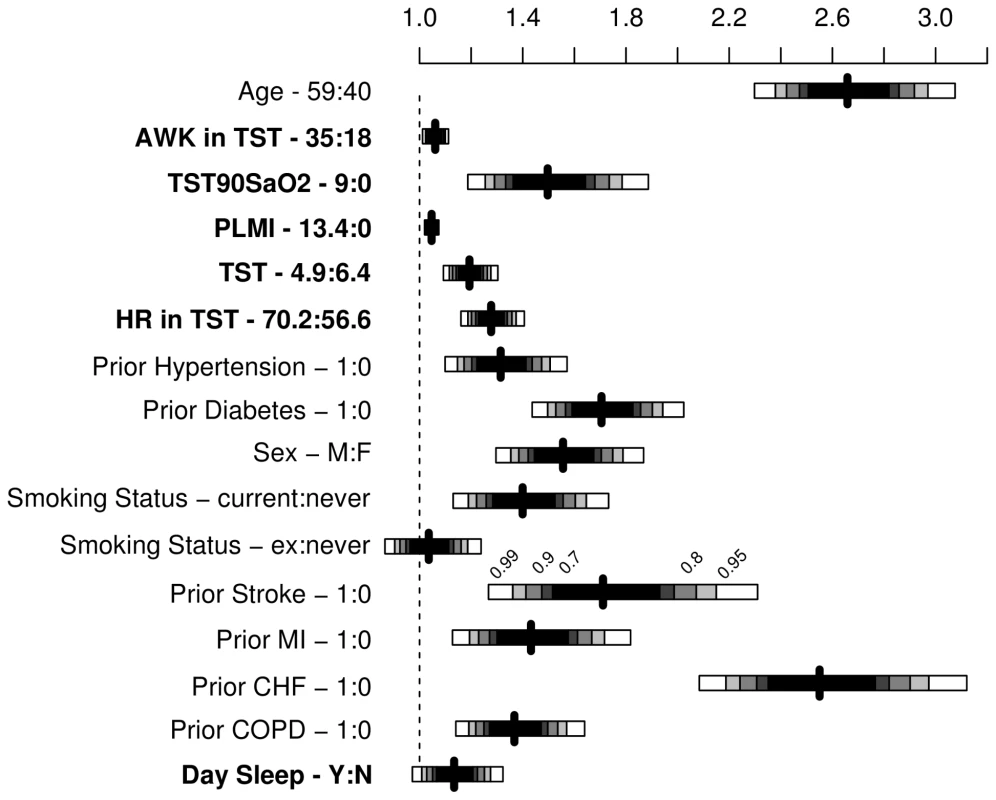
AWK, number of awakenings in TST; TST90SaO2, sleep time spent with SaO2 less than 90%; PLMI, periodic leg movement index; HR, mean heart rate during sleep; day sleep, DS, identified by a positive answer to the question “During the day, do you ever fall asleep unintentionally?”. Fig. 5. Predicted survival curves to show the effect of oxygen saturation 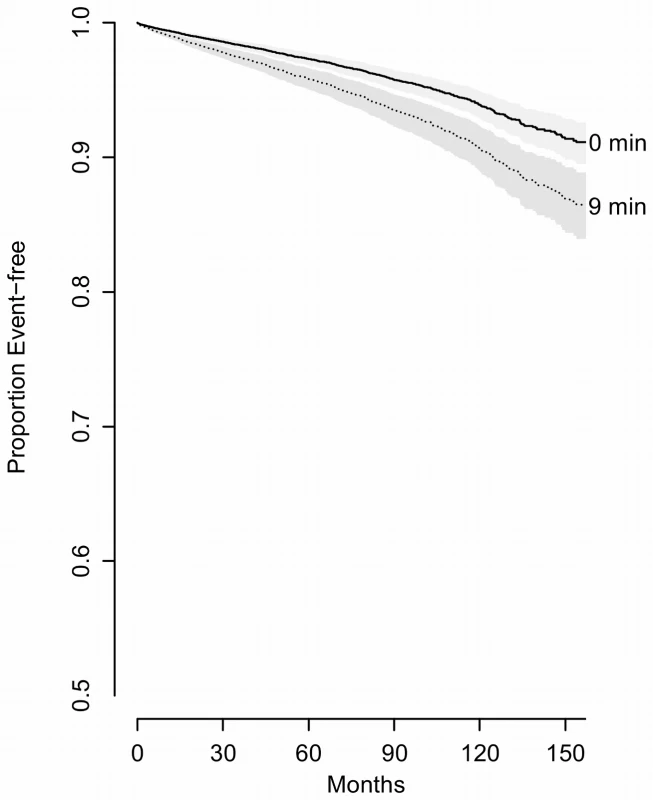
(comparing 75th percentile [9 min] to 25th percentile [0 min]) controlling for potential confounders (BMI = 29, age = 50, sex = men, never smoked, without prior hypertension, diabetes, MI, stroke or heart failure, TST = 5.8, AWK = 25, PLMI, 1.2, mean heart rate, 63, without reporting excessive DS). Tab. 2. Model fitting and effect of predictors (n = 10,149, events = 1,172). 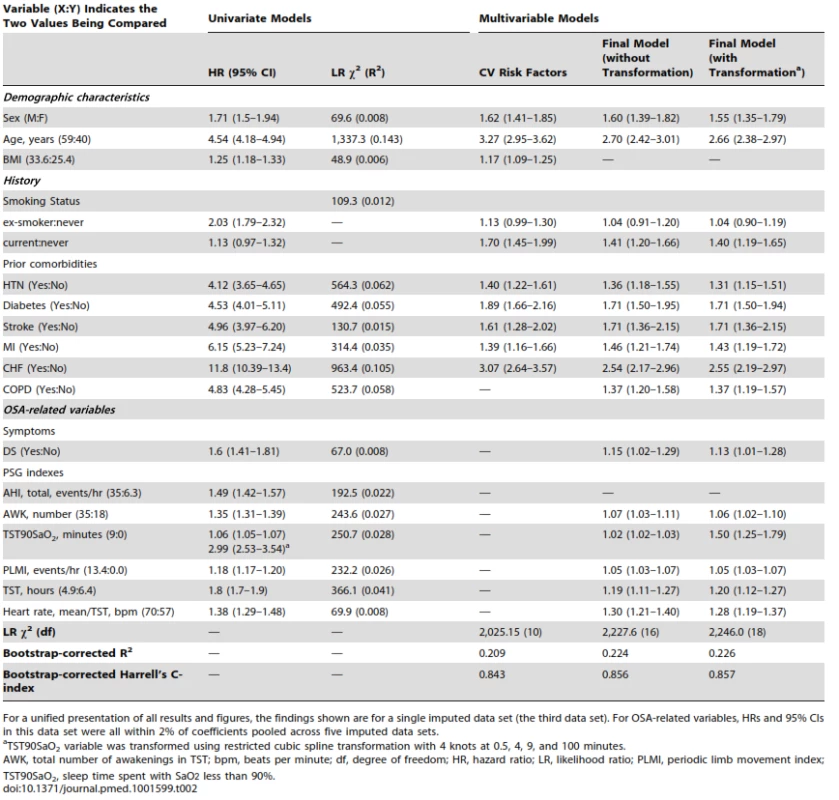
For a unified presentation of all results and figures, the findings shown are for a single imputed data set (the third data set). For OSA-related variables, HRs and 95% CIs in this data set were all within 2% of coefficients pooled across five imputed data sets. All models were well calibrated (all observed and predicted five-year survival within 5%) (Figure S2) and validated (optimism<0.004 for final model R2). The fit for the model with only traditional CV risk factors was statistically significantly worse than the final model (χ2 (8) = 220; p<0.0001), indicating that OSA-related predictors independently contribute to the development of the composite outcome.
Nomogram
Clinical nomograms estimating median survival time and the probability of 3 and 5 years event-free survival were based on the final transformed model (Figure 6). Variables with the widest point range in the nomogram provide the greatest discrimination. The points assigned to each predictor are presented in Table S3. To estimate the role of OSA-related variables using the nomogram, as an example we compared two different patients (Table 3). First, the total number of points was calculated for a relatively healthy person: male, 50 years old without signs of OSA and without comorbidities, never smoked, with mean heart rate of 70, and 7 hours of sleep. The total number of points for such a person is 75, indicating 5-year event-free survival of 0.95–0.99. The total number of points for a person with the same characteristics except with moderate-severe OSA is 99, indicating 5-year event-free survival of 0.95. The 24-point difference between the first and second person due to OSA-related predictors leads to a relatively small difference in 5-year event-free survival. However, the total number of points for a person of the same age, gender, and sleep time with comorbid illnesses, who smoked, and had a mean heart rate of 80 is 150, indicating 5-year event-free survival of 0.4–0.5 and median survival time of 4 years. For this person, a 24-point difference (attributed to OSA-related predictors) gives a total of 174, which corresponds to a 5-year event-free survival of about 0.1 and median survival time between 1 and 2 years, a clinically significant decrease.
Fig. 6. Clinical nomogram for obstructive sleep apnea patients. 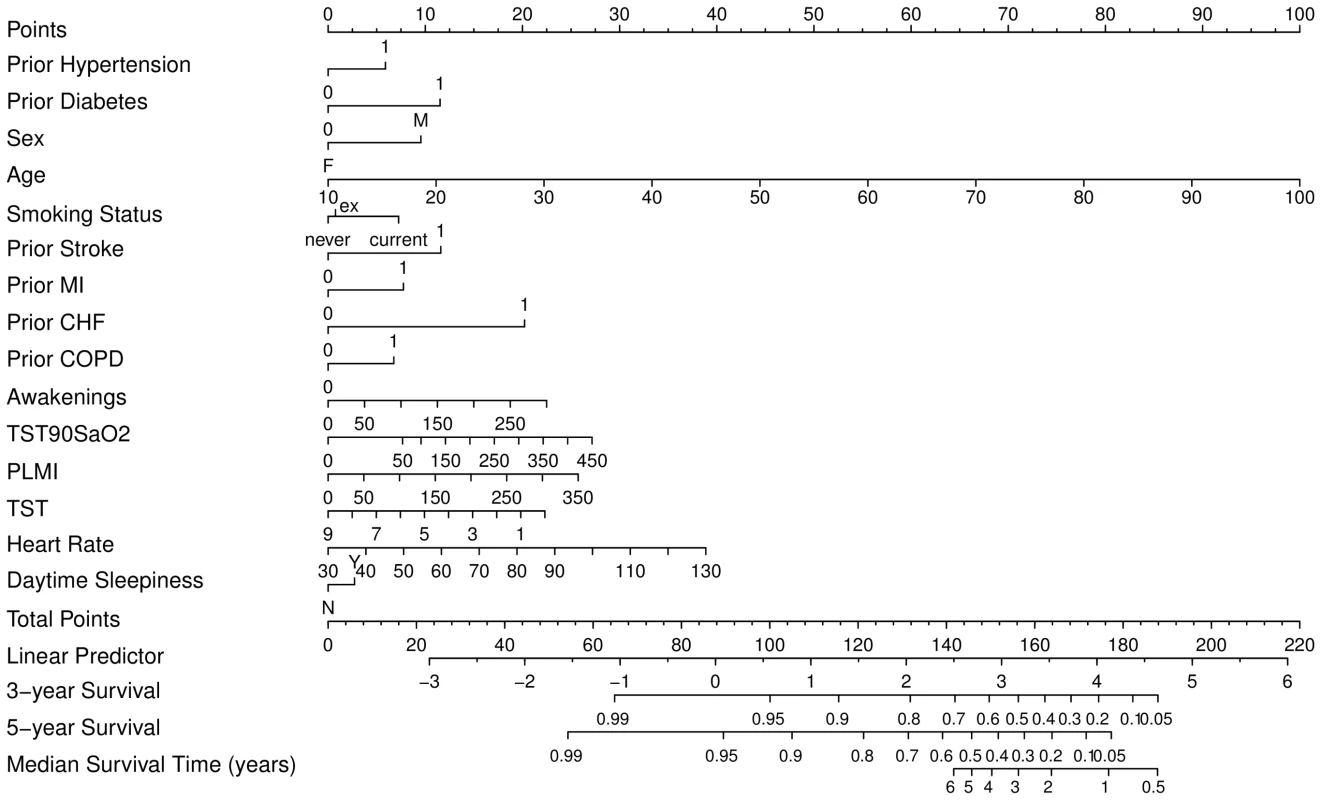
To obtain the nomogram predicted probability of three- and five-year event-free survival and to estimate median event-free survival, locate patient values at each axis, then draw a vertical line to the “Point” scale (axis) to determine how many points are attributed for each predictor. Sum the points for all predictors. Locate the sum on the “Total Points” scale. Draw a vertical line towards the “3-year Survival,” “5-year Survival,” and “Median Survival Time” axes to determine the three-year composite CV outcome-free survival, the five-year event-free survival, and to estimate median survival respectively. PLMI, periodic limb movement index; TST90SaO2, sleep time spent with SaO2 less than 90%. Tab. 3. Example of clinical nomogram (point system) usage. 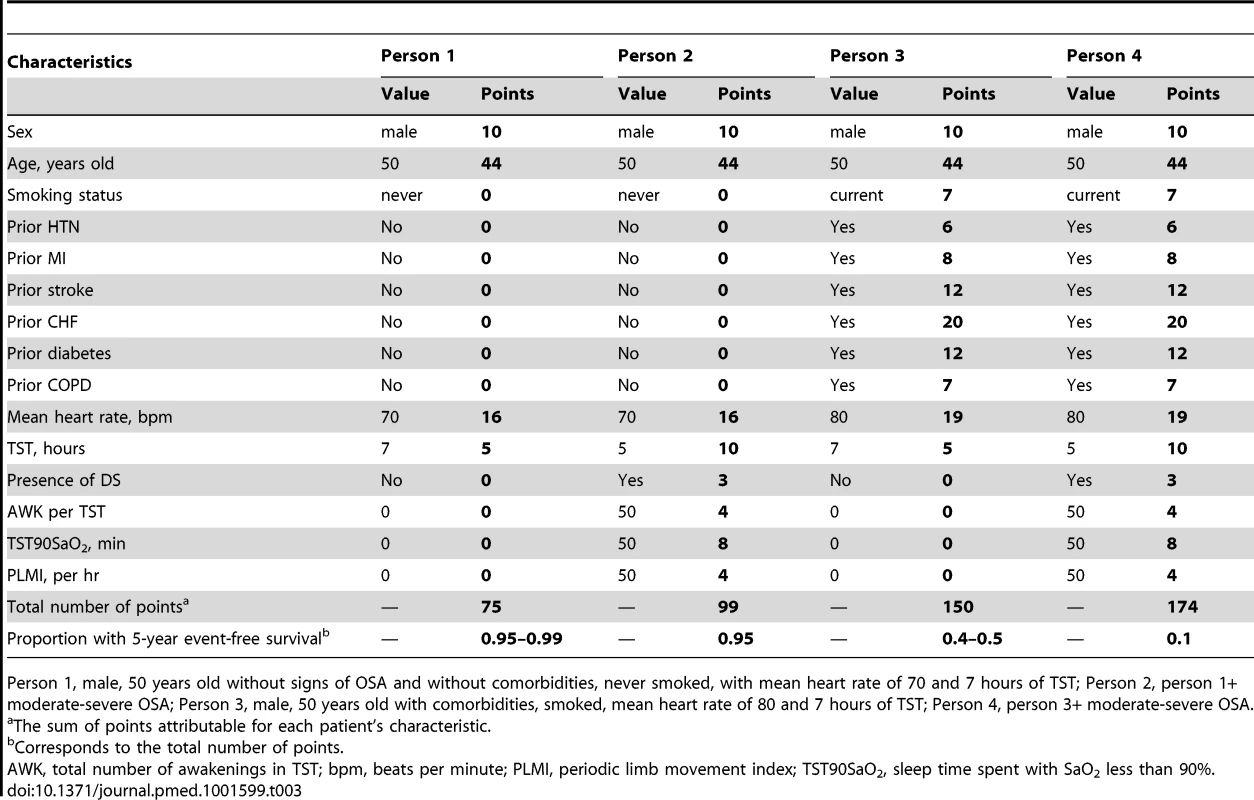
Person 1, male, 50 years old without signs of OSA and without comorbidities, never smoked, with mean heart rate of 70 and 7 hours of TST; Person 2, person 1+ moderate-severe OSA; Person 3, male, 50 years old with comorbidities, smoked, mean heart rate of 80 and 7 hours of TST; Person 4, person 3+ moderate-severe OSA. Separate Components of Composite CV Outcome
When the final model was applied to each component event, OSA-related variables were predictive of all-cause mortality (762 events), hospitalization for CHF (414 events), and stroke (100 events), but not for MI (145 events) (Table 4).
Tab. 4. Final model re-fitted for separate components of cardiovascular composite outcome (n = 10,149). 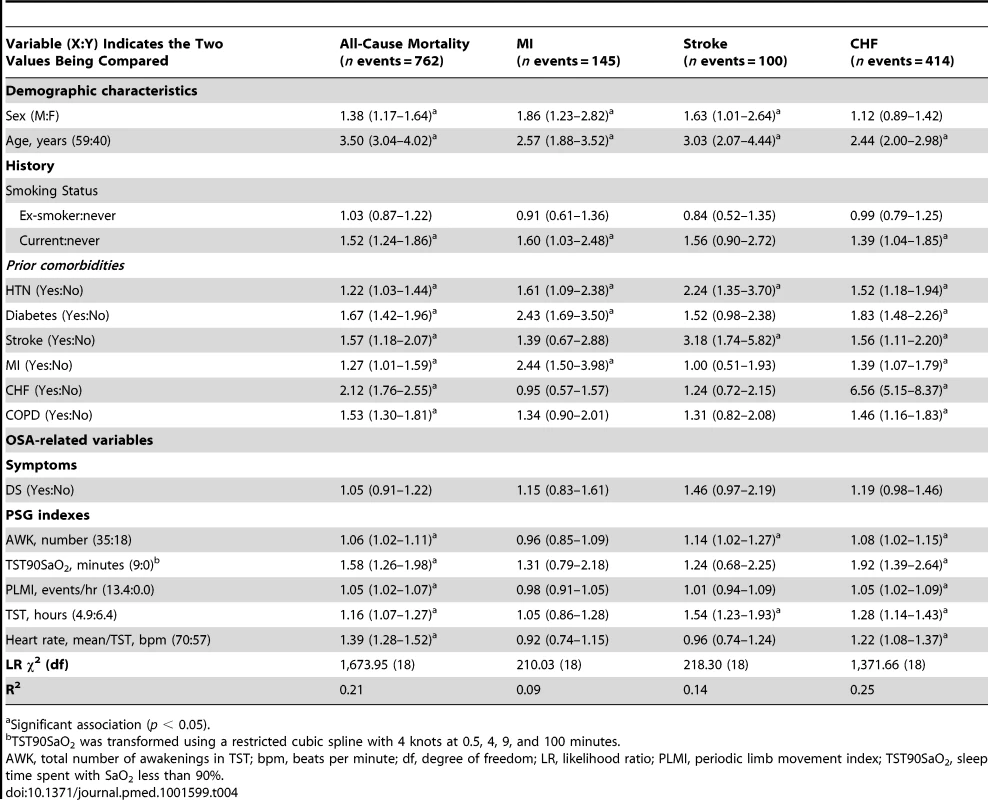
Significant association (p < 0.05). Interactions
The association between TST90SaO2 (9 versus 0 minutes) and the composite outcome was significantly (p = 0.04) stronger for females (HR = 2.21, 95% CI 1.56–3.11) than for males (HR = 1.29, 95% CI 1.05–1.57).
CPAP Treatment
Among 4,733 individuals who underwent a diagnostic sleep study between 2004 and 2010, 762 (16%) submitted a CPAP claim and 333 (7%) experienced a composite event. In our final model, a CPAP claim was not associated with risk of an event (HR = 0.85, 95% CI 0.64–1.14, p = 0.3). To assess the final model on an untreated sample, patients were censored at the time of a CPAP claim; all predictors except DS remained significantly associated with outcome (Table 5).
Tab. 5. Association between OSA-related variables and the composite CV outcome on untreated subsample versus entire cohort. 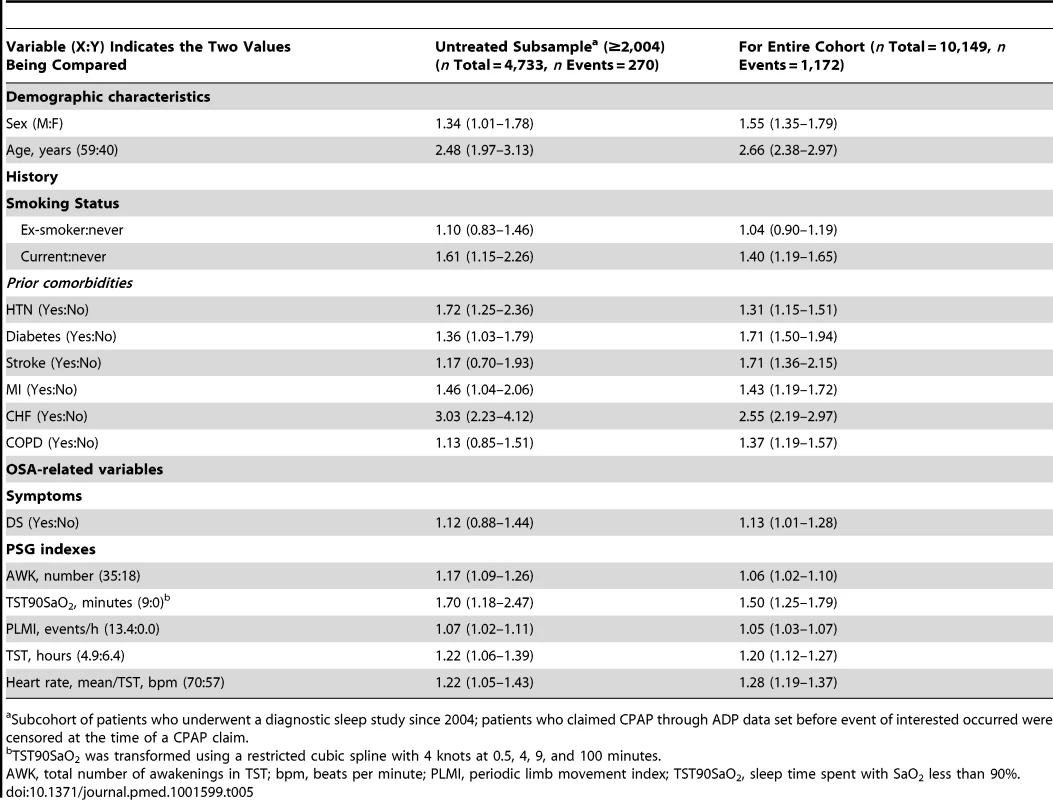
Subcohort of patients who underwent a diagnostic sleep study since 2004; patients who claimed CPAP through ADP data set before event of interested occurred were censored at the time of a CPAP claim. Discussion
In a large clinical cohort, 11.5% of individuals experienced a composite CV outcome over a median 68 months of follow-up. Our incidence rate of 2 per 100 person-years was similar to rates reported in other clinically-based sleep studies [27],[28]. While AHI was found to predict CV events in a univariate analysis, there was no significant association in a multivariable model adjusted for potential confounders. In contrast, the multivariable models identified other OSA-related factors as independent and significant predictors of the occurrence of CV events and all-cause mortality. The results obtained were driven by all-cause mortality, hospitalized heart failure and stroke, and were replicated on a subsample of untreated patients. Using a nomogram, we demonstrated that OSA-related variables independently contribute to predicted three - and five-year event-free survival and median survival time, over and above traditional risk factors. Though the difference attributed to OSA-related predictors for a relatively healthy person seems small, for individuals with high baseline risk, even a small increase in risk due to OSA will place a patient in considerably higher risk group.
The significant association between AHI and the composite CV outcome in our univariate analyses is consistent with findings of other studies. However, the persistent significance of this association in multivariable models in other studies, but not in ours, could be explained by methodological differences between our study and other studies. For example, large, community-based studies may not include potentially more important OSA-related predictors or selectively report findings from subgroup analyses [13].
Variations in the definition of hypopnea may be important. We used a definition of hypopnea that does not demand the occurrence of oxygen desaturation. The ability of AHI to predict CV disease was found to improve if 3% or 4% oxygen desaturation is required for the scoring of hypopneas [29], indicating the probable importance of hypoxia in mediating CV risk from OSA and raising the question of whether a more direct measure of hypoxia might be more predictive than AHI.
In fact, the strongest OSA-related predictor of CV events in our study was the sleep time spent with SaO2<90%. The association between TST90SaO2 and the outcome of interest remains significant for both sexes, and across a spectrum of age, BMI, DS, and comorbid CV diseases. Our finding that a measure of hypoxia predicts CV risk is consistent with emerging evidence in both animal models and humans that intermittent hypoxia may be a crucial mechanistic link whereby OSA causes oxidative stress, metabolic derangement, and endothelial damage [30],[31].
The remainder of the OSA-related variables predictive of CV risk were mainly reflective of either sleep fragmentation or sympathetic activity. Increases in the number of awakenings or periodic leg movements or decreases in TST may represent the direct effects of OSA on sleep fragmentation and deprivation that can increase risk by inducing endothelial dysfunction and sympathetic activation [32]. Despite limited evidence elsewhere on the association between the number of awakenings and CV events, the evidence is growing regarding the relationship of PLM to CV events [7],[33],[34]. Low TST may indicate insomnia, which by itself can lead to increased risk of all-cause mortality and development of CV events [35]. However, in patients with chronic insomnia, most awakenings may be caused by sleep-disordered breathing [36]. Many of the CV consequences of OSA may be mediated by activation of sympathetic activity, and represented by increased heart rate [5]. Finally, available data suggest an association between mortality (all-cause and from CV disease) and excessive DS [37].
Despite strong evidence from randomized controlled trials of a causal relationship between OSA and markers of risk of CV events, it remains to be proven whether OSA-induced HTN translates into increased morbidity and mortality [38]. A recent randomized controlled trial found that in patients with OSA without DS, the prescription of CPAP did not result in a statistically significant reduction in the incidence of a combined outcome of HTN or CV events, compared with usual care [39]. In the same trial, OSA severity, assessed by the AHI and sleep time spent with SaO2 less than 90%, was not related to the combined outcome. However, in a post hoc analysis, patients with worse oxygen desaturation at night and with CPAP adherence of less than four hours per night showed a higher rate of HTN or CV events than the control group.
Although evidence has been inconsistent on the association between OSA-related predictors and CV events in females and elderly individuals [7], we found that the significant association extended to these groups, in part due to the composition of our cohort. Our cohort included a large number of women over 50 years of age, who we assume are postmenopausal and therefore at increased risk of CV events [40],[41].
Our research addresses many limitations of previously published observational studies. Our study includes over 10,000 patients from a clinically-based sleep cohort and consequently has a large number of events, allowing us to assess many variables in the model and control for all available confounders. Data were consistently collected and used the same PSG scoring criteria over time. We included patients with a wide range of OSA severity and a relatively large number of females. We matched a high percentage of patients (89%) to administrative data, had long and complete follow-up through health administrative data, used validated algorithms to define CV outcomes and comorbidities at baseline, and, finally, used rigorous methods for missing data, model selection, calibration, and validation. The format of the nomogram used as a predictive tool allows predictions based on any combination of patient characteristics, not only from categorical, but also from continuous (possibly transformed) variables and interactions between variables. The nomogram allows the use of a complicated model in clinical prediction, improving accuracy of predictions over simpler points-based scores that require categorization of risk using arbitrary categories [42],[43].
As with any observational study, there are limitations related to availability of data. Some important confounders (e.g., level of cholesterol, race, treatment of hypertension) were not available. However, our model with CV risk factors had high predictive and discriminative ability, reflecting that the majority of important predictors were included. Assessing the sensitivity of obtained results to unmeasured confounders using approach recommended by Lin and colleagues [44], we found that unmeasured confounders (e.g., treatment for hypertension) should be really strong (i.e., increased both the hazard of composite CV outcome and the probability of severe SaO2 [75th percentile] 4-fold) to change the association between OSA and time spent with SaO2 less than 90% to non-significant. Our findings are based on patients referred to a single centre, which may reduce the generalizability of our findings. Validation of our results in other patient populations is recommended. Furthermore, there is no accepted definition of intermittent hypoxemia that includes both SaO2 variability and severity. Although oxygen desaturation index (ODI), the hourly average number of desaturation episodes, could be a useful predictor, it was not available in our study. However, while ODI reflects frequency of oxygen desaturation, time spent with SaO2<90% reflects its severity. The non-significant relationship between treatment and development of composite CV outcome that we found in the post-2004 cohort could be explained by lack of information about CPAP adherence, treatment approaches other than CPAP, and reduced power in this analysis. Moreover, the association between CPAP treatment and CV events can be attenuated because some patients were treated unnecessarily. Finally, it is naive to assume that all predictor levels will remain constant during the follow-up time [45]. Their trajectories (e.g., weight gain, OSA progression over time) were not known for our patients and not assessable at baseline when risk prediction was made.
Though we have developed a nomogram for prediction to show how the baseline predictors relate to 3 - and 5-year outcomes, we cannot strongly recommend its use in a clinical setting before it has undergone external validation. Rather, we used the nomogram to show the strength of the various factors on absolute risks of the composite CV outcome. Further, although the increments observed in c-statistics between final model and CV risk factors were small (0.857–0.843 = 0.014), it has been shown that the increase in c-statistics is very small when the baseline model's c-index is large: “good models are harder to improve upon” [46],[47].
We showed that OSA-related factors other than AHI are important predictors of the composite CV outcome. We believe a revision of the operative definition of OSA may be necessary, to reflect not simply the frequency of apneas and hypopneas, but the actual physiologic consequences that result—the severity of oxygen desaturation, sleep fragmentation, sleep deprivation, and sympathetic activation. It is these “downstream” phenomena that we have found to be more predictive of CV risk. The OSA-related predictors identified in our study could be collected using more limited recordings than PSG, potentially in the home setting.
Conclusions
AHI was significantly associated with a composite CV outcome in univariate analyses; however, this association became non-significant after controlling for potential confounders. Other OSA-related predictors, such as sleep time spent with SaO2 less than 90%, the number of awakenings, mean heart rate, TST, or presence of excessive DS, were significantly and independently associated with a 5% to 50% increased risk of development of the composite CV outcome even after controlling for known CV risk factors.
Supporting Information
Zdroje
1. PeppardPE, YoungT, BarnetJH, PaltaM, HagenEW, et al. (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol In press.
2. EpsteinLJ, KristoD, StrolloPJJr, FriedmanN, MalhotraA, et al. (2009) Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 5 : 263–276.
3. LeungRS, ComondoreVR, RyanCM, StevensD (2012) Mechanisms of sleep-disordered breathing: causes and consequences. Pflugers Arch 463 : 213–230.
4. BradleyTD, FlorasJS (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373 : 82–93.
5. KohlerM, StradlingJR (2010) Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol 7 : 677–685.
6. ChamiHA, FontesJD, VasanRS, KeaneyJFJr, O'ConnorGT, et al. (2013) Vascular inflammation and sleep disordered breathing in a community-based cohort. Sleep 36 : 763–768C.
7. KendzerskaT, MollayevaT, GershonAS, LeungRS, HawkerG, et al. (2014) Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: a systematic review. Sleep Med Rev 18 : 49–59.
8. Polysomnography in patients with obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser 6 : 1–38.
9. EdwardsBA, WellmanA, OwensRL (2013) PSGs: more than just the AHI. J Clin Sleep Med 9 : 527–528.
10. EckertDJ, JordanAS, MalhotraA, WhiteDP, WellmanA (2013) Defining phenotypic causes of obstructive sleep apnea: Identification of novel therapeutic targets. Am J Respir Crit Care Med 188 : 996–1004.
11. PunjabiNM, CaffoBS, GoodwinJL, GottliebDJ, NewmanAB, et al. (2009) Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 6: e1000132.
12. YoungT, FinnL, PeppardPE, Szklo-CoxeM, AustinD, et al. (2008) Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 31 : 1071–1078.
13. GottliebDJ, YenokyanG, NewmanAB, O'ConnorGT, PunjabiNM, et al. (2010) Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 122 : 352–360.
14. RedlineS, YenokyanG, GottliebDJ, ShaharE, O'ConnorGT, et al. (2010) Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 182 : 269–277.
15. (2005) Improving health care data in Ontario. ICES investigative report Toronto: Institute for Clinical Evaluative Sciences.
16. Policies and procedures manual for the assistive devices program. Ministry of Health and Long-Term Care 1–121.
17. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22 : 667–689.
18. FleethamJ, AyasN, BradleyD, FergusonK, FitzpatrickM, et al. (2006) Canadian Thoracic Society guidelines: diagnosis and treatment of sleep disordered breathing in adults. Can Respir J 13 : 387–392.
19. Harrell FE (2001) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer. xxii, 568 .
20. GrambschP, TherneauT (1994) Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81 : 515–526.
21. AzurMJ, StuartEA, FrangakisC, LeafPJ (2011) Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 20 : 40–49.
22. Van BuurenS, Groothuis-OudshoornK (2011) MICE: multivariate imputation by chained equations in R. Journal of Statistical Software 45 : 1–67.
23. Rubin DB (1987) Multiple imputation for nonresponse in surveys. New York: Wiley. xxix, 258 p.
24. AtkinsonAC (1980) A note on the generalized information criterion for choice of a model. Biometrika 67 : 413–418.
25. ChiaYC (2011) Review of tools of cardiovascular disease risk stratification: interpretation, customisation and application in clinical practice. Singapore Med J 52 : 116–123.
26. Harrell FE (2013) RMS: regression modeling strategies, R package verson 4.0-0. http://CRANR-projectorg[mgn]package = rms.
27. MarinJM, CarrizoSJ, VicenteE, AgustiAG (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365 : 1046–1053.
28. BuchnerNJ, SannerBM, BorgelJ, RumpLC (2007) Continuous positive airway pressure treatment of mild to moderate obstructive sleep apnea reduces cardiovascular risk. Am J Respir Crit Care Med 176 : 1274–1280.
29. PunjabiNM, NewmanAB, YoungTB, ResnickHE, SandersMH (2008) Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med 177 : 1150–1155.
30. JunJ, ReinkeC, BedjaD, BerkowitzD, Bevans-FontiS, et al. (2010) Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 209 : 381–386.
31. FosterGE, HanlyPJ, AhmedSB, BeaudinAE, PialouxV, et al. (2010) Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension 56 : 369–377.
32. AtkesonA, JelicS (2008) Mechanisms of endothelial dysfunction in obstructive sleep apnea. Vasc Health Risk Manag 4 : 1327–1335.
33. KooBB, BlackwellT, Ancoli-IsraelS, StoneKL, StefanickML, et al. (2011) Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation 124 : 1223–1231.
34. WaltersAS, RyeDB (2010) Evidence continues to mount on the relationship of restless legs syndrome/periodic limb movements in sleep to hypertension, cardiovascular disease, and stroke. Sleep 33 : 287.
35. VgontzasAN, LiaoD, PejovicS, CalhounS, KaratarakiM, et al. (2010) Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep 33 : 1159–1164.
36. KrakowB, RomeroE, UlibarriVA, KiktaS (2012) Prospective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: a pilot study of subjective and objective causes. Sleep 35 : 1685–1692.
37. Kendzerska T, Shapiro C. (2013) Associations and consequences of hypersomnias: morbidity and mortality. Kushida CA, editor. The encyclopedia of sleep. Waltham (Massachusetts): Academic Press 2: : 460–468.
38. GottliebDJ, CraigSE, Lorenzi-FilhoG, HeeleyE, RedlineS, et al. (2013) Sleep apnea cardiovascular clinical trials – current status and steps forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep 36 : 975–980.
39. BarbeF, Duran-CantollaJ, Sanchez-de-la-TorreM, Martinez-AlonsoM, CarmonaC, et al. (2012) Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 307 : 2161–2168.
40. YoungT, FinnL, AustinD, PetersonA (2003) Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 167 : 1181–1185.
41. PialouxV, BrownAD, LeighR, FriedenreichCM, PoulinMJ (2009) Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension 54 : 1014–1020.
42. EasthamJA, KattanMW, ScardinoPT (2002) Nomograms as predictive models. Semin Urol Oncol 20 : 108–115.
43. IasonosA, SchragD, RajGV, PanageasKS (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26 : 1364–1370.
44. LinDY, PsatyBM, KronmalRA (1998) Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 54 : 948–963.
45. HandDJ (2006) Classifier technology and the illusion of progress. Stat Sci 21 : 1–14.
46. TzoulakiI, LiberopoulosG, IoannidisJP (2009) Assessment of claims of improved prediction beyond the Framingham risk score. JAMA 302 : 2345–2352.
47. PencinaMJ, D'AgostinoRB, VasanRS (2010) Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 48 : 1703–1711.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 2- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Data Access for the Open Access Literature: PLOS's Data Policy
- Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap
- Effect of Water, Sanitation, and Hygiene on the Prevention of Trachoma: A Systematic Review and Meta-Analysis
- Biomarker Profiling by Nuclear Magnetic Resonance Spectroscopy for the Prediction of All-Cause Mortality: An Observational Study of 17,345 Persons
- Physicians as Fundraisers: Medical Philanthropy and the Doctor-Patient Relationship
- Molecular Diagnostics for Gonorrhoea: Implications for Antimicrobial Resistance and the Threat of Untreatable Gonorrhoea
- Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis
- Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study
- Quantitative Measurement of Melanoma Spread in Sentinel Lymph Nodes and Survival
- Effect of an Educational Toolkit on Quality of Care: A Pragmatic Cluster Randomized Trial
- Patterns of Obesity Development before the Diagnosis of Type 2 Diabetes: The Whitehall II Cohort Study
- Long-Term Survival and Dialysis Dependency Following Acute Kidney Injury in Intensive Care: Extended Follow-up of a Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Obstructive Sleep Apnea and Risk of Cardiovascular Events and All-Cause Mortality: A Decade-Long Historical Cohort Study
- Living Systematic Reviews: An Emerging Opportunity to Narrow the Evidence-Practice Gap
- Quantitative Measurement of Melanoma Spread in Sentinel Lymph Nodes and Survival
- Physicians as Fundraisers: Medical Philanthropy and the Doctor-Patient Relationship
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



